User login
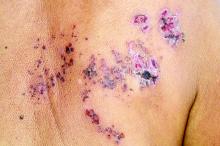
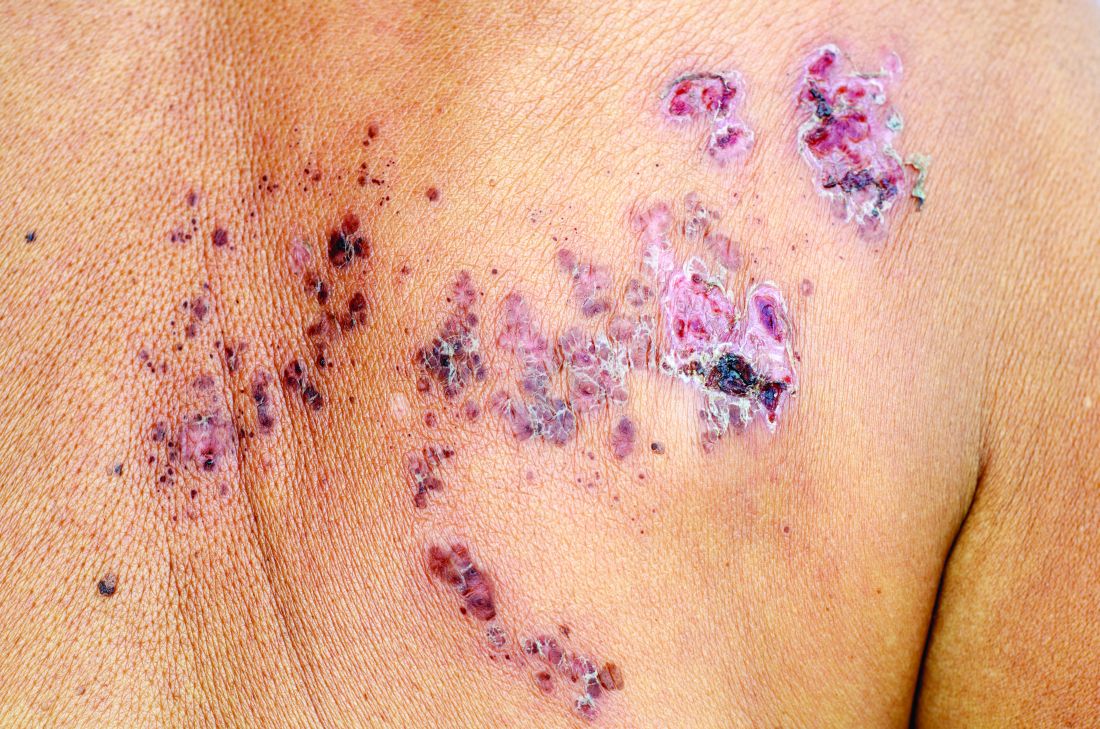
Herpes zoster risk increased with some psoriasis, psoriatic arthritis treatments
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Rituximab boosts survival in primary CNS lymphoma
For patients with primary central nervous system lymphoma (PCNSL), adding rituximab to combination high-dose methotrexate and temozolomide significantly boosted the 5-year overall survival rate, according to a retrospective study.
The triplet combination could be a safe and effective first-line option for patients with PCNSL, particularly the frail and elderly, who may have issues with toxicity when receiving current standard care, reported lead author Cui Chen, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China, and his colleagues.
“An increasing number of studies and meta‐analyses have investigated the effect of rituximab in PCNSL, indicating that rituximab can robustly enhance the response rate and possibly improve survival,” the investigators wrote in Cancer Medicine. “However, data regarding the addition of rituximab to [methotrexate and temozolomide] for PCNSL are limited, and no study has directly compared the efficacy of [rituximab/high-dose methotrexate/temozolomide] to that of [high-dose methotrexate/temozolomide].”
The study involved 62 patients with untreated PCNSL who were diagnosed between 2005 and 2015. Out of the 62 patients, 32 received rituximab/high-dose methotrexate/temozolomide (RMT) and 30 received high-dose methotrexate/temozolomide (MT). Patients received up to eight cycles of therapy, with discontinuation upon disease progression or toxicity.
The results showed that patients treated with RMT had significantly better outcomes than those who received MT, first marked by objective response rates, which were 93.7% for RMT and 69.0% for MT.
Survival rates also showed the advantage of rituximab. For the RMT group, 2-year and 5-year progression-free survival rates were 81.3% and 53.3%, respectively, compared with 46.5% and 29.1% for patients receiving MT.
Most importantly, rituximab boosted overall survival to a significant and notable extent, with higher rates at 2 years (82.3% vs. 65.7%) and 5 years (82.3% vs. 50.0%).
Efficacy did not diminish safety, as no significant differences in toxicity were found between treatment types. The most common grade 3-4 toxicities were hematologic; most commonly, this entailed neutropenia, which occurred in about one-quarter of patients.
“Given its outstanding efficacy and favorable toxicity, we consider RMT to be a feasible and safe therapeutic approach as a first‐line treatment for PCNSL. Moreover, RMT is an ideal regimen for elderly patients and frail populations who may not tolerate [whole‐brain radiation therapy] or [autologous stem‐cell transplantation],” the researchers wrote.
