User login
MedPAC eyes scholarships, loan forgiveness to boost primary care ranks
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
gtwachtman@mdedge.com
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
gtwachtman@mdedge.com
WASHINGTON – according to a proposal presented at a meeting of the Medicare Payment Advisory Commission.
“A Medicare[-based] program would have a specific objective to encourage more physicians to enter primary care and provide primary care to beneficiaries,” MedPAC staffer Ariel Winter said. “By reducing educational debt, a Medicare-specific program would provide a financial incentive for physicians to choose primary care.”
Any program would face some challenges, Mr. Winter noted. Based on evidence, “it’s difficult to predict how physicians would respond if they were offered debt reduction in exchange for a commitment to practice primary care,” as financial considerations are not the only reason why physicians choose a specific career track.
Financing the program would also need to be considered. MedPAC staff recommended using a separate recommendation, one to end the Merit-based Incentive Payment System and use its $500 million put aside as MIPS bonuses to pay for any Medicare-based program.
Staff proposed a pilot program to “test the impact of different design choices on program operations, physician participation, and career choices,” he said. “Policymakers could use the results to improve the program and decide whether to expand it.”
MedPAC Vice Chairman Jon Christianson, PhD, suggested any program be tied to “physicians who practiced in areas where Medicare beneficiaries don’t have adequate access” to primary care doctors.
However, Mr. Winter noted that he is not aware of “any off-the-shelf system that identifies areas where there’s a problem, where there’s a shortage of clinicians for Medicare beneficiaries specifically. I am not sure how you would do that.”
MedPAC member Kathy Buto, former vice president of global health policy at Johnson & Johnson, questioned whether nurse practitioners and physician assistants should be included in the program, as they “are beginning to subspecialize and get out of primary care.” Mr. Winter said it is open for consideration.
MedPAC member Pat Wang, president and CEO of Healthfirst in New York, questioned whether a new program was needed or whether fixing of existing programs, “making them work better” is the way to go given the evidence that the effect of student debt on decision making is mixed.
She suggested that rather than targeting loan forgiveness, maybe the program should be structured more as a bonus payment rather than debt forgiveness as a means of incentivizing people who may not be concerned with debt forgiveness.
Ms. Buto added that questions of autonomy might also need to be addressed. “Physicians often feel like they don’t have control in Medicare, that they’re required to do a lot of things, and that they are subject to the fee schedule. If there were some way to grant more autonomy, control, and convey status that way, whether it has to do with greater flexibility in whatever, payment models and so on, that’s where I think you can begin to shift the status within primary care.”
MedPAC Chairman Francis Crosson, MD, recalled his time at Kaiser Permanente and noted their programs showed success because of the combination of a significant amount of money and time commitment (10 years).
The time commitment became an important part because after that long, physicians became a part of their communities and tended to stay.
“Two or 3 years, from my perspective and my experience, doesn’t work very well,” Dr. Crosson said. “But a significant period of time does, and a significant amount of money does seem to work.”
gtwachtman@mdedge.com
REPORTING FROM A MEDPAC MEETING
Addressing insulin price spikes will require supply chain reform
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
WASHINGTON – panelists said at a House Committee on Energy & Commerce hearing on insulin affordability.
“Each member of the supply chain has a responsibility to help solve this problem,” said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Center at Vanderbilt University, who was speaking on behalf of the Endocrine Society during the April 2 hearing of the committee’s oversight & investigations subcommittee.
Dr. Powers identified all members – manufacturers, payers, pharmacy benefit managers, patients, providers, and Congress – as having a role in developing a solution that will encourage more access to the treatment.
The hearing was the first of two in a series specifically examining the price of insulin. This one focused on the role pricing issues play in terms of access to insulin and patient outcomes.
To highlight the pricing issues, it was noted that a vial of Humalog (insulin lispro) cost $21 when it was launched by Eli Lilly in 1996. It now costs $275 even though it has gone through no changes in formulation or innovation during that time.
