User login
Addressing anxiety helps youth with functional abdominal pain disorders
MILWAUKEE – A stepped-care approach to youth with functional abdominal pain disorders may be effective in targeting those with comorbid anxiety, according to ongoing research.
A study of 79 pediatric patients with a functional abdominal pain disorder (FAPD) and co-occurring anxiety found that those who received cognitive behavioral therapy (CBT) that included a component to address anxiety had less functional disability and anxiety than those who received treatment as usual. Pain scores also dropped, though the difference was not statistically significant.
The patients, aged 9-14 years and mostly white and female, were randomized to treatment allocation. Functional disability scores were significantly lower post-treatment for those who received the stepped therapy compared with the treatment as usual group (P less than .05, Cohen’s D = .49). This indicates a moderate effect size, said Natoshia Cunningham, PhD, speaking at the scientific meeting of the American Pain Society.
Mean scores on an anxiety rating scale also dropped below the threshold for clinical anxiety for those receiving the stepped therapy; on average, the treatment as usual group still scored above the clinical anxiety threshold after treatment (P for difference = .05).
The study, part of ongoing research, tests a hybrid online intervention, dubbed Aim to Decrease Anxiety and Pain Treatment, or ADAPT. The ADAPT program includes some common elements of CBT for anxiety that were not previously included in the pediatric pain CBT in use for the FAPD patients, she said.
The hybrid program began with two in-person sessions, each lasting one hour. These were followed by up to four web-based sessions. Patients viewed videos, read some material online, and complete activities with follow-up assessments. The web-based component was structured so that providers can see how patients fare on assessments – and even see which activities had been opened or completed. This, said Dr. Cunningham, allowed the treating provider to tailor what’s addressed in the associated weekly phone checks that accompany the online content.
Parents were also given practical, evidence-based advice to help manage their child’s FAPD. These include encouraging children to be independent in pain management, stopping “status checks,” encouraging normal school and social activities, and avoiding special privileges when pain interferes with activities.
Overall, up to 40% of pediatric functional abdominal pain patients may not respond to CBT, the most efficacious treatment known, said Dr. Cunningham, a pediatric psychologist at the University of Cincinnati. Her research indicates that comorbid anxiety may predict poor response, and that addressing anxiety improves pain and disability in this complex, common disorder.
With a brief psychosocial screening that identifies patients with anxiety, Dr. Cunningham and her colleagues can implement the targeted, partially web-based therapy strategy that tackles anxiety along with CBT for functional abdominal pain.
“Anxiety is common and related to poor outcomes,” noted Dr. Cunningham, She added that overall, half or more of individuals with chronic pain also have anxiety. Among children with FAPD, “Clinical anxiety predicts disability and poor treatment response.”
The first step, she said, was identifying the patients with FAPD who had anxiety, including those with subclinical anxiety.
At intake, children coming to the Cincinnati Children’s Hospital’s gastroenterology clinic complete anxiety screening via the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Depress Anxiety. 2000;12[2]:85-91). Disability and pain are assessed by the Functional Disability Inventory and the Numeric Rating Scale (J Pediatr Psychol. 1991 Feb;16[1]:39-58).
In earlier research, Dr. Cunningham and her collaborators found a significant association between anxiety and both higher pain levels and more disability. And, clinically significant anxiety was more likely among the FAPD patients with persistent disability after six months of treatment.
A surprising finding from the screenings, said Dr. Cunningham, is that youth endorsed more anxiety symptoms in self-assessment than their parents observed. “Children are often their own best informants of their internalizing symptoms,” she said. “Not only do their parents not notice it, it may not be obvious to their providers, either.”
Since many children with FAPD have anxiety, the next question was “How do we better enhance their treatments?” she continued. To answer that question, she took one step back: “How do these youth respond to our current best practice?”
Looking at Cincinnati Children’s patients with FAPD who did – or did not – have anxiety, Dr. Cunningham found that “those who have clinical levels of anxiety don’t respond as well to CBT.” Pain-directed therapy alone, she said, “is insufficient to treat these patients.”
Together with brief screening, stepped therapy delivered via ADAPT offers promise to boost the efficacy of FAPD treatment, perhaps even in a primary care setting, said Dr. Cunningham. She and her collaborators are continuing to study comorbid anxiety and pain in youth; current work is using functional magnetic resonance imaging to examine cognitive and affective changes in patients receiving the ADAPT intervention.
The study was funded by the American Pain Society Sharon S. Keller Chronic Pain Research Grant, Cincinnati Children’s Hospital, and the National Institutes of Health. Dr. Cunningham reported no relevant conflicts of interest.
koakes@mdedge.com
SOURCE: Cunningham N. et al. APS 2019.
MILWAUKEE – A stepped-care approach to youth with functional abdominal pain disorders may be effective in targeting those with comorbid anxiety, according to ongoing research.
A study of 79 pediatric patients with a functional abdominal pain disorder (FAPD) and co-occurring anxiety found that those who received cognitive behavioral therapy (CBT) that included a component to address anxiety had less functional disability and anxiety than those who received treatment as usual. Pain scores also dropped, though the difference was not statistically significant.
The patients, aged 9-14 years and mostly white and female, were randomized to treatment allocation. Functional disability scores were significantly lower post-treatment for those who received the stepped therapy compared with the treatment as usual group (P less than .05, Cohen’s D = .49). This indicates a moderate effect size, said Natoshia Cunningham, PhD, speaking at the scientific meeting of the American Pain Society.
