User login
Sperm counts largely stable after adjuvant treatment of clinical stage I testicular cancer
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
FROM ANNALS OF ONCOLOGY
Diet appears to play an important role in response to anti-PD-1 cancer immunotherapy
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines
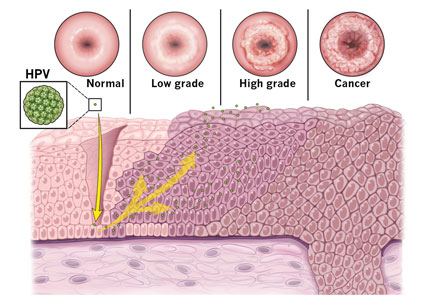
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
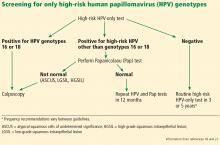
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
KEY POINTS
- Immunization against HPV can prevent up to 70% of HPV-related cervical cancer cases.
- Gardasil 9 is the only HPV vaccine currently available in the United States and is now approved for use in males and females between the ages of 9 and 45.
- In girls and boys younger than 15, a 2-dose schedule is recommended; patients ages 15 through 45 require 3 doses.
- Vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions.
- Regular cervical cancer screening is an important preventive tool and should be performed using the Papanicolaou (Pap) test, the high-risk HPV-only test, or the Pap-HPV cotest.
Charity case
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Melatonin update, Part 2
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Three neglected numbers in the CBC: The RDW, MPV, and NRBC count
The complete blood cell count (CBC) is one of the most frequently ordered laboratory tests in both the inpatient and outpatient settings. Not long ago, the CBC required peering through a microscope and counting the red blood cells, white blood cells, and platelets. These 3 numbers are still the primary purpose of the test.
Now, with automated counters, the CBC report also contains other numbers that delineate characteristics of each cell type. For example:
The mean corpuscular volume is the average volume of red blood cells. Providers use it to classify anemia as either microcytic, normocytic, or macrocytic, each with its own differential diagnosis.
The differential white blood cell count provides absolute counts and relative percentages of each type of leukocyte. For example, the absolute neutrophil count is an important measure of immunocompetence.
But other values in the CBC may be overlooked, even though they can provide important information. Here, we highlight 3 of them:
- The red blood cell distribution width (RDW)
- The mean platelet volume (MPV)
- The nucleated red blood cell (NRBC) count.
In addition to describing their diagnostic utility, we also discuss emerging evidence of their potential prognostic significance in hematologic and nonhematologic disorders. By incorporating an awareness of their value in clinical practice, providers can maximize the usefulness of the CBC.
RED BLOOD CELL DISTRIBUTION WIDTH
The RDW is a measure of variation (anisocytosis) in the size of the circulating red cells. The term “width” is misleading, as the value is not derived from the width of the red blood cell, but rather from the width of the distribution curve of the corpuscular volume (Figure 1). Therefore, a normal RDW means that the cells are all about the same size, while a high RDW means they vary widely in size.
The RDW can be calculated either as a coefficient of variation, with a reference range of 11% to 16% depending on the laboratory, or, less often, as a standard deviation, with a reference range of 39 to 46 fL.
The RDW can differentiate between causes of anemia
A high RDW is often found in nutritional deficiencies of iron, vitamin B12, and folate. This information is helpful in differentiating the cause of microcytic anemia, as a high RDW suggests iron-deficiency anemia while a normal RDW suggests thalassemia.1 In iron deficiency, the RDW often rises before the mean corpuscular volume falls, serving as an early diagnostic clue.
The RDW can also be high after recent hemorrhage or rapid hemolysis, as the acute drop in hemoglobin results in increased production of reticulocytes, which are larger than mature erythrocytes.
Because a range of disorders can elevate the RDW, reviewing the peripheral blood smear is an important next step in the diagnostic evaluation, specifically looking for reticulocytes, microspherocytes, and other abnormal red blood cells contributing to the RDW elevation.
A normal RDW is less diagnostically useful. It indicates the red blood cells are of uniform size, but they may be uniformly small or large depending on how long the anemia has persisted. Since red cells circulate for only about 120 days, patients who have severe iron-deficiency anemia for months to years are expected to have a normal rather than a high RDW, as their red cells of normal size have all been replaced by microcytes.
A low RDW is not consistently associated with any hematologic disorder.
RDW may have prognostic value
Emerging data suggest that the RDW may also have prognostic value in nonhematologic diseases. In a retrospective study of 15,852 adult participants in the Third National Health and Nutrition Examination Survey (1988–1994), a higher RDW was associated with a higher risk of death, with the all-cause mortality rate increasing by 23% for every 1% increment in RDW.2
This correlation is particularly prominent in cardiac disorders. In 2 large retrospective studies of patients with symptomatic heart failure, a higher RDW was a strong predictor of morbidity and death (hazard ratio 1.17 per 1-standard deviation increase, P < .001), even stronger than more commonly used variables such as ejection fraction, New York Heart Association functional class, and renal function.3
In a retrospective analysis of 4,111 patients with myocardial infarction, the degree of RDW elevation correlated with the risk of repeat nonfatal myocardial infarction, coronary death, new symptomatic heart failure, and stroke.4
It is hypothesized that high RDW may reflect poor cell membrane integrity from altered cholesterol content, which in turn has deleterious effects on multiple organ systems and is therefore associated with adverse outcomes.5
Currently, using the RDW to assess prognosis remains investigational, and how best to interpret it in daily practice requires further study.
MEAN PLATELET VOLUME
The MPV, ie, the average size of platelets, is reported in femtoliters (fL). Because the MPV varies depending on the instrument used, each laboratory has a unique reference range, usually about 8 to 12 fL. The MPV must be interpreted in conjunction with the platelet count; the product of the MPV and platelet count is called the total platelet mass.
Using the MPV to find the cause of thrombocytopenia
The MPV can be used to help narrow the differential diagnosis of thrombocytopenia. For example, it is high in thrombocytopenia resulting from peripheral destruction, as in immune thrombocytopenic purpura. This is because as platelets are lost, thrombopoietin production increases and new, larger platelets are released from healthy megakaryocytes in an attempt to increase the total platelet mass.
In contrast, the MPV is low in patients with thrombocytopenia due to megakaryocyte hypoplasia, as malfunctioning megakaryocytes cannot maintain the total platelet mass, and any platelets produced remain small. This distinction can be obscured in the setting of splenomegaly, as larger platelets are more easily sequestered in the spleen and the MPV may therefore be low or normal.
The MPV can also be used to differentiate congenital thrombocytopenic disorders, which can be characterized by either a high MPV (eg, gray platelet syndrome, Bernard-Soulier syndrome) or a low MPV (eg, Wiskott-Aldrich syndrome) (Figure 2).
MPV may have prognostic value
Evidence suggests that the MPV also has potential prognostic value, particularly in vascular disease, as larger platelets are hypothesized to have increased hemostatic potential.
In a large meta-analysis of patients with coronary artery disease, a high MPV was associated with worse outcomes; the risk of death or myocardial infarction was 17% higher in those with a high MPV (the threshold ranged from 8.4 to 11.7 fL in the different studies) than in those with a low MPV.6
In a study of 213 patients with non-ST-segment elevation myocardial infarction, the risk of significant coronary artery disease was 4.18 times higher in patients with a high MPV and a high troponin level than in patients with a normal MPV and a high troponin.7 The authors suggested that a high MPV may help identify patients at highest risk of significant coronary artery disease who would benefit from invasive studies (ie, coronary angiography).
This correlation has also been observed in other forms of vascular disease. In 261 patients who underwent carotid angioplasty and stenting, an MPV higher than 10.1 fL was associated with a risk of in-stent restenosis more than 3 times higher.8
The MPV has also been found to be higher in patients with type 2 diabetes than in controls, particularly in those with microvascular complications such as retinopathy or microalbuminuria.9
Conversely, in patients with cancer, a low MPV appears to be associated with a poor prognosis. In a retrospective analysis of 236 patients with esophageal cancer, those who had an MPV of 7.4 fL or less had significantly shorter overall survival than patients with an MPV higher than 7.4 fL.10
A low MPV has also been associated with an increased risk of venous thromoboembolism in patients with cancer. In a prospective observational cohort study of 1,544 patients, the 2-year probability of venous thromboembolism was 9% in patients with an MPV less than 10.8 fL, compared with 5.5% in those with higher MPV values. The 2-year overall survival rate was also higher in patients with high MPV than in those with low MPV, at 64.7% vs 55.7%, respectively (P = .001).11
But the MPV is far from a perfect clinical metric. Since its measurement is subject to significant laboratory variation, an abnormal value should always be confirmed with evaluation of a peripheral blood smear. Furthermore, it is unclear why a high MPV portends poor prognosis in patients without cancer, whereas the opposite is true in patients with cancer. Therefore, its role in prognostication remains investigational, and further studies are essential to determine its appropriate usefulness in clinical practice.12
NUCLEATED RED BLOOD CELL COUNT
NRBCs are immature red blood cell precursors not present in the circulation of healthy adults. During erythropoiesis, the common myeloid progenitor cell first differentiates into a proerythroblast; subsequently, the chromatin in the nucleus of the proerythroblast gradually condenses until it becomes an orthochromatic erythroblast, also known as a nucleated red cell (Figure 2). Once the nucleus is expelled, the cell is known as a reticulocyte, which ultimately becomes a mature erythrocyte.
Healthy newborns have circulating NRBCs that rapidly disappear within a few weeks of birth. However, NRBCs can return to the circulation in a variety of disease states.
Causes of NRBCs
Brisk hemolysis or rapid blood loss can cause NRBCs to be released into the blood as erythropoiesis increases in an attempt to compensate for acute anemia.
Damage or stress to the bone marrow also causes NRBCs to be released into the peripheral blood, as is often the case in hematologic diseases. In a study of 478 patients with hematologic diseases, the frequency of NRBC positivity at diagnosis was highest in patients with chronic myeloid leukemia (100%), acute leukemia (62%), and myelodysplastic syndromes (45%).13 NRBCs also appeared at higher frequencies during chemotherapy in other hematologic conditions, such as hemophagocytic lymphohistiocytosis.
The mechanism by which NRBCs are expelled from the bone marrow is unclear, though studies have suggested that inflammation or hypoxia or both cause increased hematopoietic stress, resulting in the release of immature red cells. Increased concentrations of inflammatory cytokines (interleukin 6 and interleukin 3) and erythropoietin in the plasma and decreased arterial oxygen partial tension have been reported in patients with circulating NRBCs.14,15
Because they are associated with hematologic disorders, the finding of NRBCs should prompt evaluation of a peripheral smear to assess for abnormalities in other cell lines.
The NRBC count and prognosis
In critically ill patients, peripheral NRBCs can also indicate life-threatening conditions.
In a study of 421 adult intensive care patients, the in-hospital mortality rate was 42% in those with peripheral NRBCs vs 5.9% in those without them.16 Further, the higher the NRBC count and the more days that NRBCs were reported in the CBC, the higher the risk of death.
