User login
Brachytherapy access may mediate poor cervical cancer survival in blacks
HONOLULU – , according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Stephanie Alimena, MD, of Brigham and Women’s Hospital in Boston, presented a large, retrospective study showing that use of brachytherapy mediated survival differences by race.
In the absence of brachytherapy, black patients had a significantly higher risk of death than did non-black patients (P = .013). However, when brachytherapy was used, black and non-black patients had a similar risk of death (P = .83).
“[W]e know that use of a brachytherapy boost is associated with improved patient outcomes, including improved cancer-specific and overall survival,” Dr. Alimena said. “We also know that African-American women have one of the highest incidences of cervical cancer in the United States and also have worse mortality from cervical cancer.”
“Studies have reached varying conclusions about the impact of race on brachytherapy utilization, with several smaller studies suggesting that minority women may be less likely to receive brachytherapy services compared to white women. No studies have specifically examined the interaction between race, radiation, and survival.”
Dr. Alimena and her colleagues decided to examine the interaction using data from the National Cancer Database. The researchers evaluated 15,411 women diagnosed with cervical cancer from 2004 to 2014. The patients had stage IB2 to IVA disease, their mean age was 54 years (range, 19-90), 58% had received brachytherapy, and 19% were black.
“Race was defined as black or non-black race, given that previous data had shown similar and even increased survival rates for Hispanic and Asian-American women compared to white patients diagnosed with cervical cancer,” Dr. Alimena noted.
Differences by race
The researchers found that black patients were significantly less likely to receive brachytherapy than were non-black patients: 52.5% vs. 59.0%, respectively (P less than .001).
Black patients were significantly more likely to have stage III disease (42.7% vs. 37.6%; P less than .001) and less likely to have stage IVA disease (6.8% vs. 7.3%; P less than .001).
Black patients were significantly more likely to have government insurance (57.0% vs. 49.1%; P less than .001) and less likely to have private insurance (27.6% vs. 36.7%; P less than .001).
And black patients were significantly more likely to have annual incomes below $38,000 (49.4% vs. 22.6%; P less than .001).
Factors associated with brachytherapy
In a multivariate analysis, black race was significantly associated with a reduced likelihood of receiving brachytherapy. The odds ratio (OR) was 0.86 (P = .003).
Other factors significantly associated with a reduced likelihood of receiving brachytherapy were:
- Being older than 70 years (OR = 0.59; P less than .001)
- Having government insurance (OR = 0.89; P = .008) or no insurance/unknown insurance status (OR = 0.75; P less than .001)
- Having stage III disease (OR = 0.47; P less than .001) or stage IVA disease (OR = 0.20; P less than .001)
- Being treated in southern states (OR = 0.67; P less than .001) or western states (OR = 0.86; P = .02)
- Having a Charlson/Deyo score of 2 or more (OR = 0.73; P less than .001).
Race, brachytherapy, and survival
“Consistent with prior data, we found that black patients had a significant decrease in overall survival, compared to non-black women,” Dr. Alimena said. “Furthermore, we found survival differences by race were mediated by brachytherapy use.”
The median overall survival was 52.5 months among black patients and 65.3 months among non-black patients (P less than .001).
Among patients who did not receive brachytherapy, black patients had a significantly higher risk of death than non-black patients (adjusted hazard ratio = 1.11; P = .013).
However, among patients who did receive brachytherapy, black and non-black patients had a similar risk of death (adjusted hazard ratio = 1.01; P = .83). The interaction term comparing these survival curves was statistically significant (P = .043).
“This is the first study, to our knowledge, to show such an interaction between race and survival being mediated by one particular treatment modality,” Dr. Alimena said.
“While not directly tested in this study, the most likely hypothesis why black patients may be less likely to receive brachytherapy is having poor access to brachytherapy services. This suggests that reducing racial disparities in survival is possible by increasing access to brachytherapy for black patients.”
Dr. Alimena had no financial disclosures.
SOURCE: Alimena S et al. SGO 2019. Abstract 11.
HONOLULU – , according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Stephanie Alimena, MD, of Brigham and Women’s Hospital in Boston, presented a large, retrospective study showing that use of brachytherapy mediated survival differences by race.
In the absence of brachytherapy, black patients had a significantly higher risk of death than did non-black patients (P = .013). However, when brachytherapy was used, black and non-black patients had a similar risk of death (P = .83).
“[W]e know that use of a brachytherapy boost is associated with improved patient outcomes, including improved cancer-specific and overall survival,” Dr. Alimena said. “We also know that African-American women have one of the highest incidences of cervical cancer in the United States and also have worse mortality from cervical cancer.”
“Studies have reached varying conclusions about the impact of race on brachytherapy utilization, with several smaller studies suggesting that minority women may be less likely to receive brachytherapy services compared to white women. No studies have specifically examined the interaction between race, radiation, and survival.”
Dr. Alimena and her colleagues decided to examine the interaction using data from the National Cancer Database. The researchers evaluated 15,411 women diagnosed with cervical cancer from 2004 to 2014. The patients had stage IB2 to IVA disease, their mean age was 54 years (range, 19-90), 58% had received brachytherapy, and 19% were black.
“Race was defined as black or non-black race, given that previous data had shown similar and even increased survival rates for Hispanic and Asian-American women compared to white patients diagnosed with cervical cancer,” Dr. Alimena noted.
Differences by race
The researchers found that black patients were significantly less likely to receive brachytherapy than were non-black patients: 52.5% vs. 59.0%, respectively (P less than .001).
Black patients were significantly more likely to have stage III disease (42.7% vs. 37.6%; P less than .001) and less likely to have stage IVA disease (6.8% vs. 7.3%; P less than .001).
Black patients were significantly more likely to have government insurance (57.0% vs. 49.1%; P less than .001) and less likely to have private insurance (27.6% vs. 36.7%; P less than .001).
And black patients were significantly more likely to have annual incomes below $38,000 (49.4% vs. 22.6%; P less than .001).
Factors associated with brachytherapy
In a multivariate analysis, black race was significantly associated with a reduced likelihood of receiving brachytherapy. The odds ratio (OR) was 0.86 (P = .003).
Other factors significantly associated with a reduced likelihood of receiving brachytherapy were:
- Being older than 70 years (OR = 0.59; P less than .001)
- Having government insurance (OR = 0.89; P = .008) or no insurance/unknown insurance status (OR = 0.75; P less than .001)
- Having stage III disease (OR = 0.47; P less than .001) or stage IVA disease (OR = 0.20; P less than .001)
- Being treated in southern states (OR = 0.67; P less than .001) or western states (OR = 0.86; P = .02)
- Having a Charlson/Deyo score of 2 or more (OR = 0.73; P less than .001).
Race, brachytherapy, and survival
“Consistent with prior data, we found that black patients had a significant decrease in overall survival, compared to non-black women,” Dr. Alimena said. “Furthermore, we found survival differences by race were mediated by brachytherapy use.”
The median overall survival was 52.5 months among black patients and 65.3 months among non-black patients (P less than .001).
Among patients who did not receive brachytherapy, black patients had a significantly higher risk of death than non-black patients (adjusted hazard ratio = 1.11; P = .013).
However, among patients who did receive brachytherapy, black and non-black patients had a similar risk of death (adjusted hazard ratio = 1.01; P = .83). The interaction term comparing these survival curves was statistically significant (P = .043).
“This is the first study, to our knowledge, to show such an interaction between race and survival being mediated by one particular treatment modality,” Dr. Alimena said.
“While not directly tested in this study, the most likely hypothesis why black patients may be less likely to receive brachytherapy is having poor access to brachytherapy services. This suggests that reducing racial disparities in survival is possible by increasing access to brachytherapy for black patients.”
Dr. Alimena had no financial disclosures.
SOURCE: Alimena S et al. SGO 2019. Abstract 11.
HONOLULU – , according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Stephanie Alimena, MD, of Brigham and Women’s Hospital in Boston, presented a large, retrospective study showing that use of brachytherapy mediated survival differences by race.
In the absence of brachytherapy, black patients had a significantly higher risk of death than did non-black patients (P = .013). However, when brachytherapy was used, black and non-black patients had a similar risk of death (P = .83).
“[W]e know that use of a brachytherapy boost is associated with improved patient outcomes, including improved cancer-specific and overall survival,” Dr. Alimena said. “We also know that African-American women have one of the highest incidences of cervical cancer in the United States and also have worse mortality from cervical cancer.”
“Studies have reached varying conclusions about the impact of race on brachytherapy utilization, with several smaller studies suggesting that minority women may be less likely to receive brachytherapy services compared to white women. No studies have specifically examined the interaction between race, radiation, and survival.”
