User login
New Frontiers in High-Value Care Education and Innovation: When Less is Not More
In this issue of the Journal of Hospital Medicine®, Drs. Arora and Moriates highlight an important deficiency in quality improvement efforts designed to reduce overuse of tests and treatments: the potential for trainees—and by extension, more seasoned clinicians—to rationalize minimizing under the guise of high-value care.1 This insightful perspective from the Co-Directors of Costs of Care should serve as a catalyst for further robust and effective care redesign efforts to optimize the use of all medical resources, including tests, treatments, procedures, consultations, emergency department (ED) visits, and hospital admissions. The formula to root out minimizers is not straightforward and requires an evaluation of wasteful practices in a nuanced and holistic manner that considers not only the frequency that the overused test (or treatment) is ordered but also the collateral impact of not ordering it. This principle has implications for measuring, paying for, and studying high-value care.
Overuse of tests and treatments increases costs and carries a risk of harm, from unnecessary use of creatine kinase–myocardial band (CK-MB) in suspected acute coronary syndrome2 to unwarranted administration of antibiotics for asymptomatic bacteriuria3 to over-administration of blood transfusions.4 However, decreasing the use of a commonly ordered test is not always clinically appropriate. To illustrate this point, we consider the evidence-based algorithm to deliver best practice in the work-up of pulmonary embolism (PE) by Raja et al; which integrates pretest probability, PERC assessment, and appropriate use of D-dimer and pulmonary CT angiography (CTA).5 Avoiding D-dimer testing is appropriate in patients with very low pretest probability who pass a pulmonary embolism rule-out criteria (PERC) clinical assessment and is also appropriate in patients who have sufficiently high clinical probability for PE to justify CTA regardless of a D-dimer result. On the other hand, avoiding D-dimer testing by attributing a patient’s symptoms to anxiety (as a minimizer might do) would increase patient risk, and could potentially increase cost if that patient ends up in intensive care after delayed diagnosis. Following diagnostic algorithms that include physician decision-making and evidence-based guidelines can prevent overuse and underuse, thereby maximizing efficiency and effectiveness. Engaging trainees in the development of such algorithms and decision support tools will serve to ingrain these principles into their practice.
Arora and Moriates highlight the importance of caring for a patient along a continuum rather than simply optimizing practice with respect to a single management decision or an isolated care episode. This approach is fundamental to the quality of care we provide, the public trust our profession still commands, and the total cost of care (TCOC). The two largest contributors to debilitating patient healthcare debt are not overuse of tests and treatments, but ED visits and hospitalizations.6 Thus, high-value quality improvement needs to anticipate future healthcare needs, including those that may result from delayed or missed diagnoses. Furthermore, excessive focus on the minutiae of high-value care (fewer daily basic metabolic panels) can lead to change fatigue and divert attention from higher impact utilization. We endorse a holistic approach in which the lens is shifted from the test—and even from the encounter or episode of care—to the entire continuum of care so that we can safeguard against inappropriate minimization. This approach has started to gain traction with policymakers. For example, the state of
Research is needed to guide best practice from this global perspective; as such, value improvement projects aimed at optimizing use of tests and treatments should include rigorous methodology, measures of downstream outcomes and costs, and balancing safety measures.8 For example, the ROMICAT II randomized trial evaluated two diagnostic approaches in emergency department patients with suspected acute coronary syndrome: early coronary computed tomography angiogram (CCTA) and standard ED evaluation.9 In addition to outcomes related to the ED visit itself, downstream testing and outcomes for 28 days after the episode were studied. In the acute setting, CCTA decreased time to diagnosis, reduced mean hospital length of stay by 7.6 hours, and resulted in 47% of patients being discharged within 8.6 hours as opposed to only 12% of the standard evaluation cohort. No cases of ACS were missed, and the CCTA cohort has slightly fewer cardiovascular adverse events (P = .18). However, the CCTA patients received significantly more diagnostic and functional testing and higher radiation exposure than the standard evaluation cohort, and underwent modestly higher rates of coronary angiography and percutaneous coronary intervention. The TCOC over the 28-day period was similar at $4,289 for CCTA versus $4,060 for standard care (P = .65).9
Reducing the TCOC is imperative to protect patients from the burden of healthcare debt, but concerns have been raised about the ethics of high-value care if decision-making is driven by cost considerations.10 A recent viewpoint proposed a framework where high-value care recommendations are categorized as obligatory (protecting patients from harm), permissible (call for shared decision-making), or suspect (entirely cost-driven). By reframing care redesign as thoughtful, responsible care delivery, we can better incentivize physicians to exercise professionalism and maintain medical practice as a public trust.
High-value champions have a great deal of work ahead to redesign care to improve health, reduce TCOC, and investigate outcomes of care redesign. We applaud Drs. Arora and Moriates for once again leading the charge in preparing medical students and residents to deliver higher-value healthcare by emphasizing that effective patient care is not measured by a single episode or clinical decision, but is defined through a lifelong partnership between the patient and the healthcare system. As the country moves toward improved holistic models of care and financing, physician leadership in care redesign is essential to ensure that quality, safety, and patient well-being are not sacrificed at the altar of cost savings.
Disclosures
Dr. Johnson is a Consultant and Advisory Board Member at Oliver Wyman, receives salary support from an AHRQ grant, and has pending potential royalties from licensure of evidence-based appropriate use guidelines/criteria to AgilMD (Agile is a clinical decision support company). The other authors have no relevant disclosures. Dr. Johnson and Dr. Pahwa are Co-directors, High Value Practice Academic Alliance, www.hvpaa.org
1. Arora V, Moriates C. Tackling the minimizers behind high value care. J Hos Med. 2019: 14(5):318-319. doi: 10.12788/jhm.3104 PubMed
2. Alvin MD, Jaffe AS, Ziegelstein RC, Trost JC. Eliminating creatine kinase-myocardial band testing in suspected acute coronary syndrome: a value-based quality improvement. JAMA Intern Med. 2017;177(10):1508-1512. doi: 10.1001/jamainternmed.2017.3597. PubMed
3. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 018;178(2):271-276. doi: 10.1001/jamainternmed.2017.7290. PubMed
4. Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178(1):116-122. doi: 10.1001/jamainternmed.2017.6369. PubMed
5. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: Best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. doi: 10.7326/M14-1772 PubMed
6. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. https://www.kff.org/health-costs/report/the-burden-of-medical-debt-results-from-the-kaiser-family-foundationnew-york-times-medical-bills-survey/. Accessed December 2, 2018.
7. Maryland Total Cost of Care Model. https://innovation.cms.gov/initiatives/md-tccm/. Accessed December 2, 2018
8. Grady D, Redberg RF, O’Malley PG. Quality improvement for quality improvement studies. JAMA Intern Med. 2018;178(2):187. doi: 10.1001/jamainternmed.2017.6875. PubMed
9. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299-308. doi: 10.1056/NEJMoa1201161. PubMed
10. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. doi: 10.1136/medethics-2016-103880. PubMed
In this issue of the Journal of Hospital Medicine®, Drs. Arora and Moriates highlight an important deficiency in quality improvement efforts designed to reduce overuse of tests and treatments: the potential for trainees—and by extension, more seasoned clinicians—to rationalize minimizing under the guise of high-value care.1 This insightful perspective from the Co-Directors of Costs of Care should serve as a catalyst for further robust and effective care redesign efforts to optimize the use of all medical resources, including tests, treatments, procedures, consultations, emergency department (ED) visits, and hospital admissions. The formula to root out minimizers is not straightforward and requires an evaluation of wasteful practices in a nuanced and holistic manner that considers not only the frequency that the overused test (or treatment) is ordered but also the collateral impact of not ordering it. This principle has implications for measuring, paying for, and studying high-value care.
Overuse of tests and treatments increases costs and carries a risk of harm, from unnecessary use of creatine kinase–myocardial band (CK-MB) in suspected acute coronary syndrome2 to unwarranted administration of antibiotics for asymptomatic bacteriuria3 to over-administration of blood transfusions.4 However, decreasing the use of a commonly ordered test is not always clinically appropriate. To illustrate this point, we consider the evidence-based algorithm to deliver best practice in the work-up of pulmonary embolism (PE) by Raja et al; which integrates pretest probability, PERC assessment, and appropriate use of D-dimer and pulmonary CT angiography (CTA).5 Avoiding D-dimer testing is appropriate in patients with very low pretest probability who pass a pulmonary embolism rule-out criteria (PERC) clinical assessment and is also appropriate in patients who have sufficiently high clinical probability for PE to justify CTA regardless of a D-dimer result. On the other hand, avoiding D-dimer testing by attributing a patient’s symptoms to anxiety (as a minimizer might do) would increase patient risk, and could potentially increase cost if that patient ends up in intensive care after delayed diagnosis. Following diagnostic algorithms that include physician decision-making and evidence-based guidelines can prevent overuse and underuse, thereby maximizing efficiency and effectiveness. Engaging trainees in the development of such algorithms and decision support tools will serve to ingrain these principles into their practice.
