User login
Malpractice suits are less frequent – but more costly
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”
Lawsuits against physicians declined across virtually all specialties by more than a quarter over a 10-year span, but the cost to manage legal challenges went up, a recent analysis finds.
From 2007 to 2016, the rate of claims dropped by 27% per 100 doctors from 5.1 to 3.7, according to a review of 124,000 cases by CRICO Strategies, a division of CRICO, the medical liability insurance provider for the Harvard medical community. CRICO’s database of claims contains about 30% of legal cases filed against health providers across the U.S.
For internists, the rate of lawsuits decreased by 35% between 2007 and 2016, according to CRICO data provided to MDedge News. Ob.gyns. saw a 44% drop in claims over the 10-year period, and surgeons experienced a 23% rate decrease. The analysis did not break down the rate of claims by other single subspecialists. Claims decreased by a combined 29% for cardiologists, dermatologists, endocrinologists, family physicians, gastroenterologists, hematologists/oncologists, hospitalists, infectious disease specialists, internists, nephrologists, neurologists, pulmonologists, and rheumatologists/immunologists, according to the report published in February 2019 on CRICO’s website.
The findings are consistent with prior research on claim trends, said Seth Seabury, PhD, a medical liability researcher and director of the Keck-Schaeffer Initiative for Population Health Policy at the University of Southern California, Los Angeles.
“Malpractice claim frequency has been falling pretty steadily for a while now, reflecting a number of factors including the widespread adoption of tort reform and other measures to shield physicians from malpractice risk,” Dr. Seabury said in an interview. “Interestingly, the decline seems greatest in the claims with lower potential stakes, as you see average indemnity holding flat or rising. Some of this likely reflects the unwillingness of attorneys to take cases with lower potential payouts, because of the high cost of litigating a malpractice case.”
While frequency went down, the cost to manage a legal claim went up, according to CRICO data. The price of defending a malpractice lawsuit rose an average of 3.5% annually over the 10-year period from $36,000 to $46,000. For cases that ended with no payment (indemnity) to plaintiffs, the cost to manage a case rose an average of 5% annually.
The upward trends in case management expenses are striking, particularly since the time to resolve cases has decreased, said Michelle Mello, PhD, a health research and policy professor at Stanford (Calif.) University. From 2007 to 2016, the average time to resolve a case dropped from 29 to 27 months, the CRICO report found.
“CRICO nods to disclosure and apology approaches as perhaps underlying the more encouraging trend in time to resolution, but it was surprising to me that such approaches have not translated into lower defense costs,” Dr. Mello said in an interview. “In particular, a lot is still being spent to manage cases that never result in a payment to the patient. My hope was that, as hospitals got better at communicating with patients about adverse events, including the fact that about three-quarters of them are not due to substandard care, there would be fewer claims involving such events and also less money spent dealing with such claims when they do arise.”
For cases that do end in payment, high payouts are on the rise. Cases that ended in payments of $1 million or more increased 4% over the 10-year time frame, while payments of $3 million to $11 million increased 7% annually, according to the CRICO report. Cases that ended in payment lower than $1 million dropped over the 10-year span.
The reasons behind increasing plaintiff payouts is uncertain, Dr. Seabury said.
“It’s hard to say exactly why high payouts are on the rise, as payout levels reflect a number of factors – [such as] economic damages, clinical severity, pain and suffering – that can be difficult to disentangle,” he said. “But it is probably concerning for doctors in the sense that, while claims are becoming less likely, when they do happen, it could be more catastrophic in the sense of having large damages that exceed the policy limit.”
March 2019 Combatting Public Pathogens
Click here to access March 2019 Combatting Public Pathogens
Table of Contents
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population
Significant HbA1c Lowering in Patients Achieving a Hepatitis C Virus Cure

Click here to access March 2019 Combatting Public Pathogens
Table of Contents
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population
Significant HbA1c Lowering in Patients Achieving a Hepatitis C Virus Cure

Click here to access March 2019 Combatting Public Pathogens
Table of Contents
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population
Significant HbA1c Lowering in Patients Achieving a Hepatitis C Virus Cure

Ublituximab depletes B cells in phase 2 trial
DALLAS – (MS), according to phase 2 trial results presented at ACTRIMS Forum 2019. Patients treated with the investigational therapy had reduced MRI activity and relapse rates during the 48-week trial, and the treatment was well tolerated, researchers said.
The monoclonal antibody targets a unique epitope on the CD20 antigen and is glycoengineered for enhanced B-cell targeting through antibody-dependent cellular cytotoxicity, said presenting author Edward Fox, MD, PhD, director of the MS Clinic of Central Texas, Round Rock. Ublituximab’s potency “may offer a benefit over currently available anti-CD20s in terms of lower doses and shorter infusion times,” Dr. Fox and his research colleagues said.
