User login
ICU admissions raise chronic condition risk
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
SAN DIEGO – The research showed rising likelihood of conditions such as depression, diabetes, and heart disease.
By merging two existing databases, the researchers were able to capture a more comprehensive picture of post-ICU patients. “We were able to include almost the entire country,” Ilse van Beusekom, a PhD candidate in health sciences at the University of Amsterdam and data manager at the National Intensive Care Evaluation (NICE) foundation, said in an interview.
Ms. van Beusekom presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published in Critical Care Medicine.
The work compared 56,760 ICU survivors from 81 facilities across the Netherlands to 75,232 age-, sex-, and socioeconomic status–matched controls. The mean age was 65 years and 60% of the population was male. “The types of chronic conditions are the same, only the prevalences are different,” said Ms. van Beusekom.
The researchers compared chronic conditions in the year before ICU admission and the year after, based on data pulled from the NICE national quality database, which includes data describing the first 24 hours of ICU admission, and the Vektis insurance claims database, which includes information on medical treatment. Before ICU admission, 45% of the ICU population was free of chronic conditions, as were 62% of controls. One chronic condition was present in 36% of ICU patients, versus 29% of controls, and two or more conditions were present in 19% versus 9% of controls.
The ICU population was more likely to have high cholesterol (16% vs. 14%), heart disease (14% vs. 6%), chronic obstructive pulmonary disease (8% vs. 3%), type II diabetes (8% vs. 6%), type I diabetes (6% vs. 3%), and depression (6% vs. 4%).
The ICU population also was at greater risk of developing one or more new chronic conditions during the year following their stay. The risk was three- to fourfold higher throughout age ranges.
The study suggests the need for greater follow-up after an ICU admission in order to help patients cope with lingering problems. Ms. van Beusekom noted that there are follow-up programs in the Netherlands for several patient groups, but none for ICU survivors. One possibility would be to have the patient return to the ICU 3 months or so after release to discuss their diagnosis, treatment, and any lingering concerns. “A lot of people don’t know that their complaints are linked with the ICU visit,” said Ms. van Beusekom.
Timothy G. Buchman, MD, professor of surgery at Emory University, Atlanta, who moderated the session, wondered why the ICU seems to be an inflection point for developing new chronic conditions. Could it simply be because patients are sicker to begin with and have reached an inflection point of their illness, or could the treatments in ICU be contributing to or exposing those conditions? Ms. van Beusekom believed it was likely a combination of factors, and she referred to data she had not presented showing that even control patients who had been to the hospital (though not the ICU) during the study period were at lower risk of new chronic conditions than ICU patients.
Ms. van Beusekom’s group plans to investigate ICU-related variables that might be associated with risk of chronic conditions.
The study was not funded. Ms. van Beusekom had no relevant disclosures.
SOURCE: van Beusekom I et al. CCC48, Abstract Crit Care Med. 2019;47:324-30.
REPORTING FROM CCC48
Commentary: Should AVFs be ligated after kidney transplant?
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
Hemodynamic complications of arteriovenous (AV) access are uncommon but can be potentially life threatening. Fistulas and grafts can cause a decrease in systemic vascular resistance and secondary increase in cardiac output in patients who may already have myocardial dysfunction secondary to their end-stage renal disease.1 This increased cardiac output is usually insignificant but in rare cases can result in clinically significant cardiac failure. Patients with high-output fistulas with volume flow greater than 2 L/min may be at increased risk of heart failure but volume flow less than 2 L/min does not preclude this complication.2
In patients with AV access–related heart failure, optimal medical management and reduction of fistula flow or ligation of the dialysis access should be considered. If continued hemodialysis is necessary, loss of a functioning dialysis access is problematic and difficult management decisions must be made. Following successful renal transplantation, ligation of vascular access in the presence of symptomatic heart failure may represent a straightforward decision. Nonetheless, there is no clear consensus of how to manage patent fistulas or grafts in patients following renal transplantation in the absence of significant cardiac symptoms with particular concern to the important issues of transplant survival and long-term cardiac prognosis. Yaffe and Greenstein3 recommend preservation of almost all fistulas after transplantation in the absence of significant complications such as venous hypertension, pseudoaneurysm, significant high-output cardiac failure or hand ischemia. They recommend taking into account the 10-year adjusted renal transplantation graft survival rates and the relative paucity of donors, recognizing the possibility that the patient may have to return to dialysis at some point in the future. They also reference the lack of information regarding the beneficial impact of fistula ligation on cardiac morphology and function as a rationale for access preservation.
A recent presentation at the American Heart Association Scientific Sessions by Michael B. Stokes, MD,4 from the department of cardiology at Royal Adelaide Hospital in Australia, suggests that cardiovascular disease is responsible for 40% of deaths among kidney transplant recipients and that left ventricular (LV) mass is strongly associated with cardiovascular mortality.
He states that, although there is no guideline consensus on the management of an AV fistula following successful renal transplantation, the fistula continues to contribute adversely to cardiac remodeling and function. The lack of previous randomized controlled trials in this area led Dr. Stokes and his colleagues to randomly assign 64 patients at least 1 year following successful kidney transplantation with stable renal function and a functioning AV fistula to either fistula ligation or no intervention. All patients underwent cardiac MRI at baseline and 6 months.
The primary endpoint of decrease in LV mass at 6 months was significant in the ligation group but not in the control group. The ligation group also had significant decrease in LV end diastolic volume, LV end systolic volume, and multiple other parameters. In addition, NT-proBNP levels and left atrial volume were significantly reduced in the ligation group when compared with the control group. Complications in the ligation group included six patients with thrombosis of their fistula vein and two infections, all of which resolved with outpatient anti-inflammatory or antimicrobial therapy.
Dr. Stokes believes that control patients in his study face “persisting and substantial deleterious cardiac remodeling” and that “further investigation would clarify the impact of AV fistula ligation on clinical outcomes following kidney transplantation.”
I believe this is important information and represents the first randomized controlled data regarding the long-term adverse cardiac effects of a patent fistula after renal transplantation. Unfortunately, information regarding baseline fistula volume flow is not provided in this abstract. As discussed earlier, patients with high-flow fistulas may be at increased risk of heart failure and hemodynamic data can be critical in establishing an algorithm for managing these challenging patients.
Ligation of a functioning and asymptomatic access in a patient with a successful renal transplant should be recommended only after informed discussion with the patient weighing the ongoing potential negative effects on cardiac function of continued access patency versus the potential need for future hemodialysis. Dr. Stokes presents interesting data that must be considered in this controversy. I believe that, in the absence of a universally applicable algorithm, the clinical decision to recommend AV fistula ligation after successful kidney transplantation should be individualized and based on ongoing assessment of cardiac and renal function and fistula complications and hemodynamics.
References
1. Eur Heart J 2017;38:1913-23.
2. Nephrol Dial Transplant 2008;23:282-7.
3. J Vasc Access 2012;13:405-8.
4. Stokes MB, et al. LBS.05 – Late Breaking Clinical Trial: Hot News in HF. Presented at American Heart Association Scientific Sessions. 2018 Nov 10-12. Chicago.
