User login
Fundamentals of Gene Therapy: Addressing Gaps in Physician Education
Click here to read supplement.
Gene therapy is a contemporary therapeutic intervention with recent positive results and regulatory approvals either completed or expected in the next several years for various conditions. In this supplement, learn more about:
- Basic principles of gene therapy
- In vivo vs ex vivo methods of gene transfer
- Vector types
- Clinical Considerations
About the Author
Click here to read supplement.
Click here to read supplement.
Gene therapy is a contemporary therapeutic intervention with recent positive results and regulatory approvals either completed or expected in the next several years for various conditions. In this supplement, learn more about:
- Basic principles of gene therapy
- In vivo vs ex vivo methods of gene transfer
- Vector types
- Clinical Considerations
About the Author
Click here to read supplement.
Click here to read supplement.
Gene therapy is a contemporary therapeutic intervention with recent positive results and regulatory approvals either completed or expected in the next several years for various conditions. In this supplement, learn more about:
- Basic principles of gene therapy
- In vivo vs ex vivo methods of gene transfer
- Vector types
- Clinical Considerations
About the Author
Click here to read supplement.
PA Specific Program Scheduled for VAM
The PA Section steering committee has been hard at work developing session programming for the 2019 Vascular Annual Meeting. Vascular PAs can look forward to a four-hour session on Thursday, June 13, completely dedicated to them. The program will focus on a variety of topics that include optimal team practice, vascular diagnostics, venous disease and wound management, an “Ask the Expert” portion and much more. Know a vascular PA who needs to become an SVS member? Encourage them to apply today.
The PA Section steering committee has been hard at work developing session programming for the 2019 Vascular Annual Meeting. Vascular PAs can look forward to a four-hour session on Thursday, June 13, completely dedicated to them. The program will focus on a variety of topics that include optimal team practice, vascular diagnostics, venous disease and wound management, an “Ask the Expert” portion and much more. Know a vascular PA who needs to become an SVS member? Encourage them to apply today.
The PA Section steering committee has been hard at work developing session programming for the 2019 Vascular Annual Meeting. Vascular PAs can look forward to a four-hour session on Thursday, June 13, completely dedicated to them. The program will focus on a variety of topics that include optimal team practice, vascular diagnostics, venous disease and wound management, an “Ask the Expert” portion and much more. Know a vascular PA who needs to become an SVS member? Encourage them to apply today.
SVSConnect – Save the Date!
The SVS is excited to announce that within your online community, SVSConnect, there will be an “Ask Us Anything” session at 2 p.m. CDT on April 8. Drs. Daniel McDevitt and William Shutze will be available in real time to answer questions about building relationships in a competitive environment. SVSConnect users will be able to chime in during the session to ask them anything. If you can’t make it to the live Q&A on April 8, all the questions and responses will be available in the SVSConnect library. More information will come soon.
The SVS is excited to announce that within your online community, SVSConnect, there will be an “Ask Us Anything” session at 2 p.m. CDT on April 8. Drs. Daniel McDevitt and William Shutze will be available in real time to answer questions about building relationships in a competitive environment. SVSConnect users will be able to chime in during the session to ask them anything. If you can’t make it to the live Q&A on April 8, all the questions and responses will be available in the SVSConnect library. More information will come soon.
The SVS is excited to announce that within your online community, SVSConnect, there will be an “Ask Us Anything” session at 2 p.m. CDT on April 8. Drs. Daniel McDevitt and William Shutze will be available in real time to answer questions about building relationships in a competitive environment. SVSConnect users will be able to chime in during the session to ask them anything. If you can’t make it to the live Q&A on April 8, all the questions and responses will be available in the SVSConnect library. More information will come soon.
