User login
How to cope with patients who get under your skin
DENVER – If you get the impression you’re dealing with more angry and manipulative patients than usual in the past several years, you’re not alone.
In 1999, as many as 15% of patient encounters were rated as difficult by health care providers, according to Tina S. Alster, MD (Arch Intern Med. 1999;159:1069-75). Today, upward of 30% of patient encounters are deemed difficult (Int J Res Med Sci. 2016;4[8]:3554-62). “That means one in three patients that you see have a problem that goes beyond the scope of our official training,” said Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “If it was limited to a medical problem, we could handle it; that’s what we’re trained to do. The interpersonal issues and psychosocial implications of treatment are much more difficult.”
At the annual conference of the American Society for Laser Medicine and Surgery, Dr. Alster said that difficult patients put a strain on your practice and your relationship with them. “These patients put you on your heels. They often point out a problem that may or may not be related to something that you’re responsible for. It’s not usually because you are running late on the day of their visit and you make them late for something else. That situation is one you can prepare for, because you know you’re running late. It’s the other stuff you don’t realize that’s going on, which can cause problems.”
Setting limits
Being proactive can help lessen the ripple effect from patients who rock the boat. “You want to set limits with those patients before you even see them,” Dr. Alster said. “There are written contracts and policies that you should have in place. Since I perform mostly cosmetic procedures in my office, it is important that patients are made aware that payment is expected at the time of service and that my office does not bill nor accept insurance payments in order to prevent misunderstandings at check out.” She also declines requests to provide expert testimony for legal cases. “I’ve been in this business a long time, and every day I get requests to be an expert or to review a case involving a provider who may or may not have made a mistake. I really hate going down that rabbit hole.”
Another solution to keeping difficult patients in check is to collaborate with your entire office staff on how to best deal with them. “You need to present a united front with these patients: They’re going to divide and conquer, with complaints like, ‘How dare the doctor be so late. I’ve been waiting here for 30 minutes. Doesn’t she know I’m busy?’ ” Dr. Alster said. “You’re going to have to give them the tools to set limits as well. During our staff meetings, we review the upcoming patient schedule and identify potentially difficult situations in order to make sure the team is on the same page. Because my office is located in the power corridor of Washington [three blocks away from the White House] and my patient base is populated by prevalent personalities, expectations are extraordinarily high. As such, it is important that we set limits and rules that everyone can play by.”
Getting a sense of whether a patient is angry or manipulative can also help. An angry patient often holds an expectation that has been unmet, or harbors fears related to the treatment itself, she said, while a manipulative patient may engage in bullying, excessive flattery, or veiled threats. “They often expect specific treatments that have only worked for them in the past, even though it may be opposed to the treatment you recommend. They know better than you, even though you’re the expert.”
Communicating effectively with these two types of patients requires a slightly different approach. “With an angry patient, you want to share decision making,” Dr. Alster said. “Have them voice their concerns and come up with a plan together going forward. You’re not going to make that person less angry by telling them what to do.” The manipulative patient, meanwhile, requires a team approach. For example, she may ask your medical assistant for opinions on the treatment option you recommended during your office consultation with her, or second-guess your recommendation with that person altogether. “Everybody needs to know who the manipulative patients are and approach them as a team.”
The art of nonconfrontation
Dr. Alster brings a nonconfrontational approach to interactions with difficult patients. “You can apologize if you’ve kept them waiting, but you can’t apologize for everything all the time,” she said. “I may say something like, ‘I appreciate that your visit is running late. I apologize for the delay and want you to know that we take as much time as necessary for each patient and that unforeseen circumstances beyond our control sometimes arise.’ ” Another phrase she may use is, “I understand that this has been a stressful visit, but I want to talk to you about your experience and identify how we can improve subsequent appointments.”
Showing empathy never hurts. “Repeat back to them what you heard, and establish the fact that you understand,” Dr. Alster said. “Lower your voice, talk slowly, don’t get caught up in emotion. Otherwise, you’re going down in a sinkhole with them. Be wrong to be right. This encourages negotiation. You also want to document all patient interactions. Put every correspondence in the patient’s EMR.”
Dr. Alster advises clinicians to provide an outline of office policies and procedures to all patients, as well as written and verbal instructions related to their care. She also phones or emails patients undergoing a treatment for the first time. “Even if they’ve been in the practice for several years, if they received filler injections for the first time [instead of Botox], we still check in with those patients when they receive a first-time treatment to make sure they’re doing okay,” she said. “We’ll call them that evening or at the very least early the next morning to make sure that they don’t have any questions or concerns.”
If problems persist despite your best efforts, sometimes your best option is to dismiss difficult patients from your practice. “That’s only when everything else fails,” Dr. Alster said. “A concise termination letter should state a ‘breakdown in physician-patient relationship.’ I call it my ‘Dear John’ letter, and since 1990, I’ve only written six of these. A detailed explanation is usually not needed, but may be advisable depending on your state, to protect yourself from a liability standpoint. I instruct patients to contact the state medical society for referral to another provider and inform them that upon their written request, their medical records will be forwarded to their new provider. I also set up a reasonable timeline during which I will continue to see them for emergency visits to ensure that there is continuity of care, even when it is a cosmetic situation.”
Dr. Alster reported having no financial disclosures related to her presentation.
DENVER – If you get the impression you’re dealing with more angry and manipulative patients than usual in the past several years, you’re not alone.
In 1999, as many as 15% of patient encounters were rated as difficult by health care providers, according to Tina S. Alster, MD (Arch Intern Med. 1999;159:1069-75). Today, upward of 30% of patient encounters are deemed difficult (Int J Res Med Sci. 2016;4[8]:3554-62). “That means one in three patients that you see have a problem that goes beyond the scope of our official training,” said Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “If it was limited to a medical problem, we could handle it; that’s what we’re trained to do. The interpersonal issues and psychosocial implications of treatment are much more difficult.”
At the annual conference of the American Society for Laser Medicine and Surgery, Dr. Alster said that difficult patients put a strain on your practice and your relationship with them. “These patients put you on your heels. They often point out a problem that may or may not be related to something that you’re responsible for. It’s not usually because you are running late on the day of their visit and you make them late for something else. That situation is one you can prepare for, because you know you’re running late. It’s the other stuff you don’t realize that’s going on, which can cause problems.”
Setting limits
Being proactive can help lessen the ripple effect from patients who rock the boat. “You want to set limits with those patients before you even see them,” Dr. Alster said. “There are written contracts and policies that you should have in place. Since I perform mostly cosmetic procedures in my office, it is important that patients are made aware that payment is expected at the time of service and that my office does not bill nor accept insurance payments in order to prevent misunderstandings at check out.” She also declines requests to provide expert testimony for legal cases. “I’ve been in this business a long time, and every day I get requests to be an expert or to review a case involving a provider who may or may not have made a mistake. I really hate going down that rabbit hole.”
Another solution to keeping difficult patients in check is to collaborate with your entire office staff on how to best deal with them. “You need to present a united front with these patients: They’re going to divide and conquer, with complaints like, ‘How dare the doctor be so late. I’ve been waiting here for 30 minutes. Doesn’t she know I’m busy?’ ” Dr. Alster said. “You’re going to have to give them the tools to set limits as well. During our staff meetings, we review the upcoming patient schedule and identify potentially difficult situations in order to make sure the team is on the same page. Because my office is located in the power corridor of Washington [three blocks away from the White House] and my patient base is populated by prevalent personalities, expectations are extraordinarily high. As such, it is important that we set limits and rules that everyone can play by.”
Getting a sense of whether a patient is angry or manipulative can also help. An angry patient often holds an expectation that has been unmet, or harbors fears related to the treatment itself, she said, while a manipulative patient may engage in bullying, excessive flattery, or veiled threats. “They often expect specific treatments that have only worked for them in the past, even though it may be opposed to the treatment you recommend. They know better than you, even though you’re the expert.”
Communicating effectively with these two types of patients requires a slightly different approach. “With an angry patient, you want to share decision making,” Dr. Alster said. “Have them voice their concerns and come up with a plan together going forward. You’re not going to make that person less angry by telling them what to do.” The manipulative patient, meanwhile, requires a team approach. For example, she may ask your medical assistant for opinions on the treatment option you recommended during your office consultation with her, or second-guess your recommendation with that person altogether. “Everybody needs to know who the manipulative patients are and approach them as a team.”
The art of nonconfrontation
Dr. Alster brings a nonconfrontational approach to interactions with difficult patients. “You can apologize if you’ve kept them waiting, but you can’t apologize for everything all the time,” she said. “I may say something like, ‘I appreciate that your visit is running late. I apologize for the delay and want you to know that we take as much time as necessary for each patient and that unforeseen circumstances beyond our control sometimes arise.’ ” Another phrase she may use is, “I understand that this has been a stressful visit, but I want to talk to you about your experience and identify how we can improve subsequent appointments.”
Showing empathy never hurts. “Repeat back to them what you heard, and establish the fact that you understand,” Dr. Alster said. “Lower your voice, talk slowly, don’t get caught up in emotion. Otherwise, you’re going down in a sinkhole with them. Be wrong to be right. This encourages negotiation. You also want to document all patient interactions. Put every correspondence in the patient’s EMR.”
Dr. Alster advises clinicians to provide an outline of office policies and procedures to all patients, as well as written and verbal instructions related to their care. She also phones or emails patients undergoing a treatment for the first time. “Even if they’ve been in the practice for several years, if they received filler injections for the first time [instead of Botox], we still check in with those patients when they receive a first-time treatment to make sure they’re doing okay,” she said. “We’ll call them that evening or at the very least early the next morning to make sure that they don’t have any questions or concerns.”
If problems persist despite your best efforts, sometimes your best option is to dismiss difficult patients from your practice. “That’s only when everything else fails,” Dr. Alster said. “A concise termination letter should state a ‘breakdown in physician-patient relationship.’ I call it my ‘Dear John’ letter, and since 1990, I’ve only written six of these. A detailed explanation is usually not needed, but may be advisable depending on your state, to protect yourself from a liability standpoint. I instruct patients to contact the state medical society for referral to another provider and inform them that upon their written request, their medical records will be forwarded to their new provider. I also set up a reasonable timeline during which I will continue to see them for emergency visits to ensure that there is continuity of care, even when it is a cosmetic situation.”
Dr. Alster reported having no financial disclosures related to her presentation.
DENVER – If you get the impression you’re dealing with more angry and manipulative patients than usual in the past several years, you’re not alone.
In 1999, as many as 15% of patient encounters were rated as difficult by health care providers, according to Tina S. Alster, MD (Arch Intern Med. 1999;159:1069-75). Today, upward of 30% of patient encounters are deemed difficult (Int J Res Med Sci. 2016;4[8]:3554-62). “That means one in three patients that you see have a problem that goes beyond the scope of our official training,” said Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “If it was limited to a medical problem, we could handle it; that’s what we’re trained to do. The interpersonal issues and psychosocial implications of treatment are much more difficult.”
At the annual conference of the American Society for Laser Medicine and Surgery, Dr. Alster said that difficult patients put a strain on your practice and your relationship with them. “These patients put you on your heels. They often point out a problem that may or may not be related to something that you’re responsible for. It’s not usually because you are running late on the day of their visit and you make them late for something else. That situation is one you can prepare for, because you know you’re running late. It’s the other stuff you don’t realize that’s going on, which can cause problems.”
Setting limits
Being proactive can help lessen the ripple effect from patients who rock the boat. “You want to set limits with those patients before you even see them,” Dr. Alster said. “There are written contracts and policies that you should have in place. Since I perform mostly cosmetic procedures in my office, it is important that patients are made aware that payment is expected at the time of service and that my office does not bill nor accept insurance payments in order to prevent misunderstandings at check out.” She also declines requests to provide expert testimony for legal cases. “I’ve been in this business a long time, and every day I get requests to be an expert or to review a case involving a provider who may or may not have made a mistake. I really hate going down that rabbit hole.”
Another solution to keeping difficult patients in check is to collaborate with your entire office staff on how to best deal with them. “You need to present a united front with these patients: They’re going to divide and conquer, with complaints like, ‘How dare the doctor be so late. I’ve been waiting here for 30 minutes. Doesn’t she know I’m busy?’ ” Dr. Alster said. “You’re going to have to give them the tools to set limits as well. During our staff meetings, we review the upcoming patient schedule and identify potentially difficult situations in order to make sure the team is on the same page. Because my office is located in the power corridor of Washington [three blocks away from the White House] and my patient base is populated by prevalent personalities, expectations are extraordinarily high. As such, it is important that we set limits and rules that everyone can play by.”
Getting a sense of whether a patient is angry or manipulative can also help. An angry patient often holds an expectation that has been unmet, or harbors fears related to the treatment itself, she said, while a manipulative patient may engage in bullying, excessive flattery, or veiled threats. “They often expect specific treatments that have only worked for them in the past, even though it may be opposed to the treatment you recommend. They know better than you, even though you’re the expert.”
Communicating effectively with these two types of patients requires a slightly different approach. “With an angry patient, you want to share decision making,” Dr. Alster said. “Have them voice their concerns and come up with a plan together going forward. You’re not going to make that person less angry by telling them what to do.” The manipulative patient, meanwhile, requires a team approach. For example, she may ask your medical assistant for opinions on the treatment option you recommended during your office consultation with her, or second-guess your recommendation with that person altogether. “Everybody needs to know who the manipulative patients are and approach them as a team.”
