User login
Planning for change in hospitalist practice management
At Sunday’s HM19 pre-course “Oh, the Places We’ll Go! Practice Management Tools for Navigating the Changing Role of Your Hospital Medicine Group,” the theme was how to anticipate and embrace changing roles as hospital medicine groups are being asked to take on more responsibility.
“The scope of hospitalist practice is evolving rapidly, both clinically and in terms of all of the other things that hospitalists are being asked to do,” said Leslie Flores, MHA, SFHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., and course co-director, in an interview before the pre-course. “Our goals with this program are to help leaders position their hospitalist groups for success with this changing environment that they’re living in and the changing roles of hospitalists.”
In an audience poll at the beginning of the pre-course, attendees – a majority of whom were practicing hospitalists and managers of hospitalist groups – said their biggest challenge areas were related to compensation or workflows that have not evolved to match their changing role, and disagreements over who should admit patients.
One of the goals of the session was to give hospitalist leaders ideas to address these issues, which included information on how to implement better team-based care and interdisciplinary care models within their groups, as well as how to adjust their compensation, scheduling, and staffing models to prepare for this “new world of hospitalist medicine,” said Ms. Flores.
“One of the biggest sources of contention and stress that we see in hospitalist groups is that there’s just so much change, and it’s happening so rapidly, and people are having a hard time really figuring out how to deal with all of that,” she said.
The day began with John Nelson, MD, MHM, outlining the “Trends in Scope of Practice Evolution.” Dr. Nelson, a partner at Nelson Flores Hospital Medicine Consultants, medical director of Overlake Medical Center in Bellevue, Wash., and course co-director, said hospitalists are increasingly working more in outpatient care, post-acute care, and other specialty facilities. In addition, as group size increases, the likelihood a hospitalist group will be responsible for an observation or short stay unit increases, while a larger group is less likely to have a clinical responsibility for a code blue, cardiac arrest, or rapid response team.
Other topics in the pre-course focused on how to change the culture in a group to an environment where team members are empowered to ask questions or voice concerns, improve patient flow by removing reasons for delays in discharge, recruit the right team members to a group, handle transitions of care, and anticipate change in a group. In addition, the speakers participated in discussions where they shared their biggest successes and failures in practice as leaders and participated in a lightning round where they provided “off-the-cuff” responses to questions from Ms. Flores.
Although hospitalists did not create the current environment that is expanding their role in the health care system, they can position themselves to decide what the scope of their role is, said Dr. Nelson.
“What we should do is navigate our group through these changes in the way that’s going to be most effective for ourselves, the providers in our group, and our organization,” he said. “Those groups that try to dig their heels in or resist all change, they fail. . . and they frustrate themselves. So instead, if you engage in planning for changes in the scope of your practice, you have a chance to make it go the way you’d like it to go, and you’re going to be more satisfied.”
At Sunday’s HM19 pre-course “Oh, the Places We’ll Go! Practice Management Tools for Navigating the Changing Role of Your Hospital Medicine Group,” the theme was how to anticipate and embrace changing roles as hospital medicine groups are being asked to take on more responsibility.
“The scope of hospitalist practice is evolving rapidly, both clinically and in terms of all of the other things that hospitalists are being asked to do,” said Leslie Flores, MHA, SFHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., and course co-director, in an interview before the pre-course. “Our goals with this program are to help leaders position their hospitalist groups for success with this changing environment that they’re living in and the changing roles of hospitalists.”
In an audience poll at the beginning of the pre-course, attendees – a majority of whom were practicing hospitalists and managers of hospitalist groups – said their biggest challenge areas were related to compensation or workflows that have not evolved to match their changing role, and disagreements over who should admit patients.
One of the goals of the session was to give hospitalist leaders ideas to address these issues, which included information on how to implement better team-based care and interdisciplinary care models within their groups, as well as how to adjust their compensation, scheduling, and staffing models to prepare for this “new world of hospitalist medicine,” said Ms. Flores.
“One of the biggest sources of contention and stress that we see in hospitalist groups is that there’s just so much change, and it’s happening so rapidly, and people are having a hard time really figuring out how to deal with all of that,” she said.
The day began with John Nelson, MD, MHM, outlining the “Trends in Scope of Practice Evolution.” Dr. Nelson, a partner at Nelson Flores Hospital Medicine Consultants, medical director of Overlake Medical Center in Bellevue, Wash., and course co-director, said hospitalists are increasingly working more in outpatient care, post-acute care, and other specialty facilities. In addition, as group size increases, the likelihood a hospitalist group will be responsible for an observation or short stay unit increases, while a larger group is less likely to have a clinical responsibility for a code blue, cardiac arrest, or rapid response team.
Other topics in the pre-course focused on how to change the culture in a group to an environment where team members are empowered to ask questions or voice concerns, improve patient flow by removing reasons for delays in discharge, recruit the right team members to a group, handle transitions of care, and anticipate change in a group. In addition, the speakers participated in discussions where they shared their biggest successes and failures in practice as leaders and participated in a lightning round where they provided “off-the-cuff” responses to questions from Ms. Flores.
Although hospitalists did not create the current environment that is expanding their role in the health care system, they can position themselves to decide what the scope of their role is, said Dr. Nelson.
“What we should do is navigate our group through these changes in the way that’s going to be most effective for ourselves, the providers in our group, and our organization,” he said. “Those groups that try to dig their heels in or resist all change, they fail. . . and they frustrate themselves. So instead, if you engage in planning for changes in the scope of your practice, you have a chance to make it go the way you’d like it to go, and you’re going to be more satisfied.”
At Sunday’s HM19 pre-course “Oh, the Places We’ll Go! Practice Management Tools for Navigating the Changing Role of Your Hospital Medicine Group,” the theme was how to anticipate and embrace changing roles as hospital medicine groups are being asked to take on more responsibility.
“The scope of hospitalist practice is evolving rapidly, both clinically and in terms of all of the other things that hospitalists are being asked to do,” said Leslie Flores, MHA, SFHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., and course co-director, in an interview before the pre-course. “Our goals with this program are to help leaders position their hospitalist groups for success with this changing environment that they’re living in and the changing roles of hospitalists.”
In an audience poll at the beginning of the pre-course, attendees – a majority of whom were practicing hospitalists and managers of hospitalist groups – said their biggest challenge areas were related to compensation or workflows that have not evolved to match their changing role, and disagreements over who should admit patients.
One of the goals of the session was to give hospitalist leaders ideas to address these issues, which included information on how to implement better team-based care and interdisciplinary care models within their groups, as well as how to adjust their compensation, scheduling, and staffing models to prepare for this “new world of hospitalist medicine,” said Ms. Flores.
“One of the biggest sources of contention and stress that we see in hospitalist groups is that there’s just so much change, and it’s happening so rapidly, and people are having a hard time really figuring out how to deal with all of that,” she said.
The day began with John Nelson, MD, MHM, outlining the “Trends in Scope of Practice Evolution.” Dr. Nelson, a partner at Nelson Flores Hospital Medicine Consultants, medical director of Overlake Medical Center in Bellevue, Wash., and course co-director, said hospitalists are increasingly working more in outpatient care, post-acute care, and other specialty facilities. In addition, as group size increases, the likelihood a hospitalist group will be responsible for an observation or short stay unit increases, while a larger group is less likely to have a clinical responsibility for a code blue, cardiac arrest, or rapid response team.
Other topics in the pre-course focused on how to change the culture in a group to an environment where team members are empowered to ask questions or voice concerns, improve patient flow by removing reasons for delays in discharge, recruit the right team members to a group, handle transitions of care, and anticipate change in a group. In addition, the speakers participated in discussions where they shared their biggest successes and failures in practice as leaders and participated in a lightning round where they provided “off-the-cuff” responses to questions from Ms. Flores.
Although hospitalists did not create the current environment that is expanding their role in the health care system, they can position themselves to decide what the scope of their role is, said Dr. Nelson.
“What we should do is navigate our group through these changes in the way that’s going to be most effective for ourselves, the providers in our group, and our organization,” he said. “Those groups that try to dig their heels in or resist all change, they fail. . . and they frustrate themselves. So instead, if you engage in planning for changes in the scope of your practice, you have a chance to make it go the way you’d like it to go, and you’re going to be more satisfied.”