The study was funded by the Natural Science Foundation of Guangdong Province. The researchers reported having no conflicts of interest.
SOURCE: Chen C et al. Cancer Med. 2019 Mar 1. doi: 10.1002/cam4.1906.
For patients with primary central nervous system lymphoma (PCNSL), adding rituximab to combination high-dose methotrexate and temozolomide significantly boosted the 5-year overall survival rate, according to a retrospective study.
The triplet combination could be a safe and effective first-line option for patients with PCNSL, particularly the frail and elderly, who may have issues with toxicity when receiving current standard care, reported lead author Cui Chen, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China, and his colleagues.
“An increasing number of studies and meta‐analyses have investigated the effect of rituximab in PCNSL, indicating that rituximab can robustly enhance the response rate and possibly improve survival,” the investigators wrote in Cancer Medicine. “However, data regarding the addition of rituximab to [methotrexate and temozolomide] for PCNSL are limited, and no study has directly compared the efficacy of [rituximab/high-dose methotrexate/temozolomide] to that of [high-dose methotrexate/temozolomide].”
The study involved 62 patients with untreated PCNSL who were diagnosed between 2005 and 2015. Out of the 62 patients, 32 received rituximab/high-dose methotrexate/temozolomide (RMT) and 30 received high-dose methotrexate/temozolomide (MT). Patients received up to eight cycles of therapy, with discontinuation upon disease progression or toxicity.
The results showed that patients treated with RMT had significantly better outcomes than those who received MT, first marked by objective response rates, which were 93.7% for RMT and 69.0% for MT.
Survival rates also showed the advantage of rituximab. For the RMT group, 2-year and 5-year progression-free survival rates were 81.3% and 53.3%, respectively, compared with 46.5% and 29.1% for patients receiving MT.
Most importantly, rituximab boosted overall survival to a significant and notable extent, with higher rates at 2 years (82.3% vs. 65.7%) and 5 years (82.3% vs. 50.0%).
Efficacy did not diminish safety, as no significant differences in toxicity were found between treatment types. The most common grade 3-4 toxicities were hematologic; most commonly, this entailed neutropenia, which occurred in about one-quarter of patients.
“Given its outstanding efficacy and favorable toxicity, we consider RMT to be a feasible and safe therapeutic approach as a first‐line treatment for PCNSL. Moreover, RMT is an ideal regimen for elderly patients and frail populations who may not tolerate [whole‐brain radiation therapy] or [autologous stem‐cell transplantation],” the researchers wrote.
The study was funded by the Natural Science Foundation of Guangdong Province. The researchers reported having no conflicts of interest.
SOURCE: Chen C et al. Cancer Med. 2019 Mar 1. doi: 10.1002/cam4.1906.
For patients with primary central nervous system lymphoma (PCNSL), adding rituximab to combination high-dose methotrexate and temozolomide significantly boosted the 5-year overall survival rate, according to a retrospective study.
The triplet combination could be a safe and effective first-line option for patients with PCNSL, particularly the frail and elderly, who may have issues with toxicity when receiving current standard care, reported lead author Cui Chen, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China, and his colleagues.
“An increasing number of studies and meta‐analyses have investigated the effect of rituximab in PCNSL, indicating that rituximab can robustly enhance the response rate and possibly improve survival,” the investigators wrote in Cancer Medicine. “However, data regarding the addition of rituximab to [methotrexate and temozolomide] for PCNSL are limited, and no study has directly compared the efficacy of [rituximab/high-dose methotrexate/temozolomide] to that of [high-dose methotrexate/temozolomide].”
The study involved 62 patients with untreated PCNSL who were diagnosed between 2005 and 2015. Out of the 62 patients, 32 received rituximab/high-dose methotrexate/temozolomide (RMT) and 30 received high-dose methotrexate/temozolomide (MT). Patients received up to eight cycles of therapy, with discontinuation upon disease progression or toxicity.
The results showed that patients treated with RMT had significantly better outcomes than those who received MT, first marked by objective response rates, which were 93.7% for RMT and 69.0% for MT.
Survival rates also showed the advantage of rituximab. For the RMT group, 2-year and 5-year progression-free survival rates were 81.3% and 53.3%, respectively, compared with 46.5% and 29.1% for patients receiving MT.
Most importantly, rituximab boosted overall survival to a significant and notable extent, with higher rates at 2 years (82.3% vs. 65.7%) and 5 years (82.3% vs. 50.0%).
Efficacy did not diminish safety, as no significant differences in toxicity were found between treatment types. The most common grade 3-4 toxicities were hematologic; most commonly, this entailed neutropenia, which occurred in about one-quarter of patients.
“Given its outstanding efficacy and favorable toxicity, we consider RMT to be a feasible and safe therapeutic approach as a first‐line treatment for PCNSL. Moreover, RMT is an ideal regimen for elderly patients and frail populations who may not tolerate [whole‐brain radiation therapy] or [autologous stem‐cell transplantation],” the researchers wrote.
The study was funded by the Natural Science Foundation of Guangdong Province. The researchers reported having no conflicts of interest.
SOURCE: Chen C et al. Cancer Med. 2019 Mar 1. doi: 10.1002/cam4.1906.