Larry A. Scher, MD, is a vascular surgeon at the Montefiore Greene Medical Arts Pavilion, New York, and an associate medical editor for Vascular Specialist.
No survival benefit from systematic lymphadenectomy in ovarian cancer
Lymphadenectomy in women with advanced ovarian cancer and normal lymph nodes does not appear to improve overall or progression-free survival, according to a randomized trial of 647 women with newly-diagnosed advanced ovarian cancer who were undergoing macroscopically complete resection.
The women were randomized during the resection to either undergo systematic pelvic and para-aortic lymphadenectomy or no lymphadenectomy. The study excluded women with obvious node involvement.
The median overall survival rates were similar between the two groups; 65.5 months in the lymphadenectomy group and 69.2 months in the no-lymphadenectomy group (HR 1.06, P = .65). There was also no significant difference between the two groups in median progression-free survival, which was 25.5 months in both.
While overall quality of life was similar between the two groups, there were some significant points of difference. Patients who underwent lymphadenectomy experienced significantly longer surgical times, and greater median blood loss, which in turn led to a higher rate of blood transfusions and higher rate of postoperative admission to intensive care.
The 60-day mortality rates were also significantly higher among the lymphadenectomy group – 3.1% vs. 0.9% (P = .049) – as was the rate of repeat laparotomies for complications (12.4% vs. 6.5%, P = .01), mainly due to bowel leakage or fistula.
While systematic pelvic and para-aortic lymphadenectomy is often used in patients with advanced ovarian cancer, there is limited evidence in its favor from randomized clinical trials, wrote Philipp Harter, MD, of the department of gynecology and gynecologic oncology at Kliniken Essen-Mitte, Germany, and his coauthors. The report is in the New England Journal of Medicine
“In this trial, patients with advanced ovarian cancer who underwent macroscopically complete resection did not benefit from systematic lymphadenectomy,” the authors wrote. “In contrast, lymphadenectomy resulted in treatment burden and harm to patients.”
The research group also tried to account for the level of surgical experience in each of the 52 centers involved in the study, and found no difference in treatment outcomes between high-recruiting centers and low-recruiting centers. All the centers also had to demonstrate their proficiency with the lymphadenectomy procedure before participating in the study.
“Accordingly, the quality of surgery and the numbers of resected lymph nodes were higher than in previous gynecologic oncologic clinical trials analyzing this issue,” they wrote.
The study was supported by the Deutsche Forschungsgemeinschaft and the Austrian Science Fund. Six authors declared a range of fees and support from the pharmaceutical industry.
SOURCE: Harter P et al. N Engl J Med. 2019 Feb 27 doi: 10.1056/NEJMoa1808424.
Pelvic and aortic lymph nodes can often contain microscopic ovarian cancer metastases even when they appear normal, so there has been some debate as to whether these should be systematically removed during primary surgery to eliminate this potential sanctuary for cancer cells.
While a number of previous studies have suggested a survival benefit, there were concerns about potential confounders that may have influenced those findings. This study avoids many of the criticisms leveled at previous trials; for example, by ensuring surgical center quality, by excluding women with obvious node involvement, and by conducting the lymphadenectomy only after complete macroscopic resection.
The findings are consistent with the notion that the most frequent cause of ovarian cancer-related illness and death is the inability to control intra-abdominal disease.
Dr. Eric L. Eisenhauer is from Massachusetts General Hospital in Boston and Dr. Dennis S. Chi is from Memorial Sloan Kettering Cancer Center in New York. These comments are adapted from their accompanying editorial (N Engl J Med. 2019 Feb 27. doi: 10.1056/NEJMe1900044). Both authors declared financial and other support, including advisory board positions, from private industry.
Pelvic and aortic lymph nodes can often contain microscopic ovarian cancer metastases even when they appear normal, so there has been some debate as to whether these should be systematically removed during primary surgery to eliminate this potential sanctuary for cancer cells.
While a number of previous studies have suggested a survival benefit, there were concerns about potential confounders that may have influenced those findings. This study avoids many of the criticisms leveled at previous trials; for example, by ensuring surgical center quality, by excluding women with obvious node involvement, and by conducting the lymphadenectomy only after complete macroscopic resection.
The findings are consistent with the notion that the most frequent cause of ovarian cancer-related illness and death is the inability to control intra-abdominal disease.
Dr. Eric L. Eisenhauer is from Massachusetts General Hospital in Boston and Dr. Dennis S. Chi is from Memorial Sloan Kettering Cancer Center in New York. These comments are adapted from their accompanying editorial (N Engl J Med. 2019 Feb 27. doi: 10.1056/NEJMe1900044). Both authors declared financial and other support, including advisory board positions, from private industry.
Pelvic and aortic lymph nodes can often contain microscopic ovarian cancer metastases even when they appear normal, so there has been some debate as to whether these should be systematically removed during primary surgery to eliminate this potential sanctuary for cancer cells.
While a number of previous studies have suggested a survival benefit, there were concerns about potential confounders that may have influenced those findings. This study avoids many of the criticisms leveled at previous trials; for example, by ensuring surgical center quality, by excluding women with obvious node involvement, and by conducting the lymphadenectomy only after complete macroscopic resection.
The findings are consistent with the notion that the most frequent cause of ovarian cancer-related illness and death is the inability to control intra-abdominal disease.
Dr. Eric L. Eisenhauer is from Massachusetts General Hospital in Boston and Dr. Dennis S. Chi is from Memorial Sloan Kettering Cancer Center in New York. These comments are adapted from their accompanying editorial (N Engl J Med. 2019 Feb 27. doi: 10.1056/NEJMe1900044). Both authors declared financial and other support, including advisory board positions, from private industry.
Lymphadenectomy in women with advanced ovarian cancer and normal lymph nodes does not appear to improve overall or progression-free survival, according to a randomized trial of 647 women with newly-diagnosed advanced ovarian cancer who were undergoing macroscopically complete resection.
The women were randomized during the resection to either undergo systematic pelvic and para-aortic lymphadenectomy or no lymphadenectomy. The study excluded women with obvious node involvement.
The median overall survival rates were similar between the two groups; 65.5 months in the lymphadenectomy group and 69.2 months in the no-lymphadenectomy group (HR 1.06, P = .65). There was also no significant difference between the two groups in median progression-free survival, which was 25.5 months in both.
While overall quality of life was similar between the two groups, there were some significant points of difference. Patients who underwent lymphadenectomy experienced significantly longer surgical times, and greater median blood loss, which in turn led to a higher rate of blood transfusions and higher rate of postoperative admission to intensive care.
The 60-day mortality rates were also significantly higher among the lymphadenectomy group – 3.1% vs. 0.9% (P = .049) – as was the rate of repeat laparotomies for complications (12.4% vs. 6.5%, P = .01), mainly due to bowel leakage or fistula.
