User login
CDC: United States has hit a plateau with HIV
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.
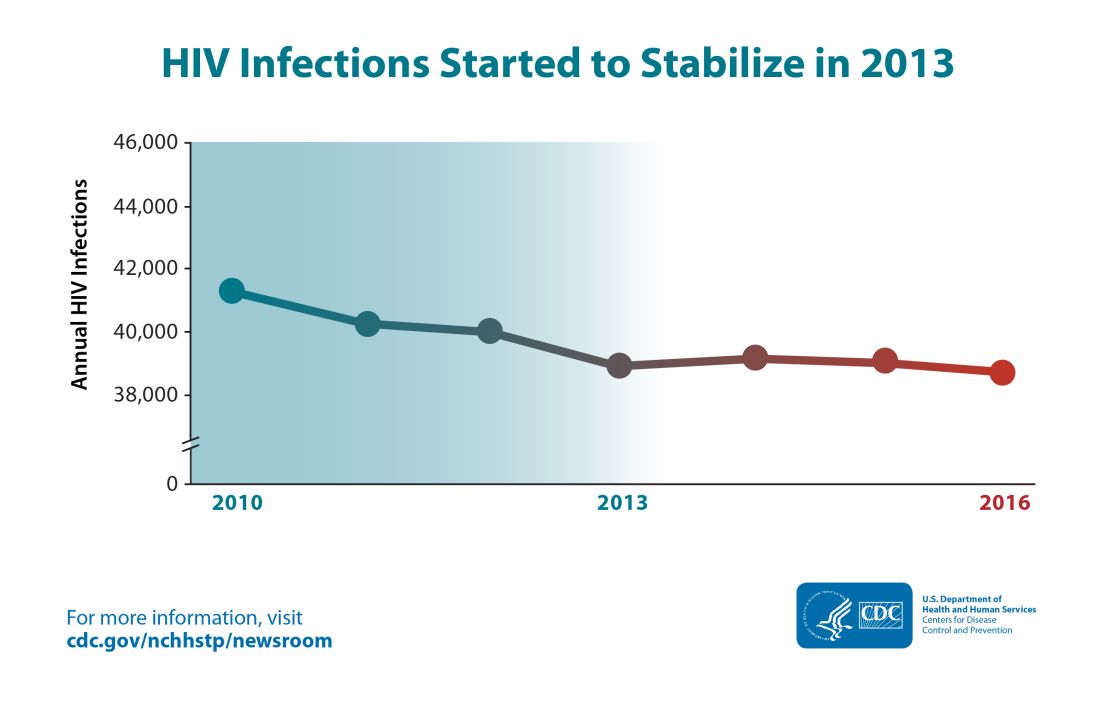
Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.

Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
The annual number of new HIV infections has remained stable in recent years, and the Centers for Disease Control and Prevention says that’s not good – but solutions are at hand.

Though the estimated number of new HIV infections declined from just under 42,000 per year in 2010 to about 39,000 annually in 2013, that figure was essentially unchanged by 2016, with 38,700 new HIV infections seen that year.
“CDC estimates that the decline in HIV infections has plateaued because effective HIV prevention and treatment are not adequately reaching those who could most benefit from them. These gaps remain particularly troublesome in rural areas and in the South and among disproportionately affected populations like African Americans and Latinos,” said the CDC in a press release accompanying the report.
The report comes soon after President Trump’s State of the Union address, which announced a new multiagency initiative to eliminate the HIV epidemic in the United States, with the goal of reducing new HIV infections by 90% over the next 10 years. The multipronged initiative will implement geographically targeted HIV elimination teams in areas with high HIV prevalence, pulling together federal agencies, local and state governments, and community-level resources.
The initiative, called “Ending the Epidemic: A Plan for America” will combine an intensified approach to early diagnosis and treatment with efforts to boost uptake of pre-exposure prophylaxis for individuals at high risk for HIV infection.
The new CDC report used CD4 counts reported to the National HIV Surveillance System at the time of diagnosis to identify new (incident) cases and to track prevalence. Much of the report is devoted to finely detailed reporting of HIV incidence across sex, age, race/ethnicity, and transmission mode.
Though some groups, such as people who inject drugs, have seen a decrease of about 30% in the annual rate of new HIV cases, new cases have jumped for other groups. In particular, Latino gay and bisexual men saw new cases climb from 6,400 per year in 2010 to 8,300 in 2016. The incidence rate has stayed high and stable among African American gay and bisexual men, with 9,800 new cases reported in 2010; the same number was seen in 2016.