The art of nonconfrontation
Dr. Alster brings a nonconfrontational approach to interactions with difficult patients. “You can apologize if you’ve kept them waiting, but you can’t apologize for everything all the time,” she said. “I may say something like, ‘I appreciate that your visit is running late. I apologize for the delay and want you to know that we take as much time as necessary for each patient and that unforeseen circumstances beyond our control sometimes arise.’ ” Another phrase she may use is, “I understand that this has been a stressful visit, but I want to talk to you about your experience and identify how we can improve subsequent appointments.”
Showing empathy never hurts. “Repeat back to them what you heard, and establish the fact that you understand,” Dr. Alster said. “Lower your voice, talk slowly, don’t get caught up in emotion. Otherwise, you’re going down in a sinkhole with them. Be wrong to be right. This encourages negotiation. You also want to document all patient interactions. Put every correspondence in the patient’s EMR.”
Dr. Alster advises clinicians to provide an outline of office policies and procedures to all patients, as well as written and verbal instructions related to their care. She also phones or emails patients undergoing a treatment for the first time. “Even if they’ve been in the practice for several years, if they received filler injections for the first time [instead of Botox], we still check in with those patients when they receive a first-time treatment to make sure they’re doing okay,” she said. “We’ll call them that evening or at the very least early the next morning to make sure that they don’t have any questions or concerns.”
If problems persist despite your best efforts, sometimes your best option is to dismiss difficult patients from your practice. “That’s only when everything else fails,” Dr. Alster said. “A concise termination letter should state a ‘breakdown in physician-patient relationship.’ I call it my ‘Dear John’ letter, and since 1990, I’ve only written six of these. A detailed explanation is usually not needed, but may be advisable depending on your state, to protect yourself from a liability standpoint. I instruct patients to contact the state medical society for referral to another provider and inform them that upon their written request, their medical records will be forwarded to their new provider. I also set up a reasonable timeline during which I will continue to see them for emergency visits to ensure that there is continuity of care, even when it is a cosmetic situation.”
Dr. Alster reported having no financial disclosures related to her presentation.
EXPERT ANALYSIS FROM ASLMS 2019
Highlights from the 2019 Society of Gynecologic Surgeons Scientific Meeting

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota

- Rising to the challenges in gynecologic surgical care
- Anterior, apical, posterior: Vaginal anatomy for the gynecologic surgeon
- Beyond enhanced recovery after surgery
B. Star Hampton, MD
Professor
Department of Obstetrics and Gynecology
Division of Urogynecology and Reconstructive
Pelvic Surgery
The Warren Albert Medical School
of Brown University
Women & Infants Hospital
Providence, Rhode Island
Peter C. Jeppson, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of New Mexico School of Medicine
Albuquerque, New Mexico
Audra Jolyn Hill, MD
Assistant Professor
Division of Urogynecology
Department of Obstetrics and Gynecology
University of Utah School of Medicine
Salt Lake City, Utah
Sunil Balgobin, MD
Assistant Professor
Department of Obstetrics and Gynecology
Division of Female Pelvic Medicine
and Reconstructive Surgery
University of Texas Southwestern Medical Center
Dallas, Texas
Sean C. Dowdy, MD
Professor and Chair
Division of Gynecologic Oncology
Mayo Clinic
Rochester, Minnesota
Management of Castration-Resistant Prostate Cancer
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
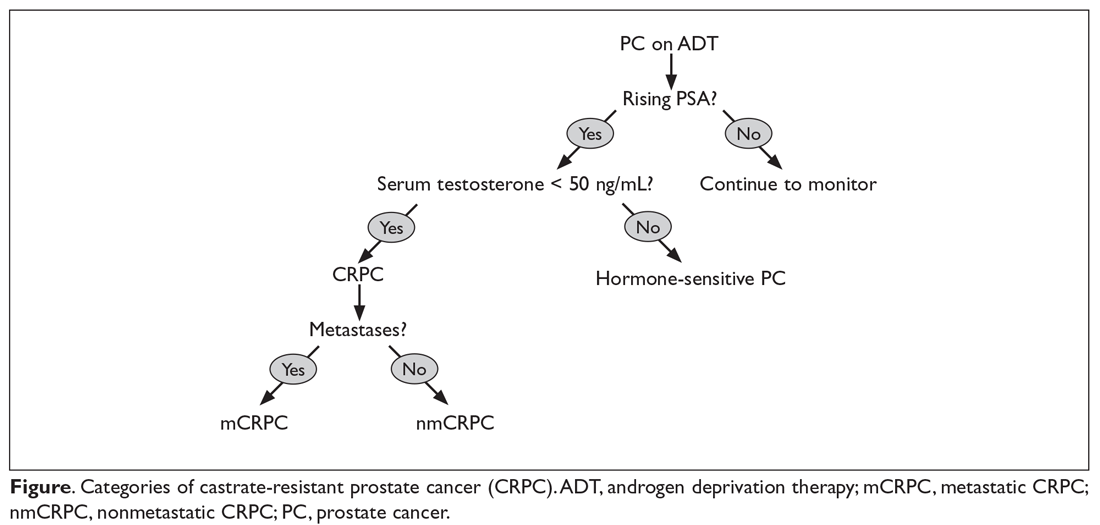
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
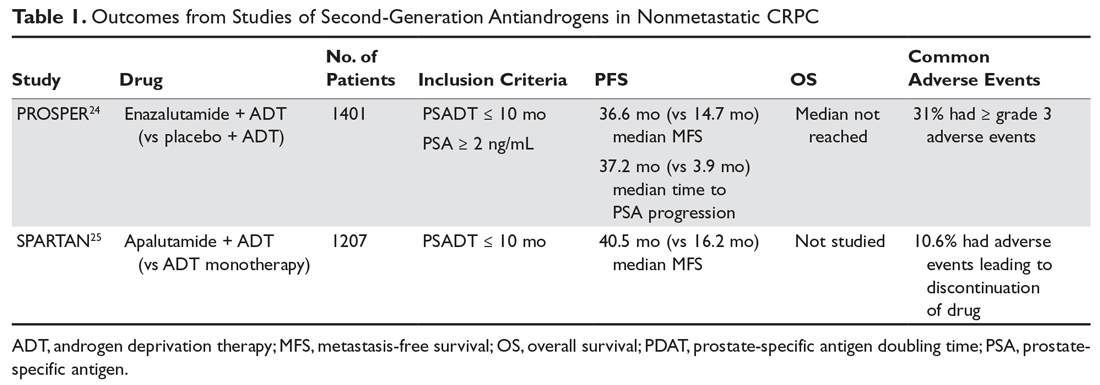
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
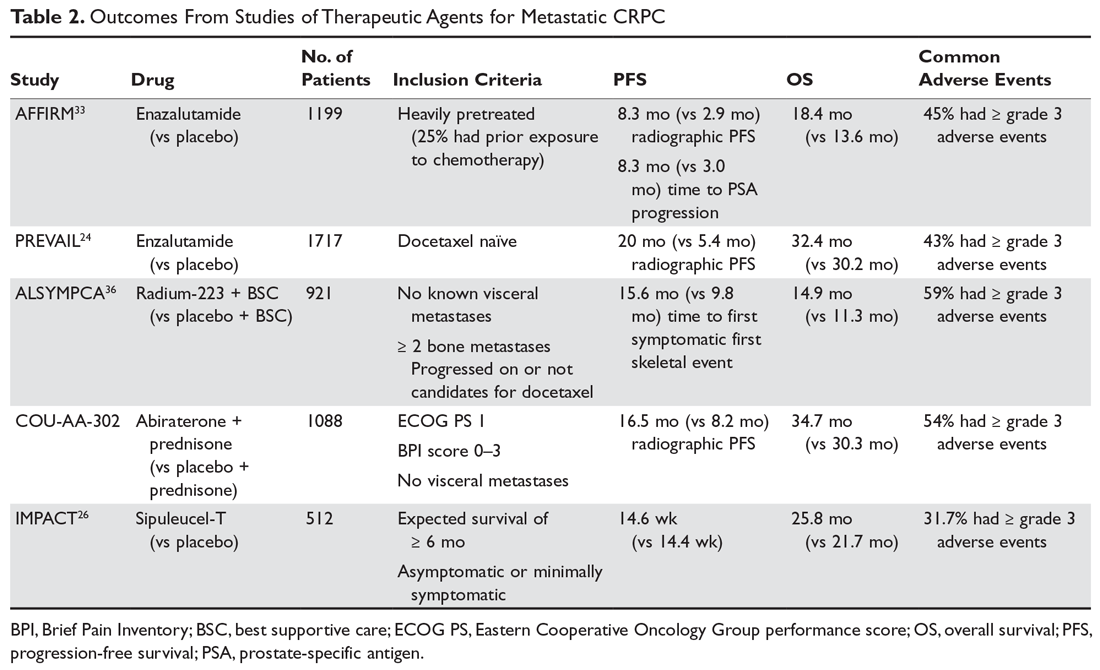
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
Prostate cancer is the most common malignancy in men, with an estimated 165,000 new prostate cancer diagnoses and 29,000 prostate cancer deaths occurring in the United States in 2018.1 Due to the widespread use of screening prostate-specific antigen (PSA), prostate cancer has been mainly diagnosed when the tumor is confined to the prostate. Despite definitive treatment of localized prostate cancer, some men develop systemic disease, either biochemical failure, as defined by rising PSA level, or metastatic disease.1 Several factors have been demonstrated to predict risk of relapse, including higher pretreatment PSA, higher Gleason score, and a greater anatomic extent of disease.2 In addition, the incidence of de novo metastatic prostate cancer was recently noted to be increasing. This may be due to changes in the United States Preventive Services Task Force prostate cancer screening guidelines in 2012, which recommended against screening for prostate cancer in men of any age. The updated 2018 guidelines recommend a discussion of the risks versus benefits of screening for prostate cancer for all men aged 55 to 69 years,recommend against screening for men older than 70 years, and do not have recommendations for high-risk subgroups.3
Androgen deprivation therapy (ADT) has been the cornerstone of therapy since 1941 for men with hormone-sensitive systemic disease, both in biochemically relapsed and metastatic disease.4,5 While more than 90% of patients respond to initial ADT, castration resistance is inevitable in some men.6,7 Prostate cancer will become castration-resistant typically after 18 to 24 months of ADT, with the majority of patients developing castration-resistant prostate cancer (CRPC) within 5 years of initiation of ADT.8
Pathogenesis
CRPC (previously called androgen independent prostate cancer) is defined as progression of disease despite serum total testosterone levels less than 50 ng/dL. CRPC is characterized by a rising PSA level and/or radiographic progression. One mechanism of castration resistance is genetic modification of the androgen receptor (AR), including increased expression of the wild-type AR.9 Alternatively, mutations of the steroid-binding domain may play a role in the development of castration resistance by allowing the AR to become activated by non-androgen steroid hormones or even paradoxically by antiandrogens. Studies suggest, however, that AR mutations may be seen in only 10% of prostate cancers that have developed castration resistance.10 The AR-V7 splice variant of the AR lacks an androgen binding site altogether, and may play an important role in castration resistance. In one study, the presence of this splice variant in circulating prostate cancer tumor cells predicted resistance to enzalutamide and abiraterone as well as poor outcomes.11 Intratumoral androgen synthesis also may play a role in the development of CRPC.12,13
CRPC can be broadly categorized into 2 categories, metastatic (mCRPC) and nonmetastatic (nmCRPC; Figure). The exact proportion of patients entering CRPC at a nonmetastatic stage (M0) is largely unknown.14 In one study of patients at the time of diagnosis of CRPC, ≥ 84% of patients were shown to have metastases.8 In this article, we review key aspects of management of CRPC, including selection of first- and second-line therapy, and briefly discuss upcoming clinical trials.
Treatment of Nonmetastatic CRPC (M0 Disease)
Early identification of M0 CRPC is important because patients with nonmetastatic CRPC are at risk for metastasis, as demonstrated by Smith and colleagues.15 In this study that evaluated data from patients with nmCRPC in the placebo group (n = 331) of a randomized controlled trial, at 2 years 46% had developed ≥ 1 bone metastasis, 20% had died, and the median bone metastasis-free survival (MFS) was 25 months.15
Rapid PSA doubling time (PSADT) is linked to shorter time to metastasis in this group of patients. Patients with a PSADT of < 10 months had a risk for bone metastasis 12 times greater and a risk for death 4 times greater than patients with a PSADT of ≥ 10 months.16 Accordingly, observation should be reserved for those patients with a PSADT of ≥ 10 months.
Options for secondary hormonal therapy in those with a PSADT of ≤ 10 months include a first-generation antiandrogen (bicalutamide, flutamide, nilutamide), ketoconazole with hydrocortisone, and more recently second-generation antiandrogens (apalutamide or enzalutamide).