While systematic pelvic and para-aortic lymphadenectomy is often used in patients with advanced ovarian cancer, there is limited evidence in its favor from randomized clinical trials, wrote Philipp Harter, MD, of the department of gynecology and gynecologic oncology at Kliniken Essen-Mitte, Germany, and his coauthors. The report is in the New England Journal of Medicine
“In this trial, patients with advanced ovarian cancer who underwent macroscopically complete resection did not benefit from systematic lymphadenectomy,” the authors wrote. “In contrast, lymphadenectomy resulted in treatment burden and harm to patients.”
The research group also tried to account for the level of surgical experience in each of the 52 centers involved in the study, and found no difference in treatment outcomes between high-recruiting centers and low-recruiting centers. All the centers also had to demonstrate their proficiency with the lymphadenectomy procedure before participating in the study.
“Accordingly, the quality of surgery and the numbers of resected lymph nodes were higher than in previous gynecologic oncologic clinical trials analyzing this issue,” they wrote.
The study was supported by the Deutsche Forschungsgemeinschaft and the Austrian Science Fund. Six authors declared a range of fees and support from the pharmaceutical industry.
SOURCE: Harter P et al. N Engl J Med. 2019 Feb 27 doi: 10.1056/NEJMoa1808424.
Lymphadenectomy in women with advanced ovarian cancer and normal lymph nodes does not appear to improve overall or progression-free survival, according to a randomized trial of 647 women with newly-diagnosed advanced ovarian cancer who were undergoing macroscopically complete resection.
The women were randomized during the resection to either undergo systematic pelvic and para-aortic lymphadenectomy or no lymphadenectomy. The study excluded women with obvious node involvement.
The median overall survival rates were similar between the two groups; 65.5 months in the lymphadenectomy group and 69.2 months in the no-lymphadenectomy group (HR 1.06, P = .65). There was also no significant difference between the two groups in median progression-free survival, which was 25.5 months in both.
While overall quality of life was similar between the two groups, there were some significant points of difference. Patients who underwent lymphadenectomy experienced significantly longer surgical times, and greater median blood loss, which in turn led to a higher rate of blood transfusions and higher rate of postoperative admission to intensive care.
The 60-day mortality rates were also significantly higher among the lymphadenectomy group – 3.1% vs. 0.9% (P = .049) – as was the rate of repeat laparotomies for complications (12.4% vs. 6.5%, P = .01), mainly due to bowel leakage or fistula.
While systematic pelvic and para-aortic lymphadenectomy is often used in patients with advanced ovarian cancer, there is limited evidence in its favor from randomized clinical trials, wrote Philipp Harter, MD, of the department of gynecology and gynecologic oncology at Kliniken Essen-Mitte, Germany, and his coauthors. The report is in the New England Journal of Medicine
“In this trial, patients with advanced ovarian cancer who underwent macroscopically complete resection did not benefit from systematic lymphadenectomy,” the authors wrote. “In contrast, lymphadenectomy resulted in treatment burden and harm to patients.”
The research group also tried to account for the level of surgical experience in each of the 52 centers involved in the study, and found no difference in treatment outcomes between high-recruiting centers and low-recruiting centers. All the centers also had to demonstrate their proficiency with the lymphadenectomy procedure before participating in the study.
“Accordingly, the quality of surgery and the numbers of resected lymph nodes were higher than in previous gynecologic oncologic clinical trials analyzing this issue,” they wrote.
The study was supported by the Deutsche Forschungsgemeinschaft and the Austrian Science Fund. Six authors declared a range of fees and support from the pharmaceutical industry.
SOURCE: Harter P et al. N Engl J Med. 2019 Feb 27 doi: 10.1056/NEJMoa1808424.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: No survival benefits are associated with systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer.
Major finding: Median overall and progression-free survival did not improve after systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer.
Study details: Randomized controlled trial of 647 women with newly-diagnosed advanced ovarian cancer.
Disclosures: The study was supported by the Deutsche Forschungsgemeinschaft and the Austrian Science Fund. Six authors declared a range of fees and support from the pharmaceutical industry.
Source: Harter P et al. N Eng J Med. 2019 Feb 27. doi: 10.1056/NEJMoa1808424.
Stroke thrombolysis looks safe 31+ days after prior stroke
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
HONOLULU – , based on a review of more than 40,000 U.S. stroke patients.
Current U.S. stroke management guidelines say that thrombolytic therapy with tissue plasminogen activator (tPA; alteplase; Activase) is contraindicated for index stroke patients who had a prior stroke within the previous 3 months (Stroke. 2018 Mar;49[3]:e46-99). But analysis of 293 U.S. patients who received thrombolytic treatment for an index acute ischemic stroke despite having had a recent, prior stroke showed no increased risk for adverse outcomes when the prior stroke occurred more than 30 days before, Shreyansh Shah, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The risk of symptomatic intracranial hemorrhage [ICH] after thrombolysis was highest among those with a history of prior ischemic stroke within the past 14 days,” said Dr. Shah, a neurologist at Duke University in Durham, N.C.
“Even after many adjustments we still saw a high risk of symptomatic ICH within the first 2 weeks, suggesting that these patients are at especially high risk” from treatment with tissue plasminogen activator for the index stroke. These findings “are very important because I don’t see a randomized trial happening to test the hypothesis,” Dr. Shah said in an interview.
He also suggested that prior treatment with tPA was not an important factor, just the occurrence of a recent, prior ischemic stoke that left blood vessels in the affected brain region “friable and at high risk for hemorrhage,” he said.
His study used data from 40,396 patients with an acute ischemic stroke who presented at and received treatment with tPA at any of 1,522 hospitals that participated in the Get With the Guidelines-Stroke program during 2009-2015. The analysis focused on 30,655 of these patients with no prior stroke history who served as the controls, and 293 who had a prior ischemic stroke within the preceding 90 days. These 293 patients further broke into 43 who received thrombolysis within 14 days of their prior stroke, 47 who had the treatment 15-30 days after their prior stroke, and 203 who underwent thrombolysis 31-90 days after their prior stroke. Patients ages’ in both the no-stroke history and recent-stroke subgroups each averaged 80 years.
A comparison between all 293 patients who had a prior stroke within 90 days and the controls showed no statistically significant difference in the rate of symptomatic ICH: 5% among those with no stroke history and 8% in those with a recent stroke. There was also no significant difference in the rate of in-hospital mortality, occurring in 9% of those without a prior stroke, compared with 13% of those with a recent prior stroke. But the patients with no stroke history fared better by other measures, with a significantly lower rate of in-hospital death or discharge to a hospice, and also a significantly higher rate of 0-1 scores on the modified Rankin Scale, compared with patients with a history of prior stroke.
A more granular analysis of the timing of the prior stroke showed that most of risk from thrombolysis clustered in patients with a very recent prior stroke. The 43 patients with a prior stroke within the preceding 14 days had a symptomatic ICH rate of 16% after thrombolysis, 3.7-fold higher than the control patients in an adjusted analysis. Once the patients with a prior stroke within the past 14 days were pulled out, the remaining patients with prior strokes 15-30 days before as well as those with a prior stroke 31-90 days previously had symptomatic ICH rates that were not significantly different from the controls, Dr. Shah reported.