Algorithm ruled out PE, averts radiation exposure in pregnant women
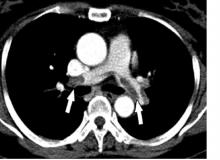
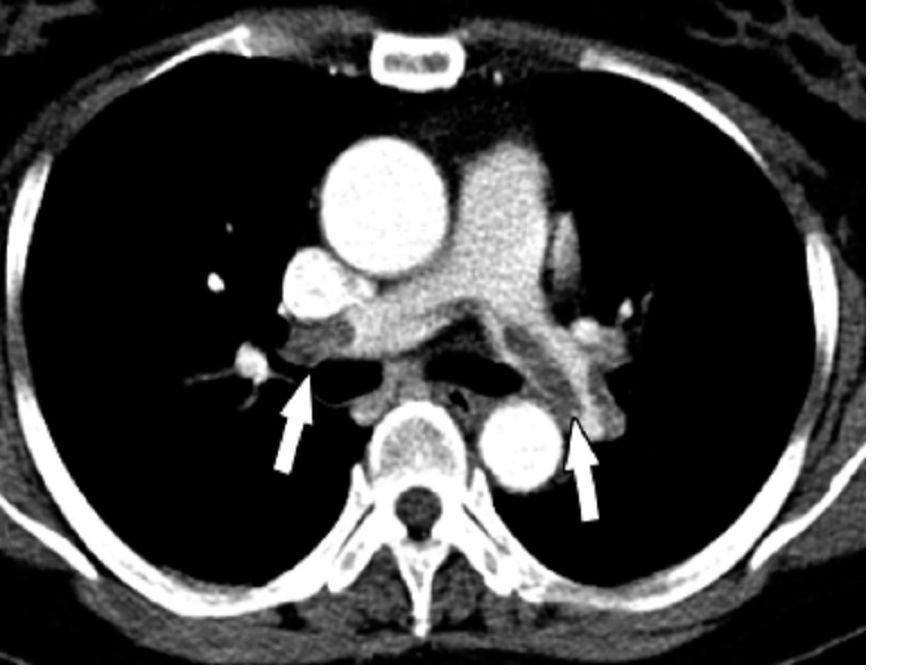
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
FROM The New England Journal of Medicine
Increased sudden death risk in HIV linked to cardiac fibrosis
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
REPORTING FROM CROI 2019
Expert calls for more ‘ethnocentric’ research in MS
DALLAS – The way Lilyana Amezcua, MD, sees it, researchers should look beyond using race and ethnicity only as demographic variables when reporting results from multiple sclerosis studies.
“As a demographic variable we see it all the time: white versus non-white, and the methods to arrive at a category are seldom discussed,” Dr. Amezcua said at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis. “We need to think about ethnocentric research, where the method for determination for race and ethnicity becomes important. This includes examining self-identity (ethnicity), along with physical characteristics and medical records, and confirming that beyond the individual.”
The goal, she continued, is to identify who is at risk for inferior health, such as trying to sort out biological and genetic explanations from non-biological explanations. Ethnocentric research also helps to address health care disparities. “But there’s a broad intersection between race and ethnicity, and depending on the question, genetic ancestry could help,” said Dr. Amezcua, a neurologist at the Keck School of Medicine at the University of Southern California, Los Angeles. “Race tries to infer biological differences, ethnicity infers societal differences, and ancestry infers genetic variations.”
While genetic and biologic features are often used to evaluate how race and ethnicity affects those with MS, Dr. Amezcua noted that several additional factors could influence outcomes. These include access to care as well as individual and community factors that relate to social determinants of health, such as poverty, exposures, and environmental stress. “These could be contributing to worse outcomes,” she said. “So could modifiable factors such as illness beliefs, health literacy, illness management, and acculturation.”
In terms of health literacy, there are reports suggesting that there is a general lack of adequate education and understanding about MS treatment and realistic expectations in African Americans and Hispanics, she said.
In addition, research has shown there is a lower probability of being under the care of a neurologist if you lack health insurance (odds ratio = 0.38) or are African American (OR = 0.52) or Hispanic (OR = 0.61), based on nationally representative data from the 2006-2013 Medical Expenditure Panel Survey (Neurology. 2017;88[24]:2268-75). “Just being African American or Hispanic lowered the probability of seeing a neurologist,” she said.
Published evidence also exists to suggest that illness beliefs drive some people away from MS treatment. “These are beliefs embedded in social and cultural factors known as cultural idioms,” Dr. Amezcua explained. “In a study that was able to capture qualitative and quantitative data, researchers found that social and cultural factors were more frequently reported in immigrant groups, alluding to the fact that we need to look beyond whether they are African American or Hispanic, and look at acculturation” (Int J MS Care. 2017;19[3]:131-9).
Then there’s the issue of Food and Drug Administration-approved disease-modifying therapies in MS and minorities. In an exploratory post hoc analysis of the Evidence of Interferon Dose-Response: European North American Comparative Efficacy (EVIDENCE) study, researchers found that African-American subjects experienced more exacerbations and were less likely to remain exacerbation free, compared with whites (Arch Neurol. 2005;62[11]:1681-3). The African-American subjects also developed more new MS lesions on T2-weighted brain MRI at 48 weeks (P = .04).
“There are a lot of unanswered questions, but understanding the effect of race/ethnicity is crucial to understanding MS disparities,” Dr. Amezcua said. “To better understand genetic variation in the context of health disparities, using ‘genetic ancestry’ could help with precision medicine. We must remember that minorities with MS face barriers related to access and education in MS care much more so than whites.”
She concluded her remarks by underscoring the importance of increasing minority participation in research and clinical trials. “But today, clinical trial participation by minorities is less than 10%. As we progress, and as we get closer to precision medicine, the health disparities will widen.”
She reported having no financial disclosures.
DALLAS – The way Lilyana Amezcua, MD, sees it, researchers should look beyond using race and ethnicity only as demographic variables when reporting results from multiple sclerosis studies.
“As a demographic variable we see it all the time: white versus non-white, and the methods to arrive at a category are seldom discussed,” Dr. Amezcua said at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis. “We need to think about ethnocentric research, where the method for determination for race and ethnicity becomes important. This includes examining self-identity (ethnicity), along with physical characteristics and medical records, and confirming that beyond the individual.”
The goal, she continued, is to identify who is at risk for inferior health, such as trying to sort out biological and genetic explanations from non-biological explanations. Ethnocentric research also helps to address health care disparities. “But there’s a broad intersection between race and ethnicity, and depending on the question, genetic ancestry could help,” said Dr. Amezcua, a neurologist at the Keck School of Medicine at the University of Southern California, Los Angeles. “Race tries to infer biological differences, ethnicity infers societal differences, and ancestry infers genetic variations.”
While genetic and biologic features are often used to evaluate how race and ethnicity affects those with MS, Dr. Amezcua noted that several additional factors could influence outcomes. These include access to care as well as individual and community factors that relate to social determinants of health, such as poverty, exposures, and environmental stress. “These could be contributing to worse outcomes,” she said. “So could modifiable factors such as illness beliefs, health literacy, illness management, and acculturation.”
In terms of health literacy, there are reports suggesting that there is a general lack of adequate education and understanding about MS treatment and realistic expectations in African Americans and Hispanics, she said.
In addition, research has shown there is a lower probability of being under the care of a neurologist if you lack health insurance (odds ratio = 0.38) or are African American (OR = 0.52) or Hispanic (OR = 0.61), based on nationally representative data from the 2006-2013 Medical Expenditure Panel Survey (Neurology. 2017;88[24]:2268-75). “Just being African American or Hispanic lowered the probability of seeing a neurologist,” she said.
Published evidence also exists to suggest that illness beliefs drive some people away from MS treatment. “These are beliefs embedded in social and cultural factors known as cultural idioms,” Dr. Amezcua explained. “In a study that was able to capture qualitative and quantitative data, researchers found that social and cultural factors were more frequently reported in immigrant groups, alluding to the fact that we need to look beyond whether they are African American or Hispanic, and look at acculturation” (Int J MS Care. 2017;19[3]:131-9).
Then there’s the issue of Food and Drug Administration-approved disease-modifying therapies in MS and minorities. In an exploratory post hoc analysis of the Evidence of Interferon Dose-Response: European North American Comparative Efficacy (EVIDENCE) study, researchers found that African-American subjects experienced more exacerbations and were less likely to remain exacerbation free, compared with whites (Arch Neurol. 2005;62[11]:1681-3). The African-American subjects also developed more new MS lesions on T2-weighted brain MRI at 48 weeks (P = .04).
“There are a lot of unanswered questions, but understanding the effect of race/ethnicity is crucial to understanding MS disparities,” Dr. Amezcua said. “To better understand genetic variation in the context of health disparities, using ‘genetic ancestry’ could help with precision medicine. We must remember that minorities with MS face barriers related to access and education in MS care much more so than whites.”
She concluded her remarks by underscoring the importance of increasing minority participation in research and clinical trials. “But today, clinical trial participation by minorities is less than 10%. As we progress, and as we get closer to precision medicine, the health disparities will widen.”
She reported having no financial disclosures.
DALLAS – The way Lilyana Amezcua, MD, sees it, researchers should look beyond using race and ethnicity only as demographic variables when reporting results from multiple sclerosis studies.