FROM CANCER MEDICINE
FDA: Programmable heart failure device approved
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
. Specifically, these patients are unsuited for other treatments, have marked physical limitations related to their heart failure, and have remained symptomatic despite optimal medical therapy. They also have a regular heart rhythm, are not candidates for resynchronization, and possess a left ventricular ejection fraction of 25%-45%.
The cardiac contractility modulation system is indicated to improve 6-minute hall walk distance, quality of life, and functional status in these patients.
The system is made up of several components, including the implantable pulse generator, a programmer, and software. The pulse generator is connected to three leads that have been implanted in the heart, after which the device is tested and programmed to deliver pulses during normal heartbeats, which improves the heart’s squeezing capability. In randomized, multicenter clinical trials, the system plus optimal medical therapy demonstrated improvements in distance during 6-minute walking tests and standard assessments of heart failure symptoms when compared with optimal medical therapy alone.
The Breakthrough Device designation means this system treats a life-threatening disease and addresses unmet medical needs among some patients. “The FDA recognized the unmet need for these patients and worked with the manufacturer through our Breakthrough Device Program to efficiently bring this product to market, while ensuring it meets our regulatory requirements for safety and effectiveness,” Bram Zuckerman, MD, the director of the division of cardiovascular devices in the FDA’s Center for Devices and Radiological Health said in a news release from the agency.
Don’t miss baby scabies
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
FDA chief calls for stricter scrutiny of electronic health records
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
BP control slowed brain damage in elderly hypertensives
NEW ORLEANS – Hypertensive elderly patients treated to maintain an ambulatory systolic blood pressure of 130 mm Hg had significantly slower progression of white matter lesions in their brains than did control hypertensive patients maintained at an ambulatory systolic pressure of about 145 mm Hg during 3 years of follow-up in a randomized, single-center study with 199 patients.
The results also showed similar rates of death, syncope episodes, and falls in the intensively and less rigorously treated subgroups, and the patients treated to a systolic of 130 mm Hg also had significantly fewer nonfatal cardiovascular disease events, further documenting the safety and efficacy in elderly patients of a more aggressive blood pressure goal like the one promoted in current guidelines from the American College of Cardiology and American Heart Association, William B. White, MD, said at the annual meeting of the American College of Cardiology.
The study’s findings also showed that in one measure of cognitive function, the serial reaction time task, the patients treated to a systolic pressure of 130 mm Hg had an average 23 millisecond improvement in their reaction time from baseline to their 3-year follow-up, while patients in the control group treated to a systolic pressure of 145 mm Hg had a 33 millisecond increase in their average reaction time during follow-up. This 56 millisecond between-group difference from baseline in average change in reaction time over 3 years was both statistically significant and represents a clinically meaningful difference for a measure of both processing speed and executive function, said Dr. White, professor of medicine at the University of Connecticut in Farmington. However, the participants also underwent assessment by five other clinical measures of cognitive function and in none of the other five tests did more intensive blood pressure control link with an improvement, compared with the results in control patients.
The study had two primary endpoints. One was progression of white matter hyperintensity on brain MR images, which is a measure of neuron necrosis in the brain, and this analysis showed that the growth of white matter occurred at a 40% reduced rate among 99 patients treated to an average ambulatory systolic blood pressure of 130 mm Hg, compared with the average progression among 100 controls treated to an average ambulatory systolic of 145 mm Hg. The second measure was improvement during 3 years, compared with controls, in any of six different measures of mobility, including gait speed. The results showed no significant differences between the treatment arms in any of these measures. The average progression of white matter disease among control patients after 3 years was of a magnitude that would trigger concern in a neurologist who saw these scans, said Dr. White. The researchers could already begin to see a between-group difference in the accumulation of white matter hyperintensity on the MR scans of patients at 18 months in the study, he added.
During his presentation, Dr. White suggested that the absence of discerned improvements in mobility from more aggressive blood pressure control despite the observed slowed progression of white matter disease may have resulted from the study’s relatively brief follow-up.
The INFINITY (Intensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional Decline in the Elderly) study enrolled hypertensive patients at least 75 years old who already showed visible evidence of white matter hypertrophy on their brain MR scan at baseline but also had normal mobility and mental function (their baseline score on the mini mental state examination had to be within the normal range, with an average score of 28 among enrolled patients), and they had no history of any chronic neurological condition (Am Heart J. 2013 Mar;165[3]:258-65). The median age of enrolled patients was 80 years. They had an average of 15 years of education, indicating a study cohort with a high level of education and function, Dr. White noted. The inclusion and exclusion criteria led to a study population that was substantially older but without as much comorbidity as patients enrolled in the SPRINT MIND study (JAMA. 2019 Jan 28;321[6]:553-61), he said. The study exclusively used 24-hour ambulatory monitoring for baseline and follow-up blood pressure measurements.
The participating clinicians successfully maintained patients in each of the treatment groups at close to their goal systolic blood pressures. At 18 months, the actual average systolic pressures among patients in the two study groups were 132 mm Hg and 146 mm Hg, and at 36 months their pressures averaged 131 mm Hg and 146 mm Hg for 163 patients who remained in the study out to 36-months. Maintenance of the lower pressure generally required treatment with one additional antihypertensive medication, compared with the control patients’ treatment, Dr. White said.
