User login
Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
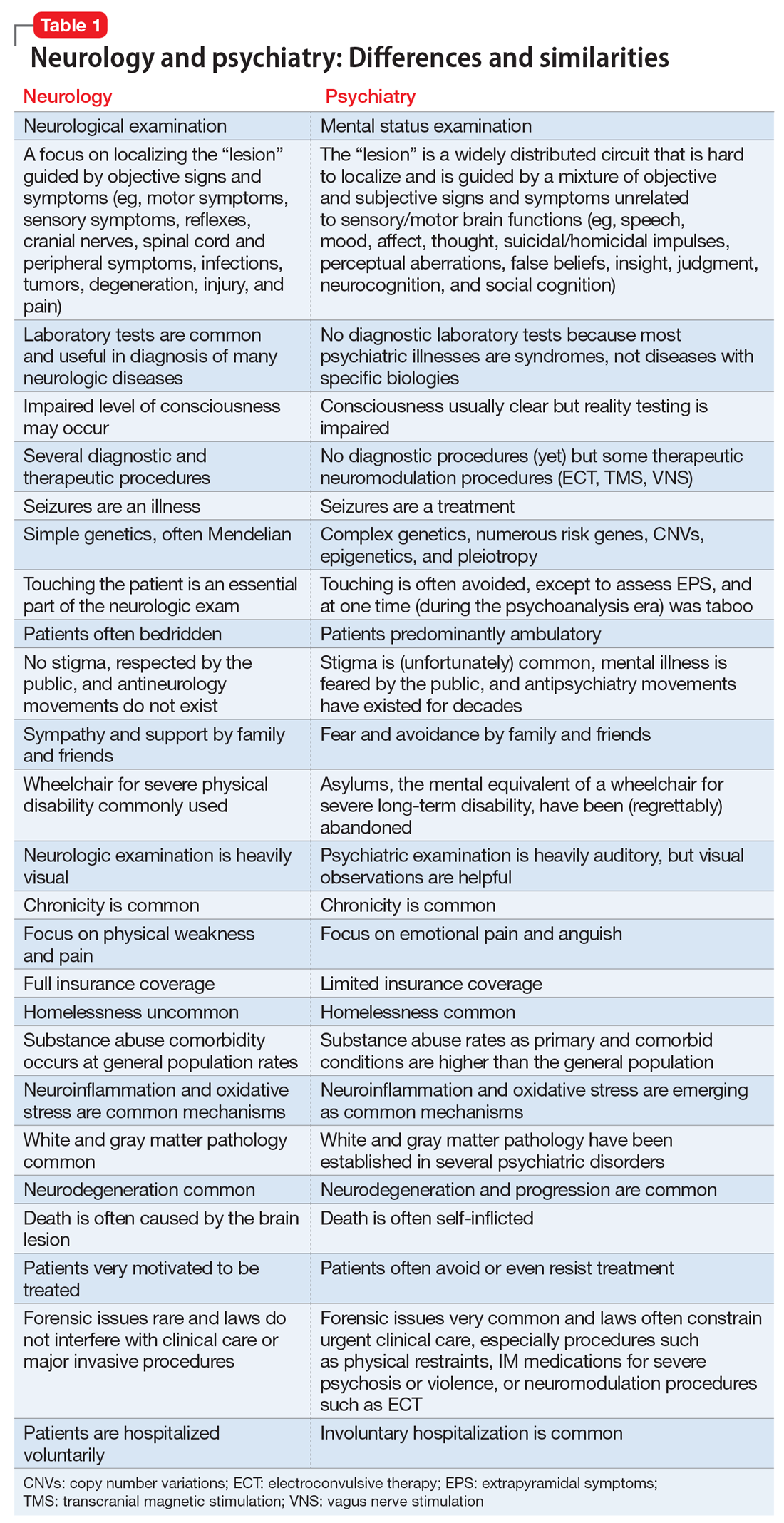
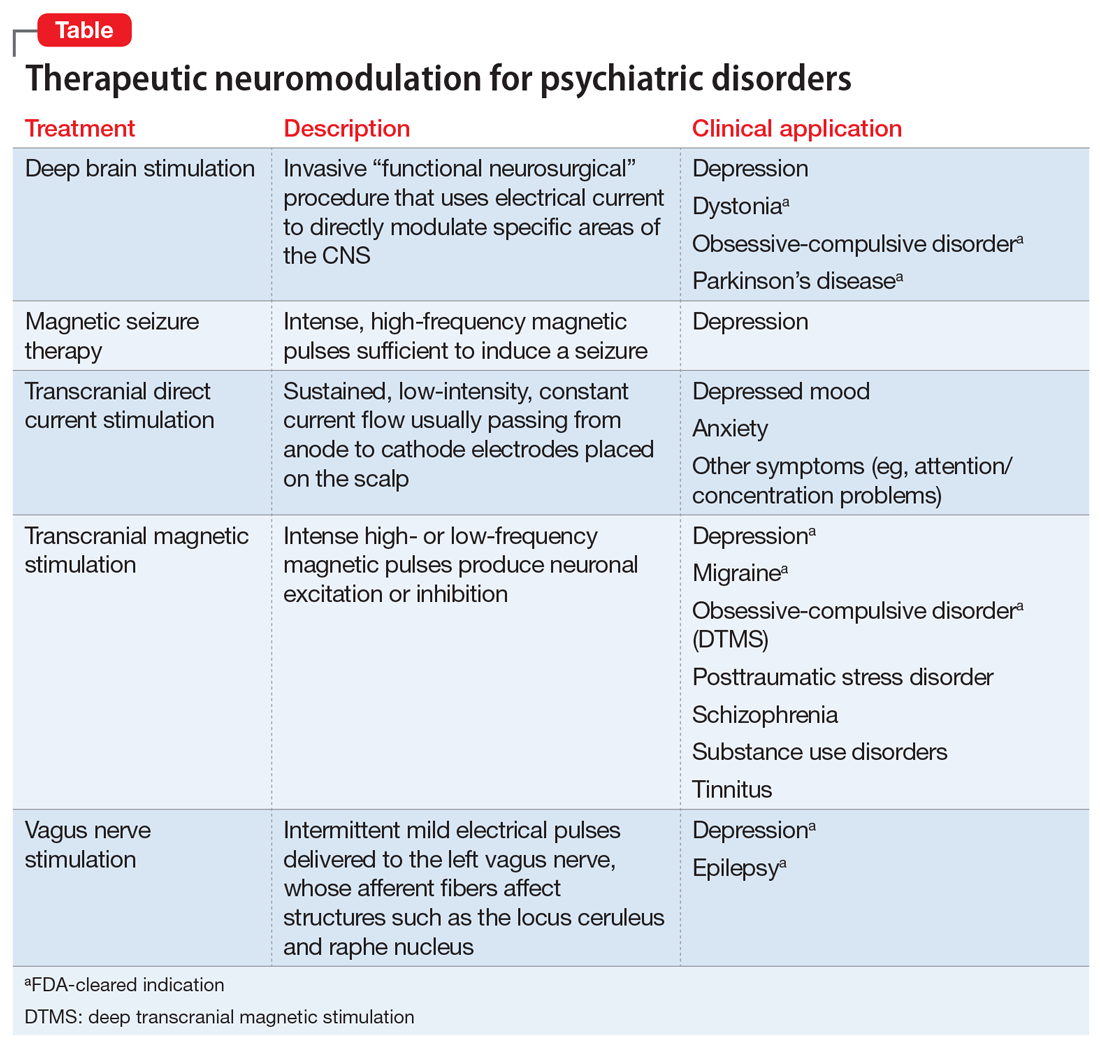
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.
Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.
Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.
Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Aerospace medicine and psychiatry
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
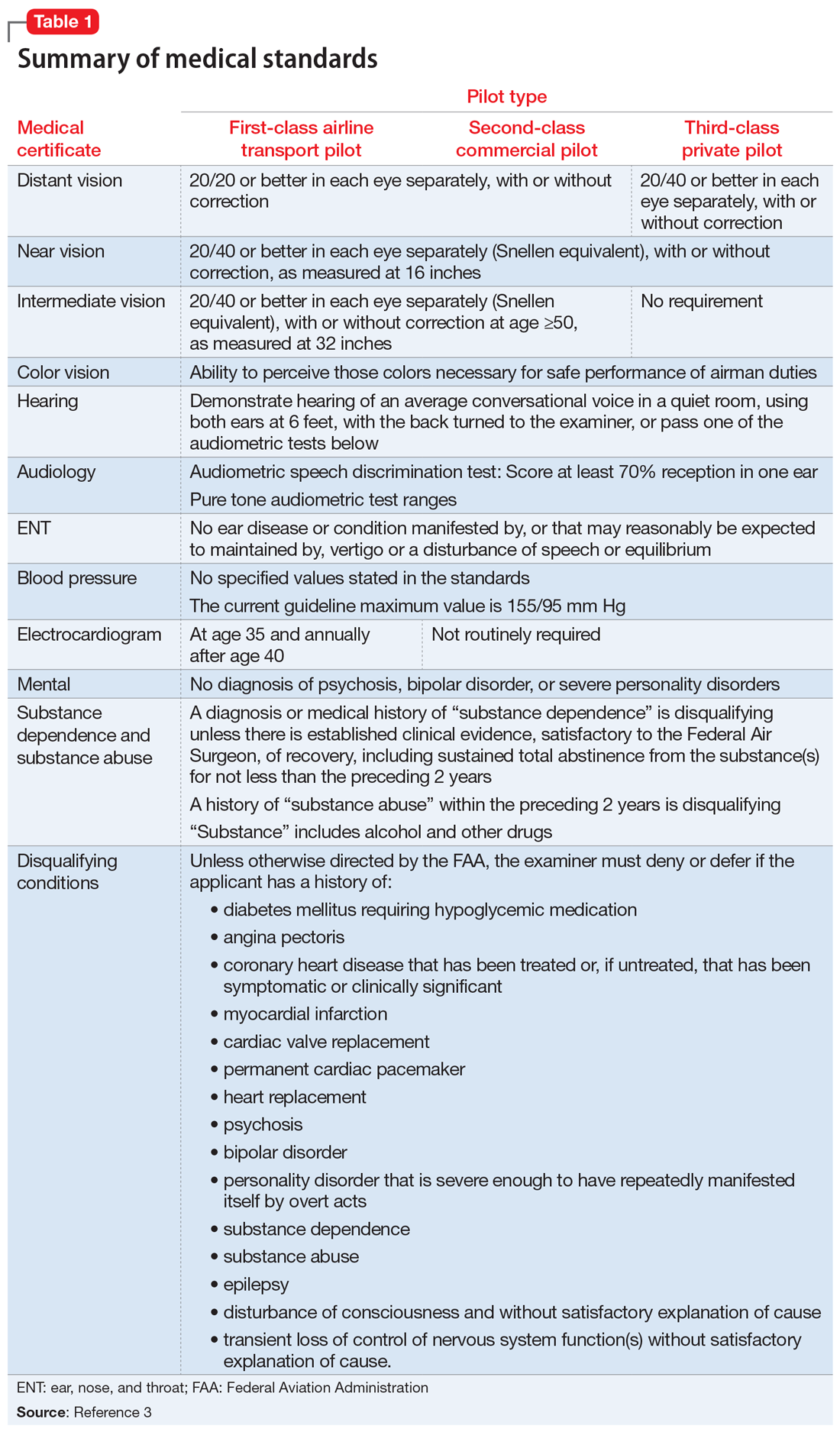
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.