To assess the optimal dose, infusion time, safety, and tolerability of ublituximab in relapsing MS, investigators conducted a phase 2, multicenter study. The trial included 48 patients with relapsing MS; 65% were female. Patients’ average age was 40 years and average disease duration was 7.7 years. The researchers included patients with one or more confirmed relapse in the past year, two relapses in the past 2 years, or at least one active gadolinium-enhancing T1 lesion. The primary endpoint was the percentage of patients with at least a 95% reduction in peripheral CD19+ B cells within 2 weeks after the second infusion on day 15.
For their first infusions, patients received 150 mg of ublituximab over an infusion time of 1, 2, 3, or 4 hours. On day 15, patients received 450 mg or 600 mg of ublituximab over an infusion time of 1, 1.5, or 3 hours. At week 24, patients received 450 mg or 650 mg of ublituximab infused over 1 hour or 1.5 hours.
All patients met the primary endpoint of greater than 95% B-cell depletion between baseline and week 4. Median B-cell depletion was 99% at week 4, and this effect was maintained at weeks 24 and 48.
The researchers detected no T1 gadolinium-enhancing lesions at week 24 or week 48, and total T2 lesion volume decreased by 10.6% between baseline and week 48.
The most frequent adverse events were infusion-related reactions, which occurred in 48% of patients and were more common with the first infusion, particularly when the infusion time was less than 4 hours. All of the infusion-related reactions were grade 1 or 2. One grade 3 serious adverse event of fatigue was considered possibly related to ublituximab. No patients withdrew from the study because of drug-related adverse events. At week 48, 93% of the patients were relapse free, 7% had 24-week confirmed disability progression, and 17% had confirmed disability improvement.
TG Therapeutics, the company developing ublituximab, is evaluating the therapy in phase 3 trials known as ULTIMATE I and 2. The phase 3 trials are using the 450-mg dose with a first dose of 150 mg delivered over 4 hours.
Dr. Fox has disclosed research support from TG Therapeutics and other pharmaceutical companies and working as a consultant and speaker for TG Therapeutics and other companies.
SOURCE: Fox E et al. ACTRIMS Forum 2019, Abstract 66.
DALLAS – (MS), according to phase 2 trial results presented at ACTRIMS Forum 2019. Patients treated with the investigational therapy had reduced MRI activity and relapse rates during the 48-week trial, and the treatment was well tolerated, researchers said.
The monoclonal antibody targets a unique epitope on the CD20 antigen and is glycoengineered for enhanced B-cell targeting through antibody-dependent cellular cytotoxicity, said presenting author Edward Fox, MD, PhD, director of the MS Clinic of Central Texas, Round Rock. Ublituximab’s potency “may offer a benefit over currently available anti-CD20s in terms of lower doses and shorter infusion times,” Dr. Fox and his research colleagues said.
To assess the optimal dose, infusion time, safety, and tolerability of ublituximab in relapsing MS, investigators conducted a phase 2, multicenter study. The trial included 48 patients with relapsing MS; 65% were female. Patients’ average age was 40 years and average disease duration was 7.7 years. The researchers included patients with one or more confirmed relapse in the past year, two relapses in the past 2 years, or at least one active gadolinium-enhancing T1 lesion. The primary endpoint was the percentage of patients with at least a 95% reduction in peripheral CD19+ B cells within 2 weeks after the second infusion on day 15.
For their first infusions, patients received 150 mg of ublituximab over an infusion time of 1, 2, 3, or 4 hours. On day 15, patients received 450 mg or 600 mg of ublituximab over an infusion time of 1, 1.5, or 3 hours. At week 24, patients received 450 mg or 650 mg of ublituximab infused over 1 hour or 1.5 hours.
All patients met the primary endpoint of greater than 95% B-cell depletion between baseline and week 4. Median B-cell depletion was 99% at week 4, and this effect was maintained at weeks 24 and 48.
The researchers detected no T1 gadolinium-enhancing lesions at week 24 or week 48, and total T2 lesion volume decreased by 10.6% between baseline and week 48.
The most frequent adverse events were infusion-related reactions, which occurred in 48% of patients and were more common with the first infusion, particularly when the infusion time was less than 4 hours. All of the infusion-related reactions were grade 1 or 2. One grade 3 serious adverse event of fatigue was considered possibly related to ublituximab. No patients withdrew from the study because of drug-related adverse events. At week 48, 93% of the patients were relapse free, 7% had 24-week confirmed disability progression, and 17% had confirmed disability improvement.
TG Therapeutics, the company developing ublituximab, is evaluating the therapy in phase 3 trials known as ULTIMATE I and 2. The phase 3 trials are using the 450-mg dose with a first dose of 150 mg delivered over 4 hours.