Kasia Lipska, MD, of Yale University School of Medicine noted that a summer 2017 survey conducted by the Yale Diabetes Center found that one in four patients took less than the prescribed dose of insulin specifically because of the cost of insulin.
William Cefalu, MD, chief scientific, medical, and mission officer at the American Diabetes Association, echoed comments from Dr. Powers about pricing and suggested that simply going after list price is not a complete solution.
“There is also no guarantee that if the list price drops there [will] be substantive changes throughout the supply chain,” Dr. Cefalu said, adding that there needs to be a move away from a system based on high list prices and rebates and toward a system that ensures that any negotiated rebate or discount will find its way to the patient at the pharmacy counter.
“That’s what is not happening now,” Dr. Cefalu added. “Unless you can control what happens downstream in the intermediaries and what happens to the patient, there is no guarantee that just dropping list prices ... is going to get the job done.”
Aaron Kowalski, PhD, chief mission officer of JDRF, an organization that funds research into type 1 diabetes, also called out insurers as a part of the problem.
“What we are seeing in the community is people being switched [from their prescribed insulin for nonmedical reasons] by their insurance companies, not by the choice of their physician or the patient, which is just not the right way to practice medicine.”
He relayed an anecdote about a woman who went from having her blood sugar well controlled to dealing with severe cases of hyperglycemia because of changes in the medical coverage of her insulin. It took 8 hours on the phone with the insurance company, not to mention countless hours spent by the physician, to get the situation corrected and to get the proper insulin covered.
“This is a broken part of the system,” Dr. Kowalski said.
Dr. Cefalu noted that data are needed on the medical impact of switching for nonmedical reasons, such as changes to insurance coverage.
Christel Marchand Aprigliano, chief executive officer of the Diabetes Patient Advocacy Coalition, also relayed an anecdote of a friend who had suffered medical consequences of nonmedical switching of his insulin and then having to deal with his insurer’s fail-first policy before they would cover his original, medically effective insulin.
“Insurance has been denied twice because they believe that insulins are interchangeable, which they aren’t,” she said.
Michael Burgess, MD, (R-Texas) asked rhetorically during the hearing whether it would make sense for payers to simply provide insulin at no cost to patients, given the cost of medical complications resulting from lack of proper use as a result of pricing likely is much higher than covering insulin completely.
While specific legislative proposals were not discussed during the hearing, one thing that the panelists agreed would help to clarify all the factors that are contributing to the pricing increases is clear, transparent information about the finances surrounding the insulin as the product moves through the supply chain.
The Food and Drug Administration is also doing its part. Although the agency was not a participant in the hearing, the agency’s commissioner, Scott Gottlieb, MD, released a statement on the same day as the hearing in which he touted efforts in the biosimilar space that could spur competition.
“Once an interchangeable insulin product is approved and available on the market, it can be substituted for the reference product at the pharmacy, potentially leading to increased access [to insulin] and lower costs for patients,” he said in the statement. “The FDA anticipates that biosimilar and interchangeable insulin products will bring the competition that’s needed to help [deliver] affordable treatment options to patients.”
Dr Gottlieb did not say when a biosimilar insulin might be available on the market.
The second hearing in this series has not been scheduled, but is expected to take place the week of April 8 and will feature representatives from three insulin manufacturers and other participants in the supply chain.
REPORTING FROM A HOUSE ENERGY & COMMERCE SUBCOMMITTEE HEARING
Novel chemo/PARP inhibitor strategy promising for advanced pancreatic cancer
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
REPORTING FROM AACR 2019
LGBT youth: Affirmation in your practice
Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.
Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.
Join us Wednesday, April 3, 2019, at 6:00 pm EST, for a Twitter discussion on caring for LGBT youth. Two pediatricians that are passionate about the LGBT community and children will be joining the conversation: Dr. Gayathri Chelvakumar and Dr. Gerald T. Montano.