_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
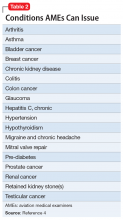
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.
_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.
_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
Antipsychotics and seizures: What are the risks?
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
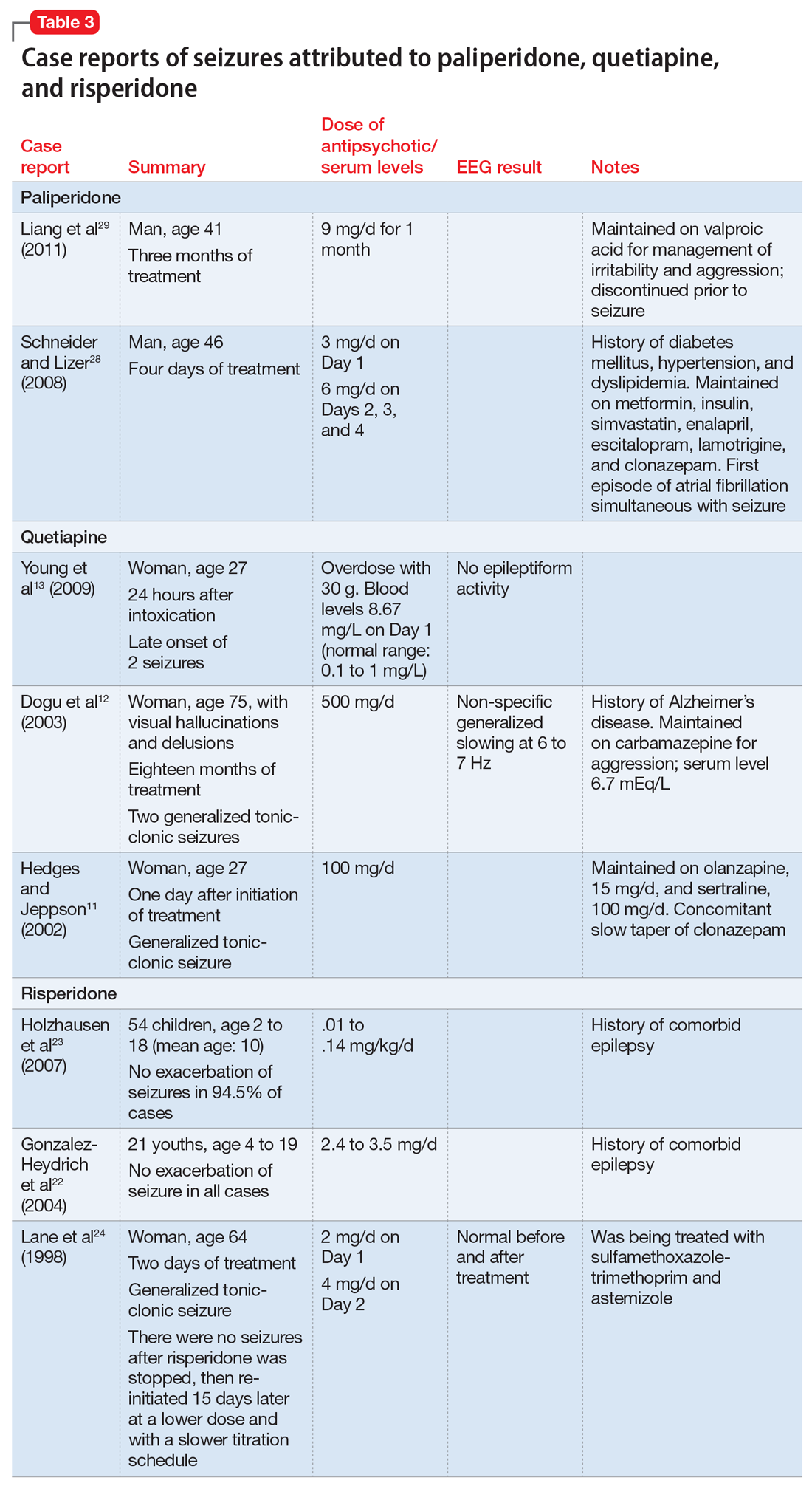
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
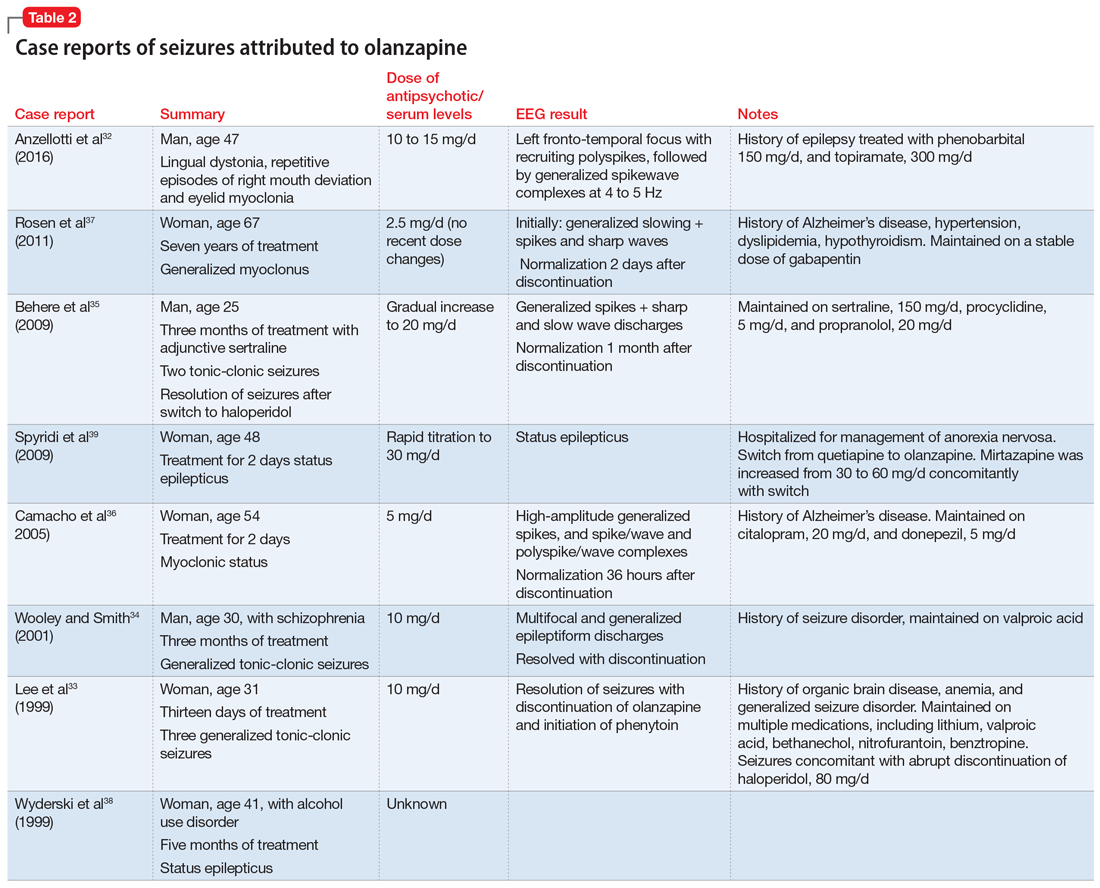
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
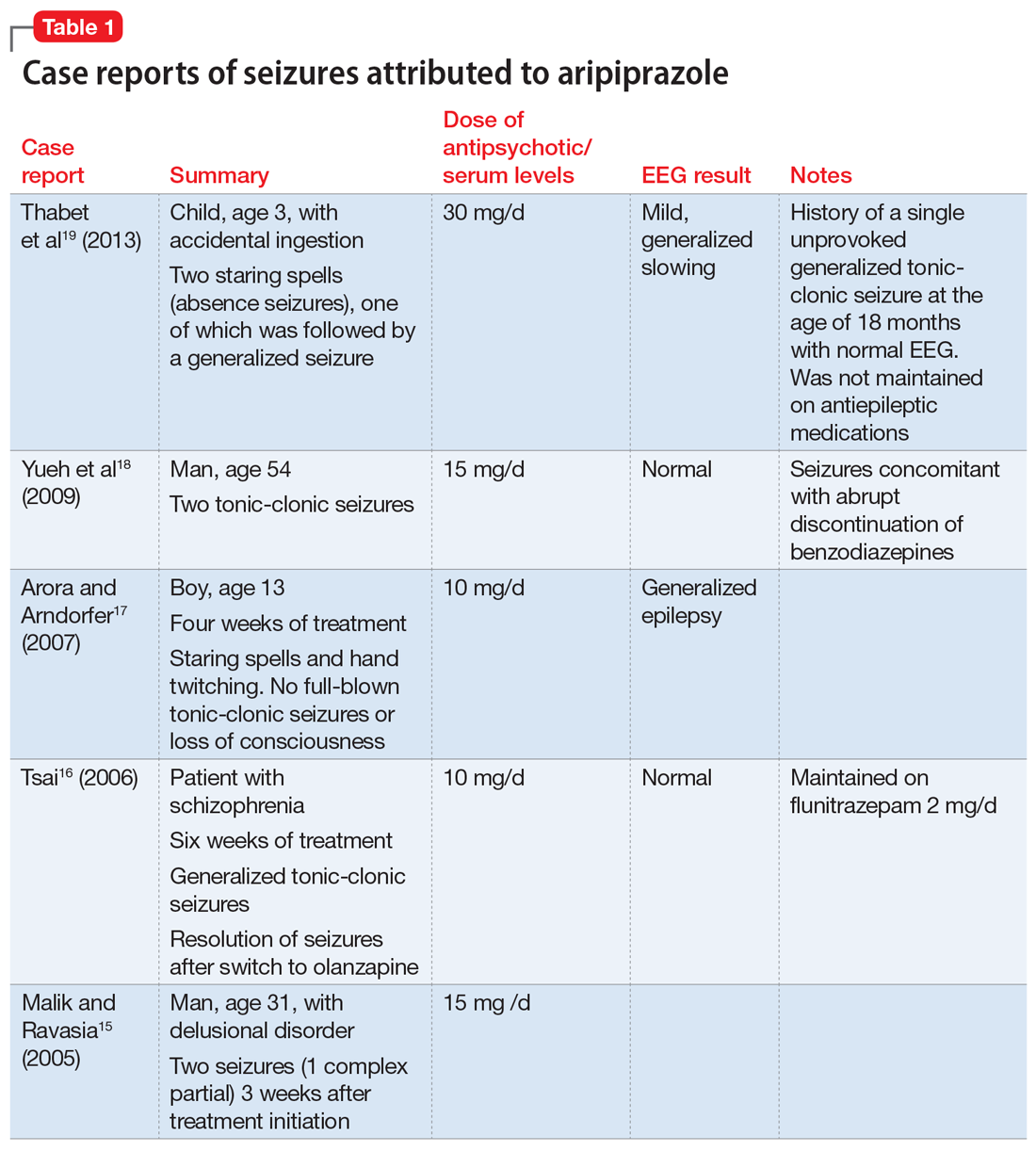
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
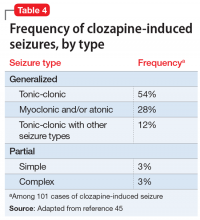
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
What’s new in transcranial magnetic stimulation
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
AGA Clinical Practice Update: Surgical risk assessment and perioperative management in cirrhosis
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
The Poison Squad
This month I am reading The Poison Squad, by Deborah Blum. It’s a fascinating book about Harvey Wiley, the first commissioner of the FDA and head chemist for 29 years (until 1912). He spearheaded passage of the Pure Food and Drug Act of 1906, the first legislation to regulate what could be put into our food and drink. Prior to (and even subsequent to) passage, hundreds of deaths, mostly children, were linked to toxic additives or adulteration of