“As a demographic variable we see it all the time: white versus non-white, and the methods to arrive at a category are seldom discussed,” Dr. Amezcua said at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis. “We need to think about ethnocentric research, where the method for determination for race and ethnicity becomes important. This includes examining self-identity (ethnicity), along with physical characteristics and medical records, and confirming that beyond the individual.”
The goal, she continued, is to identify who is at risk for inferior health, such as trying to sort out biological and genetic explanations from non-biological explanations. Ethnocentric research also helps to address health care disparities. “But there’s a broad intersection between race and ethnicity, and depending on the question, genetic ancestry could help,” said Dr. Amezcua, a neurologist at the Keck School of Medicine at the University of Southern California, Los Angeles. “Race tries to infer biological differences, ethnicity infers societal differences, and ancestry infers genetic variations.”
While genetic and biologic features are often used to evaluate how race and ethnicity affects those with MS, Dr. Amezcua noted that several additional factors could influence outcomes. These include access to care as well as individual and community factors that relate to social determinants of health, such as poverty, exposures, and environmental stress. “These could be contributing to worse outcomes,” she said. “So could modifiable factors such as illness beliefs, health literacy, illness management, and acculturation.”
In terms of health literacy, there are reports suggesting that there is a general lack of adequate education and understanding about MS treatment and realistic expectations in African Americans and Hispanics, she said.
In addition, research has shown there is a lower probability of being under the care of a neurologist if you lack health insurance (odds ratio = 0.38) or are African American (OR = 0.52) or Hispanic (OR = 0.61), based on nationally representative data from the 2006-2013 Medical Expenditure Panel Survey (Neurology. 2017;88[24]:2268-75). “Just being African American or Hispanic lowered the probability of seeing a neurologist,” she said.
Published evidence also exists to suggest that illness beliefs drive some people away from MS treatment. “These are beliefs embedded in social and cultural factors known as cultural idioms,” Dr. Amezcua explained. “In a study that was able to capture qualitative and quantitative data, researchers found that social and cultural factors were more frequently reported in immigrant groups, alluding to the fact that we need to look beyond whether they are African American or Hispanic, and look at acculturation” (Int J MS Care. 2017;19[3]:131-9).
Then there’s the issue of Food and Drug Administration-approved disease-modifying therapies in MS and minorities. In an exploratory post hoc analysis of the Evidence of Interferon Dose-Response: European North American Comparative Efficacy (EVIDENCE) study, researchers found that African-American subjects experienced more exacerbations and were less likely to remain exacerbation free, compared with whites (Arch Neurol. 2005;62[11]:1681-3). The African-American subjects also developed more new MS lesions on T2-weighted brain MRI at 48 weeks (P = .04).
“There are a lot of unanswered questions, but understanding the effect of race/ethnicity is crucial to understanding MS disparities,” Dr. Amezcua said. “To better understand genetic variation in the context of health disparities, using ‘genetic ancestry’ could help with precision medicine. We must remember that minorities with MS face barriers related to access and education in MS care much more so than whites.”
She concluded her remarks by underscoring the importance of increasing minority participation in research and clinical trials. “But today, clinical trial participation by minorities is less than 10%. As we progress, and as we get closer to precision medicine, the health disparities will widen.”
She reported having no financial disclosures.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2019
Smartphone technology helps to detect, track eye changes in MS
DALLAS – A battery of smartphone tests have been developed to help clinicians detect and monitor eye changes in MS patients.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, Randy H. Kardon, MD, PhD, said that there are two main high priority gaps to fill when it comes to better understanding the effects of MS on vision. “One, I think we need a way for early detection of visual pathway disturbances after either an acute clinical event or a subclinical event,” said Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City. “Two, we need to monitor changes in the visual pathway over time in MS patients and capture variations due to changes in nerve conduction. The idea is, can we have a suite of smartphone tests that you can use in the clinic, but the patient can also use at home unsupervised, to get a time domain, so if there are fluctuations of core body temperature due to myelination, or subclinical changes going on, could we detect it earlier and monitor these patients? That’s the motivation.”
Although use of smartphone technology and mobile devices are emerging in health care settings, most of this technology is used sparingly in vision testing, mostly due to a lack of rigorous calibration of instruments and validation, said Dr. Kardon, who also directs the Iowa City VA Center for Prevention and Treatment of Visual Loss. To make a visual smartphone test viable, he continued, the visual output of the device must match the intended input in terms of multiple parameters (technical validation). Important parameters for vision tests include brightness, luminance, spatial resolution, and temporal resolution. Confounding variables include ambient lighting and viewing distance.
In his work with researchers from Aalborg University in Denmark, Dr. Kardon has developed four smartphone visual tests intended to be intuitive for patients. “We didn’t want something that was going to take more than 15 seconds,” he said. The visual tests include:
1. Critical flicker fusion, a test of optic nerve conduction. “This tests how well you can see a light flickering at a given temporal frequency at different levels of contrast, or how fast it can flicker before you don’t see a flicker anymore,” Dr. Kardon said. “The user sees spots that are flickering and just touches the ones they perceive to be flickering; they eliminate them by touching them.” When the test ends, the software “brackets what they did to a finer scale, and it finds the contrast at which that flicker wasn’t perceived anymore.”
2. The Landolt C visual acuity test. For this, the user must indicate the direction of the gap in the ring in a forced-choice task. “The user touches which arrow on the screen is pointing to where the location of the break in the Landolt C is perceived,” Dr. Kardon said. The Landolt C becomes progressively smaller until the location of the break can no longer be seen. “It’s pretty simple, and it finds the smallest size of the ‘C’ at which you’re making mistakes about 50% of the time, which is the threshold value for visual acuity,” he said.
3. Contrast sensitivity test at a fixed spatial frequency. “In this test, we fix the size of the letter to a large size, so spatial frequency is not at play, and we vary the contrast,” he said. “Users push the arrow wherever they see the break.” The contrast between the “C” and the background is sequentially reduced until a threshold is determined for the lowest contrast at which the location of the break in the “C” can still be observed.
4. Contrast sensitivity test at different spatial frequencies. This measure, also known as a vanishing optotype, is a line drawing of an object on a smooth, diffuse grayscale background. By altering the line properties used to define the shape of the vanishing optotype, one can vary its spatial frequency and contrast independent of target size. “This makes it an easy test because what the patient is asked to do is to touch wherever they see an object on the screen to eliminate it from the series of optotypes on the screen,” Dr. Kardon said. “The test is very fast, very intuitive.”
The researchers piloted use of these tests in a study of 104 age-matched control subjects and 117 MS patients. Of the 117 MS patients, 74 had a history of optic neuritis and 43 did not. The four tests were used in conjunction with standardized assessments, including the near-contrast acuity card test at 2.5%, the distant Early Treatment Diabetic Retinopathy Study (ETDRS) acuity test, as well as optical coherence tomography (OCT) of the retinal nerve fiber layer and ganglion cell layer thickness. Dr. Kardon and his colleagues found that when clinicians used a large target and varied the contrast, the test “did very well at differentiating normal from eyes with either previous optic neuritis or no previous optic neuritis,” he said. “It also differentiated eyes with previous optic neuritis and those with no optic neuritis. The visual acuity test didn’t perform as well because this is a near test. What we discovered afterward is that even at a fixed distance, many people who are presbyopic, or don’t have their optimal near correction, don’t do so well, because you’re testing spatial acuity. That’s a warning sign for future tests. You have to be careful as to how these are interpreted if they don’t have their best correction at near.”
Results from the critical flicker fusions tests were significant, except that they didn’t differentiate eyes affected by optic neuritis from those that weren’t. “The reason is that conduction was down in all of those eyes, so the combination of using contrast sensitivity and flicker fusion may not only help you diagnose MS, but whether that eye had been involved with optic neuritis before,” Dr. Kardon said. To date, he and his colleagues have completed technical validation of temporal frequency and contrast parameters. They’ve also completed preliminary investigations for determining test-retest variability, blurring effects, binocular summation effects, and quantification of normative ranges and abnormal subject data.
“A benefit of smartphone testing in MS is that visual dysfunction can be detected, leading to earlier interventions,” Dr. Kardon concluded. “We can study this on a time scale that wasn’t previously available. Wouldn’t you like to know on a daily or even a weekly basis what the fluctuations are in a home environment for MS patients? It’s low-cost, large-scale, and enables you to study genotype-phenotype comparisons.”
Going forward, Dr. Kardon and his colleagues have developed video cameras that go around the periphery of the iPad that can assess pupil and ocular motility, as well as eyelid and facial features in real time. He disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone tests have been developed to help clinicians detect and monitor eye changes in MS patients.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, Randy H. Kardon, MD, PhD, said that there are two main high priority gaps to fill when it comes to better understanding the effects of MS on vision. “One, I think we need a way for early detection of visual pathway disturbances after either an acute clinical event or a subclinical event,” said Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City. “Two, we need to monitor changes in the visual pathway over time in MS patients and capture variations due to changes in nerve conduction. The idea is, can we have a suite of smartphone tests that you can use in the clinic, but the patient can also use at home unsupervised, to get a time domain, so if there are fluctuations of core body temperature due to myelination, or subclinical changes going on, could we detect it earlier and monitor these patients? That’s the motivation.”
