User login
What does 'Medicare for all' mean?
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.
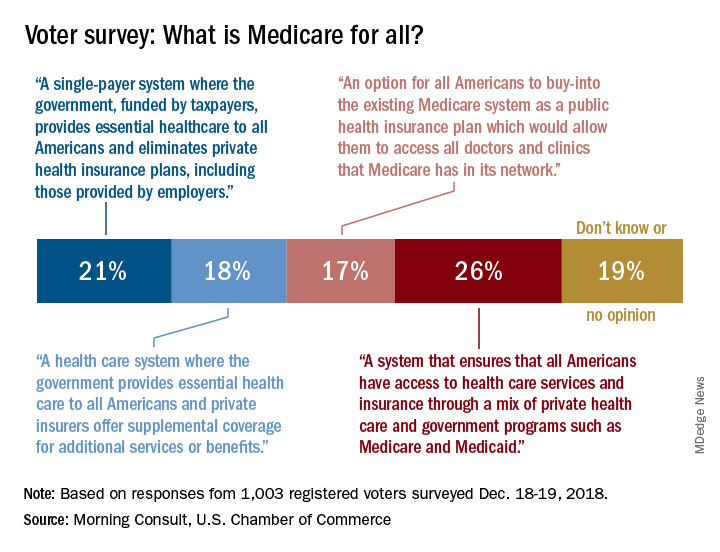
Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.

Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.

Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Amyloid PET may help facilitate diagnosis of inclusion body myositis
Amyloid PET imaging may help to accurately identify inclusion body myositis and distinguish it from polymyositis, which could potentially avoid misdiagnosis and unnecessary immunosuppressive medication, according to findings from a small prospective cohort study.
The study, which found significantly greater uptake of the imaging agent [18F]florbetapir (Amyvid) in muscle from patients with inclusion body myositis (IBM) than in those with polymyositis (PM), builds on previous research showing that the intramuscular beta-amyloid seen in histopathologic analysis of IBM can help distinguish it from PM, but this approach has a low sensitivity along with high diagnostic specificity. The latest diagnostic criteria for IBM have also shifted away from strict histopathologic analysis toward identifying its characteristic clinical pattern of muscle weakness, first author James B. Lilleker, PhD, of the Centre for Musculoskeletal Research at the University of Manchester (England) and his colleagues wrote in Annals of the Rheumatic Diseases.
“While this has improved sensitivity, clinically detectable weakness implies that significant and irreversible muscle damage has occurred, reducing the likelihood that novel treatments will be effective,” the researchers wrote.
Thomas E. Lloyd II, MD, PhD, codirector of the myositis center at Johns Hopkins University, Baltimore, said in an interview that IBM is difficult to diagnose because of a lack of awareness of the early clinical signs and symptoms of the disease among primary care physicians, neurologists, and rheumatologists.
“Typically, a myositis specialist can make the diagnosis based on a combination of history and exam [slowly progressive weakness affecting distal finger flexors and knee extensors] and muscle biopsy features [endomysial inflammation with rimmed vacuoles and protein aggregates],” said Dr. Lloyd, who was not involved in the current study. “However, early in the course of disease, some patients may be misdiagnosed with polymyositis due to having atypical clinical or pathological features, especially if the muscle biopsy lacks rimmed vacuoles or the patient lacks obvious finger flexor weakness.”
But amyloid PET is promising and needs further analysis comparing it with expert diagnosis, Dr. Lloyd said. “I think this imaging method has greatest potential utility to be helpful diagnostically early in the course of disease, when diagnosis of IBM can be most challenging. This approach may also have potential to identify presymptomatic patients, especially if amyloid imaging becomes used for screening for Alzheimer’s disease in the future.”
Dr. Lilleker and colleagues set out to determine whether amyloid PET with [18F]florbetapir could distinguish between IBM and PM. They identified 10 patients with IBM and 6 patients with PM and scanned each patient from shoulders to ankles with CT and PET, followed by a same-day, whole-body MRI scan. Overall, regions of interest included the left arm, right and left forearms, right and left thighs, and right and left calves. The researchers calculated the [18F]florbetapir standard uptake values (SUVs) for each region of interest while SUV ratios (SUVRs) were calculated using the lumbar fat pad reference region.
The IBM patients (9 men, 1 woman) had a mean age of 68.3 years at the time of the scan and a mean disease duration of 4 years, and they were not currently taking immunosuppressive treatments; however, patients had previously taken prednisolone (3 of 10 patients), azathioprine (1 of 10 patients), and mycophenolate (1 of 10 patients). In the PM group (4 men, 2 women), patients’ mean age was 59.7 years, with a mean disease duration of 1.5 years.
The researchers found significantly increased overall [18F]florbetapir SUVRs among all regions of interest for IBM patients (median total SUVR, 1.45; interquartile range, 1.28-2.05), compared with PM patients (total SUVR, 1.01; IQR, 0.80-1.22; P = .005). In addition, when total [18F]florbetapir SUVR was 1.28 or greater, the diagnostic sensitivity for IBM was 80% and specificity was 100%, with an area under the curve of 0.93. There were also no significant associations between total [18F]florbetapir SUVR and age at the time of the scan, disease duration, or clinical outcome measurements such as manual muscle testing of 26 muscles (MMT26), Health Assessment Questionnaire disability index, and IBM Functional Rating Scale.