Arora and Moriates highlight the importance of caring for a patient along a continuum rather than simply optimizing practice with respect to a single management decision or an isolated care episode. This approach is fundamental to the quality of care we provide, the public trust our profession still commands, and the total cost of care (TCOC). The two largest contributors to debilitating patient healthcare debt are not overuse of tests and treatments, but ED visits and hospitalizations.6 Thus, high-value quality improvement needs to anticipate future healthcare needs, including those that may result from delayed or missed diagnoses. Furthermore, excessive focus on the minutiae of high-value care (fewer daily basic metabolic panels) can lead to change fatigue and divert attention from higher impact utilization. We endorse a holistic approach in which the lens is shifted from the test—and even from the encounter or episode of care—to the entire continuum of care so that we can safeguard against inappropriate minimization. This approach has started to gain traction with policymakers. For example, the state of
Research is needed to guide best practice from this global perspective; as such, value improvement projects aimed at optimizing use of tests and treatments should include rigorous methodology, measures of downstream outcomes and costs, and balancing safety measures.8 For example, the ROMICAT II randomized trial evaluated two diagnostic approaches in emergency department patients with suspected acute coronary syndrome: early coronary computed tomography angiogram (CCTA) and standard ED evaluation.9 In addition to outcomes related to the ED visit itself, downstream testing and outcomes for 28 days after the episode were studied. In the acute setting, CCTA decreased time to diagnosis, reduced mean hospital length of stay by 7.6 hours, and resulted in 47% of patients being discharged within 8.6 hours as opposed to only 12% of the standard evaluation cohort. No cases of ACS were missed, and the CCTA cohort has slightly fewer cardiovascular adverse events (P = .18). However, the CCTA patients received significantly more diagnostic and functional testing and higher radiation exposure than the standard evaluation cohort, and underwent modestly higher rates of coronary angiography and percutaneous coronary intervention. The TCOC over the 28-day period was similar at $4,289 for CCTA versus $4,060 for standard care (P = .65).9
Reducing the TCOC is imperative to protect patients from the burden of healthcare debt, but concerns have been raised about the ethics of high-value care if decision-making is driven by cost considerations.10 A recent viewpoint proposed a framework where high-value care recommendations are categorized as obligatory (protecting patients from harm), permissible (call for shared decision-making), or suspect (entirely cost-driven). By reframing care redesign as thoughtful, responsible care delivery, we can better incentivize physicians to exercise professionalism and maintain medical practice as a public trust.
High-value champions have a great deal of work ahead to redesign care to improve health, reduce TCOC, and investigate outcomes of care redesign. We applaud Drs. Arora and Moriates for once again leading the charge in preparing medical students and residents to deliver higher-value healthcare by emphasizing that effective patient care is not measured by a single episode or clinical decision, but is defined through a lifelong partnership between the patient and the healthcare system. As the country moves toward improved holistic models of care and financing, physician leadership in care redesign is essential to ensure that quality, safety, and patient well-being are not sacrificed at the altar of cost savings.
Disclosures
Dr. Johnson is a Consultant and Advisory Board Member at Oliver Wyman, receives salary support from an AHRQ grant, and has pending potential royalties from licensure of evidence-based appropriate use guidelines/criteria to AgilMD (Agile is a clinical decision support company). The other authors have no relevant disclosures. Dr. Johnson and Dr. Pahwa are Co-directors, High Value Practice Academic Alliance, www.hvpaa.org
In this issue of the Journal of Hospital Medicine®, Drs. Arora and Moriates highlight an important deficiency in quality improvement efforts designed to reduce overuse of tests and treatments: the potential for trainees—and by extension, more seasoned clinicians—to rationalize minimizing under the guise of high-value care.1 This insightful perspective from the Co-Directors of Costs of Care should serve as a catalyst for further robust and effective care redesign efforts to optimize the use of all medical resources, including tests, treatments, procedures, consultations, emergency department (ED) visits, and hospital admissions. The formula to root out minimizers is not straightforward and requires an evaluation of wasteful practices in a nuanced and holistic manner that considers not only the frequency that the overused test (or treatment) is ordered but also the collateral impact of not ordering it. This principle has implications for measuring, paying for, and studying high-value care.
Overuse of tests and treatments increases costs and carries a risk of harm, from unnecessary use of creatine kinase–myocardial band (CK-MB) in suspected acute coronary syndrome2 to unwarranted administration of antibiotics for asymptomatic bacteriuria3 to over-administration of blood transfusions.4 However, decreasing the use of a commonly ordered test is not always clinically appropriate. To illustrate this point, we consider the evidence-based algorithm to deliver best practice in the work-up of pulmonary embolism (PE) by Raja et al; which integrates pretest probability, PERC assessment, and appropriate use of D-dimer and pulmonary CT angiography (CTA).5 Avoiding D-dimer testing is appropriate in patients with very low pretest probability who pass a pulmonary embolism rule-out criteria (PERC) clinical assessment and is also appropriate in patients who have sufficiently high clinical probability for PE to justify CTA regardless of a D-dimer result. On the other hand, avoiding D-dimer testing by attributing a patient’s symptoms to anxiety (as a minimizer might do) would increase patient risk, and could potentially increase cost if that patient ends up in intensive care after delayed diagnosis. Following diagnostic algorithms that include physician decision-making and evidence-based guidelines can prevent overuse and underuse, thereby maximizing efficiency and effectiveness. Engaging trainees in the development of such algorithms and decision support tools will serve to ingrain these principles into their practice.
Arora and Moriates highlight the importance of caring for a patient along a continuum rather than simply optimizing practice with respect to a single management decision or an isolated care episode. This approach is fundamental to the quality of care we provide, the public trust our profession still commands, and the total cost of care (TCOC). The two largest contributors to debilitating patient healthcare debt are not overuse of tests and treatments, but ED visits and hospitalizations.6 Thus, high-value quality improvement needs to anticipate future healthcare needs, including those that may result from delayed or missed diagnoses. Furthermore, excessive focus on the minutiae of high-value care (fewer daily basic metabolic panels) can lead to change fatigue and divert attention from higher impact utilization. We endorse a holistic approach in which the lens is shifted from the test—and even from the encounter or episode of care—to the entire continuum of care so that we can safeguard against inappropriate minimization. This approach has started to gain traction with policymakers. For example, the state of
Research is needed to guide best practice from this global perspective; as such, value improvement projects aimed at optimizing use of tests and treatments should include rigorous methodology, measures of downstream outcomes and costs, and balancing safety measures.8 For example, the ROMICAT II randomized trial evaluated two diagnostic approaches in emergency department patients with suspected acute coronary syndrome: early coronary computed tomography angiogram (CCTA) and standard ED evaluation.9 In addition to outcomes related to the ED visit itself, downstream testing and outcomes for 28 days after the episode were studied. In the acute setting, CCTA decreased time to diagnosis, reduced mean hospital length of stay by 7.6 hours, and resulted in 47% of patients being discharged within 8.6 hours as opposed to only 12% of the standard evaluation cohort. No cases of ACS were missed, and the CCTA cohort has slightly fewer cardiovascular adverse events (P = .18). However, the CCTA patients received significantly more diagnostic and functional testing and higher radiation exposure than the standard evaluation cohort, and underwent modestly higher rates of coronary angiography and percutaneous coronary intervention. The TCOC over the 28-day period was similar at $4,289 for CCTA versus $4,060 for standard care (P = .65).9
Reducing the TCOC is imperative to protect patients from the burden of healthcare debt, but concerns have been raised about the ethics of high-value care if decision-making is driven by cost considerations.10 A recent viewpoint proposed a framework where high-value care recommendations are categorized as obligatory (protecting patients from harm), permissible (call for shared decision-making), or suspect (entirely cost-driven). By reframing care redesign as thoughtful, responsible care delivery, we can better incentivize physicians to exercise professionalism and maintain medical practice as a public trust.