The rates of total falls and falls causing injury were virtually identical in the two treatment groups. The incidence of nonfatal cardiovascular disease events over 3 years, including MI, strokes, and cardiovascular disease hospitalizations, was 4 cases in the intensively-treated patients and 17 among those treated to a higher systolic pressure, a statistically significant and unexpected difference, Dr. White reported.
This is another dataset showing that blood pressure reduction in elderly people with hypertension is safe and extremely important. Clinicians today often exclude elderly patients from aggressive blood pressure control because of an unrealized fear of causing hypotension and falls. These new data add to what’s already been reported in support of the American College of Cardiology and American Heart Association blood pressure treatment target of less than 130/80 mm Hg for noninstitutionalized, ambulatory, community-dwelling adults who are aged at least 65 years (Hypertension. 2018 June;71[6]:e13-e115). Many clinicians continue to have concerns about what this guideline says about treating older patients. These new findings support the idea that blood pressure can safely be treated to the level the guideline recommends while producing signals of beneficial changes in brain health and in cognitive function.
Providers worry a lot about the potential for harm from treatment. These findings add to the data that say clinicians can safely follow the blood pressure management guideline to benefit even very old patients.
Eileen Handberg, PhD , is a research professor of medicine and director of the Cardiovascular Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
This is another dataset showing that blood pressure reduction in elderly people with hypertension is safe and extremely important. Clinicians today often exclude elderly patients from aggressive blood pressure control because of an unrealized fear of causing hypotension and falls. These new data add to what’s already been reported in support of the American College of Cardiology and American Heart Association blood pressure treatment target of less than 130/80 mm Hg for noninstitutionalized, ambulatory, community-dwelling adults who are aged at least 65 years (Hypertension. 2018 June;71[6]:e13-e115). Many clinicians continue to have concerns about what this guideline says about treating older patients. These new findings support the idea that blood pressure can safely be treated to the level the guideline recommends while producing signals of beneficial changes in brain health and in cognitive function.
Providers worry a lot about the potential for harm from treatment. These findings add to the data that say clinicians can safely follow the blood pressure management guideline to benefit even very old patients.
Eileen Handberg, PhD , is a research professor of medicine and director of the Cardiovascular Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
This is another dataset showing that blood pressure reduction in elderly people with hypertension is safe and extremely important. Clinicians today often exclude elderly patients from aggressive blood pressure control because of an unrealized fear of causing hypotension and falls. These new data add to what’s already been reported in support of the American College of Cardiology and American Heart Association blood pressure treatment target of less than 130/80 mm Hg for noninstitutionalized, ambulatory, community-dwelling adults who are aged at least 65 years (Hypertension. 2018 June;71[6]:e13-e115). Many clinicians continue to have concerns about what this guideline says about treating older patients. These new findings support the idea that blood pressure can safely be treated to the level the guideline recommends while producing signals of beneficial changes in brain health and in cognitive function.
Providers worry a lot about the potential for harm from treatment. These findings add to the data that say clinicians can safely follow the blood pressure management guideline to benefit even very old patients.
Eileen Handberg, PhD , is a research professor of medicine and director of the Cardiovascular Clinical Trials Program at the University of Florida in Gainesville. She had no relevant disclosures. She made these comments in an interview.
NEW ORLEANS – Hypertensive elderly patients treated to maintain an ambulatory systolic blood pressure of 130 mm Hg had significantly slower progression of white matter lesions in their brains than did control hypertensive patients maintained at an ambulatory systolic pressure of about 145 mm Hg during 3 years of follow-up in a randomized, single-center study with 199 patients.
The results also showed similar rates of death, syncope episodes, and falls in the intensively and less rigorously treated subgroups, and the patients treated to a systolic of 130 mm Hg also had significantly fewer nonfatal cardiovascular disease events, further documenting the safety and efficacy in elderly patients of a more aggressive blood pressure goal like the one promoted in current guidelines from the American College of Cardiology and American Heart Association, William B. White, MD, said at the annual meeting of the American College of Cardiology.
The study’s findings also showed that in one measure of cognitive function, the serial reaction time task, the patients treated to a systolic pressure of 130 mm Hg had an average 23 millisecond improvement in their reaction time from baseline to their 3-year follow-up, while patients in the control group treated to a systolic pressure of 145 mm Hg had a 33 millisecond increase in their average reaction time during follow-up. This 56 millisecond between-group difference from baseline in average change in reaction time over 3 years was both statistically significant and represents a clinically meaningful difference for a measure of both processing speed and executive function, said Dr. White, professor of medicine at the University of Connecticut in Farmington. However, the participants also underwent assessment by five other clinical measures of cognitive function and in none of the other five tests did more intensive blood pressure control link with an improvement, compared with the results in control patients.
The study had two primary endpoints. One was progression of white matter hyperintensity on brain MR images, which is a measure of neuron necrosis in the brain, and this analysis showed that the growth of white matter occurred at a 40% reduced rate among 99 patients treated to an average ambulatory systolic blood pressure of 130 mm Hg, compared with the average progression among 100 controls treated to an average ambulatory systolic of 145 mm Hg. The second measure was improvement during 3 years, compared with controls, in any of six different measures of mobility, including gait speed. The results showed no significant differences between the treatment arms in any of these measures. The average progression of white matter disease among control patients after 3 years was of a magnitude that would trigger concern in a neurologist who saw these scans, said Dr. White. The researchers could already begin to see a between-group difference in the accumulation of white matter hyperintensity on the MR scans of patients at 18 months in the study, he added.
