User login
Subscribe to SVS Student Newsletters
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
More Items Added to ‘Spectacular’ Auction
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.
SVSConnect App is Ready
You can now log in to SVSConnect conveniently from your phone and tablet. This gives you easy access to field-related discussions, resources from other users and the SVS member directory. You can do everything on the app that you can on your desktop and it only takes a few minutes to set up. Follow the steps in this flier to download. If you encounter difficulties, email communications@vascularsociety.org or call 312-334-2300.
You can now log in to SVSConnect conveniently from your phone and tablet. This gives you easy access to field-related discussions, resources from other users and the SVS member directory. You can do everything on the app that you can on your desktop and it only takes a few minutes to set up. Follow the steps in this flier to download. If you encounter difficulties, email communications@vascularsociety.org or call 312-334-2300.
You can now log in to SVSConnect conveniently from your phone and tablet. This gives you easy access to field-related discussions, resources from other users and the SVS member directory. You can do everything on the app that you can on your desktop and it only takes a few minutes to set up. Follow the steps in this flier to download. If you encounter difficulties, email communications@vascularsociety.org or call 312-334-2300.
VAM Registration Opens Soon
The time has almost come to register for the 2019 Vascular Annual Meeting. This month, you’ll be able to register for the can’t-miss event of the year for vascular care professionals. This year’s event will be held on June 12-15 in National Harbor, Md., (near Washington, D.C.) As always, attendees will learn about cutting-edge vascular research, attend innovative education sessions, have the opportunity to network with other thought leaders in the field and do much more. Reservations for VAM housing open at the same time. For more information about VAM registration and housing, visit our meeting website.
The time has almost come to register for the 2019 Vascular Annual Meeting. This month, you’ll be able to register for the can’t-miss event of the year for vascular care professionals. This year’s event will be held on June 12-15 in National Harbor, Md., (near Washington, D.C.) As always, attendees will learn about cutting-edge vascular research, attend innovative education sessions, have the opportunity to network with other thought leaders in the field and do much more. Reservations for VAM housing open at the same time. For more information about VAM registration and housing, visit our meeting website.
The time has almost come to register for the 2019 Vascular Annual Meeting. This month, you’ll be able to register for the can’t-miss event of the year for vascular care professionals. This year’s event will be held on June 12-15 in National Harbor, Md., (near Washington, D.C.) As always, attendees will learn about cutting-edge vascular research, attend innovative education sessions, have the opportunity to network with other thought leaders in the field and do much more. Reservations for VAM housing open at the same time. For more information about VAM registration and housing, visit our meeting website.
Antibiotic delay is deadly in elderly patients with UTI
A pilot study of smartphone-based visual tests shows promise in multiple sclerosis. Myeloma therapies raise cardiovascular risks. And sperm counts are largely stable after adjuvant treatment of early testicular cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A pilot study of smartphone-based visual tests shows promise in multiple sclerosis. Myeloma therapies raise cardiovascular risks. And sperm counts are largely stable after adjuvant treatment of early testicular cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A pilot study of smartphone-based visual tests shows promise in multiple sclerosis. Myeloma therapies raise cardiovascular risks. And sperm counts are largely stable after adjuvant treatment of early testicular cancer.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
CDC Expands Assessment Study of Toxic Chemicals Near Military Bases
Per- and polyfluoroalkyl substances (PFAS) are manmade chemicals used in industry and consumer products, such as nonstick cookware, water-repellent clothing, and stain-resistant fabrics. Studies have shown that exposure to PFAS can—among other things—affect growth, learning, and behavior of infants and children; reduce a woman’s chance of getting pregnant; affect the immune system; and increase the risk of cancer.
The 2018 National Defense Authorization Act allowed the CDC and the Agency for Toxic Substances and Disease Registry (ATSDR) to look at PFAS exposure in communities near current or former military bases that are known to have had PFAS in the drinking water. In a pilot study, researchers conducted assessments in Bucks and Montgomery counties in Pennsylvania (near Horsham Air Guard Station and former Naval Air Warfare Center), and in Westhampton, New York (near Gabreski Air National Guard Base).