Dr. Alimena and her colleagues decided to examine the interaction using data from the National Cancer Database. The researchers evaluated 15,411 women diagnosed with cervical cancer from 2004 to 2014. The patients had stage IB2 to IVA disease, their mean age was 54 years (range, 19-90), 58% had received brachytherapy, and 19% were black.
“Race was defined as black or non-black race, given that previous data had shown similar and even increased survival rates for Hispanic and Asian-American women compared to white patients diagnosed with cervical cancer,” Dr. Alimena noted.
Differences by race
The researchers found that black patients were significantly less likely to receive brachytherapy than were non-black patients: 52.5% vs. 59.0%, respectively (P less than .001).
Black patients were significantly more likely to have stage III disease (42.7% vs. 37.6%; P less than .001) and less likely to have stage IVA disease (6.8% vs. 7.3%; P less than .001).
Black patients were significantly more likely to have government insurance (57.0% vs. 49.1%; P less than .001) and less likely to have private insurance (27.6% vs. 36.7%; P less than .001).
And black patients were significantly more likely to have annual incomes below $38,000 (49.4% vs. 22.6%; P less than .001).
Factors associated with brachytherapy
In a multivariate analysis, black race was significantly associated with a reduced likelihood of receiving brachytherapy. The odds ratio (OR) was 0.86 (P = .003).
Other factors significantly associated with a reduced likelihood of receiving brachytherapy were:
- Being older than 70 years (OR = 0.59; P less than .001)
- Having government insurance (OR = 0.89; P = .008) or no insurance/unknown insurance status (OR = 0.75; P less than .001)
- Having stage III disease (OR = 0.47; P less than .001) or stage IVA disease (OR = 0.20; P less than .001)
- Being treated in southern states (OR = 0.67; P less than .001) or western states (OR = 0.86; P = .02)
- Having a Charlson/Deyo score of 2 or more (OR = 0.73; P less than .001).
Race, brachytherapy, and survival
“Consistent with prior data, we found that black patients had a significant decrease in overall survival, compared to non-black women,” Dr. Alimena said. “Furthermore, we found survival differences by race were mediated by brachytherapy use.”
The median overall survival was 52.5 months among black patients and 65.3 months among non-black patients (P less than .001).
Among patients who did not receive brachytherapy, black patients had a significantly higher risk of death than non-black patients (adjusted hazard ratio = 1.11; P = .013).
However, among patients who did receive brachytherapy, black and non-black patients had a similar risk of death (adjusted hazard ratio = 1.01; P = .83). The interaction term comparing these survival curves was statistically significant (P = .043).
“This is the first study, to our knowledge, to show such an interaction between race and survival being mediated by one particular treatment modality,” Dr. Alimena said.
“While not directly tested in this study, the most likely hypothesis why black patients may be less likely to receive brachytherapy is having poor access to brachytherapy services. This suggests that reducing racial disparities in survival is possible by increasing access to brachytherapy for black patients.”
Dr. Alimena had no financial disclosures.
SOURCE: Alimena S et al. SGO 2019. Abstract 11.
REPORTING FROM SGO 2019
Study explores third-line trabectedin plus PLD for BRCA1/2 ovarian cancer
HONOLULU – Adding trabectedin to pegylated liposomal doxorubicin (PLD) significantly prolongs overall and progression-free survival in BRCA1/2-mutated patients with advanced-relapsed epithelial ovarian cancer after two prior lines of platinum-based therapy, according to prespecified subgroup analyses of a randomized, open-label, phase 3 trial.
Patients with a platinum-free interval (PFI) of 6-12 months also experienced significantly improved progression-free survival (PFS), and those with both a BRCA mutation and PFI of 6-12 months experienced significantly improved overall survival (OS) and PFS with combined trabectedin and PLD vs. PLD alone, Bradley J. Monk, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Of 576 patients enrolled in the ET743-OVC-3006 trial, 289 received the combination regimen, and 287 received PLD alone. The study completed enrollment but was discontinued on January 18, 2018, after an interim analysis showed the futility threshold for OS and observed toxicity in the combination therapy group was exceeded.
OS was 21.5 and 22.2 months (hazard ratio = 1.13) in the combination and monotherapy groups, respectively. However, the subgroups analysis of patients with a germline BRCA1/2 mutation showed a median OS of 34.2 months with combination therapy and 20.9 months with PLD monotherapy, for a median survival benefit with combination therapy of 13.3 months (HR = 0.54), said Dr. Monk, professor and director of the division of gynecologic oncology at Creighton University School of Medicine, St. Joseph’s Hospital and Medical Center, Phoenix, Ariz.
A subgroup of 60 patients with both a BRCA mutation and a PFI of 6-12 months had even greater improvement in OS with combination therapy when compared with PLD monotherapy (31.5 vs. 14.9 months; HR = 0.37). An effect on PFS was also detected in this subgroup (10.1 vs. 6.1 months; HR = 0.48), he said.
No difference in PFS was found in the overall unselected study population, Dr. Monk said, but among all patients with 6-12-month PFI alone, combination therapy led to significantly longer PFS than with monotherapy (7.5 vs. 5.5 months; HR = 0.72, P = .0388) and a positive trend in OS for the combination group (24.8 vs. 17.4 months; HR = 0.69; P = .565).
“I get it – this suffers from multiple comparisons, and this [analysis] in particular was ad hoc,” he said.
Still, given historical findings with respect to trabectedin in combination with PLD, the findings are provocative, he said.
Trabectedin is “a complicated anticancer cytotoxic medication” approved by the Food and Drug Administration in 2015 based on findings of treatment benefit in liposarcoma and leiomyosarcoma (including uterine leiomyosarcoma), he explained.
The agent has also been studied widely in other tumors, including in ovarian cancer. In the OVA-301 trial, for example, Dr. Monk and his colleagues looked at trabectedin in the second-line setting in patients with recurrent ovarian cancer.
That trial, which met its primary endpoint of an overall PFS benefit with trabectedin and generated the hypothesis that the agent might have particular benefit in BRCA-mutated patients, led to regulatory approval of trabectedin in Europe and elsewhere as an option for treatment in patients with partially platinum-sensitive recurrences – but not in the United States, he said.
Since OVA-301 did not lead to FDA approval, he and his colleagues designed the current study to look more closely at trabectedin with and without PLD, using the same dosing regimens as in OVA-301, but in the third-line setting and using OS as the primary endpoint.
Based on the intriguing OVA-301 findings in BRCA-mutated patients, the BRCA1/2-mutated subgroup analysis in the ET743-OVC-3006 trial was prespecified, he noted.
Study participants were women with advanced-relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer who responded to two prior lines of platinum-based therapy and who were at least 6 months platinum-free. They were randomly assigned 1:1 to trabectedin plus PLD or PLD alone. Dosing in the combination arm was 1.1 mg/m2 of intravenous trabectedin over 3 hours and 30 mg/m2 of IV PLD given over 90 minutes every 3 weeks, and in the monotherapy arm PLD was given at a dose of 50 mg/m2 IV over 90 minutes every 4 weeks.
The groups were well balanced with respect to age, race, and performance status, and each included “a smattering of histological subtypes,” he said.
Of the 576 enrolled, 155 were BRCA1 or BRCA2-positive, with about two-thirds carrying a BRCA1 mutation.
Very few were exposed to bevacizumab or poly(ADP ribose) polymerase (PARP) inhibitors, as the study was initiated before those were developed.
Adverse events (AEs) of all grades occurred at similar rates in the two arms and occurred in most patients. Most were drug-related, but grade 3-4 AE rates were higher in the combination arm (79% vs. 54%). Drug-related AEs leading to treatment discontinuation occurred in 32.5% and 16% of the combination and monotherapy arm patients, respectively, Dr. Monk said.
“Most of those treatment-related AEs were cytopenia and/or transaminitis. There were also almost twice as many deaths in the combination arm: 3.5% vs. 1.6%,” he said, adding that the deaths were thought to be related to tumor progression rather than to the drugs.
Although the addition of trabectedin to PLD did not prolong OS, compared with PLD alone in unselected patients with advanced, third-line, recurrent ovarian cancer, the combination does appear to benefit patients with germline BRCA mutations, a 6-12 month platinum-free interval, or both, he said, concluding that “the results of this phase 3 subanalysis are consistent with the previous observations in OVA-301 that trabectedin alone or with PLD displays selective antitumor activity in this BRCA subgroup.
“It adds more toxicity, but no new safety signals were identified,” he added.
Asked during a panel discussion if he thinks these results mark “then end of the road” for trabectedin, Dr. Monk said he does not.