High-value champions have a great deal of work ahead to redesign care to improve health, reduce TCOC, and investigate outcomes of care redesign. We applaud Drs. Arora and Moriates for once again leading the charge in preparing medical students and residents to deliver higher-value healthcare by emphasizing that effective patient care is not measured by a single episode or clinical decision, but is defined through a lifelong partnership between the patient and the healthcare system. As the country moves toward improved holistic models of care and financing, physician leadership in care redesign is essential to ensure that quality, safety, and patient well-being are not sacrificed at the altar of cost savings.
Disclosures
Dr. Johnson is a Consultant and Advisory Board Member at Oliver Wyman, receives salary support from an AHRQ grant, and has pending potential royalties from licensure of evidence-based appropriate use guidelines/criteria to AgilMD (Agile is a clinical decision support company). The other authors have no relevant disclosures. Dr. Johnson and Dr. Pahwa are Co-directors, High Value Practice Academic Alliance, www.hvpaa.org
1. Arora V, Moriates C. Tackling the minimizers behind high value care. J Hos Med. 2019: 14(5):318-319. doi: 10.12788/jhm.3104 PubMed
2. Alvin MD, Jaffe AS, Ziegelstein RC, Trost JC. Eliminating creatine kinase-myocardial band testing in suspected acute coronary syndrome: a value-based quality improvement. JAMA Intern Med. 2017;177(10):1508-1512. doi: 10.1001/jamainternmed.2017.3597. PubMed
3. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 018;178(2):271-276. doi: 10.1001/jamainternmed.2017.7290. PubMed
4. Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178(1):116-122. doi: 10.1001/jamainternmed.2017.6369. PubMed
5. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: Best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. doi: 10.7326/M14-1772 PubMed
6. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. https://www.kff.org/health-costs/report/the-burden-of-medical-debt-results-from-the-kaiser-family-foundationnew-york-times-medical-bills-survey/. Accessed December 2, 2018.
7. Maryland Total Cost of Care Model. https://innovation.cms.gov/initiatives/md-tccm/. Accessed December 2, 2018
8. Grady D, Redberg RF, O’Malley PG. Quality improvement for quality improvement studies. JAMA Intern Med. 2018;178(2):187. doi: 10.1001/jamainternmed.2017.6875. PubMed
9. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299-308. doi: 10.1056/NEJMoa1201161. PubMed
10. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. doi: 10.1136/medethics-2016-103880. PubMed
1. Arora V, Moriates C. Tackling the minimizers behind high value care. J Hos Med. 2019: 14(5):318-319. doi: 10.12788/jhm.3104 PubMed
2. Alvin MD, Jaffe AS, Ziegelstein RC, Trost JC. Eliminating creatine kinase-myocardial band testing in suspected acute coronary syndrome: a value-based quality improvement. JAMA Intern Med. 2017;177(10):1508-1512. doi: 10.1001/jamainternmed.2017.3597. PubMed
3. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 018;178(2):271-276. doi: 10.1001/jamainternmed.2017.7290. PubMed
4. Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178(1):116-122. doi: 10.1001/jamainternmed.2017.6369. PubMed
5. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: Best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. doi: 10.7326/M14-1772 PubMed
6. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. https://www.kff.org/health-costs/report/the-burden-of-medical-debt-results-from-the-kaiser-family-foundationnew-york-times-medical-bills-survey/. Accessed December 2, 2018.
7. Maryland Total Cost of Care Model. https://innovation.cms.gov/initiatives/md-tccm/. Accessed December 2, 2018
8. Grady D, Redberg RF, O’Malley PG. Quality improvement for quality improvement studies. JAMA Intern Med. 2018;178(2):187. doi: 10.1001/jamainternmed.2017.6875. PubMed
9. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299-308. doi: 10.1056/NEJMoa1201161. PubMed
10. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. doi: 10.1136/medethics-2016-103880. PubMed
Tackling the Minimizers Hiding Behind High-Value Care
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
© 2019 Society of Hospital Medicine
Pharmacologic Management of Malignant Bowel Obstruction: When Surgery Is Not an Option
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
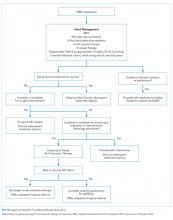
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
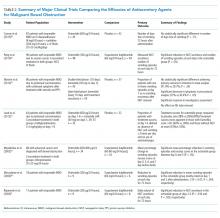
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
Malignant bowel obstruction (MBO) is a catastrophic complication of cancer that often requires hospitalization and a multidisciplinary approach in its management. Hospitalists frequently collaborate with such specialties as Hematology/Oncology, Surgery, Palliative Medicine, and Interventional Radiology in arriving at a treatment plan.
Initial management is focused on hydration, bowel rest and decompression via nasogastric (NG) tube. Surgical resection or endoscopic stenting should be considered early.1 However, patients who present in the terminal stages may be poor candidates for these options due to diminished functional status, multiple areas of obstruction, complicated anatomy limiting intervention, or an associated large volume of ascites.
Presence of inoperable MBO portends a poor prognosis, often measured in weeks.2 Presentation often occurs in the context of a sentinel hospitalization, signifying a shift in disease course.3,4 It is essential for hospitalists to be familiar with noninvasive therapies for inoperable MBO given the increasing role of hospitalists in providing inpatient palliative care. Palliative pharmacologic management of MBO can reduce symptom burden during these terminal stages and will be the focus of this paper.
BACKGROUND AND PATHOPHYSIOLOGY
Malignant bowel obstruction occurs in about 3%-15% of patients with cancer.2 A consensus definition of MBO established the following specific criteria: (1) clinical evidence of bowel obstruction, (2) obstruction distal to the ligament of Treitz, and (3) the presence of primary intra-abdominal cancer with incurable disease or extra-abdominal cancer with peritoneal involvement.5 The most common malignancies are gastric, colorectal, and ovarian in origin.1,2 The most common extra-abdominal malignancies associated with MBO are breast, melanoma, and lung. MBO is most frequently diagnosed during the advanced stages of cancer.2 The obstruction can involve a partial or total blockage of the small or large intestine from either an intrinsic or extrinsic source. Peristalsis may also be impaired via direct tumor infiltration of the intestinal walls or within the enteric nervous system or celiac plexus. Other etiologies of MBO include peritoneal carcinomatosis and radiation-induced fibrosis.1,6 The obstruction can occur at a single level or involve multiple areas, which usually precludes surgical intervention.2
Symptoms of MBO can be insidious in onset and take several weeks to manifest. The most prevalent symptoms are nausea, vomiting, constipation, abdominal pain, and distension.2,6 The intermittent pattern of symptoms may evolve into continuous episodes with spontaneous remission in between. The etiology of symptoms can be attributed to distension proximal to the site of obstruction with concomitantly increased gastrointestinal and pancreaticobiliary secretions.
The distension creates a “hypertensive state” in the intestinal lumen causing enterochromaffin cells to release serotonin which activates the enteric nervous system and its effectors including substance P, nitric oxide, acetylcholine, somatostatin, and vasoactive intestinal peptide (VIP). These neurotransmitters stimulate the secretomotor actions that cause hypersecretion of mucus from cells of the intestinal crypts. Additional water and sodium secretions accumulate due to the expanded surface area of the bowel.1,2 Overloaded with luminal contents, the bowel attempts to overcome the obstruction by contracting, which leads to colicky abdominal pain. Tumor burden can also damage the intestinal epithelium and cause continuous pain.
INITIAL MANAGEMENT
Fluid resuscitation, electrolyte repletion, and a trial of NG tube decompression are part of the initial management of MBO (Figure ). While studies have shown that moderate intravenous hydration can minimize nausea and drowsiness, excessive fluids may worsen bowel edema and exacerbate vomiting.1,8 NG tube decompression is most effective in patients with proximal obstructions but some studies suggest it can decrease vomiting in patients with colonic obstructions as well.9 Computed tomography imaging can identify the extent of the tumor, the transition point of the obstruction, and any distant metastases. Surgery, Gastroenterology, and/or Interventional Radiology consultation should be obtained early to evaluate options for direct decompression. Hematology/Oncology and Radiation/Oncology referral may help delineate prognosis and achievable outcomes. Emergent exploratory surgery may be required in cases of bowel perforation or ischemia. Otherwise, a planned surgical resection should be considered in those with an isolated resectable lesion and acceptable perioperative risk. Colorectal or duodenal stents may be an option for those who are not surgical candidates or as a bridge to surgery.