The results also showed an increased rate of in-hospital mortality or discharge to a hospice clustered in patients treated either within 14 days or during 15-30 days after a prior stroke. In both subgroups, the rate of this outcome was about triple the control rate. In the subgroup treated with thrombolysis 31-90 days after a prior stroke, the rate of in-hospital mortality or discharge to a hospice was about the same as the controls.
“It appears that some patients could benefit from tPA; there is a potential safety signal. It allows for some discretion when using thrombolytic treatment” in patients with a recent, prior stroke, Dr. Shah suggested. “This is by far the largest analysis ever reported” for thrombolytic treatment of patients following a recent, prior stroke, noted Ying Xian, MD, PhD, a Duke neurologist and study coauthor.
But Gregg C. Fonarow, MD, another coauthor, cautioned against immediately applying this finding to practice. “The findings of Dr. Shah’s study suggest that selected patients with prior stroke within a 14- to 90-day window may be considered for tPA treatment. However, further study is warranted given the relatively small number of patients,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Shah and Dr. Xian had no disclosures. Dr. Fonarow had no relevant disclosures.
SOURCE: Shah S et al. Stroke. 2019 Feb;50(Suppl_1): Abstract 35.
REPORTING FROM ISC 2019
Frontal Fibrosing Alopecia Demographics: A Survey of 29 Patients
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
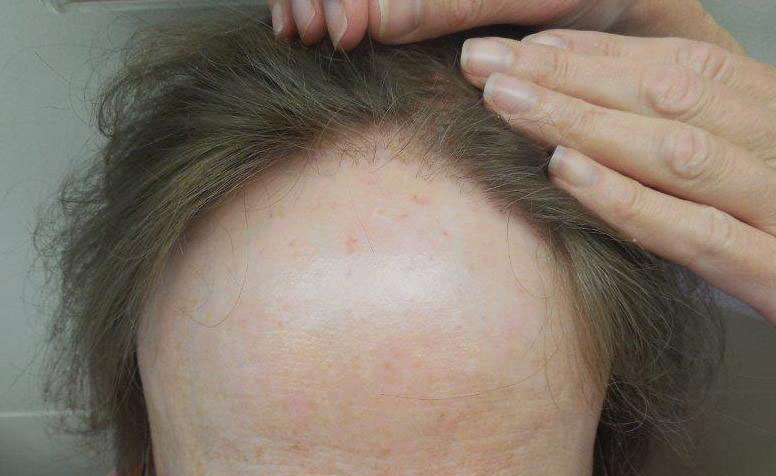
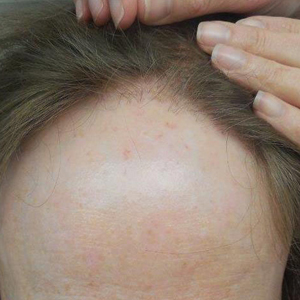
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.
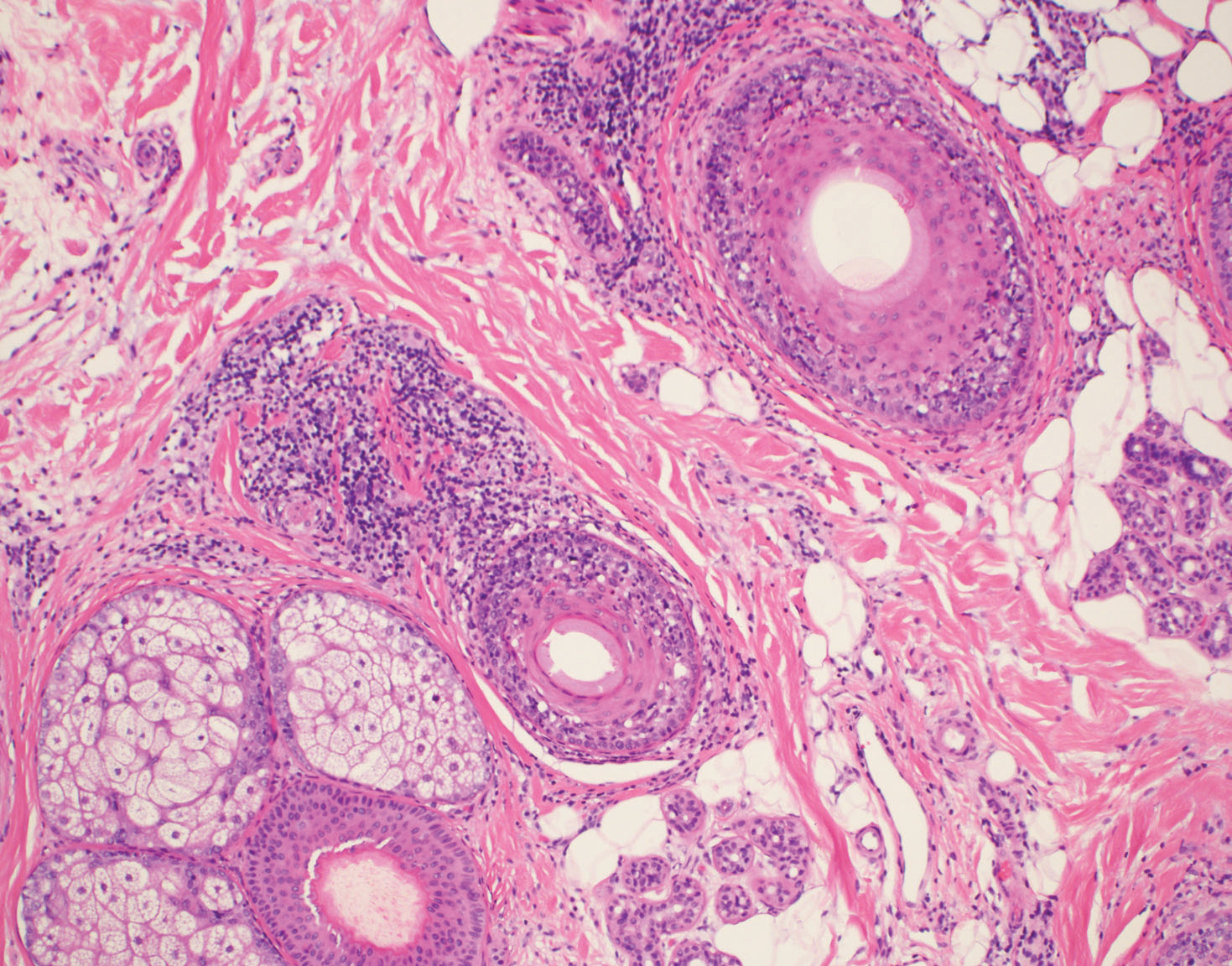
Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.
Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
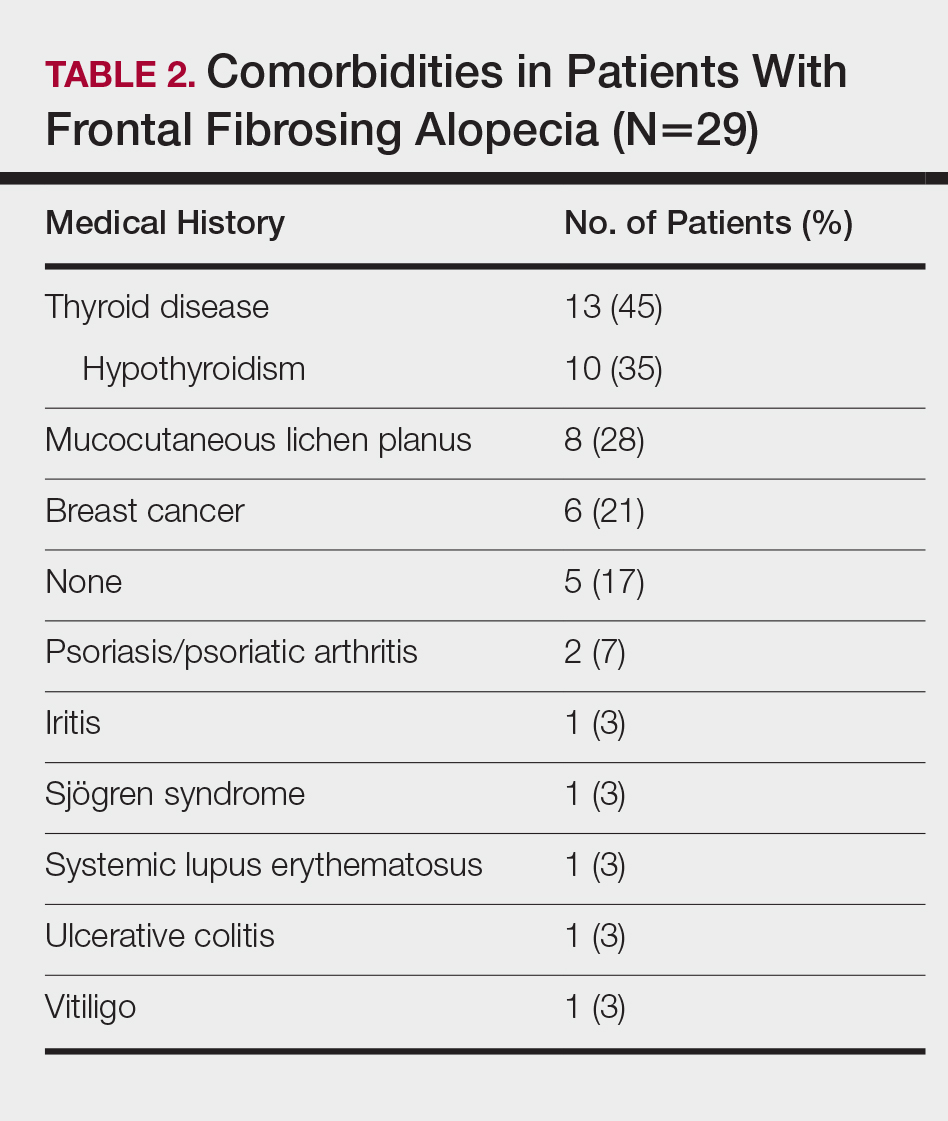
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.
A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
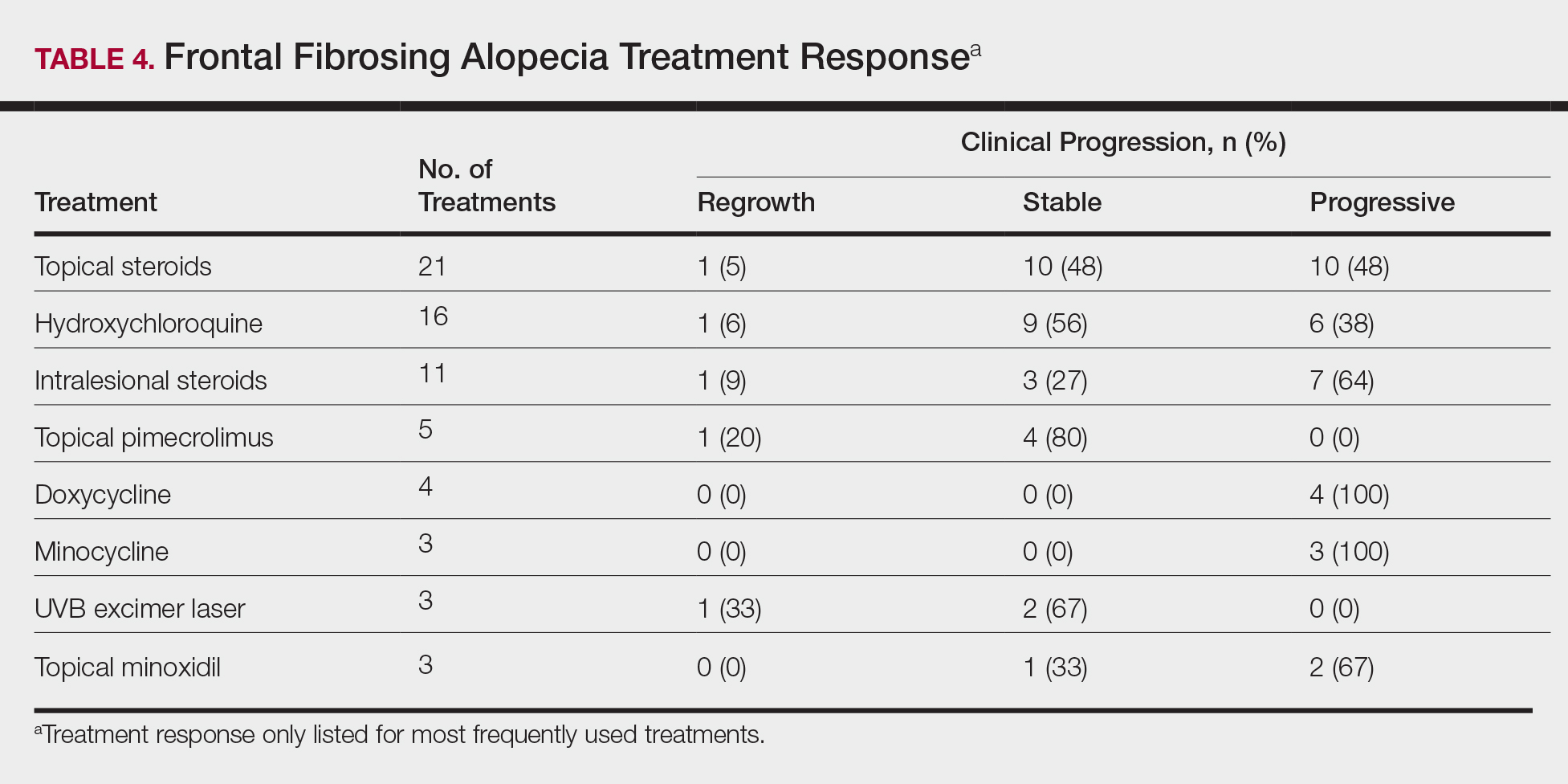
Treatments
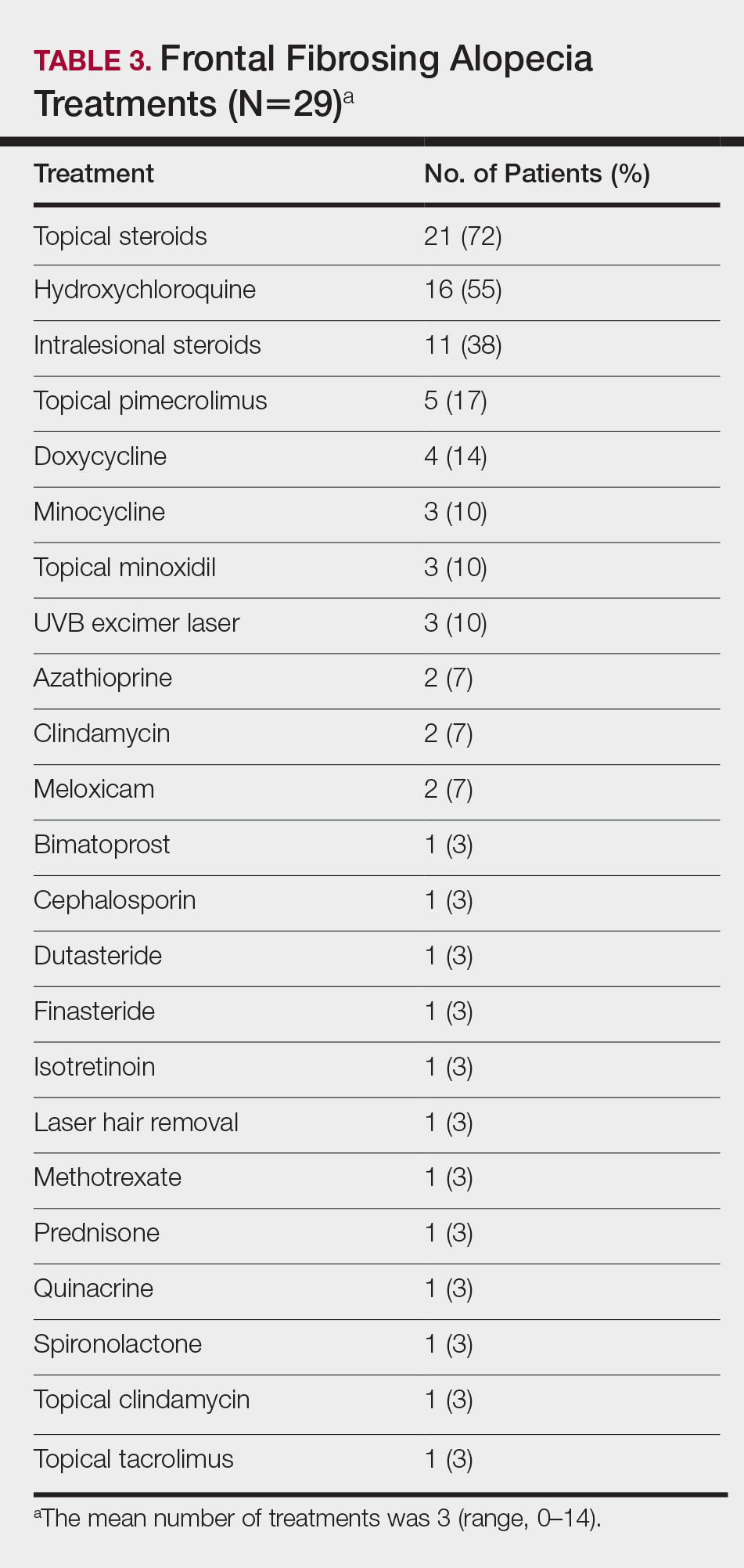
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.
Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.
Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.
Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.
A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
Treatments
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.
Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.
Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.
Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.
A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
Treatments
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.
Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
Practice Points
- Frontal fibrosing alopecia (FFA) may be associated with other autoimmune conditions, and patients should be screened accordingly.
- The most efficacious treatments for FFA include topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy.
- A stressful precipitating event or metal dental implants/fillings are 2 possible environmental triggers for this condition.
Big pharma says it can’t drop drug list prices alone
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
REPORTING FROM SENATE FINANCE COMMITTEE HEARING
Much still unknown about inflammation’s role in RA patients’ CVD risk
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
A variety of trials, some recent and some a decade old, have highlighted the role of inflammation on cardiovascular disease risk in both patients with and without rheumatoid arthritis, spurring greater interest in alleviating inflammation across a wide range of patients, Jon T. Giles, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
However, questions remain about the unique contributions of inflammation to CVD risk in RA patients and the effect of RA treatments on that risk, which future studies hope to answer.
Hints of inflammation’s effects in non-RA patients