Now, CDC/ATSDR have expanded the assessments to 8 other communities:
- Berkeley County (WV) near Shepherd Field Air National Guard Base
- El Paso County (CO) near Peterson Air Force Base
- Fairbanks North Star Borough (AK) near Eielson Air Force Base
- Hampden County (MA) near Barnes Air National Guard Base
- Lubbock County (TX) near Reese Technology Center
- Orange County (NY) near Stewart Air National Guard Base
- New Castle County (DE) near New Castle Air National Guard Base
- Spokane County (WA) near Fairchild Air Force Base
The researchers will randomly select people in each community to participate by having their PFAS levels checked in blood and urine samples. The sampling results will provide researchers and public health professionals with information about community-level exposure but also be used to help communities understand the level of risk and how to reduce PFAS exposure.
The assessments, expected to begin this year and continue through 2020, will also “lay the groundwork,” the CDC says, for a multisite health study that will examine the relationship between PFAS exposure and health outcomes.
For more information about PFAS and the Exposure Assessment, visit https://www.atsdr.cdc.gov/pfas/index.html.
Per- and polyfluoroalkyl substances (PFAS) are manmade chemicals used in industry and consumer products, such as nonstick cookware, water-repellent clothing, and stain-resistant fabrics. Studies have shown that exposure to PFAS can—among other things—affect growth, learning, and behavior of infants and children; reduce a woman’s chance of getting pregnant; affect the immune system; and increase the risk of cancer.
The 2018 National Defense Authorization Act allowed the CDC and the Agency for Toxic Substances and Disease Registry (ATSDR) to look at PFAS exposure in communities near current or former military bases that are known to have had PFAS in the drinking water. In a pilot study, researchers conducted assessments in Bucks and Montgomery counties in Pennsylvania (near Horsham Air Guard Station and former Naval Air Warfare Center), and in Westhampton, New York (near Gabreski Air National Guard Base).
Now, CDC/ATSDR have expanded the assessments to 8 other communities:
- Berkeley County (WV) near Shepherd Field Air National Guard Base
- El Paso County (CO) near Peterson Air Force Base
- Fairbanks North Star Borough (AK) near Eielson Air Force Base
- Hampden County (MA) near Barnes Air National Guard Base
- Lubbock County (TX) near Reese Technology Center
- Orange County (NY) near Stewart Air National Guard Base
- New Castle County (DE) near New Castle Air National Guard Base
- Spokane County (WA) near Fairchild Air Force Base
The researchers will randomly select people in each community to participate by having their PFAS levels checked in blood and urine samples. The sampling results will provide researchers and public health professionals with information about community-level exposure but also be used to help communities understand the level of risk and how to reduce PFAS exposure.
The assessments, expected to begin this year and continue through 2020, will also “lay the groundwork,” the CDC says, for a multisite health study that will examine the relationship between PFAS exposure and health outcomes.
For more information about PFAS and the Exposure Assessment, visit https://www.atsdr.cdc.gov/pfas/index.html.
Per- and polyfluoroalkyl substances (PFAS) are manmade chemicals used in industry and consumer products, such as nonstick cookware, water-repellent clothing, and stain-resistant fabrics. Studies have shown that exposure to PFAS can—among other things—affect growth, learning, and behavior of infants and children; reduce a woman’s chance of getting pregnant; affect the immune system; and increase the risk of cancer.
The 2018 National Defense Authorization Act allowed the CDC and the Agency for Toxic Substances and Disease Registry (ATSDR) to look at PFAS exposure in communities near current or former military bases that are known to have had PFAS in the drinking water. In a pilot study, researchers conducted assessments in Bucks and Montgomery counties in Pennsylvania (near Horsham Air Guard Station and former Naval Air Warfare Center), and in Westhampton, New York (near Gabreski Air National Guard Base).
Now, CDC/ATSDR have expanded the assessments to 8 other communities:
- Berkeley County (WV) near Shepherd Field Air National Guard Base
- El Paso County (CO) near Peterson Air Force Base
- Fairbanks North Star Borough (AK) near Eielson Air Force Base
- Hampden County (MA) near Barnes Air National Guard Base
- Lubbock County (TX) near Reese Technology Center
- Orange County (NY) near Stewart Air National Guard Base
- New Castle County (DE) near New Castle Air National Guard Base
- Spokane County (WA) near Fairchild Air Force Base
The researchers will randomly select people in each community to participate by having their PFAS levels checked in blood and urine samples. The sampling results will provide researchers and public health professionals with information about community-level exposure but also be used to help communities understand the level of risk and how to reduce PFAS exposure.
The assessments, expected to begin this year and continue through 2020, will also “lay the groundwork,” the CDC says, for a multisite health study that will examine the relationship between PFAS exposure and health outcomes.