During his presentation, Dr. White suggested that the absence of discerned improvements in mobility from more aggressive blood pressure control despite the observed slowed progression of white matter disease may have resulted from the study’s relatively brief follow-up.
The INFINITY (Intensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional Decline in the Elderly) study enrolled hypertensive patients at least 75 years old who already showed visible evidence of white matter hypertrophy on their brain MR scan at baseline but also had normal mobility and mental function (their baseline score on the mini mental state examination had to be within the normal range, with an average score of 28 among enrolled patients), and they had no history of any chronic neurological condition (Am Heart J. 2013 Mar;165[3]:258-65). The median age of enrolled patients was 80 years. They had an average of 15 years of education, indicating a study cohort with a high level of education and function, Dr. White noted. The inclusion and exclusion criteria led to a study population that was substantially older but without as much comorbidity as patients enrolled in the SPRINT MIND study (JAMA. 2019 Jan 28;321[6]:553-61), he said. The study exclusively used 24-hour ambulatory monitoring for baseline and follow-up blood pressure measurements.
The participating clinicians successfully maintained patients in each of the treatment groups at close to their goal systolic blood pressures. At 18 months, the actual average systolic pressures among patients in the two study groups were 132 mm Hg and 146 mm Hg, and at 36 months their pressures averaged 131 mm Hg and 146 mm Hg for 163 patients who remained in the study out to 36-months. Maintenance of the lower pressure generally required treatment with one additional antihypertensive medication, compared with the control patients’ treatment, Dr. White said.
The rates of total falls and falls causing injury were virtually identical in the two treatment groups. The incidence of nonfatal cardiovascular disease events over 3 years, including MI, strokes, and cardiovascular disease hospitalizations, was 4 cases in the intensively-treated patients and 17 among those treated to a higher systolic pressure, a statistically significant and unexpected difference, Dr. White reported.
NEW ORLEANS – Hypertensive elderly patients treated to maintain an ambulatory systolic blood pressure of 130 mm Hg had significantly slower progression of white matter lesions in their brains than did control hypertensive patients maintained at an ambulatory systolic pressure of about 145 mm Hg during 3 years of follow-up in a randomized, single-center study with 199 patients.
The results also showed similar rates of death, syncope episodes, and falls in the intensively and less rigorously treated subgroups, and the patients treated to a systolic of 130 mm Hg also had significantly fewer nonfatal cardiovascular disease events, further documenting the safety and efficacy in elderly patients of a more aggressive blood pressure goal like the one promoted in current guidelines from the American College of Cardiology and American Heart Association, William B. White, MD, said at the annual meeting of the American College of Cardiology.
The study’s findings also showed that in one measure of cognitive function, the serial reaction time task, the patients treated to a systolic pressure of 130 mm Hg had an average 23 millisecond improvement in their reaction time from baseline to their 3-year follow-up, while patients in the control group treated to a systolic pressure of 145 mm Hg had a 33 millisecond increase in their average reaction time during follow-up. This 56 millisecond between-group difference from baseline in average change in reaction time over 3 years was both statistically significant and represents a clinically meaningful difference for a measure of both processing speed and executive function, said Dr. White, professor of medicine at the University of Connecticut in Farmington. However, the participants also underwent assessment by five other clinical measures of cognitive function and in none of the other five tests did more intensive blood pressure control link with an improvement, compared with the results in control patients.
The study had two primary endpoints. One was progression of white matter hyperintensity on brain MR images, which is a measure of neuron necrosis in the brain, and this analysis showed that the growth of white matter occurred at a 40% reduced rate among 99 patients treated to an average ambulatory systolic blood pressure of 130 mm Hg, compared with the average progression among 100 controls treated to an average ambulatory systolic of 145 mm Hg. The second measure was improvement during 3 years, compared with controls, in any of six different measures of mobility, including gait speed. The results showed no significant differences between the treatment arms in any of these measures. The average progression of white matter disease among control patients after 3 years was of a magnitude that would trigger concern in a neurologist who saw these scans, said Dr. White. The researchers could already begin to see a between-group difference in the accumulation of white matter hyperintensity on the MR scans of patients at 18 months in the study, he added.
During his presentation, Dr. White suggested that the absence of discerned improvements in mobility from more aggressive blood pressure control despite the observed slowed progression of white matter disease may have resulted from the study’s relatively brief follow-up.
The INFINITY (Intensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional Decline in the Elderly) study enrolled hypertensive patients at least 75 years old who already showed visible evidence of white matter hypertrophy on their brain MR scan at baseline but also had normal mobility and mental function (their baseline score on the mini mental state examination had to be within the normal range, with an average score of 28 among enrolled patients), and they had no history of any chronic neurological condition (Am Heart J. 2013 Mar;165[3]:258-65). The median age of enrolled patients was 80 years. They had an average of 15 years of education, indicating a study cohort with a high level of education and function, Dr. White noted. The inclusion and exclusion criteria led to a study population that was substantially older but without as much comorbidity as patients enrolled in the SPRINT MIND study (JAMA. 2019 Jan 28;321[6]:553-61), he said. The study exclusively used 24-hour ambulatory monitoring for baseline and follow-up blood pressure measurements.