The JUPITER trial published more than a decade ago, for example, tested the effects of statins in nearly 18,000 older adults without rheumatoid arthritis (RA) who had elevated levels of inflammation, defined as a C-reactive protein (CRP) level of greater than 2 mg/L and low-density lipoprotein (LDL) cholesterol less than 130 mg/dL. Such patients would otherwise be considered low risk and not eligible for statin therapy, said Dr. Giles, a rheumatologist, epidemiologist, and clinical researcher in the division of rheumatology at Columbia University, New York.
A marked decrease in the incidence of cardiovascular disease (CVD) events was seen in those treated with statins, compared with those who received placebo, and all patient subgroups benefited; the number needed to treat to prevent one event was 32 at 5 years (N Engl J Med. 2008;359:2195-207).
The trial was remarkable in that it was stopped early for efficacy, he noted.
“So the question is: Should we be thinking about systemic inflammation as the real target here? And should RA patients who have elevated persistent levels of CRP really be the people that we’re thinking about?” he asked. “Obviously this needs to be tested; we don’t know.”
The more recent CANTOS trial looking at secondary CVD prevention in more than 10,000 non-RA patients with a prior myocardial infarction also highlighted the role of inflammation and provided “some support that decreasing inflammatory cytokines may be important for reducing [CVD] events,” he said (N Engl J Med. 2017;377:1119-31).
Participants were treated with the interleukin-1 inhibitor canakinumab (Ilaris) or placebo, and canakinumab was associated with about a 15% reduction in CVD events, providing “more proof of concept to look at the inflammatory innate immune contribution to CVD risk,” Dr. Giles said.
Treated patients had more infections, but they also had less gout, less arthritis, and less cancer than did those who received placebo, he noted.
Effect of RA treatments on CVD risk
The effects of existing treatments for RA also highlight the importance of inflammation in CVD risk in RA patients, he said, noting that data support a role for immunomodulators for risk reduction.
“There’s a lot of observational epidemiology in this space – mostly for methotrexate and [tumor necrosis factor (TNF)] inhibitors,” he said.
One analysis showed that across 8 cohort studies involving methotrexate, the disease-modifying antirheumatic agent reduced the risk of CVD events by 28%, and that across 16 cohort studies, TNF inhibitors reduced the risk by 30% (Ann Rheum Dis. 2015 Mar;74[3]:480-9).