Among gay and bisexual men overall, the rate has also stayed stable, with about 26,000 new HIV infections reported at the beginning and end of the studied period. White heterosexual women saw about 1,000 new cases per year in 2010 and in 2016.
Some groups saw declines in new cases: African American and Latina heterosexual women each saw a falling incidence of new HIV cases. For the former group, new cases fell from 4,700 to 4,000, while the latter group of women saw new cases drop from 1,200 to 980 per year from 2010 to 2016.
Within these broad groups, HIV incidence also rose among some age groups and fell among others. Decreases were seen for younger African American gay and bisexual men (those aged 13-24 years), but rates increased by about two-thirds for men in this group aged 25-34 years. A similar increase was seen for Latino men in the 25-34 years age group, a change which drove the overall 30% increase in new infections for Latino gay and bisexual men.
White gay and bisexual men saw across-the-board decreases in new infections, though the overall decrease was less than 20%.
For heterosexual individuals as a group, new infections dropped by about 17%, from 10,900 to 9,100 annually. This change was driven mostly by decreases in women identifying as heterosexual.
“After a decades-long struggle, the path to eliminate America’s HIV epidemic is clear,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention, in the press release. “Expanding efforts across the country will close gaps, overcome threats, and turn around troublesome trends.”
The press release cited local work in Washington and New York as evidence that targeted resources can make a difference in reducing new HIV cases. In these two areas, new infections dropped by 23% and 40% respectively from 2010 to 2016.
SOURCE: Centers for Disease Control. CDC Report: www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
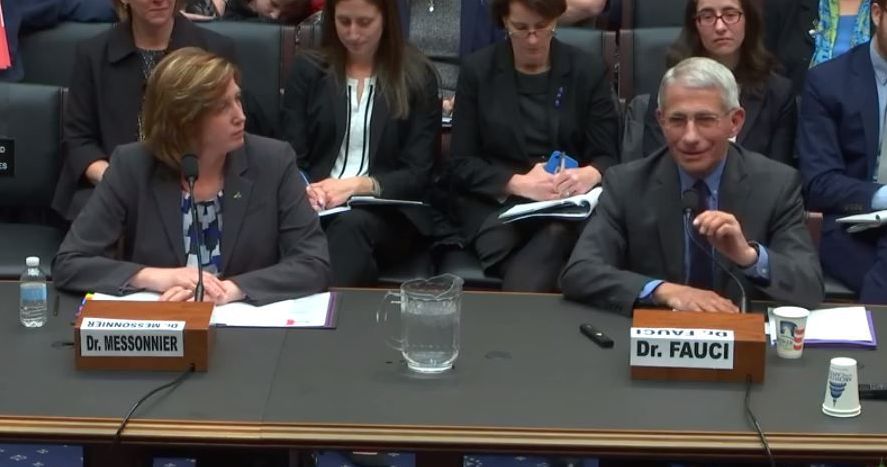
Fauci, Messonnier testify on measles outbreaks
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
REPORTING FROM HOUSE COMMITTEE HEARING
CLL, GVHD may raise risk for skin cancer after allo-HCT
Previously unknown risk factors for secondary skin cancer linked with allogeneic hematopoietic cell transplantation (HCT) have been identified, researchers report after a retrospective analysis.
“We confirmed [graft-versus-host disease] as a risk factor, identified [chronic lymphocytic leukemia] as an additional risk factor, and found that patients who received myeloablative transplants in adulthood had fewer [basal cell carcinomas] than their counterparts,” Peggy A. Wu, MD, of the Beth Israel Deaconess Medical Center in Boston, and her colleagues wrote in the Journal of Investigative Dermatology.
The team analyzed 1,974 patients who underwent transplantation for various types of hematologic cancer and survived for a minimum of 100 days following transplant. Among this cohort, 119 patients developed various forms of skin cancer, including basal and squamous cell carcinoma.
Reports of skin malignancy were confirmed using physician records and pathology reports. Dr. Wu and her colleagues excluded patients whose indication for transplant was a primary immunodeficiency or Fanconi anemia.
“Reflecting advances that allow older patients to be eligible for HCT, the median age at transplantation of our cohort was one of the oldest (51.1 years) in the literature,” the researchers wrote.