The participating clinicians successfully maintained patients in each of the treatment groups at close to their goal systolic blood pressures. At 18 months, the actual average systolic pressures among patients in the two study groups were 132 mm Hg and 146 mm Hg, and at 36 months their pressures averaged 131 mm Hg and 146 mm Hg for 163 patients who remained in the study out to 36-months. Maintenance of the lower pressure generally required treatment with one additional antihypertensive medication, compared with the control patients’ treatment, Dr. White said.
The rates of total falls and falls causing injury were virtually identical in the two treatment groups. The incidence of nonfatal cardiovascular disease events over 3 years, including MI, strokes, and cardiovascular disease hospitalizations, was 4 cases in the intensively-treated patients and 17 among those treated to a higher systolic pressure, a statistically significant and unexpected difference, Dr. White reported.
REPORTING FROM ACC 19
Study: Racial disparities in gyn-onc trial enrollment persist
HONOLULU – Racial disparities in phase 1 gynecologic oncology clinical trial enrollment is nothing new, but the gap between African American and Caucasian enrollment widened in recent years, according to a review of studies conducted since 1985.
The literature review identified 357 relevant phase 1 studies involving 9,492 patients published between 1985 and 2018. However, only 83 of the studies (23%) included a racial breakdown; of the 2,483 patients in those 83 trials, 79% were Caucasian, 5% were African American, and 16% were of “other” race, Elias Awad reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Ovarian cancer was the most common tumor type studied, but among studies listing a racial breakdown, endometrial cancer studies had the highest enrollment of African American patients at 12%, said Mr. Awad, a 4th-year medical student at the University of South Alabama, Mobile. The remaining studies all had less than 10% participation of black patients.
“Also of note is that other races exceeded black patients in 55% of trials across all tumor types,” he said. “Despite the focus on increasing diversity in clinical trials over the years, the percentage of black patients has declined 1.8-fold from 11% in 1995-1999 to 6% in 2015-2018.”
Based on Centers for Disease Control and Prevention age-adjusted incidence for race, expected and observed ratios of racial participation were calculated – and were found to be less than expected for each tumor site, Mr. Awad said.
For example, the enrollment ratio in ovarian cancer studies was 0.04:1 for black and white patients, respectively, while the ratios for endometrial and cervical cancer were 0.004:1 and 0.025:1, respectively.
These ratios represent a 1,750% lower than expected enrollment of black patients in ovarian cancer trials, 2,080% lower than expected enrollment for endometrial cancer trials, and 5,300% lower than expected enrollment for cervical cancer, he said.
This lack of minority participation in early-phase clinical trials likely contributes to cancer health disparities, Mr. Awad added, explaining that the underrepresentation of minorities in trials results in agents that work in majority populations.
“Thus, it is paramount to have therapeutic agents specifically effective in minority populations,” he said. “Considering that phase 1 trials are the entry point for therapeutic success, these results further compound the lack of adequate clinical trials for our black patients who continue to suffer the consequences of these inequities.
“Accordingly, it is time to enact strategies to enhance equity in the enrollment of black patients on clinical trials in order to assist in eliminating racial disparities in gynecologic malignancies,” he concluded.
Mr. Awad reported having no financial disclosures.
SOURCE: Awad E et al. SGO 2019, Abstract 22.
HONOLULU – Racial disparities in phase 1 gynecologic oncology clinical trial enrollment is nothing new, but the gap between African American and Caucasian enrollment widened in recent years, according to a review of studies conducted since 1985.
The literature review identified 357 relevant phase 1 studies involving 9,492 patients published between 1985 and 2018. However, only 83 of the studies (23%) included a racial breakdown; of the 2,483 patients in those 83 trials, 79% were Caucasian, 5% were African American, and 16% were of “other” race, Elias Awad reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Ovarian cancer was the most common tumor type studied, but among studies listing a racial breakdown, endometrial cancer studies had the highest enrollment of African American patients at 12%, said Mr. Awad, a 4th-year medical student at the University of South Alabama, Mobile. The remaining studies all had less than 10% participation of black patients.
“Also of note is that other races exceeded black patients in 55% of trials across all tumor types,” he said. “Despite the focus on increasing diversity in clinical trials over the years, the percentage of black patients has declined 1.8-fold from 11% in 1995-1999 to 6% in 2015-2018.”
Based on Centers for Disease Control and Prevention age-adjusted incidence for race, expected and observed ratios of racial participation were calculated – and were found to be less than expected for each tumor site, Mr. Awad said.
For example, the enrollment ratio in ovarian cancer studies was 0.04:1 for black and white patients, respectively, while the ratios for endometrial and cervical cancer were 0.004:1 and 0.025:1, respectively.
These ratios represent a 1,750% lower than expected enrollment of black patients in ovarian cancer trials, 2,080% lower than expected enrollment for endometrial cancer trials, and 5,300% lower than expected enrollment for cervical cancer, he said.
This lack of minority participation in early-phase clinical trials likely contributes to cancer health disparities, Mr. Awad added, explaining that the underrepresentation of minorities in trials results in agents that work in majority populations.
“Thus, it is paramount to have therapeutic agents specifically effective in minority populations,” he said. “Considering that phase 1 trials are the entry point for therapeutic success, these results further compound the lack of adequate clinical trials for our black patients who continue to suffer the consequences of these inequities.