All of the methotrexate studies showed a reduction, and almost all of the TNF inhibitor trials showed a reduction, Dr. Giles noted.
With respect to other non-TNF biologics, claims data suggest that abatacept (Orencia) is similar to the TNF inhibitor etanercept (Enbrel) with respect to CVD risk, and in a head-to-head, randomized clinical trial of more than 3,000 RA patients presented as a late-breaking abstract at the ACR annual meeting in 2016, Dr. Giles and his colleagues found similar cardiovascular safety between the anti-IL-6 receptor blocker tocilizumab (Actemra) and etanercept.
“I think we’ll know more about this in the near future,” he said.
As for the mechanisms of these agents, early data and animal models suggest that abatacept may play “a special role” in atherosclerosis reduction related to its effects on T cell CTLA-4 over-expression, and methotrexate also seems to have a number of “potential mechanistic benefits” that render it atheroprotective, he said.
The disappointing findings from the recently reported CIRT trial, which showed no benefit of methotrexate for secondary CVD prevention in non-RA patients (N Engl J Med. 2019;380:752-62), has dampened enthusiasm regarding methotrexate’s role here, but it is important to note that patients enrolled in CIRT, unlike those in JUPITER and CANTOS, were not enrolled based on elevated levels of CRP, Dr. Giles said.
Various studies of TNF inhibitors have shown atheroprotective effects through reductions in macrophage-derived inflammatory cytokines, downregulation of adhesion molecules on endothelial cells, improving the function of high-density lipoprotein, stabilizing atherosclerotic plaque remodeling, and reducing procoagulant states.
The TARGET trial
In a recent study of 17 patients with RA, Dr. Giles and his colleagues showed that TNF inhibitor therapy with either adalimumab (Humira) or etanercept significantly reduced aortic inflammation as measured by baseline and 8-week fluorodeoxyglucose (FDG) PET-CT.
“Is this proof that this helps? It’s not proof; there’s no control group, we don’t know that this is not the natural progression of vascular inflammation in these patients,” he said.
However, the findings were suggestive enough to prompt the launch of the TARGET trial, which is now enrolling patients at centers in the United States and Canada, Dr. Giles said.
The TARGET trial is a project involving his team at Columbia University along with researchers from Brigham and Women’s Hospital, Boston. They plan to enroll 200 RA patients without CVD who have an inadequate response to methotrexate. Participants will be randomized to receive an added TNF inhibitor or added triple therapy, and the primary outcome will be changes in inflammation in the aortic and carotid arteries on FDG PET-CT at 6 months versus baseline.
“So stay tuned and hopefully we’ll have some good information about the effect of two different types of treatments for rheumatoid arthritis on vascular inflammation,” Dr. Giles said.
A final question he addressed is whether the RA-CVD risk link is really a problem that has already been solved – one that “we’re just learning about after the fact.”
“The answer is partially yes and partially no,” he said.
The most up-to-date estimate of whether RA patients have a problem with CVD comes from a Swedish population-based study of more than 15,700 RA patients and nearly 70,900 comparators, which was published in 2018 and showed across-the-board declines in CVD rates over time.
RA and non-RA patients experienced an overall 40% reduction in acute coronary syndromes between 1997 and 2014, but the relative difference in event rates between the groups persisted (Ann Rheum Dis. 2017;76:1642-7).
“There is still a gap ... so we haven’t answered this question yet,” he said, adding that “the rates have been reduced, but we want those rates to be equal or maybe even less.
“Why can’t RA patients have less cardiovascular disease if we’re using drugs that are so effective for treating the inflammatory component of atherogenesis?” he asked.
The authors of the study noted that most RA patients in Sweden are in low disease activity by 3-6 months, so they were “a little confounded by why there is no equalization of these rates as of yet,” he said.
“I think we still have more to learn about this problem, and it is still a problem in our patients,” Dr. Giles said.
Dr. Giles is a consultant for Genentech, Lilly, Horizon, Bristol-Myers Squibb, and UCB, and he has received grant support from Pfizer.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Brilinta reduces MACE in THEMIS trial
The maker of ticagrelor has released top-line results from the phase 3 trial known as THEMIS.
AstraZeneca announced that THEMIS met its primary endpoint of reduction of major adverse cardiovascular events (MACE) among patients with coronary artery disease (CAD) and type 2 diabetes with no history of heart attack or stroke. (MACE is a composite of cardiovascular death, heart attack, and stroke.)
The trial (NCT01991795) explored these risks by comparing ticagrelor (Brilinta) plus aspirin versus aspirin alone. The oral, reversibly binding, direct-acting P2Y12 receptor antagonist is currently indicated for reducing MACE among patients with acute coronary syndrome or a history of MI. According to the press release, the multinational, randomized, double-blind trial is exploring the use of ticagrelor/aspirin among patients with CAD and type 2 diabetes because of this population’s high risk and the lack of treatment options.
Gabriel Steg, MD, THEMIS cochair and professor at Université Paris–Diderot, said in the release that “patients who have both stable coronary artery disease and diabetes are a sizable group which remains at particularly high risk of major adverse cardiac events. The optimal long-term antiplatelet therapy in that group is not fully established.” He added that the full results from the THEMIS trial will be presented later this year.
Ticagrelor comes with risks of significant and sometimes fatal bleeding; as such it is contraindicated for patients with pathological bleeding risk or history of intracranial hemorrhage. Its use is also discouraged among patients with severe hepatic impairment and in patients who are breastfeeding. Although usually self-limiting when related to ticagrelor use, dyspnea was reported in about 14% of patients taking the drug. Dyspnea and bleeding were among the most common adverse reactions seen with ticagrelor.
The maker of ticagrelor has released top-line results from the phase 3 trial known as THEMIS.
AstraZeneca announced that THEMIS met its primary endpoint of reduction of major adverse cardiovascular events (MACE) among patients with coronary artery disease (CAD) and type 2 diabetes with no history of heart attack or stroke. (MACE is a composite of cardiovascular death, heart attack, and stroke.)
The trial (NCT01991795) explored these risks by comparing ticagrelor (Brilinta) plus aspirin versus aspirin alone. The oral, reversibly binding, direct-acting P2Y12 receptor antagonist is currently indicated for reducing MACE among patients with acute coronary syndrome or a history of MI. According to the press release, the multinational, randomized, double-blind trial is exploring the use of ticagrelor/aspirin among patients with CAD and type 2 diabetes because of this population’s high risk and the lack of treatment options.
Gabriel Steg, MD, THEMIS cochair and professor at Université Paris–Diderot, said in the release that “patients who have both stable coronary artery disease and diabetes are a sizable group which remains at particularly high risk of major adverse cardiac events. The optimal long-term antiplatelet therapy in that group is not fully established.” He added that the full results from the THEMIS trial will be presented later this year.
Ticagrelor comes with risks of significant and sometimes fatal bleeding; as such it is contraindicated for patients with pathological bleeding risk or history of intracranial hemorrhage. Its use is also discouraged among patients with severe hepatic impairment and in patients who are breastfeeding. Although usually self-limiting when related to ticagrelor use, dyspnea was reported in about 14% of patients taking the drug. Dyspnea and bleeding were among the most common adverse reactions seen with ticagrelor.