Dr. Lilleker and his colleagues noted the small study size as a potential limitation, but said other factors such as age and disease severity, which differed between groups, were unlikely to affect the [18F]florbetapir SUVR.
The rarity of PM may make it difficult to determine amyloid PET imaging’s effectiveness in diagnosing IBM when compared with more traditional methods, Dr. Lloyd noted.
“Whether PET imaging is more sensitive than expert diagnosis using traditional methods [careful physical exam of individual distal interphalangeal finger flexor muscles, MRI imaging of thighs, detailed pathological analysis of muscle biopsy including immunostaining for p62/SQSTM1, detailed serum autoantibody testing, etc.] remains to be determined,” he said.
This study was supported by grants from the National Institute for Health Research Manchester Musculoskeletal Biomedical Research Centre, the Medical Research Council, and an award from the Centre for Imaging Sciences at the University of Manchester. The authors reported no conflicts of interest. Dr. Lloyd reported being a consultant for Acceleron and principal investigator for IBM clinical trials sponsored by Orphazyme and Regeneron.
SOURCE: Lilleker JB et al. Ann Rheum Dis. 2019 Feb 13. doi: 10.1136/annrheumdis-2018-214644.
Amyloid PET imaging may help to accurately identify inclusion body myositis and distinguish it from polymyositis, which could potentially avoid misdiagnosis and unnecessary immunosuppressive medication, according to findings from a small prospective cohort study.
The study, which found significantly greater uptake of the imaging agent [18F]florbetapir (Amyvid) in muscle from patients with inclusion body myositis (IBM) than in those with polymyositis (PM), builds on previous research showing that the intramuscular beta-amyloid seen in histopathologic analysis of IBM can help distinguish it from PM, but this approach has a low sensitivity along with high diagnostic specificity. The latest diagnostic criteria for IBM have also shifted away from strict histopathologic analysis toward identifying its characteristic clinical pattern of muscle weakness, first author James B. Lilleker, PhD, of the Centre for Musculoskeletal Research at the University of Manchester (England) and his colleagues wrote in Annals of the Rheumatic Diseases.
“While this has improved sensitivity, clinically detectable weakness implies that significant and irreversible muscle damage has occurred, reducing the likelihood that novel treatments will be effective,” the researchers wrote.
Thomas E. Lloyd II, MD, PhD, codirector of the myositis center at Johns Hopkins University, Baltimore, said in an interview that IBM is difficult to diagnose because of a lack of awareness of the early clinical signs and symptoms of the disease among primary care physicians, neurologists, and rheumatologists.
“Typically, a myositis specialist can make the diagnosis based on a combination of history and exam [slowly progressive weakness affecting distal finger flexors and knee extensors] and muscle biopsy features [endomysial inflammation with rimmed vacuoles and protein aggregates],” said Dr. Lloyd, who was not involved in the current study. “However, early in the course of disease, some patients may be misdiagnosed with polymyositis due to having atypical clinical or pathological features, especially if the muscle biopsy lacks rimmed vacuoles or the patient lacks obvious finger flexor weakness.”
But amyloid PET is promising and needs further analysis comparing it with expert diagnosis, Dr. Lloyd said. “I think this imaging method has greatest potential utility to be helpful diagnostically early in the course of disease, when diagnosis of IBM can be most challenging. This approach may also have potential to identify presymptomatic patients, especially if amyloid imaging becomes used for screening for Alzheimer’s disease in the future.”
Dr. Lilleker and colleagues set out to determine whether amyloid PET with [18F]florbetapir could distinguish between IBM and PM. They identified 10 patients with IBM and 6 patients with PM and scanned each patient from shoulders to ankles with CT and PET, followed by a same-day, whole-body MRI scan. Overall, regions of interest included the left arm, right and left forearms, right and left thighs, and right and left calves. The researchers calculated the [18F]florbetapir standard uptake values (SUVs) for each region of interest while SUV ratios (SUVRs) were calculated using the lumbar fat pad reference region.
The IBM patients (9 men, 1 woman) had a mean age of 68.3 years at the time of the scan and a mean disease duration of 4 years, and they were not currently taking immunosuppressive treatments; however, patients had previously taken prednisolone (3 of 10 patients), azathioprine (1 of 10 patients), and mycophenolate (1 of 10 patients). In the PM group (4 men, 2 women), patients’ mean age was 59.7 years, with a mean disease duration of 1.5 years.
The researchers found significantly increased overall [18F]florbetapir SUVRs among all regions of interest for IBM patients (median total SUVR, 1.45; interquartile range, 1.28-2.05), compared with PM patients (total SUVR, 1.01; IQR, 0.80-1.22; P = .005). In addition, when total [18F]florbetapir SUVR was 1.28 or greater, the diagnostic sensitivity for IBM was 80% and specificity was 100%, with an area under the curve of 0.93. There were also no significant associations between total [18F]florbetapir SUVR and age at the time of the scan, disease duration, or clinical outcome measurements such as manual muscle testing of 26 muscles (MMT26), Health Assessment Questionnaire disability index, and IBM Functional Rating Scale.