“Accordingly, it is time to enact strategies to enhance equity in the enrollment of black patients on clinical trials in order to assist in eliminating racial disparities in gynecologic malignancies,” he concluded.
Mr. Awad reported having no financial disclosures.
SOURCE: Awad E et al. SGO 2019, Abstract 22.
HONOLULU – Racial disparities in phase 1 gynecologic oncology clinical trial enrollment is nothing new, but the gap between African American and Caucasian enrollment widened in recent years, according to a review of studies conducted since 1985.
The literature review identified 357 relevant phase 1 studies involving 9,492 patients published between 1985 and 2018. However, only 83 of the studies (23%) included a racial breakdown; of the 2,483 patients in those 83 trials, 79% were Caucasian, 5% were African American, and 16% were of “other” race, Elias Awad reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Ovarian cancer was the most common tumor type studied, but among studies listing a racial breakdown, endometrial cancer studies had the highest enrollment of African American patients at 12%, said Mr. Awad, a 4th-year medical student at the University of South Alabama, Mobile. The remaining studies all had less than 10% participation of black patients.
“Also of note is that other races exceeded black patients in 55% of trials across all tumor types,” he said. “Despite the focus on increasing diversity in clinical trials over the years, the percentage of black patients has declined 1.8-fold from 11% in 1995-1999 to 6% in 2015-2018.”
Based on Centers for Disease Control and Prevention age-adjusted incidence for race, expected and observed ratios of racial participation were calculated – and were found to be less than expected for each tumor site, Mr. Awad said.
For example, the enrollment ratio in ovarian cancer studies was 0.04:1 for black and white patients, respectively, while the ratios for endometrial and cervical cancer were 0.004:1 and 0.025:1, respectively.
These ratios represent a 1,750% lower than expected enrollment of black patients in ovarian cancer trials, 2,080% lower than expected enrollment for endometrial cancer trials, and 5,300% lower than expected enrollment for cervical cancer, he said.
This lack of minority participation in early-phase clinical trials likely contributes to cancer health disparities, Mr. Awad added, explaining that the underrepresentation of minorities in trials results in agents that work in majority populations.
“Thus, it is paramount to have therapeutic agents specifically effective in minority populations,” he said. “Considering that phase 1 trials are the entry point for therapeutic success, these results further compound the lack of adequate clinical trials for our black patients who continue to suffer the consequences of these inequities.
“Accordingly, it is time to enact strategies to enhance equity in the enrollment of black patients on clinical trials in order to assist in eliminating racial disparities in gynecologic malignancies,” he concluded.
Mr. Awad reported having no financial disclosures.
SOURCE: Awad E et al. SGO 2019, Abstract 22.
REPORTING FROM SGO 2019
Patients with OCD/hoarding differ from those with OCD/nonhoarding
More autism spectrum disorder symptoms identified as distinguishing factor
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
More autism spectrum disorder symptoms identified as distinguishing factor
More autism spectrum disorder symptoms identified as distinguishing factor
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
Hoarding symptoms are frequently present in patients with obsessive-compulsive disorder (OCD), and significant differences can be found between them and patients with OCD and no hoarding symptoms, a study of 407 patients shows.
The study also identified links to autism spectrum disorder (ASD) among patients with OCD/hoarding symptoms. “The most relevant outcome of this study was the association between persons with OCD/hoarding and the increased severity of autism symptoms,” wrote Yentl E. Boerema of the Amsterdam Public Health Research Institute, and associates. The study was published in the Journal of Affective Disorders.
To conduct the study, the investigators used cross-sectional baseline data from the Netherlands Obsessive Compulsive Disorder Association (NOCDA) study. Participants in the NOCDA sample were aged 18-79 years with current or remitted DSM-IV-TR criteria for OCD. Hoarding symptoms were determined via the adapted version of the Yale-Brown Obsessive Compulsive Scale. A total of 58 patients were found to have both OCD/hoarding symptoms, compared with 349 who did not, the investigators reported.
OCD/hoarding was associated with earlier age of onset (P less than .05) and more severe OCD symptoms (P less than .001), as well as higher scores on all OCD subtypes. It was associated with living without a partner (P less than .05), being less conscientious (P less than .05), and severity of autism symptoms (P less than .001). The investigators speculated that ASD factors might “be responsible for hoarding behavior, including (a) having intense or focused interests, which can lead to collecting of items, and/or (b) having a lack of seeking shared enjoyment, interests, and activity with other people.”
Meanwhile, the investigators found that coexisting OCD/hoarding was not associated with childhood trauma, posttraumatic stress disorder or attention-deficit/hyperactivity disorder – inattentive type or hyperactive type.
“Clinical implications of our findings are to have the treatment follow a more intensive and structured course (i.e., avoid surprises),” having more attention for affective education, and making more use of visual illustrations, the investigators said. “Taken together, this knowledge provides a better understanding of persons with OCD/hoarding and has the potential to improve treatment.”
The study authors reported no conflicts of interest.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
FDA halts enrollment in trial of venetoclax for multiple myeloma
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
The Food and Drug Administration has halted enrollment in trials of venetoclax (Venclexta) for multiple myeloma.