In adults with acute respiratory distress syndrome, the finding of any NRBCs in the peripheral blood was an independent risk factor for death, and an NRBC count higher than 220 cells/µL was associated with a more than 3-fold higher risk of death.17
Daily screening in patients in surgical intensive care units revealed that NRBCs appeared an average of 9 days before death, consistent with an early marker of impending decline.18
In another study,19 the risk of death within 90 days of hospital discharge was higher in NRBC-positive patients, reaching 21.9% in those who had a count higher than 200 cells/µL. The risk of unplanned hospital readmission within 30 days was also increased.
Leukoerythroblastosis
The combination of NRBCs and immature white blood cells (eg, myelocytes, metamyelocytes) is called leukoerythroblastosis.
Leukoerythroblastosis is classically seen in myelophthisic anemias in which hematopoietic cells in the marrow are displaced by fibrosis, tumor, or other space-occupying processes, but it can also occur in any situation of acute marrow stress, including critical illness.
In addition, leukoerythroblastosis appears in a rare complication of sickle cell hemoglobinopathies: bone marrow necrosis with fat embolism syndrome.20,21 As the marrow necroses, fat emboli are released in the systemic circulation causing micro- and macrovascular occlusions and multiorgan failure. The largest case series in the literature reports 58 patients with bone marrow necrosis with fat embolism syndrome.22
At our institution, we have seen 18 patients with this condition in the past 8 years, with the frequency of diagnosis increasing with heightened awareness of the disorder. We have found that leukoerythroblastosis is often an early marker of this unrecognized syndrome and can prompt emergency red cell exchange, which is considered to be lifesaving in this condition.22
These examples and many others show that the presence of NRBCs in the CBC can serve as an important clinical warning.
OLD TESTS CAN STILL BE USEFUL
The CBC provides much more than simple cell counts; it is a rich collection of information related to each blood cell. These days, with new diagnostic tests and prognostic tools based on molecular analysis, it is important to not overlook the value of the tests clinicians have been ordering for generations.
The RDW, MPV, and NRBC count will not likely provide definitive or flawless diagnostic or prognostic information, but when understood and used correctly, they provide readily available, cost-effective, and useful data that can supplement and guide clinical decision-making. By understanding the CBC more fully, providers can maximize the truly complete nature of this routine laboratory test.
- Lima CS, Reis AR, Grotto HZ, Saad ST, Costa FF. Comparison of red cell distribution width and a red cell discriminant function incorporating volume dispersion for distinguishing iron deficiency from beta thalassemia trait in patients with microcytosis. Sao Paulo Med J 1996; 114(5):1265–1269. pmid:9239926
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009; 169(6):588–594. doi:10.1001/archinternmed.2009.55
- Felker GM, Allen LA, Pocock SJ, et al; CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007; 50(1):40–47. doi:10.1016/j.jacc.2007.02.067
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117(2):163–168. doi:10.1161/CIRCULATIONAHA.107.727545
- Goldstein MR, Mascitelli L, Pezzetta F. Is red cell distribution width a marker of overall membrane integrity? [Letter] Arch Intern Med 2009; 169(16):1539–1540. doi:10.1001/archinternmed.2009.275
- Sansanaydhu N, Numthavaj P, Muntham D, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015; 114(6):1299–1309. doi:10.1160/TH15-04-0280
- Taskesen T, Sekhon H, Wroblewski I, et al. Usefulness of mean platelet volume to predict significant coronary artery disease in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol 2017; 119(2):192–196. doi:10.1016/j.amjcard.2016.09.042
- Dai Z, Gao J, Li S, et al. Mean platelet volume as a predictor for restenosis after carotid angioplasty and stenting. Stroke 2018; 49(4):872–876. doi:10.1161/STROKEAHA.117.019748
- Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15(8):475–478. doi:10.1080/0953710042000267707
- Shen W, Cui MM, Wang X, Wang RT. Reduced mean platelet volume is associated with poor prognosis in esophageal cancer. Cancer Biomark 2018; 22(3):559–563. doi:10.3233/CBM-181231
- Riedl J, Kaider A, Reitter EM, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS). Thromb Haemost 2014; 111(4):670–678. doi:10.1160/TH13-07-0603
- Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost 2003; 9(3):177–190. pmid:14507105
- Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2011; 50(2):357–360. doi:10.1515/CCLM.2011.766
- Stachon A, Bolulul O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock 2005; 24(1):34–39. pmid:15988318
- Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med 2011; 49(2):257–263. doi:10.1515/CCLM.2011.041
- Stachon A, Holland-Letz T, Krieg M. In-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med 2004; 42(8):933–938. doi:10.1515/CCLM.2004.151
- Menk M, Giebelhäuser L, Vorderwülbecke G, et al. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensive Care 2018; 8(1):42. doi:10.1186/s13613-018-0387-5
- Stachon A, Kempf R, Holland-Letz T, Friese J, Becker A, Krieg M. Daily monitoring of nucleated red blood cells in the blood of surgical intensive care patients. Clin Chim Acta 2006; 366(1–2):329–335. doi:10.1016/j.cca.2005.11.022
- Purtle SW, Horkan CM, Moromizato T, Gibbons FK, Christopher KB. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: a cohort study. Crit Care 2017; 21(1):154. doi:10.1186/s13054-017-1724-z
- May J, Sullivan JC, LaVie D, LaVie K, Marques MB. Inside out: bone marrow necrosis and fat embolism complicating sickle-beta+ thalassemia. Am J Med 2016; 129(12):e321–e324. doi:10.1016/j.amjmed.2016.05.027
- Gangaraju R, Reddy VV, Marques MB. Fat embolism syndrome secondary to bone marrow necrosis in patients with hemoglobinopathies. South Med J 2016; 109(9):549–553. doi:10.14423/SMJ.0000000000000520
- Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 2014; 28(1):23–30. doi:10.1016/j.blre.2013.12.002
The complete blood cell count (CBC) is one of the most frequently ordered laboratory tests in both the inpatient and outpatient settings. Not long ago, the CBC required peering through a microscope and counting the red blood cells, white blood cells, and platelets. These 3 numbers are still the primary purpose of the test.
Now, with automated counters, the CBC report also contains other numbers that delineate characteristics of each cell type. For example:
The mean corpuscular volume is the average volume of red blood cells. Providers use it to classify anemia as either microcytic, normocytic, or macrocytic, each with its own differential diagnosis.
The differential white blood cell count provides absolute counts and relative percentages of each type of leukocyte. For example, the absolute neutrophil count is an important measure of immunocompetence.
But other values in the CBC may be overlooked, even though they can provide important information. Here, we highlight 3 of them:
- The red blood cell distribution width (RDW)
- The mean platelet volume (MPV)
- The nucleated red blood cell (NRBC) count.
In addition to describing their diagnostic utility, we also discuss emerging evidence of their potential prognostic significance in hematologic and nonhematologic disorders. By incorporating an awareness of their value in clinical practice, providers can maximize the usefulness of the CBC.
RED BLOOD CELL DISTRIBUTION WIDTH
The RDW is a measure of variation (anisocytosis) in the size of the circulating red cells. The term “width” is misleading, as the value is not derived from the width of the red blood cell, but rather from the width of the distribution curve of the corpuscular volume (Figure 1). Therefore, a normal RDW means that the cells are all about the same size, while a high RDW means they vary widely in size.
The RDW can be calculated either as a coefficient of variation, with a reference range of 11% to 16% depending on the laboratory, or, less often, as a standard deviation, with a reference range of 39 to 46 fL.
The RDW can differentiate between causes of anemia
A high RDW is often found in nutritional deficiencies of iron, vitamin B12, and folate. This information is helpful in differentiating the cause of microcytic anemia, as a high RDW suggests iron-deficiency anemia while a normal RDW suggests thalassemia.1 In iron deficiency, the RDW often rises before the mean corpuscular volume falls, serving as an early diagnostic clue.
The RDW can also be high after recent hemorrhage or rapid hemolysis, as the acute drop in hemoglobin results in increased production of reticulocytes, which are larger than mature erythrocytes.
Because a range of disorders can elevate the RDW, reviewing the peripheral blood smear is an important next step in the diagnostic evaluation, specifically looking for reticulocytes, microspherocytes, and other abnormal red blood cells contributing to the RDW elevation.
A normal RDW is less diagnostically useful. It indicates the red blood cells are of uniform size, but they may be uniformly small or large depending on how long the anemia has persisted. Since red cells circulate for only about 120 days, patients who have severe iron-deficiency anemia for months to years are expected to have a normal rather than a high RDW, as their red cells of normal size have all been replaced by microcytes.
A low RDW is not consistently associated with any hematologic disorder.
RDW may have prognostic value
Emerging data suggest that the RDW may also have prognostic value in nonhematologic diseases. In a retrospective study of 15,852 adult participants in the Third National Health and Nutrition Examination Survey (1988–1994), a higher RDW was associated with a higher risk of death, with the all-cause mortality rate increasing by 23% for every 1% increment in RDW.2
This correlation is particularly prominent in cardiac disorders. In 2 large retrospective studies of patients with symptomatic heart failure, a higher RDW was a strong predictor of morbidity and death (hazard ratio 1.17 per 1-standard deviation increase, P < .001), even stronger than more commonly used variables such as ejection fraction, New York Heart Association functional class, and renal function.3
In a retrospective analysis of 4,111 patients with myocardial infarction, the degree of RDW elevation correlated with the risk of repeat nonfatal myocardial infarction, coronary death, new symptomatic heart failure, and stroke.4
It is hypothesized that high RDW may reflect poor cell membrane integrity from altered cholesterol content, which in turn has deleterious effects on multiple organ systems and is therefore associated with adverse outcomes.5
Currently, using the RDW to assess prognosis remains investigational, and how best to interpret it in daily practice requires further study.
MEAN PLATELET VOLUME
The MPV, ie, the average size of platelets, is reported in femtoliters (fL). Because the MPV varies depending on the instrument used, each laboratory has a unique reference range, usually about 8 to 12 fL. The MPV must be interpreted in conjunction with the platelet count; the product of the MPV and platelet count is called the total platelet mass.
Using the MPV to find the cause of thrombocytopenia
The MPV can be used to help narrow the differential diagnosis of thrombocytopenia. For example, it is high in thrombocytopenia resulting from peripheral destruction, as in immune thrombocytopenic purpura. This is because as platelets are lost, thrombopoietin production increases and new, larger platelets are released from healthy megakaryocytes in an attempt to increase the total platelet mass.
In contrast, the MPV is low in patients with thrombocytopenia due to megakaryocyte hypoplasia, as malfunctioning megakaryocytes cannot maintain the total platelet mass, and any platelets produced remain small. This distinction can be obscured in the setting of splenomegaly, as larger platelets are more easily sequestered in the spleen and the MPV may therefore be low or normal.
The MPV can also be used to differentiate congenital thrombocytopenic disorders, which can be characterized by either a high MPV (eg, gray platelet syndrome, Bernard-Soulier syndrome) or a low MPV (eg, Wiskott-Aldrich syndrome) (Figure 2).