Mean scores on an anxiety rating scale also dropped below the threshold for clinical anxiety for those receiving the stepped therapy; on average, the treatment as usual group still scored above the clinical anxiety threshold after treatment (P for difference = .05).
The study, part of ongoing research, tests a hybrid online intervention, dubbed Aim to Decrease Anxiety and Pain Treatment, or ADAPT. The ADAPT program includes some common elements of CBT for anxiety that were not previously included in the pediatric pain CBT in use for the FAPD patients, she said.
The hybrid program began with two in-person sessions, each lasting one hour. These were followed by up to four web-based sessions. Patients viewed videos, read some material online, and complete activities with follow-up assessments. The web-based component was structured so that providers can see how patients fare on assessments – and even see which activities had been opened or completed. This, said Dr. Cunningham, allowed the treating provider to tailor what’s addressed in the associated weekly phone checks that accompany the online content.
Parents were also given practical, evidence-based advice to help manage their child’s FAPD. These include encouraging children to be independent in pain management, stopping “status checks,” encouraging normal school and social activities, and avoiding special privileges when pain interferes with activities.
Overall, up to 40% of pediatric functional abdominal pain patients may not respond to CBT, the most efficacious treatment known, said Dr. Cunningham, a pediatric psychologist at the University of Cincinnati. Her research indicates that comorbid anxiety may predict poor response, and that addressing anxiety improves pain and disability in this complex, common disorder.
With a brief psychosocial screening that identifies patients with anxiety, Dr. Cunningham and her colleagues can implement the targeted, partially web-based therapy strategy that tackles anxiety along with CBT for functional abdominal pain.
“Anxiety is common and related to poor outcomes,” noted Dr. Cunningham, She added that overall, half or more of individuals with chronic pain also have anxiety. Among children with FAPD, “Clinical anxiety predicts disability and poor treatment response.”
The first step, she said, was identifying the patients with FAPD who had anxiety, including those with subclinical anxiety.
At intake, children coming to the Cincinnati Children’s Hospital’s gastroenterology clinic complete anxiety screening via the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Depress Anxiety. 2000;12[2]:85-91). Disability and pain are assessed by the Functional Disability Inventory and the Numeric Rating Scale (J Pediatr Psychol. 1991 Feb;16[1]:39-58).
In earlier research, Dr. Cunningham and her collaborators found a significant association between anxiety and both higher pain levels and more disability. And, clinically significant anxiety was more likely among the FAPD patients with persistent disability after six months of treatment.
A surprising finding from the screenings, said Dr. Cunningham, is that youth endorsed more anxiety symptoms in self-assessment than their parents observed. “Children are often their own best informants of their internalizing symptoms,” she said. “Not only do their parents not notice it, it may not be obvious to their providers, either.”
Since many children with FAPD have anxiety, the next question was “How do we better enhance their treatments?” she continued. To answer that question, she took one step back: “How do these youth respond to our current best practice?”
Looking at Cincinnati Children’s patients with FAPD who did – or did not – have anxiety, Dr. Cunningham found that “those who have clinical levels of anxiety don’t respond as well to CBT.” Pain-directed therapy alone, she said, “is insufficient to treat these patients.”
Together with brief screening, stepped therapy delivered via ADAPT offers promise to boost the efficacy of FAPD treatment, perhaps even in a primary care setting, said Dr. Cunningham. She and her collaborators are continuing to study comorbid anxiety and pain in youth; current work is using functional magnetic resonance imaging to examine cognitive and affective changes in patients receiving the ADAPT intervention.
The study was funded by the American Pain Society Sharon S. Keller Chronic Pain Research Grant, Cincinnati Children’s Hospital, and the National Institutes of Health. Dr. Cunningham reported no relevant conflicts of interest.
koakes@mdedge.com
SOURCE: Cunningham N. et al. APS 2019.
MILWAUKEE – A stepped-care approach to youth with functional abdominal pain disorders may be effective in targeting those with comorbid anxiety, according to ongoing research.
A study of 79 pediatric patients with a functional abdominal pain disorder (FAPD) and co-occurring anxiety found that those who received cognitive behavioral therapy (CBT) that included a component to address anxiety had less functional disability and anxiety than those who received treatment as usual. Pain scores also dropped, though the difference was not statistically significant.
The patients, aged 9-14 years and mostly white and female, were randomized to treatment allocation. Functional disability scores were significantly lower post-treatment for those who received the stepped therapy compared with the treatment as usual group (P less than .05, Cohen’s D = .49). This indicates a moderate effect size, said Natoshia Cunningham, PhD, speaking at the scientific meeting of the American Pain Society.
Mean scores on an anxiety rating scale also dropped below the threshold for clinical anxiety for those receiving the stepped therapy; on average, the treatment as usual group still scored above the clinical anxiety threshold after treatment (P for difference = .05).
The study, part of ongoing research, tests a hybrid online intervention, dubbed Aim to Decrease Anxiety and Pain Treatment, or ADAPT. The ADAPT program includes some common elements of CBT for anxiety that were not previously included in the pediatric pain CBT in use for the FAPD patients, she said.