“I don’t think we should be penalized because we enrolled all those non-BRCA patients. When you look at the BRCA cohort I think there is an opportunity to get this to our patients,” he said, noting that “the major challenge” is that those patients had not been given a PARP inhibitor. “So I think that there might be an opportunity for licensing, but the confirmatory trial would have to [look at whether] the drug still works after failure of a PARP.”
The ET743-OVC-3006 trial was sponsored by Janssen Research & Development. Dr. Monk reported financial relationships (consulting, honoraria/reimbursement, and/or speaker’s bureau participation) with Janssen/Johnson & Johnson and more than 20 other pharmaceutical companies.
SOURCE: Monk B et al., SGO 2019: Abstract 20.
HONOLULU – Adding trabectedin to pegylated liposomal doxorubicin (PLD) significantly prolongs overall and progression-free survival in BRCA1/2-mutated patients with advanced-relapsed epithelial ovarian cancer after two prior lines of platinum-based therapy, according to prespecified subgroup analyses of a randomized, open-label, phase 3 trial.
Patients with a platinum-free interval (PFI) of 6-12 months also experienced significantly improved progression-free survival (PFS), and those with both a BRCA mutation and PFI of 6-12 months experienced significantly improved overall survival (OS) and PFS with combined trabectedin and PLD vs. PLD alone, Bradley J. Monk, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Of 576 patients enrolled in the ET743-OVC-3006 trial, 289 received the combination regimen, and 287 received PLD alone. The study completed enrollment but was discontinued on January 18, 2018, after an interim analysis showed the futility threshold for OS and observed toxicity in the combination therapy group was exceeded.
OS was 21.5 and 22.2 months (hazard ratio = 1.13) in the combination and monotherapy groups, respectively. However, the subgroups analysis of patients with a germline BRCA1/2 mutation showed a median OS of 34.2 months with combination therapy and 20.9 months with PLD monotherapy, for a median survival benefit with combination therapy of 13.3 months (HR = 0.54), said Dr. Monk, professor and director of the division of gynecologic oncology at Creighton University School of Medicine, St. Joseph’s Hospital and Medical Center, Phoenix, Ariz.
A subgroup of 60 patients with both a BRCA mutation and a PFI of 6-12 months had even greater improvement in OS with combination therapy when compared with PLD monotherapy (31.5 vs. 14.9 months; HR = 0.37). An effect on PFS was also detected in this subgroup (10.1 vs. 6.1 months; HR = 0.48), he said.
No difference in PFS was found in the overall unselected study population, Dr. Monk said, but among all patients with 6-12-month PFI alone, combination therapy led to significantly longer PFS than with monotherapy (7.5 vs. 5.5 months; HR = 0.72, P = .0388) and a positive trend in OS for the combination group (24.8 vs. 17.4 months; HR = 0.69; P = .565).
“I get it – this suffers from multiple comparisons, and this [analysis] in particular was ad hoc,” he said.
Still, given historical findings with respect to trabectedin in combination with PLD, the findings are provocative, he said.
Trabectedin is “a complicated anticancer cytotoxic medication” approved by the Food and Drug Administration in 2015 based on findings of treatment benefit in liposarcoma and leiomyosarcoma (including uterine leiomyosarcoma), he explained.
The agent has also been studied widely in other tumors, including in ovarian cancer. In the OVA-301 trial, for example, Dr. Monk and his colleagues looked at trabectedin in the second-line setting in patients with recurrent ovarian cancer.
That trial, which met its primary endpoint of an overall PFS benefit with trabectedin and generated the hypothesis that the agent might have particular benefit in BRCA-mutated patients, led to regulatory approval of trabectedin in Europe and elsewhere as an option for treatment in patients with partially platinum-sensitive recurrences – but not in the United States, he said.
Since OVA-301 did not lead to FDA approval, he and his colleagues designed the current study to look more closely at trabectedin with and without PLD, using the same dosing regimens as in OVA-301, but in the third-line setting and using OS as the primary endpoint.
Based on the intriguing OVA-301 findings in BRCA-mutated patients, the BRCA1/2-mutated subgroup analysis in the ET743-OVC-3006 trial was prespecified, he noted.
Study participants were women with advanced-relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer who responded to two prior lines of platinum-based therapy and who were at least 6 months platinum-free. They were randomly assigned 1:1 to trabectedin plus PLD or PLD alone. Dosing in the combination arm was 1.1 mg/m2 of intravenous trabectedin over 3 hours and 30 mg/m2 of IV PLD given over 90 minutes every 3 weeks, and in the monotherapy arm PLD was given at a dose of 50 mg/m2 IV over 90 minutes every 4 weeks.
The groups were well balanced with respect to age, race, and performance status, and each included “a smattering of histological subtypes,” he said.
Of the 576 enrolled, 155 were BRCA1 or BRCA2-positive, with about two-thirds carrying a BRCA1 mutation.
Very few were exposed to bevacizumab or poly(ADP ribose) polymerase (PARP) inhibitors, as the study was initiated before those were developed.
Adverse events (AEs) of all grades occurred at similar rates in the two arms and occurred in most patients. Most were drug-related, but grade 3-4 AE rates were higher in the combination arm (79% vs. 54%). Drug-related AEs leading to treatment discontinuation occurred in 32.5% and 16% of the combination and monotherapy arm patients, respectively, Dr. Monk said.
“Most of those treatment-related AEs were cytopenia and/or transaminitis. There were also almost twice as many deaths in the combination arm: 3.5% vs. 1.6%,” he said, adding that the deaths were thought to be related to tumor progression rather than to the drugs.
Although the addition of trabectedin to PLD did not prolong OS, compared with PLD alone in unselected patients with advanced, third-line, recurrent ovarian cancer, the combination does appear to benefit patients with germline BRCA mutations, a 6-12 month platinum-free interval, or both, he said, concluding that “the results of this phase 3 subanalysis are consistent with the previous observations in OVA-301 that trabectedin alone or with PLD displays selective antitumor activity in this BRCA subgroup.
“It adds more toxicity, but no new safety signals were identified,” he added.
Asked during a panel discussion if he thinks these results mark “then end of the road” for trabectedin, Dr. Monk said he does not.
“I don’t think we should be penalized because we enrolled all those non-BRCA patients. When you look at the BRCA cohort I think there is an opportunity to get this to our patients,” he said, noting that “the major challenge” is that those patients had not been given a PARP inhibitor. “So I think that there might be an opportunity for licensing, but the confirmatory trial would have to [look at whether] the drug still works after failure of a PARP.”
The ET743-OVC-3006 trial was sponsored by Janssen Research & Development. Dr. Monk reported financial relationships (consulting, honoraria/reimbursement, and/or speaker’s bureau participation) with Janssen/Johnson & Johnson and more than 20 other pharmaceutical companies.
SOURCE: Monk B et al., SGO 2019: Abstract 20.
HONOLULU – Adding trabectedin to pegylated liposomal doxorubicin (PLD) significantly prolongs overall and progression-free survival in BRCA1/2-mutated patients with advanced-relapsed epithelial ovarian cancer after two prior lines of platinum-based therapy, according to prespecified subgroup analyses of a randomized, open-label, phase 3 trial.
Patients with a platinum-free interval (PFI) of 6-12 months also experienced significantly improved progression-free survival (PFS), and those with both a BRCA mutation and PFI of 6-12 months experienced significantly improved overall survival (OS) and PFS with combined trabectedin and PLD vs. PLD alone, Bradley J. Monk, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Of 576 patients enrolled in the ET743-OVC-3006 trial, 289 received the combination regimen, and 287 received PLD alone. The study completed enrollment but was discontinued on January 18, 2018, after an interim analysis showed the futility threshold for OS and observed toxicity in the combination therapy group was exceeded.
OS was 21.5 and 22.2 months (hazard ratio = 1.13) in the combination and monotherapy groups, respectively. However, the subgroups analysis of patients with a germline BRCA1/2 mutation showed a median OS of 34.2 months with combination therapy and 20.9 months with PLD monotherapy, for a median survival benefit with combination therapy of 13.3 months (HR = 0.54), said Dr. Monk, professor and director of the division of gynecologic oncology at Creighton University School of Medicine, St. Joseph’s Hospital and Medical Center, Phoenix, Ariz.
A subgroup of 60 patients with both a BRCA mutation and a PFI of 6-12 months had even greater improvement in OS with combination therapy when compared with PLD monotherapy (31.5 vs. 14.9 months; HR = 0.37). An effect on PFS was also detected in this subgroup (10.1 vs. 6.1 months; HR = 0.48), he said.
No difference in PFS was found in the overall unselected study population, Dr. Monk said, but among all patients with 6-12-month PFI alone, combination therapy led to significantly longer PFS than with monotherapy (7.5 vs. 5.5 months; HR = 0.72, P = .0388) and a positive trend in OS for the combination group (24.8 vs. 17.4 months; HR = 0.69; P = .565).