MPV may have prognostic value
Evidence suggests that the MPV also has potential prognostic value, particularly in vascular disease, as larger platelets are hypothesized to have increased hemostatic potential.
In a large meta-analysis of patients with coronary artery disease, a high MPV was associated with worse outcomes; the risk of death or myocardial infarction was 17% higher in those with a high MPV (the threshold ranged from 8.4 to 11.7 fL in the different studies) than in those with a low MPV.6
In a study of 213 patients with non-ST-segment elevation myocardial infarction, the risk of significant coronary artery disease was 4.18 times higher in patients with a high MPV and a high troponin level than in patients with a normal MPV and a high troponin.7 The authors suggested that a high MPV may help identify patients at highest risk of significant coronary artery disease who would benefit from invasive studies (ie, coronary angiography).
This correlation has also been observed in other forms of vascular disease. In 261 patients who underwent carotid angioplasty and stenting, an MPV higher than 10.1 fL was associated with a risk of in-stent restenosis more than 3 times higher.8
The MPV has also been found to be higher in patients with type 2 diabetes than in controls, particularly in those with microvascular complications such as retinopathy or microalbuminuria.9
Conversely, in patients with cancer, a low MPV appears to be associated with a poor prognosis. In a retrospective analysis of 236 patients with esophageal cancer, those who had an MPV of 7.4 fL or less had significantly shorter overall survival than patients with an MPV higher than 7.4 fL.10
A low MPV has also been associated with an increased risk of venous thromoboembolism in patients with cancer. In a prospective observational cohort study of 1,544 patients, the 2-year probability of venous thromboembolism was 9% in patients with an MPV less than 10.8 fL, compared with 5.5% in those with higher MPV values. The 2-year overall survival rate was also higher in patients with high MPV than in those with low MPV, at 64.7% vs 55.7%, respectively (P = .001).11
But the MPV is far from a perfect clinical metric. Since its measurement is subject to significant laboratory variation, an abnormal value should always be confirmed with evaluation of a peripheral blood smear. Furthermore, it is unclear why a high MPV portends poor prognosis in patients without cancer, whereas the opposite is true in patients with cancer. Therefore, its role in prognostication remains investigational, and further studies are essential to determine its appropriate usefulness in clinical practice.12
NUCLEATED RED BLOOD CELL COUNT
NRBCs are immature red blood cell precursors not present in the circulation of healthy adults. During erythropoiesis, the common myeloid progenitor cell first differentiates into a proerythroblast; subsequently, the chromatin in the nucleus of the proerythroblast gradually condenses until it becomes an orthochromatic erythroblast, also known as a nucleated red cell (Figure 2). Once the nucleus is expelled, the cell is known as a reticulocyte, which ultimately becomes a mature erythrocyte.
Healthy newborns have circulating NRBCs that rapidly disappear within a few weeks of birth. However, NRBCs can return to the circulation in a variety of disease states.
Causes of NRBCs
Brisk hemolysis or rapid blood loss can cause NRBCs to be released into the blood as erythropoiesis increases in an attempt to compensate for acute anemia.
Damage or stress to the bone marrow also causes NRBCs to be released into the peripheral blood, as is often the case in hematologic diseases. In a study of 478 patients with hematologic diseases, the frequency of NRBC positivity at diagnosis was highest in patients with chronic myeloid leukemia (100%), acute leukemia (62%), and myelodysplastic syndromes (45%).13 NRBCs also appeared at higher frequencies during chemotherapy in other hematologic conditions, such as hemophagocytic lymphohistiocytosis.
The mechanism by which NRBCs are expelled from the bone marrow is unclear, though studies have suggested that inflammation or hypoxia or both cause increased hematopoietic stress, resulting in the release of immature red cells. Increased concentrations of inflammatory cytokines (interleukin 6 and interleukin 3) and erythropoietin in the plasma and decreased arterial oxygen partial tension have been reported in patients with circulating NRBCs.14,15
Because they are associated with hematologic disorders, the finding of NRBCs should prompt evaluation of a peripheral smear to assess for abnormalities in other cell lines.
The NRBC count and prognosis
In critically ill patients, peripheral NRBCs can also indicate life-threatening conditions.
In a study of 421 adult intensive care patients, the in-hospital mortality rate was 42% in those with peripheral NRBCs vs 5.9% in those without them.16 Further, the higher the NRBC count and the more days that NRBCs were reported in the CBC, the higher the risk of death.
In adults with acute respiratory distress syndrome, the finding of any NRBCs in the peripheral blood was an independent risk factor for death, and an NRBC count higher than 220 cells/µL was associated with a more than 3-fold higher risk of death.17
Daily screening in patients in surgical intensive care units revealed that NRBCs appeared an average of 9 days before death, consistent with an early marker of impending decline.18
In another study,19 the risk of death within 90 days of hospital discharge was higher in NRBC-positive patients, reaching 21.9% in those who had a count higher than 200 cells/µL. The risk of unplanned hospital readmission within 30 days was also increased.
Leukoerythroblastosis
The combination of NRBCs and immature white blood cells (eg, myelocytes, metamyelocytes) is called leukoerythroblastosis.
Leukoerythroblastosis is classically seen in myelophthisic anemias in which hematopoietic cells in the marrow are displaced by fibrosis, tumor, or other space-occupying processes, but it can also occur in any situation of acute marrow stress, including critical illness.
In addition, leukoerythroblastosis appears in a rare complication of sickle cell hemoglobinopathies: bone marrow necrosis with fat embolism syndrome.20,21 As the marrow necroses, fat emboli are released in the systemic circulation causing micro- and macrovascular occlusions and multiorgan failure. The largest case series in the literature reports 58 patients with bone marrow necrosis with fat embolism syndrome.22
At our institution, we have seen 18 patients with this condition in the past 8 years, with the frequency of diagnosis increasing with heightened awareness of the disorder. We have found that leukoerythroblastosis is often an early marker of this unrecognized syndrome and can prompt emergency red cell exchange, which is considered to be lifesaving in this condition.22
These examples and many others show that the presence of NRBCs in the CBC can serve as an important clinical warning.
OLD TESTS CAN STILL BE USEFUL
The CBC provides much more than simple cell counts; it is a rich collection of information related to each blood cell. These days, with new diagnostic tests and prognostic tools based on molecular analysis, it is important to not overlook the value of the tests clinicians have been ordering for generations.
The RDW, MPV, and NRBC count will not likely provide definitive or flawless diagnostic or prognostic information, but when understood and used correctly, they provide readily available, cost-effective, and useful data that can supplement and guide clinical decision-making. By understanding the CBC more fully, providers can maximize the truly complete nature of this routine laboratory test.
The complete blood cell count (CBC) is one of the most frequently ordered laboratory tests in both the inpatient and outpatient settings. Not long ago, the CBC required peering through a microscope and counting the red blood cells, white blood cells, and platelets. These 3 numbers are still the primary purpose of the test.
Now, with automated counters, the CBC report also contains other numbers that delineate characteristics of each cell type. For example:
The mean corpuscular volume is the average volume of red blood cells. Providers use it to classify anemia as either microcytic, normocytic, or macrocytic, each with its own differential diagnosis.
The differential white blood cell count provides absolute counts and relative percentages of each type of leukocyte. For example, the absolute neutrophil count is an important measure of immunocompetence.
But other values in the CBC may be overlooked, even though they can provide important information. Here, we highlight 3 of them:
- The red blood cell distribution width (RDW)
- The mean platelet volume (MPV)
- The nucleated red blood cell (NRBC) count.
In addition to describing their diagnostic utility, we also discuss emerging evidence of their potential prognostic significance in hematologic and nonhematologic disorders. By incorporating an awareness of their value in clinical practice, providers can maximize the usefulness of the CBC.
RED BLOOD CELL DISTRIBUTION WIDTH
The RDW is a measure of variation (anisocytosis) in the size of the circulating red cells. The term “width” is misleading, as the value is not derived from the width of the red blood cell, but rather from the width of the distribution curve of the corpuscular volume (Figure 1). Therefore, a normal RDW means that the cells are all about the same size, while a high RDW means they vary widely in size.
The RDW can be calculated either as a coefficient of variation, with a reference range of 11% to 16% depending on the laboratory, or, less often, as a standard deviation, with a reference range of 39 to 46 fL.
The RDW can differentiate between causes of anemia
A high RDW is often found in nutritional deficiencies of iron, vitamin B12, and folate. This information is helpful in differentiating the cause of microcytic anemia, as a high RDW suggests iron-deficiency anemia while a normal RDW suggests thalassemia.1 In iron deficiency, the RDW often rises before the mean corpuscular volume falls, serving as an early diagnostic clue.
The RDW can also be high after recent hemorrhage or rapid hemolysis, as the acute drop in hemoglobin results in increased production of reticulocytes, which are larger than mature erythrocytes.
Because a range of disorders can elevate the RDW, reviewing the peripheral blood smear is an important next step in the diagnostic evaluation, specifically looking for reticulocytes, microspherocytes, and other abnormal red blood cells contributing to the RDW elevation.
A normal RDW is less diagnostically useful. It indicates the red blood cells are of uniform size, but they may be uniformly small or large depending on how long the anemia has persisted. Since red cells circulate for only about 120 days, patients who have severe iron-deficiency anemia for months to years are expected to have a normal rather than a high RDW, as their red cells of normal size have all been replaced by microcytes.
A low RDW is not consistently associated with any hematologic disorder.
RDW may have prognostic value
Emerging data suggest that the RDW may also have prognostic value in nonhematologic diseases. In a retrospective study of 15,852 adult participants in the Third National Health and Nutrition Examination Survey (1988–1994), a higher RDW was associated with a higher risk of death, with the all-cause mortality rate increasing by 23% for every 1% increment in RDW.2
This correlation is particularly prominent in cardiac disorders. In 2 large retrospective studies of patients with symptomatic heart failure, a higher RDW was a strong predictor of morbidity and death (hazard ratio 1.17 per 1-standard deviation increase, P < .001), even stronger than more commonly used variables such as ejection fraction, New York Heart Association functional class, and renal function.3
In a retrospective analysis of 4,111 patients with myocardial infarction, the degree of RDW elevation correlated with the risk of repeat nonfatal myocardial infarction, coronary death, new symptomatic heart failure, and stroke.4
It is hypothesized that high RDW may reflect poor cell membrane integrity from altered cholesterol content, which in turn has deleterious effects on multiple organ systems and is therefore associated with adverse outcomes.5
Currently, using the RDW to assess prognosis remains investigational, and how best to interpret it in daily practice requires further study.
MEAN PLATELET VOLUME
The MPV, ie, the average size of platelets, is reported in femtoliters (fL). Because the MPV varies depending on the instrument used, each laboratory has a unique reference range, usually about 8 to 12 fL. The MPV must be interpreted in conjunction with the platelet count; the product of the MPV and platelet count is called the total platelet mass.