In univariable models, the researchers found that prior chronic lymphocytic leukemia (CLL) (hazard ratio, 2.2; 95% CI, 1.3-3.7), chronic graft-versus-host disease (GVHD) (HR, 3.1; 95% CI, 1.7-5.4), and age at transplant of more than 60 years (HR, 10.8; 95% CI, 3.3-35.6) were all linked to an increased risk for squamous cell carcinomas. A multivariable analysis found that these factors continued as significant risk factors.
For basal cell carcinomas, the risk factors identified were prior CLL (HR, 3.5; 95% CI, 2.0-6.4), acute GVHD (HR, 1.9; 95% CI, 1.1-3.3), and chronic GVHD (HR, 3.2; 95% CI, 1.6-6.5) using univariable models. These factors all continued to be significant in multivariable analysis.
Additionally, the researchers found that a myeloablative conditioning regimen and total body irradiation were protective against development of basal cell carcinomas in univariable models. However, the protective effect continued for myeloablative condition in the multivariable model only.
“To our knowledge, previously unreported risk factors in this contemporary cohort include prior CLL for squamous cell carcinoma and basal cell carcinoma and reduced-intensity conditioning for basal cell carcinoma,” the researchers wrote.
The study was supported by the Skin Cancer Foundation, Women’s Dermatologic Society, Harvard Catalyst, and Harvard University. The authors reported having no conflicts of interest.
SOURCE: Wu PA et al. J Invest Dermatol. 2019 Mar;139(3):591-9.
Previously unknown risk factors for secondary skin cancer linked with allogeneic hematopoietic cell transplantation (HCT) have been identified, researchers report after a retrospective analysis.
“We confirmed [graft-versus-host disease] as a risk factor, identified [chronic lymphocytic leukemia] as an additional risk factor, and found that patients who received myeloablative transplants in adulthood had fewer [basal cell carcinomas] than their counterparts,” Peggy A. Wu, MD, of the Beth Israel Deaconess Medical Center in Boston, and her colleagues wrote in the Journal of Investigative Dermatology.
The team analyzed 1,974 patients who underwent transplantation for various types of hematologic cancer and survived for a minimum of 100 days following transplant. Among this cohort, 119 patients developed various forms of skin cancer, including basal and squamous cell carcinoma.
Reports of skin malignancy were confirmed using physician records and pathology reports. Dr. Wu and her colleagues excluded patients whose indication for transplant was a primary immunodeficiency or Fanconi anemia.
“Reflecting advances that allow older patients to be eligible for HCT, the median age at transplantation of our cohort was one of the oldest (51.1 years) in the literature,” the researchers wrote.
In univariable models, the researchers found that prior chronic lymphocytic leukemia (CLL) (hazard ratio, 2.2; 95% CI, 1.3-3.7), chronic graft-versus-host disease (GVHD) (HR, 3.1; 95% CI, 1.7-5.4), and age at transplant of more than 60 years (HR, 10.8; 95% CI, 3.3-35.6) were all linked to an increased risk for squamous cell carcinomas. A multivariable analysis found that these factors continued as significant risk factors.
For basal cell carcinomas, the risk factors identified were prior CLL (HR, 3.5; 95% CI, 2.0-6.4), acute GVHD (HR, 1.9; 95% CI, 1.1-3.3), and chronic GVHD (HR, 3.2; 95% CI, 1.6-6.5) using univariable models. These factors all continued to be significant in multivariable analysis.
Additionally, the researchers found that a myeloablative conditioning regimen and total body irradiation were protective against development of basal cell carcinomas in univariable models. However, the protective effect continued for myeloablative condition in the multivariable model only.
“To our knowledge, previously unreported risk factors in this contemporary cohort include prior CLL for squamous cell carcinoma and basal cell carcinoma and reduced-intensity conditioning for basal cell carcinoma,” the researchers wrote.
The study was supported by the Skin Cancer Foundation, Women’s Dermatologic Society, Harvard Catalyst, and Harvard University. The authors reported having no conflicts of interest.
SOURCE: Wu PA et al. J Invest Dermatol. 2019 Mar;139(3):591-9.
Previously unknown risk factors for secondary skin cancer linked with allogeneic hematopoietic cell transplantation (HCT) have been identified, researchers report after a retrospective analysis.