Dr. Fox has disclosed research support from TG Therapeutics and other pharmaceutical companies and working as a consultant and speaker for TG Therapeutics and other companies.
SOURCE: Fox E et al. ACTRIMS Forum 2019, Abstract 66.
DALLAS – (MS), according to phase 2 trial results presented at ACTRIMS Forum 2019. Patients treated with the investigational therapy had reduced MRI activity and relapse rates during the 48-week trial, and the treatment was well tolerated, researchers said.
The monoclonal antibody targets a unique epitope on the CD20 antigen and is glycoengineered for enhanced B-cell targeting through antibody-dependent cellular cytotoxicity, said presenting author Edward Fox, MD, PhD, director of the MS Clinic of Central Texas, Round Rock. Ublituximab’s potency “may offer a benefit over currently available anti-CD20s in terms of lower doses and shorter infusion times,” Dr. Fox and his research colleagues said.
To assess the optimal dose, infusion time, safety, and tolerability of ublituximab in relapsing MS, investigators conducted a phase 2, multicenter study. The trial included 48 patients with relapsing MS; 65% were female. Patients’ average age was 40 years and average disease duration was 7.7 years. The researchers included patients with one or more confirmed relapse in the past year, two relapses in the past 2 years, or at least one active gadolinium-enhancing T1 lesion. The primary endpoint was the percentage of patients with at least a 95% reduction in peripheral CD19+ B cells within 2 weeks after the second infusion on day 15.
For their first infusions, patients received 150 mg of ublituximab over an infusion time of 1, 2, 3, or 4 hours. On day 15, patients received 450 mg or 600 mg of ublituximab over an infusion time of 1, 1.5, or 3 hours. At week 24, patients received 450 mg or 650 mg of ublituximab infused over 1 hour or 1.5 hours.
All patients met the primary endpoint of greater than 95% B-cell depletion between baseline and week 4. Median B-cell depletion was 99% at week 4, and this effect was maintained at weeks 24 and 48.
The researchers detected no T1 gadolinium-enhancing lesions at week 24 or week 48, and total T2 lesion volume decreased by 10.6% between baseline and week 48.
The most frequent adverse events were infusion-related reactions, which occurred in 48% of patients and were more common with the first infusion, particularly when the infusion time was less than 4 hours. All of the infusion-related reactions were grade 1 or 2. One grade 3 serious adverse event of fatigue was considered possibly related to ublituximab. No patients withdrew from the study because of drug-related adverse events. At week 48, 93% of the patients were relapse free, 7% had 24-week confirmed disability progression, and 17% had confirmed disability improvement.
TG Therapeutics, the company developing ublituximab, is evaluating the therapy in phase 3 trials known as ULTIMATE I and 2. The phase 3 trials are using the 450-mg dose with a first dose of 150 mg delivered over 4 hours.
Dr. Fox has disclosed research support from TG Therapeutics and other pharmaceutical companies and working as a consultant and speaker for TG Therapeutics and other companies.
SOURCE: Fox E et al. ACTRIMS Forum 2019, Abstract 66.
REPORTING FROM ACTRIMS FORUM 2019
CSF biomarker clusters correlate with MS severity
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
DALLAS – Patients with multiple sclerosis (MS) have elevated levels of specific clusters of cerebrospinal fluid (CSF) biomarkers related to astrocytes and microglia that correlated with disease severity in a blinded analysis of more than 1,000 proteins from the CSF of more than 400 patients with neuroimmunologic disease and healthy volunteers.
Previous studies have indicated that aberrant activation of astrocytes and microglia underlies disability progression in older patients with MS, but researchers have lacked biomarkers of these processes in living subjects. In a presentation at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Ruturaj R. Masvekar, PhD, described developing biomarkers of CNS cell–specific processes and examining how they relate to MS disability progression. Dr. Masvekar, a researcher at the National Institute of Allergy and Infectious Diseases, and his coinvestigators used a modified DNA aptamer assay to measure proteins in the CSF of 431 patients with neuroimmunologic diseases and healthy volunteers, followed by variable cluster analysis and in vitro modeling to define 64 clusters of CSF biomarkers that relate to CNS cell types.
The study included 42 healthy donors, 20 patients with clinically isolated syndrome, 57 patients with noninflammatory neurologic disorders, 127 patients with relapsing-remitting MS, 72 patients with secondary progressive MS, and 113 patients with primary progressive MS. In a training cohort of 217 participants, the researchers assessed how biomarkers differed between the diagnostic categories. The researchers then validated the results in an independent cohort of 214 participants.
