User login
NIH to undertake first in-human trial of universal influenza vaccine
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
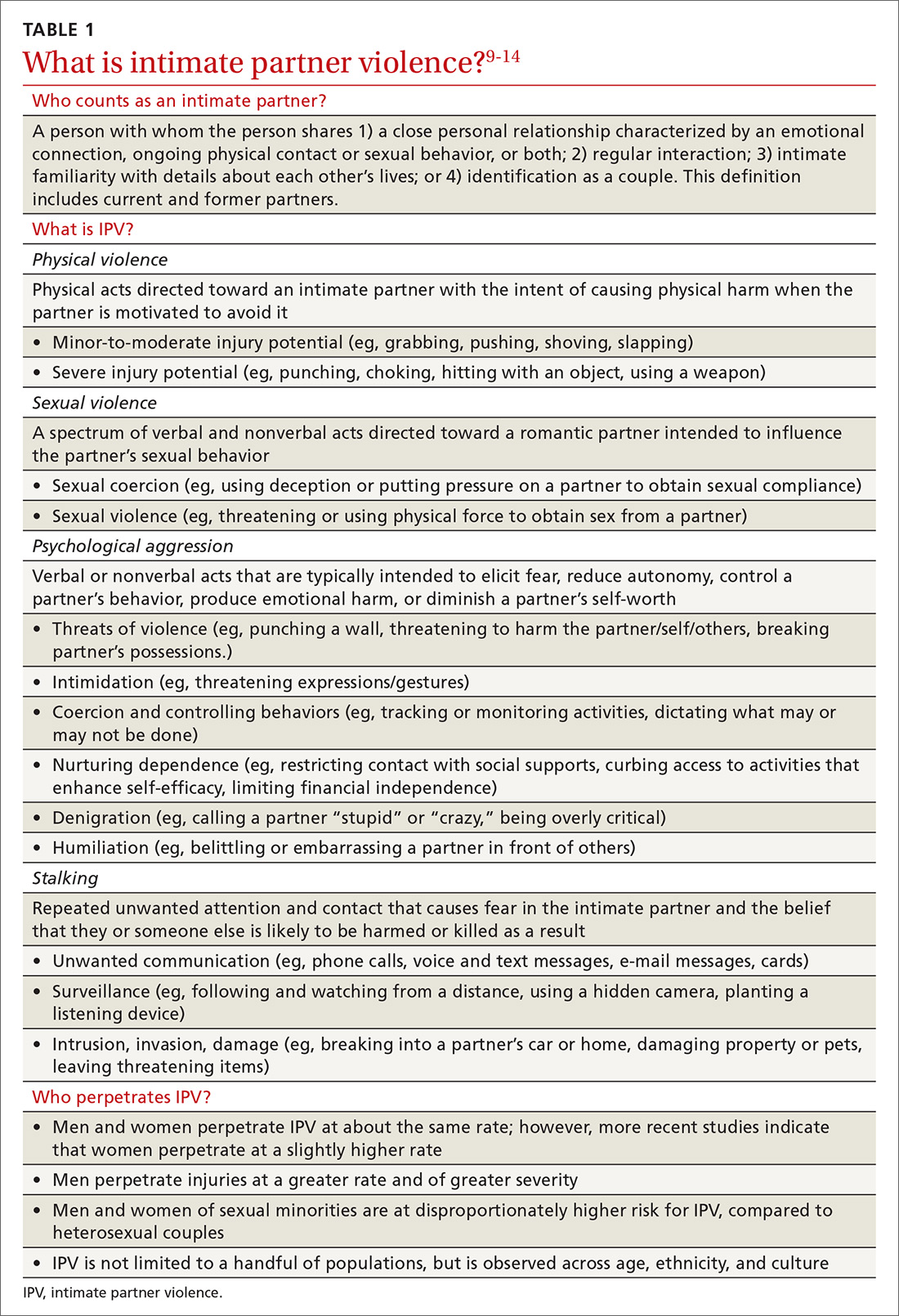
Leukemia Cutis–Associated Leonine Facies and Eyebrow Loss
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
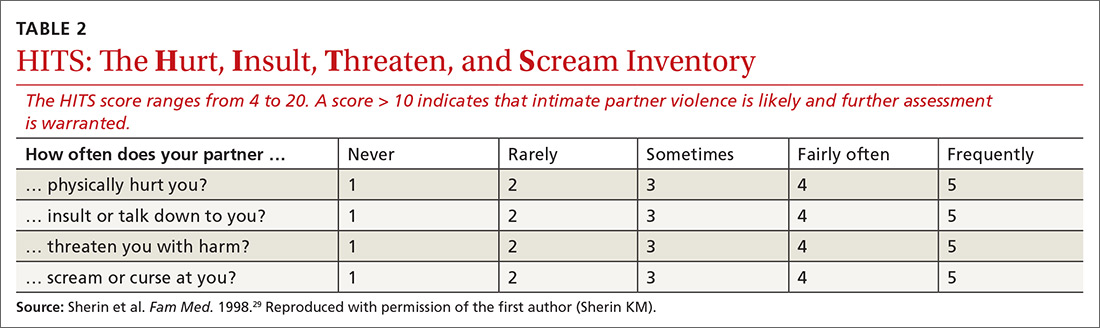
Clinical Pearl: Kinesiology Tape for Onychocryptosis
Practice Gap
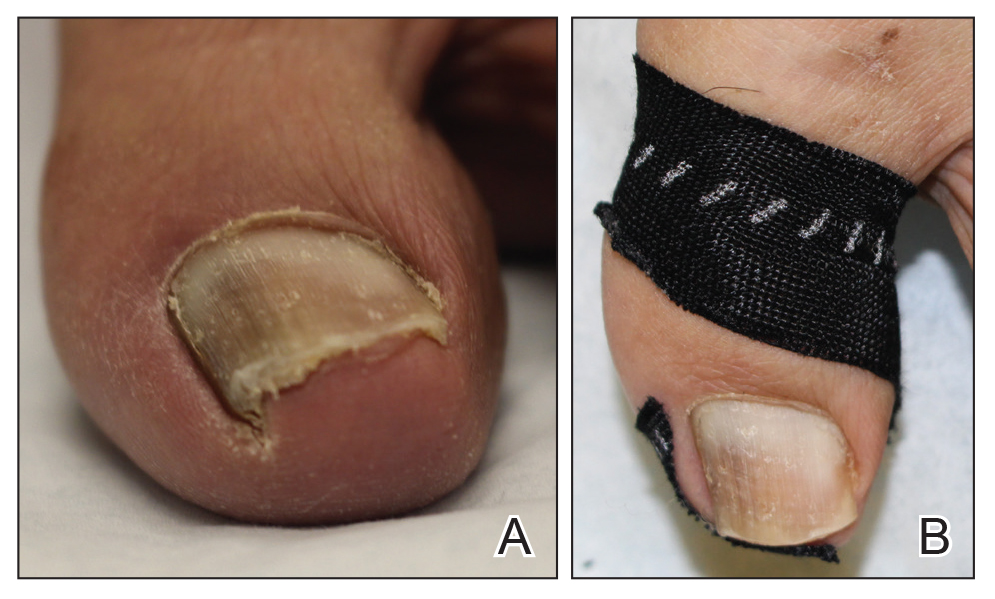
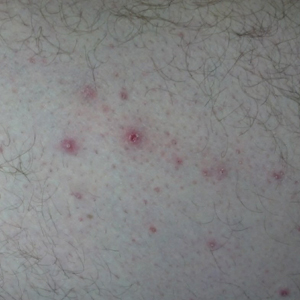
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
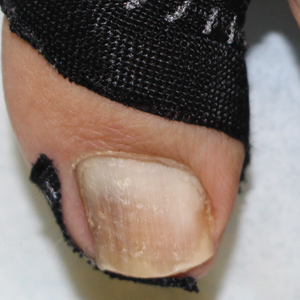
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Aspirin for primary prevention: USPSTF recommendations for CVD and colorectal cancer
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