Questions will include:
- How do people become aware they are gay, lesbian, bisexual, or transgender?
- How can we address specific health concerns of LGBT youth?
- How can we make our practices a safe space for LGBT youth?
- What is gender dysphoria, and how is it treated?
LGBT Youth Consult, by Dr. Gayathri Chelvakumar
- A primer on sexuality and gender identity
- Creating safe spaces for LGBTQ youth, families in health care settings
- How we can support our LGBTQ patients
- New research on health-related behaviors of sexual minority youth
- Promoting mental well-being in LGBTQ youth
New to Twitter Chats? Steps to join the conversation are below.
UNITY-NHL: Interim findings show activity, tolerability of umbralisib for R/R MZL
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
REPORTING FROM AACR 2019
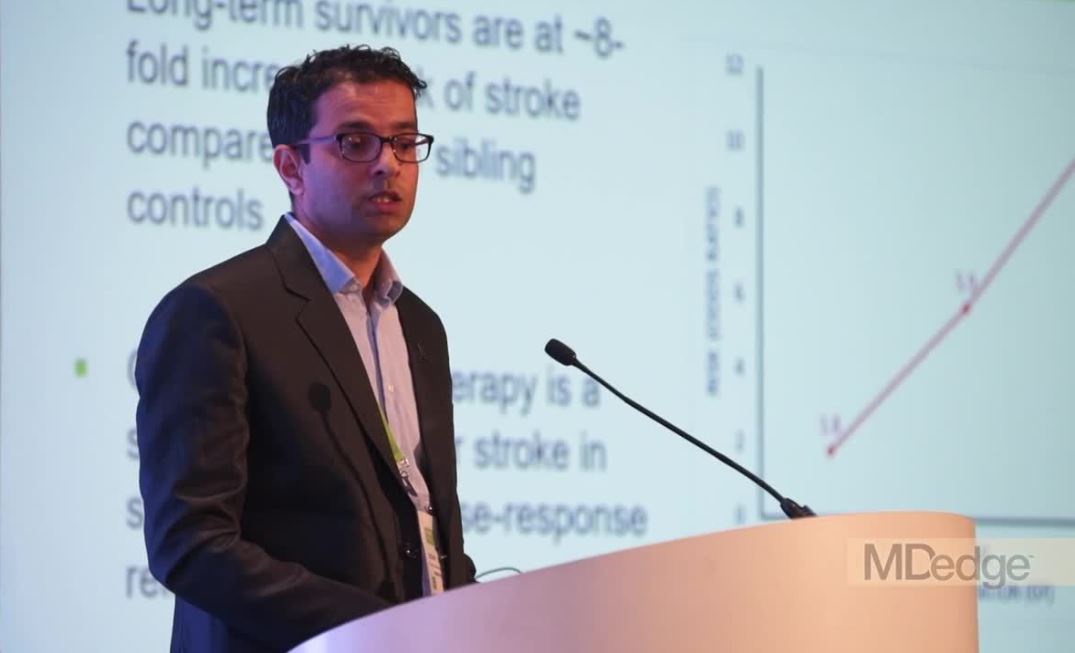
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
2019 Update on prenatal exome sequencing
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
Prenatal diagnosis of genetic anomalies is important for diagnosing lethal genetic conditions before birth. It can provide information for parents regarding pregnancy options and allow for recurrence risk counseling and the potential use of preimplantation genetic testing in the next pregnancy. For decades, a karyotype was used to analyze amniocentesis and chorionic villus sampling specimens; in recent years, chromosomal microarray analysis provides more information about significant chromosomal abnormalities, including microdeletions and microduplications. However, microarrays also have limitations, as they do not identify base pair changes associated with single-gene disorders.