Bicalutamide competitively inhibits dihydrotestosterone and testosterone binding to the AR and is generally well-tolerated; it is given in conjunction with a GnRH agonist/castration.17 The use of other first-generation antiandrogens is limited mainly due to their toxicity profile. When compared to flutamide in a randomized, double-blinded control study, bicalutamide had significantly improved time to treatment failure.18 Due to promiscuous binding to AR, withdrawal of first-generation antiandrogen therapy has been associated with a biochemical response in a small proportion of patients, with response typically seen after 5 to 7 half-lives of the drug have elapsed.19
Although traditionally used as an antifungal agent, ketoconazole also inhibits androgen synthesis in the adrenal glands and acts as a direct cytotoxin to cancer cells.20 Ketoconazole (with hydrocortisone) has been considered as a treatment option, typically at the time of antiandrogen withdrawal. However, ketoconazole offers no survival benefit, and with the approval of abiraterone in M1 CRPC, its use has declined significantly.21 Additionally, ketoconazole poses a risk for severe hepatotoxicity and QT prolongation, and has significant interactions with numerous drugs, thereby limiting its use. Given the typically short duration of response to first-generation antiandrogens, the second-generation antiandrogens were developed and are associated with a significantly greater progression-free survival (PFS) in M0 CRPC.22,23
The second-generation antiandrogens enzalutamide and apalutamide not only competitively bind to the AR, inhibiting formation of the androgen/AR complex, but they also inhibit androgen/AR complex nuclear translocation and binding to nuclear DNA. In the PROSPER trial, enzalutamide significantly increased radiographic PFS and improved quality of life compared to placebo in chemotherapy-naive patients (Table 1).24 Apalutamide significantly increased MFS as well as PFS and time to PSA progression compared to placebo in the phase 3 SPARTAN trial.25 Apalutamide is generally well tolerated, with hypertension and rash being the most common severe adverse effects. Apalutamide also has less potential for central nervous system toxicities than enzalutamide. The recent approval of these agents is likely to change responses to subsequent treatments, especially in the metastatic setting.
Treatment of Metastatic CRPC (M1 Disease)
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.
Future of CRPC Treatment
Patients with CRPC should be considered for clinical trials when available. These patients’ tumors should be assessed with next-generation sequencing for analysis of microsatellite instability (MSI) or mismatch repair (MMR) as well as the presence of other potentially targetable mutations, as this information may bring into consideration additional investigational as well as FDA-approved treatment options. As of May 2017, immunotherapy with pembrolizumab is approved for patients whose prostate cancer is deficient in MMR or has a high MSI burden based on a study of 12 solid tumor types (including prostate cancer) with deficient MMR.40 Additionally, for patients whose tumor has ≥ 1% programmed death ligand 1 (PD-L1) expression, pembrolizumab has a 17% overall response rate and confers stability of disease in 35%, with a median response duration of 13.5 months.41 Cabozantinib is a mesenchymal epithelial transition (MET) kinase and vascular endothelial growth factor receptor (VEGF-R) inhibitor. When used in heavily pre-treated patients with mCRPC, it showed a radiographic PFS benefit but no survival benefit over prednisone monotherapy.42 One study showed that for patients whose mCRPC had a homozygous deletion and/or a deleterious mutation in the homologous recombination repair genes BRCA1/2, ATM, and CHEK2 or the Fanconi anemia genes, the response rate to the poly ADP ribose polymerase (PARP) inhibitor olaparib was 88%, with a 100% response rate in those with BRCA2 mutations.43 Furthermore, mutations in these DNA repair genes predict increased sensitivity to platinum-based chemotherapy.
Supportive Care
Zoledronic acid or denosumab are FDA approved for men with CRPC and bone metastasis based on the ability of these agents to delay skeletal-related events, including pathologic fracture and spinal cord compression.46 Bisphosphonates, however, do not decrease the incidence of bone metastases.47 And while denosumab does delay the time to first bone metastasis in nmCRPC (particularly in patients with a PSADT of ≤ 6 months), it does not improve OS.48 Other supportive measures include exercise and nutrition. Moderate aerobic exercise for 150 minutes in addition to 2 or 3 strength training sessions per week is recommended by the American College of Sport Medicine to combat cancer-related fatigue.49 There are currently no dietary changes that are routinely recommended to improve the outcome of prostate cancer, but a study noted a shorter biochemical failure–free survival in men with prostate cancer who were obese and consumed a diet high in saturated fat.50
Conclusion
Prostate cancer affects more men in the United States than any other cancer. Once a patient is started on hormone therapy, in all likelihood their prostate cancer will become castration-resistant. Once prostate cancer has developed hormone resistance, there are a host of further treatment options available, including further hormone therapy, chemotherapy, immunotherapy, radiation therapy, bone-targeting agents, and clinical trials. Determining the appropriate sequence in which to use these therapies requires knowledge of the natural history of CRPC, the indications for changing therapies, the mechanism of action and adverse event profile of each treatment, and the optimal time to enroll in a clinical trial.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395-406.
2. Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643-647.
3. US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901-1913.
4. Huggins C, Hodges CV. Studies on prostatic cancer. I: The effects of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297.
5. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. II: The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209-223.
6. Pomerantz M, Kantoff P. Clinical progression to castration recurrent prostate cancer. In: Tindall DJ, James M, eds. Androgen Action in Prostate Cancer. New York: Springer; 2009:57-72.
7. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192.
8. Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985-989.
9. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
10. Taplin ME, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678.
11. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 (AR-V7) mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149-2156.
12. Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588-595.
13. Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3:849-861
14. Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012;23(suppl 10):251-258
15. Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant non-metastatic prostate cancer. Cancer. 2011;117:2077-2085.
16. Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761-768
17. Akaza H, Yamaguchi A, Matsuda T, et al. Superior anti-tumor efficacy of bicalutamide 80mg in combination with luteinizing hormone-releasing hormone (LHRH) against versus LHRH agonist monotherapy as first line treatment for advanced prostate cancer: Interim results of a randomized study in Japanese patients. J Clin Oncol. 2004;34:20-28
18. Schellhammer P, Patterson AL, Sharifi R, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology. 1995;45(5):745-752.
19. Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group Trial (SWOG 9426). Cancer. 2008;112:2393-2400.
20. Eichenberger T, Trachtenberg J, Toor P, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141:190-191.
21. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033.
22. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098-2106.
23. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomized, double-blind, phase 2 study. Lancet Oncol. 2016;199:147-154.
24. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
25. Smith MR, Saad F, Chowdhury S; SPARTAN Investigators, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
26. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
27. Ryan CJ, Smith MR, de Bono JS, et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N Engl J Med. 2013;368:138-148
28. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242-245.
29. Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189-3197.
30. Eisenberger M, Hardy-Bessard A-C, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol. 2017;35:3198-3206.
31. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):P1147-1154.
32. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-1512.
33. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147-1156.
34. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
35. Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
36. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double blind, randomized trial. Lancet Oncol. 2014;15:738-746.
37. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213-223.
38. Turo R, Smolski M, Esler R, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14.
39. Venkitaraman R, Lorente D, Murthy V. A randomized phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol. 2015 67:673-679.
40. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
41. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807-1813.
42. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
43. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
44. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70:161-168.
45. Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34:3005-3013.
46. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
47. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67:482-491.
48. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastases-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39-46.
49. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426.
50. Strom SS, Yamamura Y, Flores-Sandoval FN, et al. Prostate cancer in Mexican-Americans: identification of risk factors. Prostate. 2008;68:563-570.
Nivolumab-Induced Lichen Planus Pemphigoides
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
Nivolumab, an immune checkpoint modulator, acts by binding to the programmed cell death 1 (PD-1) receptor on T cells, which blocks the inhibition of T cells. Nivolumab ultimately leads to stimulation of the T-cell response1 and overcomes evasive adaptations of certain cancers. Cutaneous adverse events (AEs) have been reported in approximately 20% to 40% of patients treated with the anti–PD-1 class of drugs, including nivolumab.2-4 The most common cutaneous AEs include pruritus; vitiligo; and various forms of rash, such as lichenoid dermatitis, psoriasiform eruptions, and bullous pemphigoid.1-3,5-7 We report a patient with non–small cell lung cancer being treated with nivolumab who developed a bullous lichenoid eruption consistent with the diagnosis of lichen planus pemphigoides (LPP).
Case Report
An 87-year-old woman presented with a pruritic rash on the trunk and extremities of 3 weeks’ duration. Her medical history included stage IV non–small cell lung cancer, congestive heart failure, coronary artery disease, chronic kidney disease, and hypertension. Her long-term medications were ipratropium-albuterol, alendronate, amlodipine, aspirin, carvedilol, colesevelam, probiotic granules, and bumetanide. She was previously treated with carboplatin and docetaxel, which were discontinued secondary to fatigue, diarrhea, poor appetite, loss of taste, and a nonspecific rash. Six months later (approximately 3 months prior to the onset of cutaneous symptoms), she was started on nivolumab monotherapy every 14 days for a total of 9 infusions.
At the current presentation, physical examination revealed erythematous crusted erosions on the trunk and extremities and 1 flaccid bulla on the back. A punch biopsy revealed lichenoid dermatitis. The patient returned 2 weeks later with worsening of cutaneous manifestations, including more blisters and erosions. Figure 1 shows the clinical appearance of the eruption on the patient’s leg. At this time, additional biopsies revealed a subepidermal bullous lichenoid eruption with eosinophils (Figure 2). Direct immunofluorescence (DIF) was negative; however, indirect immunofluorescence (IIF) revealed weak linear staining for IgG antibodies along the basement membrane zone on monkey esophagus substrate. Examination of salt-split skin was noncontributory. The patient improved with a 2-week oral prednisone taper (starting at 40 mg daily). The dose was decreased incrementally over the course of 2 weeks from 40 mg to 20 mg to 0 mg. Because of the presumed grade 3 (severe) cutaneous drug eruption linked to nivolumab and further discussion with the medical oncology team, the patient decided to cease therapy. Since cessation of therapy, she has been seen twice for follow-up. At 2-month follow-up, she presented with drastic improvement of the eruption, and at 1 year she has continued to forego any further treatment for the stable and nonprogressing malignancy.
Widespread coalescent lesions with crusted and hemorrhagic bullae were present on the thigh and knee.
Comment
Immunotherapy
The interaction between the PD-1 receptor and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2, is an immune checkpoint.8,9 Under normal physiologic conditions, this checkpoint serves to prevent autoimmunity.10 When the PD-1 receptor is left unbound, T cells are more inclined to mount an immune response. If the receptor is ligand bound, the response of T cells is suppressed via mechanisms such as anergy or apoptosis.8 Tumor cells are known to produce PD-L1 as an adaptive resistance mechanism to evade immunity.8 Nivolumab is a human monoclonal antibody that targets the PD-1 receptor, thereby preventing the interaction with its ligand and allowing for unsuppressed activity of T cells.10 This therapy ultimately blocks the tumor’s local immune suppression mechanisms, which allows T cells to recognize cancer antigens.10
Adverse Events
Dermatologic AEs are among the most common with nivolumab treatment. In a pooled retrospective analysis of melanoma patients, Weber et al9 found that 34% of 576 patients experience cutaneous any-grade AEs associated with nivolumab treatment, most commonly pruritus. It has been well documented that anti–PD-1 therapy AEs of the skin as well as other organ systems have a delayed onset of at least 1 month.9 The average time of onset for bullous eruptions associated with anti–PD-1 therapy has been reported to be approximately 12 weeks, with a range of 7 to 16.1 weeks.11 Our patient had a bullous eruption with an onset of 12 weeks following initiation of treatment.
Although lichenoid reactions appear to be relatively common AEs of anti–PD-1 therapy,2,5,6 only a small number of cases of bullous pemphigoid eruptions have been reported.7 It has been hypothesized that blockade of the PD-1/PD-L1 pathway increases production of hemidesmosomal protein BP180 autoantibody, which is involved in the pathogenesis of LPP.7 Bullous eruptions have not been reported in the use of anticytotoxic T-lymphocyte–associated protein 4 agents, which could indicate that such eruptions are specific to the anti–PD-1 class of drugs.7
Diagnosis
Our patient represents a rare drug reaction involving both lichenoid and bullous components. Our differential diagnosis included drug-induced bullous lichen planus (BLP) and drug-induced LPP. Differentiation of these diagnoses can be difficult. In fact, in 2017 Fujii et al12 found reason to reprise the hypothesis that BLP is a transitional step toward LPP. The histologic evaluation of LPP differs depending on the type of lesion biopsied and can be indistinguishable from BLP as well as bullous pemphigoid. Therefore, clinical history and immunofluorescence should be used to make a diagnosis. Lichen planus pemphigoides typically will have linear IgG deposition along the basement membrane zone on both DIF and IIF, findings that will be negative in patients with BLP.13 Direct immunofluorescence findings in BLP include shaggy deposits of fibrin along the basement membrane zone. In this patient, DIF was negative, which may have been caused by variability among lesions in LPP, but IIF was positive. Given the clinicopathologic correlation, the diagnosis of LPP was made. Further studies, such as immunoblot and enzyme-linked immunosorbent assay, also can be used to aid diagnosis.
A similar presentation has been documented in a patient with metastatic melanoma.14 The diagnosis in this patient was LPP induced by pembrolizumab, which is another agent within the anti–PD-1 class. The Naranjo probability scale scored our patient’s eruption as a possible adverse drug reaction.15 Thus, other etiologies, such as a paraneoplastic process, cannot be completely ruled out. However, our patient has not had recurrence after 1 year, and the timing of the eruption appeared to be related to drug therapy, making alternative etiologies less likely.