As bowel obstruction is often a late manifestation of advanced malignancy, many patients may not be appropriate candidates for operative/interventional treatment due to malnutrition, comorbid conditions, or anatomic considerations. For these individuals, pharmacologic management is the mainstay of treatment. Additionally, the pharmacologic approaches detailed below may provide benefit as adjunctive therapy for patients undergoing procedural intervention.7 Consultation for early palliative care can improve symptom control as well as clarify goals of care.
PHARMACOLOGIC MANAGEMENT
Given the pathophysiology of MBO, pharmacologic therapies are focused on controlling nausea and pain while reducing bowel edema and secretions.
Antiemetic Agents
Nausea and vomiting in MBO are due to activation of vagal nerve fibers in the gastric wall and stimulation of the chemoreceptor trigger zone (CTZ).10 Dopamine antagonists have started to gain favor for MBO compared to more commonly used antiemetics such as the serotonin antagonists. Haloperidol should be considered as a first-line antiemetic in patients with MBO. Its potent D2-receptor antagonistic properties block receptors in the CTZ. The high affinity of the drug for only the D2-receptor makes it preferable to alternative agents in the same class such as chlorpromazine. However, haloperidol may cause or worsen QT prolongation and should be avoided in patients with Parkinson’s disease. The medication has less sedative and unwanted anticholinergic side effects due to its limited interaction with histaminergic and acetylcholine receptors.11 Haloperidol has been shown in the past to be efficacious for post-operative nausea but there are few randomized controlled trials in the terminally ill.12 Nonetheless, recent consensus guidelines from the Multinational Association of Supportive Care recommended haloperidol as the initial treatment of nausea for individuals with MBO based on available systematic reviews.10
Other dopamine antagonists remain good options, though they may cause additional side effects due to actions on other receptor types. Metoclopramide, another D2-receptor antagonist, has been shown to be effective in the treatment of nausea and vomiting due to advanced cancer.13 However as a prokinetic agent, this medication should be avoided in those with complete MBO and only considered in those with partial MBO.10,14
Olanzapine, an atypical antipsychotic, may also have a role in controlling nausea in patients with MBO. It functions as a 5-HT2A and D2-receptor antagonist, with a slightly greater affinity for the 5-HT2A receptor. Olanzapine thus can target two critical receptors playing a role in nausea and vomiting. A study of patients with incomplete bowel obstruction found the addition of olanzapine significantly decreased nausea and vomiting in patients who were refractory to other treatments including steroids and haloperidol.15 Olanzapine has the added advantage of single-day dosing as well as an oral disintegrating formulation.16
Intravenous and sublingual preparations of 5-HT3 receptor antagonists such as ondansetron are commonly used in the inpatient setting. These medications are potent antiemetics that exhibit their effects via pathways where serotonin acts as a neurotransmitter.17 An alternative agent, tropisetron, has shown promise when used alone or in conjunction with metoclopramide but is not currently available in the US.18 Granisetron is available in a transdermal formulation, which can be very convenient for patients with bowel obstruction. Its mechanism of action differs from ondansetron as it is an allosteric inhibitor rather than a competitive inhibitor.19 Granisetron needs more specific study with regards to its role in MBO.
Although haloperidol remains the initial choice, combination therapy can help to decrease the risk of extrapyramidal symptoms seen with higher doses of dopaminergic monotherapy.
Analgesics
Pain control is an essential part of the palliative treatment of MBO as bowel distention, secretions, and edema can cause rapid onset of pain. Parenteral step three opioids remain the optimal initial choice since patients are unable to take medications orally and may have compromised absorption. Opioids address both the colicky and continuous aspects of MBO pain.
Short-acting intravenous opioids such as morphine or hydromorphone may be scheduled every four hours with breakthrough dosing every hour in between. Alternatively, analgesics can be administered via a patient-controlled analgesia (PCA) pump.1 Although doses vary across patients, opioid-naïve patients can be initiated on a low dose therapy such as hydromorphone 0.2 mg IV/SC or morphine 1 mg IV/SC every four hours as needed for pain control.
Ongoing pain management for patients with MBO requires coordination of care. Many patients will elect to receive hospice care following discharge. Direct communication with palliative consultants and hospice providers can help facilitate a smooth transition. In patients for whom bowel obstruction resolves, transition to oral opioids based on morphine equivalent daily dose is indicated with further dose adjustment as patients may have reduced pain at this stage.
Options for patients with unresolved obstruction include transdermal and sublingual preparations as well as outpatient PCA with hospice support. Transdermal fentanyl patch can be useful but onset of peak levels occur within 8-12 hours.20 The patch is usually exchanged every 72 hours and is most effective when applied to areas containing adipose tissue which may limit its use in cachectic patients. The liquid preparation of methadone can be useful even in patients with unresolved MBO. Its lipophilic properties allow for ease of absorption.21 A baseline electrocardiogram (EKG) is recommended prior to methadone initiation due to the potential for QT prolongation. Methadone should not be a first-line option for opioid-naïve individuals due to its longer onset of action which limits rapid dose titration. Close collaboration with palliative medicine is highly recommended when using longer acting opioids.
Antisecretory Agents
Antisecretory agents are a mainstay of the pharmacologic management of inoperable MBO. Medications that reduce secretions and bowel edema include: somatostatin analogs, H2-blockers, proton pump inhibitors (PPIs), steroids, and anticholinergic agents. Table 2 summarizes the major studies comparing various antisecretory medications.
Octreotide, a somatostatin analog, has been increasingly used for the palliative treatment of MBO. The mechanism of action involves splanchnic vasoconstriction, reduction of intestinal and pancreatic secretions (via inhibition of VIP), decrease in gastric emptying, and slowing of smooth muscle contractions.22 Octreotide comes in an immediate-release formulation with an initial subcutaneous dose of 100 µg three or four times per day. Most patients will require 300-800 µg/day, maximum dose being up to 1 mg/day.22,23 A long-acting formulation, lanreotide, exists but can be difficult to obtain and may not provide the immediate relief needed in an acute care setting.
Initiation of octreotide should be considered in the presence of persistent symptoms. Studies have suggested that the benefit of octreotide is most apparent in the first three days of treatment (range 1-5 days).6,22,24 The medication should be discontinued if there is no clinical improvement such as reduction of NG tube output. Octreotide has been shown to be more efficacious than anticholinergic agents in reducing secretions as well as frequency of nausea and vomiting.8,25-28 Octreotide expedites NG tube removal, recovery of bowel function, and improvement in quality of life.29-32 The medication should also be considered in cases of recurrent MBO that previously responded to the medication.
Octreotide is considered the first-line agent in the palliative treatment of MBO, however the medication is costly. Recent studies suggest combination therapy with steroids and H2-blockers or PPIs may be an equally effective and less expensive alternative. The primary rationale for the use of steroids in MBO is their ability to decrease peritumoral edema and promote salt and water absorption from the intestine.1,2 PPIs and H2-blockers decrease distension, pain, and vomiting by reducing the volume of gastric secretions.33 A recent meta-analysis of phase 3 trials found both PPIs and H2-blockers to be effective in lowering volumes of gastric aspirates with ranitidine being slightly superior.34
Initial research into the utility of steroids in MBO garnered mixed results. One study showed marginal benefit for steroid plus octreotide combination therapy compared to octreotide, in a cohort of 27 patients.35 A subsequent review of practice patterns in the management of terminal MBO in Japan found that patients given steroids in combination with octreotide compared to octreotide alone were more likely to undergo early NG tube removal.36 A 1999 systematic review of corticosteroid treatment of MBO concluded low morbidity associated with the medications with a trend toward benefit that was not statistically significant.37 A 2015 study by Currow showed the addition of octreotide in patients already on a regime of dexamethasone and ranitidine did not improve the number of days free from vomiting but did reduce vomiting episodes in those with the most refractory symptoms.38
Collectively, the studies suggest that combination therapy with steroid and PPI or H2 blocker could be a less expensive option in the initial management of MBO. Alternatively, steroids may provide additional relief in patients with continued symptoms on octreotide and H2-blockers. Dexamethasone is preferable given its longer half-life and decreased propensity for sodium retention. Dosing of dexamethasone should be 8 mg IV once a day.38
Anticholinergic agents also reduce secretions. However, they are considered second-line therapy given their lower efficacy compared to other treatment options as well as their propensity to worsen cognitive function.1,2 Anticholinergics may benefit patients with continued symptoms who cannot tolerate the side effects of other treatments. Scopolamine, also known as hyoscine hydrobromide in the US, should be avoided as it crosses the blood-brain barrier. The quaternary formulation, scopolamine butylbromide (hyoscine butylbromide), does not pass this barrier but is currently not available in the US. Glycopyrrolate may be considered as it is also a quaternary ammonium compound that does not cross the blood-brain barrier. Several case reports have described its effectiveness in the resolution of refractory nausea and vomiting in combination with haloperidol and hydromorphone for symptom control.39 Effective oral care is imperative if anticholinergics are used in order to prevent the unpleasant feeling of dry mouth.