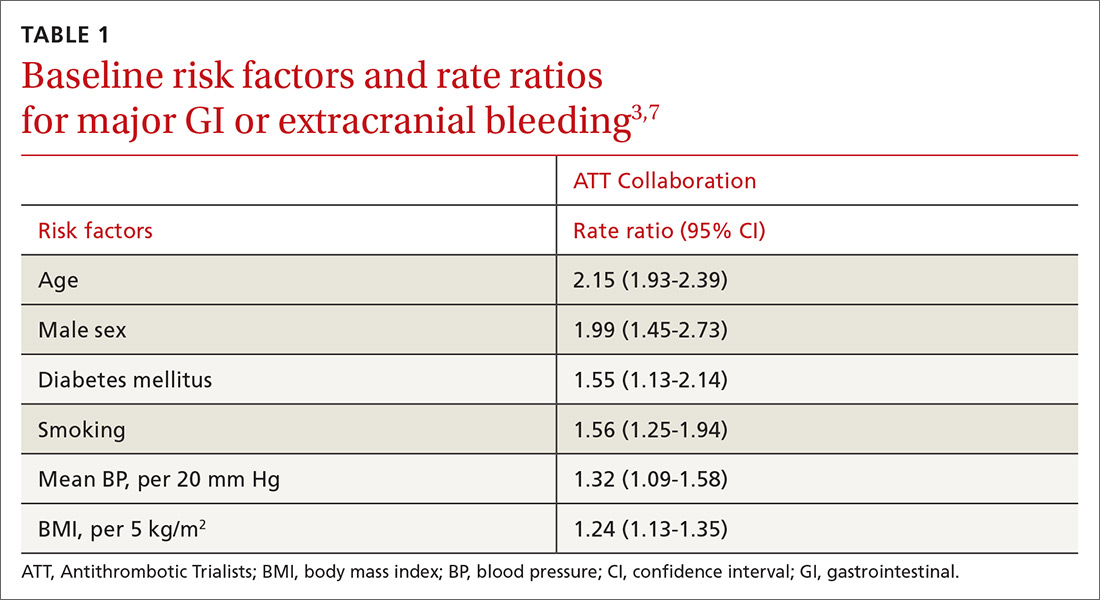
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
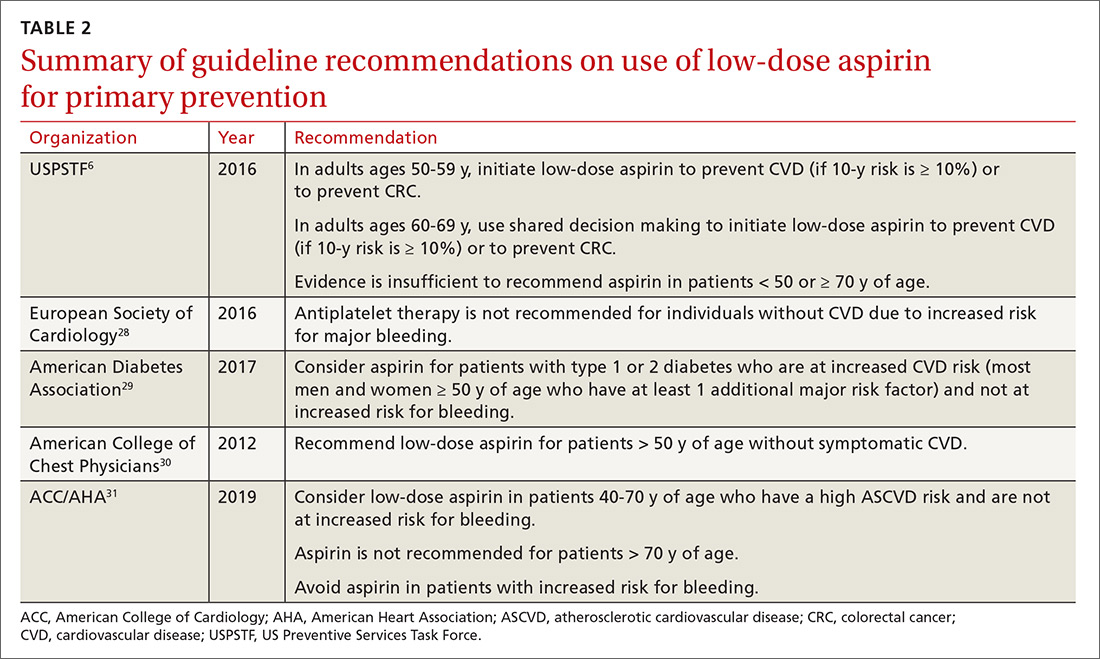
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
Which patients are likely to benefit from using aspirin for primary prevention? In this article, we review the evidence to date, summarized for primary care settings in guidelines issued by the US Preventive Services Task Force (USPSTF). We supplement this summary with a rundown of the risks associated with aspirin use. And then we wrap up by identifying a clinical decision tool that is available to help make personalized decisions in a busy clinic setting, where determining an individual’s potential cardiovascular benefits and bleeding risk can be challenging.