“We confirmed [graft-versus-host disease] as a risk factor, identified [chronic lymphocytic leukemia] as an additional risk factor, and found that patients who received myeloablative transplants in adulthood had fewer [basal cell carcinomas] than their counterparts,” Peggy A. Wu, MD, of the Beth Israel Deaconess Medical Center in Boston, and her colleagues wrote in the Journal of Investigative Dermatology.
The team analyzed 1,974 patients who underwent transplantation for various types of hematologic cancer and survived for a minimum of 100 days following transplant. Among this cohort, 119 patients developed various forms of skin cancer, including basal and squamous cell carcinoma.
Reports of skin malignancy were confirmed using physician records and pathology reports. Dr. Wu and her colleagues excluded patients whose indication for transplant was a primary immunodeficiency or Fanconi anemia.
“Reflecting advances that allow older patients to be eligible for HCT, the median age at transplantation of our cohort was one of the oldest (51.1 years) in the literature,” the researchers wrote.
In univariable models, the researchers found that prior chronic lymphocytic leukemia (CLL) (hazard ratio, 2.2; 95% CI, 1.3-3.7), chronic graft-versus-host disease (GVHD) (HR, 3.1; 95% CI, 1.7-5.4), and age at transplant of more than 60 years (HR, 10.8; 95% CI, 3.3-35.6) were all linked to an increased risk for squamous cell carcinomas. A multivariable analysis found that these factors continued as significant risk factors.
For basal cell carcinomas, the risk factors identified were prior CLL (HR, 3.5; 95% CI, 2.0-6.4), acute GVHD (HR, 1.9; 95% CI, 1.1-3.3), and chronic GVHD (HR, 3.2; 95% CI, 1.6-6.5) using univariable models. These factors all continued to be significant in multivariable analysis.
Additionally, the researchers found that a myeloablative conditioning regimen and total body irradiation were protective against development of basal cell carcinomas in univariable models. However, the protective effect continued for myeloablative condition in the multivariable model only.
“To our knowledge, previously unreported risk factors in this contemporary cohort include prior CLL for squamous cell carcinoma and basal cell carcinoma and reduced-intensity conditioning for basal cell carcinoma,” the researchers wrote.
The study was supported by the Skin Cancer Foundation, Women’s Dermatologic Society, Harvard Catalyst, and Harvard University. The authors reported having no conflicts of interest.
SOURCE: Wu PA et al. J Invest Dermatol. 2019 Mar;139(3):591-9.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Today’s Care Must Extend Beyond the Exam Room
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
In May 2014, a 70-year-old retiree underwent repair of a fracture of her left ankle. The procedure was performed at a local hospital. A splint was applied to the ankle, and a nurse provided crutches.
Following discharge from the hospital, the patient hailed a taxi to take her home. As she was exiting the taxi at her residence, the patient fell and sustained comminuted fractures to the distal radius and distal ulna of her right (dominant) wrist and a trimalleolar fracture to her repaired left ankle.
The plaintiff was transported back to the hospital via ambulance. She underwent closed reduction of her wrist fractures and 11 days later was transferred to another facility for open reduction and internal fixation of her left ankle fracture. Her hospitalizations totaled 13 days and were followed by a course of inpatient rehabilitative therapy; the latter lasted until late August 2014, with a brief interruption in June when she underwent open reduction and internal fixation of her wrist fractures. When she returned home in August, the patient required the assistance of visiting aides and 3 additional months of rehabilitative therapy.
At trial, the plaintiff claimed that her left ankle and her right wrist remained painful, that she sustained a mild residual diminution of each area’s range of motion, and that these residual effects hindered her performance of basic physical activities (eg, cleaning and cooking).
The plaintiff alleged that her fall while exiting the taxi resulted from unsteadiness, which was a lingering effect of morphine that was administered during the repair of her fracture. She sought recovery of damages for past and future pain and suffering from the hospital’s operator. The lawsuit alleged that the nurse had failed to provide instructions on the proper use of crutches, that the nurse had failed to undertake measures that would have diminished the plaintiff’s likelihood of falling, that the nurse’s failures constituted malpractice and negligence, and that the hospital operator was vicariously liable for the nurse’s actions.
The plaintiff claimed that she repeatedly warned that she did not believe that she could safely use the crutches provided by the nurse. She claimed that she was unsteady and lightheaded, and that when she requested a wheelchair, an escort, or an ambulance, the nurse rejected the request. The nursing standards expert for the plaintiff opined that the request should have been satisfied or alternatively, that the nurse should have explained the manner in which a crutch-dependent person could safely enter and exit a vehicle.