“I get it – this suffers from multiple comparisons, and this [analysis] in particular was ad hoc,” he said.
Still, given historical findings with respect to trabectedin in combination with PLD, the findings are provocative, he said.
Trabectedin is “a complicated anticancer cytotoxic medication” approved by the Food and Drug Administration in 2015 based on findings of treatment benefit in liposarcoma and leiomyosarcoma (including uterine leiomyosarcoma), he explained.
The agent has also been studied widely in other tumors, including in ovarian cancer. In the OVA-301 trial, for example, Dr. Monk and his colleagues looked at trabectedin in the second-line setting in patients with recurrent ovarian cancer.
That trial, which met its primary endpoint of an overall PFS benefit with trabectedin and generated the hypothesis that the agent might have particular benefit in BRCA-mutated patients, led to regulatory approval of trabectedin in Europe and elsewhere as an option for treatment in patients with partially platinum-sensitive recurrences – but not in the United States, he said.
Since OVA-301 did not lead to FDA approval, he and his colleagues designed the current study to look more closely at trabectedin with and without PLD, using the same dosing regimens as in OVA-301, but in the third-line setting and using OS as the primary endpoint.
Based on the intriguing OVA-301 findings in BRCA-mutated patients, the BRCA1/2-mutated subgroup analysis in the ET743-OVC-3006 trial was prespecified, he noted.
Study participants were women with advanced-relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer who responded to two prior lines of platinum-based therapy and who were at least 6 months platinum-free. They were randomly assigned 1:1 to trabectedin plus PLD or PLD alone. Dosing in the combination arm was 1.1 mg/m2 of intravenous trabectedin over 3 hours and 30 mg/m2 of IV PLD given over 90 minutes every 3 weeks, and in the monotherapy arm PLD was given at a dose of 50 mg/m2 IV over 90 minutes every 4 weeks.
The groups were well balanced with respect to age, race, and performance status, and each included “a smattering of histological subtypes,” he said.
Of the 576 enrolled, 155 were BRCA1 or BRCA2-positive, with about two-thirds carrying a BRCA1 mutation.
Very few were exposed to bevacizumab or poly(ADP ribose) polymerase (PARP) inhibitors, as the study was initiated before those were developed.
Adverse events (AEs) of all grades occurred at similar rates in the two arms and occurred in most patients. Most were drug-related, but grade 3-4 AE rates were higher in the combination arm (79% vs. 54%). Drug-related AEs leading to treatment discontinuation occurred in 32.5% and 16% of the combination and monotherapy arm patients, respectively, Dr. Monk said.
“Most of those treatment-related AEs were cytopenia and/or transaminitis. There were also almost twice as many deaths in the combination arm: 3.5% vs. 1.6%,” he said, adding that the deaths were thought to be related to tumor progression rather than to the drugs.
Although the addition of trabectedin to PLD did not prolong OS, compared with PLD alone in unselected patients with advanced, third-line, recurrent ovarian cancer, the combination does appear to benefit patients with germline BRCA mutations, a 6-12 month platinum-free interval, or both, he said, concluding that “the results of this phase 3 subanalysis are consistent with the previous observations in OVA-301 that trabectedin alone or with PLD displays selective antitumor activity in this BRCA subgroup.
“It adds more toxicity, but no new safety signals were identified,” he added.
Asked during a panel discussion if he thinks these results mark “then end of the road” for trabectedin, Dr. Monk said he does not.
“I don’t think we should be penalized because we enrolled all those non-BRCA patients. When you look at the BRCA cohort I think there is an opportunity to get this to our patients,” he said, noting that “the major challenge” is that those patients had not been given a PARP inhibitor. “So I think that there might be an opportunity for licensing, but the confirmatory trial would have to [look at whether] the drug still works after failure of a PARP.”
The ET743-OVC-3006 trial was sponsored by Janssen Research & Development. Dr. Monk reported financial relationships (consulting, honoraria/reimbursement, and/or speaker’s bureau participation) with Janssen/Johnson & Johnson and more than 20 other pharmaceutical companies.
SOURCE: Monk B et al., SGO 2019: Abstract 20.
REPORTING FROM SGO 2019
No birth rate gains from levothyroxine in pregnancy
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
FROM ENDO 2019
Key clinical point:
Major finding: Pregnancy rates and outcomes were similar in women treated with levothyroxine and those treated with placebo.
Study details: Double-blind, randomized, placebo-controlled trial in 952 women.
Disclosures: The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
Source: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
H3N2 putting a damper on flu season’s departure
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.
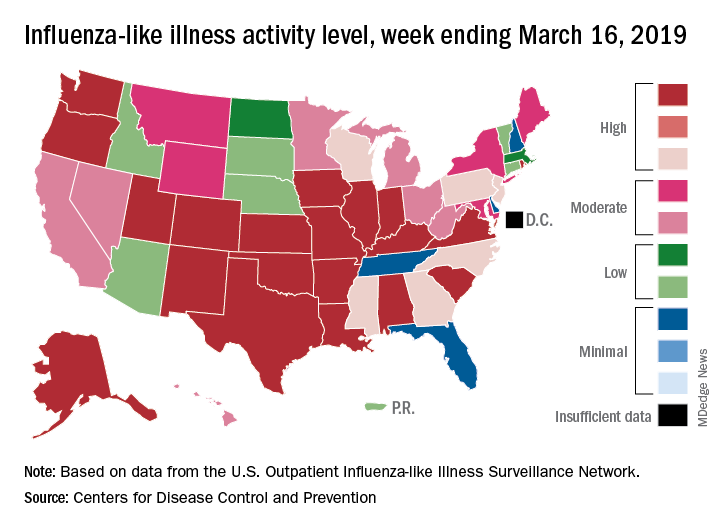
Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.

Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.

Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
Biologics boost work outcomes in axial spondyloarthritis
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
ACC launches new recertification pathway
Cardiologists now have an alternative option to maintain their board certification through a new pathway developed jointly by the American College of Cardiology and the American Board of Internal Medicine.
The new avenue, announced at the ACC’s annual meeting, focuses on assessment of specific study areas over the course of 5 years, rather than a single, broader test every 10 years. The Collaborative Maintenance Pathway (CMP) begins in 2019 with a cardiovascular disease CMP option that includes engagement with the ACC’s Adult Clinical Cardiology Self-Assessment Program as a prerequisite to qualify for a performance assessment later in the year.
The new pathway was developed based on feedback from cardiologists who expressed interest in focusing their study on specific areas, said Timothy W. Attebery, ACC CEO.
“The new CMP leverages the respective expertise of the ACC and ABIM to create a literal ‘pathway’ that meets the ongoing learning needs of cardiologists, while also giving patients, the public, and other stakeholders confidence that the care provided by their physicians is of the highest quality,” Mr. Attebery said in a statement. “We appreciate ABIM working with us on what we believe is a win-win solution for cardiologists and the patients they serve.”
As part of the pathway, new performance assessments will be available annually, with each covering 20% of the field of cardiovascular disease. Ultimately, the breadth of general cardiology will be covered in a span of 5 years, according to a summary of the option. The 2019 performance assessment will focus on arrhythmias, which means physicians planning to enter the CMP option in 2019 can begin studying the arrhythmia section of the education materials now in preparation for the fall 2019 performance assessment. The ACC expects to launch CMPs in clinical cardiac electrophysiology, interventional cardiology and advanced heart failure, and transplant cardiology in 2020. The pathways are being developed in collaboration with the Heart Rhythm Society, the Society for Cardiovascular Angiography and Interventions, and the Heart Failure Society of America.
ABIM’s traditional 10-year maintenance of certification exam and the 2-year knowledge check-in assessment will remain available to diplomates who choose not to participate in the CMP option.
ABIM President Richard J. Baron, MD, said the organization is proud to continue the evolution of its MOC program to better meet the needs of physicians and patients.
“This new offering increases choice, flexibility, and relevance for board-certified cardiologists while also keeping a performance standard that gives patients confidence that their physician possesses the current medical knowledge necessary to deliver high-quality care,” Dr. Baron said in a statement. “We appreciate ACC’s expertise and partnership throughout this journey to co-create an innovative new assessment option for cardiologists.”
A summary of the new pathway and how physicians can apply is provided on the ACC website.
Lawsuit against ABIM continues
Not all cardiologists welcome the change.
The CMP option is nothing different and its development is “one of the most shameful money grabs from U.S. cardiologists and cardiac electrophysiologists imaginable, “ Chicago-based cardiologist Wes Fisher, MD, wrote in his blog.