Although use of smartphone technology and mobile devices are emerging in health care settings, most of this technology is used sparingly in vision testing, mostly due to a lack of rigorous calibration of instruments and validation, said Dr. Kardon, who also directs the Iowa City VA Center for Prevention and Treatment of Visual Loss. To make a visual smartphone test viable, he continued, the visual output of the device must match the intended input in terms of multiple parameters (technical validation). Important parameters for vision tests include brightness, luminance, spatial resolution, and temporal resolution. Confounding variables include ambient lighting and viewing distance.
In his work with researchers from Aalborg University in Denmark, Dr. Kardon has developed four smartphone visual tests intended to be intuitive for patients. “We didn’t want something that was going to take more than 15 seconds,” he said. The visual tests include:
1. Critical flicker fusion, a test of optic nerve conduction. “This tests how well you can see a light flickering at a given temporal frequency at different levels of contrast, or how fast it can flicker before you don’t see a flicker anymore,” Dr. Kardon said. “The user sees spots that are flickering and just touches the ones they perceive to be flickering; they eliminate them by touching them.” When the test ends, the software “brackets what they did to a finer scale, and it finds the contrast at which that flicker wasn’t perceived anymore.”
2. The Landolt C visual acuity test. For this, the user must indicate the direction of the gap in the ring in a forced-choice task. “The user touches which arrow on the screen is pointing to where the location of the break in the Landolt C is perceived,” Dr. Kardon said. The Landolt C becomes progressively smaller until the location of the break can no longer be seen. “It’s pretty simple, and it finds the smallest size of the ‘C’ at which you’re making mistakes about 50% of the time, which is the threshold value for visual acuity,” he said.
3. Contrast sensitivity test at a fixed spatial frequency. “In this test, we fix the size of the letter to a large size, so spatial frequency is not at play, and we vary the contrast,” he said. “Users push the arrow wherever they see the break.” The contrast between the “C” and the background is sequentially reduced until a threshold is determined for the lowest contrast at which the location of the break in the “C” can still be observed.
4. Contrast sensitivity test at different spatial frequencies. This measure, also known as a vanishing optotype, is a line drawing of an object on a smooth, diffuse grayscale background. By altering the line properties used to define the shape of the vanishing optotype, one can vary its spatial frequency and contrast independent of target size. “This makes it an easy test because what the patient is asked to do is to touch wherever they see an object on the screen to eliminate it from the series of optotypes on the screen,” Dr. Kardon said. “The test is very fast, very intuitive.”
The researchers piloted use of these tests in a study of 104 age-matched control subjects and 117 MS patients. Of the 117 MS patients, 74 had a history of optic neuritis and 43 did not. The four tests were used in conjunction with standardized assessments, including the near-contrast acuity card test at 2.5%, the distant Early Treatment Diabetic Retinopathy Study (ETDRS) acuity test, as well as optical coherence tomography (OCT) of the retinal nerve fiber layer and ganglion cell layer thickness. Dr. Kardon and his colleagues found that when clinicians used a large target and varied the contrast, the test “did very well at differentiating normal from eyes with either previous optic neuritis or no previous optic neuritis,” he said. “It also differentiated eyes with previous optic neuritis and those with no optic neuritis. The visual acuity test didn’t perform as well because this is a near test. What we discovered afterward is that even at a fixed distance, many people who are presbyopic, or don’t have their optimal near correction, don’t do so well, because you’re testing spatial acuity. That’s a warning sign for future tests. You have to be careful as to how these are interpreted if they don’t have their best correction at near.”
Results from the critical flicker fusions tests were significant, except that they didn’t differentiate eyes affected by optic neuritis from those that weren’t. “The reason is that conduction was down in all of those eyes, so the combination of using contrast sensitivity and flicker fusion may not only help you diagnose MS, but whether that eye had been involved with optic neuritis before,” Dr. Kardon said. To date, he and his colleagues have completed technical validation of temporal frequency and contrast parameters. They’ve also completed preliminary investigations for determining test-retest variability, blurring effects, binocular summation effects, and quantification of normative ranges and abnormal subject data.
“A benefit of smartphone testing in MS is that visual dysfunction can be detected, leading to earlier interventions,” Dr. Kardon concluded. “We can study this on a time scale that wasn’t previously available. Wouldn’t you like to know on a daily or even a weekly basis what the fluctuations are in a home environment for MS patients? It’s low-cost, large-scale, and enables you to study genotype-phenotype comparisons.”
Going forward, Dr. Kardon and his colleagues have developed video cameras that go around the periphery of the iPad that can assess pupil and ocular motility, as well as eyelid and facial features in real time. He disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone tests have been developed to help clinicians detect and monitor eye changes in MS patients.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, Randy H. Kardon, MD, PhD, said that there are two main high priority gaps to fill when it comes to better understanding the effects of MS on vision. “One, I think we need a way for early detection of visual pathway disturbances after either an acute clinical event or a subclinical event,” said Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City. “Two, we need to monitor changes in the visual pathway over time in MS patients and capture variations due to changes in nerve conduction. The idea is, can we have a suite of smartphone tests that you can use in the clinic, but the patient can also use at home unsupervised, to get a time domain, so if there are fluctuations of core body temperature due to myelination, or subclinical changes going on, could we detect it earlier and monitor these patients? That’s the motivation.”
Although use of smartphone technology and mobile devices are emerging in health care settings, most of this technology is used sparingly in vision testing, mostly due to a lack of rigorous calibration of instruments and validation, said Dr. Kardon, who also directs the Iowa City VA Center for Prevention and Treatment of Visual Loss. To make a visual smartphone test viable, he continued, the visual output of the device must match the intended input in terms of multiple parameters (technical validation). Important parameters for vision tests include brightness, luminance, spatial resolution, and temporal resolution. Confounding variables include ambient lighting and viewing distance.
In his work with researchers from Aalborg University in Denmark, Dr. Kardon has developed four smartphone visual tests intended to be intuitive for patients. “We didn’t want something that was going to take more than 15 seconds,” he said. The visual tests include:
1. Critical flicker fusion, a test of optic nerve conduction. “This tests how well you can see a light flickering at a given temporal frequency at different levels of contrast, or how fast it can flicker before you don’t see a flicker anymore,” Dr. Kardon said. “The user sees spots that are flickering and just touches the ones they perceive to be flickering; they eliminate them by touching them.” When the test ends, the software “brackets what they did to a finer scale, and it finds the contrast at which that flicker wasn’t perceived anymore.”
2. The Landolt C visual acuity test. For this, the user must indicate the direction of the gap in the ring in a forced-choice task. “The user touches which arrow on the screen is pointing to where the location of the break in the Landolt C is perceived,” Dr. Kardon said. The Landolt C becomes progressively smaller until the location of the break can no longer be seen. “It’s pretty simple, and it finds the smallest size of the ‘C’ at which you’re making mistakes about 50% of the time, which is the threshold value for visual acuity,” he said.
3. Contrast sensitivity test at a fixed spatial frequency. “In this test, we fix the size of the letter to a large size, so spatial frequency is not at play, and we vary the contrast,” he said. “Users push the arrow wherever they see the break.” The contrast between the “C” and the background is sequentially reduced until a threshold is determined for the lowest contrast at which the location of the break in the “C” can still be observed.
4. Contrast sensitivity test at different spatial frequencies. This measure, also known as a vanishing optotype, is a line drawing of an object on a smooth, diffuse grayscale background. By altering the line properties used to define the shape of the vanishing optotype, one can vary its spatial frequency and contrast independent of target size. “This makes it an easy test because what the patient is asked to do is to touch wherever they see an object on the screen to eliminate it from the series of optotypes on the screen,” Dr. Kardon said. “The test is very fast, very intuitive.”
The researchers piloted use of these tests in a study of 104 age-matched control subjects and 117 MS patients. Of the 117 MS patients, 74 had a history of optic neuritis and 43 did not. The four tests were used in conjunction with standardized assessments, including the near-contrast acuity card test at 2.5%, the distant Early Treatment Diabetic Retinopathy Study (ETDRS) acuity test, as well as optical coherence tomography (OCT) of the retinal nerve fiber layer and ganglion cell layer thickness. Dr. Kardon and his colleagues found that when clinicians used a large target and varied the contrast, the test “did very well at differentiating normal from eyes with either previous optic neuritis or no previous optic neuritis,” he said. “It also differentiated eyes with previous optic neuritis and those with no optic neuritis. The visual acuity test didn’t perform as well because this is a near test. What we discovered afterward is that even at a fixed distance, many people who are presbyopic, or don’t have their optimal near correction, don’t do so well, because you’re testing spatial acuity. That’s a warning sign for future tests. You have to be careful as to how these are interpreted if they don’t have their best correction at near.”