One astrocyte-related cluster (MMP7, SERPINA3, GZMA, and CLIC1) and one microglia-related cluster (DSG2 and TNFRSF25) was significantly elevated in all MS subgroups, compared with healthy controls and patients with noninflammatory neurologic disorders.
In addition, these clusters “significantly correlated with clinical measures of disability, CNS tissue destruction, and MS severity,” Dr. Masvekar said.
The microglial cluster was significantly elevated in all MS subgroups, whereas neuronal endothelial, astrocytic, and oligodendroglial biomarker clusters were elevated only in patients with progressive MS.
“Microglial activation is present in all stages of MS, while toxic astrogliosis increases with MS duration, concomitantly with neuronal and oligodendroglial degeneration,” Dr. Masvekar said. “Microglial activation and toxic astrogliosis likely partake in CNS tissue destruction and enhance MS severity.”
This study, which was recently published in Multiple Sclerosis and Related Disorders (2019 Feb;28:34-43), was supported by the intramural research program at NIAID.
jremaly@mdedge.com
SOURCE: Masvekar RR et al. ACTRIMS Forum 2019, Abstract 281.
REPORTING FROM ACTRIMS FORUM 2019
Just over half of MS patients get DMD therapy
The finding comes from a retrospective analysis of claims data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This rate of DMD treatment merits further exploration given that evidence suggests the importance of early DMD treatment initiation in patients with a confirmed diagnosis of relapsing forms of MS to help optimize the benefits of treatment,” Amy L. Phillips, PharmD, a study author, said in an interview in advance of the meeting.
Little is known about current DMD treatment patterns in U.S. patients with multiple sclerosis following an increase in the number of available DMDs in recent years, according to Dr. Phillips of the department of health economics and outcomes research at EMD Serono.
“Most previous studies have focused on self-injectable DMDs or self-injectable and infusion DMDs,” she said. “Information about the treatment patterns including oral DMDs is scarce, particularly in large population samples. The objective of our study was to describe treatment patterns and sequences of therapy among U.S. patients with MS newly initiating DMD treatment between Jan. 1, 2011 and June 30, 2015.”
Dr. Phillips, lead study author Jacqueline A. Nicholas, MD, MPH, of the OhioHealth Multiple Sclerosis Center, and their colleagues used data from the IQVIA RWD Adjudicated Claims database to identify patients who had at least two medical claims with a MS diagnosis and at least one DMD claim during the study period. Other eligibility criteria included continuous eligibility with commercial insurance 1 year before (baseline period) and 2 years after (follow-up period) initiation of the DMD, no evidence of DMD use during the baseline period, and being aged between 18 and 63 years.
Of 63,946 diagnostically eligible patients, 36,175 (57%) had a claim for a DMD. The researchers reported findings from 8,251 patients who met all of the eligibility criteria. Their mean age was 43 years, 76% were female, and the mean number of DMDs over 2 years among newly treated patients was 1.27.
The most common first-line DMD therapy was glatiramer acetate (GA, 38%), followed by intramuscular interferon beta (IM IFNb-1a, 14%), subcutaneous interferon beta (SC IFNb-1a, 14%), dimethyl fumarate (DMF, 14%), and fingolimod (9%). DMF was the most common second-line therapy (36%), followed by fingolimod (17%), GA (17%), SC IFNb-1a (8%), and IM IFNb-1a (7%).
“Numerous DMD treatment patterns observed in this study highlight the diverse patient and treatment needs,” Dr. Phillips said. “DMD treatment patterns in MS vary due to the heterogeneity of the disease, physician preferences, and patient needs and treatment goals. Patient-centered care and shared decision making has been shown to improve patient satisfaction and to encourage treatment adherence in MS.”
She acknowledged certain limitations of the study, including the fact that the analysis presents only the most common DMD treatment sequences observed in this patient population.
“Future analyses might examine less common DMD treatment sequences,” she said. “Also, more research is needed to understand how DMD treatment patterns and sequences change over time, and the factors that may be associated with DMD switching and treatment discontinuation.”
She also noted that the administrative claims data studied represent mostly patients with commercial health insurance, limiting the generalizability of the findings. Further, ICD-9 and ICD-10 codes do not distinguish between MS types.
Funding for the study was provided by EMD Serono, the biopharmaceutical business of Merck KGaA, Darmstadt, Germany, in the United States and Canada. Dr. Phillips is an employee of the company.
SOURCE: Phillips A et al. ACTRIMS FORUM 2019, Abstract 001.
The finding comes from a retrospective analysis of claims data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This rate of DMD treatment merits further exploration given that evidence suggests the importance of early DMD treatment initiation in patients with a confirmed diagnosis of relapsing forms of MS to help optimize the benefits of treatment,” Amy L. Phillips, PharmD, a study author, said in an interview in advance of the meeting.