The advent of next-generation sequencing has substantially reduced the cost of DNA sequencing. Whole genome sequencing (WGS) can sequence the entire genome— both the coding (exonic) and noncoding (intronic) regions—while exome sequencing analyzes only the protein-coding exons, which make up 1% to 2% of the genome and about 85% of the protein-coding genes associated with known human disease. Exome sequencing increasingly is used in cases of suspected genetic disorders when other tests have been unrevealing.
In this Update, we review recent reports of prenatal exome sequencing, including studies exploring the yield in fetuses with structural anomalies; the importance of prenatal phenotyping; the perspectives of parents and health care professionals who were involved in prenatal exome sequencing studies; and a summary of a joint position statement from 3 societies regarding prenatal sequencing.
Prenatal whole exome sequencing has potential utility, with some limitations
Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
Exome sequencing has been shown to identify an underlying genetic cause in 25% to 30% of children with an undiagnosed suspected genetic disorder. Two studies recently published in the Lancet sought to determine the incremental diagnostic yield of prenatal whole exome sequencing (WES) in the setting of fetal structural anomalies when karyotype and microarray results were normal.
Continue to: Details of the studies...
Details of the studies
In a prospective cohort study by Petrovski and colleagues, DNA samples from 234 fetuses with a structural anomaly (identified on ultrasonography) and both parents (parent-fetus "trios") were used for analysis. WES identified diagnostic genetic variants in 24 trios (10%). An additional 46 (20%) had variants that indicated pathogenicity but without sufficient evidence to be considered diagnostic.
The anomalies with the highest frequency of a genetic diagnosis were lymphatic, 24%; skeletal, 24%; central nervous system, 22%; and renal, 16%; while cardiac anomalies had the lowest yield at 5%.
In another prospective cohort study, known as the Prenatal Assessment of Genomes and Exomes (PAGE), Lord and colleagues sequenced DNA samples from 610 parent-fetus trios, but they restricted sequencing to a predefined list of 1,628 genes. Diagnostic genetic variants were identified in 52 fetuses (8.5%), while 24 (3.9%) had a variant of uncertain significance that was thought to be of potential clinical usefulness.
Fetuses with multiple anomalies had the highest genetic yield (15.4%), followed by skeletal (15.4%) and cardiac anomalies (11.1%), with the lowest yield in fetuses with isolated increased nuchal translucency (3.2%).
Diagnostic yield is high, but prenatal utility is limited
Both studies showed a clinically significant diagnostic yield of 8% to 10% for prenatal exome sequencing in cases of fetal structural anomalies with normal karyotype and microarray testing. While this yield demonstrates the utility of prenatal exome sequencing, it is significantly lower than what has been reported in postnatal studies. One of the reasons for this is the inherent limitation of prenatal phenotyping (discussed below).
The cohort studies by both Petrovski and Lord and their colleagues show the feasibility and potential diagnostic utility of exome sequencing in cases of fetal structural anomalies where karyotype and microarray are not diagnostic. However, the lower yield found in these studies compared with those in postnatal studies highlights in part the limitations of prenatal phenotyping.
The importance of prenatal phenotyping
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In postnatal exome sequencing, the physical exam, imaging findings, and laboratory results are components of the phenotype that are used to interpret the sequencing data. Prenatal phenotyping, however, is limited to the use of fetal ultrasonography and, occasionally, the addition of magnetic resonance imaging. Prenatal phenotyping is without the benefit of an exam to detect more subtle anomalies or functional status, such as developmental delay, seizures, or failure to thrive.
When a structural anomaly is identified on prenatal ultrasonography, it is especially important that detailed imaging be undertaken to detect other anomalies, including more subtle facial features and dysmorphology.
Value of reanalyzing exome sequencing data
Aarabi and colleagues conducted a retrospective study of 20 fetuses with structural anomalies and normal karyotype and microarray. They performed trio exome sequencing first using information available only prenatally and then conducted a reanalysis using information available after delivery.
With prenatal phenotyping only, the investigators identified no pathogenic, or likely pathogenic, variants. On reanalysis of combined prenatal and postnatal findings, however, they identified pathogenic variants in 20% of cases.