Results from the critical flicker fusions tests were significant, except that they didn’t differentiate eyes affected by optic neuritis from those that weren’t. “The reason is that conduction was down in all of those eyes, so the combination of using contrast sensitivity and flicker fusion may not only help you diagnose MS, but whether that eye had been involved with optic neuritis before,” Dr. Kardon said. To date, he and his colleagues have completed technical validation of temporal frequency and contrast parameters. They’ve also completed preliminary investigations for determining test-retest variability, blurring effects, binocular summation effects, and quantification of normative ranges and abnormal subject data.
“A benefit of smartphone testing in MS is that visual dysfunction can be detected, leading to earlier interventions,” Dr. Kardon concluded. “We can study this on a time scale that wasn’t previously available. Wouldn’t you like to know on a daily or even a weekly basis what the fluctuations are in a home environment for MS patients? It’s low-cost, large-scale, and enables you to study genotype-phenotype comparisons.”
Going forward, Dr. Kardon and his colleagues have developed video cameras that go around the periphery of the iPad that can assess pupil and ocular motility, as well as eyelid and facial features in real time. He disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2019
Study eyes serious adverse events from long-term rituximab use in MS
DALLAS – An analysis of two large cohorts treated with rituximab (Rituxan) showed that the rate of serious infections was 8.1%, yet no life-threatening infusion reactions occurred.
The finding comes from a retrospective chart review of 500 patients treated at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado at Denver, Aurora, and 269 treated at the New York University Multiple Sclerosis Comprehensive Care Center.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, one of the study authors, Brandi L. Vollmer, MPH, noted that while rituximab is used off-label to treat multiple sclerosis (MS) and related disorders, limited long-term data for MS patients exist. Ms. Vollmer, a professional research assistant in the department of neurology in the division of neuroimmunology at the University of Colorado at Denver, and her associates identified all patients at the two MS centers who received at least one dose of rituximab. They reviewed patient charts from rituximab start date until 12 months after the last rituximab end date, or last ocrelizumab (Ocrevus) infusion in cases where patients switched to ocrelizumab without interruption with any other disease-modifying therapy. Data were abstracted from each chart using a case report form that systematically queried for demographic and clinical characteristics, serious adverse events, and significant laboratory abnormalities. The researchers used descriptive statistics to describe the sample group.
Key outcomes of interest were infusion reactions that were life-threatening or resulted in a hospitalization; infections that resulted in hospitalization, the need for IV antibiotics, or extended antibiotics; abnormal lab values; new diagnosis of malignant cancer; new diagnosis of autoimmune disease; diagnosis of serious thromboembolism; and mortality within 12 months of last infusion. At baseline, the mean age of the 769 patients was 43 years, 69% were female, 64% were white, and 16% were black. More than half (58%) had a diagnosis of relapsing-remitting MS, followed by secondary progressive MS (20%), primary progressive MS (11%), neuromyelitis optica spectrum disorder (9%), or other, and their mean disease duration was 8 years.
The researchers reported that the mean cumulative rituximab dose was 4,124 mg (median of 3,000 mg), the mean dose of ocrelizumab for ocrelizumab-switchers was 1,087 mg, and the mean time of follow-up was 33 months. Infections while on rituximab/ocrelizumab resulting in a hospitalization were observed in 50 patients (6.5%), which were primarily urinary tract infections (UTIs; 19 cases), pneumonia (12 cases), and sepsis (12 cases), while 7 patients (0.9%) experienced an infection that resulted in extended dosing antibiotics, including 5 recurrent UTIs, 1 case of bacteremia, and 1 case of osteomyelitis.
Ms. Vollmer and her colleagues also found that five patients (0.7%) experienced an infection that resulted in IV antibiotics without hospitalization, including one case each of pneumonia, cellulitis, UTI, infected wound, and aspiration pneumonia with respiratory syncytial virus. Serious de novo diagnoses while on rituximab were reported for 18 patients (2%), including autoimmune disease in 4, non-skin neoplasm in 7, and serious thromboembolic events in 7.
No patient experienced an infusion reaction requiring hospitalization. However, one patient received epinephrine after seeking care at an emergency department post infusion. Twelve patients (2%) died within 12 months of their last rituximab dose, although none were deemed by the treating clinician to be related to rituximab. Significant neutropenia was observed in 12 (2%), lymphopenia in 46 (6%), and IgG values below 500 mg/dL in 28 (4%).
“So far, this is just a descriptive analysis,” Ms. Vollmer said. “We want to look further into how abnormal lab values correlate with serious infections in particular.”
She acknowledged certain limitations of the study, including its retrospective design. Ms. Vollmer reported having no financial disclosures. Many of her coauthors reporting having numerous financial ties to the pharmaceutical industry.
SOURCE: Vollmer B et al. ACTRIMS Forum 2019, Poster 113
DALLAS – An analysis of two large cohorts treated with rituximab (Rituxan) showed that the rate of serious infections was 8.1%, yet no life-threatening infusion reactions occurred.
The finding comes from a retrospective chart review of 500 patients treated at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado at Denver, Aurora, and 269 treated at the New York University Multiple Sclerosis Comprehensive Care Center.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, one of the study authors, Brandi L. Vollmer, MPH, noted that while rituximab is used off-label to treat multiple sclerosis (MS) and related disorders, limited long-term data for MS patients exist. Ms. Vollmer, a professional research assistant in the department of neurology in the division of neuroimmunology at the University of Colorado at Denver, and her associates identified all patients at the two MS centers who received at least one dose of rituximab. They reviewed patient charts from rituximab start date until 12 months after the last rituximab end date, or last ocrelizumab (Ocrevus) infusion in cases where patients switched to ocrelizumab without interruption with any other disease-modifying therapy. Data were abstracted from each chart using a case report form that systematically queried for demographic and clinical characteristics, serious adverse events, and significant laboratory abnormalities. The researchers used descriptive statistics to describe the sample group.
Key outcomes of interest were infusion reactions that were life-threatening or resulted in a hospitalization; infections that resulted in hospitalization, the need for IV antibiotics, or extended antibiotics; abnormal lab values; new diagnosis of malignant cancer; new diagnosis of autoimmune disease; diagnosis of serious thromboembolism; and mortality within 12 months of last infusion. At baseline, the mean age of the 769 patients was 43 years, 69% were female, 64% were white, and 16% were black. More than half (58%) had a diagnosis of relapsing-remitting MS, followed by secondary progressive MS (20%), primary progressive MS (11%), neuromyelitis optica spectrum disorder (9%), or other, and their mean disease duration was 8 years.
The researchers reported that the mean cumulative rituximab dose was 4,124 mg (median of 3,000 mg), the mean dose of ocrelizumab for ocrelizumab-switchers was 1,087 mg, and the mean time of follow-up was 33 months. Infections while on rituximab/ocrelizumab resulting in a hospitalization were observed in 50 patients (6.5%), which were primarily urinary tract infections (UTIs; 19 cases), pneumonia (12 cases), and sepsis (12 cases), while 7 patients (0.9%) experienced an infection that resulted in extended dosing antibiotics, including 5 recurrent UTIs, 1 case of bacteremia, and 1 case of osteomyelitis.
Ms. Vollmer and her colleagues also found that five patients (0.7%) experienced an infection that resulted in IV antibiotics without hospitalization, including one case each of pneumonia, cellulitis, UTI, infected wound, and aspiration pneumonia with respiratory syncytial virus. Serious de novo diagnoses while on rituximab were reported for 18 patients (2%), including autoimmune disease in 4, non-skin neoplasm in 7, and serious thromboembolic events in 7.
No patient experienced an infusion reaction requiring hospitalization. However, one patient received epinephrine after seeking care at an emergency department post infusion. Twelve patients (2%) died within 12 months of their last rituximab dose, although none were deemed by the treating clinician to be related to rituximab. Significant neutropenia was observed in 12 (2%), lymphopenia in 46 (6%), and IgG values below 500 mg/dL in 28 (4%).
“So far, this is just a descriptive analysis,” Ms. Vollmer said. “We want to look further into how abnormal lab values correlate with serious infections in particular.”
She acknowledged certain limitations of the study, including its retrospective design. Ms. Vollmer reported having no financial disclosures. Many of her coauthors reporting having numerous financial ties to the pharmaceutical industry.
SOURCE: Vollmer B et al. ACTRIMS Forum 2019, Poster 113
DALLAS – An analysis of two large cohorts treated with rituximab (Rituxan) showed that the rate of serious infections was 8.1%, yet no life-threatening infusion reactions occurred.