The “roadmap” from the guidelines. In 2014, after performing a review of the literature, the US Food and Drug Administration recommended against the routine use of aspirin for primary prevention of cardiovascular disease (CVD).1 In 2016, the USPSTF published 4 separate systematic reviews along with a decision analysis using a microsimulation model, which informed their position statement on aspirin for primary prevention.2-6 These USPSTF reviews and recommendations incorporated both CVD and colorectal cancer (CRC) benefits with the bleeding risks from aspirin. Generally, for individuals 50 to 59 years old, the benefits are deemed to outweigh the harms; shared decision making is advised with those 60 to 69 years of age. For patients younger than 50 or 70 and older, evidence is inconclusive.
The benefits of primary prevention with aspirin
Cardiovascular disease
The Antithrombotic Trialists’ (ATT) Collaboration was one of the first meta-analyses that addressed the benefit-to-harm balance and called into question the routine use of aspirin for primary prevention.7 The USPSTF systematic review included the studies from the ATT Collaboration as well as trials performed after its publication, bringing the total number of eligible randomized controlled trials reviewed to 11.2
The benefit of aspirin for primary prevention of nonfatal myocardial infarction (MI) has been shown in multiple randomized controlled trials. The USPSTF systematic review showed a statistically significant relative risk reduction of 17% in patients taking low-dose aspirin (≤ 100 mg; relative risk [RR] = 0.83; confidence interval [95% CI], 0.74-0.94), although the heterogeneity of the studies was high. The same low dose of aspirin showed a statistically significant reduction in nonfatal stroke (RR = 0.86; 95% CI, 0.76-0.98), although the same benefit was not observed when all doses of aspirin were included. Cardiovascular disease mortality and all-cause mortality were not statistically different for patients taking low-dose aspirin when compared with placebo (RR = 0.97; 95% CI, 0.85-1.10 for CVD mortality; RR = 0.95; 95% CI, 0.89-1.01 for all-cause mortality).2
One study of more than 14,000 older (≥ 60 years) Japanese patients showed a statistically significant reduction in nonfatal MI (hazard ratio [HR] = 0.53; 95% CI, 0.31-0.91, P = .02) and nonfatal strokes (HR = 0.57; 95% CI, 0.32-0.99; P = .04). The study was stopped early because at 5 years of follow-up there was no statistically significant difference in a composite primary outcome, which included death from cardiovascular causes, nonfatal MI, and nonfatal stroke (HR = 0.94; 95% CI, 0.77-1.15; P = .54).8
Several recent landmark studies have called into question the benefit of aspirin for cardiovascular primary prevention, especially in obese individuals, patients with diabetes, and the elderly. A meta-analysis of 10 trials showed that the effectiveness of aspirin doses between 75 mg and 100 mg for primary prevention decreased as weight increased; patients weighing 70 kg or more received no benefit.9 The ASCEND (A Study of Cardiovascular Events in Diabetes) trial included more than 15,000 patients with diabetes but no cardiovascular disease. Patients randomized to receive the low-dose aspirin did have fewer serious vascular events (incidence rate ratio [IRR] = 0.88; 95% CI, 0.79-0.97; P = .01), but they also had high risk of major bleeding events (IRR = 1.29; 95% CI, 1.09-1.52; P = .003).10 The ASPREE (Aspirin in Reducing Events in the Elderly) trial included more than 19,000 patients ages 70 years and older with no cardiovascular disease and compared low-dose aspirin to placebo. There was no statistically significant cardiovascular benefit, although there was an increase of major hemorrhage (HR = 1.38; 95% CI, 1.18-1.62; P < .001).11 The ARRIVE (A Randomized Trial of Induction Versus Expectant Management) trial included 12,546 moderate atherosclerotic CVD (ASCVD) risk patients. Although a per-protocol analysis showed a decrease in rates of fatal and nonfatal MI (HR = 0.53; 95% CI, 0.36-0.79; P = .0014), the more reliable intention-to-treat analysis showed no improvement for any outcomes.12
[polldaddy:10286821]
Colorectal cancer
The literature base on prevention of cancer has been growing rapidly. However, the deluge of findings over the past 2 decades of trials and analyses has also introduced ambiguity and, often, conflicting results. The first journal article suggesting aspirin for primary prevention of cancer, published in 1988, was a case-control study wherein a population with CRC was matched to controls to look for potential protective factors.13 The most notable finding was the CRC risk reduction for those taking aspirin or aspirin-containing medications. Since then numerous studies and analyses have explored aspirin’s potential in primary prevention of many types of cancer, with overall unclear findings as denoted in the 2016 USPSTF systemic reviews and recommendations.