Using the MPV to find the cause of thrombocytopenia
The MPV can be used to help narrow the differential diagnosis of thrombocytopenia. For example, it is high in thrombocytopenia resulting from peripheral destruction, as in immune thrombocytopenic purpura. This is because as platelets are lost, thrombopoietin production increases and new, larger platelets are released from healthy megakaryocytes in an attempt to increase the total platelet mass.
In contrast, the MPV is low in patients with thrombocytopenia due to megakaryocyte hypoplasia, as malfunctioning megakaryocytes cannot maintain the total platelet mass, and any platelets produced remain small. This distinction can be obscured in the setting of splenomegaly, as larger platelets are more easily sequestered in the spleen and the MPV may therefore be low or normal.
The MPV can also be used to differentiate congenital thrombocytopenic disorders, which can be characterized by either a high MPV (eg, gray platelet syndrome, Bernard-Soulier syndrome) or a low MPV (eg, Wiskott-Aldrich syndrome) (Figure 2).
MPV may have prognostic value
Evidence suggests that the MPV also has potential prognostic value, particularly in vascular disease, as larger platelets are hypothesized to have increased hemostatic potential.
In a large meta-analysis of patients with coronary artery disease, a high MPV was associated with worse outcomes; the risk of death or myocardial infarction was 17% higher in those with a high MPV (the threshold ranged from 8.4 to 11.7 fL in the different studies) than in those with a low MPV.6
In a study of 213 patients with non-ST-segment elevation myocardial infarction, the risk of significant coronary artery disease was 4.18 times higher in patients with a high MPV and a high troponin level than in patients with a normal MPV and a high troponin.7 The authors suggested that a high MPV may help identify patients at highest risk of significant coronary artery disease who would benefit from invasive studies (ie, coronary angiography).
This correlation has also been observed in other forms of vascular disease. In 261 patients who underwent carotid angioplasty and stenting, an MPV higher than 10.1 fL was associated with a risk of in-stent restenosis more than 3 times higher.8
The MPV has also been found to be higher in patients with type 2 diabetes than in controls, particularly in those with microvascular complications such as retinopathy or microalbuminuria.9
Conversely, in patients with cancer, a low MPV appears to be associated with a poor prognosis. In a retrospective analysis of 236 patients with esophageal cancer, those who had an MPV of 7.4 fL or less had significantly shorter overall survival than patients with an MPV higher than 7.4 fL.10
A low MPV has also been associated with an increased risk of venous thromoboembolism in patients with cancer. In a prospective observational cohort study of 1,544 patients, the 2-year probability of venous thromboembolism was 9% in patients with an MPV less than 10.8 fL, compared with 5.5% in those with higher MPV values. The 2-year overall survival rate was also higher in patients with high MPV than in those with low MPV, at 64.7% vs 55.7%, respectively (P = .001).11
But the MPV is far from a perfect clinical metric. Since its measurement is subject to significant laboratory variation, an abnormal value should always be confirmed with evaluation of a peripheral blood smear. Furthermore, it is unclear why a high MPV portends poor prognosis in patients without cancer, whereas the opposite is true in patients with cancer. Therefore, its role in prognostication remains investigational, and further studies are essential to determine its appropriate usefulness in clinical practice.12
NUCLEATED RED BLOOD CELL COUNT
NRBCs are immature red blood cell precursors not present in the circulation of healthy adults. During erythropoiesis, the common myeloid progenitor cell first differentiates into a proerythroblast; subsequently, the chromatin in the nucleus of the proerythroblast gradually condenses until it becomes an orthochromatic erythroblast, also known as a nucleated red cell (Figure 2). Once the nucleus is expelled, the cell is known as a reticulocyte, which ultimately becomes a mature erythrocyte.
Healthy newborns have circulating NRBCs that rapidly disappear within a few weeks of birth. However, NRBCs can return to the circulation in a variety of disease states.
Causes of NRBCs
Brisk hemolysis or rapid blood loss can cause NRBCs to be released into the blood as erythropoiesis increases in an attempt to compensate for acute anemia.
Damage or stress to the bone marrow also causes NRBCs to be released into the peripheral blood, as is often the case in hematologic diseases. In a study of 478 patients with hematologic diseases, the frequency of NRBC positivity at diagnosis was highest in patients with chronic myeloid leukemia (100%), acute leukemia (62%), and myelodysplastic syndromes (45%).13 NRBCs also appeared at higher frequencies during chemotherapy in other hematologic conditions, such as hemophagocytic lymphohistiocytosis.
The mechanism by which NRBCs are expelled from the bone marrow is unclear, though studies have suggested that inflammation or hypoxia or both cause increased hematopoietic stress, resulting in the release of immature red cells. Increased concentrations of inflammatory cytokines (interleukin 6 and interleukin 3) and erythropoietin in the plasma and decreased arterial oxygen partial tension have been reported in patients with circulating NRBCs.14,15
Because they are associated with hematologic disorders, the finding of NRBCs should prompt evaluation of a peripheral smear to assess for abnormalities in other cell lines.
The NRBC count and prognosis
In critically ill patients, peripheral NRBCs can also indicate life-threatening conditions.
In a study of 421 adult intensive care patients, the in-hospital mortality rate was 42% in those with peripheral NRBCs vs 5.9% in those without them.16 Further, the higher the NRBC count and the more days that NRBCs were reported in the CBC, the higher the risk of death.
In adults with acute respiratory distress syndrome, the finding of any NRBCs in the peripheral blood was an independent risk factor for death, and an NRBC count higher than 220 cells/µL was associated with a more than 3-fold higher risk of death.17
Daily screening in patients in surgical intensive care units revealed that NRBCs appeared an average of 9 days before death, consistent with an early marker of impending decline.18
In another study,19 the risk of death within 90 days of hospital discharge was higher in NRBC-positive patients, reaching 21.9% in those who had a count higher than 200 cells/µL. The risk of unplanned hospital readmission within 30 days was also increased.
Leukoerythroblastosis
The combination of NRBCs and immature white blood cells (eg, myelocytes, metamyelocytes) is called leukoerythroblastosis.
Leukoerythroblastosis is classically seen in myelophthisic anemias in which hematopoietic cells in the marrow are displaced by fibrosis, tumor, or other space-occupying processes, but it can also occur in any situation of acute marrow stress, including critical illness.
In addition, leukoerythroblastosis appears in a rare complication of sickle cell hemoglobinopathies: bone marrow necrosis with fat embolism syndrome.20,21 As the marrow necroses, fat emboli are released in the systemic circulation causing micro- and macrovascular occlusions and multiorgan failure. The largest case series in the literature reports 58 patients with bone marrow necrosis with fat embolism syndrome.22
At our institution, we have seen 18 patients with this condition in the past 8 years, with the frequency of diagnosis increasing with heightened awareness of the disorder. We have found that leukoerythroblastosis is often an early marker of this unrecognized syndrome and can prompt emergency red cell exchange, which is considered to be lifesaving in this condition.22
These examples and many others show that the presence of NRBCs in the CBC can serve as an important clinical warning.
OLD TESTS CAN STILL BE USEFUL
The CBC provides much more than simple cell counts; it is a rich collection of information related to each blood cell. These days, with new diagnostic tests and prognostic tools based on molecular analysis, it is important to not overlook the value of the tests clinicians have been ordering for generations.
The RDW, MPV, and NRBC count will not likely provide definitive or flawless diagnostic or prognostic information, but when understood and used correctly, they provide readily available, cost-effective, and useful data that can supplement and guide clinical decision-making. By understanding the CBC more fully, providers can maximize the truly complete nature of this routine laboratory test.
- Lima CS, Reis AR, Grotto HZ, Saad ST, Costa FF. Comparison of red cell distribution width and a red cell discriminant function incorporating volume dispersion for distinguishing iron deficiency from beta thalassemia trait in patients with microcytosis. Sao Paulo Med J 1996; 114(5):1265–1269. pmid:9239926
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009; 169(6):588–594. doi:10.1001/archinternmed.2009.55
- Felker GM, Allen LA, Pocock SJ, et al; CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007; 50(1):40–47. doi:10.1016/j.jacc.2007.02.067
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117(2):163–168. doi:10.1161/CIRCULATIONAHA.107.727545
- Goldstein MR, Mascitelli L, Pezzetta F. Is red cell distribution width a marker of overall membrane integrity? [Letter] Arch Intern Med 2009; 169(16):1539–1540. doi:10.1001/archinternmed.2009.275
- Sansanaydhu N, Numthavaj P, Muntham D, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015; 114(6):1299–1309. doi:10.1160/TH15-04-0280
- Taskesen T, Sekhon H, Wroblewski I, et al. Usefulness of mean platelet volume to predict significant coronary artery disease in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol 2017; 119(2):192–196. doi:10.1016/j.amjcard.2016.09.042
- Dai Z, Gao J, Li S, et al. Mean platelet volume as a predictor for restenosis after carotid angioplasty and stenting. Stroke 2018; 49(4):872–876. doi:10.1161/STROKEAHA.117.019748
- Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15(8):475–478. doi:10.1080/0953710042000267707
- Shen W, Cui MM, Wang X, Wang RT. Reduced mean platelet volume is associated with poor prognosis in esophageal cancer. Cancer Biomark 2018; 22(3):559–563. doi:10.3233/CBM-181231
- Riedl J, Kaider A, Reitter EM, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS). Thromb Haemost 2014; 111(4):670–678. doi:10.1160/TH13-07-0603
- Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost 2003; 9(3):177–190. pmid:14507105
- Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2011; 50(2):357–360. doi:10.1515/CCLM.2011.766
- Stachon A, Bolulul O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock 2005; 24(1):34–39. pmid:15988318
- Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med 2011; 49(2):257–263. doi:10.1515/CCLM.2011.041
- Stachon A, Holland-Letz T, Krieg M. In-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med 2004; 42(8):933–938. doi:10.1515/CCLM.2004.151
- Menk M, Giebelhäuser L, Vorderwülbecke G, et al. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensive Care 2018; 8(1):42. doi:10.1186/s13613-018-0387-5
- Stachon A, Kempf R, Holland-Letz T, Friese J, Becker A, Krieg M. Daily monitoring of nucleated red blood cells in the blood of surgical intensive care patients. Clin Chim Acta 2006; 366(1–2):329–335. doi:10.1016/j.cca.2005.11.022
- Purtle SW, Horkan CM, Moromizato T, Gibbons FK, Christopher KB. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: a cohort study. Crit Care 2017; 21(1):154. doi:10.1186/s13054-017-1724-z
- May J, Sullivan JC, LaVie D, LaVie K, Marques MB. Inside out: bone marrow necrosis and fat embolism complicating sickle-beta+ thalassemia. Am J Med 2016; 129(12):e321–e324. doi:10.1016/j.amjmed.2016.05.027
- Gangaraju R, Reddy VV, Marques MB. Fat embolism syndrome secondary to bone marrow necrosis in patients with hemoglobinopathies. South Med J 2016; 109(9):549–553. doi:10.14423/SMJ.0000000000000520
- Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 2014; 28(1):23–30. doi:10.1016/j.blre.2013.12.002