Management
Cessation of nivolumab therapy and a short course of oral corticosteroid therapy led to marked improvement of symptoms. Given the emergent treatment of our patient, the resolution of her symptoms cannot be solely attributed to the cessation of nivolumab or to treatment with prednisone. Oral rather than topical corticosteroids were chosen because of the severity of the eruption. Topical corticosteroids and oral antihistamines can provide relief in less severe cases of bullous reactions to anti–PD-1 therapy.7,11 This regimen also has proven to be effective in lichenoid dermatitis induced by anti–PD-1.2
Conclusion
We hope this case report will contribute to the growing body of evidence regarding recognition and management of unique reactions to cancer immunotherapies.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72:221-236; quiz 237-238.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25.
- Abdel-Rahman O, El Halawani H, Fouad M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol. 2015;11:2471-2484.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort [published online January 12, 2016]. J Am Acad Dermatol. 2016;74:455-461.e1.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263.
- Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383-389.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792.
- Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306:511-519.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43:688-696.
- Fujii M, Takahashi I, Honma M, et al. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol. 2017;44:E124-E125.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15:742-745.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Practice Points
- Dermatologists should be aware that lichen planus pemphigoides is within the spectrum of toxicity for patients treated with nivolumab.
- Bullous eruptions related to anti–programmed cell death 1 agents tend to appear 4 months after initiation of therapy.
- A severe cutaneous toxicity of a checkpoint inhibitor should be managed using oral corticosteroids with consideration of withdrawing the offending agent.
Survivors offer ‘mass shooting grief 101’
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
People coping with the aftermath of mass shootings might find some solace from Sandy and Lonnie Phillips. The couple, whose daughter was gunned down in the 2012 slaughter in an Aurora, Colo., movie theater, travels the country to help those whose pain is raw begin processing their grief. Mr. and Mrs. Phillips, driven by compassion, hope that sharing their experience will help others. They have started a nonprofit organization called Survivors Empowered to offer advice and kinship in the wake of mass shootings.
Emergency department visits can prove disorienting for patients with physical illnesses. But for those with mental illness, such visits can feel not only disorienting but disconcerting – particularly if sedation or restraints are involved. “If you are living with schizophrenia or bipolar disorder, that is a really tough way to begin that road to recovery,” said Jack Rozel, MD, president of the American Association for Emergency Psychiatry (AAEP), in an interview with CNN. At about 100 locations across the country, psychiatric EDs staffed by psychiatrists and other physicians, nurses, and social workers are triaging and treating patients with mental illness. The goal is treatment and either release or referral to more specialized care within 24 hours. The approach is the brainchild of Scott Zeller, MD, vice president of acute psychiatry at Vituity and a past president of the AAEP. The physician-led organization provides staffing and consulting services to medical centers nationwide. Dr. Zeller hatched the idea of a psychiatric ED while working as chief of psychiatric emergency services at John George Psychiatric Hospital in San Leandro, Calif. “We need to treat people at the emergency level of care,” he said. “The vast majority of psychiatric emergencies can be resolved in less than 24 hours.” Dr. Zeller took the reins in transforming the center from a traditional ward, including the use of restraints, to a setting more like a living room that was supportive rather than institutional. The results included improved patient outcomes and cost savings – in part because of the reduced time spent in the ED. CNN.
In Buffalo, N.Y., a new memorial to be installed in the Buffalo and Erie County Naval and Military Park will honor veterans who were lost to suicide. Ground was broken recently for the Battle Within Memorial. The 8-feet-tall sculpture will portray one soldier carrying the empty outline of another soldier as a metaphor for help between comrades and the losses that can be tied to the fallout from combat. “It’s a compelling piece of steel,” because it is designed in such a way that allows viewers to see through it, said Paul Marzello, the park’s president and chief executive officer, in an interview with WBFO, a National Public Radio affiliate in Buffalo. “By looking through it you’ll be able to look, as you see through it, someone’s soul.” Among veterans and active duty personnel, an estimated 20 commit suicide each day, according to the military publication Stars and Stripes. One hope of supporters of The Battle Within Foundation, which is leading the project, is that the memorial will inspire those experiencing psychic pain to seek help.“It is our sincerest hope that this monument will in some way help build public awareness of this ongoing tragedy, provide a lifeline for the suffering, and honor our heroes for their service, no matter where they died,” said Mark Donnelly, PhD, president of the Battle Within Foundation. The unveiling is scheduled for May 27, 2019. WBFO.
The field of psychiatry needs to do a better job of helping some patients come off of psychiatric drugs, said Allen Frances, MD, chairperson of DSM-IV task force, in an interview with the New Yorker. This process of removing drugs, called “deprescribing,” “requires a great deal more skill, time, commitment, and knowledge of the patient than prescribing does.” Another psychiatrist quoted in the article, Giovanni A. Fava, MD, said he has struggled to publish research looking at what happens to patients when they stop taking antidepressants. Yet another psychiatrist quoted in the article, Swapnil Gupta, MD, MBBS, said that, for many patients, coming off their medications “is a loss of identity, a different way of living. Suddenly, everything that you are doing is yours – and not necessarily your medication.” The article chronicles the experiences of Laura Delano, a woman diagnosed with bipolar disorder and prescribed valproic acid (Depakote) while in high school. Over the next several years, Ms. Delano found herself taking numerous medications and with a different diagnosis. Eventually, she removed herself from her many medications, started a blog, and launched a website called The Withdrawal Project. The New Yorker.
The National Council for Behavioral Health and Lady Gaga’s Born This Way Foundation have teamed up to develop a Teen Mental Health First Aid pilot program designed to “enhance the mental health of young people,” according to a report on Washington’s WTOP radio. Eight U.S. high schools have been chosen to participate in the program, which will teach students about mental illnesses and addictions. It also will help teenagers respond to friends who might be struggling with a problem with mental health or addiction. The Teen Mental Health First Aid program, a five-step action plan, was adapted from an evidence-based training program from Australia. Researchers at Johns Hopkins University, Baltimore, will evaluate the pilot program to assess its effectiveness. The National Alliance on Mental Illness has reported than 20% of American adults and the same percentage of U.S. teens are living with mental health challenges. After the pilot study results are analyzed, the training will be made available to the public. WTOP.
Relapsing Polychondritis in Human Immunodeficiency Virus
Relapsing polychondritis (RP) is a recurrent inflammatory condition involving primarily cartilaginous structures. The disease, first described as a clinical entity in 1960 by Pearson et al,1 is rare with an estimated incidence of 3.5 cases per 1 million individuals.2 The pathogenesis of RP is widely accepted as being autoimmune in nature, largely due to the identification of circulating autoantibodies seen in the sera of patients with similar clinical pictures.3
Although in most patients the primary process involves inflammation of cartilage, a subset of patients experience involvement of noncartilaginous sites.4 The degree of systemic involvement varies from none to notable, affecting the cardiovascular and respiratory systems and potentially leading to life-threatening complications, including cardiac valve compromise and airway collapse. Relapsing polychondritis is considered to be a progressive disease with the ultimate potential to be life-threatening.5
Human immunodeficiency virus (HIV) infection leads to a profound state of immune dysregulation, affecting innate, adaptive, and natural killer components of the immune system.6 There is variability in the development of autoimmune disease in HIV patients depending on the stage of infection. The frequency of rheumatologic disease in HIV patients might be as high as 60%.6 Relapsing polychondritis is rare in patients with HIV.7-9 Of 4 reported cases, 2 patients had other coexisting autoimmune disease—sarcoidosis and Behçet disease.8,9
Case Report
A 36-year-old man presented to the clinic with a concern of recurrent ear pain and swelling of approximately 2 years’ duration. Onset was sudden without inciting event. Symptoms initially involved the right ear with eventual progression to both ears. Additional symptoms included an auditory perception of underwater submersion, intermittent vertigo, and 3 episodes of throat closure sensation.
The patient’s medical history was notable for asthma; gastritis; depression; and HIV infection, which was diagnosed 4 years earlier and adequately managed with highly active antiretroviral therapy. His family history was notable for systemic lupus erythematosus in his mother, maternal aunt, and maternal cousin.
At presentation, the patient’s CD4 count was 799 cells/mm3 with an undetectable viral load. Medications included abacavir-dolutegravir-lamivudine, hydroxyzine, meclizine, mometasone, and quetiapine. Physical examination showed erythema, swelling, and tenderness of the left and right auricles with sparing of the earlobe that was more noticeable on the left ear (Figure 1). Bacterial culture from the external auditory meatus was positive for methicillin-resistant Staphylococcus aureus. Biopsy revealed chronic inflammatory perichondritis with mild to moderate fibrosis and chronic lymphocytic inflammation at the dermal cartilaginous junction (Figure 2). A direct immunofluorescent biopsy was unremarkable, but subsequent type II collagen antibodies were positive (35.5 endotoxin units/mL [reference range, <20 endotoxin units/mL]).
Comment
Relapsing polychondritis is an uncommon progressive disease characterized by recurrent inflammatory insults to cartilaginous and proteoglycan-rich structures.4 The most consistent clinical features of RP are ear inflammation that involves the auricle and spares the lobe, nasal chondritis, and arthralgia.10 Laryngotracheal compromise may occur from tracheal cartilage inflammation. The involvement of these specific structures is due to commonality between their component collagens.5 Although any organ system can be affected, as many as 50% of patients have respiratory tract involvement, which may affect any portion of the respiratory tree.11 If involving the larynx, this inflammation can lead to severe edema warranting intubation. Cardiovascular involvement is present in 24% to 52% of patients,10 which most commonly manifests as valvular impairment affecting the aortic valve more frequently than the mitral valve.5
Pathogenesis
Although the etiology of RP remains undetermined, multiple hypotheses have been proposed. One is that a certain subset of patients is predisposed to autoimmunity, and a secondary triggering event in the form of infection, malignancy, or medication catalyzes development of RP. A second hypothesis is that mechanical trauma to cartilage exposes the immune system to certain antigens that would have otherwise remained hidden, prompting autosensitization.12,13
Regardless of cause, an autoimmune pathogenesis is favored based on the following observations: RP is frequently associated with other autoimmune diseases in the same patient, glucocorticosteroids and other immunosuppressive therapies are effective for treatment, and histopathologic findings include an infiltrate of CD4+ T lymphocytes with detection of immunoglobulins and plasma cells in different lesions.5 The detection of autoantibodies against collagen in the serum of patients with RP further supports an autoimmune pathogenesis.3 The earliest identified autoantibodies in patients with RP were against type II collagen. Subsequent studies have identified autoantibodies against type IV and type XI collagens as well as other cartilage-related proteins such as matrilin 114 and cartilage oligomeric matrix proteins.15 Although circulating antibodies to type II collagen are present in a variable number of patients with the disease (30%–70%), levels likely correlate with disease activity and are highest at times of acute inflammation.3 Additionally, titers of type II collagen antibodies have been shown to decrease upon institution of immunosuppressive therapy.16
Although a humoral response dominates the picture of RP, there also is an associated T cell–mediated response.13 Histopathologically, biopsy of an active lesion of auricular cartilage shows a mixed inflammatory infiltrate composed primarily of lymphocytes, with variable numbers of polymorphonuclear cells, monocytes, and plasma cells. Loss of basophilia of the cartilage matrix can be observed, thought to be the result of proteoglycan depletion.13 Later, lesions classically display apoptosis of chondrocytes, focal calcification, or fibrosis.5
Diagnosis
Relapsing polychondritis acts classically as an autoimmune disease with a variable presentation, making diagnosis a challenge. Many sets of diagnostic criteria have been proposed. The most referenced remains the original criteria described by McAdam et al.17 In 2012, the Relapsing Polychondritis Disease Activity Index modified criteria set forth by Michet et al18 and might serve as the standard for diagnosis going forward.19
McAdam et al17 proposed that 3 of 6 clinical features are necessary for diagnosis: bilateral auricular chondritis, nonerosive seronegative inflammatory polyarthritis, nasal chondritis, ocular inflammation, respiratory tract chondritis, and audiovestibular damage. Michet et al18 proposed that 1 of 2 conditions are necessary for diagnosis of RP: (1) proven inflammation in 2 of 3 of the auricular, nasal, or laryngotracheal cartilages; or (2) proven inflammation in 1 of 3 of the auricular, nasal, or laryngotracheal cartilages, plus 2 other signs, including ocular inflammation, vestibular dysfunction, seronegative inflammatory arthritis, and hearing loss.
These criteria were proposed originally in 197617 and modified in 1986.18 No further updates have been offered since then. As such, serologic findings, such as antibodies against type II collagen, are not included in the diagnostic criteria. Additionally, these antibodies are not specific for RP and can be seen in other conditions such as rheumatoid arthritis.20
More recently, imaging analysis has been employed in conjunction with clinical and serologic data to diagnose the disease and evaluate its severity. The use of imaging modalities for these purposes is most beneficial in patients with notable disease and respiratory involvement.21
Although the clinical picture is typified by the classic findings described above, the clinician must be aware of more subtle clues to diagnosis,11 which is of particular importance to the dermatologist because 35% of patients with RP alone will have skin manifestations that can precede onset of chondritis.10 Most commonly, dermatologic manifestations are nonspecific and can include nodules on the limbs, purpura, and urticarial lesions.22 Individual case reports have noted the coexistence of RP with erythema multiforme,18 erythema annulare centrifugum,23 pyoderma gangrenosum,24 and panniculitis,18 among other disorders.