Continue to: One major limiting factor...
One major limiting factor is that most data come from CVD prevention trials, and only a limited number of trials have focused specifically on cancer prevention. For the USPSTF, these data showed no statistically significant risk reduction in overall cancer mortality (RR = 0.96; 95% CI, 0.87-1.06) or in total cancer incidence (RR = 0.98; 95% CI, 0.93-1.04).4 Other ongoing trials may yield more definitive data.14
The particular interest in CRC was due to it being the first cancer found to be preventable with aspirin therapy. The USPSTF, while acknowledging the homogeneous nature of supporting studies, noted that their significant number and resulting evidence made CRC the only cancer warranting evaluation. Population studies have now shown more benefit than the few randomized control trials. The Women’s Health Study and the Physicians’ Health Study were both limited by their duration. But such studies conducted over a longer period revealed notable benefits in the second decade of use, with a statistically significant lower CRC incidence (RR = 0.60; 95% CI, 0.47-0.76). Additionally, CRC mortality at 20 years was decreased in patients taking aspirin regularly (RR = 0.67; 95% CI, 0.52-0.86).4 Multiple studies are in progress to better establish aspirin’s CRC benefit.
While not directly applicable to the general population, use of aspirin for patients with Lynch syndrome to prevent CRC has strong supporting evidence.15 Beyond CRC, there is nascent evidence from limited observational studies that aspirin may have a preventive effect on melanoma and ovarian and pancreatic cancers.16-18 Further studies or compilations of data would be needed to draw more significant conclusions on other types of cancers. Larger studies would prove more difficult to do, given the smaller incidences of these cancers.
Interestingly, a recent study showed that for individuals 70 years and older, aspirin might increase the risk for all-cause mortality, primarily due to increased cancer mortality across all types.19 Although this result was unexpected, caution should be used when prescribing aspirin particularly for patients 70 or older with active cancer.
A look at the harms associated with aspirin use
Aspirin has long been known to cause clinically significant bleeding. Aspirin inhibits platelet-derived cyclooxygenase-1 (COX-1), a potent vasoconstrictor, and thereby decreases platelet aggregation, reducing thromboembolic potential and prolonging bleeding time. These effects can confer health benefits but also carry the potential for risks. A decision to initiate aspirin therapy for primary prevention relies on an understanding of the benefit-to-harm balance.
Continue to: Initial aspirin studies...
Initial aspirin studies did not show a statistically significant increase in bleeding, likely due to too few events and inadequate powering. Subsequent meta-analyses from multiple evaluations have consistently shown bleeding to be a risk.3,7 The risk for bleeding with aspirin has also been examined in multiple cohort studies, which has helped elucidate the risk in greater detail.
Gastrointestinal bleeding
Epidemiologic data show that among patients who do not use nonsteroidal anti-inflammatory drugs (NSAIDs), the rate of upper gastrointestinal (GI) complications is 1 case per 1000 patient-years.20 Multiple studies have consistently shown that aspirin use increases the rate of significant upper GI bleeding over baseline risk (odds ratio [OR] = 1.54-1.58).3,21,22 Interestingly, these increases seem not to be influenced by other factors, such as comorbidities that increase the risk for ASCVD. Analysis of cancer prevention studies showed similar epidemiologic trends, with aspirin use exceeding a baseline bleeding risk of 0.7 cases of upper GI complications per 1000 patient-years (
Other risk factors. Evaluation of risk factors for bleeding primarily comes from 2 studies.3,7 Most data concern the impact of individual factors on significant GI bleeding, with fewer data available for evaluating risk for intracerebral hemorrhage (ICH). Initial analysis of individual prospective studies showed little or no correlation between risk for bleeding and such factors as gender, age, or history of hypertension or ASCVD.21 Subsequent analysis of meta-data and large cohorts did show statistically significant impact on rates of bleeding across several factors (TABLE 13,7).
Of note is a large heterogeneous cohort study conducted in Spain. Data showed significant increases in baseline risk for GI bleeding in older men with a history of GI bleeding and NSAID use. The absolute risk for GI bleed in this group was potentially as high as 150 cases per 1000 patient-years, well above the risk level assumed for the average patient.24 A seemingly small OR of 1.5 could dramatically increase the absolute risk for bleeding in such patients, and it suggests that a generalized risk for bleeding probably shouldn’t be applied to all patients. Individuals may be better served by a baseline risk calculation reflecting multiple factors.
Intracerebral hemorrhage
Due to the comparatively uncommon nature of ICH, fewer data are available to support definitive conclusions about its increased risk with aspirin use. Aspirin use appeared to increase the risk for ICH with ratios between 1.27 and 1.32 in meta-analyses (measured as an OR or as an RR),3,7,21 with an IRR of 1.54 in a cohort study.22 The only statistically significant factors suspected to increase the risk of ICH at baseline were smoking (RR = 2.18) and mean BP > 20 mm Hg over normal (OR = 2.18). Age, gender, and diabetes all showed a nonsignificant trend toward risk increase.7
Continue to: Risk based on dose and formulation
Risk based on dose and formulation
The effect of aspirin dose and formulation on bleeding risk is uncertain. Some studies have shown an increased risk for bleeding with daily doses of aspirin ≥ 300 mg, while others have shown no significant increase in rates for bleeding with differing doses.21,25 Enteric coating does appear to lower the rates of gastric mucosal injury, although there are few data on the effect toward reducing clinically significant bleeding.26 Currently, several prospective studies are underway to help clarify the evidence.27
Putting it all together
For the general population, the evidence shows that the benefits and harms of aspirin for primary prevention are relatively even. The USPSTF guidelines are the first to recommend aspirin for both CVD and cancer prevention while taking into account the bleeding risk. According to the findings of the USPSTF, the balance of benefits and harms of aspirin use is contingent on 4 factors: age, baseline CVD risk, risk for bleeding, and preferences about taking aspirin.6 The complete recommendations from the USPSTF, along with other leading organizations, are outlined in TABLE 2.6,28-31
Applying the evidence and varying guidelines in practice can feel daunting. Some practical tools have been developed to help clinicians understand patients’ bleeding risk and potential benefits with aspirin use. One such tool is highlighted below. Others are also available, and each has its own strengths and weaknesses.