Dr. Lilleker and his colleagues noted the small study size as a potential limitation, but said other factors such as age and disease severity, which differed between groups, were unlikely to affect the [18F]florbetapir SUVR.
The rarity of PM may make it difficult to determine amyloid PET imaging’s effectiveness in diagnosing IBM when compared with more traditional methods, Dr. Lloyd noted.
“Whether PET imaging is more sensitive than expert diagnosis using traditional methods [careful physical exam of individual distal interphalangeal finger flexor muscles, MRI imaging of thighs, detailed pathological analysis of muscle biopsy including immunostaining for p62/SQSTM1, detailed serum autoantibody testing, etc.] remains to be determined,” he said.
This study was supported by grants from the National Institute for Health Research Manchester Musculoskeletal Biomedical Research Centre, the Medical Research Council, and an award from the Centre for Imaging Sciences at the University of Manchester. The authors reported no conflicts of interest. Dr. Lloyd reported being a consultant for Acceleron and principal investigator for IBM clinical trials sponsored by Orphazyme and Regeneron.
SOURCE: Lilleker JB et al. Ann Rheum Dis. 2019 Feb 13. doi: 10.1136/annrheumdis-2018-214644.
Amyloid PET imaging may help to accurately identify inclusion body myositis and distinguish it from polymyositis, which could potentially avoid misdiagnosis and unnecessary immunosuppressive medication, according to findings from a small prospective cohort study.
The study, which found significantly greater uptake of the imaging agent [18F]florbetapir (Amyvid) in muscle from patients with inclusion body myositis (IBM) than in those with polymyositis (PM), builds on previous research showing that the intramuscular beta-amyloid seen in histopathologic analysis of IBM can help distinguish it from PM, but this approach has a low sensitivity along with high diagnostic specificity. The latest diagnostic criteria for IBM have also shifted away from strict histopathologic analysis toward identifying its characteristic clinical pattern of muscle weakness, first author James B. Lilleker, PhD, of the Centre for Musculoskeletal Research at the University of Manchester (England) and his colleagues wrote in Annals of the Rheumatic Diseases.
“While this has improved sensitivity, clinically detectable weakness implies that significant and irreversible muscle damage has occurred, reducing the likelihood that novel treatments will be effective,” the researchers wrote.
Thomas E. Lloyd II, MD, PhD, codirector of the myositis center at Johns Hopkins University, Baltimore, said in an interview that IBM is difficult to diagnose because of a lack of awareness of the early clinical signs and symptoms of the disease among primary care physicians, neurologists, and rheumatologists.
“Typically, a myositis specialist can make the diagnosis based on a combination of history and exam [slowly progressive weakness affecting distal finger flexors and knee extensors] and muscle biopsy features [endomysial inflammation with rimmed vacuoles and protein aggregates],” said Dr. Lloyd, who was not involved in the current study. “However, early in the course of disease, some patients may be misdiagnosed with polymyositis due to having atypical clinical or pathological features, especially if the muscle biopsy lacks rimmed vacuoles or the patient lacks obvious finger flexor weakness.”
But amyloid PET is promising and needs further analysis comparing it with expert diagnosis, Dr. Lloyd said. “I think this imaging method has greatest potential utility to be helpful diagnostically early in the course of disease, when diagnosis of IBM can be most challenging. This approach may also have potential to identify presymptomatic patients, especially if amyloid imaging becomes used for screening for Alzheimer’s disease in the future.”
Dr. Lilleker and colleagues set out to determine whether amyloid PET with [18F]florbetapir could distinguish between IBM and PM. They identified 10 patients with IBM and 6 patients with PM and scanned each patient from shoulders to ankles with CT and PET, followed by a same-day, whole-body MRI scan. Overall, regions of interest included the left arm, right and left forearms, right and left thighs, and right and left calves. The researchers calculated the [18F]florbetapir standard uptake values (SUVs) for each region of interest while SUV ratios (SUVRs) were calculated using the lumbar fat pad reference region.
The IBM patients (9 men, 1 woman) had a mean age of 68.3 years at the time of the scan and a mean disease duration of 4 years, and they were not currently taking immunosuppressive treatments; however, patients had previously taken prednisolone (3 of 10 patients), azathioprine (1 of 10 patients), and mycophenolate (1 of 10 patients). In the PM group (4 men, 2 women), patients’ mean age was 59.7 years, with a mean disease duration of 1.5 years.
The researchers found significantly increased overall [18F]florbetapir SUVRs among all regions of interest for IBM patients (median total SUVR, 1.45; interquartile range, 1.28-2.05), compared with PM patients (total SUVR, 1.01; IQR, 0.80-1.22; P = .005). In addition, when total [18F]florbetapir SUVR was 1.28 or greater, the diagnostic sensitivity for IBM was 80% and specificity was 100%, with an area under the curve of 0.93. There were also no significant associations between total [18F]florbetapir SUVR and age at the time of the scan, disease duration, or clinical outcome measurements such as manual muscle testing of 26 muscles (MMT26), Health Assessment Questionnaire disability index, and IBM Functional Rating Scale.
Dr. Lilleker and his colleagues noted the small study size as a potential limitation, but said other factors such as age and disease severity, which differed between groups, were unlikely to affect the [18F]florbetapir SUVR.