Significance of the findings
This study highlights both the importance of a careful, detailed fetal ultrasonography study and the possible additional benefit of a postnatal examination (such as an autopsy) in order to yield improved results. In addition, the authors noted that the development of a prenatal phenotype-genotype database would significantly help exome sequencing interpretation in the prenatal setting.
Careful prenatal ultrasonography is crucial to help in the interpretation of prenatal exome sequencing. Patients who have undergone prenatal clinical exome sequencing may benefit from reanalysis of the genetic data based on detailed postnatal findings.
Social impact of WES: Parent and provider perspectives
Wou K, Weitz T, McCormack C, et al. Parental perceptions of prenatal whole exome sequencing (PPPWES) study. Prenat Diagn. 2018;38:801-811.
Horn R, Parker M. Health professionals' and researchers' perspectives on prenatal whole genome and exome sequencing: 'We can't shut the door now, the genie's out, we need to refine it.' PLoS One. 2018;13:e0204158.
As health care providers enter a new era of prenatal genetic testing with exome sequencing, it is crucial to the path forward that we obtain perspectives from the parents and providers who participated in these studies. Notably, in both of the previously discussed Lancet reports, the authors interviewed the participants to discuss the challenges involved and identify strategies for improving future testing.
Continue to: What parents want...
What parents want
To ascertain the perceptions of couples who underwent prenatal WES, Wou and colleagues conducted semi-structured interviews with participants from the Fetal Sequencing Study regarding their experience. They interviewed 29 parents from 17 pregnancies, including a mix of those who had pathogenic prenatal results, terminated prior to receiving the results, and had normal results.
Expressed feelings and desires. Parents recalled feelings of anxiety and stress around the time of diagnosis and the need for help with coping while awaiting results. The majority of parents reported that they would like to be told about uncertain results, but that desire decreased as the certainty of results decreased.
Parents were overall satisfied with the prenatal genetic testing experience, but they added that they would have liked to receive written materials beforehand and a written report of the test results (including negative cases). They also would like to have connected with other families with similar experiences, to have received results sooner, and to have an in-person meeting after telephone disclosure of the results.
Health professionals articulate complexity of prenatal genomics
In a qualitative interview study to explore critical issues involved in the clinical practice use of prenatal genomics, Horn and Parker conducted interviews with 20 health care professionals who were involved in the previously described PAGE trial. Patient recruiters, midwives, genetic counselors, research assistants, and laboratory staff were included.
Interviewees cited numerous challenges involved in their day-to-day work with prenatal whole genome and exome sequencing, including:
- the complexity of achieving valid parental consent at a time of vulnerability
- management of parent expectations
- transmitting and comprehending complex information
- the usefulness of information
- the difficulty of a long turnaround time for study results.
All the interviewees agreed that prenatal exome sequencing studies contribute to knowledge generation and the advancement of technology.
The authors concluded that an appropriate next step would be the development of appropriate guidelines for good ethical practice that address the concerns encountered in genomics clinical practice.
The prenatal experience can be overwhelming for parents. Pretest and posttest counseling on genetic testing and results are of the utmost importance, as is finding ways to help support parents through this anxious time.
Societies offer guidance on using genome and exome sequencing
International Society for Prenatal Diagnosis, Society for Maternal and Fetal Medicine, Perinatal Quality Foundation. Joint Position Statement from the International Society for Prenatal Diagnosis (ISPD), the Society for Maternal Fetal Medicine (SMFM), and the Perinatal Quality Foundation (PQF) on the use of genome-wide sequencing for fetal diagnosis. Prenat Diagn. 2018;38:6-9.
In response to the rapid integration of exome sequencing for genetic diagnosis, several professional societies—the International Society for Prenatal Diagnosis, Society for Maternal Fetal Medicine, and Perinatal Quality Foundation—issued a joint statement addressing the clinical use of prenatal diagnostic genome wide sequencing, including exome sequencing.