The finding comes from a retrospective chart review of 500 patients treated at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado at Denver, Aurora, and 269 treated at the New York University Multiple Sclerosis Comprehensive Care Center.
At a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis, one of the study authors, Brandi L. Vollmer, MPH, noted that while rituximab is used off-label to treat multiple sclerosis (MS) and related disorders, limited long-term data for MS patients exist. Ms. Vollmer, a professional research assistant in the department of neurology in the division of neuroimmunology at the University of Colorado at Denver, and her associates identified all patients at the two MS centers who received at least one dose of rituximab. They reviewed patient charts from rituximab start date until 12 months after the last rituximab end date, or last ocrelizumab (Ocrevus) infusion in cases where patients switched to ocrelizumab without interruption with any other disease-modifying therapy. Data were abstracted from each chart using a case report form that systematically queried for demographic and clinical characteristics, serious adverse events, and significant laboratory abnormalities. The researchers used descriptive statistics to describe the sample group.
Key outcomes of interest were infusion reactions that were life-threatening or resulted in a hospitalization; infections that resulted in hospitalization, the need for IV antibiotics, or extended antibiotics; abnormal lab values; new diagnosis of malignant cancer; new diagnosis of autoimmune disease; diagnosis of serious thromboembolism; and mortality within 12 months of last infusion. At baseline, the mean age of the 769 patients was 43 years, 69% were female, 64% were white, and 16% were black. More than half (58%) had a diagnosis of relapsing-remitting MS, followed by secondary progressive MS (20%), primary progressive MS (11%), neuromyelitis optica spectrum disorder (9%), or other, and their mean disease duration was 8 years.
The researchers reported that the mean cumulative rituximab dose was 4,124 mg (median of 3,000 mg), the mean dose of ocrelizumab for ocrelizumab-switchers was 1,087 mg, and the mean time of follow-up was 33 months. Infections while on rituximab/ocrelizumab resulting in a hospitalization were observed in 50 patients (6.5%), which were primarily urinary tract infections (UTIs; 19 cases), pneumonia (12 cases), and sepsis (12 cases), while 7 patients (0.9%) experienced an infection that resulted in extended dosing antibiotics, including 5 recurrent UTIs, 1 case of bacteremia, and 1 case of osteomyelitis.
Ms. Vollmer and her colleagues also found that five patients (0.7%) experienced an infection that resulted in IV antibiotics without hospitalization, including one case each of pneumonia, cellulitis, UTI, infected wound, and aspiration pneumonia with respiratory syncytial virus. Serious de novo diagnoses while on rituximab were reported for 18 patients (2%), including autoimmune disease in 4, non-skin neoplasm in 7, and serious thromboembolic events in 7.
No patient experienced an infusion reaction requiring hospitalization. However, one patient received epinephrine after seeking care at an emergency department post infusion. Twelve patients (2%) died within 12 months of their last rituximab dose, although none were deemed by the treating clinician to be related to rituximab. Significant neutropenia was observed in 12 (2%), lymphopenia in 46 (6%), and IgG values below 500 mg/dL in 28 (4%).
“So far, this is just a descriptive analysis,” Ms. Vollmer said. “We want to look further into how abnormal lab values correlate with serious infections in particular.”
She acknowledged certain limitations of the study, including its retrospective design. Ms. Vollmer reported having no financial disclosures. Many of her coauthors reporting having numerous financial ties to the pharmaceutical industry.
SOURCE: Vollmer B et al. ACTRIMS Forum 2019, Poster 113
REPORTING FROM ACTRIMS 2019
NIH director updates study enrolling one million participants
NEW ORLEANS – It is not too late to enroll your patients or yourself into the largest longitudinal cohort study ever initiated, according to Francis S. Collins, MD, PhD, who is director of the National Institutes of Health (NIH).
Since May 2018, when it was initiated, the NIH-funded All of Us Research Program has already enrolled 200,000 of the planned goal of one million participants in the United States. Of these, approximately half have already provided baseline demographics and health information as well as their consent to use the slew of health data that is being collected.
“The only way to do this kind of thing is to have data – a lot of it,” said Dr. Collins, explaining the premise of the All of Us Research Program in an interview conducted at the annual meeting of the Endocrine Society.
The data are not limited to medical records: Blood samples, whole genome sequencing, wearable activity monitors, and subject-completed questionnaires are among a long list of sources of information to be collected from participants, who are expected to be followed indefinitely.
According to Dr. Collins, who delivered a plenary address at the meeting, these data will become more valuable over time, one of the most important goals of this study is to prepare the way for precision medicine. As opposed to the traditional one-size-fits-all approach to treating disease, he believes that this large dataset will allow researchers to understand differences in common diseases at the individual level.
In relation to endocrinology, Dr. Collins said that a cohort of one million participants would be expected to have close to 100,000 individuals with diabetes mellitus.
“This is going to be transformative,” said Dr. Collins, who emphasized that the enrollment is specifically designed to capture participants from diverse ethnic and racial groups.
All of the data collected will be made broadly available to research initiatives of all kinds, many of which have not yet even been envisioned.
Information on enrollment is available on line: joinallofus.org.
NEW ORLEANS – It is not too late to enroll your patients or yourself into the largest longitudinal cohort study ever initiated, according to Francis S. Collins, MD, PhD, who is director of the National Institutes of Health (NIH).
Since May 2018, when it was initiated, the NIH-funded All of Us Research Program has already enrolled 200,000 of the planned goal of one million participants in the United States. Of these, approximately half have already provided baseline demographics and health information as well as their consent to use the slew of health data that is being collected.
“The only way to do this kind of thing is to have data – a lot of it,” said Dr. Collins, explaining the premise of the All of Us Research Program in an interview conducted at the annual meeting of the Endocrine Society.
The data are not limited to medical records: Blood samples, whole genome sequencing, wearable activity monitors, and subject-completed questionnaires are among a long list of sources of information to be collected from participants, who are expected to be followed indefinitely.
According to Dr. Collins, who delivered a plenary address at the meeting, these data will become more valuable over time, one of the most important goals of this study is to prepare the way for precision medicine. As opposed to the traditional one-size-fits-all approach to treating disease, he believes that this large dataset will allow researchers to understand differences in common diseases at the individual level.
In relation to endocrinology, Dr. Collins said that a cohort of one million participants would be expected to have close to 100,000 individuals with diabetes mellitus.
“This is going to be transformative,” said Dr. Collins, who emphasized that the enrollment is specifically designed to capture participants from diverse ethnic and racial groups.
All of the data collected will be made broadly available to research initiatives of all kinds, many of which have not yet even been envisioned.
Information on enrollment is available on line: joinallofus.org.
NEW ORLEANS – It is not too late to enroll your patients or yourself into the largest longitudinal cohort study ever initiated, according to Francis S. Collins, MD, PhD, who is director of the National Institutes of Health (NIH).
Since May 2018, when it was initiated, the NIH-funded All of Us Research Program has already enrolled 200,000 of the planned goal of one million participants in the United States. Of these, approximately half have already provided baseline demographics and health information as well as their consent to use the slew of health data that is being collected.
“The only way to do this kind of thing is to have data – a lot of it,” said Dr. Collins, explaining the premise of the All of Us Research Program in an interview conducted at the annual meeting of the Endocrine Society.
The data are not limited to medical records: Blood samples, whole genome sequencing, wearable activity monitors, and subject-completed questionnaires are among a long list of sources of information to be collected from participants, who are expected to be followed indefinitely.
According to Dr. Collins, who delivered a plenary address at the meeting, these data will become more valuable over time, one of the most important goals of this study is to prepare the way for precision medicine. As opposed to the traditional one-size-fits-all approach to treating disease, he believes that this large dataset will allow researchers to understand differences in common diseases at the individual level.
In relation to endocrinology, Dr. Collins said that a cohort of one million participants would be expected to have close to 100,000 individuals with diabetes mellitus.
“This is going to be transformative,” said Dr. Collins, who emphasized that the enrollment is specifically designed to capture participants from diverse ethnic and racial groups.
All of the data collected will be made broadly available to research initiatives of all kinds, many of which have not yet even been envisioned.
Information on enrollment is available on line: joinallofus.org.
REPORTING FROM ENDO 2019
Syncope session showcases latest research and guidance for practice
Syncope is a common problem, but an area in which practice likely varies by region and provider, according to Carrie Herzke, MD, SFHM, of Johns Hopkins University in Baltimore.
The variation in practice also suggests opportunities to safely cut costs, Dr. Herzke said in an interview.
Dr. Herzke will review the recent literature and summarize the current guidelines related to the treatment of syncope in her session, “SyncopE – Effective, Efficient and Economic Evaluations,” on Monday, March 25, at HM19.