- Lima CS, Reis AR, Grotto HZ, Saad ST, Costa FF. Comparison of red cell distribution width and a red cell discriminant function incorporating volume dispersion for distinguishing iron deficiency from beta thalassemia trait in patients with microcytosis. Sao Paulo Med J 1996; 114(5):1265–1269. pmid:9239926
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009; 169(6):588–594. doi:10.1001/archinternmed.2009.55
- Felker GM, Allen LA, Pocock SJ, et al; CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007; 50(1):40–47. doi:10.1016/j.jacc.2007.02.067
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117(2):163–168. doi:10.1161/CIRCULATIONAHA.107.727545
- Goldstein MR, Mascitelli L, Pezzetta F. Is red cell distribution width a marker of overall membrane integrity? [Letter] Arch Intern Med 2009; 169(16):1539–1540. doi:10.1001/archinternmed.2009.275
- Sansanaydhu N, Numthavaj P, Muntham D, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015; 114(6):1299–1309. doi:10.1160/TH15-04-0280
- Taskesen T, Sekhon H, Wroblewski I, et al. Usefulness of mean platelet volume to predict significant coronary artery disease in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol 2017; 119(2):192–196. doi:10.1016/j.amjcard.2016.09.042
- Dai Z, Gao J, Li S, et al. Mean platelet volume as a predictor for restenosis after carotid angioplasty and stenting. Stroke 2018; 49(4):872–876. doi:10.1161/STROKEAHA.117.019748
- Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15(8):475–478. doi:10.1080/0953710042000267707
- Shen W, Cui MM, Wang X, Wang RT. Reduced mean platelet volume is associated with poor prognosis in esophageal cancer. Cancer Biomark 2018; 22(3):559–563. doi:10.3233/CBM-181231
- Riedl J, Kaider A, Reitter EM, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS). Thromb Haemost 2014; 111(4):670–678. doi:10.1160/TH13-07-0603
- Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost 2003; 9(3):177–190. pmid:14507105
- Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2011; 50(2):357–360. doi:10.1515/CCLM.2011.766
- Stachon A, Bolulul O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock 2005; 24(1):34–39. pmid:15988318
- Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med 2011; 49(2):257–263. doi:10.1515/CCLM.2011.041
- Stachon A, Holland-Letz T, Krieg M. In-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med 2004; 42(8):933–938. doi:10.1515/CCLM.2004.151
- Menk M, Giebelhäuser L, Vorderwülbecke G, et al. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensive Care 2018; 8(1):42. doi:10.1186/s13613-018-0387-5
- Stachon A, Kempf R, Holland-Letz T, Friese J, Becker A, Krieg M. Daily monitoring of nucleated red blood cells in the blood of surgical intensive care patients. Clin Chim Acta 2006; 366(1–2):329–335. doi:10.1016/j.cca.2005.11.022
- Purtle SW, Horkan CM, Moromizato T, Gibbons FK, Christopher KB. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: a cohort study. Crit Care 2017; 21(1):154. doi:10.1186/s13054-017-1724-z
- May J, Sullivan JC, LaVie D, LaVie K, Marques MB. Inside out: bone marrow necrosis and fat embolism complicating sickle-beta+ thalassemia. Am J Med 2016; 129(12):e321–e324. doi:10.1016/j.amjmed.2016.05.027
- Gangaraju R, Reddy VV, Marques MB. Fat embolism syndrome secondary to bone marrow necrosis in patients with hemoglobinopathies. South Med J 2016; 109(9):549–553. doi:10.14423/SMJ.0000000000000520
- Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 2014; 28(1):23–30. doi:10.1016/j.blre.2013.12.002
KEY POINTS
- The RDW can help differentiate the cause of anemia: eg, a high RDW suggests iron-deficiency anemia, while a normal RDW suggests thalassemia. Studies also suggest that a high RDW may be associated with an increased rate of all-cause mortality and may predict a poor prognosis in several cardiac diseases.
- The MPV can be used in the evaluation of thrombocytopenia. Furthermore, emerging evidence suggests that high MPV is associated with worse outcomes in cardiovascular disorders.
- An elevated NRBC count may predict poor outcomes in a number of critical care settings. It can also indicate a serious underlying hematologic disorder.
Assessing liver fibrosis without biopsy in patients with HCV or NAFLD
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
KEY POINTS
- Liver biopsy remains the gold standard for determining fibrosis stage but is expensive and entails risk of complications.
- For patients infected with HCV, fibrosis stage should be determined with transient elastography, a transthoracic ultrasonographic technique that measures shear-wave velocity.
- For patients with cirrhosis, transient elastography combined with a platelet count can detect developing portal hypertension and determine whether to screen for esophageal varices.
- For NAFLD, combined elastography and NAFLD fibrosis score—which incorporates patient characteristics and laboratory test results—should be used to determine the need for liver biopsy.
Laboratory tests in rheumatology: A rational approach
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
Laboratory tests are often ordered inappropriately for patients in whom a rheumatologic illness is suspected; this occurs in both primary and secondary care.1 Some tests are available both singly and as part of a battery of tests screening healthy people without symptoms.
The problem: negative test results are by no means always reassuring, and false-positive results raise the risks of unnecessary anxiety for patients and clinicians, needless referrals, and potential morbidity due to further unnecessary testing and exposure to wrong treatments.2 Clinicians should be aware of the pitfalls of these tests in order to choose them wisely and interpret the results correctly.
This article provides practical guidance on requesting and interpreting some common tests in rheumatology, with the aid of case vignettes.
RHEUMATOID FACTOR AND ANTICITRULLINATED PEPTIDE ANTIBODY
A 41-year-old woman, previously in good health, presents to her primary care practitioner with a 6-week history of pain and swelling in her hands and early morning stiffness lasting about 2 hours. She denies having any extraarticular symptoms. Physical examination reveals synovitis across her right metacarpophalangeal joints, proximal interphalangeal joint of the left middle finger, and left wrist. The primary care physician is concerned that her symptoms might be due to rheumatoid arthritis.
Would testing for rheumatoid factor and anticitrullinated peptide antibody be useful in this patient?
Rheumatoid factor is an antibody (immunoglobulin M, IgG, or IgA) targeted against the Fc fragment of IgG.3 It was so named because it was originally detected in patients with rheumatoid arthritis, but it is neither sensitive nor specific for this condition. A meta-analysis of more than 5,000 patients with rheumatoid arthritis reported that rheumatoid factor testing had a sensitivity of 69% and specificity of 85%.4
Anticitrullinated peptide antibody, on the other hand, is much more specific for rheumatoid arthritis (95%), as it is seldom seen in other conditions, but its sensitivity is similar to that of rheumatoid factor (68%).4–6 A positive result would thus lend strength to the diagnosis of rheumatoid arthritis, but a negative result would not exclude it.
Approach to early arthritis
When faced with a patient with early arthritis, some key questions to ask include7,8:
Is this an inflammatory or a mechanical problem? Inflammatory arthritis is suggested by joint swelling that is not due to trauma or bony hypertrophy, early morning stiffness lasting longer than 30 minutes, and elevated inflammatory markers (erythrocyte sedimentation rate or C-reactive protein). Involvement of the small joints of the hands and feet may be suggested by pain on compression of the metacarpophalangeal and metatarsophalangeal joints, respectively.
Is there a definite identifiable underlying cause for the inflammatory arthritis? The pattern of development of joint symptoms or the presence of extraarticular symptoms may suggest an underlying problem such as gout, psoriatic arthritis, systemic lupus erythematosus, or sarcoidosis.
If the arthritis is undifferentiated (ie, there is no definite identifiable cause), is it likely to remit or persist? This is perhaps the most important question to ask in order to prognosticate. Patients with risk factors for persistent disease, ie, for development of rheumatoid arthritis, should be referred to a rheumatologist early for timely institution of disease-modifying antirheumatic drug therapy.9 Multiple studies have shown that patients in whom this therapy is started early have much better clinical, functional, and radiologic outcomes than those in whom it is delayed.10–12
The revised American College of Rheumatology and European League Against Rheumatism criteria13 include the following factors as predictors of persistence:
- Number of involved joints (with greater weight given to involvement of small joints)
- Duration of symptoms 6 weeks or longer
- Elevated acute-phase response (erythrocyte sedimentation rate or C-reactive protein level)
- A positive serologic test (either rheumatoid factor or anticitrullinated peptide antibody).
If both rheumatoid factor and anticitrullinated peptide antibody are positive in a patient with early undifferentiated arthritis, the risk of progression to rheumatoid arthritis is almost 100%, thus underscoring the importance of testing for these antibodies.5,6 Referral to a rheumatologist should, however, not be delayed in patients with negative test results (more than one-third of patients with rheumatoid arthritis may be negative for both), and should be considered in those with inflammatory joint symptoms persisting longer than 6 weeks, especially with involvement of the small joints (sparing the distal interphalangeals) and elevated acute-phase response.
Rheumatoid factor in healthy people without symptoms
In some countries, testing for rheumatoid factor is offered as part of a battery of screening tests in healthy people who have no symptoms, a practice that should be strongly discouraged.
Multiple studies, both prospective and retrospective, have demonstrated that both rheumatoid factor and anticitrullinated peptide antibody may be present several years before the clinical diagnosis of rheumatoid arthritis.6,14–16 But the risk of developing rheumatoid arthritis for asymptomatic individuals who are rheumatoid factor-positive depends on the rheumatoid factor titer, positive family history of rheumatoid arthritis in first-degree relatives, and copresence of anticitrullinated peptide antibody. The absolute risk, nevertheless, is still very small. In some, there might be an alternative explanation such as undiagnosed Sjögren syndrome or hepatitis C.
In any event, no strategy is currently available that is proven to prevent the development of rheumatoid arthritis, and there is no role for disease-modifying therapy during the preclinical phase.16
Back to our patient
Blood testing in our patient reveals normal complete blood cell counts, aminotransferase levels, and serum creatinine concentration; findings on urinalysis are normal. Her erythrocyte sedimentation rate is 56 mm/hour (reference range 0–15), and her C-reactive protein level is 26 mg/dL (normal < 3). Testing is negative for rheumatoid factor and anticitrullinated peptide antibody.
Although her rheumatoid factor and anticitrullinated peptide antibody tests are negative, she is referred to a rheumatologist because she has predictors of persistent disease, ie, symptom duration of 6 weeks, involvement of the small joints of the hands, and elevated erythrocyte sedimentation rate and C-reactive protein. The rheumatologist checks her parvovirus serology, which is negative.
The patient is given parenteral depot corticosteroid therapy, to which she responds briefly. Because her symptoms persist and continue to worsen, methotrexate treatment is started after an additional 6 weeks.
ANTINUCLEAR ANTIBODY
A 37-year-old woman presents to her primary care physician with the complaint of tiredness. She has a family history of systemic lupus erythematosus in her sister and maternal aunt. She is understandably worried about lupus because of the family history and is asking to be tested for it.