The hybrid program began with two in-person sessions, each lasting one hour. These were followed by up to four web-based sessions. Patients viewed videos, read some material online, and complete activities with follow-up assessments. The web-based component was structured so that providers can see how patients fare on assessments – and even see which activities had been opened or completed. This, said Dr. Cunningham, allowed the treating provider to tailor what’s addressed in the associated weekly phone checks that accompany the online content.
Parents were also given practical, evidence-based advice to help manage their child’s FAPD. These include encouraging children to be independent in pain management, stopping “status checks,” encouraging normal school and social activities, and avoiding special privileges when pain interferes with activities.
Overall, up to 40% of pediatric functional abdominal pain patients may not respond to CBT, the most efficacious treatment known, said Dr. Cunningham, a pediatric psychologist at the University of Cincinnati. Her research indicates that comorbid anxiety may predict poor response, and that addressing anxiety improves pain and disability in this complex, common disorder.
With a brief psychosocial screening that identifies patients with anxiety, Dr. Cunningham and her colleagues can implement the targeted, partially web-based therapy strategy that tackles anxiety along with CBT for functional abdominal pain.
“Anxiety is common and related to poor outcomes,” noted Dr. Cunningham, She added that overall, half or more of individuals with chronic pain also have anxiety. Among children with FAPD, “Clinical anxiety predicts disability and poor treatment response.”
The first step, she said, was identifying the patients with FAPD who had anxiety, including those with subclinical anxiety.
At intake, children coming to the Cincinnati Children’s Hospital’s gastroenterology clinic complete anxiety screening via the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Depress Anxiety. 2000;12[2]:85-91). Disability and pain are assessed by the Functional Disability Inventory and the Numeric Rating Scale (J Pediatr Psychol. 1991 Feb;16[1]:39-58).
In earlier research, Dr. Cunningham and her collaborators found a significant association between anxiety and both higher pain levels and more disability. And, clinically significant anxiety was more likely among the FAPD patients with persistent disability after six months of treatment.
A surprising finding from the screenings, said Dr. Cunningham, is that youth endorsed more anxiety symptoms in self-assessment than their parents observed. “Children are often their own best informants of their internalizing symptoms,” she said. “Not only do their parents not notice it, it may not be obvious to their providers, either.”
Since many children with FAPD have anxiety, the next question was “How do we better enhance their treatments?” she continued. To answer that question, she took one step back: “How do these youth respond to our current best practice?”
Looking at Cincinnati Children’s patients with FAPD who did – or did not – have anxiety, Dr. Cunningham found that “those who have clinical levels of anxiety don’t respond as well to CBT.” Pain-directed therapy alone, she said, “is insufficient to treat these patients.”
Together with brief screening, stepped therapy delivered via ADAPT offers promise to boost the efficacy of FAPD treatment, perhaps even in a primary care setting, said Dr. Cunningham. She and her collaborators are continuing to study comorbid anxiety and pain in youth; current work is using functional magnetic resonance imaging to examine cognitive and affective changes in patients receiving the ADAPT intervention.
The study was funded by the American Pain Society Sharon S. Keller Chronic Pain Research Grant, Cincinnati Children’s Hospital, and the National Institutes of Health. Dr. Cunningham reported no relevant conflicts of interest.
koakes@mdedge.com
SOURCE: Cunningham N. et al. APS 2019.
REPORTING FROM APS 2019
International registry of laser surgery outcomes in the works
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
dbrunk@mdedge.com
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
dbrunk@mdedge.com
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
dbrunk@mdedge.com
REPORTING FROM ASLMS 2019
MedPAC to begin work on Part D redesign
WASHINGTON – The Medicare Payment Advisory Commission will begin work on developing a plan to overhaul the Medicare Part D program.

Commission members, after hearing about a pair of options related to affordability of specialty drugs and biologics, instructed staff to pursue the option that would essentially amount to an overhaul to the program.
Commission staff presented two options to the commission at a meeting on April 5. Gaining no traction was the easier of the two fixes that could be implemented rapidly – setting a maximum out-of-pocket limit on each specialty tier prescription. The staff used the lesser of a 33% coinsurance (which is generally calculated against the list price of the drug) or $200 for a 30-day supply as the limit.
That first option gained little traction with commission members, who instead gravitated to the option that would restructure the Part D prescription drug benefit to provide stronger formulary and pricing incentives.
As proposed, the second option would replace the coverage gap discount with a manufacturer “cap discount” and restructure the catastrophic benefit.
Commission staff members said that this would help provide stronger incentives for the use of generics, increase affordability for enrollees and the Medicare program, and provide stronger incentives for plans to manage spending. Staff members also said it could provide a disincentive for manufacturers to set high launch prices and/or increase prices rapidly.
The basic plan design would have 25% coinsurance after the deductible is met, up to a to-be-determined out-of-pocket threshold. This would be followed by catastrophic coverage, with details about who pays and how much still to be determined. Currently, the basic structure of the Part D program calls for plan members to pay a 5% coinsurance.
While speaking favorably about the concept of redesign, MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted that it might be a tough sell.
“The difficulty we are going to have, or Medicare will have, in moving to something like this is that people are now entrenched in the current [program],” she said. “As much as I like this, I think it’s daunting moving from the current system.”
She said that if there is a focus on getting the benefits right, it will be a much better approach that could result in lower drug prices.
MedPAC member Amy Bricker, vice president of supply chain strategy at Express Scripts, called for consideration of additional reform to the current program’s six protected classes of drugs, within which plans are required to cover all or substantially all products.