The rarity of PM may make it difficult to determine amyloid PET imaging’s effectiveness in diagnosing IBM when compared with more traditional methods, Dr. Lloyd noted.
“Whether PET imaging is more sensitive than expert diagnosis using traditional methods [careful physical exam of individual distal interphalangeal finger flexor muscles, MRI imaging of thighs, detailed pathological analysis of muscle biopsy including immunostaining for p62/SQSTM1, detailed serum autoantibody testing, etc.] remains to be determined,” he said.
This study was supported by grants from the National Institute for Health Research Manchester Musculoskeletal Biomedical Research Centre, the Medical Research Council, and an award from the Centre for Imaging Sciences at the University of Manchester. The authors reported no conflicts of interest. Dr. Lloyd reported being a consultant for Acceleron and principal investigator for IBM clinical trials sponsored by Orphazyme and Regeneron.
SOURCE: Lilleker JB et al. Ann Rheum Dis. 2019 Feb 13. doi: 10.1136/annrheumdis-2018-214644.
FROM ANNALS OF THE RHEUMATIC DISEASES
Consider therapeutic drug monitoring in stubborn IBD cases
LAS VEGAS – An estimated 10%-30% of patients with inflammatory bowel disease (IBD) don’t respond to biologics, leaving physicians with a big question: What now? Evidence suggests the best strategy is an approach grounded in therapeutic drug monitoring, appropriate disease monitoring, and other strategies, a gastroenterologist told colleagues.
“We can’t look at any one of these tools in isolation,” said Edward V. Loftus Jr., MD, professor of medicine at the Mayo Clinic in Rochester, Minn. He spoke about a reactive approach to stubborn cases of IBD at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Loftus offered several tips and tidbits about managing IBD, especially when patients fail to appropriately respond to biologics.
- Many other conditions can cause symptoms that may appear to suggest lack of response to medication in IBD. These can include celiac disease, bacterial overgrowth, bile salt diarrhea, irritable bowel syndrome, hypersensitivity colitis, short bowel syndrome, and carbohydrate malabsorption. As a result, “before you make big changes in biologics or immunomodulators, make sure you’re actually confirming the presence of inflammation,” Dr. Loftus advised. Appropriate tests may be a colonoscopy/ileoscopy, CT or MR enterography, or a simple fecal calprotectin test; he also recommended that physicians exclude complications such as stricture, fistula, and abscess.
- The definition of lack of response in IBD has evolved over the years, Dr. Loftus said. The definition ideally refers to clinical response after an appropriate time period, such as 14 weeks of treatment (infliximab) or 8-12 weeks of treatment (injectable anti–tumor necrosis factor [TNF] drugs).
- Factors linked to primary nonresponse include disease duration of 2 years or more, smoking, and elevated C-reactive protein.
- Secondary lack of response – when drugs lose effectiveness over time even though they previously were effective – is common in IBD, with research suggesting it may affect about 20% of patients on infliximab or adalimumab.
- When patients fail to fully respond to a therapeutic level of an anti-TNF drug, research suggests it may be more effective to switch to another one rather than increase the dose, Dr. Loftus said.
- In cases of secondary lack of response in patients taking anti-TNF drugs, AGA guidelines suggest therapeutic drug monitoring may be appropriate, Dr. Loftus said. However, the AGA doesn’t make any recommendations regarding therapeutic drug monitoring in cases where IBD is dormant while patients are on anti-TNF drugs.
- Research suggests that a treat-to-target approach – designed to reach specific testing targets – may boost the effectiveness of therapeutic drug monitoring, Dr. Loftus said, and algorithm-based treatment might be even better.
“Therapeutic drug management in isolation will only get you so far,” he advised. “But don’t be confused. The drug level is not the target. The absence of inflammation is the target.”
Dr. Loftus reported recent relationships (research support and consultant) with multiple drugmakers, including AbbVie, Bristol-Myers Squibb, Pfizer, Gilead, Celgene, Eli Lilly, and several others.
LAS VEGAS – An estimated 10%-30% of patients with inflammatory bowel disease (IBD) don’t respond to biologics, leaving physicians with a big question: What now? Evidence suggests the best strategy is an approach grounded in therapeutic drug monitoring, appropriate disease monitoring, and other strategies, a gastroenterologist told colleagues.
“We can’t look at any one of these tools in isolation,” said Edward V. Loftus Jr., MD, professor of medicine at the Mayo Clinic in Rochester, Minn. He spoke about a reactive approach to stubborn cases of IBD at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Loftus offered several tips and tidbits about managing IBD, especially when patients fail to appropriately respond to biologics.
- Many other conditions can cause symptoms that may appear to suggest lack of response to medication in IBD. These can include celiac disease, bacterial overgrowth, bile salt diarrhea, irritable bowel syndrome, hypersensitivity colitis, short bowel syndrome, and carbohydrate malabsorption. As a result, “before you make big changes in biologics or immunomodulators, make sure you’re actually confirming the presence of inflammation,” Dr. Loftus advised. Appropriate tests may be a colonoscopy/ileoscopy, CT or MR enterography, or a simple fecal calprotectin test; he also recommended that physicians exclude complications such as stricture, fistula, and abscess.