Little is known about current DMD treatment patterns in U.S. patients with multiple sclerosis following an increase in the number of available DMDs in recent years, according to Dr. Phillips of the department of health economics and outcomes research at EMD Serono.
“Most previous studies have focused on self-injectable DMDs or self-injectable and infusion DMDs,” she said. “Information about the treatment patterns including oral DMDs is scarce, particularly in large population samples. The objective of our study was to describe treatment patterns and sequences of therapy among U.S. patients with MS newly initiating DMD treatment between Jan. 1, 2011 and June 30, 2015.”
Dr. Phillips, lead study author Jacqueline A. Nicholas, MD, MPH, of the OhioHealth Multiple Sclerosis Center, and their colleagues used data from the IQVIA RWD Adjudicated Claims database to identify patients who had at least two medical claims with a MS diagnosis and at least one DMD claim during the study period. Other eligibility criteria included continuous eligibility with commercial insurance 1 year before (baseline period) and 2 years after (follow-up period) initiation of the DMD, no evidence of DMD use during the baseline period, and being aged between 18 and 63 years.
Of 63,946 diagnostically eligible patients, 36,175 (57%) had a claim for a DMD. The researchers reported findings from 8,251 patients who met all of the eligibility criteria. Their mean age was 43 years, 76% were female, and the mean number of DMDs over 2 years among newly treated patients was 1.27.
The most common first-line DMD therapy was glatiramer acetate (GA, 38%), followed by intramuscular interferon beta (IM IFNb-1a, 14%), subcutaneous interferon beta (SC IFNb-1a, 14%), dimethyl fumarate (DMF, 14%), and fingolimod (9%). DMF was the most common second-line therapy (36%), followed by fingolimod (17%), GA (17%), SC IFNb-1a (8%), and IM IFNb-1a (7%).
“Numerous DMD treatment patterns observed in this study highlight the diverse patient and treatment needs,” Dr. Phillips said. “DMD treatment patterns in MS vary due to the heterogeneity of the disease, physician preferences, and patient needs and treatment goals. Patient-centered care and shared decision making has been shown to improve patient satisfaction and to encourage treatment adherence in MS.”
She acknowledged certain limitations of the study, including the fact that the analysis presents only the most common DMD treatment sequences observed in this patient population.
“Future analyses might examine less common DMD treatment sequences,” she said. “Also, more research is needed to understand how DMD treatment patterns and sequences change over time, and the factors that may be associated with DMD switching and treatment discontinuation.”
She also noted that the administrative claims data studied represent mostly patients with commercial health insurance, limiting the generalizability of the findings. Further, ICD-9 and ICD-10 codes do not distinguish between MS types.
Funding for the study was provided by EMD Serono, the biopharmaceutical business of Merck KGaA, Darmstadt, Germany, in the United States and Canada. Dr. Phillips is an employee of the company.
SOURCE: Phillips A et al. ACTRIMS FORUM 2019, Abstract 001.
The finding comes from a retrospective analysis of claims data presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “This rate of DMD treatment merits further exploration given that evidence suggests the importance of early DMD treatment initiation in patients with a confirmed diagnosis of relapsing forms of MS to help optimize the benefits of treatment,” Amy L. Phillips, PharmD, a study author, said in an interview in advance of the meeting.
Little is known about current DMD treatment patterns in U.S. patients with multiple sclerosis following an increase in the number of available DMDs in recent years, according to Dr. Phillips of the department of health economics and outcomes research at EMD Serono.
“Most previous studies have focused on self-injectable DMDs or self-injectable and infusion DMDs,” she said. “Information about the treatment patterns including oral DMDs is scarce, particularly in large population samples. The objective of our study was to describe treatment patterns and sequences of therapy among U.S. patients with MS newly initiating DMD treatment between Jan. 1, 2011 and June 30, 2015.”
Dr. Phillips, lead study author Jacqueline A. Nicholas, MD, MPH, of the OhioHealth Multiple Sclerosis Center, and their colleagues used data from the IQVIA RWD Adjudicated Claims database to identify patients who had at least two medical claims with a MS diagnosis and at least one DMD claim during the study period. Other eligibility criteria included continuous eligibility with commercial insurance 1 year before (baseline period) and 2 years after (follow-up period) initiation of the DMD, no evidence of DMD use during the baseline period, and being aged between 18 and 63 years.
Of 63,946 diagnostically eligible patients, 36,175 (57%) had a claim for a DMD. The researchers reported findings from 8,251 patients who met all of the eligibility criteria. Their mean age was 43 years, 76% were female, and the mean number of DMDs over 2 years among newly treated patients was 1.27.