SUBSEQUENT SUPPORTIVE CARE
While initial management of MBO often requires placement of an NG tube, prolonged placement can increase the risk for erosions, aspiration, and sinus infections. Removal of the NG tube is most successful when secretions are minimal, but this may not happen unless the obstruction resolves. Some patients may elect to keep an NG tube if symptoms cannot be otherwise controlled by medications.
A venting gastrostomy tube can be considered as an alternative to prolonged NG tube placement. The tube may help alleviate distressing symptoms and can enhance the quality of life of patients by allowing the sensation of oral intake, though it will not allow for absorption of nutrients.40 Although a low risk procedure, patients may be too frail to undergo the procedure and may have postprocedure pain and complications. Anatomic abnormalities such as overlying bowel may also prevent the noninvasive percutaneous approach.
In patients with unresolved obstruction, oral intake should be reinitiated with caution with the patient’s wishes taken into account at all times. Some patients may prioritize the comfort derived from eating small amounts over any associated risks of increased nausea and vomiting.
Parenteral nutrition should be avoided in those with inoperable MBO in the advanced stages. The risks of infection, refeeding syndrome, and the discomfort of an intravenous line and intermittent testing may outweigh any benefits given the overall prognosis.41,42
CONCLUSION
Hospitalists are often involved in the initial care of patients with advanced malignancy who present with MBO. When interventions or surgeries to directly alleviate the obstruction are not possible, pharmacologic options are essential in managing burdensome symptoms and improving quality of life. Early Palliative Care referral can also assist with symptom management, emotional support, clarification of goals of care, and transition to the outpatient setting. While patients with inoperable MBO have a poor prognosis, hospitalists can play a vital role in alleviation of suffering in this devastating complication of advanced cancer.
Disclosures
The authors have nothing to disclose.
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
1. Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105-1115. doi: 10.1016/j.ejca.2008.02.028. PubMed
2. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. doi: 10.2147/CMAR.S29297. PubMed
3. Meier DE. Palliative care in hospitals. J Hosp Med. 2006;1(1):21-28. doi: 10.1002/jhm.3. PubMed
4. Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160. PubMed
5. Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34(1 Suppl):S49-S59. doi: 10.1016/j.jpainsymman.2007.04.011. PubMed
6. Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(1):75-91. doi: 10.1016/j.jpainsymman.2013.08.022. PubMed
7. Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg. 2015;4(3):264-270. doi: 10.1016/j.amsu.2015.07.018. PubMed
8. Ripamonti C, Mercadante S, Groff L, et al. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: A prospective randomized trial. J Pain Symptom Manage. 2000;19(1):23-34. doi: 10.1016/S0885-3924(99)00147-5. PubMed
9. Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26(4):423-429. doi: 10.1007/s00384-010-1093-4. PubMed
10. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333-340. doi: 10.1007/s00520-016-3371-3. PubMed
11. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;11(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. PubMed
12. Digges M, Hussein A, Wilcock A, et al. Pharmacovigilance in hospice/palliative care: Net effect of haloperidol for nausea or vomiting. J Palliat Med. 2018;21(1):37-43. doi: 10.1089/jpm.2017.0159. PubMed
13. Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427-435. doi: 10.1016/S0885-3924(00)00138-X. PubMed
14. Gupta M, Davis M, LeGrand S, Walsh D, Lagman R. Nausea and vomiting in advanced cancer: the Cleveland clinic protocol. J Support Oncol. 2013;11(1):8-13. doi: 10.1016/j.suponc.2012.10.002. PubMed
15. Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604-607. doi: 10.1016/j.jpainsymman.2011.10.023. PubMed
16. Prommer E. Olanzapine: palliative medicine update. Am J Hosp Palliat Care. 2013;30(1):75-82. doi: 10.1177/1049909112441241. PubMed
17. Currow DC, Coughlan M, Fardell B, Cooney NJ. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13(5):302-307. doi: 10.1016/S0885-3924(97)00079-1. PubMed
18. Mystakidou K
19. Tuca A, Roca R, Sala C, et al. Efficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: A phase II clinical trial. J Pain Symptom Manage. 2009;37(2):259-270. doi: 10.1016/j.jpainsymman.2008.01.014. PubMed
20. Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12(10):947-954. doi: 10.1089/jpm.2009.0051. PubMed
21. Shaiova LL, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176. doi: 10.1017/S1478951508000254. PubMed
22. Murphy E, Prommer EE, Mihalyo M, Wilcock A. Octreotide. J Pain Symptom Manage. 2010;40(1):142-148. doi: 10.1016/j.jpainsymman.2010.05.002. PubMed
23. Prommer EE. Established and potential therapeutic applications of octreotide in palliative care. Support Care Cancer. 2008;16(10):1117-1123. doi: 10.1007/s00520-007-0399-4. PubMed
24. Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage. 2004;28(4):412-416. doi: 10.1016/j.jpainsymman.2004.01.007. PubMed
25. Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol. 2015;13:50. doi: 10.1186/s12957-015-0455-3. PubMed
26. Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer. 2000;8(3):188-191. doi: 10.1007/s005200050283. PubMed
27. Mystakidou K, Tsilika E, Kalaidopoulou O, et al. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res. 2002;22(2B):1187-1192. PubMed
28. Obita GP, Boland EG, Currow DC, Johnson MJ, Boland JW. Somatostatin analogues compared with placebo and other pharmacologic agents in the management of symptoms of inoperable malignant bowel obstruction: a systematic review. J Pain Symptom Manage. 2016;52(6):901-919. doi: 10.1016/j.jpainsymman.2016.05.032. PubMed
29. Watari H, Hosaka M, Wakui Y, et al. A prospective study on the efficacy of octreotide in the management of malignant bowel obstruction in gynecologic cancer. Int J Gynecol Cancer. 2012;22(4):692-696. doi: 10.1097/IGC.0b013e318244ce93. PubMed
30. Hisanaga T, Shinjo T, Morita T, et al. Multicenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstruction. Jpn J Clin Oncol. 2010;40(8):739-745. doi: 10.1093/jjco/hyq048. PubMed
31. Laval G, Rousselot H, Toussaint-Martel S, et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99(2):E1-E9. doi: 10.1684/bdc.2011.1535. PubMed
32. Mariani PP, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2012;30(35):4337-4343. doi: 10.1200/JCO.2011.40.5712. PubMed
33. Clark K, Lam L, Currow D. Reducing gastric secretions--a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction? Support Care Cancer. 2009;17(12):1463-1468. doi: 10.1007/s00520-009-0609-3. PubMed
34. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27-37. doi: 10.5009/gnl15502. PubMed
35. Murakami H, Matsumoto H, Nakamura M, Hirai T, Yamaguchi Y. Octreotide acetate-steroid combination therapy for malignant gastrointestinal obstruction. Anticancer Res. 2013;33(12):5557-5560. PubMed
36. Minoura T, Takeuchi M, Morita T, Kawakami K. Practice patterns of medications for patients with malignant bowel obstruction using a nationwide claims database and the association between treatment outcomes and concomitant use of H2-blockers/proton pump inhibitors and corticosteroids with octreotide. J Pain Symptom Manage. 2018;55(2):413-419. doi: 10.1016/j.jpainsymman.2017.10.019. PubMed
37. Feuer DJ, Broadley KE. Systematic review and meta-analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Systematic Review Steering Committee. Ann Oncol. 1999;10(9):1035-1041. doi: 10.1023/A:1008361102808. PubMed
38. Currow DC, Quinn S, Agar M, et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49(5):814-821. doi: 10.1016/j.jpainsymman.2014.09.013. PubMed
39. Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage. 1999;18(3):153-154. PubMed
40. Zucchi E, Fornasarig M, Martella L, et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(7):2877-2882. doi: 10.1007/s00520-016-3102-9. PubMed
41. Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825-837. doi: 10.1016/j.clnu.2014.09.010. PubMed
42. O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother. 2011;12(14):2205-2214. doi: 10.1517/14656566.2011.597382. PubMed
© 2019 Society of Hospital Medicine
Update in Hospital Medicine: Practical Lessons from Current Literature
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
© 2019 Society of Hospital Medicine
Direct-to-consumer telemedicine visits may lead to pediatric antibiotic overprescribing
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
FROM PEDIATRICS
Key clinical point: For children diagnosed with acute respiratory infections, antibiotic prescribing was higher and guideline-concordant antibiotic management was lower at direct-to-consumer (DTC) telemedicine visits.