Treatment
Standardized guidelines for treatment do not exist. Treatments should be chosen based on severity of disease. Mild disease, presenting with recurrent chondritis and arthritis without evidence of systemic involvement, can be treated with nonsteroidal anti-inflammatory drugs, dapsone, or colchicine. Refractory disease often requires high-dose systemic corticosteroids.5
Severe systemic involvement leads to increased mortality and warrants more aggressive treatment.22 Commonly used agents include the immunosuppressants cyclophosphamide, cyclosporine, and methotrexate. Tumor necrosis factor α inhibitors have been the most widely utilized immunomodulatory agent for treatment of RP.25,26 Abatacept and rituximab also have been used with variable efficacy in patients with severe disease. Recently, the IL-6 receptor blocker tocilizumab has been used with some success.27
Prognosis
The prognosis for patients with RP largely depends on the severity of disease and degree of internal involvement. With improved management, largely due to awareness and recognition of disease, the survival rate among RP patients has increased from 55% at 10 years to 94% at the end of 8 years.18 The main cause of death in RP patients is airway complications related to laryngotracheal involvement.10 The second most common cause of death is cardiovascular complications in which valvular disease predominates.5
Concomitant Illness
Thirty-five percent of RP patients have coexisting autoimmune disease, the most common being antineutrophil cytoplasmic antibody–associated vasculitis.5,28 Although this association with autoimmune disease is well described, reports of RP occurring in other states of immune dysfunction are sparse. One case of RP has been reported in a child with common variable immunodeficiency thought to be related to underlying abnormal immune regulation and immunodeficiency.29 Relapsing polychondritis has been described in 4 patients with HIV, 2 of whom had concomitant autoimmune disease.7-9
Human immunodeficiency virus infection is a well-established cause of immune dysregulation and has variable association with autoimmunity. This variability depends largely on the stage of infection. When divided into stages, autoimmune diseases develop predominantly in stage I, during acute infection with an intact immune system; in stage III, with immunosuppression, a low CD4 count, and development of AIDS; and in stage IV, when the immune system is restored with the institution of highly active antiretroviral therapy.6 The interplay between HIV infection and development of autoimmune disease is complex, and pathogenesis remains speculative.
Conclusion
Our patient represents a case of RP in an HIV-positive patient. Additionally, our patient had no other identifiable autoimmune conditions but did have a strong family history of them. It is important for providers to be aware of the potential for development of RP as well as other autoimmune disease in the setting of HIV infection. The implications of a missed diagnosis could be dire because the disease course of RP is progressive and has the potential to decrease survival.
- Pearson CM, Kline HM, Newcomer VD. Relapsing polychondritis. N Engl J Med. 1960;263:51-58.
- Kent PD, Michet CJ Jr, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol. 2004;16:56-61.
- Ebringer R, Rook G, Swana GT, et al. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis. 1981;40:473-479.
- Sharma A, Law AD, Bambery P, et al. Relapsing polychondritis: clinical presentations, disease activity and outcomes. Orphanet J Rare Dis. 2014;9:198.
- Vitale A, Sota J, Rigante D, et al. Relapsing polychondritis: an update on pathogenesis, clinical features, diagnostic tools, and therapeutic perspectives. Curr Rheumatol Rep. 2016;18:3.
- Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
- Dolev JC, Maurer TA, Reddy SG, et al. Relapsing polychondritis in HIV-infected patients: a report of two cases. J Am Acad Dermatol. 2004;51:1023-1025.
- Zandman-Goddard G, Peeva E, Barland P. Combined autoimmune disease in a patient with AIDS. Clin Rheumatol. 2002;21:70-72.
- Belzunegui J, Cancio J, Pego JM, et al. Relapsing polychondritis and Behc¸et’s syndrome in a patient with HIV infection. Ann Rheum Dis. 1995;54:780.
- Sharma A, Gnanapandithan K, Sharma K, et al. Relapsing polychondritis: a review. Clin Rheumatol. 2013;32:1575-1583.
- Cantarini L, Vitale A, Brizi MG, et al. Diagnosis and classification of relapsing polychondritis. J Autoimmun. 2014;48-49:53-59.
- Cañas CA, Bonilla Abadía F. Local cartilage trauma as a pathogenic factor in autoimmunity (one hypothesis based on patients with relapsing polychondritis triggered by cartilage trauma). Autoimmune Dis. 2012;2012:453698.
- Ouchi N, Uzuki M, Kamataki A, et al. Cartilage destruction is partly induced by the internal proteolytic enzymes and apoptotic phenomenon of chondrocytes in relapsing polychondritis. J Rheumatol. 2011;38:730-737.
- Buckner JH, Wu JJ, Reife RA, et al. Autoreactivity against matrilin-1 in a patient with relapsing polychondritis. Arthritis Rheum. 2000;43:939-943.
- Kempta Lekpa F, Piette JC, Bastuji-Garin S, et al. Serum cartilage oligomeric matrix protein (COMP) is a marker of disease activity in relapsing polychondritis. Clin Exp Rheumatol. 2010;28:553-555.
- Foidart JM, Abe S, Martin GR, et al. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med. 1978;299:1203-1207.
- McAdam LP, O’Hanlan MA, Bluestone R, et al. Relapsing polychondritis: prospective study of 23 patients and review of the literature. Medicine (Baltimore). 1976;55:193-215.
- Michet CJ, McKenna CH, Luthra HS, et al. Relapsing polychondritis: survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78.
- Arnaud L, Devilliers H, Peng SL, et al. The Relapsing Polychondritis Disease Activity Index: development of a disease activity score for relapsing polychondritis. Autoimmun Rev. 2012;12:204-209.
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3-18.
- Thaiss WM, Nikolaou K, Spengler W, et al. Imaging diagnosis in relapsing polychondritis and correlation with clinical and serological data. Skeletal Radiol. 2015;5:339-346.
- Lahmer T, Treiber M, von Werder A, et al. Relapsing polychondritis: an autoimmune disease with many faces. Autoimmun Rev. 2010;9:540-546.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Francès C, el Rassi R, Laporte JL, et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine (Baltimore). 2001;80:173-179.
- Chopra R, Chaudhary N, Kay J. Relapsing polychondritis. Rheum Dis Clin North Am. 2013;39:263-276.
- Moulis G, Sailler L, Pugnet G, et al. Biologics in relapsing polychondritis: a case series. Clin Exp Rheumatol. 2013;31:937-939.
- Henes CJ, Xenitidis T, Horger M. Tocilizumab for refractory relapsing polychondritis—long-term response monitoring by magnetic resonance imaging. Joint Bone Spine. 2016;83:365-366.
- Weinberger A, Myers AR. Relapsing polychondritis associated with cutaneous vasculitis. Arch Dermatol. 1979;115:980-981.
- Karaca NE, Aksu G, Yildiz B, et al. Relapsing polychondritis in a child with common variable immunodeficiency. Int J Dermatol. 2009;48:525-528.
Relapsing polychondritis (RP) is a recurrent inflammatory condition involving primarily cartilaginous structures. The disease, first described as a clinical entity in 1960 by Pearson et al,1 is rare with an estimated incidence of 3.5 cases per 1 million individuals.2 The pathogenesis of RP is widely accepted as being autoimmune in nature, largely due to the identification of circulating autoantibodies seen in the sera of patients with similar clinical pictures.3
Although in most patients the primary process involves inflammation of cartilage, a subset of patients experience involvement of noncartilaginous sites.4 The degree of systemic involvement varies from none to notable, affecting the cardiovascular and respiratory systems and potentially leading to life-threatening complications, including cardiac valve compromise and airway collapse. Relapsing polychondritis is considered to be a progressive disease with the ultimate potential to be life-threatening.5
Human immunodeficiency virus (HIV) infection leads to a profound state of immune dysregulation, affecting innate, adaptive, and natural killer components of the immune system.6 There is variability in the development of autoimmune disease in HIV patients depending on the stage of infection. The frequency of rheumatologic disease in HIV patients might be as high as 60%.6 Relapsing polychondritis is rare in patients with HIV.7-9 Of 4 reported cases, 2 patients had other coexisting autoimmune disease—sarcoidosis and Behçet disease.8,9
Case Report
A 36-year-old man presented to the clinic with a concern of recurrent ear pain and swelling of approximately 2 years’ duration. Onset was sudden without inciting event. Symptoms initially involved the right ear with eventual progression to both ears. Additional symptoms included an auditory perception of underwater submersion, intermittent vertigo, and 3 episodes of throat closure sensation.
The patient’s medical history was notable for asthma; gastritis; depression; and HIV infection, which was diagnosed 4 years earlier and adequately managed with highly active antiretroviral therapy. His family history was notable for systemic lupus erythematosus in his mother, maternal aunt, and maternal cousin.
At presentation, the patient’s CD4 count was 799 cells/mm3 with an undetectable viral load. Medications included abacavir-dolutegravir-lamivudine, hydroxyzine, meclizine, mometasone, and quetiapine. Physical examination showed erythema, swelling, and tenderness of the left and right auricles with sparing of the earlobe that was more noticeable on the left ear (Figure 1). Bacterial culture from the external auditory meatus was positive for methicillin-resistant Staphylococcus aureus. Biopsy revealed chronic inflammatory perichondritis with mild to moderate fibrosis and chronic lymphocytic inflammation at the dermal cartilaginous junction (Figure 2). A direct immunofluorescent biopsy was unremarkable, but subsequent type II collagen antibodies were positive (35.5 endotoxin units/mL [reference range, <20 endotoxin units/mL]).
Comment
Relapsing polychondritis is an uncommon progressive disease characterized by recurrent inflammatory insults to cartilaginous and proteoglycan-rich structures.4 The most consistent clinical features of RP are ear inflammation that involves the auricle and spares the lobe, nasal chondritis, and arthralgia.10 Laryngotracheal compromise may occur from tracheal cartilage inflammation. The involvement of these specific structures is due to commonality between their component collagens.5 Although any organ system can be affected, as many as 50% of patients have respiratory tract involvement, which may affect any portion of the respiratory tree.11 If involving the larynx, this inflammation can lead to severe edema warranting intubation. Cardiovascular involvement is present in 24% to 52% of patients,10 which most commonly manifests as valvular impairment affecting the aortic valve more frequently than the mitral valve.5
Pathogenesis
Although the etiology of RP remains undetermined, multiple hypotheses have been proposed. One is that a certain subset of patients is predisposed to autoimmunity, and a secondary triggering event in the form of infection, malignancy, or medication catalyzes development of RP. A second hypothesis is that mechanical trauma to cartilage exposes the immune system to certain antigens that would have otherwise remained hidden, prompting autosensitization.12,13
Regardless of cause, an autoimmune pathogenesis is favored based on the following observations: RP is frequently associated with other autoimmune diseases in the same patient, glucocorticosteroids and other immunosuppressive therapies are effective for treatment, and histopathologic findings include an infiltrate of CD4+ T lymphocytes with detection of immunoglobulins and plasma cells in different lesions.5 The detection of autoantibodies against collagen in the serum of patients with RP further supports an autoimmune pathogenesis.3 The earliest identified autoantibodies in patients with RP were against type II collagen. Subsequent studies have identified autoantibodies against type IV and type XI collagens as well as other cartilage-related proteins such as matrilin 114 and cartilage oligomeric matrix proteins.15 Although circulating antibodies to type II collagen are present in a variable number of patients with the disease (30%–70%), levels likely correlate with disease activity and are highest at times of acute inflammation.3 Additionally, titers of type II collagen antibodies have been shown to decrease upon institution of immunosuppressive therapy.16
Although a humoral response dominates the picture of RP, there also is an associated T cell–mediated response.13 Histopathologically, biopsy of an active lesion of auricular cartilage shows a mixed inflammatory infiltrate composed primarily of lymphocytes, with variable numbers of polymorphonuclear cells, monocytes, and plasma cells. Loss of basophilia of the cartilage matrix can be observed, thought to be the result of proteoglycan depletion.13 Later, lesions classically display apoptosis of chondrocytes, focal calcification, or fibrosis.5
Diagnosis
Relapsing polychondritis acts classically as an autoimmune disease with a variable presentation, making diagnosis a challenge. Many sets of diagnostic criteria have been proposed. The most referenced remains the original criteria described by McAdam et al.17 In 2012, the Relapsing Polychondritis Disease Activity Index modified criteria set forth by Michet et al18 and might serve as the standard for diagnosis going forward.19
McAdam et al17 proposed that 3 of 6 clinical features are necessary for diagnosis: bilateral auricular chondritis, nonerosive seronegative inflammatory polyarthritis, nasal chondritis, ocular inflammation, respiratory tract chondritis, and audiovestibular damage. Michet et al18 proposed that 1 of 2 conditions are necessary for diagnosis of RP: (1) proven inflammation in 2 of 3 of the auricular, nasal, or laryngotracheal cartilages; or (2) proven inflammation in 1 of 3 of the auricular, nasal, or laryngotracheal cartilages, plus 2 other signs, including ocular inflammation, vestibular dysfunction, seronegative inflammatory arthritis, and hearing loss.