food. Formaldehyde, for example, was routinely added to milk as a preservative and was linked to dozens of children’s deaths. This single, dedicated scientist fought governmental corruption and big business to protect the public.
The book describes dark money corrupting senators, fake news, denigration of science, suppression of FDA scientific studies that ran counter to administration goals, solicitation of “scientists” who would publicly denounce test results, advocates of states’ rights who fought federal overreach, those that predicted regulation would “ruin American business” and other themes that parallel what we encounter in current news. There are even examples of policy by Executive Order (related to purity of whiskey of all things). One could easily be reading about tobacco, climate change, or vaccines and encounter the same themes. “Those who fail to learn from history are doomed to repeat it” (Santayana 1905 and Churchill 1948).
We are covering a number of important articles this issue. Our cover stories concern surgery in patients with cirrhosis, post colonoscopy FIT testing, and liver transplant in patients with alcoholic liver disease. Another important story reminds us to help our IBD patients with reproductive counseling.
Just a few months to go before Digestive Disease Week® (DDW) in San Diego. Registration is open and hotels are filling; visit www.DDW.org/registration for more information. This year’s scientific lineup is stellar.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This month I am reading The Poison Squad, by Deborah Blum. It’s a fascinating book about Harvey Wiley, the first commissioner of the FDA and head chemist for 29 years (until 1912). He spearheaded passage of the Pure Food and Drug Act of 1906, the first legislation to regulate what could be put into our food and drink. Prior to (and even subsequent to) passage, hundreds of deaths, mostly children, were linked to toxic additives or adulteration of food. Formaldehyde, for example, was routinely added to milk as a preservative and was linked to dozens of children’s deaths. This single, dedicated scientist fought governmental corruption and big business to protect the public.
The book describes dark money corrupting senators, fake news, denigration of science, suppression of FDA scientific studies that ran counter to administration goals, solicitation of “scientists” who would publicly denounce test results, advocates of states’ rights who fought federal overreach, those that predicted regulation would “ruin American business” and other themes that parallel what we encounter in current news. There are even examples of policy by Executive Order (related to purity of whiskey of all things). One could easily be reading about tobacco, climate change, or vaccines and encounter the same themes. “Those who fail to learn from history are doomed to repeat it” (Santayana 1905 and Churchill 1948).