“At the end of this session I am hopeful attendees will have a better understanding of the current guidelines, as well as which tests are most likely to be high value in the evaluation of syncope,” Dr. Herzke said. But before testing, clinicians should keep in mind that a history and physical examination are key components to evaluating syncope, she noted.
The European Society of Cardiology Guidelines for Syncope, published in 2018, feature several new concepts for evaluating and managing syncope in clinical settings and testing, including tilt testing, a greater role for prolonged ECG monitoring, use of video recording for suspected syncope, consideration of adenosine sensitive syncope, and consideration of neurological causes of syncope.
The ESC guidelines include using algorithms to determine the appropriate therapy for reflex syncope based on age, severity, and clinical forms. The guidelines also address diagnostic tests, monitoring in and out of the hospital, and treatment options including lifestyle changes and education as well as pharmacotherapy.
Dr. Herzke also will review the 2017 ACC/AHA/HRS Guideline for Patients With Syncope, published in 2017 in the journal Circulation. This guideline has an algorithm for initial evaluation of patients with syncope that includes a history, physical, and ECG. If the cause is known, patients should be assessed for risk and treated; syncope of unknown cause requires further evaluation, and the guideline presents recommendations for additional assessment through means including cardiac imaging, stress testing, blood testing, neurological testing, and tilt table testing.
“The selection of a given diagnostic test, after the initial history, physical examination, and baseline ECG, is a clinical decision based on the patient’s clinical presentation, risk stratification, and a clear understanding of diagnostic and prognostic value of any further testing,” according to the guideline authors.
The 2017 ACC/AHA/HRS Guideline for Patients With Syncope also provides recommendations for the management of cardiovascular conditions, including arrhythmic and structural conditions.
Dr. Herzke said she thinks that the topic of pulmonary embolism and syncope may prompt the liveliest discussion, and she will include several recent articles on this topic in her literature review.
“There has been some debate about how often PE is present in patients presenting after a syncopal episode,” she said. A recent study published in JAMA Internal Medicine found a prevalence of PE from 0.06% to 0.55% among all adults presenting to an ED with syncope and a prevalence of PE from 0.14% to 0.83%.
SyncopE – Effective, Efficient and Economic Evaluations
Monday 1:10 pm
Maryland BD/4-6
Syncope is a common problem, but an area in which practice likely varies by region and provider, according to Carrie Herzke, MD, SFHM, of Johns Hopkins University in Baltimore.
The variation in practice also suggests opportunities to safely cut costs, Dr. Herzke said in an interview.
Dr. Herzke will review the recent literature and summarize the current guidelines related to the treatment of syncope in her session, “SyncopE – Effective, Efficient and Economic Evaluations,” on Monday, March 25, at HM19.
“At the end of this session I am hopeful attendees will have a better understanding of the current guidelines, as well as which tests are most likely to be high value in the evaluation of syncope,” Dr. Herzke said. But before testing, clinicians should keep in mind that a history and physical examination are key components to evaluating syncope, she noted.
The European Society of Cardiology Guidelines for Syncope, published in 2018, feature several new concepts for evaluating and managing syncope in clinical settings and testing, including tilt testing, a greater role for prolonged ECG monitoring, use of video recording for suspected syncope, consideration of adenosine sensitive syncope, and consideration of neurological causes of syncope.
The ESC guidelines include using algorithms to determine the appropriate therapy for reflex syncope based on age, severity, and clinical forms. The guidelines also address diagnostic tests, monitoring in and out of the hospital, and treatment options including lifestyle changes and education as well as pharmacotherapy.
Dr. Herzke also will review the 2017 ACC/AHA/HRS Guideline for Patients With Syncope, published in 2017 in the journal Circulation. This guideline has an algorithm for initial evaluation of patients with syncope that includes a history, physical, and ECG. If the cause is known, patients should be assessed for risk and treated; syncope of unknown cause requires further evaluation, and the guideline presents recommendations for additional assessment through means including cardiac imaging, stress testing, blood testing, neurological testing, and tilt table testing.
“The selection of a given diagnostic test, after the initial history, physical examination, and baseline ECG, is a clinical decision based on the patient’s clinical presentation, risk stratification, and a clear understanding of diagnostic and prognostic value of any further testing,” according to the guideline authors.
The 2017 ACC/AHA/HRS Guideline for Patients With Syncope also provides recommendations for the management of cardiovascular conditions, including arrhythmic and structural conditions.
Dr. Herzke said she thinks that the topic of pulmonary embolism and syncope may prompt the liveliest discussion, and she will include several recent articles on this topic in her literature review.
“There has been some debate about how often PE is present in patients presenting after a syncopal episode,” she said. A recent study published in JAMA Internal Medicine found a prevalence of PE from 0.06% to 0.55% among all adults presenting to an ED with syncope and a prevalence of PE from 0.14% to 0.83%.
SyncopE – Effective, Efficient and Economic Evaluations
Monday 1:10 pm
Maryland BD/4-6
Syncope is a common problem, but an area in which practice likely varies by region and provider, according to Carrie Herzke, MD, SFHM, of Johns Hopkins University in Baltimore.
The variation in practice also suggests opportunities to safely cut costs, Dr. Herzke said in an interview.
Dr. Herzke will review the recent literature and summarize the current guidelines related to the treatment of syncope in her session, “SyncopE – Effective, Efficient and Economic Evaluations,” on Monday, March 25, at HM19.
“At the end of this session I am hopeful attendees will have a better understanding of the current guidelines, as well as which tests are most likely to be high value in the evaluation of syncope,” Dr. Herzke said. But before testing, clinicians should keep in mind that a history and physical examination are key components to evaluating syncope, she noted.
The European Society of Cardiology Guidelines for Syncope, published in 2018, feature several new concepts for evaluating and managing syncope in clinical settings and testing, including tilt testing, a greater role for prolonged ECG monitoring, use of video recording for suspected syncope, consideration of adenosine sensitive syncope, and consideration of neurological causes of syncope.
The ESC guidelines include using algorithms to determine the appropriate therapy for reflex syncope based on age, severity, and clinical forms. The guidelines also address diagnostic tests, monitoring in and out of the hospital, and treatment options including lifestyle changes and education as well as pharmacotherapy.
Dr. Herzke also will review the 2017 ACC/AHA/HRS Guideline for Patients With Syncope, published in 2017 in the journal Circulation. This guideline has an algorithm for initial evaluation of patients with syncope that includes a history, physical, and ECG. If the cause is known, patients should be assessed for risk and treated; syncope of unknown cause requires further evaluation, and the guideline presents recommendations for additional assessment through means including cardiac imaging, stress testing, blood testing, neurological testing, and tilt table testing.
“The selection of a given diagnostic test, after the initial history, physical examination, and baseline ECG, is a clinical decision based on the patient’s clinical presentation, risk stratification, and a clear understanding of diagnostic and prognostic value of any further testing,” according to the guideline authors.
The 2017 ACC/AHA/HRS Guideline for Patients With Syncope also provides recommendations for the management of cardiovascular conditions, including arrhythmic and structural conditions.
Dr. Herzke said she thinks that the topic of pulmonary embolism and syncope may prompt the liveliest discussion, and she will include several recent articles on this topic in her literature review.
“There has been some debate about how often PE is present in patients presenting after a syncopal episode,” she said. A recent study published in JAMA Internal Medicine found a prevalence of PE from 0.06% to 0.55% among all adults presenting to an ED with syncope and a prevalence of PE from 0.14% to 0.83%.
SyncopE – Effective, Efficient and Economic Evaluations
Monday 1:10 pm
Maryland BD/4-6
Simple screening for risk of falling in elderly can guide prevention
NEW ORLEANS – , according to an update on this field at the annual meeting at the Endocrine Society.
“The risk of falling in older adults is very high, but risk can be evaluated, and there are effective strategies for risk reduction,” reported Kenton R. Kaufman, PhD, codirector of the Biomechanics and Motion Analysis Laboratory at the Mayo Clinic, Rochester, Minn.
There is not much debate that aging individuals are at an increased risk of falls, but Dr. Kaufman presented his own set of data to reinforce this point. In a longitudinal study of 125 individuals over the age of 65 years who were followed for a year at his institution, 59% had at least one fall even though all were healthy and functional when enrolled.
“It was more common to fall in summer than in winter, and most occurred on a level surface,” said Dr. Kaufman citing data from a study published 2 years ago (Arch Gerontol Geriatr. 2017;73:240-7). About half of the falls occurred at home.
Only 20%-30% of falls lead to moderate to severe injuries, but this is enough to make fall prevention an appropriate and important focus of public health initiatives to reduce morbidity and lower health costs, according to Dr. Kaufman, citing data suggesting that the medical costs total in the billions of dollars.
As a result of a substantial body of research in this area, there are now multiple clinical tests, such as grip strength, the functional reach test, and the 5-minute walk, that provide some degree of predictive value for identifying elderly individuals at risk for falls.