Major finding: Children at DTC telemedicine visits were prescribed antibiotics for respiratory infections 52% of the time, compared with 42% at urgent care visits and 31% at primary care provider visits.
Study details: A retrospective cohort study of DTC telemedicine, urgent care, and primary care provider visits for acute respiratory infections and subsequent antibiotic prescriptions.
Disclosures: The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
Source: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
Deuterium-altered ruxolitinib may be an effective treatment for alopecia areata
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
REPORTING FROM AAD 19
Targeting parasitic histones may improve outcomes in cerebral malaria
GLASGOW – Targeting circulating parasitic histones may hold promise for patients with cerebral malaria (CM), according to investigators.
A retrospective study, involving over 300 individuals, compared parasitic histone concentrations among patients with various forms of malaria and non-malarial illnesses, in addition to healthy controls, finding that elevated histone levels were associated with malarial disease severity and death, reported Simon Abrams, PhD, of the University of Liverpool, UK, a coauthor of the study. He noted that this research could guide the development of treatment strategies for hundreds of thousands of patients each year, particularly children.
“Cerebral malaria is the most severe form of Plasmodium falciparum infection, and despite effective anti-malarial therapy, between 10% and 20% of children that develop cerebral malaria die,” Dr. Abrams said during his presentation at the annual meeting of the British Society for Haematology. “This accounts for a huge amount of deaths per annum. Around 400,000 malarial deaths are in children in subSaharan Africa, and death typically occurs within 24 hours of hospital admission.”
In CM, the blood-brain barrier deteriorates, leading to brain swelling, hemorrhaging, clot formation, and in many cases, death, Dr. Abrams said. CM patients with the worst outcomes typically have retinal abnormalities on fundic exam, granting the disease subtype “retinopathy-positive.”
Aided by colleagues in Malawi, the investigators gathered over 300 patient samples for analysis. They found that patients with retinopathy-positive CM had higher mean extracellular histone levels than retinopathy-negative CM patients and healthy controls (22.6 mcg/ml, 6.31 mcg/ml, and 0.33 mcg/ml, respectively). In addition, retinopathy-positive CM patients who died had significantly higher levels of circulating histones, compared with similar patients who survived (35.7 mcg/ml vs. 21.6 mcg/ml).
These findings translated to predictive capability, as the investigators showed that patients with CM who had elevated histones when admitted to the hospital were at a higher risk of death than those with normal histone levels (P = .04). Unlike patients with CM, patients with uncomplicated malaria had relatively low histone levels (0.57 mcg/ml), as did patients with mild non-malarial febrile illness (1.73 mcg/ml) and non-malarial coma (1.73 mcg/ml).
During his presentation, Dr. Abrams elaborated on the origins of these histones and how they contribute to poor outcomes in patients.
“Histones are small positively charged proteins that bind to negatively charged DNA,” Dr. Abrams said. “Typically, they are found within the cell nucleus, where they are involved in the packaging of DNA. However, during cell death and cell damage, histones are released from the nucleus, extracellularly, and we find that they are very much elevated in critically ill patients that have undergone huge amounts of cell death and damage.”
Once in circulation, histones can make a bad situation even worse.
“Work by ourselves and others around the globe have found that when circulating histones are elevated in these critically ill patients, they’re extremely toxic,” Dr. Abrams said. “Histones can induce endothelial damage and vascular permeability.” In addition, he pointed out that histones are pro-inflammatory and pro-coagulant. “If you bring all of these phenomena together,” he pointed out, “histones induce organ injury and mortality in critically ill patients.”
“The current hypothesis is that if you’re treating patients with these antimalarials, and it’s killing off the parasite, it may cause the histones to be released, which is actually worse for certain patients,” Dr. Abrams explained.
Based on this hypothesis, the investigators developed an anti-histone therapy.
“We’ve got a small peptide that we use to bind to the histones that reduces their toxicity,” Dr. Abrams said. “If we coincubate the serum of [CM] patients with our anti-histone reagent and then put this onto a monolayer of endothelial cells, we see that this toxicity is inhibited. Therefore, this is suggestive that a major toxic factor within these patients are the extracellular histones.”
Providing additional support for the role of histones in cerebral toxicity, postmortem brain tissue from patients with CM showed localization of histones to the endothelium, which has been tied with increased permeability of vascular tissue. In addition, “we are seeing co-localization between the histones and the sequestration of the malarial parasite itself,” Dr. Abrams said.
Concluding his presentation, he looked to the future.
“It’s difficult to get an animal model for malaria,” but he and his associates are currently working with other investigators to develop one. Once developed, the investigators plan on testing concurrent administration of anti-malarial therapy with antihistone therapy.
“What we’re hoping is that sometime in the future, maybe we’d be able to target circulating histones in this patient cohort to improve the survival of these patients,” Dr. Abrams said.
The investigators declared no conflicts of interest.
SOURCE: Moxon et al. BSH 2019. Abstract OR-034.
GLASGOW – Targeting circulating parasitic histones may hold promise for patients with cerebral malaria (CM), according to investigators.
A retrospective study, involving over 300 individuals, compared parasitic histone concentrations among patients with various forms of malaria and non-malarial illnesses, in addition to healthy controls, finding that elevated histone levels were associated with malarial disease severity and death, reported Simon Abrams, PhD, of the University of Liverpool, UK, a coauthor of the study. He noted that this research could guide the development of treatment strategies for hundreds of thousands of patients each year, particularly children.
“Cerebral malaria is the most severe form of Plasmodium falciparum infection, and despite effective anti-malarial therapy, between 10% and 20% of children that develop cerebral malaria die,” Dr. Abrams said during his presentation at the annual meeting of the British Society for Haematology. “This accounts for a huge amount of deaths per annum. Around 400,000 malarial deaths are in children in subSaharan Africa, and death typically occurs within 24 hours of hospital admission.”
In CM, the blood-brain barrier deteriorates, leading to brain swelling, hemorrhaging, clot formation, and in many cases, death, Dr. Abrams said. CM patients with the worst outcomes typically have retinal abnormalities on fundic exam, granting the disease subtype “retinopathy-positive.”
Aided by colleagues in Malawi, the investigators gathered over 300 patient samples for analysis. They found that patients with retinopathy-positive CM had higher mean extracellular histone levels than retinopathy-negative CM patients and healthy controls (22.6 mcg/ml, 6.31 mcg/ml, and 0.33 mcg/ml, respectively). In addition, retinopathy-positive CM patients who died had significantly higher levels of circulating histones, compared with similar patients who survived (35.7 mcg/ml vs. 21.6 mcg/ml).
These findings translated to predictive capability, as the investigators showed that patients with CM who had elevated histones when admitted to the hospital were at a higher risk of death than those with normal histone levels (P = .04). Unlike patients with CM, patients with uncomplicated malaria had relatively low histone levels (0.57 mcg/ml), as did patients with mild non-malarial febrile illness (1.73 mcg/ml) and non-malarial coma (1.73 mcg/ml).
During his presentation, Dr. Abrams elaborated on the origins of these histones and how they contribute to poor outcomes in patients.
“Histones are small positively charged proteins that bind to negatively charged DNA,” Dr. Abrams said. “Typically, they are found within the cell nucleus, where they are involved in the packaging of DNA. However, during cell death and cell damage, histones are released from the nucleus, extracellularly, and we find that they are very much elevated in critically ill patients that have undergone huge amounts of cell death and damage.”
Once in circulation, histones can make a bad situation even worse.
“Work by ourselves and others around the globe have found that when circulating histones are elevated in these critically ill patients, they’re extremely toxic,” Dr. Abrams said. “Histones can induce endothelial damage and vascular permeability.” In addition, he pointed out that histones are pro-inflammatory and pro-coagulant. “If you bring all of these phenomena together,” he pointed out, “histones induce organ injury and mortality in critically ill patients.”