We are covering a number of important articles this issue. Our cover stories concern surgery in patients with cirrhosis, post colonoscopy FIT testing, and liver transplant in patients with alcoholic liver disease. Another important story reminds us to help our IBD patients with reproductive counseling.
Just a few months to go before Digestive Disease Week® (DDW) in San Diego. Registration is open and hotels are filling; visit www.DDW.org/registration for more information. This year’s scientific lineup is stellar.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This month I am reading The Poison Squad, by Deborah Blum. It’s a fascinating book about Harvey Wiley, the first commissioner of the FDA and head chemist for 29 years (until 1912). He spearheaded passage of the Pure Food and Drug Act of 1906, the first legislation to regulate what could be put into our food and drink. Prior to (and even subsequent to) passage, hundreds of deaths, mostly children, were linked to toxic additives or adulteration of food. Formaldehyde, for example, was routinely added to milk as a preservative and was linked to dozens of children’s deaths. This single, dedicated scientist fought governmental corruption and big business to protect the public.
The book describes dark money corrupting senators, fake news, denigration of science, suppression of FDA scientific studies that ran counter to administration goals, solicitation of “scientists” who would publicly denounce test results, advocates of states’ rights who fought federal overreach, those that predicted regulation would “ruin American business” and other themes that parallel what we encounter in current news. There are even examples of policy by Executive Order (related to purity of whiskey of all things). One could easily be reading about tobacco, climate change, or vaccines and encounter the same themes. “Those who fail to learn from history are doomed to repeat it” (Santayana 1905 and Churchill 1948).
We are covering a number of important articles this issue. Our cover stories concern surgery in patients with cirrhosis, post colonoscopy FIT testing, and liver transplant in patients with alcoholic liver disease. Another important story reminds us to help our IBD patients with reproductive counseling.
Just a few months to go before Digestive Disease Week® (DDW) in San Diego. Registration is open and hotels are filling; visit www.DDW.org/registration for more information. This year’s scientific lineup is stellar.
John I. Allen, MD, MBA, AGAF
Editor in Chief
AGA Clinical Practice Update: Changing utility of serology and histologic measures in celiac disease
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
FROM GASTROENTEROLOGY
Myositis mimics: Clues for making the right diagnosis
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
A number of conditions can mimic myositis, but clues that can point to the correct diagnosis are often present in cases involving the mimics, according to Lisa Christopher-Stine, MD.
For example, elevated levels of certain muscle enzymes are an important source of diagnostic information, Dr. Christopher-Stine, director of the Johns Hopkins Myositis Center, Baltimore, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Isolated elevations in aldolase can be seen in connective tissue–associated interstitial lung disease or in patients with fascial edema, and aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), and creatine kinase (CK) levels can also be helpful, she explained.
The latter can also be elevated in the absence of muscle disease, for example, in healthy individuals following exercise. CK peaks at 24 hours after exercise before returning to baseline by 72 hours. In an experimental setting, a threefold increase in CK levels has been seen at 8-24 hours after exercise, Dr. Christopher-Stine said.
“HyperCKemia”
Trauma from causes such as intramuscular injection, electromyography (EMG), major surgery, or biopsy can also lead to increased CK levels. Motor neuron disease can also cause such increases. In one study, 75% of patients with amyotrophic lateral sclerosis had a mean twofold increase in CK levels, she said.
Asymptomatic CK elevations may also represent presymptomatic myopathies, type 1 or 2 macro-CK, manual labor occupations, or they may be idiopathic.
Race can play a role in CK levels as well. Black people tend to have higher CK levels than white people, she said, noting that one study of more than 10,000 adults showed that black race was strongly associated with CK, and that body composition largely explained differences in CK by age, but not by race/ethnicity (Medicine. Aug 2016;95[33]:e4344).
“So elevated CK may not herald any discernible illness,” she said.
Dr. Christopher-Stine described a case involving an otherwise healthy 30-year-old man with a CK level of 695 IU/L that was found incidentally. He had a desk job, no recent travel, and denied weakness, myalgias, joint pain, dysphagia, shortness of breath, and fevers. In this case, the elevated CK was felt to be secondary to his African American race given that other causes were ruled out.
Another case involved a 72-year-old man with left-arm pain. A cardiac event was ruled out, and CK was found to be about 4,500 IU/L. He reported “flare-ups” of diffuse swelling of the hands and feet. X-rays showed concerning signs of erosions. His transaminases and electromyogram were normal; he reported no weakness or myalgia; and an MRI showed no muscle edema. He was diagnosed with macro-CK, which refers to CK with an increased molecular weight. A clue to this diagnosis is a normal liver function test. In some cases, muscle/brain CK levels (CK-MB) are elevated and higher than total CK, she noted.