These criteria were proposed originally in 197617 and modified in 1986.18 No further updates have been offered since then. As such, serologic findings, such as antibodies against type II collagen, are not included in the diagnostic criteria. Additionally, these antibodies are not specific for RP and can be seen in other conditions such as rheumatoid arthritis.20
More recently, imaging analysis has been employed in conjunction with clinical and serologic data to diagnose the disease and evaluate its severity. The use of imaging modalities for these purposes is most beneficial in patients with notable disease and respiratory involvement.21
Although the clinical picture is typified by the classic findings described above, the clinician must be aware of more subtle clues to diagnosis,11 which is of particular importance to the dermatologist because 35% of patients with RP alone will have skin manifestations that can precede onset of chondritis.10 Most commonly, dermatologic manifestations are nonspecific and can include nodules on the limbs, purpura, and urticarial lesions.22 Individual case reports have noted the coexistence of RP with erythema multiforme,18 erythema annulare centrifugum,23 pyoderma gangrenosum,24 and panniculitis,18 among other disorders.
Treatment
Standardized guidelines for treatment do not exist. Treatments should be chosen based on severity of disease. Mild disease, presenting with recurrent chondritis and arthritis without evidence of systemic involvement, can be treated with nonsteroidal anti-inflammatory drugs, dapsone, or colchicine. Refractory disease often requires high-dose systemic corticosteroids.5
Severe systemic involvement leads to increased mortality and warrants more aggressive treatment.22 Commonly used agents include the immunosuppressants cyclophosphamide, cyclosporine, and methotrexate. Tumor necrosis factor α inhibitors have been the most widely utilized immunomodulatory agent for treatment of RP.25,26 Abatacept and rituximab also have been used with variable efficacy in patients with severe disease. Recently, the IL-6 receptor blocker tocilizumab has been used with some success.27
Prognosis
The prognosis for patients with RP largely depends on the severity of disease and degree of internal involvement. With improved management, largely due to awareness and recognition of disease, the survival rate among RP patients has increased from 55% at 10 years to 94% at the end of 8 years.18 The main cause of death in RP patients is airway complications related to laryngotracheal involvement.10 The second most common cause of death is cardiovascular complications in which valvular disease predominates.5
Concomitant Illness
Thirty-five percent of RP patients have coexisting autoimmune disease, the most common being antineutrophil cytoplasmic antibody–associated vasculitis.5,28 Although this association with autoimmune disease is well described, reports of RP occurring in other states of immune dysfunction are sparse. One case of RP has been reported in a child with common variable immunodeficiency thought to be related to underlying abnormal immune regulation and immunodeficiency.29 Relapsing polychondritis has been described in 4 patients with HIV, 2 of whom had concomitant autoimmune disease.7-9
Human immunodeficiency virus infection is a well-established cause of immune dysregulation and has variable association with autoimmunity. This variability depends largely on the stage of infection. When divided into stages, autoimmune diseases develop predominantly in stage I, during acute infection with an intact immune system; in stage III, with immunosuppression, a low CD4 count, and development of AIDS; and in stage IV, when the immune system is restored with the institution of highly active antiretroviral therapy.6 The interplay between HIV infection and development of autoimmune disease is complex, and pathogenesis remains speculative.
Conclusion
Our patient represents a case of RP in an HIV-positive patient. Additionally, our patient had no other identifiable autoimmune conditions but did have a strong family history of them. It is important for providers to be aware of the potential for development of RP as well as other autoimmune disease in the setting of HIV infection. The implications of a missed diagnosis could be dire because the disease course of RP is progressive and has the potential to decrease survival.
Relapsing polychondritis (RP) is a recurrent inflammatory condition involving primarily cartilaginous structures. The disease, first described as a clinical entity in 1960 by Pearson et al,1 is rare with an estimated incidence of 3.5 cases per 1 million individuals.2 The pathogenesis of RP is widely accepted as being autoimmune in nature, largely due to the identification of circulating autoantibodies seen in the sera of patients with similar clinical pictures.3
Although in most patients the primary process involves inflammation of cartilage, a subset of patients experience involvement of noncartilaginous sites.4 The degree of systemic involvement varies from none to notable, affecting the cardiovascular and respiratory systems and potentially leading to life-threatening complications, including cardiac valve compromise and airway collapse. Relapsing polychondritis is considered to be a progressive disease with the ultimate potential to be life-threatening.5
Human immunodeficiency virus (HIV) infection leads to a profound state of immune dysregulation, affecting innate, adaptive, and natural killer components of the immune system.6 There is variability in the development of autoimmune disease in HIV patients depending on the stage of infection. The frequency of rheumatologic disease in HIV patients might be as high as 60%.6 Relapsing polychondritis is rare in patients with HIV.7-9 Of 4 reported cases, 2 patients had other coexisting autoimmune disease—sarcoidosis and Behçet disease.8,9
Case Report
A 36-year-old man presented to the clinic with a concern of recurrent ear pain and swelling of approximately 2 years’ duration. Onset was sudden without inciting event. Symptoms initially involved the right ear with eventual progression to both ears. Additional symptoms included an auditory perception of underwater submersion, intermittent vertigo, and 3 episodes of throat closure sensation.
The patient’s medical history was notable for asthma; gastritis; depression; and HIV infection, which was diagnosed 4 years earlier and adequately managed with highly active antiretroviral therapy. His family history was notable for systemic lupus erythematosus in his mother, maternal aunt, and maternal cousin.
At presentation, the patient’s CD4 count was 799 cells/mm3 with an undetectable viral load. Medications included abacavir-dolutegravir-lamivudine, hydroxyzine, meclizine, mometasone, and quetiapine. Physical examination showed erythema, swelling, and tenderness of the left and right auricles with sparing of the earlobe that was more noticeable on the left ear (Figure 1). Bacterial culture from the external auditory meatus was positive for methicillin-resistant Staphylococcus aureus. Biopsy revealed chronic inflammatory perichondritis with mild to moderate fibrosis and chronic lymphocytic inflammation at the dermal cartilaginous junction (Figure 2). A direct immunofluorescent biopsy was unremarkable, but subsequent type II collagen antibodies were positive (35.5 endotoxin units/mL [reference range, <20 endotoxin units/mL]).
Comment
Relapsing polychondritis is an uncommon progressive disease characterized by recurrent inflammatory insults to cartilaginous and proteoglycan-rich structures.4 The most consistent clinical features of RP are ear inflammation that involves the auricle and spares the lobe, nasal chondritis, and arthralgia.10 Laryngotracheal compromise may occur from tracheal cartilage inflammation. The involvement of these specific structures is due to commonality between their component collagens.5 Although any organ system can be affected, as many as 50% of patients have respiratory tract involvement, which may affect any portion of the respiratory tree.11 If involving the larynx, this inflammation can lead to severe edema warranting intubation. Cardiovascular involvement is present in 24% to 52% of patients,10 which most commonly manifests as valvular impairment affecting the aortic valve more frequently than the mitral valve.5
Pathogenesis
Although the etiology of RP remains undetermined, multiple hypotheses have been proposed. One is that a certain subset of patients is predisposed to autoimmunity, and a secondary triggering event in the form of infection, malignancy, or medication catalyzes development of RP. A second hypothesis is that mechanical trauma to cartilage exposes the immune system to certain antigens that would have otherwise remained hidden, prompting autosensitization.12,13
Regardless of cause, an autoimmune pathogenesis is favored based on the following observations: RP is frequently associated with other autoimmune diseases in the same patient, glucocorticosteroids and other immunosuppressive therapies are effective for treatment, and histopathologic findings include an infiltrate of CD4+ T lymphocytes with detection of immunoglobulins and plasma cells in different lesions.5 The detection of autoantibodies against collagen in the serum of patients with RP further supports an autoimmune pathogenesis.3 The earliest identified autoantibodies in patients with RP were against type II collagen. Subsequent studies have identified autoantibodies against type IV and type XI collagens as well as other cartilage-related proteins such as matrilin 114 and cartilage oligomeric matrix proteins.15 Although circulating antibodies to type II collagen are present in a variable number of patients with the disease (30%–70%), levels likely correlate with disease activity and are highest at times of acute inflammation.3 Additionally, titers of type II collagen antibodies have been shown to decrease upon institution of immunosuppressive therapy.16
Although a humoral response dominates the picture of RP, there also is an associated T cell–mediated response.13 Histopathologically, biopsy of an active lesion of auricular cartilage shows a mixed inflammatory infiltrate composed primarily of lymphocytes, with variable numbers of polymorphonuclear cells, monocytes, and plasma cells. Loss of basophilia of the cartilage matrix can be observed, thought to be the result of proteoglycan depletion.13 Later, lesions classically display apoptosis of chondrocytes, focal calcification, or fibrosis.5
Diagnosis
Relapsing polychondritis acts classically as an autoimmune disease with a variable presentation, making diagnosis a challenge. Many sets of diagnostic criteria have been proposed. The most referenced remains the original criteria described by McAdam et al.17 In 2012, the Relapsing Polychondritis Disease Activity Index modified criteria set forth by Michet et al18 and might serve as the standard for diagnosis going forward.19
McAdam et al17 proposed that 3 of 6 clinical features are necessary for diagnosis: bilateral auricular chondritis, nonerosive seronegative inflammatory polyarthritis, nasal chondritis, ocular inflammation, respiratory tract chondritis, and audiovestibular damage. Michet et al18 proposed that 1 of 2 conditions are necessary for diagnosis of RP: (1) proven inflammation in 2 of 3 of the auricular, nasal, or laryngotracheal cartilages; or (2) proven inflammation in 1 of 3 of the auricular, nasal, or laryngotracheal cartilages, plus 2 other signs, including ocular inflammation, vestibular dysfunction, seronegative inflammatory arthritis, and hearing loss.
These criteria were proposed originally in 197617 and modified in 1986.18 No further updates have been offered since then. As such, serologic findings, such as antibodies against type II collagen, are not included in the diagnostic criteria. Additionally, these antibodies are not specific for RP and can be seen in other conditions such as rheumatoid arthritis.20
More recently, imaging analysis has been employed in conjunction with clinical and serologic data to diagnose the disease and evaluate its severity. The use of imaging modalities for these purposes is most beneficial in patients with notable disease and respiratory involvement.21
Although the clinical picture is typified by the classic findings described above, the clinician must be aware of more subtle clues to diagnosis,11 which is of particular importance to the dermatologist because 35% of patients with RP alone will have skin manifestations that can precede onset of chondritis.10 Most commonly, dermatologic manifestations are nonspecific and can include nodules on the limbs, purpura, and urticarial lesions.22 Individual case reports have noted the coexistence of RP with erythema multiforme,18 erythema annulare centrifugum,23 pyoderma gangrenosum,24 and panniculitis,18 among other disorders.
Treatment
Standardized guidelines for treatment do not exist. Treatments should be chosen based on severity of disease. Mild disease, presenting with recurrent chondritis and arthritis without evidence of systemic involvement, can be treated with nonsteroidal anti-inflammatory drugs, dapsone, or colchicine. Refractory disease often requires high-dose systemic corticosteroids.5
Severe systemic involvement leads to increased mortality and warrants more aggressive treatment.22 Commonly used agents include the immunosuppressants cyclophosphamide, cyclosporine, and methotrexate. Tumor necrosis factor α inhibitors have been the most widely utilized immunomodulatory agent for treatment of RP.25,26 Abatacept and rituximab also have been used with variable efficacy in patients with severe disease. Recently, the IL-6 receptor blocker tocilizumab has been used with some success.27
Prognosis
The prognosis for patients with RP largely depends on the severity of disease and degree of internal involvement. With improved management, largely due to awareness and recognition of disease, the survival rate among RP patients has increased from 55% at 10 years to 94% at the end of 8 years.18 The main cause of death in RP patients is airway complications related to laryngotracheal involvement.10 The second most common cause of death is cardiovascular complications in which valvular disease predominates.5
Concomitant Illness
Thirty-five percent of RP patients have coexisting autoimmune disease, the most common being antineutrophil cytoplasmic antibody–associated vasculitis.5,28 Although this association with autoimmune disease is well described, reports of RP occurring in other states of immune dysfunction are sparse. One case of RP has been reported in a child with common variable immunodeficiency thought to be related to underlying abnormal immune regulation and immunodeficiency.29 Relapsing polychondritis has been described in 4 patients with HIV, 2 of whom had concomitant autoimmune disease.7-9
Human immunodeficiency virus infection is a well-established cause of immune dysregulation and has variable association with autoimmunity. This variability depends largely on the stage of infection. When divided into stages, autoimmune diseases develop predominantly in stage I, during acute infection with an intact immune system; in stage III, with immunosuppression, a low CD4 count, and development of AIDS; and in stage IV, when the immune system is restored with the institution of highly active antiretroviral therapy.6 The interplay between HIV infection and development of autoimmune disease is complex, and pathogenesis remains speculative.