In addition, simple questionnaires that measure the fear of falling, such as the Activities-Specific Balance Confidence Scale (ABC test), and the Falls Efficacy Scale, also identify individuals at higher risk of falling. According to Dr. Kaufman, the predictive value of these questionnaires stems from the fact that those with more fears are more likely to fall.
Dr. Kaufman advised using these simple measures alone or in combination to screen aging patients for risk of failing. Although he singled out grip strength and the ABC test as the clinical test and the questionnaire he is most likely to employ, he believes others are also reasonable. When performed by primary care physicians, although not specialists, evaluating patients for risk of falling is Medicare-reimbursable, according to Dr. Kaufman.
There are two components to effective prophylaxis. One is improving muscle strength. The other is improving neuromuscular response, which means moving quickly enough to compensate when one’s center of gravity is disturbed. According to Dr. Kaufman, who cited two randomized trials, exercise to restore muscle strength can by itself reduce the risk of falling by 10%-20%.
Neuromuscular training is more intensive and not widely available but very effective. This involves training patients to improve their reaction time in the event of an impending fall. This approach, called postural perturbation training, employs a harness to prevent injury.
“The elderly can lose their facility for rapid recovery but this can be relearned,” said Dr. Kaufman, who cited another two randomized trials with this approach that reduced falls by 45% and 55%.
Postural perturbation training, although used to train amputees to gain comfort ambulating on artificial limbs, has so far had limited use in the elderly, but Dr. Kaufman said it might have utility in selected individuals, and he noted that there is at least one commercial device now being marketed.
Many elderly patients will not be candidates for training to reduce falls due to frailty or comorbid conditions that prevent exercise, but Dr. Kaufman encouraged clinicians to evaluate risk of falls in aging individuals who are active because there are strategies to reduce risk, and falls are a major source of morbidity and mortality.
Even for those who are not suitable for risk reduction strategies, testing for risk of falls has the ancillary benefit of raising awareness, according to Dr. Kaufman.
Dr. Kaufman reported no relevant financial relationships to disclose.
NEW ORLEANS – , according to an update on this field at the annual meeting at the Endocrine Society.
“The risk of falling in older adults is very high, but risk can be evaluated, and there are effective strategies for risk reduction,” reported Kenton R. Kaufman, PhD, codirector of the Biomechanics and Motion Analysis Laboratory at the Mayo Clinic, Rochester, Minn.
There is not much debate that aging individuals are at an increased risk of falls, but Dr. Kaufman presented his own set of data to reinforce this point. In a longitudinal study of 125 individuals over the age of 65 years who were followed for a year at his institution, 59% had at least one fall even though all were healthy and functional when enrolled.
“It was more common to fall in summer than in winter, and most occurred on a level surface,” said Dr. Kaufman citing data from a study published 2 years ago (Arch Gerontol Geriatr. 2017;73:240-7). About half of the falls occurred at home.
Only 20%-30% of falls lead to moderate to severe injuries, but this is enough to make fall prevention an appropriate and important focus of public health initiatives to reduce morbidity and lower health costs, according to Dr. Kaufman, citing data suggesting that the medical costs total in the billions of dollars.
As a result of a substantial body of research in this area, there are now multiple clinical tests, such as grip strength, the functional reach test, and the 5-minute walk, that provide some degree of predictive value for identifying elderly individuals at risk for falls.
In addition, simple questionnaires that measure the fear of falling, such as the Activities-Specific Balance Confidence Scale (ABC test), and the Falls Efficacy Scale, also identify individuals at higher risk of falling. According to Dr. Kaufman, the predictive value of these questionnaires stems from the fact that those with more fears are more likely to fall.
Dr. Kaufman advised using these simple measures alone or in combination to screen aging patients for risk of failing. Although he singled out grip strength and the ABC test as the clinical test and the questionnaire he is most likely to employ, he believes others are also reasonable. When performed by primary care physicians, although not specialists, evaluating patients for risk of falling is Medicare-reimbursable, according to Dr. Kaufman.
There are two components to effective prophylaxis. One is improving muscle strength. The other is improving neuromuscular response, which means moving quickly enough to compensate when one’s center of gravity is disturbed. According to Dr. Kaufman, who cited two randomized trials, exercise to restore muscle strength can by itself reduce the risk of falling by 10%-20%.
Neuromuscular training is more intensive and not widely available but very effective. This involves training patients to improve their reaction time in the event of an impending fall. This approach, called postural perturbation training, employs a harness to prevent injury.
“The elderly can lose their facility for rapid recovery but this can be relearned,” said Dr. Kaufman, who cited another two randomized trials with this approach that reduced falls by 45% and 55%.
Postural perturbation training, although used to train amputees to gain comfort ambulating on artificial limbs, has so far had limited use in the elderly, but Dr. Kaufman said it might have utility in selected individuals, and he noted that there is at least one commercial device now being marketed.
Many elderly patients will not be candidates for training to reduce falls due to frailty or comorbid conditions that prevent exercise, but Dr. Kaufman encouraged clinicians to evaluate risk of falls in aging individuals who are active because there are strategies to reduce risk, and falls are a major source of morbidity and mortality.
Even for those who are not suitable for risk reduction strategies, testing for risk of falls has the ancillary benefit of raising awareness, according to Dr. Kaufman.
Dr. Kaufman reported no relevant financial relationships to disclose.
NEW ORLEANS – , according to an update on this field at the annual meeting at the Endocrine Society.
“The risk of falling in older adults is very high, but risk can be evaluated, and there are effective strategies for risk reduction,” reported Kenton R. Kaufman, PhD, codirector of the Biomechanics and Motion Analysis Laboratory at the Mayo Clinic, Rochester, Minn.
There is not much debate that aging individuals are at an increased risk of falls, but Dr. Kaufman presented his own set of data to reinforce this point. In a longitudinal study of 125 individuals over the age of 65 years who were followed for a year at his institution, 59% had at least one fall even though all were healthy and functional when enrolled.
“It was more common to fall in summer than in winter, and most occurred on a level surface,” said Dr. Kaufman citing data from a study published 2 years ago (Arch Gerontol Geriatr. 2017;73:240-7). About half of the falls occurred at home.
Only 20%-30% of falls lead to moderate to severe injuries, but this is enough to make fall prevention an appropriate and important focus of public health initiatives to reduce morbidity and lower health costs, according to Dr. Kaufman, citing data suggesting that the medical costs total in the billions of dollars.
As a result of a substantial body of research in this area, there are now multiple clinical tests, such as grip strength, the functional reach test, and the 5-minute walk, that provide some degree of predictive value for identifying elderly individuals at risk for falls.
In addition, simple questionnaires that measure the fear of falling, such as the Activities-Specific Balance Confidence Scale (ABC test), and the Falls Efficacy Scale, also identify individuals at higher risk of falling. According to Dr. Kaufman, the predictive value of these questionnaires stems from the fact that those with more fears are more likely to fall.
Dr. Kaufman advised using these simple measures alone or in combination to screen aging patients for risk of failing. Although he singled out grip strength and the ABC test as the clinical test and the questionnaire he is most likely to employ, he believes others are also reasonable. When performed by primary care physicians, although not specialists, evaluating patients for risk of falling is Medicare-reimbursable, according to Dr. Kaufman.
There are two components to effective prophylaxis. One is improving muscle strength. The other is improving neuromuscular response, which means moving quickly enough to compensate when one’s center of gravity is disturbed. According to Dr. Kaufman, who cited two randomized trials, exercise to restore muscle strength can by itself reduce the risk of falling by 10%-20%.
Neuromuscular training is more intensive and not widely available but very effective. This involves training patients to improve their reaction time in the event of an impending fall. This approach, called postural perturbation training, employs a harness to prevent injury.
“The elderly can lose their facility for rapid recovery but this can be relearned,” said Dr. Kaufman, who cited another two randomized trials with this approach that reduced falls by 45% and 55%.
Postural perturbation training, although used to train amputees to gain comfort ambulating on artificial limbs, has so far had limited use in the elderly, but Dr. Kaufman said it might have utility in selected individuals, and he noted that there is at least one commercial device now being marketed.
Many elderly patients will not be candidates for training to reduce falls due to frailty or comorbid conditions that prevent exercise, but Dr. Kaufman encouraged clinicians to evaluate risk of falls in aging individuals who are active because there are strategies to reduce risk, and falls are a major source of morbidity and mortality.
Even for those who are not suitable for risk reduction strategies, testing for risk of falls has the ancillary benefit of raising awareness, according to Dr. Kaufman.
Dr. Kaufman reported no relevant financial relationships to disclose.
REPORTING FROM ENDO 2019
Patient selection for acute stroke thrombectomy stirs controversy
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
REPORTING FROM ISC 2019