Aspirin-Guide (www.aspiringuide.com) is a Web-based clinical decision support tool with an associated mobile application. It uses internal calculators (including the pooled cohort calculator prepared jointly by the American College of Cardiology and the American Heart Association) to assess CVD risk as well as bleeding risk. This tool gives clinicians patient-specific numbers-needed-to-treat and numbers-needed-to-harm when considering starting aspirin for primary prevention. It gives specific recommendations for aspirin use based on the data entered, and it also gives providers information to help guide shared decision-making with patients.32 Unfortunately, this decision support tool and others do not take into account the data from the most recent trials, so they should be used with caution.
CORRESPONDENCE
LCDR Dustin K. Smith, DO, Naval Branch Clinic Diego Garcia, PSC 466, Box 301, FPO, AP 96595; dustinksmith@yahoo.com.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
1. FDA. Use of aspirin for primary prevention of heart attack and stroke. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm390574.htm. Accessed March 22, 2019.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Chubak J, Whitlock EP, Williams SB, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:814-825.
5. Dehmer SP, Maciosek MV, Flottemesch TJ, et al. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777-786.
6. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Baigent C, Blackwell L, Colins R, et al; Antithrombotic Trialists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participation data from randomised trials. Lancet. 2009:373:1849-1860.
8. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510-2520.
9. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392:387-399.
10. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
12. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
13. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illness, operations, and medications: case control results from Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404.
14. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess. 2013;17:1-253.
15. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087.
16. Gamba CA, Swetter SM, Stefanick ML, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119:1562-1569.
17. Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431.
18. Risch H, Lu L, Streicher SA, et al. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2016;26:68-74.
19. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-1528.
20. Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55:157-163.
21. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis no 131. Rockville, MD: Agency for Healthcare Research and Quality; 2015. https://www.ncbi.nlm.nih.gov/books/NBK321623/. Accessed March 22, 2019.
22. De Berardis G, Lucisano G, D’Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294.
23. Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5-18.
24. Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
25. Huang ES, Strate LL, Ho WW, et al. Long term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011:124;426-433.
26. Walker J, Robinson J, Stewart J, et al. Does enteric-coated aspirin result in a lower incidence of gastrointestinal complications compared to normal aspirin? Interact Cardiovasc Thorac Surg. 2007:6;519-522.
27. NIH. Aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (ADAPTABLE). https://clinicaltrials.gov/ct2/show/NCT02697916. Accessed March 22, 2019.
28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315-2381.
29. ADA. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1). http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed March 22, 2019.
30. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
31. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Col Cardiol. 2019. doi: https://doi.org/10.1016/j.jacc.2019.03.010. Accessed March 22, 2019.
32. Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med. 2016;176:1195-1204.
PRACTICE RECOMMENDATIONS
› Consider aspirin for patients 50 to 59 years of age who have a 10-year cardiovascular disease (CVD) risk of ≥ 10% and low bleeding risk. C
› Discuss prophylactic aspirin (using a shared decision-making model) with patients 60 to 69 years of age who have a 10-year CVD risk of ≥ 10% and low bleeding risk. C
› Avoid using aspirin for primary prevention in patients ≥ 70 years of age. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What’s Eating You? Millipede Burns
Clinical Presentation
Millipedes secrete a noxious toxin implicated in millipede burns. The toxic substance is benzoquinone, a strong irritant secreted from the repugnatorial glands contained in each segment of the arthropod (Figure 1). This compound serves as a natural insect repellant, acting as the millipede’s defense mechanism from potential predators.1 On human skin, benzoquinone causes localized pigmentary changes most commonly presenting on the feet and toes. Local lesions may be associated with pain or burning, but there are no known reports of adverse systemic effects.2 Affected patients experience cutaneous pigmentary changes, which may be dark red, blue, or black, and spontaneously resolve over time.2 The degree of pigment change may be associated with duration of skin contact with the toxin. The affected areas may resemble burns, dermatitis, or skin necrosis. More distal lesions may present similarly to blue toe syndrome or acute arterial occlusion but can be differentiated by the presence of intact peripheral pulses and lack of temperature discrepancy between the feet.3,4 Histologic evaluation of the lesions generally reveals nonspecific full-thickness epidermal necrosis, making clinical suspicion and physical examination paramount to the diagnosis of millipede burns.5
Diagnostic Difficulties
Accurate diagnosis of millipede burns is more difficult when the burn involves an unusual site. The most common site of involvement is the foot (Figure 2), followed by other commonly exposed areas such as the arms, face, and eyes.2,3,6,7 Covered parts of the body are much less commonly affected, requiring the arthropod to gain access via infiltration of clothing, often when hanging on a clothesline. In these cases, burns may be mistaken for child abuse, especially if certain areas of the body are involved, such as the groin and genitals.2 The well-defined arcuate lesions of the burns may resemble injuries from a wire or belt to the unsuspecting observer.
Conclusion
Although millipedes often are regarded as harmless, they are capable of causing adverse reactions through the secretion of toxic chemicals. Millipede burns cause localized pigmentary changes that may be associated with pain or burning in some patients. Because these burns may resemble child abuse in pediatric patients, physicians should be aware of this diagnosis when unusual parts of the body are involved.