She also suggested providing Part D plans with the ability to exclude high-priced drug launches from their formularies, something they cannot do now.
“Having the ability to exclude a product at launch is the single biggest tool that a commercial plan has and manufacturers fear,” she said, noting that it gives plans leverage, especially if there is little or no competition to that product.
Ms. Bricker came out against the consensus of other members of the commission and supported the out-of-pocket cap in addition to the overhauled Part D proposal.
She noted that the background material provided to the commission offered a suggestion that coinsurance would provide pressure on manufacturers to lower list prices because of the difficulty it puts on beneficiaries. “It doesn’t,” she said. “You can get a headline in the Wall Street Journal. You can hear about these stories in pockets. But it does not impact the decisions of the manufacturer.”
MedPAC staff will be working in the coming year to work out the details of the restructured proposal.
WASHINGTON – The Medicare Payment Advisory Commission will begin work on developing a plan to overhaul the Medicare Part D program.

Commission members, after hearing about a pair of options related to affordability of specialty drugs and biologics, instructed staff to pursue the option that would essentially amount to an overhaul to the program.
Commission staff presented two options to the commission at a meeting on April 5. Gaining no traction was the easier of the two fixes that could be implemented rapidly – setting a maximum out-of-pocket limit on each specialty tier prescription. The staff used the lesser of a 33% coinsurance (which is generally calculated against the list price of the drug) or $200 for a 30-day supply as the limit.
That first option gained little traction with commission members, who instead gravitated to the option that would restructure the Part D prescription drug benefit to provide stronger formulary and pricing incentives.
As proposed, the second option would replace the coverage gap discount with a manufacturer “cap discount” and restructure the catastrophic benefit.
Commission staff members said that this would help provide stronger incentives for the use of generics, increase affordability for enrollees and the Medicare program, and provide stronger incentives for plans to manage spending. Staff members also said it could provide a disincentive for manufacturers to set high launch prices and/or increase prices rapidly.
The basic plan design would have 25% coinsurance after the deductible is met, up to a to-be-determined out-of-pocket threshold. This would be followed by catastrophic coverage, with details about who pays and how much still to be determined. Currently, the basic structure of the Part D program calls for plan members to pay a 5% coinsurance.
While speaking favorably about the concept of redesign, MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted that it might be a tough sell.
“The difficulty we are going to have, or Medicare will have, in moving to something like this is that people are now entrenched in the current [program],” she said. “As much as I like this, I think it’s daunting moving from the current system.”
She said that if there is a focus on getting the benefits right, it will be a much better approach that could result in lower drug prices.
MedPAC member Amy Bricker, vice president of supply chain strategy at Express Scripts, called for consideration of additional reform to the current program’s six protected classes of drugs, within which plans are required to cover all or substantially all products.
She also suggested providing Part D plans with the ability to exclude high-priced drug launches from their formularies, something they cannot do now.
“Having the ability to exclude a product at launch is the single biggest tool that a commercial plan has and manufacturers fear,” she said, noting that it gives plans leverage, especially if there is little or no competition to that product.
Ms. Bricker came out against the consensus of other members of the commission and supported the out-of-pocket cap in addition to the overhauled Part D proposal.
She noted that the background material provided to the commission offered a suggestion that coinsurance would provide pressure on manufacturers to lower list prices because of the difficulty it puts on beneficiaries. “It doesn’t,” she said. “You can get a headline in the Wall Street Journal. You can hear about these stories in pockets. But it does not impact the decisions of the manufacturer.”
MedPAC staff will be working in the coming year to work out the details of the restructured proposal.
WASHINGTON – The Medicare Payment Advisory Commission will begin work on developing a plan to overhaul the Medicare Part D program.

Commission members, after hearing about a pair of options related to affordability of specialty drugs and biologics, instructed staff to pursue the option that would essentially amount to an overhaul to the program.
Commission staff presented two options to the commission at a meeting on April 5. Gaining no traction was the easier of the two fixes that could be implemented rapidly – setting a maximum out-of-pocket limit on each specialty tier prescription. The staff used the lesser of a 33% coinsurance (which is generally calculated against the list price of the drug) or $200 for a 30-day supply as the limit.
That first option gained little traction with commission members, who instead gravitated to the option that would restructure the Part D prescription drug benefit to provide stronger formulary and pricing incentives.
As proposed, the second option would replace the coverage gap discount with a manufacturer “cap discount” and restructure the catastrophic benefit.
Commission staff members said that this would help provide stronger incentives for the use of generics, increase affordability for enrollees and the Medicare program, and provide stronger incentives for plans to manage spending. Staff members also said it could provide a disincentive for manufacturers to set high launch prices and/or increase prices rapidly.
The basic plan design would have 25% coinsurance after the deductible is met, up to a to-be-determined out-of-pocket threshold. This would be followed by catastrophic coverage, with details about who pays and how much still to be determined. Currently, the basic structure of the Part D program calls for plan members to pay a 5% coinsurance.
While speaking favorably about the concept of redesign, MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, noted that it might be a tough sell.
“The difficulty we are going to have, or Medicare will have, in moving to something like this is that people are now entrenched in the current [program],” she said. “As much as I like this, I think it’s daunting moving from the current system.”
She said that if there is a focus on getting the benefits right, it will be a much better approach that could result in lower drug prices.