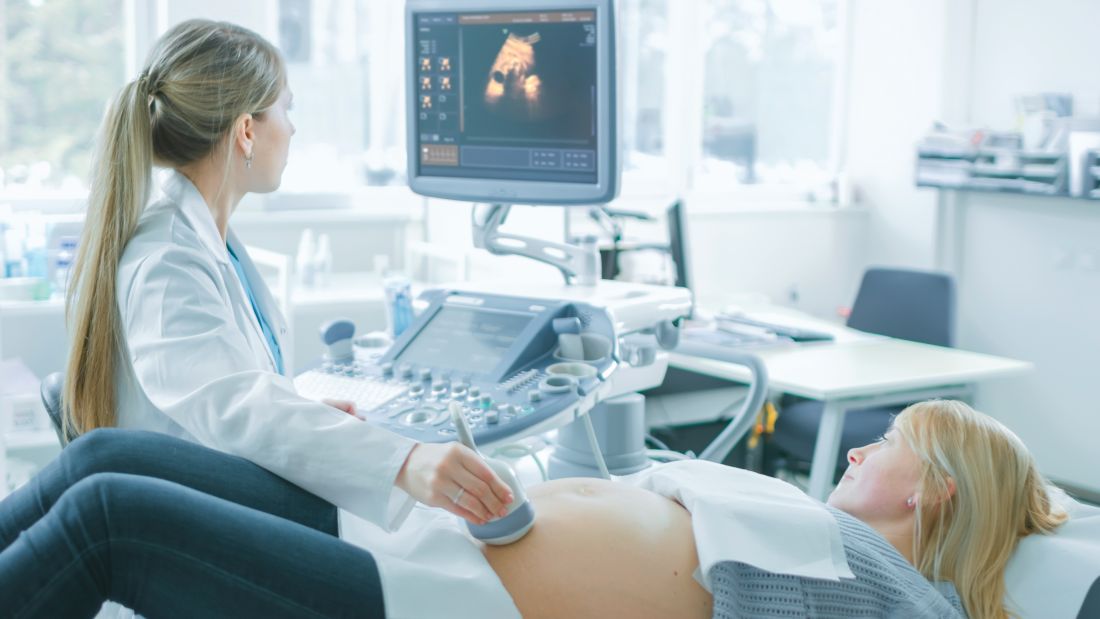
Reframing the conversation around the ‘gender-reveal’ ultrasound
How revealing fetal anatomy, not gender, is inclusive
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
How revealing fetal anatomy, not gender, is inclusive
How revealing fetal anatomy, not gender, is inclusive
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
Every ob.gyn. expects that the topic of gender will come up at some point in a patient’s pregnancy. “When will I find out the gender?” asks the 24-year-old at her first prenatal visit. “We want the gender to be a surprise!” exclaims the couple at their anatomy scan for their second in vitro fertilization pregnancy. “Do you know what you’re having?” asks the obstetrician anticipating an imminent delivery.
The topic of gender is in fact so ingrained in our practice that we don’t bat an eye when approached with questions about fetal gender. But what exactly are we talking about when we discuss the gender of an unborn baby?
As we established in our previous column, gender identity is an internal experience of gender that one feels to be a part of oneself. So, then, what does an ultrasound actually reveal? Objectively, ultrasound can show the provider the presence or absence of a hyperechoic anatomical structure between the fetal legs that may become a penis, a vagina, or an ambiguous form of genitalia. While ultrasound is an incredible tool for anatomical and other forms of antenatal testing, ultrasound cannot detect identity characteristics because identities are, by definition, socially and internally experienced without respect to anatomy.
The distinction between gender identity and sex assigned at birth in discussions of antenatal ultrasonography is more than just a simple problem of semantics or vocabulary. To describe a fetus as a boy or a girl based on the presence/absence of a projection between the fetal legs seen on ultrasound is to reinforce the idea that gender identity and sex assigned at birth are equivalent. This conflation also erases nonbinary, genderqueer, and many other groups that identify with genders other than “boy” or “girl.” To be clear, unborn fetuses do not have a gender identity. Studies have shown that children begin to self-label their gender as early as 18-24 months of age, and similarly those who grow up to inhabit gender-nonconforming identities usually already are starting to show signs of their nonconformity starting at age 2 years.1 Some of the deepest traumas that trans and gender-nonconforming people experience are rated to the enforcement of unwritten gender laws during early childhood that are applied based on the sex assigned at birth.