Continue to: Guidance at a glance...
Guidance at a glance
The societies' recommendations are summarized as follows:
- Exome sequencing is best done as a trio analysis, with fetal and both parental samples sequenced and analyzed together.
- Extensive pretest education, counseling, and informed consent, as well as posttest counseling, are essential. This should include:
—the types of results to be conveyed (variants that are pathogenic, likely pathogenic, of uncertain significance, likely benign, and benign)
—the possibility that results will not be obtained or may not be available before the birth of the fetus
—realistic expectations regarding the likelihood that a significant result will be obtained
—the timeframe to results
—the option to include or exclude in the results incidental or secondary findings (such as an unexpected childhood disorder, cancer susceptibility genes, adult-onset disorders)
—the possibility of uncovering nonpaternity or consanguinity
—the potential reanalysis of results over time
—how data are stored, who has access, and for what purpose.
- Fetal sequencing may be beneficial in the following scenarios:
—multiple fetal anomalies or a single major anomaly suggestive of a genetic disorder, when the microarray is negative
—no microarray result is available, but the fetus exhibits a pattern of anomalies strongly suggestive of a single-gene disorder
—a prior undiagnosed fetus (or child) with anomalies suggestive of a genetic etiology, and with similar anomalies in the current pregnancy, with normal karyotype or microarray. Providers also can consider sequencing samples from both parents prior to preimplantation genetic testing to check for shared carrier status for autosomal recessive mutations, although obtaining exome sequencing from the prior affected fetus (or child) is ideal.
—history of recurrent stillbirths of unknown etiology, with a recurrent pattern of anomalies in the current pregnancy, with normal karyotype or microarray.
- Interpretation of results should be done using a multidisciplinary team-based approach, including clinical scientists, geneticists, genetic counselors, and experts in prenatal diagnosis.
- Where possible and after informed consent, reanalysis of results should be undertaken if a future pregnancy is planned or ongoing, and a significant amount of time has elapsed since the time the result was last reported.
- Parents should be given a written report of test results.
Three professional societies have convened to issue consensus opinion that includes current indications for prenatal exome sequencing and important factors to include in the consent process. We follow these guidelines in our own practice.
Summary
Exome sequencing is increasingly becoming mainstream in postnatal genetic testing, and it is emerging as the newest diagnostic frontier in prenatal genetic testing. However, there are limitations to prenatal exome sequencing, including issues with consent at a vulnerable time for parents, limited information available regarding the phenotype, and results that may not be available before the birth of a fetus. Providers should be familiar with the indications for testing, the possible results, the limitations of prenatal phenotyping, and the implications for future pregnancies.
What is the association of menopausal HT use and risk of Alzheimer disease?
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
Amyloid brain imaging changed clinical management in 60% of MCI and dementia patients
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
Current clinical practice does not routinely include biomarkers, and if given a choice, most patients would prefer brain imaging to spinal fluid-based testing, so IDEAS may be making imaging-based biomarker characterization a real possibility in the future.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He made these comments in an interview.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
.
Diagnoses changed from Alzheimer’s disease to non–Alzheimer’s disease in 25% of 11,409 patients and from non–Alzheimer’s disease to Alzheimer’s disease in 10.5%, reported Gil Rabinovici, MD, and his colleagues. The use of Alzheimer’s disease drugs doubled in amyloid-positive MCI patients, and increased by a third in amyloid-positive dementia patients. Physicians involved in the study said the scans provided key clinical information in 82% of cases with post-scan management changes.
Scans also benefited amyloid-negative patients. Before the scan, 71% of these carried an Alzheimer’s disease diagnosis; afterward, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
The study was powered to detect a 30% or greater change in the MCI and dementia groups. The 60% change emphasize how useful amyloid PET scans could be in clinical practice, Dr. Rabinovici, the study’s lead author and principal investigator, said in a press statement.