MedPAC member Amy Bricker, vice president of supply chain strategy at Express Scripts, called for consideration of additional reform to the current program’s six protected classes of drugs, within which plans are required to cover all or substantially all products.
She also suggested providing Part D plans with the ability to exclude high-priced drug launches from their formularies, something they cannot do now.
“Having the ability to exclude a product at launch is the single biggest tool that a commercial plan has and manufacturers fear,” she said, noting that it gives plans leverage, especially if there is little or no competition to that product.
Ms. Bricker came out against the consensus of other members of the commission and supported the out-of-pocket cap in addition to the overhauled Part D proposal.
She noted that the background material provided to the commission offered a suggestion that coinsurance would provide pressure on manufacturers to lower list prices because of the difficulty it puts on beneficiaries. “It doesn’t,” she said. “You can get a headline in the Wall Street Journal. You can hear about these stories in pockets. But it does not impact the decisions of the manufacturer.”
MedPAC staff will be working in the coming year to work out the details of the restructured proposal.
REPORTING FROM MEDPAC
HM19: Lessons from the Update in Hospital Medicine session

In the second of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss more of their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala share practice-changing takeaways from the Update in Hospital Medicine session.

In the second of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss more of their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala share practice-changing takeaways from the Update in Hospital Medicine session.

In the second of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss more of their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala share practice-changing takeaways from the Update in Hospital Medicine session.
REPORTING FROM HM19
HM19: Key takeaways on quality and innovation

In the first of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala review key points from sessions on quality and patient safety, caring for the complex medically ill, using data analytics to drive clinical change, and the best studies from the Research and Innovation poster competition.

In the first of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala review key points from sessions on quality and patient safety, caring for the complex medically ill, using data analytics to drive clinical change, and the best studies from the Research and Innovation poster competition.

In the first of two episodes, Amith Skandhan, MD, FHM, of Southeast Alabama Medical Center, Dothan, Ala., and Raman Palabindala, MD, SFHM, of the University of Mississippi Medical Center, Jackson, Miss., discuss their favorite lessons from the annual meeting of the Society of Hospital Medicine. Dr. Skandhan and Dr. Palabindala review key points from sessions on quality and patient safety, caring for the complex medically ill, using data analytics to drive clinical change, and the best studies from the Research and Innovation poster competition.
REPORTING FROM HM19
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass

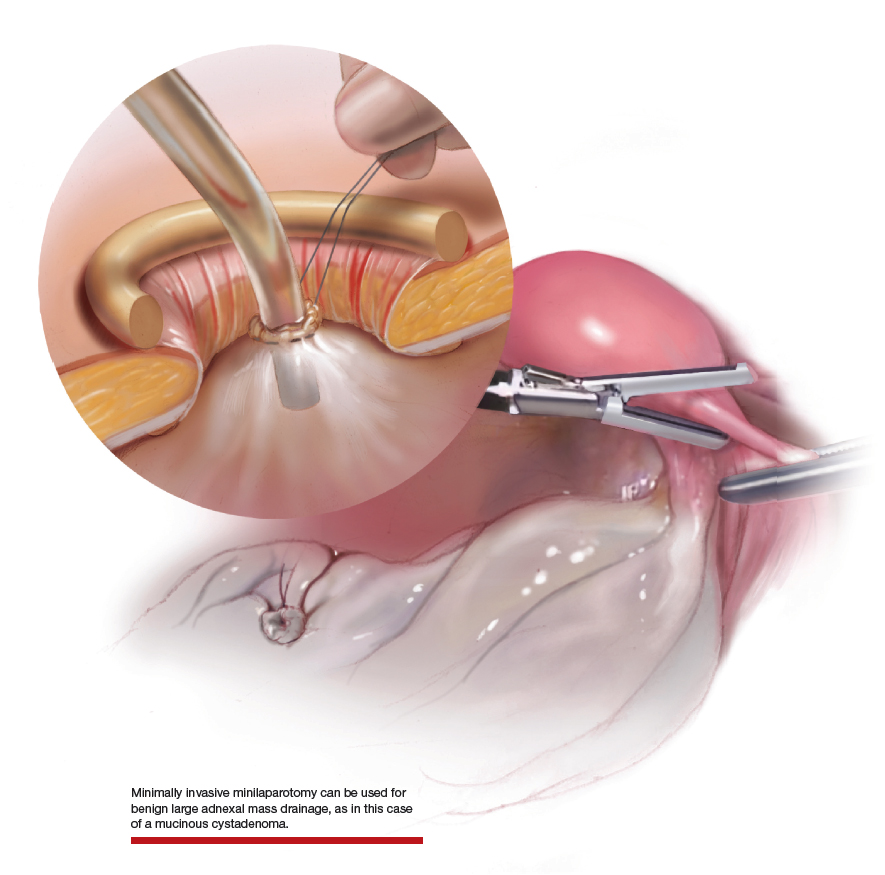
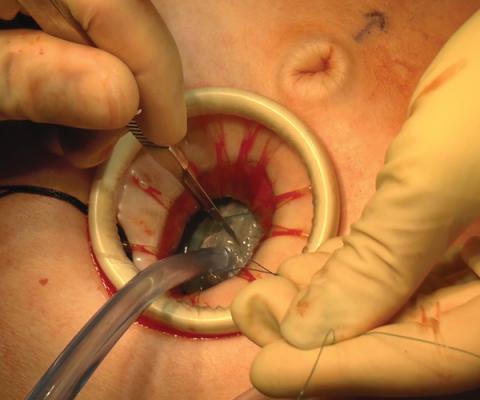
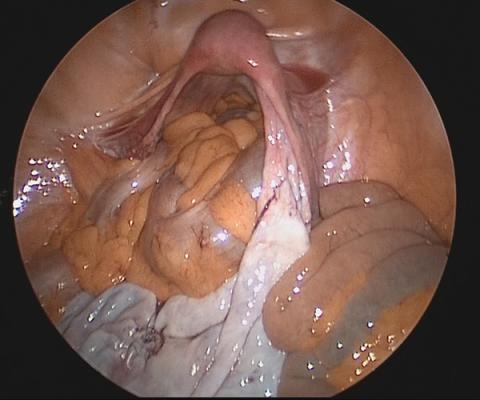
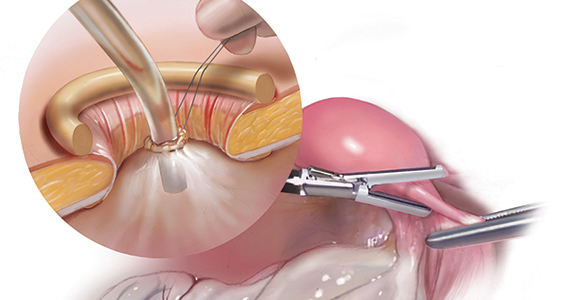
Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.


Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues


Large adnexal masses traditionally are removed surgically via laparotomy through a midline vertical incision to achieve adequate exposure and to avoid spillage of cyst contents. However, large laparotomies carry significant morbidity compared with minimally invasive techniques. Minilaparotomy is a minimally invasive approach that is associated with shorter operating times and lower estimated blood loss compared with laparoscopy in gynecologic surgery.1 The procedure also provides adequate exposure and can be used for carefully selected patients with a large adnexal mass.2,3 Preoperative assessment for the risk of malignancy typically includes an evaluation of risk factors, physical examination, imaging, and tumor markers.4
In this video, we illustrate a minimally invasive technique for the removal of a massively enlarged adnexal mass through laparoscopic bilateral salpingo-oophorectomy with minilaparotomy assistance. We conclude that this procedure is a safe and feasible option for women with a large benign adnexal mass, such as the highlighted patient whose final pathology resulted in a mucinous cystadenoma. Careful patient selection and preoperative assessment of malignancy risk is critical.5,6
We hope that you find this innovative approach useful in your clinical practice.
>> Dr. Arnold P. Advincula and colleagues
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.
- Kumar A, Pearl M. Mini-laparotomy versus laparoscopy for gynecologic conditions. J Minim Invasive Gynecol. 2014;21:109-114.
- Pelosi MA. A novel minilaparotomy approach for large ovarian cysts. OBG Manag. 2004;16(2):17-30.
- Rhode JM, Advincula AP, Reynolds RK, et al. A minimally invasive technique for management of the large adnexal mass. J Minim Invasive Gynecol. 2006;13:476-479.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins--Gynecology. Practice Bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210-e226.
- Roman LD, Muderspach LI, Stein SM, et al. Pelvic examination, tumor marker level, and gray-scale and Doppler sonography in the prediction of pelvic cancer. Obstet Gynecol. 1997;89:493-500.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012:126:157-166.
CAR T cells home in on HER2 in advanced sarcomas
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
ATLANTA – A novel chimeric antigen receptor (CAR) T-cell construct centered on HER2 as the target antigen was safe and showed early promise in the treatment of advanced sarcomas of bone and soft tissues in a phase I trial.
One patient, a 16-year-old girl with advanced osteosarcoma metastatic to her lungs, had a complete response to the therapy that is ongoing out to nearly 3 years, reported Shoba A. Navai, MD, from Baylor College of Medicine in Houston.
A second patient, an 8-year-old boy with rhabdomyosarcoma metastatic to bone marrow, had a complete response lasting 12 months. Upon relapse he was re-enrolled, received additional CAR T-cell infusions, and had a second complete response that has been ongoing for 17 months.
“HER2 CAR T cells can induce objective clinical responses in some patients with sarcoma, and engagement of endogenous immunity may aid in generation of tumor responses. We are currently working to validate these findings in other patients who were treated,” she said at a briefing at the annual meeting of the American Association for Cancer Research.
HER2 is a member of the human epidermal growth factor receptor family that is primarily expressed on the surface of tumor cells but is largely absent from nonmalignant tissues. HER2 can be expressed in a variety of sarcomas, including osteosarcoma, and HER2 expression in osteosarcoma correlates with worse overall survival.
Unlike HER2-positive breast cancers, however, HER2 expression levels in osteosarcoma are too low to be effectively targeted by anti-HER2 agents such as trastuzumab (Hereceptin).
But as Dr. Navai and colleagues have found, HER2 appears to be a valid target for CAR T-cell therapy in otherwise antigenically “cold” tumors – that is, tumors with few targetable antigens.
Old target, new weapon
They have developed a CAR T-cell construct using a HER2-directed antibody coupled with CD28 as the costimulatory molecule. As with other CAR T therapies, the patient’s T cells or selected T cell subsets are collected, transfected to express the antigen, and are then expanded and returned to the patient following lymphodepletion with either fludarabine alone or with cyclophosphamide.