“More importantly, the ACC leverages (ties) CMP to the threat to a physician’s ABIM board certification status, and therefore their right to work, and I believe represents a restriction of trade and is in violation of U.S. antitrust and racketeering laws,” Dr. Fisher wrote. “It is truly unbelievable that the ACC and the Heart Rhythm Society would do this to their own membership, but then again, given their prolific financial balance sheets, maybe it’s not so unbelievable after all.”
Dr. Fisher declined to comment for this story. He and his fellow physicians with the Practicing Physicians of America (PPA) are funding a lawsuit against ABIM in an effort to invalidate its MOC process.
The legal challenge, filed Dec. 6, 2018, in Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims
In a motion filed March 18, attorneys for ABIM asked a judge to dismiss the suit. The plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust the claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Dr. Baron said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing, More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by PPA to pay for the plaintiffs’ legal costs.
Cardiologists now have an alternative option to maintain their board certification through a new pathway developed jointly by the American College of Cardiology and the American Board of Internal Medicine.
The new avenue, announced at the ACC’s annual meeting, focuses on assessment of specific study areas over the course of 5 years, rather than a single, broader test every 10 years. The Collaborative Maintenance Pathway (CMP) begins in 2019 with a cardiovascular disease CMP option that includes engagement with the ACC’s Adult Clinical Cardiology Self-Assessment Program as a prerequisite to qualify for a performance assessment later in the year.
The new pathway was developed based on feedback from cardiologists who expressed interest in focusing their study on specific areas, said Timothy W. Attebery, ACC CEO.
“The new CMP leverages the respective expertise of the ACC and ABIM to create a literal ‘pathway’ that meets the ongoing learning needs of cardiologists, while also giving patients, the public, and other stakeholders confidence that the care provided by their physicians is of the highest quality,” Mr. Attebery said in a statement. “We appreciate ABIM working with us on what we believe is a win-win solution for cardiologists and the patients they serve.”
As part of the pathway, new performance assessments will be available annually, with each covering 20% of the field of cardiovascular disease. Ultimately, the breadth of general cardiology will be covered in a span of 5 years, according to a summary of the option. The 2019 performance assessment will focus on arrhythmias, which means physicians planning to enter the CMP option in 2019 can begin studying the arrhythmia section of the education materials now in preparation for the fall 2019 performance assessment. The ACC expects to launch CMPs in clinical cardiac electrophysiology, interventional cardiology and advanced heart failure, and transplant cardiology in 2020. The pathways are being developed in collaboration with the Heart Rhythm Society, the Society for Cardiovascular Angiography and Interventions, and the Heart Failure Society of America.
ABIM’s traditional 10-year maintenance of certification exam and the 2-year knowledge check-in assessment will remain available to diplomates who choose not to participate in the CMP option.
ABIM President Richard J. Baron, MD, said the organization is proud to continue the evolution of its MOC program to better meet the needs of physicians and patients.
“This new offering increases choice, flexibility, and relevance for board-certified cardiologists while also keeping a performance standard that gives patients confidence that their physician possesses the current medical knowledge necessary to deliver high-quality care,” Dr. Baron said in a statement. “We appreciate ACC’s expertise and partnership throughout this journey to co-create an innovative new assessment option for cardiologists.”
A summary of the new pathway and how physicians can apply is provided on the ACC website.
Lawsuit against ABIM continues
Not all cardiologists welcome the change.
The CMP option is nothing different and its development is “one of the most shameful money grabs from U.S. cardiologists and cardiac electrophysiologists imaginable, “ Chicago-based cardiologist Wes Fisher, MD, wrote in his blog.
“More importantly, the ACC leverages (ties) CMP to the threat to a physician’s ABIM board certification status, and therefore their right to work, and I believe represents a restriction of trade and is in violation of U.S. antitrust and racketeering laws,” Dr. Fisher wrote. “It is truly unbelievable that the ACC and the Heart Rhythm Society would do this to their own membership, but then again, given their prolific financial balance sheets, maybe it’s not so unbelievable after all.”
Dr. Fisher declined to comment for this story. He and his fellow physicians with the Practicing Physicians of America (PPA) are funding a lawsuit against ABIM in an effort to invalidate its MOC process.
The legal challenge, filed Dec. 6, 2018, in Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims
In a motion filed March 18, attorneys for ABIM asked a judge to dismiss the suit. The plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust the claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Dr. Baron said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing, More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by PPA to pay for the plaintiffs’ legal costs.
Cardiologists now have an alternative option to maintain their board certification through a new pathway developed jointly by the American College of Cardiology and the American Board of Internal Medicine.
The new avenue, announced at the ACC’s annual meeting, focuses on assessment of specific study areas over the course of 5 years, rather than a single, broader test every 10 years. The Collaborative Maintenance Pathway (CMP) begins in 2019 with a cardiovascular disease CMP option that includes engagement with the ACC’s Adult Clinical Cardiology Self-Assessment Program as a prerequisite to qualify for a performance assessment later in the year.
The new pathway was developed based on feedback from cardiologists who expressed interest in focusing their study on specific areas, said Timothy W. Attebery, ACC CEO.
“The new CMP leverages the respective expertise of the ACC and ABIM to create a literal ‘pathway’ that meets the ongoing learning needs of cardiologists, while also giving patients, the public, and other stakeholders confidence that the care provided by their physicians is of the highest quality,” Mr. Attebery said in a statement. “We appreciate ABIM working with us on what we believe is a win-win solution for cardiologists and the patients they serve.”
As part of the pathway, new performance assessments will be available annually, with each covering 20% of the field of cardiovascular disease. Ultimately, the breadth of general cardiology will be covered in a span of 5 years, according to a summary of the option. The 2019 performance assessment will focus on arrhythmias, which means physicians planning to enter the CMP option in 2019 can begin studying the arrhythmia section of the education materials now in preparation for the fall 2019 performance assessment. The ACC expects to launch CMPs in clinical cardiac electrophysiology, interventional cardiology and advanced heart failure, and transplant cardiology in 2020. The pathways are being developed in collaboration with the Heart Rhythm Society, the Society for Cardiovascular Angiography and Interventions, and the Heart Failure Society of America.
ABIM’s traditional 10-year maintenance of certification exam and the 2-year knowledge check-in assessment will remain available to diplomates who choose not to participate in the CMP option.
ABIM President Richard J. Baron, MD, said the organization is proud to continue the evolution of its MOC program to better meet the needs of physicians and patients.
“This new offering increases choice, flexibility, and relevance for board-certified cardiologists while also keeping a performance standard that gives patients confidence that their physician possesses the current medical knowledge necessary to deliver high-quality care,” Dr. Baron said in a statement. “We appreciate ACC’s expertise and partnership throughout this journey to co-create an innovative new assessment option for cardiologists.”
A summary of the new pathway and how physicians can apply is provided on the ACC website.
Lawsuit against ABIM continues
Not all cardiologists welcome the change.
The CMP option is nothing different and its development is “one of the most shameful money grabs from U.S. cardiologists and cardiac electrophysiologists imaginable, “ Chicago-based cardiologist Wes Fisher, MD, wrote in his blog.
“More importantly, the ACC leverages (ties) CMP to the threat to a physician’s ABIM board certification status, and therefore their right to work, and I believe represents a restriction of trade and is in violation of U.S. antitrust and racketeering laws,” Dr. Fisher wrote. “It is truly unbelievable that the ACC and the Heart Rhythm Society would do this to their own membership, but then again, given their prolific financial balance sheets, maybe it’s not so unbelievable after all.”
Dr. Fisher declined to comment for this story. He and his fellow physicians with the Practicing Physicians of America (PPA) are funding a lawsuit against ABIM in an effort to invalidate its MOC process.
The legal challenge, filed Dec. 6, 2018, in Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims
In a motion filed March 18, attorneys for ABIM asked a judge to dismiss the suit. The plaintiffs fail to prove that board certification – initial certification and continuing certification – are two separate products that ABIM is unlawfully tying, and for that reason, their antitrust the claims are invalid, according to the motion.
“Plaintiffs may disagree with ABIM and members of the medical community on whether ABIM certification provides them value, but their claims have no basis in the law,” Dr. Baron said in a statement. “With advances in medical science and technology occurring constantly, periodic assessments are critical to ensure internists are staying current and continuing to meet high performance standards in their field.”
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing, More than $200,000 has been raised by doctors and their supporters nationwide through a GoFundMe campaign launched by PPA to pay for the plaintiffs’ legal costs.
FDA approves solriamfetol for daytime sleepiness treatment
The. It is the first dopamine and norepinephrine reuptake inhibitor approved to treat those conditions.