Obstetricians can help to break the cycle of inappropriate gender assignment by correctly using the terms “gender” and “sex assigned at birth.” One opportunity for addressing patients’ questions about fetal gender might be to avoid the term “gender” altogether when discussing fetal sex assigned at birth, emphasizing instead what fetal ultrasound is actually able to do: Give us information about the appearance of external genitalia to help predict what sex will be assigned at birth.2 We have used this strategy when performing anatomy scans, and our experience has been that patients often will make their own assumptions about what it means to see certain external genitalia on ultrasound between the fetal legs. Motivated providers who want to go the extra mile may use a patient’s exclamation about their understanding of the fetus’s gender as an opportunity to educate the patient on the distinction between gender and sex assigned at birth, but even just smiling and moving onto the next part of the scan is an appropriate way of maintaining an atmosphere of inclusion and respect.
One of the roots of gender-based violence and gender dysphoria later in life is the conflation of gender and sex assigned at birth. While there is an entire social and political framework that enforces and polices gender after birth, the obstetrician can take steps to break the cycle starting before the birth has even occurred. Obstetricians are tasked with the unique challenge of providing care for the mother-fetus dyad, and much of the work is in setting up the fetus for the best possible life. Our scope of inclusion should be sufficiently wide to account for nonanatomical variations that could develop later in life in the infants we deliver.
Dr. Bahng is a PGY-1 resident physician in the gynecology & obstetrics residency program at Emory University, Atlanta. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner is an assistant professor at Emory University and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Bahng and Dr. Joyner reported no financial disclosures.
References
1. Horm Behav. 2013 Jul;64(2):288-97.
2. Obstet Gynecol Surv. 2009 Jan;64(1):50-7.
Valproate, topiramate prescribed in young women despite known teratogenicity risks
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
FROM JAMA NEUROLOGY
Key clinical point: Both valproate and topiramate are prescribed relatively often in women of childbearing age despite known teratogenic risks.
Major finding: Topiramate was the second- or third-most prescribed AED in the analyses. Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy.
Study details: Retrospective cohort study including nearly 47,000 women of childbearing age enrolled in claims databases between 2009 and 2013.
Disclosures: UCB Pharma sponsored the study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
Source: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
Gene mutations may predispose women to alopecia subtype
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Women with central centrifugal cicatricial alopecia (CCCA) were more likely to have a mutation of the PAD13 gene than unaffected women.
Major finding: Researchers identified six mutations in PAD13 that appeared in 24% of 58 women with CCCA.
Study details: The data come from 58 adult women with African ancestry who had a diagnosis of CCCA.
Disclosures: The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
Source: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
Psoriatic Arthritis Journal Scan: March 2019
A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: From the Medical Board of the National Psoriasis Foundation.
Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. J Am Acad Dermatol. 2019 Mar 15
A systematic literature search was performed of HZ in patients with PsO/PsA. HZ vaccination guidelines were reviewed and the medical board of the National Psoriasis Foundation made consensus recommendations in PsO/PsA patients based on graded evidence. HZ risk depends on disease severity and treatment class. Recombinant zoster vaccine should be given to all PsO/PsA patients >50 years old and younger patients at increased risk.
Ultrasonographic and Clinical Assessment of Peripheral Enthesitis in Patients with Psoriatic Arthritis, Psoriasis, and Fibromyalgia Syndrome: The ULISSE Study.
Macchioni P, Salvarani C, Possemato N, et al. J Rheumatol. 2019 Mar 15.
The ULISSE study indicated that enthesitis is a common feature in patients with PsA, those with psoriasis, and in those with FMS if only clinical examination is used. US entheseal assessment showed findings more consistent with the 3 disorders.
The development of a modified Psoriatic Arthritis Disease Activity Score (mPASDAS) using SF-12 as a measure of quality of life.
Perruccio AV, Got M, Li S, Ye Y, Gladman DD, Chandran V. Arthritis Care Res (Hoboken). 2019 Mar 15.