The most common first-line DMD therapy was glatiramer acetate (GA, 38%), followed by intramuscular interferon beta (IM IFNb-1a, 14%), subcutaneous interferon beta (SC IFNb-1a, 14%), dimethyl fumarate (DMF, 14%), and fingolimod (9%). DMF was the most common second-line therapy (36%), followed by fingolimod (17%), GA (17%), SC IFNb-1a (8%), and IM IFNb-1a (7%).
“Numerous DMD treatment patterns observed in this study highlight the diverse patient and treatment needs,” Dr. Phillips said. “DMD treatment patterns in MS vary due to the heterogeneity of the disease, physician preferences, and patient needs and treatment goals. Patient-centered care and shared decision making has been shown to improve patient satisfaction and to encourage treatment adherence in MS.”
She acknowledged certain limitations of the study, including the fact that the analysis presents only the most common DMD treatment sequences observed in this patient population.
“Future analyses might examine less common DMD treatment sequences,” she said. “Also, more research is needed to understand how DMD treatment patterns and sequences change over time, and the factors that may be associated with DMD switching and treatment discontinuation.”
She also noted that the administrative claims data studied represent mostly patients with commercial health insurance, limiting the generalizability of the findings. Further, ICD-9 and ICD-10 codes do not distinguish between MS types.
Funding for the study was provided by EMD Serono, the biopharmaceutical business of Merck KGaA, Darmstadt, Germany, in the United States and Canada. Dr. Phillips is an employee of the company.
SOURCE: Phillips A et al. ACTRIMS FORUM 2019, Abstract 001.
REPORTING FROM ACTRIMS FORUM 2019
Flu season shows signs of peaking
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.
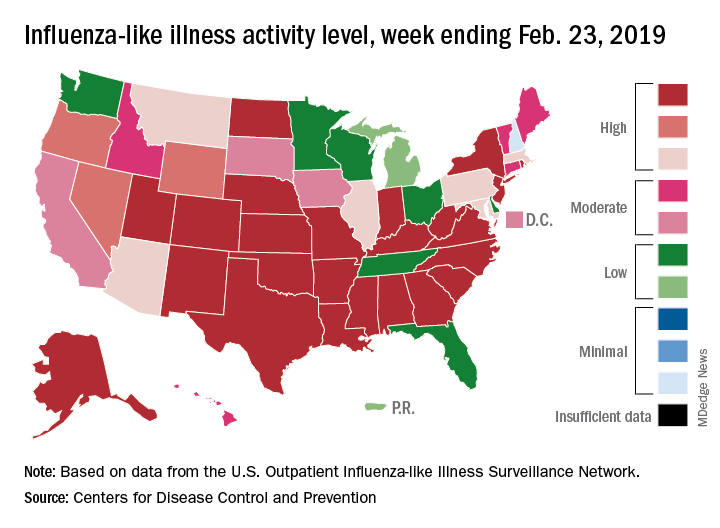
Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
Watch for depression symptom trajectory in high-risk young adults
Severity, variability of symptoms may be only predictor of suicide attempts.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
Severity, variability of symptoms may be only predictor of suicide attempts.
Severity, variability of symptoms may be only predictor of suicide attempts.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
Among the trajectories of clinical predictors of suicide attempt, depression symptoms were the only ones linked with an increased risk of suicide attempt in young adults whose parents have mood disorders, according to a longitudinal study.
Psychiatric diagnoses are well established as predictors of suicidal behavior; however, symptoms and risk can vary over the course of illness, and it is important to identify symptoms that can change over time, wrote Nadine M. Melhem, PhD, associate professor of psychiatry at the University of Pittsburgh, and her associates. The report is in JAMA Psychiatry.
Between July 15, 1997, and Sept. 6, 2005, 663 adolescents and young adults (mean age, 23.8 years) whose parents have mood disorders were recruited and followed until Jan. 21, 2014. All participants were assessed at baseline and every year for up to 12 years (median follow-up, 8.1 years) for lifetime and current psychiatric disorders as well as suicidal ideation. In addition, participants were assessed at baseline and at each follow-up for the trajectory of depression symptoms, hopelessness, impulsivity, aggression, impulsive aggression, and irritability.
After the study period, participants were analyzed for all trajectories and separated into classes based on mean scores and variability. All trajectories except for depression had two classes, in which participants in class 2 had higher mean scores and variability; for depression, patients were separated into three classes, in which class 3 had the highest mean score and variability.
Over the study period, 71 of the 663 patients attempted suicide (10.7%), with 51 patients attempting suicide for the first time. The mean number of attempts was 1.2, and the median time from the last assessment to the attempt was 45 weeks.