Approval was based on results from TONES (Treatment of Obstructive Sleep Apnea and Narcolepsy Excessive Sleepiness), a phase 3 study that combined four randomized, placebo-controlled trials assessing solriamfetol at various doses, compared with a placebo. After 12 weeks, 68%-74% of patients taking solriamfetol at 75 mg and 78%-90% of those taking solriamfetol at 150 mg reported improvement as assessed by the Patient Global Impression of Change scale.
Solriamfetol is approved at 75 mg and 150 mg for patients with narcolepsy and at 37.5 mg, 75 mg, and 150 mg for patients with obstructive sleep apnea. The most common adverse events associated with solriamfetol are headache, nausea, decreased appetite, and anxiety.
“Excessive daytime sleepiness can negatively impact the daily lives of people living with narcolepsy or obstructive sleep apnea at work, at home, or in daily activities. With this approval, a new, daytime medicine that can provide sustained wakefulness throughout the day will be available for patients,” Bruce Cozadd, chairman and CEO of Jazz Pharmaceuticals, said in the press release.
Find the full press release on the Jazz Pharmaceuticals website.
The. It is the first dopamine and norepinephrine reuptake inhibitor approved to treat those conditions.
Approval was based on results from TONES (Treatment of Obstructive Sleep Apnea and Narcolepsy Excessive Sleepiness), a phase 3 study that combined four randomized, placebo-controlled trials assessing solriamfetol at various doses, compared with a placebo. After 12 weeks, 68%-74% of patients taking solriamfetol at 75 mg and 78%-90% of those taking solriamfetol at 150 mg reported improvement as assessed by the Patient Global Impression of Change scale.
Solriamfetol is approved at 75 mg and 150 mg for patients with narcolepsy and at 37.5 mg, 75 mg, and 150 mg for patients with obstructive sleep apnea. The most common adverse events associated with solriamfetol are headache, nausea, decreased appetite, and anxiety.
“Excessive daytime sleepiness can negatively impact the daily lives of people living with narcolepsy or obstructive sleep apnea at work, at home, or in daily activities. With this approval, a new, daytime medicine that can provide sustained wakefulness throughout the day will be available for patients,” Bruce Cozadd, chairman and CEO of Jazz Pharmaceuticals, said in the press release.
Find the full press release on the Jazz Pharmaceuticals website.
The. It is the first dopamine and norepinephrine reuptake inhibitor approved to treat those conditions.
Approval was based on results from TONES (Treatment of Obstructive Sleep Apnea and Narcolepsy Excessive Sleepiness), a phase 3 study that combined four randomized, placebo-controlled trials assessing solriamfetol at various doses, compared with a placebo. After 12 weeks, 68%-74% of patients taking solriamfetol at 75 mg and 78%-90% of those taking solriamfetol at 150 mg reported improvement as assessed by the Patient Global Impression of Change scale.
Solriamfetol is approved at 75 mg and 150 mg for patients with narcolepsy and at 37.5 mg, 75 mg, and 150 mg for patients with obstructive sleep apnea. The most common adverse events associated with solriamfetol are headache, nausea, decreased appetite, and anxiety.
“Excessive daytime sleepiness can negatively impact the daily lives of people living with narcolepsy or obstructive sleep apnea at work, at home, or in daily activities. With this approval, a new, daytime medicine that can provide sustained wakefulness throughout the day will be available for patients,” Bruce Cozadd, chairman and CEO of Jazz Pharmaceuticals, said in the press release.
Find the full press release on the Jazz Pharmaceuticals website.
Special Programming for Trainees at VAM set
This year’s Vascular Annual Meeting on June 12-15 will introduce a dedicated program for vascular trainees. The Vascular Residents and Fellows Program will help these trainees make the transition from training to practice, and to explore leadership development. Programming is set for 2 to 6 p.m. Thursday, June 13, with a reception from 6 to 7 p.m. and dinner and keynote speaker from 7 to 9:30 p.m. Attendance is open to all vascular residents and fellows currently enrolled in vascular fellowship or 0+5 residency programs. Be sure to contact education@vascularsociety.org for more information
This year’s Vascular Annual Meeting on June 12-15 will introduce a dedicated program for vascular trainees. The Vascular Residents and Fellows Program will help these trainees make the transition from training to practice, and to explore leadership development. Programming is set for 2 to 6 p.m. Thursday, June 13, with a reception from 6 to 7 p.m. and dinner and keynote speaker from 7 to 9:30 p.m. Attendance is open to all vascular residents and fellows currently enrolled in vascular fellowship or 0+5 residency programs. Be sure to contact education@vascularsociety.org for more information
This year’s Vascular Annual Meeting on June 12-15 will introduce a dedicated program for vascular trainees. The Vascular Residents and Fellows Program will help these trainees make the transition from training to practice, and to explore leadership development. Programming is set for 2 to 6 p.m. Thursday, June 13, with a reception from 6 to 7 p.m. and dinner and keynote speaker from 7 to 9:30 p.m. Attendance is open to all vascular residents and fellows currently enrolled in vascular fellowship or 0+5 residency programs. Be sure to contact education@vascularsociety.org for more information
The past and future of hospital medicine
Challenges faced, and overcome
While I hope I’ll still be doing much the same work for many more years, I’m clearly at a stage of life that most of my career is behind me. So I guess it’s natural that I think about the past a little more than I used to. And one of the things that makes me smile is how I’m like George Costanza in The Comeback episode of “Seinfeld.”
In 1997 I had just delivered a presentation about what the future might hold for hospitalists to the roughly 110 attendees at the first in-person meeting of SHM (then known as the National Association of Inpatient Physicians). During the Q&A that followed, someone asked me what clinical content I would include in the hospitalist-specific test or board exam that I had speculated might be in our future. I took his tone and body language to suggest his main intent was to convey that I was crazy to think that such a test might ever be worthwhile.
A pregnant silence followed his question, after which I gave a tentative response that I worried made me sound dumb. So like George in “Seinfeld,” I continued to think about this, and days later came up with what I’m sure would have been a terrific comeback that would have gotten a robust laugh from the audience without being demeaning to the questioner. For the last 22 years I’ve been waiting for someone to ask me the same question so I can finally deliver my winner of a response.
There have been other missed opportunities, but when I think about the past and future of our field and our Society, I’m reminded of many past accomplishments and a promising future.
When Dr. Win Whitcomb and I founded SHM, I had the idea that, among its most important roles, would be serving as a forum for exchange of ideas among hospitalists and providing robust practice management resources for hospitalist groups. Through the efforts of so many people, including Angela Musial, the first SHM staff person, and so many other staff and members, we now have dozens of active special interest groups, informative publications, an active online discussion forum, and blogs. And our annual conference has grown a lot from that first meeting of 110 people; HM19 will bring together nearly 5,000 of us to educate, inspire, and support one another. Collectively, there are a lot of ideas being exchanged through SHM.
When SHM was brand new I had hope that it would grow. But I never guessed that hospital medicine would become the fastest-growing field in the history of U.S. health care.
I also never guessed that the term “nocturnist” would become a standard part of our field’s lexicon. I used it solely as a reliable way to get a laugh and find it really funny and delightful that it caught on.
And OB hospitalists? Neurohospitalists? I never saw these and the many other variations coming at all. But I see it as validating an idea first adopted by medicine and pediatrics. But dermatology hospitalists? Yep, that’s a thing too. The hospitalist model has been adopted, in at least a few places, by nearly every specialty in medicine.
And it is terrific that March 7, 2019, is the first National Hospitalist Day. SHM made this happen too.
I also think about the future of our field and see some pretty big challenges, though our past success as a field makes me confident we’ll navigate them effectively.
The burden of administrative, regulatory, and EHR-related tasks just keeps growing for hospitalists. This often means it is difficult or impossible see as many patients in a day as might have been reasonable in the past. In the near term, the only solution might be to reduce patient loads, but that isn’t a sustainable solution in the long term. I’m convinced we need to offload much of the work we do today that isn’t purely clinical, so that a typical hospitalist in the future can see more patients each day without working harder or longer.
I imagine a future in which the typical hospitalist goes home after seeing 20 or more patients in a day and isn’t completely exhausted and stressed, but sees it as a good day at work. I’m not sure exactly how we’ll get there, but it will probably include things like no longer having to devote any time or attention to whether the patient is inpatient or observation status, or whether they have had a qualifying 3-midnight stay so Medicare will cover a skilled nursing facility. I’m excited to see how this will evolve.
Dr. Nelson is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses.
Challenges faced, and overcome
Challenges faced, and overcome
While I hope I’ll still be doing much the same work for many more years, I’m clearly at a stage of life that most of my career is behind me. So I guess it’s natural that I think about the past a little more than I used to. And one of the things that makes me smile is how I’m like George Costanza in The Comeback episode of “Seinfeld.”