The Psoriatic Arthritis Disease Activity Score (PASDAS) is a composite measure of psoriatic arthritis (PsA) disease activity. The length of its patient-reported components raises concern about questionnaire burden. The PASDAS includes the SF-36 measure. The study investigated the agreement between PASDAS and a modified PASDAS (mPASDAS) which substituted the SF-36 with the shortened SF-12.
The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study.
Adawi M, Damiani G, Bragazzi NL, et al. Nutrients. 2019 Mar 12;11(3).
Fasting was found to be a predictor of a decrease in PsA disease activity scores (DAPSA, BASDAI, LEI, DSS) even after adjustment for weight loss. IL-17 therapy was found to be an independent predictor of decreases in LEI after fasting.
Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition.
Scher JU, Ogdie A, Merola JF, Ritchlin C. Nat Rev Rheumatol. 2019 Mar;15(3):153-166.
The events responsible for progression to PsA are currently unclear. Genetic and clinical–demographic risk factors (most notably familial aggregation and psoriasis sub-phenotypes) provide relevant insights into the variables that promote transition.
A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: From the Medical Board of the National Psoriasis Foundation.
Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. J Am Acad Dermatol. 2019 Mar 15
A systematic literature search was performed of HZ in patients with PsO/PsA. HZ vaccination guidelines were reviewed and the medical board of the National Psoriasis Foundation made consensus recommendations in PsO/PsA patients based on graded evidence. HZ risk depends on disease severity and treatment class. Recombinant zoster vaccine should be given to all PsO/PsA patients >50 years old and younger patients at increased risk.
Ultrasonographic and Clinical Assessment of Peripheral Enthesitis in Patients with Psoriatic Arthritis, Psoriasis, and Fibromyalgia Syndrome: The ULISSE Study.
Macchioni P, Salvarani C, Possemato N, et al. J Rheumatol. 2019 Mar 15.
The ULISSE study indicated that enthesitis is a common feature in patients with PsA, those with psoriasis, and in those with FMS if only clinical examination is used. US entheseal assessment showed findings more consistent with the 3 disorders.
The development of a modified Psoriatic Arthritis Disease Activity Score (mPASDAS) using SF-12 as a measure of quality of life.
Perruccio AV, Got M, Li S, Ye Y, Gladman DD, Chandran V. Arthritis Care Res (Hoboken). 2019 Mar 15.
The Psoriatic Arthritis Disease Activity Score (PASDAS) is a composite measure of psoriatic arthritis (PsA) disease activity. The length of its patient-reported components raises concern about questionnaire burden. The PASDAS includes the SF-36 measure. The study investigated the agreement between PASDAS and a modified PASDAS (mPASDAS) which substituted the SF-36 with the shortened SF-12.
The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study.
Adawi M, Damiani G, Bragazzi NL, et al. Nutrients. 2019 Mar 12;11(3).
Fasting was found to be a predictor of a decrease in PsA disease activity scores (DAPSA, BASDAI, LEI, DSS) even after adjustment for weight loss. IL-17 therapy was found to be an independent predictor of decreases in LEI after fasting.
Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition.
Scher JU, Ogdie A, Merola JF, Ritchlin C. Nat Rev Rheumatol. 2019 Mar;15(3):153-166.
The events responsible for progression to PsA are currently unclear. Genetic and clinical–demographic risk factors (most notably familial aggregation and psoriasis sub-phenotypes) provide relevant insights into the variables that promote transition.
A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: From the Medical Board of the National Psoriasis Foundation.
Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. J Am Acad Dermatol. 2019 Mar 15
A systematic literature search was performed of HZ in patients with PsO/PsA. HZ vaccination guidelines were reviewed and the medical board of the National Psoriasis Foundation made consensus recommendations in PsO/PsA patients based on graded evidence. HZ risk depends on disease severity and treatment class. Recombinant zoster vaccine should be given to all PsO/PsA patients >50 years old and younger patients at increased risk.
Ultrasonographic and Clinical Assessment of Peripheral Enthesitis in Patients with Psoriatic Arthritis, Psoriasis, and Fibromyalgia Syndrome: The ULISSE Study.