The maker of ticagrelor has released top-line results from the phase 3 trial known as THEMIS.
AstraZeneca announced that THEMIS met its primary endpoint of reduction of major adverse cardiovascular events (MACE) among patients with coronary artery disease (CAD) and type 2 diabetes with no history of heart attack or stroke. (MACE is a composite of cardiovascular death, heart attack, and stroke.)
The trial (NCT01991795) explored these risks by comparing ticagrelor (Brilinta) plus aspirin versus aspirin alone. The oral, reversibly binding, direct-acting P2Y12 receptor antagonist is currently indicated for reducing MACE among patients with acute coronary syndrome or a history of MI. According to the press release, the multinational, randomized, double-blind trial is exploring the use of ticagrelor/aspirin among patients with CAD and type 2 diabetes because of this population’s high risk and the lack of treatment options.
Gabriel Steg, MD, THEMIS cochair and professor at Université Paris–Diderot, said in the release that “patients who have both stable coronary artery disease and diabetes are a sizable group which remains at particularly high risk of major adverse cardiac events. The optimal long-term antiplatelet therapy in that group is not fully established.” He added that the full results from the THEMIS trial will be presented later this year.
Ticagrelor comes with risks of significant and sometimes fatal bleeding; as such it is contraindicated for patients with pathological bleeding risk or history of intracranial hemorrhage. Its use is also discouraged among patients with severe hepatic impairment and in patients who are breastfeeding. Although usually self-limiting when related to ticagrelor use, dyspnea was reported in about 14% of patients taking the drug. Dyspnea and bleeding were among the most common adverse reactions seen with ticagrelor.
Minilaparotomy: Minimally invasive approach to abdominal myomectomy
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
Helping quality improvement teams succeed
QI coaches may be the answer
Hospitalists understand the need for quality improvement (QI) as an important part of health care, and they take active roles in – or personally drive – many of the QI efforts at their own facilities. But too often the results are inconsistent and the adoption of new practices slow.
Help can come from a QI Coach, according to a recent paper describing a model of successful coaching. “We wanted to be able to help novice QI teams to be successful,” said the paper’s lead author Danielle Olds, PhD. “Unfortunately, most QI projects are not successful for a variety of reasons including inadequate project planning, a lack of QI skills, a lack of leadership and stakeholder buy-in, and inappropriate measures and methods.”
The coaching model outlined comes from the VAQS program, launched in 1998 to provide structured training around QI and the care of veterans. The seven-step process outlined in the paper provides a road map to overcoming typical QI stumbling blocks and create more successful projects.
“Improvement should be a part of everyone’s practice, however most clinicians have not been trained in how to successfully lead a formal QI project,” said Dr. Olds, who is based at the University of Kansas Medical Center in Kansas City. “Hospitals can bridge this gap by providing QI coaches as a resource to guide teams through the process.”
The model offers a new way for hospitalists to take the lead on QI. “Hospitalists who may have extensive experience in conducting QI could use a model, such as ours, to guide their coaching of teams within their facility,” she said. “Because of the nature of hospitalist practice, they are in an ideal position to understand improvement needs at a systems level within their facility. I would strongly encourage hospitalists to engage in QI because of the wealth of knowledge and experience that they could bring.”
Reference
Olds DM et al. “VA Quality Scholars Quality Improvement Coach Model to Facilitate Learning and Success.” Qual Manag Healthcare. 2018;27(2):87-92. doi: 10.1097/QMH.0000000000000164. Accessed 2018 Jun 11.
QI coaches may be the answer
QI coaches may be the answer
Hospitalists understand the need for quality improvement (QI) as an important part of health care, and they take active roles in – or personally drive – many of the QI efforts at their own facilities. But too often the results are inconsistent and the adoption of new practices slow.
Help can come from a QI Coach, according to a recent paper describing a model of successful coaching. “We wanted to be able to help novice QI teams to be successful,” said the paper’s lead author Danielle Olds, PhD. “Unfortunately, most QI projects are not successful for a variety of reasons including inadequate project planning, a lack of QI skills, a lack of leadership and stakeholder buy-in, and inappropriate measures and methods.”
The coaching model outlined comes from the VAQS program, launched in 1998 to provide structured training around QI and the care of veterans. The seven-step process outlined in the paper provides a road map to overcoming typical QI stumbling blocks and create more successful projects.
“Improvement should be a part of everyone’s practice, however most clinicians have not been trained in how to successfully lead a formal QI project,” said Dr. Olds, who is based at the University of Kansas Medical Center in Kansas City. “Hospitals can bridge this gap by providing QI coaches as a resource to guide teams through the process.”
The model offers a new way for hospitalists to take the lead on QI. “Hospitalists who may have extensive experience in conducting QI could use a model, such as ours, to guide their coaching of teams within their facility,” she said. “Because of the nature of hospitalist practice, they are in an ideal position to understand improvement needs at a systems level within their facility. I would strongly encourage hospitalists to engage in QI because of the wealth of knowledge and experience that they could bring.”
Reference
Olds DM et al. “VA Quality Scholars Quality Improvement Coach Model to Facilitate Learning and Success.” Qual Manag Healthcare. 2018;27(2):87-92. doi: 10.1097/QMH.0000000000000164. Accessed 2018 Jun 11.
Hospitalists understand the need for quality improvement (QI) as an important part of health care, and they take active roles in – or personally drive – many of the QI efforts at their own facilities. But too often the results are inconsistent and the adoption of new practices slow.
Help can come from a QI Coach, according to a recent paper describing a model of successful coaching. “We wanted to be able to help novice QI teams to be successful,” said the paper’s lead author Danielle Olds, PhD. “Unfortunately, most QI projects are not successful for a variety of reasons including inadequate project planning, a lack of QI skills, a lack of leadership and stakeholder buy-in, and inappropriate measures and methods.”
The coaching model outlined comes from the VAQS program, launched in 1998 to provide structured training around QI and the care of veterans. The seven-step process outlined in the paper provides a road map to overcoming typical QI stumbling blocks and create more successful projects.
“Improvement should be a part of everyone’s practice, however most clinicians have not been trained in how to successfully lead a formal QI project,” said Dr. Olds, who is based at the University of Kansas Medical Center in Kansas City. “Hospitals can bridge this gap by providing QI coaches as a resource to guide teams through the process.”
The model offers a new way for hospitalists to take the lead on QI. “Hospitalists who may have extensive experience in conducting QI could use a model, such as ours, to guide their coaching of teams within their facility,” she said. “Because of the nature of hospitalist practice, they are in an ideal position to understand improvement needs at a systems level within their facility. I would strongly encourage hospitalists to engage in QI because of the wealth of knowledge and experience that they could bring.”
Reference
Olds DM et al. “VA Quality Scholars Quality Improvement Coach Model to Facilitate Learning and Success.” Qual Manag Healthcare. 2018;27(2):87-92. doi: 10.1097/QMH.0000000000000164. Accessed 2018 Jun 11.