(22.9% with vs. 27 without), class 2 impulsivity (38.8% vs. 21.7%), class 2 aggression (29.0% vs. 15.6%), class 2 impulsive aggression (76.5% vs. 52.2%), and class 2 irritability (39.4% vs. 22.7%). However, after adjustment for demographics, parental suicide attempts, and additional clinical characteristics, only class 3 depression remained associated with suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Other significant predictors of suicide attempts were younger age (OR, 0.82; 95% CI, 0.74-0.90; P less than .001), lifetime history of unipolar disorder (OR, 4.71; 95% CI, 1.63-13.58; P = .004), lifetime history of bipolar disorder (OR, 3.4; 95% CI, 0.96-12.04; P = .06), history of childhood abuse (OR, 2.98; 95% CI, 1.40-6.38; P = .01), and parental suicide attempt (OR, 2.24; 95% CI, 1.06-4.75; P = .04).
The investigators concluded that clinicians should “pay particular attention to the severity of both current and past depression and the variability in these symptoms, and monitor and treat depression symptoms over time to reduce symptom severity and fluctuation, and thus the likelihood for suicide attempt, in high-risk young adults.”
Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
SOURCE: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
FROM JAMA PSYCHIATRY
Key clinical point: Only depression symptoms were associated with a higher suicide attempt risk in young adults whose parents have mood disorders.
Major finding: The depression symptom trajectory with the highest mean scores and variability over time was the only measured trajectory that predicted suicide attempts (odds ratio, 4.72; 95% confidence interval, 1.47-15.21; P = .01).
Study details: A longitudinal study of 663 adolescents and younger adults whose parents have mood disorders.
Disclosures: Dr. Melhem reported receiving research support from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the American Foundation for Suicide Prevention. Several other coauthors also reported conflicts of interest.
Source: Melhem NM et al. JAMA Psychiatry. 2019 Feb 27. doi: 10.1001/jamapsychiatry.2018.4513.
Biologic aging is associated with MS disability progression
DALLAS – according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Shorter telomere length is associated with increased MS disability in cross-sectional and longitudinal analyses, said Kristen M. Krysko, MD, clinical fellow in neurology at University of California, San Francisco. The results suggest that biologic aging may contribute to neurologic injury in MS and that “targeting aging-related mechanisms may be a potential therapeutic strategy,” said Dr. Krysko.
If validated, telomere length may be a biomarker that neurologists could use to guide decisions about MS treatment, said principal investigator Jennifer Graves, MD, PhD, also of UCSF.
“Factors leading to MS progression are not fully understood,” Dr. Krysko said. “But consistently, older chronological age has been associated with a faster time to disability milestones.” Aging may reduce remyelination capacity and alter immunologic responses. Telomere shortening, a marker of cellular aging, is “the ultimate biological clock.” It has been associated with cardiovascular disease, dementia, and autoimmune diseases, and one study found that patients with primary progressive MS have shorter telomere length, compared with controls.
To assess whether LTL is associated with clinical disability and brain volume in patients with MS, the researchers analyzed data from 516 adults with MS or clinically isolated syndrome in the EPIC cohort study at UCSF. Telomere length was measured on stored baseline DNA samples and expressed as the ratio of telomere to a single-copy gene.
The patients had an average age of 43 years, median disease duration of 6 years, and median Expanded Disability Status Scale (EDSS) score of 1.5; about 70% were women. The average telomere length was 0.97.
Older age and longer disease duration were associated with shorter LTL. For every 0.2-unit decrease in telomere length, EDSS score increased by 0.41. After adjusting for age, disease duration, and sex, every 0.2-unit decrease in telomere length was associated with a score increase of 0.27 on the EDSS. LTL also was associated with total brain volume and total white matter volume.
In addition, the investigators conducted a case control study that included a subset of 23 patients who developed secondary progressive MS during follow-up and had DNA available at multiple time points. The researchers matched these patients with 23 patients who continued to have relapsing MS. Patients were matched by age, sex, and disease duration. An adjusted analysis found that change in LTL was predictive of change in EDSS over 10 years such that every 0.2-unit decrease in LTL was associated with a 0.34-unit increase in EDSS.
Longitudinal analyses found that baseline LTL predicted higher levels of disability over time.
The study was funded by the National Multiple Sclerosis Society.
SOURCE: Krysko KM et al. ACTRIMS Forum 2019, Abstract 289.
DALLAS – according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Shorter telomere length is associated with increased MS disability in cross-sectional and longitudinal analyses, said Kristen M. Krysko, MD, clinical fellow in neurology at University of California, San Francisco. The results suggest that biologic aging may contribute to neurologic injury in MS and that “targeting aging-related mechanisms may be a potential therapeutic strategy,” said Dr. Krysko.
If validated, telomere length may be a biomarker that neurologists could use to guide decisions about MS treatment, said principal investigator Jennifer Graves, MD, PhD, also of UCSF.