Macchioni P, Salvarani C, Possemato N, et al. J Rheumatol. 2019 Mar 15.
The ULISSE study indicated that enthesitis is a common feature in patients with PsA, those with psoriasis, and in those with FMS if only clinical examination is used. US entheseal assessment showed findings more consistent with the 3 disorders.
The development of a modified Psoriatic Arthritis Disease Activity Score (mPASDAS) using SF-12 as a measure of quality of life.
Perruccio AV, Got M, Li S, Ye Y, Gladman DD, Chandran V. Arthritis Care Res (Hoboken). 2019 Mar 15.
The Psoriatic Arthritis Disease Activity Score (PASDAS) is a composite measure of psoriatic arthritis (PsA) disease activity. The length of its patient-reported components raises concern about questionnaire burden. The PASDAS includes the SF-36 measure. The study investigated the agreement between PASDAS and a modified PASDAS (mPASDAS) which substituted the SF-36 with the shortened SF-12.
The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study.
Adawi M, Damiani G, Bragazzi NL, et al. Nutrients. 2019 Mar 12;11(3).
Fasting was found to be a predictor of a decrease in PsA disease activity scores (DAPSA, BASDAI, LEI, DSS) even after adjustment for weight loss. IL-17 therapy was found to be an independent predictor of decreases in LEI after fasting.
Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition.
Scher JU, Ogdie A, Merola JF, Ritchlin C. Nat Rev Rheumatol. 2019 Mar;15(3):153-166.
The events responsible for progression to PsA are currently unclear. Genetic and clinical–demographic risk factors (most notably familial aggregation and psoriasis sub-phenotypes) provide relevant insights into the variables that promote transition.
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
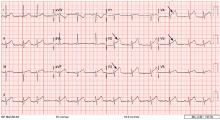
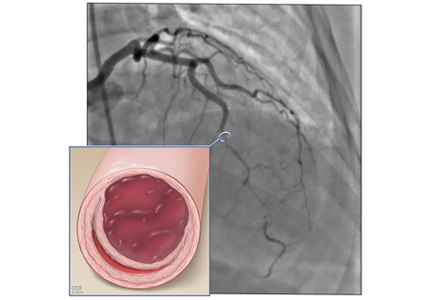
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
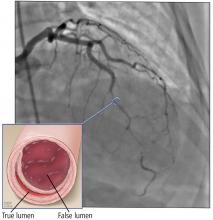
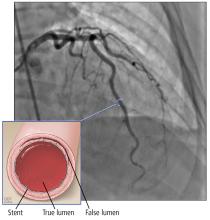
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.
Acute kidney injury after hip or knee replacement: Can we lower the risk?
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
KEY POINTS
- Using current diagnostic criteria, the incidence of acute kidney injury complicating primary total joint arthroplasty may be nearly 10%, and 25% after placement of an antibiotic-loaded cement spacer to treat infection.
- In primary total joint arthroplasty, significant risk factors include older age, higher body mass index, chronic kidney disease, comorbidity, anemia, perioperative transfusion, aminoglycoside prophylaxis and treatment, preoperative heart murmur, and renin-angiotensin-aldosterone system blockade.
- Acute kidney injury may arise from infection, systemic administration of nephrotoxic antibiotics, and elution of antibiotics from antibiotic-loaded cement.
- No randomized controlled trial aimed at reducing acute kidney injury in these settings has been published; however, suggestions for practice modification are made based on the available data.
Unusual effects of common antibiotics
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
KEY POINTS
- Piperacillin-induced encephalopathy and seizure can occur on a continuum, with progressive dysarthria, tremor, and confusion culminating in tonic-clonic seizures.
- Monocycline-induced lupus can present as myalgia, arthralgia, serositis, constitutional symptoms, and a positive antinuclear antibody titer. The effect is not dose-dependent.
- Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been reported with quinolones and sulfonamides.
- QT-interval prolongation is a class effect of quinolones and is more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, or bradycardia, or in women.