“We are impressed by the magnitude of these results, which make it clear that amyloid PET imaging can have a major impact on how we diagnose and care for patients with Alzheimer’s disease and other forms of cognitive decline,” said Dr. Rabinovici of the University of California, San Francisco.
Alzheimer’s Association leaders were similarly pleased.
“These results present highly credible, large-scale evidence that amyloid PET imaging can be a powerful tool to improve the accuracy of Alzheimer’s diagnosis and lead to better medical management, especially in difficult-to-diagnose cases,” said Maria C. Carrillo, PhD, chief science officer of the Alzheimer’s Association and a coauthor of the study. “It is important that amyloid PET imaging be more broadly accessible to those who need it.”
Next steps
Ultimately, investigators hope the nationwide-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service for those who meet the appropriate use criteria set forth by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging.
IDEAS’ second goal – showing that the scans improve health outcomes – is scheduled for 2020. These data are a key component of the CMS decision, but they might be a tough sell, Clifford R. Jack Jr., MD, and Ronald C. Petersen, MD, PhD, wrote in an accompanying editorial. Dr. Jack and Dr. Petersen are affiliated with the Mayo Clinic in Rochester, Minn.
“For CMS to cover the cost of amyloid PET, it must be demonstrated that the result of a scan has an effect on patient outcomes, not just patient care processes – and, without a disease-modifying therapy available, that might be a challenge,” they wrote.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
SOURCE: Rabinovici GD et al. JAMA. 2019 Apr 2. doi: 10.1001/jama.2019.2000.
FROM THE JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION
Pre-exposure prophylaxis for the prevention of HIV infection: Ready for prime time
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
The first cases of HIV infection in the United States were reported in 1981. Since that time, more than 700,000 individuals in our country have died of AIDS. Slightly more than 1 million persons in the United States are currently living with HIV infection; approximately 15% of them are unaware of their infection. Men who have sex with men (MSM) and African American and Hispanic/Latino men and women are disproportionately affected by HIV infection.1 Among men, MSM is the most common method of infection transmission, accounting for 83% of infections. Heterosexual contact accounts for 9.4% of new infections and injection drug use for 4.0%. Among women in the United States, heterosexual contact is the most common mechanism of transmission, accounting for about 87% of cases; injection drug use accounts for about 12%.1 Perinatal transmission rates are extremely low—less than 1%—when women receive effective treatment during pregnancy and their infants are treated in the neonatal period.1,2
The prognosis for HIV-infected patients has improved dramatically in recent years with the availability of many new and exceptionally effective highly-active antiretroviral treatment regimens. Nevertheless, the disease is not yet completely curable. Therefore, preventive measures are of great importance in reducing the enormous toll imposed by this condition.2
Evaluating effectiveness of PrEP
At the request of the US Preventive Services Task Force, Chou and colleagues recently conducted a systematic review to determine the effectiveness of pre-exposure prophylaxis (PrEP) in preventing the horizontal transmission of HIV infection.1 The authors’ secondary objectives included assessing the relationship between degree of adherence to the prophylactic regimen and degree of effectiveness and evaluating the accuracy of various screening systems for identifying patients at high risk for acquiring HIV infection.
The authors reviewed prospective, randomized controlled trials (treatment versus no treatment or treatment versus placebo) published through 2018. Pregnant women were excluded from the studies, as were women who became pregnant after enrollment.
Two different prophylactic regimens were used in the reviewed studies: 1) the combination of tenofovir disoproxil fumarate 300 mg or 245 mg plus emtricitabine 200 mg and 2) tenofovir 300 mg alone. Most trials used the combination regimen. With the exception of one trial, the medications were given daily to uninfected patients at high risk of acquiring HIV infection. In one investigation, the administration of prophylaxis was event driven (administered after a specific high-risk exposure).