“The current hypothesis is that if you’re treating patients with these antimalarials, and it’s killing off the parasite, it may cause the histones to be released, which is actually worse for certain patients,” Dr. Abrams explained.
Based on this hypothesis, the investigators developed an anti-histone therapy.
“We’ve got a small peptide that we use to bind to the histones that reduces their toxicity,” Dr. Abrams said. “If we coincubate the serum of [CM] patients with our anti-histone reagent and then put this onto a monolayer of endothelial cells, we see that this toxicity is inhibited. Therefore, this is suggestive that a major toxic factor within these patients are the extracellular histones.”
Providing additional support for the role of histones in cerebral toxicity, postmortem brain tissue from patients with CM showed localization of histones to the endothelium, which has been tied with increased permeability of vascular tissue. In addition, “we are seeing co-localization between the histones and the sequestration of the malarial parasite itself,” Dr. Abrams said.
Concluding his presentation, he looked to the future.
“It’s difficult to get an animal model for malaria,” but he and his associates are currently working with other investigators to develop one. Once developed, the investigators plan on testing concurrent administration of anti-malarial therapy with antihistone therapy.
“What we’re hoping is that sometime in the future, maybe we’d be able to target circulating histones in this patient cohort to improve the survival of these patients,” Dr. Abrams said.
The investigators declared no conflicts of interest.
SOURCE: Moxon et al. BSH 2019. Abstract OR-034.
GLASGOW – Targeting circulating parasitic histones may hold promise for patients with cerebral malaria (CM), according to investigators.
A retrospective study, involving over 300 individuals, compared parasitic histone concentrations among patients with various forms of malaria and non-malarial illnesses, in addition to healthy controls, finding that elevated histone levels were associated with malarial disease severity and death, reported Simon Abrams, PhD, of the University of Liverpool, UK, a coauthor of the study. He noted that this research could guide the development of treatment strategies for hundreds of thousands of patients each year, particularly children.
“Cerebral malaria is the most severe form of Plasmodium falciparum infection, and despite effective anti-malarial therapy, between 10% and 20% of children that develop cerebral malaria die,” Dr. Abrams said during his presentation at the annual meeting of the British Society for Haematology. “This accounts for a huge amount of deaths per annum. Around 400,000 malarial deaths are in children in subSaharan Africa, and death typically occurs within 24 hours of hospital admission.”
In CM, the blood-brain barrier deteriorates, leading to brain swelling, hemorrhaging, clot formation, and in many cases, death, Dr. Abrams said. CM patients with the worst outcomes typically have retinal abnormalities on fundic exam, granting the disease subtype “retinopathy-positive.”
Aided by colleagues in Malawi, the investigators gathered over 300 patient samples for analysis. They found that patients with retinopathy-positive CM had higher mean extracellular histone levels than retinopathy-negative CM patients and healthy controls (22.6 mcg/ml, 6.31 mcg/ml, and 0.33 mcg/ml, respectively). In addition, retinopathy-positive CM patients who died had significantly higher levels of circulating histones, compared with similar patients who survived (35.7 mcg/ml vs. 21.6 mcg/ml).
These findings translated to predictive capability, as the investigators showed that patients with CM who had elevated histones when admitted to the hospital were at a higher risk of death than those with normal histone levels (P = .04). Unlike patients with CM, patients with uncomplicated malaria had relatively low histone levels (0.57 mcg/ml), as did patients with mild non-malarial febrile illness (1.73 mcg/ml) and non-malarial coma (1.73 mcg/ml).
During his presentation, Dr. Abrams elaborated on the origins of these histones and how they contribute to poor outcomes in patients.
“Histones are small positively charged proteins that bind to negatively charged DNA,” Dr. Abrams said. “Typically, they are found within the cell nucleus, where they are involved in the packaging of DNA. However, during cell death and cell damage, histones are released from the nucleus, extracellularly, and we find that they are very much elevated in critically ill patients that have undergone huge amounts of cell death and damage.”
Once in circulation, histones can make a bad situation even worse.
“Work by ourselves and others around the globe have found that when circulating histones are elevated in these critically ill patients, they’re extremely toxic,” Dr. Abrams said. “Histones can induce endothelial damage and vascular permeability.” In addition, he pointed out that histones are pro-inflammatory and pro-coagulant. “If you bring all of these phenomena together,” he pointed out, “histones induce organ injury and mortality in critically ill patients.”
“The current hypothesis is that if you’re treating patients with these antimalarials, and it’s killing off the parasite, it may cause the histones to be released, which is actually worse for certain patients,” Dr. Abrams explained.
Based on this hypothesis, the investigators developed an anti-histone therapy.
“We’ve got a small peptide that we use to bind to the histones that reduces their toxicity,” Dr. Abrams said. “If we coincubate the serum of [CM] patients with our anti-histone reagent and then put this onto a monolayer of endothelial cells, we see that this toxicity is inhibited. Therefore, this is suggestive that a major toxic factor within these patients are the extracellular histones.”
Providing additional support for the role of histones in cerebral toxicity, postmortem brain tissue from patients with CM showed localization of histones to the endothelium, which has been tied with increased permeability of vascular tissue. In addition, “we are seeing co-localization between the histones and the sequestration of the malarial parasite itself,” Dr. Abrams said.
Concluding his presentation, he looked to the future.
“It’s difficult to get an animal model for malaria,” but he and his associates are currently working with other investigators to develop one. Once developed, the investigators plan on testing concurrent administration of anti-malarial therapy with antihistone therapy.
“What we’re hoping is that sometime in the future, maybe we’d be able to target circulating histones in this patient cohort to improve the survival of these patients,” Dr. Abrams said.
The investigators declared no conflicts of interest.
SOURCE: Moxon et al. BSH 2019. Abstract OR-034.
REPORTING FROM BSH 2019
Oscillatory ventilation reduced reintubation risk for preterm infants
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
FROM CHEST
Survey finds high rate of complications from laser tattoo removal in non-clinic settings
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
REPORTING FROM ASLMS 2019
In sickle cell disease, opioid prescribing starts early, study finds
MILWAUKEE – A new study of for them.
The Medicaid claims database analysis looked at a one-year snapshot of prescriptions filled for a variety of opioids among children and young adults in North Carolina, said Nancy Crego, PhD, in an interview at a poster session of the scientific meeting of the American Pain Society.
Dr. Crego and her colleagues at Duke University School of Nursing, Durham, N.C., studied 1,560 children and young adults aged 0-22 years with sickle cell disease who received Medicaid; in all, 586 (38%) had an opioid prescription filled during the year-long study period.
Among adolescents and young adults with sickle cell disease, outpatient opioid prescriptions were common, with increasing prescription fills seen through the middle years and young adulthood. “Opioid prescription claims were prevalent across all age groups,” wrote Dr. Crego and her associates.
Though 20% of preschoolers (87 of 428) had had a prescription filled for opioids, the rates of opioid prescribing increased with age. Of adolescents aged 15-18 years, 54% (154 of 284) had filled an opioid prescription, as had 50% (110 of 221) of those aged 19-22 years.
For the 366 school-aged children aged 6-10 years, 117 (32%) had an opioid prescription filled. The number of prescriptions filled per patient on an annual basis for this age group ranged from one to 10.
There was a wide variation in the number of prescriptions filled in all other age groups over the study period as well. For school-aged children, the range was 1 to 10, and 1 to 18 for middle schoolers aged 11-14 years. Adolescents filled from 1-30 prescriptions, and for young adults, the range was 1-24.
Though the rates of opioid prescribing increased with age, the number of doses per prescription actually fell throughout the adolescent and young adult years. In an interview at the poster presentation, Dr. Crego speculated that this decrease observed with increasing age might reflect provider concern about opioid misuse and diversion, though the study methodology didn’t allow them to examine this.
Dr. Crego said that she was surprised by the high numbers of children who were receiving opioid prescriptions in the preschool years. “I wonder what their parents are being taught about how to administer these medications” to this very young age group, she commented.
Opioids included in the claims database analysis included morphine, hydromorphone, hydrocodone, oxycodone, oxymorphone, methadone, fentanyl, codeine, and tramadol.
Children with sickle cell disease are exposed to opioids in early childhood,” Dr. Crego and her colleagues wrote in the poster, but they acknowledged that “it is unknown if this early exposure increases the risk of opioid misuse later in life in this population ... Prescribers should incorporate continuous assessments for potential misuse and abuse in all age groups.”