- Kuwahara Y, Omura H, Tanabe T. 2-Nitroethenylbenzenes as naturalproducts in millipede defense secretions. Naturwissenschaften. 2002;89:308-310.
- De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190.
- Heeren Neto AS, Bernardes Filho F, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258.
- Lima CA, Cardoso JL, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda class (“millipedes”). An Bras Dermatol. 2010;85:391-392.
- Dar NR, Raza N, Rehman SB. Millipede burn at an unusual site mimicking child abuse in an 8-year-old girl. Clin Pediatr (Phila). 2008;47:490-492.
- Hendrickson RG. Millipede exposure. Clin Toxicol (Phila). 2005;43:211-212.
- Verma AK, Bourke B. Millipede burn masquerading as trash foot in a paediatric patient [published online October 29, 2013]. ANZ J Surg. 2014;84:388-390.
Clinical Presentation
Millipedes secrete a noxious toxin implicated in millipede burns. The toxic substance is benzoquinone, a strong irritant secreted from the repugnatorial glands contained in each segment of the arthropod (Figure 1). This compound serves as a natural insect repellant, acting as the millipede’s defense mechanism from potential predators.1 On human skin, benzoquinone causes localized pigmentary changes most commonly presenting on the feet and toes. Local lesions may be associated with pain or burning, but there are no known reports of adverse systemic effects.2 Affected patients experience cutaneous pigmentary changes, which may be dark red, blue, or black, and spontaneously resolve over time.2 The degree of pigment change may be associated with duration of skin contact with the toxin. The affected areas may resemble burns, dermatitis, or skin necrosis. More distal lesions may present similarly to blue toe syndrome or acute arterial occlusion but can be differentiated by the presence of intact peripheral pulses and lack of temperature discrepancy between the feet.3,4 Histologic evaluation of the lesions generally reveals nonspecific full-thickness epidermal necrosis, making clinical suspicion and physical examination paramount to the diagnosis of millipede burns.5
Diagnostic Difficulties
Accurate diagnosis of millipede burns is more difficult when the burn involves an unusual site. The most common site of involvement is the foot (Figure 2), followed by other commonly exposed areas such as the arms, face, and eyes.2,3,6,7 Covered parts of the body are much less commonly affected, requiring the arthropod to gain access via infiltration of clothing, often when hanging on a clothesline. In these cases, burns may be mistaken for child abuse, especially if certain areas of the body are involved, such as the groin and genitals.2 The well-defined arcuate lesions of the burns may resemble injuries from a wire or belt to the unsuspecting observer.
Conclusion
Although millipedes often are regarded as harmless, they are capable of causing adverse reactions through the secretion of toxic chemicals. Millipede burns cause localized pigmentary changes that may be associated with pain or burning in some patients. Because these burns may resemble child abuse in pediatric patients, physicians should be aware of this diagnosis when unusual parts of the body are involved.
Clinical Presentation
Millipedes secrete a noxious toxin implicated in millipede burns. The toxic substance is benzoquinone, a strong irritant secreted from the repugnatorial glands contained in each segment of the arthropod (Figure 1). This compound serves as a natural insect repellant, acting as the millipede’s defense mechanism from potential predators.1 On human skin, benzoquinone causes localized pigmentary changes most commonly presenting on the feet and toes. Local lesions may be associated with pain or burning, but there are no known reports of adverse systemic effects.2 Affected patients experience cutaneous pigmentary changes, which may be dark red, blue, or black, and spontaneously resolve over time.2 The degree of pigment change may be associated with duration of skin contact with the toxin. The affected areas may resemble burns, dermatitis, or skin necrosis. More distal lesions may present similarly to blue toe syndrome or acute arterial occlusion but can be differentiated by the presence of intact peripheral pulses and lack of temperature discrepancy between the feet.3,4 Histologic evaluation of the lesions generally reveals nonspecific full-thickness epidermal necrosis, making clinical suspicion and physical examination paramount to the diagnosis of millipede burns.5
Diagnostic Difficulties
Accurate diagnosis of millipede burns is more difficult when the burn involves an unusual site. The most common site of involvement is the foot (Figure 2), followed by other commonly exposed areas such as the arms, face, and eyes.2,3,6,7 Covered parts of the body are much less commonly affected, requiring the arthropod to gain access via infiltration of clothing, often when hanging on a clothesline. In these cases, burns may be mistaken for child abuse, especially if certain areas of the body are involved, such as the groin and genitals.2 The well-defined arcuate lesions of the burns may resemble injuries from a wire or belt to the unsuspecting observer.
Conclusion
Although millipedes often are regarded as harmless, they are capable of causing adverse reactions through the secretion of toxic chemicals. Millipede burns cause localized pigmentary changes that may be associated with pain or burning in some patients. Because these burns may resemble child abuse in pediatric patients, physicians should be aware of this diagnosis when unusual parts of the body are involved.
- Kuwahara Y, Omura H, Tanabe T. 2-Nitroethenylbenzenes as naturalproducts in millipede defense secretions. Naturwissenschaften. 2002;89:308-310.
- De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190.
- Heeren Neto AS, Bernardes Filho F, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258.
- Lima CA, Cardoso JL, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda class (“millipedes”). An Bras Dermatol. 2010;85:391-392.
- Dar NR, Raza N, Rehman SB. Millipede burn at an unusual site mimicking child abuse in an 8-year-old girl. Clin Pediatr (Phila). 2008;47:490-492.
- Hendrickson RG. Millipede exposure. Clin Toxicol (Phila). 2005;43:211-212.
- Verma AK, Bourke B. Millipede burn masquerading as trash foot in a paediatric patient [published online October 29, 2013]. ANZ J Surg. 2014;84:388-390.
- Kuwahara Y, Omura H, Tanabe T. 2-Nitroethenylbenzenes as naturalproducts in millipede defense secretions. Naturwissenschaften. 2002;89:308-310.