- The definition of lack of response in IBD has evolved over the years, Dr. Loftus said. The definition ideally refers to clinical response after an appropriate time period, such as 14 weeks of treatment (infliximab) or 8-12 weeks of treatment (injectable anti–tumor necrosis factor [TNF] drugs).
- Factors linked to primary nonresponse include disease duration of 2 years or more, smoking, and elevated C-reactive protein.
- Secondary lack of response – when drugs lose effectiveness over time even though they previously were effective – is common in IBD, with research suggesting it may affect about 20% of patients on infliximab or adalimumab.
- When patients fail to fully respond to a therapeutic level of an anti-TNF drug, research suggests it may be more effective to switch to another one rather than increase the dose, Dr. Loftus said.
- In cases of secondary lack of response in patients taking anti-TNF drugs, AGA guidelines suggest therapeutic drug monitoring may be appropriate, Dr. Loftus said. However, the AGA doesn’t make any recommendations regarding therapeutic drug monitoring in cases where IBD is dormant while patients are on anti-TNF drugs.
- Research suggests that a treat-to-target approach – designed to reach specific testing targets – may boost the effectiveness of therapeutic drug monitoring, Dr. Loftus said, and algorithm-based treatment might be even better.
“Therapeutic drug management in isolation will only get you so far,” he advised. “But don’t be confused. The drug level is not the target. The absence of inflammation is the target.”
Dr. Loftus reported recent relationships (research support and consultant) with multiple drugmakers, including AbbVie, Bristol-Myers Squibb, Pfizer, Gilead, Celgene, Eli Lilly, and several others.
LAS VEGAS – An estimated 10%-30% of patients with inflammatory bowel disease (IBD) don’t respond to biologics, leaving physicians with a big question: What now? Evidence suggests the best strategy is an approach grounded in therapeutic drug monitoring, appropriate disease monitoring, and other strategies, a gastroenterologist told colleagues.
“We can’t look at any one of these tools in isolation,” said Edward V. Loftus Jr., MD, professor of medicine at the Mayo Clinic in Rochester, Minn. He spoke about a reactive approach to stubborn cases of IBD at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Loftus offered several tips and tidbits about managing IBD, especially when patients fail to appropriately respond to biologics.
- Many other conditions can cause symptoms that may appear to suggest lack of response to medication in IBD. These can include celiac disease, bacterial overgrowth, bile salt diarrhea, irritable bowel syndrome, hypersensitivity colitis, short bowel syndrome, and carbohydrate malabsorption. As a result, “before you make big changes in biologics or immunomodulators, make sure you’re actually confirming the presence of inflammation,” Dr. Loftus advised. Appropriate tests may be a colonoscopy/ileoscopy, CT or MR enterography, or a simple fecal calprotectin test; he also recommended that physicians exclude complications such as stricture, fistula, and abscess.
- The definition of lack of response in IBD has evolved over the years, Dr. Loftus said. The definition ideally refers to clinical response after an appropriate time period, such as 14 weeks of treatment (infliximab) or 8-12 weeks of treatment (injectable anti–tumor necrosis factor [TNF] drugs).
- Factors linked to primary nonresponse include disease duration of 2 years or more, smoking, and elevated C-reactive protein.
- Secondary lack of response – when drugs lose effectiveness over time even though they previously were effective – is common in IBD, with research suggesting it may affect about 20% of patients on infliximab or adalimumab.
- When patients fail to fully respond to a therapeutic level of an anti-TNF drug, research suggests it may be more effective to switch to another one rather than increase the dose, Dr. Loftus said.
- In cases of secondary lack of response in patients taking anti-TNF drugs, AGA guidelines suggest therapeutic drug monitoring may be appropriate, Dr. Loftus said. However, the AGA doesn’t make any recommendations regarding therapeutic drug monitoring in cases where IBD is dormant while patients are on anti-TNF drugs.
- Research suggests that a treat-to-target approach – designed to reach specific testing targets – may boost the effectiveness of therapeutic drug monitoring, Dr. Loftus said, and algorithm-based treatment might be even better.
“Therapeutic drug management in isolation will only get you so far,” he advised. “But don’t be confused. The drug level is not the target. The absence of inflammation is the target.”
Dr. Loftus reported recent relationships (research support and consultant) with multiple drugmakers, including AbbVie, Bristol-Myers Squibb, Pfizer, Gilead, Celgene, Eli Lilly, and several others.
EXPERT ANALYSIS FROM THE CROHN’S & COLITIS CONGRESS
Genes associated with PTSD coming into focus
LAS VEGAS – Despite recent advances in the diagnosis and treatment of posttraumatic stress disorder, researchers have yet to fully understand its pathophysiology.
“We don’t know whether there is just one type of pathophysiology or if there are different mechanisms and different etiologies that contribute to the symptomatic heterogeneity that we see,” Murray B. Stein, MD, MPH, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Many theories currently being studied are inflammatory theories, looking at autonomic dysregulation, glucocorticoid sensitivity, hippocampal volume/dysfunction, and cortical-amygdala circuit dysregulation.”