Would testing for antinuclear antibody be reasonable?
Antinuclear antibody is not a single antibody but rather a family of autoantibodies that are directed against nuclear constituents such as single- or double-stranded deoxyribonucleic acid (dsDNA), histones, centromeres, proteins complexed with ribonucleic acid (RNA), and enzymes such as topoisomerase.17,18
Protein antigens complexed with RNA and some enzymes in the nucleus are also known as extractable nuclear antigens (ENAs). They include Ro, La, Sm, Jo-1, RNP, and ScL-70 and are named after the patient in whom they were first discovered (Robert, Lavine, Smith, and John), the antigen that is targeted (ribonucleoprotein or RNP), and the disease with which they are associated (anti-ScL-70 or antitopoisomerase in diffuse cutaneous scleroderma).
Antinuclear antibody testing is commonly requested to exclude connective tissue diseases such as lupus, but the clinician needs to be aware of the following points:
Antinuclear antibody may be encountered in conditions other than lupus
These include19:
- Other autoimmune diseases such as rheumatoid arthritis, primary Sjögren syndrome, systemic sclerosis, autoimmune thyroid disease, and myasthenia gravis
- Infection with organisms that share the epitope with self-antigens (molecular mimicry)
- Cancers
- Drugs such as hydralazine, procainamide, and minocycline.
Antinuclear antibody might also be produced by the healthy immune system from time to time to clear the nuclear debris that is extruded from aging cells.
A study in healthy individuals20 reported a prevalence of positive antinuclear antibody of 32% at a titer of 1/40, 15% at a titer of 1/80, 7% at a titer of 1/160, and 3% at a titer of 1/320. Importantly, a positive result was more common among family members of patients with autoimmune connective tissue diseases.21 Hence, a positive antinuclear antibody result does not always mean lupus.
Antinuclear antibody testing is highly sensitive for lupus
With current laboratory methods, antinuclear antibody testing has a sensitivity close to 100%. Hence, a negative result virtually rules out lupus.
Two methods are commonly used to test for antinuclear antibody: indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA).22 While human epithelial (Hep2) cells are used as the source of antigen in immunofluorescence, purified nuclear antigens coated on multiple-well plates are used in ELISA.
Although ELISA is simpler to perform, immunofluorescence has a slightly better sensitivity (because the Hep2 cells express a wide range of antigens) and is still considered the gold standard. As expected, the higher sensitivity occurs at the cost of reduced specificity (about 60%), so antinuclear antibody will also be detected in all the other conditions listed above.23
To improve the specificity of antinuclear antibody testing, laboratories report titers (the highest dilution of the test serum that tested positive); a cutoff of greater than 1/80 is generally considered significant.
Do not order antinuclear antibody testing indiscriminately
To sum up, the antinuclear antibody test should be requested only in patients with involvement of multiple organ systems. Although a negative result would make it extremely unlikely that the clinical presentation is due to lupus, a positive result is insufficient on its own to make a diagnosis of lupus.
Diagnosing lupus is straightforward when patients present with a specific manifestation such as inflammatory arthritis, photosensitive skin rash, hemolytic anemia, thrombocytopenia, or nephritis, or with specific antibodies such as those against dsDNA or Sm. Patients who present with nonspecific symptoms such as arthralgia or tiredness with a positive antinuclear antibody and negative anti-dsDNA and anti-Sm may present difficulties even for the specialist.25–27
Back to our patient
Our patient denies arthralgia. She has no extraarticular symptoms such as skin rashes, oral ulcers, sicca symptoms, muscle weakness, Raynaud phenomenon, pleuritic chest pain, or breathlessness. Findings on physical examination and urinalysis are unremarkable.
Her primary care physician decides to check her complete blood cell count, erythrocyte sedimentation rate, and thyroid-stimulating hormone level. Although she is reassured that her tiredness is not due to lupus, she insists on getting an antinuclear antibody test.
Her complete blood cell counts are normal. Her erythrocyte sedimentation rate is 6 mm/hour. However, her thyroid-stimulating hormone level is elevated, and subsequent testing shows low free thyroxine and positive thyroid peroxidase antibodies. The antinuclear antibody is positive in a titer of 1/80 and negative for anti-dsDNA and anti-ENA.
We explain to her that the positive antinuclear antibody is most likely related to her autoimmune thyroid disease. She is referred to an endocrinologist.
ANTIPHOSPHOLIPID ANTIBODIES
A 24-year-old woman presents to the emergency department with acute unprovoked deep vein thrombosis in her right leg, confirmed by ultrasonography. She has no history of previous thrombosis, and the relevant family history is unremarkable. She has never been pregnant. Her platelet count is 84 × 109/L (reference range 150–400), and her baseline activated partial thromboplastin time is prolonged at 62 seconds (reference range 23.0–32.4). The rest of her blood counts and her prothrombin time, liver enzyme levels, and serum creatinine level are normal.
Should this patient be tested for antiphospholipid antibodies?
Antiphospholipid antibodies are important because of their association with thrombotic risk (both venous and arterial) and pregnancy morbidity. The name is a misnomer, as these antibodies are targeted against some proteins that are bound to phospholipids and not only to the phospholipids themselves.
According to the modified Sapporo criteria for the classification of antiphospholipid syndrome,28 antiphospholipid antibodies should remain persistently positive on at least 2 separate occasions at least 12 weeks apart for the result to be considered significant because some infections and drugs may be associated with the transient presence of antiphospholipid antibodies.
Screening for antiphospholipid antibodies should include testing for IgM and IgG anticardiolipin antibodies, lupus anticoagulant, and IgM and IgG beta-2 glycoprotein I antibodies.29,30
Anticardiolipin antibodies
Anticardiolipin (aCL) antibodies may be targeted either against beta-2 glycoprotein I (beta-2GPI) that is bound to cardiolipin (a phospholipid) or against cardiolipin alone; the former is more specific. Antibodies directed against cardiolipin alone are usually transient and are associated with infections and drugs. The result is considered significant only when anticardiolipin antibodies are present in a medium to high titer (> 40 IgG phospholipid units or IgM phospholipid units, or > 99th percentile).
Lupus anticoagulant
The antibody with “lupus anticoagulant activity” is targeted against prothrombin plus phospholipid or beta-2GPI plus phospholipid. The test for it is a functional assay involving 3 steps:
Demonstrating the prolongation of a phospholipid-dependent coagulation assay like the activated partial thromboplastin time (aPTT). (This may explain the prolongation of aPTT in the patient described in the vignette.) Although the presence of lupus anticoagulant is associated with thrombosis, it is called an “anticoagulant” because of this in vitro prolongation of phospholipid-dependent coagulation assays.
Mixing study. The phospholipid-dependent coagulation assay could be prolonged because of either the deficiency of a coagulation factor or the presence of the antiphospholipid antibodies. This can be differentiated by mixing the patient’s plasma with normal plasma (which will have all the clotting factors) in a 1:1 ratio. If the coagulation assay remains prolonged after the addition of normal plasma, clotting factor deficiency can be excluded.
Addition of a phospholipid. If the prolongation of the coagulation assay is due to the presence of an antiphospholipid antibody, addition of extra phospholipid will correct this.
Beta-2 glycoprotein I antibody (anti-beta-2GPI)
The beta-2GPI that is not bound to the cardiolipin can be detected by separately testing for beta-2GPI (the anticardiolipin test only detects the beta-2GPI that is bound to the cardiolipin). The result is considered significant if beta-2GPI is present in a medium to high titer (> 99th percentile).
Studies have shown that antiphospholipid antibodies may be present in 1% to 5% of apparently healthy people in the general population.31 These are usually low-titer anticardiolipin or anti-beta-GPI IgM antibodies that are not associated with thrombosis or adverse pregnancy outcomes. Hence, the term antiphospholipid syndrome should be reserved for those who have had at least 1 episode of thrombosis or pregnancy morbidity and persistent antiphospholipid antibodies, and not those who have asymptomatic or transient antiphospholipid antibodies.
Triple positivity (positive anticardiolipin, lupus anticoagulant, and anti-beta-2GPI) seems to be associated with the highest risk of thrombosis, with a 10-year cumulative incidence of 37.1% (95% confidence interval [CI] 19.9–54.3) for a first thrombotic event,32 and 44.2% (95% CI 38.6–49.8) for recurrent thrombosis.33
The association with thrombosis is stronger for lupus anticoagulant than with the other 2 antibodies, with different studies34 finding an odds ratio ranging from 5 to 16. A positive lupus anticoagulant test with or without a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a high-risk profile, while a moderate to high titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a moderate-risk profile. A low titer of anticardiolipin or anti-beta-2GPI IgM or IgG constitutes a low-risk profile that may not be associated with thrombosis.35
Antiphospholipid syndrome is important to recognize because of the need for long-term anticoagulation to prevent recurrence.36 It may be primary, when it occurs on its own, or secondary, when it occurs in association with another autoimmune disease such as lupus.
Venous events in antiphospholipid syndrome most commonly manifest as lower-limb deep vein thrombosis or pulmonary embolism, while arterial events most commonly manifest as stroke or transient ischemic attack.37 Obstetric manifestations may include not only miscarriage and stillbirth, but also preterm delivery, intrauterine growth retardation, and preeclampsia, all occurring due to placental insufficiency.
The frequency of antiphospholipid antibodies has been estimated as 13.5% in patients with stroke, 11% with myocardial infarction, 9.5% with deep vein thrombosis, and 6% for those with pregnancy morbidity.38
Some noncriteria manifestations have also been recognized in antiphospholipid syndrome, such as thrombocytopenia, cardiac vegetations (Libman-Sachs endocarditis), livedo reticularis, and nephropathy.
Back to our patient
Our patient’s anticardiolipin IgG test is negative, while her lupus anticoagulant and beta-2GPI IgG are positive. She has no clinical or laboratory features suggesting lupus.
She is started on warfarin. After 3 months, the warfarin is interrupted for several days, and she is retested for all 3 antiphospholipid antibodies. Her beta-2GPI I IgG and lupus anticoagulant tests are again positive. Because of the persistent antiphospholipid antibody positivity and clinical history of deep vein thrombosis, her condition is diagnosed as primary antiphospholipid syndrome. She is advised to continue anticoagulant therapy indefinitely.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY
A 34-year-old man who is an injecting drug user presents with a 2-week history of fever, malaise, and generalized arthralgia. There are no localizing symptoms of infection. Notable findings on examination include a temperature of 38.0°C (100.4°F), needle track marks in his arms, nonblanching vasculitic rash in his legs, and a systolic murmur over the precordium.
His white blood cell count is 15.3 × 109/L (reference range 3.7–11.0), and his C-reactive protein level is 234 mg/dL (normal < 3). Otherwise, results of blood cell counts, liver enzyme tests, renal function tests, urinalysis, and chest radiography are normal.