For more information about PFAS and the Exposure Assessment, visit https://www.atsdr.cdc.gov/pfas/index.html.
Click for Credit: Endometriosis surgery benefits; diabetes & aging; more
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
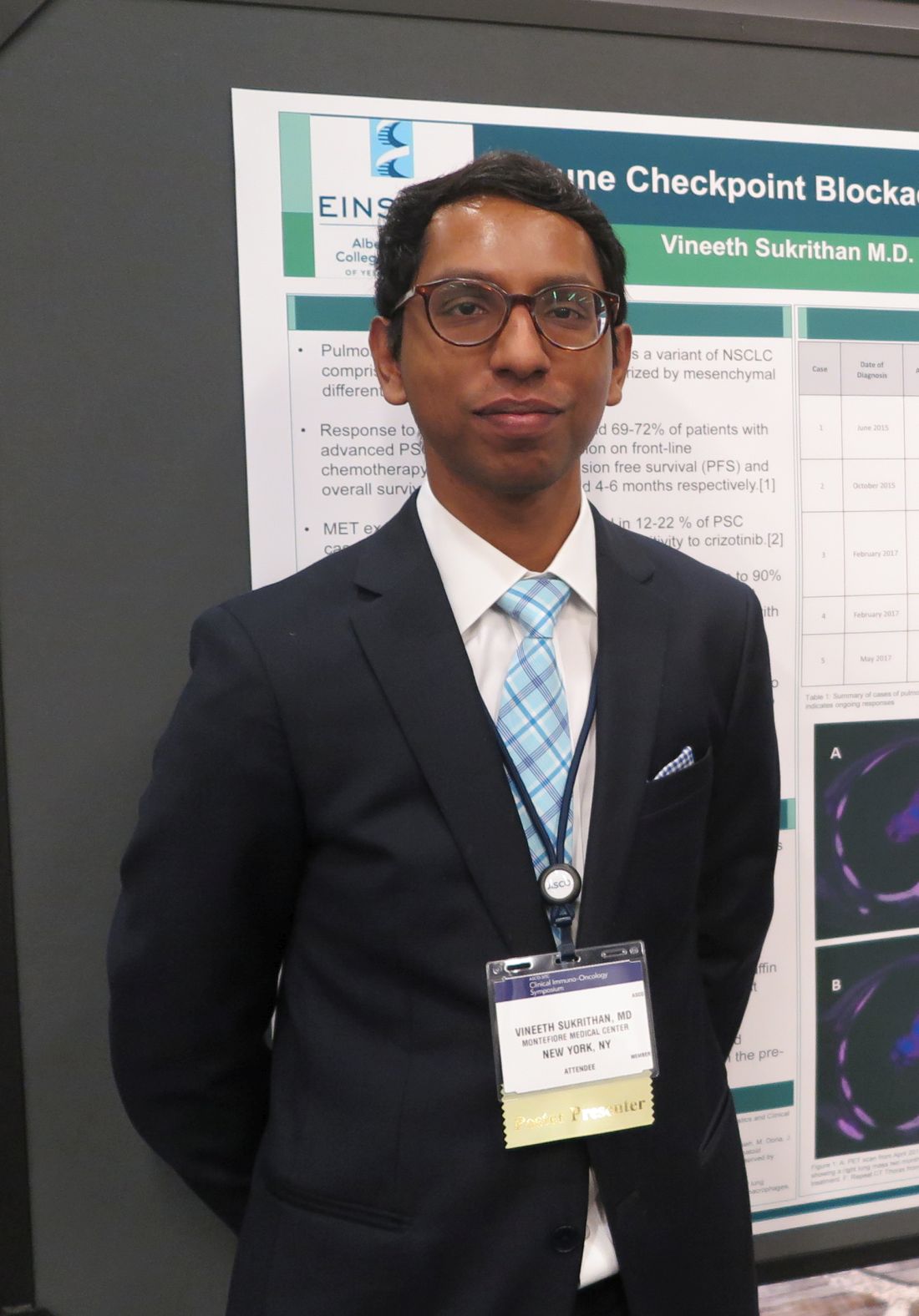
Rare, aggressive NSCLC type yields to pembrolizumab
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
REPORTING FROM ASCO-SITC
Pediatric pruritus requires distinct approach to assessment and management
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
REPORTING FROM AAD 2019
MS research: “Our patients can’t wait” for conventional techniques
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
REPORTING FROM ACTRIMS FORUM 2019