Dr. Stein expects clinicians to gain an improved understanding of PTSD and a myriad of other conditions in the coming years as the Million Veteran Program (MVP) gets underway. MVP researchers are building one of the world’s largest medical databases by asking 1 million veteran volunteers to provide a blood sample and complete surveys about their health, military experience, lifestyle, and other topics that might contribute to health and disease. They will study how genes influence diseases such as diabetes and cancer, and military-related illnesses, such as PTSD. “It’s an amazing study, where over the next decade, you’re going to hear research findings not just on PTSD but in other areas of mental health and physical health,” said Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego. So far, he said, 700,000 veterans are enrolled.
Dr. Stein shared preliminary findings from one component of the MVP study, in which participants complete the PTSD Checklist (PCL-17), a widely used 17-item self-report measure of PTSD symptoms in the past month. Using Manhattan plot analysis, Dr. Stein and his colleagues conducted a PTSD genomewide association study based on PCL-17 total scores. They found several genes significantly associated with PTSD, including LRRIQ3, TRAIP, KCNIP4, PCDHA1, MAD1L1, TSNARE1, EXD3, MGC57346-CRHR1, and TCF4. “This is the first study in the world to find this many genes [associated with PTSD],” said Dr. Stein, a staff psychiatrist with the VA San Diego Healthcare System. “The MAD1L1 gene is also one that’s come up in schizophrenia and in major depression. In fact, if we look at genetic correlations of how PTSD is genetically associated to other disorders that have been studied in this way, like major depression and schizophrenia, we find that there is shared variation. However, there are also unique features, so we are learning what we think is true about mental disorders overall, that in some ways comorbidity is explained by genetic risk, but there are also individual specific risk factors that go with specific disorders.
“We’re exploring each of those genes.”
of any of the genes in the genome. “One of the prominent theories of PTSD is that there is an increase in CRHR1 in the brain of individuals with the disorder,” Dr. Stein said. “More than by chance, it looks like the genes that are popping up in PTSD seem to be expressed in the frontal cortex, the anterior cingulate, the cortex, the hypothalamus, the amygdala, the hippocampus, the basal ganglia, and the substantia nigra. So all of a sudden, we go from having a list of genes to knowing there’s something going on in the brain of people with PTSD that involves expression in these particular regions that we might be able to target.”
In related work using the same genetic information, Dr. Stein and his colleagues have demonstrated an association between PTSD and medium spiny neurons, which are located in the basal ganglia and make up 95% of neurons in the striatum. “They also have GABAergic projection to other parts of the brain and play a key role in motivation, reward, enforcement, and aversion,” Dr. Stein said.
Dr. Stein disclosed that he has received research support from the National Institute of Mental Health, the National Institute of Alcoholism and Alcohol Abuse, the National Institute of Neurological Disorders and Stroke, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. He also has received consulting fees from Aptinyx, Bionomics, Janssen, Neurocrine, Oxeia Biopharmaceuticals, and Resilience Therapeutics.
LAS VEGAS – Despite recent advances in the diagnosis and treatment of posttraumatic stress disorder, researchers have yet to fully understand its pathophysiology.
“We don’t know whether there is just one type of pathophysiology or if there are different mechanisms and different etiologies that contribute to the symptomatic heterogeneity that we see,” Murray B. Stein, MD, MPH, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Many theories currently being studied are inflammatory theories, looking at autonomic dysregulation, glucocorticoid sensitivity, hippocampal volume/dysfunction, and cortical-amygdala circuit dysregulation.”
Dr. Stein expects clinicians to gain an improved understanding of PTSD and a myriad of other conditions in the coming years as the Million Veteran Program (MVP) gets underway. MVP researchers are building one of the world’s largest medical databases by asking 1 million veteran volunteers to provide a blood sample and complete surveys about their health, military experience, lifestyle, and other topics that might contribute to health and disease. They will study how genes influence diseases such as diabetes and cancer, and military-related illnesses, such as PTSD. “It’s an amazing study, where over the next decade, you’re going to hear research findings not just on PTSD but in other areas of mental health and physical health,” said Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego. So far, he said, 700,000 veterans are enrolled.
Dr. Stein shared preliminary findings from one component of the MVP study, in which participants complete the PTSD Checklist (PCL-17), a widely used 17-item self-report measure of PTSD symptoms in the past month. Using Manhattan plot analysis, Dr. Stein and his colleagues conducted a PTSD genomewide association study based on PCL-17 total scores. They found several genes significantly associated with PTSD, including LRRIQ3, TRAIP, KCNIP4, PCDHA1, MAD1L1, TSNARE1, EXD3, MGC57346-CRHR1, and TCF4. “This is the first study in the world to find this many genes [associated with PTSD],” said Dr. Stein, a staff psychiatrist with the VA San Diego Healthcare System. “The MAD1L1 gene is also one that’s come up in schizophrenia and in major depression. In fact, if we look at genetic correlations of how PTSD is genetically associated to other disorders that have been studied in this way, like major depression and schizophrenia, we find that there is shared variation. However, there are also unique features, so we are learning what we think is true about mental disorders overall, that in some ways comorbidity is explained by genetic risk, but there are also individual specific risk factors that go with specific disorders.
“We’re exploring each of those genes.”
of any of the genes in the genome. “One of the prominent theories of PTSD is that there is an increase in CRHR1 in the brain of individuals with the disorder,” Dr. Stein said. “More than by chance, it looks like the genes that are popping up in PTSD seem to be expressed in the frontal cortex, the anterior cingulate, the cortex, the hypothalamus, the amygdala, the hippocampus, the basal ganglia, and the substantia nigra. So all of a sudden, we go from having a list of genes to knowing there’s something going on in the brain of people with PTSD that involves expression in these particular regions that we might be able to target.”