“Factors leading to MS progression are not fully understood,” Dr. Krysko said. “But consistently, older chronological age has been associated with a faster time to disability milestones.” Aging may reduce remyelination capacity and alter immunologic responses. Telomere shortening, a marker of cellular aging, is “the ultimate biological clock.” It has been associated with cardiovascular disease, dementia, and autoimmune diseases, and one study found that patients with primary progressive MS have shorter telomere length, compared with controls.
To assess whether LTL is associated with clinical disability and brain volume in patients with MS, the researchers analyzed data from 516 adults with MS or clinically isolated syndrome in the EPIC cohort study at UCSF. Telomere length was measured on stored baseline DNA samples and expressed as the ratio of telomere to a single-copy gene.
The patients had an average age of 43 years, median disease duration of 6 years, and median Expanded Disability Status Scale (EDSS) score of 1.5; about 70% were women. The average telomere length was 0.97.
Older age and longer disease duration were associated with shorter LTL. For every 0.2-unit decrease in telomere length, EDSS score increased by 0.41. After adjusting for age, disease duration, and sex, every 0.2-unit decrease in telomere length was associated with a score increase of 0.27 on the EDSS. LTL also was associated with total brain volume and total white matter volume.
In addition, the investigators conducted a case control study that included a subset of 23 patients who developed secondary progressive MS during follow-up and had DNA available at multiple time points. The researchers matched these patients with 23 patients who continued to have relapsing MS. Patients were matched by age, sex, and disease duration. An adjusted analysis found that change in LTL was predictive of change in EDSS over 10 years such that every 0.2-unit decrease in LTL was associated with a 0.34-unit increase in EDSS.
Longitudinal analyses found that baseline LTL predicted higher levels of disability over time.
The study was funded by the National Multiple Sclerosis Society.
SOURCE: Krysko KM et al. ACTRIMS Forum 2019, Abstract 289.
DALLAS – according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Shorter telomere length is associated with increased MS disability in cross-sectional and longitudinal analyses, said Kristen M. Krysko, MD, clinical fellow in neurology at University of California, San Francisco. The results suggest that biologic aging may contribute to neurologic injury in MS and that “targeting aging-related mechanisms may be a potential therapeutic strategy,” said Dr. Krysko.
If validated, telomere length may be a biomarker that neurologists could use to guide decisions about MS treatment, said principal investigator Jennifer Graves, MD, PhD, also of UCSF.
“Factors leading to MS progression are not fully understood,” Dr. Krysko said. “But consistently, older chronological age has been associated with a faster time to disability milestones.” Aging may reduce remyelination capacity and alter immunologic responses. Telomere shortening, a marker of cellular aging, is “the ultimate biological clock.” It has been associated with cardiovascular disease, dementia, and autoimmune diseases, and one study found that patients with primary progressive MS have shorter telomere length, compared with controls.
To assess whether LTL is associated with clinical disability and brain volume in patients with MS, the researchers analyzed data from 516 adults with MS or clinically isolated syndrome in the EPIC cohort study at UCSF. Telomere length was measured on stored baseline DNA samples and expressed as the ratio of telomere to a single-copy gene.
The patients had an average age of 43 years, median disease duration of 6 years, and median Expanded Disability Status Scale (EDSS) score of 1.5; about 70% were women. The average telomere length was 0.97.
Older age and longer disease duration were associated with shorter LTL. For every 0.2-unit decrease in telomere length, EDSS score increased by 0.41. After adjusting for age, disease duration, and sex, every 0.2-unit decrease in telomere length was associated with a score increase of 0.27 on the EDSS. LTL also was associated with total brain volume and total white matter volume.
In addition, the investigators conducted a case control study that included a subset of 23 patients who developed secondary progressive MS during follow-up and had DNA available at multiple time points. The researchers matched these patients with 23 patients who continued to have relapsing MS. Patients were matched by age, sex, and disease duration. An adjusted analysis found that change in LTL was predictive of change in EDSS over 10 years such that every 0.2-unit decrease in LTL was associated with a 0.34-unit increase in EDSS.
Longitudinal analyses found that baseline LTL predicted higher levels of disability over time.
The study was funded by the National Multiple Sclerosis Society.
SOURCE: Krysko KM et al. ACTRIMS Forum 2019, Abstract 289.
REPORTING FROM ACTRIMS FORUM 2019
Tanuja Chitnis: “It’s the right time” for precision medicine in MS
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
koakes@mdedge.com
REPORTING FROM ACTRIMS FORUM 2019
Novel capsid assembly modulator shows promise in HBV
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
FROM GASTROENTEROLOGY