She presented an algorithm for the diagnostic work-up of patients presenting with elevated CK of unclear significance. Her recommended approach involves repeat CK assessment and a closer look at family history, medication, drug/toxin history, examination for weakness and neurologic abnormalities, and additional lab assessments in those whose levels remain elevated. In those in whom a diagnosis is not identified, the algorithm calls for observation every 3 months – including physical examination and labs – in asymptomatic patients with levels at less than five times the upper limit of normal, and further evaluation, including EMG with nerve-conduction velocity testing, muscle biopsy, and MRI in those with (or who later develop) marked elevation greater than five times the upper limit of normal and/or symptoms.
Patient assessment
The physical examination should involve localization and quantification of weakness, and assessment for fever, rash, atrophy/wasting/scooping of forearms, fasciculations, cranial nerve involvement, Raynaud’s phenomenon, nailfold capillary changes, arthritis, calcinosis, “mechanic’s hands,” signs of other autoimmune diseases, and lung crackles. Initial laboratory testing should include HIV and hepatitis B and C testing; measurement of CK, AST, ALT, aldolase, thyroid-stimulating hormone, and magnesium levels; a comprehensive metabolic panel and complete blood count; and measurement of erythrocyte sedimentation rate and C-reactive protein.
“Weakness may be secondary to a neuropathy, myopathy, or a problem at the neuropathic junction. Many causes of weakness can be readily identified by careful history taking, focused physical examination, and directed laboratory evaluation,” she said.
Features pointing toward a diagnosis of myositis include characteristic rashes, gradual symptom onset, proximal limb and truncal weakness, other connective tissue disease features such as Raynaud’s and arthritis, and the presence of lung disease, including interstitial lung disease or unexplained infiltrates, she said.
Features pointing away from a diagnosis of myositis include a family history of a similar illness, weakness that is associated with eating or fasting, neurologic signs, cranial nerve involvement, fasciculations, severe muscle cramping, early atrophy, and creatine phosphokinase levels that are either less than 2 times or more than 100 times the upper limit of normal.
Among the conditions to consider in the presence of the features that point away from a myositis diagnosis are muscular dystrophies, metabolic myopathies, and toxic (drug-induced) myopathies, to name a few, Dr. Christopher-Stine said.
She described a number of other cases to illustrate the need for – and to help develop – a differential diagnosis in patients presenting with apparent myositis.
Muscular dystrophies
A 38-year-old woman with limited scleroderma and anti-PM/Scl autoantibodies developed proximal weakness over 9 months and was eventually unable to walk up a flight of stairs. She had heliotrope rash and Gottron’s sign, her serum CK was 723 IU/L, and EMG showed an irritable myopathy.
Muscle biopsy showed inflammation, and she was treated with prednisone, but this led to worsening weakness. She complained of prominent fatigue and double vision at the end of the day, and these symptoms did not improve with steroids.
Anti-AChR and anti-MuSK antibodies were negative, but she had a decrement on repetitive nerve stimulation testing.
She was treated with pyridostigmine and experienced near-complete resolution of her proximal weakness and double vision. A chest CT scan showed thymic hyperplasia; thymectomy was recommended.
In another case, a 19-year-old woman who complained of leg pain after exercise was found to have intact strength but asymmetric calf hypertrophy. Her CK level was 5,000 IU/L, and she was referred to rule out acute myositis.
A quadriceps biopsy was performed and showed abnormal dystrophin immunostaining but no inflammation. A molecular genetic analysis showed deletions in Xp21 and she was diagnosed as a manifesting carrier of Duchenne muscular dystrophy. It was recommended that she be evaluated for cardiomyopathy and receive genetic counseling.
A number of other cases presented by Dr. Christopher-Stine highlighted other muscular dystrophies that can mimic myositis, such as:
- Myotonic dystrophies. These are more often type 2 than type 1. Myotonia may be subtle, cataracts are seen early in all patients, and cardiac arrhythmias are common.
- Limb girdle muscular dystrophy type 2 B (dysferlinopathy). In the legs, this often affects the gastrocnemius muscle, and this will be visible on MRI. In the arms, it most often affects the biceps, sparing the deltoids. CKs are typically very high.
- Facioscapulohumeral muscular dystrophy (FSHD). This involves facial weakness, especially obicularis oris, in 95% of cases, as well as scapular weakness and winging, inflammation on muscle biopsy in 75% of cases, and typically is endomysial or perivascular.
Metabolic myopathies
Among metabolic myopathies that can mimic myositis are disorders of carbohydrate metabolism such as McArdle’s disease, 6-phosphofructokinase deficiency, and Pompe’s disease (adult acid maltase deficiency); disorders of lipid metabolism such as carnitine deficiency and carnitine palmitoyltransferase 2 (CPT2) deficiency; and disorders of purine metabolism, such as myoadenylate deaminase deficiency.
A 27-year-old patient who complained of weakness with activity was referred for possible myositis and was found to have a CK of 3,650 IU/L that never normalized. Physical examination showed intact strength and no muscle atrophy or fasciculations, and an enzyme stain for myophosphorylase showed a normal staining pattern and complete absence of the enzyme on quadricep biopsy. A 22-year-old man with similar symptoms plus recent onset of brown/black urine after physical activity had CK of 110,000 IU/L when symptomatic, and also underwent biopsy after being referred for possible myopathy. Both patients were ultimately diagnosed with CPT2 deficiency, which is associated with risk of rhabdomyolysis triggered by prolonged exercise, diets low in carbohydrates and high in fat, or by fasting.