In related work using the same genetic information, Dr. Stein and his colleagues have demonstrated an association between PTSD and medium spiny neurons, which are located in the basal ganglia and make up 95% of neurons in the striatum. “They also have GABAergic projection to other parts of the brain and play a key role in motivation, reward, enforcement, and aversion,” Dr. Stein said.
Dr. Stein disclosed that he has received research support from the National Institute of Mental Health, the National Institute of Alcoholism and Alcohol Abuse, the National Institute of Neurological Disorders and Stroke, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. He also has received consulting fees from Aptinyx, Bionomics, Janssen, Neurocrine, Oxeia Biopharmaceuticals, and Resilience Therapeutics.
LAS VEGAS – Despite recent advances in the diagnosis and treatment of posttraumatic stress disorder, researchers have yet to fully understand its pathophysiology.
“We don’t know whether there is just one type of pathophysiology or if there are different mechanisms and different etiologies that contribute to the symptomatic heterogeneity that we see,” Murray B. Stein, MD, MPH, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Many theories currently being studied are inflammatory theories, looking at autonomic dysregulation, glucocorticoid sensitivity, hippocampal volume/dysfunction, and cortical-amygdala circuit dysregulation.”
Dr. Stein expects clinicians to gain an improved understanding of PTSD and a myriad of other conditions in the coming years as the Million Veteran Program (MVP) gets underway. MVP researchers are building one of the world’s largest medical databases by asking 1 million veteran volunteers to provide a blood sample and complete surveys about their health, military experience, lifestyle, and other topics that might contribute to health and disease. They will study how genes influence diseases such as diabetes and cancer, and military-related illnesses, such as PTSD. “It’s an amazing study, where over the next decade, you’re going to hear research findings not just on PTSD but in other areas of mental health and physical health,” said Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego. So far, he said, 700,000 veterans are enrolled.
Dr. Stein shared preliminary findings from one component of the MVP study, in which participants complete the PTSD Checklist (PCL-17), a widely used 17-item self-report measure of PTSD symptoms in the past month. Using Manhattan plot analysis, Dr. Stein and his colleagues conducted a PTSD genomewide association study based on PCL-17 total scores. They found several genes significantly associated with PTSD, including LRRIQ3, TRAIP, KCNIP4, PCDHA1, MAD1L1, TSNARE1, EXD3, MGC57346-CRHR1, and TCF4. “This is the first study in the world to find this many genes [associated with PTSD],” said Dr. Stein, a staff psychiatrist with the VA San Diego Healthcare System. “The MAD1L1 gene is also one that’s come up in schizophrenia and in major depression. In fact, if we look at genetic correlations of how PTSD is genetically associated to other disorders that have been studied in this way, like major depression and schizophrenia, we find that there is shared variation. However, there are also unique features, so we are learning what we think is true about mental disorders overall, that in some ways comorbidity is explained by genetic risk, but there are also individual specific risk factors that go with specific disorders.
“We’re exploring each of those genes.”
of any of the genes in the genome. “One of the prominent theories of PTSD is that there is an increase in CRHR1 in the brain of individuals with the disorder,” Dr. Stein said. “More than by chance, it looks like the genes that are popping up in PTSD seem to be expressed in the frontal cortex, the anterior cingulate, the cortex, the hypothalamus, the amygdala, the hippocampus, the basal ganglia, and the substantia nigra. So all of a sudden, we go from having a list of genes to knowing there’s something going on in the brain of people with PTSD that involves expression in these particular regions that we might be able to target.”
In related work using the same genetic information, Dr. Stein and his colleagues have demonstrated an association between PTSD and medium spiny neurons, which are located in the basal ganglia and make up 95% of neurons in the striatum. “They also have GABAergic projection to other parts of the brain and play a key role in motivation, reward, enforcement, and aversion,” Dr. Stein said.
Dr. Stein disclosed that he has received research support from the National Institute of Mental Health, the National Institute of Alcoholism and Alcohol Abuse, the National Institute of Neurological Disorders and Stroke, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. He also has received consulting fees from Aptinyx, Bionomics, Janssen, Neurocrine, Oxeia Biopharmaceuticals, and Resilience Therapeutics.
REPORTING FROM NPA 2019
Caring for aging HIV-infected patients requires close attention to unrelated diseases
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
FROM THE LANCET HIV
Derm MOC reboot welcomed
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Heart failure outcomes are worse in select HIV-infected individuals
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
People living with HIV (PLHIV) who have a low CD4 count or detectable viral load (VL) have an increased 30-day heart failure (HF) readmission rate as well as increased cardiovascular and all-cause mortality, compared with uninfected controls, according to Raza M. Alvi, MD, of Massachusetts General Hospital and Harvard Medical School, Boston, and his colleagues.
Overall, the 30-day HF hospital readmission rate was higher among PLHIV versus non-HIV–infected individuals (49% vs. 32%, P less than .001), according to the results of their cohort study of 2,308 individuals admitted to the hospital with decompensated HF.