In 1997 I had just delivered a presentation about what the future might hold for hospitalists to the roughly 110 attendees at the first in-person meeting of SHM (then known as the National Association of Inpatient Physicians). During the Q&A that followed, someone asked me what clinical content I would include in the hospitalist-specific test or board exam that I had speculated might be in our future. I took his tone and body language to suggest his main intent was to convey that I was crazy to think that such a test might ever be worthwhile.
A pregnant silence followed his question, after which I gave a tentative response that I worried made me sound dumb. So like George in “Seinfeld,” I continued to think about this, and days later came up with what I’m sure would have been a terrific comeback that would have gotten a robust laugh from the audience without being demeaning to the questioner. For the last 22 years I’ve been waiting for someone to ask me the same question so I can finally deliver my winner of a response.
There have been other missed opportunities, but when I think about the past and future of our field and our Society, I’m reminded of many past accomplishments and a promising future.
When Dr. Win Whitcomb and I founded SHM, I had the idea that, among its most important roles, would be serving as a forum for exchange of ideas among hospitalists and providing robust practice management resources for hospitalist groups. Through the efforts of so many people, including Angela Musial, the first SHM staff person, and so many other staff and members, we now have dozens of active special interest groups, informative publications, an active online discussion forum, and blogs. And our annual conference has grown a lot from that first meeting of 110 people; HM19 will bring together nearly 5,000 of us to educate, inspire, and support one another. Collectively, there are a lot of ideas being exchanged through SHM.
When SHM was brand new I had hope that it would grow. But I never guessed that hospital medicine would become the fastest-growing field in the history of U.S. health care.
I also never guessed that the term “nocturnist” would become a standard part of our field’s lexicon. I used it solely as a reliable way to get a laugh and find it really funny and delightful that it caught on.
And OB hospitalists? Neurohospitalists? I never saw these and the many other variations coming at all. But I see it as validating an idea first adopted by medicine and pediatrics. But dermatology hospitalists? Yep, that’s a thing too. The hospitalist model has been adopted, in at least a few places, by nearly every specialty in medicine.
And it is terrific that March 7, 2019, is the first National Hospitalist Day. SHM made this happen too.
I also think about the future of our field and see some pretty big challenges, though our past success as a field makes me confident we’ll navigate them effectively.
The burden of administrative, regulatory, and EHR-related tasks just keeps growing for hospitalists. This often means it is difficult or impossible see as many patients in a day as might have been reasonable in the past. In the near term, the only solution might be to reduce patient loads, but that isn’t a sustainable solution in the long term. I’m convinced we need to offload much of the work we do today that isn’t purely clinical, so that a typical hospitalist in the future can see more patients each day without working harder or longer.
I imagine a future in which the typical hospitalist goes home after seeing 20 or more patients in a day and isn’t completely exhausted and stressed, but sees it as a good day at work. I’m not sure exactly how we’ll get there, but it will probably include things like no longer having to devote any time or attention to whether the patient is inpatient or observation status, or whether they have had a qualifying 3-midnight stay so Medicare will cover a skilled nursing facility. I’m excited to see how this will evolve.
Dr. Nelson is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses.
While I hope I’ll still be doing much the same work for many more years, I’m clearly at a stage of life that most of my career is behind me. So I guess it’s natural that I think about the past a little more than I used to. And one of the things that makes me smile is how I’m like George Costanza in The Comeback episode of “Seinfeld.”
In 1997 I had just delivered a presentation about what the future might hold for hospitalists to the roughly 110 attendees at the first in-person meeting of SHM (then known as the National Association of Inpatient Physicians). During the Q&A that followed, someone asked me what clinical content I would include in the hospitalist-specific test or board exam that I had speculated might be in our future. I took his tone and body language to suggest his main intent was to convey that I was crazy to think that such a test might ever be worthwhile.
A pregnant silence followed his question, after which I gave a tentative response that I worried made me sound dumb. So like George in “Seinfeld,” I continued to think about this, and days later came up with what I’m sure would have been a terrific comeback that would have gotten a robust laugh from the audience without being demeaning to the questioner. For the last 22 years I’ve been waiting for someone to ask me the same question so I can finally deliver my winner of a response.
There have been other missed opportunities, but when I think about the past and future of our field and our Society, I’m reminded of many past accomplishments and a promising future.
When Dr. Win Whitcomb and I founded SHM, I had the idea that, among its most important roles, would be serving as a forum for exchange of ideas among hospitalists and providing robust practice management resources for hospitalist groups. Through the efforts of so many people, including Angela Musial, the first SHM staff person, and so many other staff and members, we now have dozens of active special interest groups, informative publications, an active online discussion forum, and blogs. And our annual conference has grown a lot from that first meeting of 110 people; HM19 will bring together nearly 5,000 of us to educate, inspire, and support one another. Collectively, there are a lot of ideas being exchanged through SHM.
When SHM was brand new I had hope that it would grow. But I never guessed that hospital medicine would become the fastest-growing field in the history of U.S. health care.
I also never guessed that the term “nocturnist” would become a standard part of our field’s lexicon. I used it solely as a reliable way to get a laugh and find it really funny and delightful that it caught on.
And OB hospitalists? Neurohospitalists? I never saw these and the many other variations coming at all. But I see it as validating an idea first adopted by medicine and pediatrics. But dermatology hospitalists? Yep, that’s a thing too. The hospitalist model has been adopted, in at least a few places, by nearly every specialty in medicine.
And it is terrific that March 7, 2019, is the first National Hospitalist Day. SHM made this happen too.
I also think about the future of our field and see some pretty big challenges, though our past success as a field makes me confident we’ll navigate them effectively.
The burden of administrative, regulatory, and EHR-related tasks just keeps growing for hospitalists. This often means it is difficult or impossible see as many patients in a day as might have been reasonable in the past. In the near term, the only solution might be to reduce patient loads, but that isn’t a sustainable solution in the long term. I’m convinced we need to offload much of the work we do today that isn’t purely clinical, so that a typical hospitalist in the future can see more patients each day without working harder or longer.
I imagine a future in which the typical hospitalist goes home after seeing 20 or more patients in a day and isn’t completely exhausted and stressed, but sees it as a good day at work. I’m not sure exactly how we’ll get there, but it will probably include things like no longer having to devote any time or attention to whether the patient is inpatient or observation status, or whether they have had a qualifying 3-midnight stay so Medicare will cover a skilled nursing facility. I’m excited to see how this will evolve.
Dr. Nelson is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses.
Combo shows promise for endometrial, ovarian cancers
HONOLULU – The combination of lenvatinib and paclitaxel was considered active and well tolerated in a phase 1 trial of patients with recurrent endometrial or ovarian cancer.
Daily lenvatinib plus weekly paclitaxel produced an objective response rate of 65% and a clinical benefit rate of 96%.
Most adverse events (AEs) were grade 1 to 2. The most common grade 3 or higher AEs were hypertension, cytopenias, fatigue, and diarrhea.
Floor J. Backes, MD, of the Ohio State University Comprehensive Cancer Center in Columbus presented these results at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial (NCT02788708) included 26 patients with a median age of 63 years (range, 45-74). All patients had adequate organ function and measurable disease.
Nineteen patients had ovarian cancer: 13 with high-grade serous, 2 with low-grade serous, 2 clear cell, 1 endometrioid, and 1 carcinosarcoma. Seven patients had endometrial cancer: 4 endometrioid and 3 serous.
The patients must have received at least one prior platinum-based therapy. In all, they had a median of 3 prior therapies (range, 1-5).
The patients received intravenous paclitaxel at 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle. They also received oral lenvatinib daily at doses of 8 mg (n = 4), 12 mg (n = 3), 16 mg (n = 13), or 20 mg (n = 6).
There were no dose-limiting toxicities (DLTs) in the 8-mg or 12-mg dose groups. There was one DLT (mucositis) at the 16-mg dose level, and two DLTs (hypertension and fatigue) occurred at the 20-mg dose level.
“[At 20 mg,] patients were unable to take more than 75% of the study drug during the first cycle, and that was considered a DLT,” Dr. Backes said. “So our recommended phase 2 dose was lenvatinib 16 mg daily and paclitaxel 80 mg.”
Safety
“Toxicities were fairly common,” Dr. Backes noted. “The majority of these were cytopenias, but we also saw some GI toxicity – mucositis, diarrhea, nausea – and the VEGF inhibitor–related toxicities that we’re familiar with – hypertension, proteinuria, epistaxis. Fortunately, the majority of toxicities were grade 1 to 2.”
The most common AEs of any grade were leukopenia (58%), anemia (50%), mucositis (46%), diarrhea (46%), lymphopenia (42%), anorexia (42%), hypertension (42%), fatigue (42%), nausea (35%), proteinuria (27%), epistaxis (27%), and hoarseness (27%).