Myalgias are common, and CK levels are normal or only mildly elevated between episodes in CPT2 deficiency, Dr. Christopher-Stine noted.
Toxic myopathies
Drug-induced myopathies are among the most common etiologies of myopathy and can range from mild myalgia to massive rhabdomyolysis. They can cause mild to severe weakness and may be chronic. The mechanism of toxic injury is direct via myotoxins such as ethyl alcohol, glucocorticoids, lipid-lowering drugs, cocaine, antimalarial drugs, antipsychotic drugs, colchicine, and Ipecac syrup.
One case described by Dr. Christopher-Stine involved “statin myopathy.”
A 55-year-old man on atorvastatin complained of myalgias and brown urine, but had no definitive weakness. He had intact strength and diffuse myalgias that weren’t reproducible. His CK was 45,000 IU/L.
Statin myopathy, as seen in this patient, is usually self-limited and is not associated with autoimmunity or with anti-HMGCR autoantibody positivity.
The mechanism is unknown, but statin myopathy has an incidence of 1.2 per 10,000 patient-years. Myalgias, myositis, rhabdomyolysis, and asymptomatic hyperCKemia are commonly seen. This is in contrast to the immune-mediated necrotizing myelitis that can be secondary to statins and is responsive to immunosuppression, she noted.
Other myositis mimics
In addition to these common myositis mimics, certain other neurologic diseases (such as ALS and cervical myelopathy), endocrinopathies (such as hypothyroidism), and infections (like toxoplasmosis) can also be mistaken for myositis, Dr. Christopher-Stine said, noting that cases illustrating these mimics underscore the need for careful consideration of possible alternate diagnoses.
“While most noninflammatory myopathies are self-limited or have no therapies available, knowing the diagnosis can be helpful for genetic counseling of the patient and family, for mitigating risk factors, and for precluding the use of unwarranted immunosuppressive agents,” she said.
Dr. Christopher-Stine reported having intellectual property interest in a novel Inova Diagnostics autoantibody assay detection for anti-HMGCR. She was also the safety officer for the JBT-101 Trial sponsored by Corbus and funded by the National Institutes of Health.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Barriers to CAR T use in the spotlight at first European meeting
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
Spinal cord atrophy found to be accelerated in subset of RRMS patients
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
DALLAS – The rate of spinal cord atrophy at the C1 level is promising as a prognostic biomarker for the future conversion to secondary progressive disease in patients with relapsing remitting multiple sclerosis (RRMS), results from a novel, single-center study suggest.
“Among all magnetic resonance imaging measures, spinal cord area shows the strongest correlations with MS disability and has been shown to discriminate progressive from relapsing remitting disease subtypes,” lead study author Antje Bischof, MD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “In our work, we used a novel method to accurately measure upper cervical cord area at C1 vertebral level from brain MRI. This enabled us to show for the first time that compared to a matched group of patients who remained relapsing remitting MS over 2 decades.”
Dr. Bischof, a postdoctoral research fellow in the department of neurology at the University of California, San Francisco, and her colleagues matched 54 RRMS patients who converted to secondary progressive MS (SPMS) during the 12-year observation period with 54 RRMS patients who remained RRMS during the observation period, based on demographic and clinical criteria. Additionally, they evaluated 54 age- and sex-matched healthy controls at baseline. From routine T1-weighted brain MRI, they analyzed brain measures and spinal cord area at C1 level over 12 years to evaluate their potential to discriminate between the two matched groups during the preconversion period.
Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001). Their data suggest that this difference exists at least 4 years before conversion to SPMS. “None of the commonly used measures of the brain including global brain volumes like white matter and gray matter, regional brain volumes like thalamus, and MS lesion volumes, discriminated between the patients with relapsing remitting MS who later converted to secondary progressive disease and the patients who remained RRMS,” Dr. Bischof said.
She acknowledged certain limitations of the study, including the small sample size and the fact that the results require confirmation in a second MS cohort in order to be generalizable. “These results suggest cervical cord atrophy rate at C1 level as a prognostic biomarker for the conversion to secondary progressive MS and could be useful for treatment decisions early in the disease course, and for the study of genetic, epidemiologic, and immune variables in MS,” Dr. Bischof concluded.
She reported having no financial disclosures.
SOURCE: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: In patients with relapsing remitting MS (RRMS), upper cervical cord atrophy, as obtained from routine T1-weighted brain MRI, is a strong indicator of impending conversion to secondary progressive MS (SPMS).
Major finding: Subjects who developed SPMS showed higher rates of spinal cord atrophy (–2.2% per year; standard error, 0.2) before conversion to a secondary progressive course, compared with their RRMS matches who did not convert to SPMS (–0.7% per year; SE, 0.2; P less than .0001).
Study details: A single-center, observational study of 54 RRMS patients who converted to SPMS during the 12-year observation and 54 RRMS patients who remained RRMS during the observation period.
Disclosures: Dr. Bischof reported having no financial disclosures.
Source: Bischof A et al. ACTRIMS Forum 2019, Abstract P157.