Conclusion
Our patient represents a case of RP in an HIV-positive patient. Additionally, our patient had no other identifiable autoimmune conditions but did have a strong family history of them. It is important for providers to be aware of the potential for development of RP as well as other autoimmune disease in the setting of HIV infection. The implications of a missed diagnosis could be dire because the disease course of RP is progressive and has the potential to decrease survival.
- Pearson CM, Kline HM, Newcomer VD. Relapsing polychondritis. N Engl J Med. 1960;263:51-58.
- Kent PD, Michet CJ Jr, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol. 2004;16:56-61.
- Ebringer R, Rook G, Swana GT, et al. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis. 1981;40:473-479.
- Sharma A, Law AD, Bambery P, et al. Relapsing polychondritis: clinical presentations, disease activity and outcomes. Orphanet J Rare Dis. 2014;9:198.
- Vitale A, Sota J, Rigante D, et al. Relapsing polychondritis: an update on pathogenesis, clinical features, diagnostic tools, and therapeutic perspectives. Curr Rheumatol Rep. 2016;18:3.
- Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
- Dolev JC, Maurer TA, Reddy SG, et al. Relapsing polychondritis in HIV-infected patients: a report of two cases. J Am Acad Dermatol. 2004;51:1023-1025.
- Zandman-Goddard G, Peeva E, Barland P. Combined autoimmune disease in a patient with AIDS. Clin Rheumatol. 2002;21:70-72.
- Belzunegui J, Cancio J, Pego JM, et al. Relapsing polychondritis and Behc¸et’s syndrome in a patient with HIV infection. Ann Rheum Dis. 1995;54:780.
- Sharma A, Gnanapandithan K, Sharma K, et al. Relapsing polychondritis: a review. Clin Rheumatol. 2013;32:1575-1583.
- Cantarini L, Vitale A, Brizi MG, et al. Diagnosis and classification of relapsing polychondritis. J Autoimmun. 2014;48-49:53-59.
- Cañas CA, Bonilla Abadía F. Local cartilage trauma as a pathogenic factor in autoimmunity (one hypothesis based on patients with relapsing polychondritis triggered by cartilage trauma). Autoimmune Dis. 2012;2012:453698.
- Ouchi N, Uzuki M, Kamataki A, et al. Cartilage destruction is partly induced by the internal proteolytic enzymes and apoptotic phenomenon of chondrocytes in relapsing polychondritis. J Rheumatol. 2011;38:730-737.
- Buckner JH, Wu JJ, Reife RA, et al. Autoreactivity against matrilin-1 in a patient with relapsing polychondritis. Arthritis Rheum. 2000;43:939-943.
- Kempta Lekpa F, Piette JC, Bastuji-Garin S, et al. Serum cartilage oligomeric matrix protein (COMP) is a marker of disease activity in relapsing polychondritis. Clin Exp Rheumatol. 2010;28:553-555.
- Foidart JM, Abe S, Martin GR, et al. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med. 1978;299:1203-1207.
- McAdam LP, O’Hanlan MA, Bluestone R, et al. Relapsing polychondritis: prospective study of 23 patients and review of the literature. Medicine (Baltimore). 1976;55:193-215.
- Michet CJ, McKenna CH, Luthra HS, et al. Relapsing polychondritis: survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78.
- Arnaud L, Devilliers H, Peng SL, et al. The Relapsing Polychondritis Disease Activity Index: development of a disease activity score for relapsing polychondritis. Autoimmun Rev. 2012;12:204-209.
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3-18.
- Thaiss WM, Nikolaou K, Spengler W, et al. Imaging diagnosis in relapsing polychondritis and correlation with clinical and serological data. Skeletal Radiol. 2015;5:339-346.
- Lahmer T, Treiber M, von Werder A, et al. Relapsing polychondritis: an autoimmune disease with many faces. Autoimmun Rev. 2010;9:540-546.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Francès C, el Rassi R, Laporte JL, et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine (Baltimore). 2001;80:173-179.
- Chopra R, Chaudhary N, Kay J. Relapsing polychondritis. Rheum Dis Clin North Am. 2013;39:263-276.
- Moulis G, Sailler L, Pugnet G, et al. Biologics in relapsing polychondritis: a case series. Clin Exp Rheumatol. 2013;31:937-939.
- Henes CJ, Xenitidis T, Horger M. Tocilizumab for refractory relapsing polychondritis—long-term response monitoring by magnetic resonance imaging. Joint Bone Spine. 2016;83:365-366.
- Weinberger A, Myers AR. Relapsing polychondritis associated with cutaneous vasculitis. Arch Dermatol. 1979;115:980-981.
- Karaca NE, Aksu G, Yildiz B, et al. Relapsing polychondritis in a child with common variable immunodeficiency. Int J Dermatol. 2009;48:525-528.
- Pearson CM, Kline HM, Newcomer VD. Relapsing polychondritis. N Engl J Med. 1960;263:51-58.
- Kent PD, Michet CJ Jr, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol. 2004;16:56-61.
- Ebringer R, Rook G, Swana GT, et al. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis. 1981;40:473-479.
- Sharma A, Law AD, Bambery P, et al. Relapsing polychondritis: clinical presentations, disease activity and outcomes. Orphanet J Rare Dis. 2014;9:198.
- Vitale A, Sota J, Rigante D, et al. Relapsing polychondritis: an update on pathogenesis, clinical features, diagnostic tools, and therapeutic perspectives. Curr Rheumatol Rep. 2016;18:3.
- Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
- Dolev JC, Maurer TA, Reddy SG, et al. Relapsing polychondritis in HIV-infected patients: a report of two cases. J Am Acad Dermatol. 2004;51:1023-1025.
- Zandman-Goddard G, Peeva E, Barland P. Combined autoimmune disease in a patient with AIDS. Clin Rheumatol. 2002;21:70-72.
- Belzunegui J, Cancio J, Pego JM, et al. Relapsing polychondritis and Behc¸et’s syndrome in a patient with HIV infection. Ann Rheum Dis. 1995;54:780.
- Sharma A, Gnanapandithan K, Sharma K, et al. Relapsing polychondritis: a review. Clin Rheumatol. 2013;32:1575-1583.
- Cantarini L, Vitale A, Brizi MG, et al. Diagnosis and classification of relapsing polychondritis. J Autoimmun. 2014;48-49:53-59.
- Cañas CA, Bonilla Abadía F. Local cartilage trauma as a pathogenic factor in autoimmunity (one hypothesis based on patients with relapsing polychondritis triggered by cartilage trauma). Autoimmune Dis. 2012;2012:453698.
- Ouchi N, Uzuki M, Kamataki A, et al. Cartilage destruction is partly induced by the internal proteolytic enzymes and apoptotic phenomenon of chondrocytes in relapsing polychondritis. J Rheumatol. 2011;38:730-737.
- Buckner JH, Wu JJ, Reife RA, et al. Autoreactivity against matrilin-1 in a patient with relapsing polychondritis. Arthritis Rheum. 2000;43:939-943.
- Kempta Lekpa F, Piette JC, Bastuji-Garin S, et al. Serum cartilage oligomeric matrix protein (COMP) is a marker of disease activity in relapsing polychondritis. Clin Exp Rheumatol. 2010;28:553-555.
- Foidart JM, Abe S, Martin GR, et al. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med. 1978;299:1203-1207.
- McAdam LP, O’Hanlan MA, Bluestone R, et al. Relapsing polychondritis: prospective study of 23 patients and review of the literature. Medicine (Baltimore). 1976;55:193-215.
- Michet CJ, McKenna CH, Luthra HS, et al. Relapsing polychondritis: survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78.
- Arnaud L, Devilliers H, Peng SL, et al. The Relapsing Polychondritis Disease Activity Index: development of a disease activity score for relapsing polychondritis. Autoimmun Rev. 2012;12:204-209.
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3-18.
- Thaiss WM, Nikolaou K, Spengler W, et al. Imaging diagnosis in relapsing polychondritis and correlation with clinical and serological data. Skeletal Radiol. 2015;5:339-346.
- Lahmer T, Treiber M, von Werder A, et al. Relapsing polychondritis: an autoimmune disease with many faces. Autoimmun Rev. 2010;9:540-546.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Francès C, el Rassi R, Laporte JL, et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine (Baltimore). 2001;80:173-179.
- Chopra R, Chaudhary N, Kay J. Relapsing polychondritis. Rheum Dis Clin North Am. 2013;39:263-276.
- Moulis G, Sailler L, Pugnet G, et al. Biologics in relapsing polychondritis: a case series. Clin Exp Rheumatol. 2013;31:937-939.
- Henes CJ, Xenitidis T, Horger M. Tocilizumab for refractory relapsing polychondritis—long-term response monitoring by magnetic resonance imaging. Joint Bone Spine. 2016;83:365-366.
- Weinberger A, Myers AR. Relapsing polychondritis associated with cutaneous vasculitis. Arch Dermatol. 1979;115:980-981.
- Karaca NE, Aksu G, Yildiz B, et al. Relapsing polychondritis in a child with common variable immunodeficiency. Int J Dermatol. 2009;48:525-528.
Practice Points
- Relapsing polychondritis (RP) is characterized by recurrent inflammatory insults to cartilaginous and proteoglycan-rich structures, most often manifesting as ear inflammation that involves the auricle but spares the lobe, nasal chondritis, and arthralgia.
- Relapsing polychondritis acts classically as an autoimmune disease with a variable presentation, making diagnosis a challenge.
- One-third of RP patients have coexisting autoimmune disease.
- Treatment of RP depends on severity of disease.
- Dermatologists must be aware of the potential for development of RP in the setting of human immunodeficiency virus infection; a missed diagnosis of this progressive disease has the potential to be life-threatening.
EHR parodies, hangover-free booze, and dubstep repellent
Epic tweets
Need a little break from seeing all your patients? Hop on Twitter for a few minutes – specifically, the Epic parody account.
A frustrated but hilarious physician somewhere on the East Coast created the account a month ago to brighten busy days and help other physicians connect through a shared hatred of Epic, the electronic health records system.
The account’s bio states: “My goal is to create confusion for doctors. I will not rest until doctors do nothing but click buttons. Eye contact is evil.” In continuing with this theme, the account dedicates many Tweets to decrying patient-doctor interaction and encouraging computer-doctor interaction instead. And it rails against the “shaming of data-entry clerks.”
If you, too, are frustrated with Epic or your own EHR system, submit them to @EPICEMRparody with the hashtag #EPICfail. Join the club.
Pour me another, bartender
All the beauty of alcohol without the soul-crushing hangover the next day? I’ll take two on the rocks, please.
For the last decade, visionary, scientist, and soon-to-be hero David Nutt, DM, has been working on a formula for synthetic alcohol that gets you nice and tipsy, but spares you the hangover. Dr. Nutt has created a compound he calls “alcosynth” that triggers a buzz but doesn’t activate the brain receptors that cause the symptoms of hangover.
Dr. Nutt is confident that his alcohol will hit liquor stores in just 5 years. As the director of neuropsychopharmacology at the Imperial College London and a wine bar owner, he knows a bit more than the average person about alcohol and brain receptors. He realized that the brain’s GABA receptors can be stimulated by alcohol and developed his product, Alcarelle, to simulate that stimulation without causing headaches, nausea, dizziness, and other hangover symptoms.
Dr. Nutt guards his process pretty tightly, however – the secret to hangover-free booze is as precious as a golden ticket to the Wonka factory. Everyone’s going to want to get their hands on it!
Don’t just do something, lie there
As the Trump administration tries to implement work requirements for food stamps and Medicaid, the Artificial Gravity Bed Rest Study offers another way to get paid for doing nothing.
This joint effort between NASA, the European Space Agency, and the German Space Agency will recreate outer space conditions right here on earth – in Cologne, Germany, to be exact – by having participants lie down for 60 days with their heads 6 degrees below their feet, causing fluids in the body to shift toward their heads and reproducing conditions experienced by astronauts in the absence of gravity.
Unsurprisingly, not just anyone will be considered: Participants have to be women aged 24-55 years with body mass indexes of 19-30 kg/m2 who speak German and don’t smoke. Test subjects have to bathe, eat (the project’s website mentions pancakes), and go to the toilet while lying down, and they will spend 30 minutes a day in the human centrifuge – that’s the artificial gravity part of the study – to counteract the effects of weightlessness. They also will receive an expense allowance of about $18,500.
So, you can earn almost 20 grand as you get waited on, stuffed with pancakes, and massaged while catching up on your reading and relaxing for 2 months. Hmm, we may have gotten this backwards with that work requirement remark. Sounds more like a trial membership into the “1%,” so be sure to keep track of your portfolios.
That’s why mosquitoes don’t go to clubs
There’s not a whole lot of positive things that can be said about mosquitoes, spreaders of such wonderful diseases such as malaria and Zika. But we’ll give them this: They have good taste in music.
Those musical standards were the subject of a study published in Acta Tropica, in which female mosquitoes were starved for 12 hours, introduced to a fresh hamster for an all-you-can-eat buffet and a single male mosquito for reproductive purposes, then subjected to either silence or some extremely loud dubstep music, courtesy of the artist Skrillex. The researchers’ goal was simple: Is loud music an “environmentally friendly” repellent alternative?