- De Capitani EM, Vieira RJ, Bucaretchi F, et al. Human accidents involving Rhinocricus spp., a common millipede genus observed in urban areas of Brazil. Clin Toxicol (Phila). 2011;49:187-190.
- Heeren Neto AS, Bernardes Filho F, Martins G. Skin lesions simulating blue toe syndrome caused by prolonged contact with a millipede. Rev Soc Bras Med Trop. 2014;47:257-258.
- Lima CA, Cardoso JL, Magela A, et al. Exogenous pigmentation in toes feigning ischemia of the extremities: a diagnostic challenge brought by arthropods of the Diplopoda class (“millipedes”). An Bras Dermatol. 2010;85:391-392.
- Dar NR, Raza N, Rehman SB. Millipede burn at an unusual site mimicking child abuse in an 8-year-old girl. Clin Pediatr (Phila). 2008;47:490-492.
- Hendrickson RG. Millipede exposure. Clin Toxicol (Phila). 2005;43:211-212.
- Verma AK, Bourke B. Millipede burn masquerading as trash foot in a paediatric patient [published online October 29, 2013]. ANZ J Surg. 2014;84:388-390.
Practice Points
- The most common site of involvement of millipede burns is the foot, followed by other commonly exposed areas such as the arms, face, and eyes. Covered parts of the body are much less commonly affected.
- Millipede burns may resemble child abuse in pediatric patients; therefore, physicians should be aware of this diagnosis when unusual parts of the body are involved.
It’s time to start asking all patients about intimate partner violence
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
PRACTICE RECOMMENDATIONS
› Perform annual screening for intimate partner violence of all female patients of childbearing age; strongly consider a pilot program of universal screening (all male and female patients, across the lifespan). B
› Establish a protocol for intimate partner violence screening and referral—possibly the most effective means of identifying intimate partner violence at early and severe stages. B
› Collaborate with the patient in the safety planning and referral process; benefits include improved likelihood that the patient will adhere to a safety plan and follow through with the referral. B
› Utilize online resources to 1) ease the process of establishing relationships with local intimate partner violence referrals and 2) facilitate warm handoffs to increase the likelihood of patient engagement. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Parabens: The 2019 Nonallergen of the Year
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
No further amplification of signal for benefit from ultrathin stent at 3 years
WASHINGTON – In the latest analysis of data from a randomized trial comparing three different thin polymer-coated drug-eluting stents, the signal at 1 year that the thinnest device might reduce risk of target lesion revascularization has been lost at 3 years, according to an update of results from the BIO-RESORT trial presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“At 3-year follow-up, all three drug-eluting stents were associated with favorable outcomes and both very thin strut polymer-coated devices showed safety and patency similar to the thin-strut durable polymer drug-eluting stent,” reported Clemons von Birgelen, MD, PhD, professor of cardiology, University of Twente, Enschede, the Netherlands.
In order of strut thickness, the stents tested in BIO-RESORT were Orsiro (60 mcm), Synergy (74 mcm), and Resolute Integrity (90 mcm). Although the study had a noninferiority design, the potential for the biodegradable polymer coatings of the two thinner stents to provide faster healing than the durable polymer of the Resolute stent was one of the driving hypotheses of the trial (Lancet. 2016 Nov 26;388[10060]:2607-17).
Some support for this hypothesis was provided by 2-year results presented at EuroPCR 2018 last year. At that time, it was reported that the risk of target lesion revascularization between the end of year 1 and end of year 2 was significantly lower for the Orsiro stent (1.3%) than the Resolute stent (2.3%). Target lesion revascularization also was lower in the Synergy stent group (1.8%), but this rate did not differ significantly from that of the other two stents in the trial.
Now, reassessed at 3 years, the target lesion revascularization rates are 2.9%, 3.3%, and 3.8% for the Orsiro, Synergy, and Resolute stents, respectively. Although the numerical hierarchy is preserved, the differences are no longer significant.
Other outcomes, including the primary outcome of target lesion failure, show the same numerical hierarchy but, again, without differences reaching significance. For target lesion failure, these rates are 8.5%, 8.8%, and 10.0%, respectively.
The difference in the rates of stent thrombosis at 3 years was even smaller with rates of less than 1% for all three stents. A catch-up phenomenon between years 2 and 3 of follow-up largely eliminated a numerical advantage seen earlier for the Orsiro stent.
The BIO-RESORT trial randomized 3,514 patients, of whom 70% had an acute coronary syndrome. Nearly one-third had an ST-elevated myocardial infarction. Dr. von Birgelen emphasized that this was “a very complex study population.” For example, roughly 20% had severely calcified lesions. Follow-up data were available on 97% of the randomized patients at 3 years.
There are differences between these stents other than thickness and the durability of the polymer. In particular, Orsiro is coated with sirolimus, Synergy with everolimus, and Resolute with zotarolimus. While the metals of the frame also differ, the estimated time to resorption of the polymer is faster with the Synergy stent (4 months) than the Orsiro stent (24 months).
Despite the loss of a difference in target vessel revascularization in the most recent follow-up, the potential for the differences in designs and materials to influence risk of late complications, including revascularization and thrombosis, persists.
“Follow-up beyond 3 years is of interest to definitely answer the question of whether one of these drug-eluting stents might improve outcome at a later stage,” Dr. von Birgelen said.
WASHINGTON – In the latest analysis of data from a randomized trial comparing three different thin polymer-coated drug-eluting stents, the signal at 1 year that the thinnest device might reduce risk of target lesion revascularization has been lost at 3 years, according to an update of results from the BIO-RESORT trial presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“At 3-year follow-up, all three drug-eluting stents were associated with favorable outcomes and both very thin strut polymer-coated devices showed safety and patency similar to the thin-strut durable polymer drug-eluting stent,” reported Clemons von Birgelen, MD, PhD, professor of cardiology, University of Twente, Enschede, the Netherlands.