PLHIV and the non-HIV control groups were both followed over 2 years with a median follow-up period of 19 months, the authors wrote in the American Heart Journal. Demographic make-up of the two groups was similar; in particular, there was no difference in blood pressure and heart rate between the PLHIV and non-HIV controls, suggesting that adherence with HF medications may be similar between groups. The cohorts differed primarily in that pulmonary artery systolic pressure was significantly higher (45 vs. 40 mm Hg; P less than .001) in the HIV group, as was cocaine use (36% vs. 19%; P less than .001) and hepatitis C virus (HCV) infection (13% vs. 7%; P less than .001).
The differing results between the two cohorts were primarily caused by the fact that, for PLHIV with HF, CD4 count and VL were risk factors for adverse outcomes among patients with all types of HF. (30-day HF readmission, cardiovascular [CV] mortality, and all-cause mortality), compared with those with CD4 count greater than or equal to 200 cells/mm3. In addition, a low CD4 count/high VL independently related to an increased 30-day HF readmission rate even after the researchers controlled for major traditional and nontraditional HF risk factors.
“Rates of 30-day HF readmission, CV mortality, and all-cause mortality are worse among individuals with a low CD4 count or nonsuppressed viral load,” the researchers concluded.
The National Institutes of Health sponsored the study. The authors reported that they had no conflicts.
SOURCE: Alvi RM et al. Am Heart J. 2019 Apr;210:39-48.
FROM THE AMERICAN HEART JOURNAL
FDA accepts supplemental New Drug Application to expand lorcaserin label
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
Rhymin’ pediatric dermatologist provides Demodex tips
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org/discussions) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Ileoclonic Crohn’s in VA patient
A 77-year-old patient with chronic kidney disease, dementia and congestive heart failure was seen to evaluate chronic diarrhea. His colonoscopy revealed active inflammation and a stricture at the anastomosis, which prevented the physician from bypassing with a pediatric colonoscope. The patient’s diarrhea improved once he was started on budesonide. The discussion in the AGA Community forum outlined next steps and the best course of treatment for this complicated patient.
2. H. pylori in a penicillin allergic patient
A patient diagnosed with H. pylori during an endoscopy has a history of a severe penicillin allergy and has used clarithromycin in the past year. Antibiotic resistance testing revealed genetic pattern suggesting resistance to clarithromycin, fluoroquinolones and metronidazole. Recommendations from GIs included combination therapy with proton pump inhibitors (PPIs), antacids and antibiotics.
3. Reintroduction of azathioprine after moderate leukopenia
This 48-year-old patient has a history of ulcerative colitis pancolitis and developed antibodies to Humira monotherapy. Her GI is adjusting her azathioprine dose and repeating lab work to recover her white blood cell counts and is soliciting advice from the practice community on using methotrexate for combination therapy.
More clinical cases and discussions are at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org/discussions) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Ileoclonic Crohn’s in VA patient
A 77-year-old patient with chronic kidney disease, dementia and congestive heart failure was seen to evaluate chronic diarrhea. His colonoscopy revealed active inflammation and a stricture at the anastomosis, which prevented the physician from bypassing with a pediatric colonoscope. The patient’s diarrhea improved once he was started on budesonide. The discussion in the AGA Community forum outlined next steps and the best course of treatment for this complicated patient.
2. H. pylori in a penicillin allergic patient
A patient diagnosed with H. pylori during an endoscopy has a history of a severe penicillin allergy and has used clarithromycin in the past year. Antibiotic resistance testing revealed genetic pattern suggesting resistance to clarithromycin, fluoroquinolones and metronidazole. Recommendations from GIs included combination therapy with proton pump inhibitors (PPIs), antacids and antibiotics.
3. Reintroduction of azathioprine after moderate leukopenia
This 48-year-old patient has a history of ulcerative colitis pancolitis and developed antibodies to Humira monotherapy. Her GI is adjusting her azathioprine dose and repeating lab work to recover her white blood cell counts and is soliciting advice from the practice community on using methotrexate for combination therapy.
More clinical cases and discussions are at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org/discussions) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses.
In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Ileoclonic Crohn’s in VA patient
A 77-year-old patient with chronic kidney disease, dementia and congestive heart failure was seen to evaluate chronic diarrhea. His colonoscopy revealed active inflammation and a stricture at the anastomosis, which prevented the physician from bypassing with a pediatric colonoscope. The patient’s diarrhea improved once he was started on budesonide. The discussion in the AGA Community forum outlined next steps and the best course of treatment for this complicated patient.
2. H. pylori in a penicillin allergic patient
A patient diagnosed with H. pylori during an endoscopy has a history of a severe penicillin allergy and has used clarithromycin in the past year. Antibiotic resistance testing revealed genetic pattern suggesting resistance to clarithromycin, fluoroquinolones and metronidazole. Recommendations from GIs included combination therapy with proton pump inhibitors (PPIs), antacids and antibiotics.
3. Reintroduction of azathioprine after moderate leukopenia
This 48-year-old patient has a history of ulcerative colitis pancolitis and developed antibodies to Humira monotherapy. Her GI is adjusting her azathioprine dose and repeating lab work to recover her white blood cell counts and is soliciting advice from the practice community on using methotrexate for combination therapy.
More clinical cases and discussions are at https://community.gastro.org/discussions.