Two sets of blood cultures are drawn. Transthoracic echocardiography and the antineutrophil cytoplasmic antibody (ANCA) test are requested, as are screening tests for human immunodeficiency virus, hepatitis B, and hepatitis C.
Was the ANCA test indicated in this patient?
ANCAs are autoantibodies against antigens located in the cytoplasmic granules of neutrophils and monocytes. They are associated with small-vessel vasculitides such as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and isolated pauciimmune crescentic glomerulonephritis, all collectively known as ANCA-associated vasculitis (AAV).39
Laboratory methods to detect ANCA include indirect immunofluorescence and antigen-specific enzyme immunoassays. Indirect immunofluorescence only tells us whether or not an antibody that is targeting a cytoplasmic antigen is present. Based on the indirect immunofluorescent pattern, ANCA can be classified as follows:
- Perinuclear or p-ANCA (if the targeted antigen is located just around the nucleus and extends into it)
- Cytoplasmic or c-ANCA (if the targeted antigen is located farther away from the nucleus)
- Atypical ANCA (if the indirect immunofluorescent pattern does not fit with either p-ANCA or c-ANCA).
Indirect immunofluorescence does not give information about the exact antigen that is targeted; this can only be obtained by performing 1 of the antigen-specific immunoassays. The target antigen for c-ANCA is usually proteinase-3 (PR3), while that for p-ANCA could be myeloperoxidase (MPO), cathepsin, lysozyme, lactoferrin, or bactericidal permeability inhibitor. Anti-PR3 is highly specific for GPA, while anti-MPO is usually associated with MPA and EGPA. Less commonly, anti-PR3 may be seen in patients with MPA and anti-MPO in those with GPA. Hence, there is an increasing trend toward classifying ANCA-associated vasculitis into PR3-associated or MPO-associated vasculitis rather than as GPA, MPA, EGPA, or renal-limited vasculitis.40
Several audits have shown that the ANCA test is widely misused and requested indiscriminately to rule out vasculitis. This results in a lower positive predictive value, possible harm to patients due to increased false-positive rates, and increased burden on the laboratory.41–43 At least 2 separate groups have demonstrated that a gating policy that refuses ANCA testing in patients without clinical evidence of systemic vasculitis can reduce the number of inappropriate requests, improve the diagnostic yield, and make it more clinically relevant and cost-effective.44,45
The clinician should bear in mind that:
Current guidelines recommend using one of the antigen-specific assays for PR3 and MPO as the primary screening method.48 Until recently, indirect immunofluorescence was used to screen for ANCA-associated vasculitis, and positive results were confirmed by ELISA to detect ANCAs specific for PR3 and MPO,49 but this is no longer recommended because of recent evidence suggesting a large variability between the different indirect immunofluorescent methods and improved diagnostic performance of the antigen-specific assays.
In a large multicenter study by Damoiseaux et al, the specificity with the different antigen-specific immunoassays was 98% to 99% for PR3-ANCA and 96% to 99% for MPO-ANCA.50
ANCA-associated vasculitis should not be considered excluded if the PR3 and MPO-ANCA are negative. In the Damoiseaux study, about 11% to 15% of patients with GPA and 8% to 24% of patients with MPA tested negative for both PR3 and MPO-ANCA.50
If the ANCA result is negative and clinical suspicion for ANCA-associated vasculitis is high, the clinician may wish to consider requesting another immunoassay method or indirect immunofluorescence. Results of indirect immunofluorescent testing results may be positive in those with a negative immunoassay, and vice versa.
Thus, the ANCA result should always be interpreted in the context of the whole clinical picture.51 Biopsy should still be considered the gold standard for the diagnosis of ANCA-associated vasculitis. The ANCA titer can help to improve clinical interpretation, because the likelihood of ANCA-associated vasculitis increases with higher levels of PR3 and MPO-ANCA.52
Back to our patient
Our patient’s blood cultures grow methicillin-sensitive Staphylococcus aureus in both sets after 48 hours. Transthoracic echocardiography reveals vegetations around the tricuspid valve, with no evidence of valvular regurgitation. The diagnosis is right-sided infective endocarditis. He is started on appropriate antibiotics.
Tests for human immunodeficiency virus, hepatitis B, and hepatitis C are negative. The ANCA test is positive for MPO-ANCA at 28 IU/mL (normal < 10).
The positive ANCA is thought to be related to the infective endocarditis. His vasculitis is most likely secondary to infective endocarditis and not ANCA-associated vasculitis. The ANCA test need not have been requested in the first place.
HUMAN LEUKOCYTE ANTIGEN-B27
A 22-year-old man presents to his primary care physician with a 4-month history of gradually worsening low back pain associated with early morning stiffness lasting more than 2 hours. He has no peripheral joint symptoms.
In the last 2 years, he has had 2 separate episodes of uveitis. There is a family history of ankylosing spondylitis in his father. Examination reveals global restriction of lumbar movements but is otherwise unremarkable. Magnetic resonance imaging (MRI) of the lumbar spine and sacroiliac joints is normal.
Should this patient be tested for human leukocyte antigen-B27 (HLA-B27)?
The major histocompatibility complex (MHC) is a gene complex that is present in all animals. It encodes proteins that help with immunologic tolerance. HLA simply refers to the human version of the MHC.53 The HLA gene complex, located on chromosome 6, is categorized into class I, class II, and class III. HLA-B is one of the 3 class I genes. Thus, a positive HLA-B27 result simply means that the particular gene is present in that person.
HLA-B27 is strongly associated with ankylosing spondylitis, also known as axial spondyloarthropathy.54 Other genes also contribute to the pathogenesis of ankylosing spondylitis, but HLA-B27 is present in more than 90% of patients with this disease and is by far considered the most important. The association is not as strong for peripheral spondyloarthropathy, with studies reporting a frequency of up to 75% for reactive arthritis and inflammatory bowel disease-associated arthritis, and up to 50% for psoriatic arthritis and uveitis.55
About 9% of healthy, asymptomatic individuals may have HLA-B27, so the mere presence of this gene is not evidence of disease.56 There may be up to a 20-fold increased risk of ankylosing spondylitis among those who are HLA-B27-positive.57
Some HLA genes have many different alleles, each of which is given a number (explaining the number 27 that follows the B). Closely related alleles that differ from one another by only a few amino-acid substitutions are then categorized together, thus accounting for more than 100 subtypes of HLA-B27 (designated from HLA-B*2701 to HLA-B*27106). These subtypes vary in frequency among different racial groups, and the population prevalence of ankylosing spondylitis parallels the frequency of HLA-B27.58 The most common subtype seen in white people and American Indians is B*2705. HLA-B27 is rare in blacks, explaining the rarity of ankylosing spondylitis in this population. Further examples include HLA-B*2704, which is seen in Asians, and HLA-B*2702, seen in Mediterranean populations. Not all subtypes of HLA-B27 are associated with disease, and some, like HLA-B*2706, may also be protective.
When should the clinician consider testing for HLA-B27?
Peripheral spondyloarthropathy may present with arthritis, enthesitis (eg, heel pain due to inflammation at the site of insertion of the Achilles tendon or plantar fascia), or dactylitis (“sausage” swelling of the whole finger or toe due to extension of inflammation beyond the margins of the joint). Other clues may include psoriasis, inflammatory bowel disease, history of preceding gastrointestinal or genitourinary infection, family history of similar conditions, and history of recurrent uveitis.
For the initial assessment of patients who have inflammatory back pain, plain radiography of the sacroiliac joints is considered the gold standard.59 If plain radiography does not show evidence of sacroiliitis, MRI of the sacroiliac joints should be considered. While plain radiography can reveal only structural changes such as sclerosis, erosions, and ankylosis, MRI is useful to evaluate for early inflammatory changes such as bone marrow edema. Imaging the lumbar spine is not necessary, as the sacroiliac joints are almost invariably involved in axial spondyloarthropathy, and lesions seldom occur in the lumbar spine in isolation.60
The diagnosis of ankylosing spondylitis previously relied on confirmatory imaging features, but based on the new International Society classification criteria,61–63 which can be applied to patients with more than 3 months of back pain and age of onset of symptoms before age 45, patients can be classified as having 1 of the following:
- Radiographic axial spondyloarthropathy, if they have evidence of sacroiliitis on imaging plus 1 other feature of spondyloarthropathy
- Nonradiographic axial spondyloarthropathy, if they have a positive HLA-B27 plus 2 other features of spondyloarthropathy (Table 7).
These new criteria have a sensitivity of 82.9% and specificity of 84.4%.62,63 The disease burden of radiographic and nonradiographic axial spondyloarthropathy has been shown to be similar, suggesting that they are part of the same disease spectrum. Thus, the HLA-B27 test is useful to make a diagnosis of axial spondyloarthropathy even in the absence of imaging features and could be requested in patients with 2 or more features of spondyloarthropathy. In the absence of imaging features and a negative HLA-B27 result, however, the patient cannot be classified as having axial spondyloarthropathy.
Back to our patient
The absence of radiographic evidence would not exclude axial spondyloarthropathy in our patient. The HLA-B27 test is requested because of the inflammatory back pain and the presence of 2 spondyloarthropathy features (uveitis and the family history) and is reported to be positive. His disease is classified as nonradiographic axial spondyloarthropathy.
He is started on regular naproxen and is referred to a physiotherapist. After 1 month, he reports significant symptomatic improvement. He asks if he can be retested for HLA-B27 to see if it has become negative. We tell him that there is no point in repeating it, as it is a gene and will not disappear.
SUMMARY: CONSIDER THE CLINICAL PICTURE
When approaching a patient suspected of having a rheumatologic disease, a clinician should first consider the clinical presentation and the intended purpose of each test. The tests, in general, might serve several purposes. They might help to:
Increase the likelihood of the diagnosis in question. For example, a positive rheumatoid factor or anticitrullinated peptide antibody can help diagnose rheumatoid arthritis in a patient with early polyarthritis, a positive HLA-B27 can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging, and a positive ANCA can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
Reduce the likelihood of the diagnosis in question. For example, a negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pains.
Monitor the condition. For example DNA antibodies can be used to monitor the activity of lupus.
Plan the treatment strategy. For example, one might consider lifelong anticoagulation if antiphospholipid antibodies are persistently positive in a patient with thrombosis.
Prognosticate. For example, positive rheumatoid factor and anticitrullinated peptide antibody increase the risk of erosive rheumatoid arthritis.
If the test was requested in the absence of a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test and not attach a diagnostic label prematurely. None of the tests can confirm or exclude a condition, so the results should always be interpreted in the context of the whole clinical picture.