Defense counsel claimed that the nurse explained proper use of the crutches, the plaintiff indicated that she understood the explanation, and the plaintiff demonstrated proper use and did not express concern. The defense’s expert contended that the nurse did not have to explain how a crutch-dependent person could safely enter and exit a vehicle and that the plaintiff’s fall resulted from her own failure to exercise appropriate caution. The defense further contended that the plaintiff achieved an excellent recovery.
Continue to: After a 7-day trial...
After a 7-day trial and 3 hours and 45 minutes’ deliberation, the jury found in favor of the plaintiff. It found that the nurse was negligent in her provision of crutches and that the act was a substantial cause of the plaintiff’s injuries. The jury also found that the nurse did not properly explain the use of crutches but determined that the error was not a substantial cause of the plaintiff’s injuries.
VERDICT
The jury awarded the plaintiff a total of $850,000 in damages. The plaintiff also recovered stipulated medical expenses.
COMMENTARY
Medical malpractice litigation involves recovery for acts or omissions that constitute a departure from the standard of care. We all recognize injurious acts—improper esophageal intubation in the emergency department, transection of a nerve in the operating room, or prescription of a contraindicated medication to an allergic patient—and acknowledge damaging omissions, such as failure to screen for colon cancer or recognize treatable diabetes.
However, some cases are disposition related; they arise from how patients are discharged, what instructions they are given, where they go, and what they do after discharge. These cases involve the patient’s medical issues engrafted on his or her transportation, job, and more generally, living environment.
The lay public expects patients to have a right of self-determination, to control the nature and course of their medical care. Yet, the modern lay public also expects the medical profession to act as an authority figure—exercising a degree of paternalism to safeguard patients from harm. This expectation is commonly articulated in retrospect, after something has gone wrong. Consequently, clinicians must be aware of what will happen to the patient after discharge.
Continue to: With all interventions...
With all interventions, weigh the post-discharge consequences. If you give an injection of hydromorphone, you cannot discharge the patient to drive home 45 minutes later. If you have diagnosed vertigo in a patient, you cannot prescribe meclizine and return that patient to her job working on scaffolding 50 ft above ground. If a frail patient lives alone and cannot safely walk, and you’ve started him on furosemide, you cannot discharge him without considering how he will get to the bathroom. Other concerns are even more difficult—for example, the homeless patient who does not have the environment or resources to follow your instructions.
It is tempting to view these concerns as not our responsibility or dismiss them as “not medicine.” Clinicians can feel frustrated at being pulled into the realm of social work, where we are ill equipped to deal with and sort out the patient’s “life problems.” For one thing, we don’t often have the resources to deal with these issues. And for another, addressing the patient’s postdischarge living situation takes time—something in short supply and intangible to the other patients in the waiting room, who are expecting your attention and wondering, “What’s the holdup?”
In the case presented, the plaintiff was a 70-year-old retiree. She was discharged from the hospital with crutches. Crutches are age-old and familiar devices. Nevertheless, crutches are for people who are able to use their arms for weight bearing and propulsion and require a fair amount of physical strength, timing, and dexterity. While a potentially debatable point, an assumption that a 70-year-old patient has the arm strength and dexterity to properly propel herself with crutches may be faulty. There was disagreement between the patient, who claimed she could not safely use the crutches, and the nurse, who said the patient accepted the crutches without concern. The safest course of action would be for discharge personnel to demonstrate the use of crutches, observe the patient using the crutches, and document that in the record.
In this case, it is unclear if the nurse demonstrated how to use the crutches or witnessed the plaintiff demonstrating she could safely use them. The jury found the nurse was negligent “in her provision” of crutches—an act they deemed a substantial cause of the plaintiff’s injuries. Interestingly, the jury did not consider the lack of explanation on the crutches’ use to be a substantial cause of injury. But the bottom line is, they faulted the nurse for the act of giving this patient crutches and awarded $850,000 in damages.
Society is changing. Fifty years ago, jurors would expect people to be familiar with crutches, and if you fell while using them, that was your own fault. Modern jurors expect hospitals and providers to get more involved in what happens to a patient after discharge. The news media has heavily publicized cases of alleged “patient dumping.”
Continue to: As a result...