In order of strut thickness, the stents tested in BIO-RESORT were Orsiro (60 mcm), Synergy (74 mcm), and Resolute Integrity (90 mcm). Although the study had a noninferiority design, the potential for the biodegradable polymer coatings of the two thinner stents to provide faster healing than the durable polymer of the Resolute stent was one of the driving hypotheses of the trial (Lancet. 2016 Nov 26;388[10060]:2607-17).
Some support for this hypothesis was provided by 2-year results presented at EuroPCR 2018 last year. At that time, it was reported that the risk of target lesion revascularization between the end of year 1 and end of year 2 was significantly lower for the Orsiro stent (1.3%) than the Resolute stent (2.3%). Target lesion revascularization also was lower in the Synergy stent group (1.8%), but this rate did not differ significantly from that of the other two stents in the trial.
Now, reassessed at 3 years, the target lesion revascularization rates are 2.9%, 3.3%, and 3.8% for the Orsiro, Synergy, and Resolute stents, respectively. Although the numerical hierarchy is preserved, the differences are no longer significant.
Other outcomes, including the primary outcome of target lesion failure, show the same numerical hierarchy but, again, without differences reaching significance. For target lesion failure, these rates are 8.5%, 8.8%, and 10.0%, respectively.
The difference in the rates of stent thrombosis at 3 years was even smaller with rates of less than 1% for all three stents. A catch-up phenomenon between years 2 and 3 of follow-up largely eliminated a numerical advantage seen earlier for the Orsiro stent.
The BIO-RESORT trial randomized 3,514 patients, of whom 70% had an acute coronary syndrome. Nearly one-third had an ST-elevated myocardial infarction. Dr. von Birgelen emphasized that this was “a very complex study population.” For example, roughly 20% had severely calcified lesions. Follow-up data were available on 97% of the randomized patients at 3 years.
There are differences between these stents other than thickness and the durability of the polymer. In particular, Orsiro is coated with sirolimus, Synergy with everolimus, and Resolute with zotarolimus. While the metals of the frame also differ, the estimated time to resorption of the polymer is faster with the Synergy stent (4 months) than the Orsiro stent (24 months).
Despite the loss of a difference in target vessel revascularization in the most recent follow-up, the potential for the differences in designs and materials to influence risk of late complications, including revascularization and thrombosis, persists.
“Follow-up beyond 3 years is of interest to definitely answer the question of whether one of these drug-eluting stents might improve outcome at a later stage,” Dr. von Birgelen said.
WASHINGTON – In the latest analysis of data from a randomized trial comparing three different thin polymer-coated drug-eluting stents, the signal at 1 year that the thinnest device might reduce risk of target lesion revascularization has been lost at 3 years, according to an update of results from the BIO-RESORT trial presented at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
“At 3-year follow-up, all three drug-eluting stents were associated with favorable outcomes and both very thin strut polymer-coated devices showed safety and patency similar to the thin-strut durable polymer drug-eluting stent,” reported Clemons von Birgelen, MD, PhD, professor of cardiology, University of Twente, Enschede, the Netherlands.
In order of strut thickness, the stents tested in BIO-RESORT were Orsiro (60 mcm), Synergy (74 mcm), and Resolute Integrity (90 mcm). Although the study had a noninferiority design, the potential for the biodegradable polymer coatings of the two thinner stents to provide faster healing than the durable polymer of the Resolute stent was one of the driving hypotheses of the trial (Lancet. 2016 Nov 26;388[10060]:2607-17).
Some support for this hypothesis was provided by 2-year results presented at EuroPCR 2018 last year. At that time, it was reported that the risk of target lesion revascularization between the end of year 1 and end of year 2 was significantly lower for the Orsiro stent (1.3%) than the Resolute stent (2.3%). Target lesion revascularization also was lower in the Synergy stent group (1.8%), but this rate did not differ significantly from that of the other two stents in the trial.
Now, reassessed at 3 years, the target lesion revascularization rates are 2.9%, 3.3%, and 3.8% for the Orsiro, Synergy, and Resolute stents, respectively. Although the numerical hierarchy is preserved, the differences are no longer significant.
Other outcomes, including the primary outcome of target lesion failure, show the same numerical hierarchy but, again, without differences reaching significance. For target lesion failure, these rates are 8.5%, 8.8%, and 10.0%, respectively.
The difference in the rates of stent thrombosis at 3 years was even smaller with rates of less than 1% for all three stents. A catch-up phenomenon between years 2 and 3 of follow-up largely eliminated a numerical advantage seen earlier for the Orsiro stent.
The BIO-RESORT trial randomized 3,514 patients, of whom 70% had an acute coronary syndrome. Nearly one-third had an ST-elevated myocardial infarction. Dr. von Birgelen emphasized that this was “a very complex study population.” For example, roughly 20% had severely calcified lesions. Follow-up data were available on 97% of the randomized patients at 3 years.
There are differences between these stents other than thickness and the durability of the polymer. In particular, Orsiro is coated with sirolimus, Synergy with everolimus, and Resolute with zotarolimus. While the metals of the frame also differ, the estimated time to resorption of the polymer is faster with the Synergy stent (4 months) than the Orsiro stent (24 months).
Despite the loss of a difference in target vessel revascularization in the most recent follow-up, the potential for the differences in designs and materials to influence risk of late complications, including revascularization and thrombosis, persists.
“Follow-up beyond 3 years is of interest to definitely answer the question of whether one of these drug-eluting stents might improve outcome at a later stage,” Dr. von Birgelen said.
REPORTING FROM CRT 2019
HM19 Day One highlights: Plenary and sepsis updates (VIDEO)

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.
Scurvy Masquerading as Reactive Arthritis
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
Practice Points
- Patients with scurvy often pose a diagnostic dilemma because their presenting symptoms can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups.
- The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency with rapid resolution upon vitamin C supplementation.