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
- American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 2002; 47(4):429–433. doi:10.1002/art.10381
- Rang M. The Ulysses syndrome. Can Med Assoc J 1972; 106(2):122–123. pmid:5058884
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013; 35(6):727–734. doi:10.1155/2013/726598
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146(11):797–808. pmid:17548411
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038. doi:10.4061/2011/815038
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48(10):2741–2749. doi:10.1002/art.11223
- Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med 2004; 97(9):421–424. doi:10.1258/jrsm.97.9.421
- Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61(4):290–297. pmid:11874828
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76(6):948–959. doi:10.1136/annrheumdis-2016-210602
- Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year follow up of a prospective double blind placebo controlled study. J Rheumatol 1995; 22(12):2208–2213. pmid:8835550
- van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med 1996; 124(8):699–707. pmid:8633829
- Andreson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9):2569–2581. doi:10.1002/art.27584
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50(2):380–386. doi:10.1002/art.20018
- del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum 1988; 31(10):1239–1244. pmid:3178905
- Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am 2010; 36(2):213–241. doi:10.1016/j.rdc.2010.02.001
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000; 124(1):71–81. doi:10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2
- Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br J Hosp Med (Lond) 2007; 68(10):538–541. doi:10.12968/hmed.2007.68.10.27324
- Illei GG, Klippel JH. Why is the ANA result positive? Bull Rheum Dis 1999; 48(1):1–4. pmid:10028188
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40(9):1601–1611. doi:10.1002/art.1780400909
- Langkilde H, Voss A, Heegaard N, Laustrup H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017; 26(7):723–728. doi:10.1177/0961203316676378
- Rondeel JM. Immunofluorescence versus ELISA for the detection of antinuclear antigens. Expert Rev Mol Diagn 2002; 2(3):226–232. doi:10.1586/14737159.2.3.226
- Solomon DH, Kavanaugh AJ, Schur PH; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002; 47(4):434–444. doi:10.1002/art.10561
- Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996; 156(13):1421–1425. pmid:8678710
- Maddison PJ. Is it SLE? Best Pract Res Clin Rheumatol 2002; 16(2):167–180. doi:10.1053/berh.2001.0219
- Price E, Walker E. Diagnostic vertigo: the journey to diagnosis in systemic lupus erythematosus. Health (London) 2014; 18(3):223–239. doi:10.1177/1363459313488008
- Blumenthal DE. Tired, aching, ANA-positive: does your patient have lupus or fibromyalgia? Cleve Clin J Med 2002; 69(2):143–146, 151–152. pmid:11990644
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157(1):47–58. doi:10.1111/j.1365-2141.2012.09037.x
- Giannakopoulos B, Passam F, Iannou Y, Krillis SA. How we diagnose the antiphospholipid syndrome. Blood 2009; 113(5):985–994. doi:10.1182/blood-2007-12-129627
- Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010; 9(5):A299–A304. doi:10.1016/j.autrev.2009.11.013
- Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118(17):4714–4718. doi:10.1182/blood-2011-03-340232
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8(2):237–242. doi:10.1111/j.1538-7836.2009.03674.x
- Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003; 101(5):1827–1832. doi:10.1182/blood-2002-02-0441
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378(21):2010–2021. doi:10.1056/NEJMra1705454
- Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122(5):817–824. doi:10.1182/blood-2013-04-496257
- Cervera R. Lessons from the “Euro-Phospholipid” project. Autoimmun Rev 2008; 7(3):174–178. doi:10.1016/j.autrev.2007.11.011
- Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013; 65(11):1869–1873. doi:10.1002/acr.22066
- Miller A, Chan M, Wiik A, Misbah SA, Luqmani RA. An approach to the diagnosis and management of systemic vasculitis. Clin Exp Immunol 2010; 160(2):143–160. doi:10.1111/j.1365-2249.2009.04078.x
- Cornec D, Cornec-Le-Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis—clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016; 12(10):570–579. doi:10.1038/nrrheum.2016.123
- Edgar JD, McMillan SA, Bruce IN, Conlan SK. An audit of ANCA in routine clinical practice. Postgrad Med J 1995; 71(840):605–612. pmid:8545289
- McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic testing in a routine clinical setting. QJM 2001; 94(11):615–621. pmid:11704691
- Mandl LA, Solomon DH, Smith EL, Lew RA, Katz JN, Shmerling RH. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch Intern Med 2002; 162(13):1509–1514. pmid:12090888
- Sinclair D, Saas M, Stevens JM. The effect of a symptom related “gated policy” on ANCA requests in routine clinical practice. J Clin Pathol 2004; 57(2):131–134. pmid:14747434
- Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol 2010; 63(8):678–680. doi:10.1136/jcp.2009.072504
- Savige J, Gills D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999; 111(4):507–513. pmid:10191771
- Robinson PC, Steele RH. Appropriateness of antineutrophil cytoplasmic antibody testing in a tertiary hospital. J Clin Pathol 2009; 62(8):743–745. doi:10.1136/jcp.2009.064485
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017; 13(11):683–692. doi:10.1038/nrrheum.2017.140
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998; 53(3):743–753. doi:10.1046/j.1523-1755.1998.00807.x
- Damoiseaux J, Csemok E, Rasmussen N, et al. Detection of antineutrophil antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen specific immunoassays. Ann Rheum Dis 2017; 76(4):647–653. doi:10.1136/annrheumdis-2016-209507
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J 2006; 82(970):483–488. doi:10.1136/pgmj.2005.042648
- Vermeersch P, Blockmans D, Bossuyt X. Use of likelihood ratios can improve the clinical usefulness of enzyme immunoassays for the diagnosis of small-vessel vasculitis. Clin Chem 2009; 55(10):1886–1888. doi:10.1373/clinchem.2009.130583
- Bowness P. HLA-B27. Annu Rev Immunol 2015; 33:29–48. doi:10.1146/annurev-immunol-032414-112110
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
- Khan MA. Thoughts concerning the early diagnosis of ankylosing spondylitis and related diseases. Clin Exp Rheumatol 2002; 20(6 suppl 28):S6–S10. pmid:12463439
- Braun J, Bollow M, Remlinger G, et al. Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41(1):58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984; 27(3):241–249. pmid:6608352
- Sheehan NJ. HLA-B27: what’s new? Rheumatology (Oxford) 2010; 49(4):621–631. doi:10.1093/rheumatology/kep450
- Baraliakos X, Maksymmowych WP. Imaging in the diagnosis and management of axial spondyloarthritis. Best Pract Res Clin Rheumatol 2016; 30(4):608–623. doi:10.1016/j.berh.2016.09.011
- Mandl P, Navarro-Compan V, Terslev L, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015; 74(7):1327–1339. doi:10.1136/annrheumdis-2014-206971
- McAllister K, Goodson N, Warburton I, Rogers G. Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 2017; 356:j839. doi:10.1136/bmj.j839
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015; 74(8):1483–1487. doi:10.1136/annrheumdis-2014-207151
- Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68(6):777–783. doi:10.1136/ard.2009.108233
KEY POINTS
- If a test was requested without a clear indication and the result is positive, it is important to bear in mind the potential pitfalls associated with that test; immunologic tests have limited specificity.
- A positive rheumatoid factor or anticitrullinated peptide antibody test can help diagnose rheumatoid arthritis in a patient with early polyarthritis.
- A positive HLA-B27 test can help diagnose ankylosing spondylitis in patients with inflammatory back pain and normal imaging.
- Positive antinuclear cytoplasmic antibody (ANCA) can help diagnose ANCA-associated vasculitis in a patient with glomerulonephritis.
- A negative antinuclear antibody test reduces the likelihood of lupus in a patient with joint pain.
The tests that we order define us
May et al discuss one of the most common laboratory tests we order, the complete blood cell count, and how to interpret and unlock additional information that we often overlook.
Singh et al explain the utility and limitations of assessing hepatic fibrosis in patients with known liver disease using specialized and increasingly available imaging techniques in patients with common diseases that may progress to liver failure.
Using several clinical scenarios, Suresh explores the limitations of serologic testing in patients with a potential “autoimmune” or systemic inflammatory syndrome (which, based on new consultations I see in my rheumatology clinic, seems to be virtually everyone who has experienced pain or fatigue).
The Journal also continues our ongoing series on Smart Testing that has focused on tests and testing strategies that have a strong evidence basis to support or discourage their utilization in specific settings. But in most real-life clinical scenarios, relatively little directly applicable evidence can be brought to bear on our decision process with a specific patient. Hence the ongoing need for each of us to refine our clinical reasoning skills, and to recognize the continuing challenges facing the incorporation of artificial intelligence and algorithmic practice into the management of the individual patient sitting or lying in front of us.
The challenge is to balance input from Watson, “Dr. Google,” our accumulated anecdotal and group experience, and specific data from the patient’s physical examination and provided history. All these sources are valuable, and I believe that how we thoughtfully and purposefully weigh and incorporate this information into practice defines us as the clinicians we are.
May et al discuss one of the most common laboratory tests we order, the complete blood cell count, and how to interpret and unlock additional information that we often overlook.
Singh et al explain the utility and limitations of assessing hepatic fibrosis in patients with known liver disease using specialized and increasingly available imaging techniques in patients with common diseases that may progress to liver failure.
Using several clinical scenarios, Suresh explores the limitations of serologic testing in patients with a potential “autoimmune” or systemic inflammatory syndrome (which, based on new consultations I see in my rheumatology clinic, seems to be virtually everyone who has experienced pain or fatigue).
The Journal also continues our ongoing series on Smart Testing that has focused on tests and testing strategies that have a strong evidence basis to support or discourage their utilization in specific settings. But in most real-life clinical scenarios, relatively little directly applicable evidence can be brought to bear on our decision process with a specific patient. Hence the ongoing need for each of us to refine our clinical reasoning skills, and to recognize the continuing challenges facing the incorporation of artificial intelligence and algorithmic practice into the management of the individual patient sitting or lying in front of us.
The challenge is to balance input from Watson, “Dr. Google,” our accumulated anecdotal and group experience, and specific data from the patient’s physical examination and provided history. All these sources are valuable, and I believe that how we thoughtfully and purposefully weigh and incorporate this information into practice defines us as the clinicians we are.
May et al discuss one of the most common laboratory tests we order, the complete blood cell count, and how to interpret and unlock additional information that we often overlook.
Singh et al explain the utility and limitations of assessing hepatic fibrosis in patients with known liver disease using specialized and increasingly available imaging techniques in patients with common diseases that may progress to liver failure.
Using several clinical scenarios, Suresh explores the limitations of serologic testing in patients with a potential “autoimmune” or systemic inflammatory syndrome (which, based on new consultations I see in my rheumatology clinic, seems to be virtually everyone who has experienced pain or fatigue).
The Journal also continues our ongoing series on Smart Testing that has focused on tests and testing strategies that have a strong evidence basis to support or discourage their utilization in specific settings. But in most real-life clinical scenarios, relatively little directly applicable evidence can be brought to bear on our decision process with a specific patient. Hence the ongoing need for each of us to refine our clinical reasoning skills, and to recognize the continuing challenges facing the incorporation of artificial intelligence and algorithmic practice into the management of the individual patient sitting or lying in front of us.
The challenge is to balance input from Watson, “Dr. Google,” our accumulated anecdotal and group experience, and specific data from the patient’s physical examination and provided history. All these sources are valuable, and I believe that how we thoughtfully and purposefully weigh and incorporate this information into practice defines us as the clinicians we are.
Flu or strep? Rapid tests can mislead
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012