“Most of the data that we have on opioid prescription claims in children usually exclude chronically ill children; they’re almost all of acutely ill children, and quite a bit of it is on postoperative care,” Dr. Crego said. The current study captures early-life prescribing “for somebody who’s going to be on opioids for a lot of their life,” she noted.
The studies of opioids used for acute pain, she said, showed that parents would often “administer opioids for inappropriate indications.” She is now conducting a qualitative study investigating pharmacologic and non-pharmacologic pain interventions for children with sickle cell disease. She’s also investigating how parents decide to administer opioids: “What did they see in their child that would prompt them to give an opioid versus giving another type of analgesic?”
There are some limitations to working with a claims database, acknowledged Dr. Crego: “We don’t know about their actual use, because we don’t know how often they are taking it, but we know it’s a filled opioid prescription.”
Dr. Crego said that more work is needed to examine how parents administer opioids to their children with sickle cell disease, and to learn more about what parents are told – and what they understand – about how their child’s pain should be managed. Also, she added, more research is needed on non-pharmacologic pain management for pediatric patients with sickle cell disease.
The study was funded by the Agency for Healthcare Research and Quality. Dr. Crego and her coauthors reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Crego, N. et al. APS 2019.
MILWAUKEE – A new study of for them.
The Medicaid claims database analysis looked at a one-year snapshot of prescriptions filled for a variety of opioids among children and young adults in North Carolina, said Nancy Crego, PhD, in an interview at a poster session of the scientific meeting of the American Pain Society.
Dr. Crego and her colleagues at Duke University School of Nursing, Durham, N.C., studied 1,560 children and young adults aged 0-22 years with sickle cell disease who received Medicaid; in all, 586 (38%) had an opioid prescription filled during the year-long study period.
Among adolescents and young adults with sickle cell disease, outpatient opioid prescriptions were common, with increasing prescription fills seen through the middle years and young adulthood. “Opioid prescription claims were prevalent across all age groups,” wrote Dr. Crego and her associates.
Though 20% of preschoolers (87 of 428) had had a prescription filled for opioids, the rates of opioid prescribing increased with age. Of adolescents aged 15-18 years, 54% (154 of 284) had filled an opioid prescription, as had 50% (110 of 221) of those aged 19-22 years.
For the 366 school-aged children aged 6-10 years, 117 (32%) had an opioid prescription filled. The number of prescriptions filled per patient on an annual basis for this age group ranged from one to 10.
There was a wide variation in the number of prescriptions filled in all other age groups over the study period as well. For school-aged children, the range was 1 to 10, and 1 to 18 for middle schoolers aged 11-14 years. Adolescents filled from 1-30 prescriptions, and for young adults, the range was 1-24.
Though the rates of opioid prescribing increased with age, the number of doses per prescription actually fell throughout the adolescent and young adult years. In an interview at the poster presentation, Dr. Crego speculated that this decrease observed with increasing age might reflect provider concern about opioid misuse and diversion, though the study methodology didn’t allow them to examine this.
Dr. Crego said that she was surprised by the high numbers of children who were receiving opioid prescriptions in the preschool years. “I wonder what their parents are being taught about how to administer these medications” to this very young age group, she commented.
Opioids included in the claims database analysis included morphine, hydromorphone, hydrocodone, oxycodone, oxymorphone, methadone, fentanyl, codeine, and tramadol.
Children with sickle cell disease are exposed to opioids in early childhood,” Dr. Crego and her colleagues wrote in the poster, but they acknowledged that “it is unknown if this early exposure increases the risk of opioid misuse later in life in this population ... Prescribers should incorporate continuous assessments for potential misuse and abuse in all age groups.”
“Most of the data that we have on opioid prescription claims in children usually exclude chronically ill children; they’re almost all of acutely ill children, and quite a bit of it is on postoperative care,” Dr. Crego said. The current study captures early-life prescribing “for somebody who’s going to be on opioids for a lot of their life,” she noted.
The studies of opioids used for acute pain, she said, showed that parents would often “administer opioids for inappropriate indications.” She is now conducting a qualitative study investigating pharmacologic and non-pharmacologic pain interventions for children with sickle cell disease. She’s also investigating how parents decide to administer opioids: “What did they see in their child that would prompt them to give an opioid versus giving another type of analgesic?”
There are some limitations to working with a claims database, acknowledged Dr. Crego: “We don’t know about their actual use, because we don’t know how often they are taking it, but we know it’s a filled opioid prescription.”
Dr. Crego said that more work is needed to examine how parents administer opioids to their children with sickle cell disease, and to learn more about what parents are told – and what they understand – about how their child’s pain should be managed. Also, she added, more research is needed on non-pharmacologic pain management for pediatric patients with sickle cell disease.
The study was funded by the Agency for Healthcare Research and Quality. Dr. Crego and her coauthors reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Crego, N. et al. APS 2019.
MILWAUKEE – A new study of for them.
The Medicaid claims database analysis looked at a one-year snapshot of prescriptions filled for a variety of opioids among children and young adults in North Carolina, said Nancy Crego, PhD, in an interview at a poster session of the scientific meeting of the American Pain Society.
Dr. Crego and her colleagues at Duke University School of Nursing, Durham, N.C., studied 1,560 children and young adults aged 0-22 years with sickle cell disease who received Medicaid; in all, 586 (38%) had an opioid prescription filled during the year-long study period.
Among adolescents and young adults with sickle cell disease, outpatient opioid prescriptions were common, with increasing prescription fills seen through the middle years and young adulthood. “Opioid prescription claims were prevalent across all age groups,” wrote Dr. Crego and her associates.
Though 20% of preschoolers (87 of 428) had had a prescription filled for opioids, the rates of opioid prescribing increased with age. Of adolescents aged 15-18 years, 54% (154 of 284) had filled an opioid prescription, as had 50% (110 of 221) of those aged 19-22 years.
For the 366 school-aged children aged 6-10 years, 117 (32%) had an opioid prescription filled. The number of prescriptions filled per patient on an annual basis for this age group ranged from one to 10.
There was a wide variation in the number of prescriptions filled in all other age groups over the study period as well. For school-aged children, the range was 1 to 10, and 1 to 18 for middle schoolers aged 11-14 years. Adolescents filled from 1-30 prescriptions, and for young adults, the range was 1-24.
Though the rates of opioid prescribing increased with age, the number of doses per prescription actually fell throughout the adolescent and young adult years. In an interview at the poster presentation, Dr. Crego speculated that this decrease observed with increasing age might reflect provider concern about opioid misuse and diversion, though the study methodology didn’t allow them to examine this.
Dr. Crego said that she was surprised by the high numbers of children who were receiving opioid prescriptions in the preschool years. “I wonder what their parents are being taught about how to administer these medications” to this very young age group, she commented.
Opioids included in the claims database analysis included morphine, hydromorphone, hydrocodone, oxycodone, oxymorphone, methadone, fentanyl, codeine, and tramadol.
Children with sickle cell disease are exposed to opioids in early childhood,” Dr. Crego and her colleagues wrote in the poster, but they acknowledged that “it is unknown if this early exposure increases the risk of opioid misuse later in life in this population ... Prescribers should incorporate continuous assessments for potential misuse and abuse in all age groups.”
“Most of the data that we have on opioid prescription claims in children usually exclude chronically ill children; they’re almost all of acutely ill children, and quite a bit of it is on postoperative care,” Dr. Crego said. The current study captures early-life prescribing “for somebody who’s going to be on opioids for a lot of their life,” she noted.
The studies of opioids used for acute pain, she said, showed that parents would often “administer opioids for inappropriate indications.” She is now conducting a qualitative study investigating pharmacologic and non-pharmacologic pain interventions for children with sickle cell disease. She’s also investigating how parents decide to administer opioids: “What did they see in their child that would prompt them to give an opioid versus giving another type of analgesic?”
There are some limitations to working with a claims database, acknowledged Dr. Crego: “We don’t know about their actual use, because we don’t know how often they are taking it, but we know it’s a filled opioid prescription.”
Dr. Crego said that more work is needed to examine how parents administer opioids to their children with sickle cell disease, and to learn more about what parents are told – and what they understand – about how their child’s pain should be managed. Also, she added, more research is needed on non-pharmacologic pain management for pediatric patients with sickle cell disease.
The study was funded by the Agency for Healthcare Research and Quality. Dr. Crego and her coauthors reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Crego, N. et al. APS 2019.
REPORTING FROM APS 2019