Grade 3 or higher AEs included hypertension (19%), neutropenia (15%), leukopenia (12%), anemia (12%), lymphopenia (8%), fatigue (8%), diarrhea (8%), mucositis (4%), vomiting (4%), hematuria (4%), rash (4%), and thrombocytopenia (4%).
“There was one patient [4%] who had a bowel perforation – small bowel perforation – at the site of previous stenosis after multiple prior bowel surgeries,” Dr. Backes noted.
Efficacy
In the 23 evaluable patients, the objective response rate was 65%, and the clinical benefit rate was 96%. One patient had a complete response, 14 had partial responses, 7 had stable disease, and 1 progressed.
The median duration of response was 10.9 months, and the median progression-free survival was 14.0 months.
The objective response rate was 71% in patients with ovarian cancer and 50% in patients with endometrial cancer. The median progression-free survival in these groups was 14.0 months and 12.8 months, respectively.
Dr. Backes said these results suggest lenvatinib plus paclitaxel is safe and active in recurrent ovarian and endometrial cancer, but these findings must be confirmed in a larger study.
This was an investigator-initiated study supported by Eisai. Dr. Backes disclosed relationships with Eisai, ImmunoGen, Clovis Oncology, Tesaro, Merck, and Agenus.
SOURCE: Backes FJ et al. SGO 2019, Abstract LBA5.
HONOLULU – The combination of lenvatinib and paclitaxel was considered active and well tolerated in a phase 1 trial of patients with recurrent endometrial or ovarian cancer.
Daily lenvatinib plus weekly paclitaxel produced an objective response rate of 65% and a clinical benefit rate of 96%.
Most adverse events (AEs) were grade 1 to 2. The most common grade 3 or higher AEs were hypertension, cytopenias, fatigue, and diarrhea.
Floor J. Backes, MD, of the Ohio State University Comprehensive Cancer Center in Columbus presented these results at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial (NCT02788708) included 26 patients with a median age of 63 years (range, 45-74). All patients had adequate organ function and measurable disease.
Nineteen patients had ovarian cancer: 13 with high-grade serous, 2 with low-grade serous, 2 clear cell, 1 endometrioid, and 1 carcinosarcoma. Seven patients had endometrial cancer: 4 endometrioid and 3 serous.
The patients must have received at least one prior platinum-based therapy. In all, they had a median of 3 prior therapies (range, 1-5).
The patients received intravenous paclitaxel at 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle. They also received oral lenvatinib daily at doses of 8 mg (n = 4), 12 mg (n = 3), 16 mg (n = 13), or 20 mg (n = 6).
There were no dose-limiting toxicities (DLTs) in the 8-mg or 12-mg dose groups. There was one DLT (mucositis) at the 16-mg dose level, and two DLTs (hypertension and fatigue) occurred at the 20-mg dose level.
“[At 20 mg,] patients were unable to take more than 75% of the study drug during the first cycle, and that was considered a DLT,” Dr. Backes said. “So our recommended phase 2 dose was lenvatinib 16 mg daily and paclitaxel 80 mg.”
Safety
“Toxicities were fairly common,” Dr. Backes noted. “The majority of these were cytopenias, but we also saw some GI toxicity – mucositis, diarrhea, nausea – and the VEGF inhibitor–related toxicities that we’re familiar with – hypertension, proteinuria, epistaxis. Fortunately, the majority of toxicities were grade 1 to 2.”
The most common AEs of any grade were leukopenia (58%), anemia (50%), mucositis (46%), diarrhea (46%), lymphopenia (42%), anorexia (42%), hypertension (42%), fatigue (42%), nausea (35%), proteinuria (27%), epistaxis (27%), and hoarseness (27%).
Grade 3 or higher AEs included hypertension (19%), neutropenia (15%), leukopenia (12%), anemia (12%), lymphopenia (8%), fatigue (8%), diarrhea (8%), mucositis (4%), vomiting (4%), hematuria (4%), rash (4%), and thrombocytopenia (4%).
“There was one patient [4%] who had a bowel perforation – small bowel perforation – at the site of previous stenosis after multiple prior bowel surgeries,” Dr. Backes noted.
Efficacy
In the 23 evaluable patients, the objective response rate was 65%, and the clinical benefit rate was 96%. One patient had a complete response, 14 had partial responses, 7 had stable disease, and 1 progressed.
The median duration of response was 10.9 months, and the median progression-free survival was 14.0 months.
The objective response rate was 71% in patients with ovarian cancer and 50% in patients with endometrial cancer. The median progression-free survival in these groups was 14.0 months and 12.8 months, respectively.
Dr. Backes said these results suggest lenvatinib plus paclitaxel is safe and active in recurrent ovarian and endometrial cancer, but these findings must be confirmed in a larger study.
This was an investigator-initiated study supported by Eisai. Dr. Backes disclosed relationships with Eisai, ImmunoGen, Clovis Oncology, Tesaro, Merck, and Agenus.
SOURCE: Backes FJ et al. SGO 2019, Abstract LBA5.
HONOLULU – The combination of lenvatinib and paclitaxel was considered active and well tolerated in a phase 1 trial of patients with recurrent endometrial or ovarian cancer.
Daily lenvatinib plus weekly paclitaxel produced an objective response rate of 65% and a clinical benefit rate of 96%.
Most adverse events (AEs) were grade 1 to 2. The most common grade 3 or higher AEs were hypertension, cytopenias, fatigue, and diarrhea.
Floor J. Backes, MD, of the Ohio State University Comprehensive Cancer Center in Columbus presented these results at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial (NCT02788708) included 26 patients with a median age of 63 years (range, 45-74). All patients had adequate organ function and measurable disease.
Nineteen patients had ovarian cancer: 13 with high-grade serous, 2 with low-grade serous, 2 clear cell, 1 endometrioid, and 1 carcinosarcoma. Seven patients had endometrial cancer: 4 endometrioid and 3 serous.
The patients must have received at least one prior platinum-based therapy. In all, they had a median of 3 prior therapies (range, 1-5).
The patients received intravenous paclitaxel at 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle. They also received oral lenvatinib daily at doses of 8 mg (n = 4), 12 mg (n = 3), 16 mg (n = 13), or 20 mg (n = 6).
There were no dose-limiting toxicities (DLTs) in the 8-mg or 12-mg dose groups. There was one DLT (mucositis) at the 16-mg dose level, and two DLTs (hypertension and fatigue) occurred at the 20-mg dose level.
“[At 20 mg,] patients were unable to take more than 75% of the study drug during the first cycle, and that was considered a DLT,” Dr. Backes said. “So our recommended phase 2 dose was lenvatinib 16 mg daily and paclitaxel 80 mg.”
Safety
“Toxicities were fairly common,” Dr. Backes noted. “The majority of these were cytopenias, but we also saw some GI toxicity – mucositis, diarrhea, nausea – and the VEGF inhibitor–related toxicities that we’re familiar with – hypertension, proteinuria, epistaxis. Fortunately, the majority of toxicities were grade 1 to 2.”
The most common AEs of any grade were leukopenia (58%), anemia (50%), mucositis (46%), diarrhea (46%), lymphopenia (42%), anorexia (42%), hypertension (42%), fatigue (42%), nausea (35%), proteinuria (27%), epistaxis (27%), and hoarseness (27%).
Grade 3 or higher AEs included hypertension (19%), neutropenia (15%), leukopenia (12%), anemia (12%), lymphopenia (8%), fatigue (8%), diarrhea (8%), mucositis (4%), vomiting (4%), hematuria (4%), rash (4%), and thrombocytopenia (4%).
“There was one patient [4%] who had a bowel perforation – small bowel perforation – at the site of previous stenosis after multiple prior bowel surgeries,” Dr. Backes noted.
Efficacy
In the 23 evaluable patients, the objective response rate was 65%, and the clinical benefit rate was 96%. One patient had a complete response, 14 had partial responses, 7 had stable disease, and 1 progressed.
The median duration of response was 10.9 months, and the median progression-free survival was 14.0 months.
The objective response rate was 71% in patients with ovarian cancer and 50% in patients with endometrial cancer. The median progression-free survival in these groups was 14.0 months and 12.8 months, respectively.
Dr. Backes said these results suggest lenvatinib plus paclitaxel is safe and active in recurrent ovarian and endometrial cancer, but these findings must be confirmed in a larger study.
This was an investigator-initiated study supported by Eisai. Dr. Backes disclosed relationships with Eisai, ImmunoGen, Clovis Oncology, Tesaro, Merck, and Agenus.
SOURCE: Backes FJ et al. SGO 2019, Abstract LBA5.
REPORTING FROM SGO 2019