In a blow to the egos of dubstep fans everywhere, not only did the mosquitoes forced to listen to Skrillex take longer to start sucking blood from the hamster, they sucked out less blood and made fewer attempts to reproduce with the lone male mosquito. According to the researchers, the so-called music’s aggressive vibrations disrupts the mosquito’s ability to fly and synchronize wing beats.
This is really a good news/bad news situation here. Good news, we’ve found an easy way to drive off a very annoying insect. Bad news, we have to suffer through loud dubstep music. Personally, we’ll take the mosquitoes.

Epic tweets
Need a little break from seeing all your patients? Hop on Twitter for a few minutes – specifically, the Epic parody account.
A frustrated but hilarious physician somewhere on the East Coast created the account a month ago to brighten busy days and help other physicians connect through a shared hatred of Epic, the electronic health records system.
The account’s bio states: “My goal is to create confusion for doctors. I will not rest until doctors do nothing but click buttons. Eye contact is evil.” In continuing with this theme, the account dedicates many Tweets to decrying patient-doctor interaction and encouraging computer-doctor interaction instead. And it rails against the “shaming of data-entry clerks.”
If you, too, are frustrated with Epic or your own EHR system, submit them to @EPICEMRparody with the hashtag #EPICfail. Join the club.
Pour me another, bartender
All the beauty of alcohol without the soul-crushing hangover the next day? I’ll take two on the rocks, please.
For the last decade, visionary, scientist, and soon-to-be hero David Nutt, DM, has been working on a formula for synthetic alcohol that gets you nice and tipsy, but spares you the hangover. Dr. Nutt has created a compound he calls “alcosynth” that triggers a buzz but doesn’t activate the brain receptors that cause the symptoms of hangover.
Dr. Nutt is confident that his alcohol will hit liquor stores in just 5 years. As the director of neuropsychopharmacology at the Imperial College London and a wine bar owner, he knows a bit more than the average person about alcohol and brain receptors. He realized that the brain’s GABA receptors can be stimulated by alcohol and developed his product, Alcarelle, to simulate that stimulation without causing headaches, nausea, dizziness, and other hangover symptoms.
Dr. Nutt guards his process pretty tightly, however – the secret to hangover-free booze is as precious as a golden ticket to the Wonka factory. Everyone’s going to want to get their hands on it!
Don’t just do something, lie there
As the Trump administration tries to implement work requirements for food stamps and Medicaid, the Artificial Gravity Bed Rest Study offers another way to get paid for doing nothing.
This joint effort between NASA, the European Space Agency, and the German Space Agency will recreate outer space conditions right here on earth – in Cologne, Germany, to be exact – by having participants lie down for 60 days with their heads 6 degrees below their feet, causing fluids in the body to shift toward their heads and reproducing conditions experienced by astronauts in the absence of gravity.
Unsurprisingly, not just anyone will be considered: Participants have to be women aged 24-55 years with body mass indexes of 19-30 kg/m2 who speak German and don’t smoke. Test subjects have to bathe, eat (the project’s website mentions pancakes), and go to the toilet while lying down, and they will spend 30 minutes a day in the human centrifuge – that’s the artificial gravity part of the study – to counteract the effects of weightlessness. They also will receive an expense allowance of about $18,500.
So, you can earn almost 20 grand as you get waited on, stuffed with pancakes, and massaged while catching up on your reading and relaxing for 2 months. Hmm, we may have gotten this backwards with that work requirement remark. Sounds more like a trial membership into the “1%,” so be sure to keep track of your portfolios.
That’s why mosquitoes don’t go to clubs
There’s not a whole lot of positive things that can be said about mosquitoes, spreaders of such wonderful diseases such as malaria and Zika. But we’ll give them this: They have good taste in music.
Those musical standards were the subject of a study published in Acta Tropica, in which female mosquitoes were starved for 12 hours, introduced to a fresh hamster for an all-you-can-eat buffet and a single male mosquito for reproductive purposes, then subjected to either silence or some extremely loud dubstep music, courtesy of the artist Skrillex. The researchers’ goal was simple: Is loud music an “environmentally friendly” repellent alternative?
In a blow to the egos of dubstep fans everywhere, not only did the mosquitoes forced to listen to Skrillex take longer to start sucking blood from the hamster, they sucked out less blood and made fewer attempts to reproduce with the lone male mosquito. According to the researchers, the so-called music’s aggressive vibrations disrupts the mosquito’s ability to fly and synchronize wing beats.
This is really a good news/bad news situation here. Good news, we’ve found an easy way to drive off a very annoying insect. Bad news, we have to suffer through loud dubstep music. Personally, we’ll take the mosquitoes.

Epic tweets
Need a little break from seeing all your patients? Hop on Twitter for a few minutes – specifically, the Epic parody account.
A frustrated but hilarious physician somewhere on the East Coast created the account a month ago to brighten busy days and help other physicians connect through a shared hatred of Epic, the electronic health records system.
The account’s bio states: “My goal is to create confusion for doctors. I will not rest until doctors do nothing but click buttons. Eye contact is evil.” In continuing with this theme, the account dedicates many Tweets to decrying patient-doctor interaction and encouraging computer-doctor interaction instead. And it rails against the “shaming of data-entry clerks.”
If you, too, are frustrated with Epic or your own EHR system, submit them to @EPICEMRparody with the hashtag #EPICfail. Join the club.
Pour me another, bartender
All the beauty of alcohol without the soul-crushing hangover the next day? I’ll take two on the rocks, please.
For the last decade, visionary, scientist, and soon-to-be hero David Nutt, DM, has been working on a formula for synthetic alcohol that gets you nice and tipsy, but spares you the hangover. Dr. Nutt has created a compound he calls “alcosynth” that triggers a buzz but doesn’t activate the brain receptors that cause the symptoms of hangover.
Dr. Nutt is confident that his alcohol will hit liquor stores in just 5 years. As the director of neuropsychopharmacology at the Imperial College London and a wine bar owner, he knows a bit more than the average person about alcohol and brain receptors. He realized that the brain’s GABA receptors can be stimulated by alcohol and developed his product, Alcarelle, to simulate that stimulation without causing headaches, nausea, dizziness, and other hangover symptoms.
Dr. Nutt guards his process pretty tightly, however – the secret to hangover-free booze is as precious as a golden ticket to the Wonka factory. Everyone’s going to want to get their hands on it!
Don’t just do something, lie there
As the Trump administration tries to implement work requirements for food stamps and Medicaid, the Artificial Gravity Bed Rest Study offers another way to get paid for doing nothing.
This joint effort between NASA, the European Space Agency, and the German Space Agency will recreate outer space conditions right here on earth – in Cologne, Germany, to be exact – by having participants lie down for 60 days with their heads 6 degrees below their feet, causing fluids in the body to shift toward their heads and reproducing conditions experienced by astronauts in the absence of gravity.
Unsurprisingly, not just anyone will be considered: Participants have to be women aged 24-55 years with body mass indexes of 19-30 kg/m2 who speak German and don’t smoke. Test subjects have to bathe, eat (the project’s website mentions pancakes), and go to the toilet while lying down, and they will spend 30 minutes a day in the human centrifuge – that’s the artificial gravity part of the study – to counteract the effects of weightlessness. They also will receive an expense allowance of about $18,500.
So, you can earn almost 20 grand as you get waited on, stuffed with pancakes, and massaged while catching up on your reading and relaxing for 2 months. Hmm, we may have gotten this backwards with that work requirement remark. Sounds more like a trial membership into the “1%,” so be sure to keep track of your portfolios.
That’s why mosquitoes don’t go to clubs
There’s not a whole lot of positive things that can be said about mosquitoes, spreaders of such wonderful diseases such as malaria and Zika. But we’ll give them this: They have good taste in music.
Those musical standards were the subject of a study published in Acta Tropica, in which female mosquitoes were starved for 12 hours, introduced to a fresh hamster for an all-you-can-eat buffet and a single male mosquito for reproductive purposes, then subjected to either silence or some extremely loud dubstep music, courtesy of the artist Skrillex. The researchers’ goal was simple: Is loud music an “environmentally friendly” repellent alternative?
In a blow to the egos of dubstep fans everywhere, not only did the mosquitoes forced to listen to Skrillex take longer to start sucking blood from the hamster, they sucked out less blood and made fewer attempts to reproduce with the lone male mosquito. According to the researchers, the so-called music’s aggressive vibrations disrupts the mosquito’s ability to fly and synchronize wing beats.
This is really a good news/bad news situation here. Good news, we’ve found an easy way to drive off a very annoying insect. Bad news, we have to suffer through loud dubstep music. Personally, we’ll take the mosquitoes.

Topical Natural Products in Managing Dermatologic Conditions: Observations and Recommendations
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Practice Points
- Patients are increasingly interested in and asking for nature-based products and formulations to manage dermatologic conditions.
- Physicians can satisfy patient interests with nature-based formulations that are as beneficial or more so than synthetic formulations because of the physiologic activity of the ingredients within these formulations.
- Physicians should have resources available to them that adequately educate on nature-based ingredients and how to recommend them.
Cutaneous Metastasis of Endometrial Carcinoma: An Unusual and Dramatic Presentation
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
Case Report
A 62-year-old woman presented with multiple large friable tumors of the abdominal panniculus. The patient also reported an unintentional 75-lb weight loss over the last 9 months as well as vaginal bleeding and fecal discharge from the vagina of 2 weeks’ duration. The patient had a surgical and medical history of a robotic-assisted hysterectomy and bilateral salpingo-oophorectomy performed 4 years prior to presentation. Final surgical pathology showed complex atypical endometrial hyperplasia with no adenocarcinoma identified.
Physical examination revealed multiple large, friable, exophytic tumors of the left side of the lower abdominal panniculus within close vicinity of the patient’s abdominal hysterectomy scars (Figure 1). The largest lesion measured approximately 6 cm in length. Laboratory values were elevated for carcinoembryonic antigen (5.9 ng/mL [reference range, <3.0 ng/mL]) and cancer antigen 125 (202 U/mL [reference range, <35 U/mL]). Computed tomography of the abdomen and pelvis revealed diffuse metastatic disease.
Comment
Incidence and Pathogenesis
Endometrial carcinoma is the most common gynecologic malignancy in the United States, but it rarely progresses to disseminated disease because of routine gynecologic examinations and the low threshold for surgical intervention. Cutaneous metastases represent one of the rarest presentations of disseminated disease, occurring in only 0.8% of those diagnosed with endometrial carcinoma.1 Cutaneous metastases occur almost exclusively in women older than 50 years and typically appear several months to years after hysterectomy. Although the exact pathogenesis is unknown, it is theorized that small foci of malignant cells may be seeded during surgery, leading to visceral and cutaneous involvement.
Clinical Presentation
Lesions vary morphologically, most commonly presenting as nonspecific, painless, hemorrhagic nodules. Lesions typically present in areas of direct local extension; prior radiotherapy; or areas of initial surgery, as was the case with our patient.2 Approximately 20 cases of umbilical involvement (Sister Mary Joseph nodule) have been reported in the literature. These cases are thought to occur from direct local spread of disease from the peritoneum.3 Hematogenous and lymphatic spread to distant sites such as the scalp and mandible also have been reported. More than 50% of patients will have underlying visceral metastatic disease at the time of diagnosis.3
Histopathologic Findings
Histopathology varies with the morphology of the underlying primary tumor, with endometrioid adenocarcinoma being the most common form associated with cutaneous metastasis, as was the case with our patient.4 Histology is characterized by dermal proliferation of atypical glandular epithelium with diffuse hemorrhage. Staining typically is positive for CK7 and negative for CK20 and CDX2.5 Histopathology and immunohistochemical staining are not specific for diagnosis and must be correlated with clinical history.
Management and Prognosis
Similar to cutaneous metastasis in other internal malignancies, prognosis is poor, as widespread dissemination of the underlying malignancy typically is present. Mean life expectancy is 4 to 12 months.6 Treatment is primarily palliative, as chemotherapy and radiotherapy are largely ineffective.
Conclusion
Our patient represents a dramatic form of cutaneous extension of a common disease. Dermatologists often are consulted because of the nonspecific nature of the lesions and must be conscious of this entity. As with other cutaneous metastases, a thorough medical and surgical history in conjunction with histopathology are necessary for an accurate diagnosis.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
- Atallah D, el Kassis N, Lutfallah F, et al. Cutaneous metastasis in endometrial cancer: once in a blue moon—case report. World J Surg Oncol. 2014;12:86.
- Temkin SM, Hellman M, Lee YC, et al. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118-121.
- Kushner DM, Lurain JR, Fu TS, et al. Endometrial adenocarcinoma metastatic to the scalp: case report and literature review. Gynecol Oncol. 1997;65:530-533.
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19-23.
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201-203
- Damewood MD, Rosenshein NB, Grumbine FC, et al. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471-1477.
Practice Points
- Cutaneous metastases of endometrial carcinoma are extremely rare and typically present in areas of direct local spread.
- As with other cutaneous metastases, lesions often are nonspecific, making history and histopathology essential for diagnosis.
Analysis of Nail-Related Content in the Basic Dermatology Curriculum
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3
Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3

Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Practice Points
- Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving.
- Education on diagnosis and management of nail conditions is deficient in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum.
- Increased efforts are needed to incorporate relevant nail education materials into the AAD Basic Dermatology Curriculum.