The move comes after a review of data from the phase 3 BELLINI trial, which pitted venetoclax against placebo in relapsed and refractory multiple myeloma patients on a background of bortezomib and low-dose dexamethasone. Venetoclax is not approved for the treatment of multiple myeloma; the agency said that patients using the drug for approved indications should continue use of the drug.
There were 41/194 deaths (21.1%) in the venetoclax arm, versus 11/97 (11.3%) in the placebo group; 13 of the deaths in the venetoclax arm (32%) and 1 death in the placebo arm (9%) were treatment related. Sepsis, pneumonia, and cardiac arrest were the most common treatment-related causes of death in the venetoclax group; 8 of the 13 deaths (62%) were due to infection.
The FDA estimated that the drug doubled the risk of death compared to placebo.
The agency warned against off-label use of venetoclax for multiple myeloma, and noted that the drug “is safe and effective for its approved uses,” which include second-line treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma in adults, as well as newly-diagnosed acute myeloid leukemia in adults age 75 years or older or who have contraindications to standard chemotherapy.
There are more than 10 trials in the United States of venetoclax for multiple myeloma, and most of them have been suspended, including BELLINI.
Patients already enrolled in the trial can remain on treatment, but they must re-consent to the trial. The FDA “will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue,” the agency said in a statement.
Abbvie, which is developing venetoclax in partnership with Roche, noted in its own press release that the drug otherwise outperformed placebo in BELLINI, both in progression-free survival (22.4 months versus 11.5 months), and in overall (82% versus 68%) and partial (59% versus 36%) response rates.
Severe grade 3-5 toxicity and serious adverse event rates were similar in the two study arms, as was the overall incidence of infections (79.8% versus 77.1%). However, the incidence of pneumonia was 20.7% with venetoclax, versus 15.6% with placebo.
“We will continue working with the FDA and worldwide regulatory agencies to determine appropriate next steps for the multiple myeloma program,” Michael Severino, MD, AbbVie vice chairman and president, said in the press release.
Venetoclax binds and inhibits the B-cell lymphoma-2 protein, which prevents some blood cancer cells from undergoing programmed cell death.
What does COMPASS mean for vascular surgeons?
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
Antithrombotic therapy with low-dose rivaroxaban plus aspirin should be considered in low–bleeding risk patients with peripheral arterial disease who are at increased risk for ischemic and/or limb events, according to an analysis of the COMPASS trial published in Current Opinion in Cardiology.
Mohamad A. Hussain, MD, of the University of Toronto and his colleagues assessed the ramifications of COMPASS to vascular surgeons. They used two clinical case studies of patients with peripheral arterial disease (PAD) to illustrate differing considerations in care.
The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial showed that low-dose rivaroxaban at 2.5 mg twice daily plus daily aspirin was superior to aspirin alone in reducing major adverse cardiovascular and cerebrovascular events, as well as major adverse limb events, among patients with stable atherosclerotic vascular disease, including those with PAD. However, the risk for major bleeding was higher with rivaroxaban plus aspirin and is a serious consideration for patient treatment.
In clinical case 1, used to illustrate the pertinence of COMPASS to patient care, Dr. Hussain and his colleagues detailed a 68-year-old man presenting with a 3-month history of intermittent claudication of bilateral calves at 10 minutes of brisk walking. His comorbidities include a MI with percutaneous coronary stenting 2 years ago, diabetes mellitus, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease on the basis of prior smoking.
Clinical case 2 was a 70-year-old woman with a small gangrenous ulcer on the dorsal part of her first toe on the left foot. She has history of coronary artery disease with coronary artery bypass graft surgery 5 years prior, diabetes mellitus, mild chronic kidney disease, and hypertension. She underwent an uneventful lower extremity bypass using a prosthetic graft and had an uncomplicated postoperative course.
Both patients were on daily 81 mg aspirin.
In order to determine the appropriate care for these patients, the authors presented a flowchart of considerations regarding risk and benefits. Patients with symptomatic PAD who had no recent major bleeds, history of stroke, congestive heart failure, chronic kidney disease, frailty, or anemia were considered for rivaroxaban treatment, otherwise they were put on single antiplatelet therapy.
The investigators recommended that, if the patients were at high limb risk or high ischemic risk, they should be treated with either rivaroxaban plus aspirin or dual antiplatelet therapy (the latter if there was a recent MI or peripheral stenting). If the patients were not at risk, they were deemed eligible for either the rivaroxaban plus aspirin therapy or single antiplatelet therapy.
With regard to the clinical case studies, the authors discussed the rationale for putting both patients on the rivaroxaban plus aspirin therapy after an assessment of the risk/benefit profile for each patient based upon the above considerations. In both cases the bleeding risk was considered low; the ischemic risk in the first patient and the limb risk in the second patient was considered high. Although the second patient had chronic kidney disease, it was not considered severe enough to preclude such treatment.
“Future data from trials such as Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization For Peripheral Artery Disease [VOYAGER PAD] will provide data with regards to the role of low-dose rivaroxaban plus aspirin following peripheral artery revascularization for PAD,” the researchers concluded.
Dr. Hussain reported having no conflicts; his coauthors reported receiving funding from various pharmaceutical companies, including Bayer, which was a sponsor of the original COMPASS trial.
SOURCE: Hussain MA et al. Curr Opin Cardiol. 2019;34:178-84.
FROM CURRENT OPINION IN CARDIOLOGY