Each patient received up to three infusions of autologous CAR T cells at a dose of 1 x 108 cells/m2, and eligible patients received up to five additional infusions without additional lymphodepletion.
Dr. Navai presented data on 10 patients treated to date, including the two mentioned before; the boy with rhabdomyosarcoma was counted as two separate patients for the purpose of the efficacy analysis.
All patients had metastatic disease, including five with osteosarcoma, three with rhabdomyosarcoma, one with Ewing sarcoma, and one with synovial sarcoma.
The lymphodepletion regimens did their job, inducing neutropenia (defined as an absolute neutrophil count less than 500 per milliliter ) for up to 14 days.
Eight patients developed grade 1 or 2 cytokine release syndrome within 24 hours of CAR T-cell infusion, and all cases completely resolved with supportive care within 5 days of onset.
In nine patients, T cells were successfully expanded, with a median peak expansion on day 7.
In all 10 patients, CAR T cells were detected by quantitative polymerase chain reaction 6 weeks after infusion.
In addition to the two patients with complete remissions already described, three patients had stable disease. The remaining patients had disease progression. At the most recent analysis, five patients were still alive, and five had died.
The infusions were safe, with no dose-limiting toxicities reported. No patient required a transfusion, and there were no opportunistic, infections, no neurotoxicities, and no lasting pulmonary or cardiac toxicities, Dr. Navai reported.
Some fare better than others
Nilofer S. Azad, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, who moderated the briefing, commented that the study had “very small numbers, but is still very exciting.”
She noted that the patients who benefited most from the therapy either had minimal residual disease or bone marrow disease without visceral disease; she asked Dr. Navai how this could be addressed going forward.
“The patients who seemed to have had responses both in this trial, as well as in our previous trial without lymphodepletion, tended to have less disease or more accessible disease. So we hypothesized that disease that’s in the bone marrow because it’s more accessible, or in the lungs, where also CAR T cells go after they are first infused, may be more amenable to treatment,” Dr. Navai said.
In contrast, larger tumors and more invasive disease may emit immune inhibitory signals that dampen the efficacy of CAR T cells, she added.
Development of the CAR T-cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai and Dr. Azad reported having no disclosures relevant to the work.
SOURCE: Navai SA et al. AACR 2019, Abstract LB-147.
REPORTING FROM AACR 2019
Flu activity falling but still elevated
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.
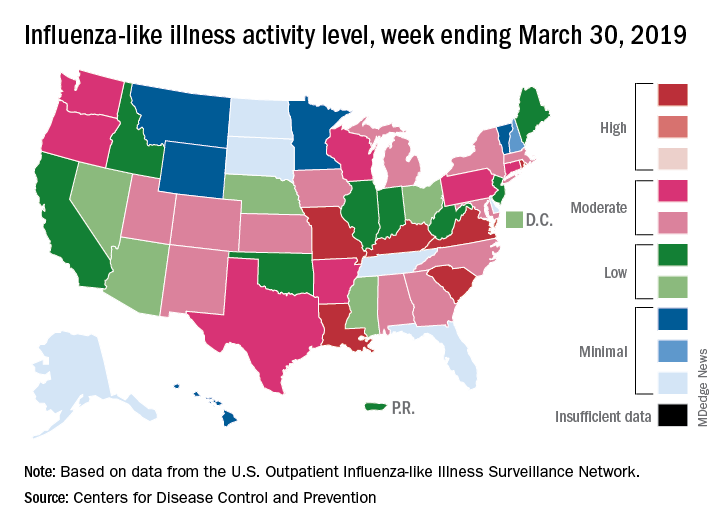
On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
DDNA19: News and advances in IBD

Dr. Stephen Brant and Dr. Nikolaos Pyrsopoulos discuss the latest news and advances in inflammatory bowel disease (IBD) at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Dr. Stephen Brant and Dr. Nikolaos Pyrsopoulos discuss the latest news and advances in inflammatory bowel disease (IBD) at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Dr. Stephen Brant and Dr. Nikolaos Pyrsopoulos discuss the latest news and advances in inflammatory bowel disease (IBD) at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
New leaders at SGO, AACR
The Society of Gynecologic Oncology (SGO) has a new leader at the helm.
Warner K. Huh, MD, of the University of Alabama at Birmingham, has been named president of the SGO. Dr. Huh began his 1-year term at the end of SGO’s Annual Meeting on Women’s Cancer, which took place in March 2019.
Dr. Huh said he plans to focus his presidency on the changing practice of gynecologic oncology, including surgery and novel therapies, clinical trial mentorship, subspecialty awareness, alternative payment models, and the role of gynecologic oncologists in benign gynecologic surgery.
Another newly installed president is Elaine R. Mardis, PhD, of Nationwide Children’s Hospital in Columbus, Ohio. Dr. Mardis was named president of the American Association for Cancer Research (AACR) for 2019-2020.
She has conducted extensive research on the genomic characterization of various cancers. She was inaugurated as AACR president during the AACR’s annual meeting, which took place March 29-April 3, 2019.
Also at the AACR annual meeting, Antoni Ribas, MD, PhD, of the University of California, Los Angeles, was inducted as president-elect of AACR. Dr. Ribas will assume the presidency in April 2020. He has conducted research focused on malignant melanoma and is said to have been “instrumental” in the development of several drugs used to treat the disease.