Key study findings
PrEP decreased HIV transmission in high-risk patients. Chou and colleagues found that high-risk patients included primarily MSM who did not use condoms consistently or who had a high number of sex partners, individuals in an HIV-serodiscordant relationship, and intravenous drug users who shared injection equipment.
In these high-risk patients, PrEP was associated with a significantly decreased risk of HIV transmission. Observations from 11 trials demonstrated a relative risk (RR) of 0.46 (95% confidence interval [CI], 0.33–0.66). The absolute risk reduction was -2.0% (95% CI, -2.8% to -1.2%). The duration of follow up ranged from 4 months to 4 years.
Continue to: Better medication adherence = greater prophylaxis effectiveness...
Better medication adherence = greater prophylaxis effectiveness. When adherence was ≥70%, the RR was 0.27 (95% CI, 0.19–0.39). When adherence was 40% to 70%, the RR was 0.51 (95% CI, 0.38–0.70). When adherence was ≤40%, the relative risk was 0.93 (95% CI, 0.72–1.20). Adherence was better with daily administration, as opposed to event-driven administration.
Although the combination prophylactic regimen (tenofovir plus emtricitabine) was most frequently used in the clinical trials, tenofovir alone was comparable in effectiveness.
PrEP resulted in more mild adverse effects. Patients who received PrEP were more likely to develop gastrointestinal adverse effects and renal function abnormalities when compared with patients in the control arms of the studies. These adverse effects were virtually always mild and did not necessitate discontinuation of treatment.
No increase in promiscuous sexual behavior with PrEP. Specifically, investigators did not document an increased incidence of new sexually transmitted infections (STIs) in treated patients.
PrEP did not increase adverse pregnancy outcomes. In women who became pregnant while on PrEP, and who then discontinued treatment, there was no increase in the frequency of spontaneous abortion, congenital anomalies, or other adverse pregnancy outcomes.
In addition, PrEP posed a low risk for causing drug resistance in patients who became infected despite prophylaxis. Finally, the authors found that screening instruments for identifying patients at highest risk for acquiring HIV infection had low to modest sensitivity.
My recommendations for practice
Based on the study by Chou and colleagues, and on a recent commentary by Marcus et al, I believe that the following actions are justified1–3:
- For prophylaxis to be effective, we must identify all infected patients. Therefore, screening of asymptomatic individuals during routine health encounters is essential.
- All patients should have access to easy-to-understand information related to risk factors for HIV infection.
- Every effort should be made to promote safe sex practices, such as use of latex condoms, avoidance of sex during menses and in the presence of ulcerative genital lesions, and avoidance of use of contaminated drug-injection needles.
- All high-risk patients, as defined above, should be offered PrEP.
- To the greatest extent possible, financial barriers to PrEP should be eliminated.
- Patients receiving PrEP should be monitored for evidence of renal dysfunction. Should they become infected despite prophylaxis, they should be evaluated carefully to detect drug-resistant viral strains.
- Although PrEP is definitely effective in reducing the risk of transmission of HIV infection, it does not prevent the transmission of other STIs, such as syphilis, gonorrhea, and chlamydia.
In my practice, I administer prophyaxis on a daily basis rather than just before, or after, a high-risk exposure. This approach enhances patient adherence and, hopefully, will lead to maximum effectiveness over time. I also use the combination of tenofovir disoproxil fumarate plus emtricitabine rather than tenofovir alone because there is more published information regarding the effectiveness of the combination regimen.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.
- Chou R, Evans C, Hoverman A, et al. Pre-exposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review for the U.S. Preventive Services Task Force. AHRQ Publication No. 18-05247-EF-1; November 2018.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, Green MF, Copel JA, Silver RM (eds). Creasy & Resnik's Maternal-Fetal Medicine. Principles and Practice (8th ed). Philadelphia, PA: Elsevier; 2019.
- Marcus JL, Katz KA, Krakower DS, et al. Risk compensation and clinical decision making--the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380:510-512.