In other news, the National Comprehensive Cancer Network (NCCN) named Ronald Walters, MD, chair of its board of directors, and Ruth O’Regan, MD, was named vice chair.
Dr. Walters, of the University of Texas MD Anderson Cancer Center in Houston, conducts research focused on health care reform and cost accounting in health care.
Dr. O’Regan, of the University of Wisconsin Carbone Cancer Center in Madison, conducts research focused on identifying mechanisms of treatment resistance and developing new therapies for breast cancer.
Finally, Giulio F. Draetta, MD, PhD, was named chief scientific officer at the University of Texas MD Anderson Cancer Center. This is a new position that “champions innovation, develops strong partnerships, and provides focused leadership on the science and clinical translation of research programs,” according to MD Anderson.
Dr. Draetta conducted “fundamental” research on the eukaryotic cell division cycle and DNA damage-induced checkpoints. He has cofounded and led biotechnology companies and headed drug discovery and development programs that led to two drug approvals, according to MD Anderson.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
The Society of Gynecologic Oncology (SGO) has a new leader at the helm.
Warner K. Huh, MD, of the University of Alabama at Birmingham, has been named president of the SGO. Dr. Huh began his 1-year term at the end of SGO’s Annual Meeting on Women’s Cancer, which took place in March 2019.
Dr. Huh said he plans to focus his presidency on the changing practice of gynecologic oncology, including surgery and novel therapies, clinical trial mentorship, subspecialty awareness, alternative payment models, and the role of gynecologic oncologists in benign gynecologic surgery.
Another newly installed president is Elaine R. Mardis, PhD, of Nationwide Children’s Hospital in Columbus, Ohio. Dr. Mardis was named president of the American Association for Cancer Research (AACR) for 2019-2020.
She has conducted extensive research on the genomic characterization of various cancers. She was inaugurated as AACR president during the AACR’s annual meeting, which took place March 29-April 3, 2019.
Also at the AACR annual meeting, Antoni Ribas, MD, PhD, of the University of California, Los Angeles, was inducted as president-elect of AACR. Dr. Ribas will assume the presidency in April 2020. He has conducted research focused on malignant melanoma and is said to have been “instrumental” in the development of several drugs used to treat the disease.
In other news, the National Comprehensive Cancer Network (NCCN) named Ronald Walters, MD, chair of its board of directors, and Ruth O’Regan, MD, was named vice chair.
Dr. Walters, of the University of Texas MD Anderson Cancer Center in Houston, conducts research focused on health care reform and cost accounting in health care.
Dr. O’Regan, of the University of Wisconsin Carbone Cancer Center in Madison, conducts research focused on identifying mechanisms of treatment resistance and developing new therapies for breast cancer.
Finally, Giulio F. Draetta, MD, PhD, was named chief scientific officer at the University of Texas MD Anderson Cancer Center. This is a new position that “champions innovation, develops strong partnerships, and provides focused leadership on the science and clinical translation of research programs,” according to MD Anderson.
Dr. Draetta conducted “fundamental” research on the eukaryotic cell division cycle and DNA damage-induced checkpoints. He has cofounded and led biotechnology companies and headed drug discovery and development programs that led to two drug approvals, according to MD Anderson.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
The Society of Gynecologic Oncology (SGO) has a new leader at the helm.
Warner K. Huh, MD, of the University of Alabama at Birmingham, has been named president of the SGO. Dr. Huh began his 1-year term at the end of SGO’s Annual Meeting on Women’s Cancer, which took place in March 2019.
Dr. Huh said he plans to focus his presidency on the changing practice of gynecologic oncology, including surgery and novel therapies, clinical trial mentorship, subspecialty awareness, alternative payment models, and the role of gynecologic oncologists in benign gynecologic surgery.
Another newly installed president is Elaine R. Mardis, PhD, of Nationwide Children’s Hospital in Columbus, Ohio. Dr. Mardis was named president of the American Association for Cancer Research (AACR) for 2019-2020.
She has conducted extensive research on the genomic characterization of various cancers. She was inaugurated as AACR president during the AACR’s annual meeting, which took place March 29-April 3, 2019.
Also at the AACR annual meeting, Antoni Ribas, MD, PhD, of the University of California, Los Angeles, was inducted as president-elect of AACR. Dr. Ribas will assume the presidency in April 2020. He has conducted research focused on malignant melanoma and is said to have been “instrumental” in the development of several drugs used to treat the disease.
In other news, the National Comprehensive Cancer Network (NCCN) named Ronald Walters, MD, chair of its board of directors, and Ruth O’Regan, MD, was named vice chair.
Dr. Walters, of the University of Texas MD Anderson Cancer Center in Houston, conducts research focused on health care reform and cost accounting in health care.
Dr. O’Regan, of the University of Wisconsin Carbone Cancer Center in Madison, conducts research focused on identifying mechanisms of treatment resistance and developing new therapies for breast cancer.
Finally, Giulio F. Draetta, MD, PhD, was named chief scientific officer at the University of Texas MD Anderson Cancer Center. This is a new position that “champions innovation, develops strong partnerships, and provides focused leadership on the science and clinical translation of research programs,” according to MD Anderson.
Dr. Draetta conducted “fundamental” research on the eukaryotic cell division cycle and DNA damage-induced checkpoints. He has cofounded and led biotechnology companies and headed drug discovery and development programs that led to two drug approvals, according to MD Anderson.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.