As a result, we see legislative changes, such as the recently passed California Senate Bill 1152, which requires that homeless patients be fed; provided weather-appropriate clothing, filled prescriptions, and vaccinations; given medical screening, examination, and evaluation that requires the “treating physician” to arrange behavioral health care; and enrolled in “any affordable health insurance coverage for which he or she is eligible.”
Whether it is appropriate to ask hospitals and clinicians to get this involved is beyond the scope of this column. What is clear is that society increasingly expects clinicians and hospitals to take responsibility for patients. This societal change has an impact on the lay public’s perception of what is expected of health care providers. Tomorrow’s juror comes to court with a belief that hospitals and clinicians owe a duty of care that extends beyond the walls of the exam room.
IN SUMMARY
Reality test your post-treatment instructions to be sure they will work for the patient and are not grossly incompatible with his or her known postdischarge environment. To the extent possible, involve discharge planning personnel in your practice. Let your record reflect that you are acting in the patient’s best interest, and evade the temptation to squint narrowly to avoid seeing circumstances in the patient’s life that prevent safe implementation of your plan.
NILE: Liquid biopsy bests tissue testing for targetable mutations in NSCLC
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
FDA approves label extension for dapagliflozin
The Food and Drug Administration has approved a label extension for Farxiga (dapagliflozin) and Xigduo XR (extended-release dapagliflozin and metformin HCl) for use in patients with type 2 diabetes and moderate renal impairment, lowering the estimated glomerular filtration rate (eGFR) threshold to 45 mL/min per 1.73 m2 from the current60 mL/min per 1.73 m2.
The update is based on results from DERIVE, a phase 3 study in patients with inadequately controlled diabetes and an eGFR of 45-59 mL/min per 1.73 m2 who received either dapagliflozin 10 mg or placebo during a 24-week period. After that time, patients who received dapagliflozin had significant reductions in glycosylated hemoglobin, compared with placebo. The safety profile was similar to that in other studies with dapagliflozin.
The most common adverse events associated with Farxiga are female genital mycotic infections, nasopharyngitis, and urinary tract infections. For Xigduo XR, the most common adverse events are female genital mycotic infection, nasopharyngitis, urinary tract infection, diarrhea, and headache.
“The DERIVE study, which further confirmed the well-established efficacy and safety profile for Farxiga and Xigduo XR, has resulted in important label changes for patients with type 2 diabetes that enable a broader population with impaired renal function to potentially benefit from these important treatment options,” Jim McDermott, PhD, vice president, U.S. medical affairs, diabetes, at AstraZeneca, said in the press release.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has approved a label extension for Farxiga (dapagliflozin) and Xigduo XR (extended-release dapagliflozin and metformin HCl) for use in patients with type 2 diabetes and moderate renal impairment, lowering the estimated glomerular filtration rate (eGFR) threshold to 45 mL/min per 1.73 m2 from the current60 mL/min per 1.73 m2.
The update is based on results from DERIVE, a phase 3 study in patients with inadequately controlled diabetes and an eGFR of 45-59 mL/min per 1.73 m2 who received either dapagliflozin 10 mg or placebo during a 24-week period. After that time, patients who received dapagliflozin had significant reductions in glycosylated hemoglobin, compared with placebo. The safety profile was similar to that in other studies with dapagliflozin.
The most common adverse events associated with Farxiga are female genital mycotic infections, nasopharyngitis, and urinary tract infections. For Xigduo XR, the most common adverse events are female genital mycotic infection, nasopharyngitis, urinary tract infection, diarrhea, and headache.
“The DERIVE study, which further confirmed the well-established efficacy and safety profile for Farxiga and Xigduo XR, has resulted in important label changes for patients with type 2 diabetes that enable a broader population with impaired renal function to potentially benefit from these important treatment options,” Jim McDermott, PhD, vice president, U.S. medical affairs, diabetes, at AstraZeneca, said in the press release.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has approved a label extension for Farxiga (dapagliflozin) and Xigduo XR (extended-release dapagliflozin and metformin HCl) for use in patients with type 2 diabetes and moderate renal impairment, lowering the estimated glomerular filtration rate (eGFR) threshold to 45 mL/min per 1.73 m2 from the current60 mL/min per 1.73 m2.
The update is based on results from DERIVE, a phase 3 study in patients with inadequately controlled diabetes and an eGFR of 45-59 mL/min per 1.73 m2 who received either dapagliflozin 10 mg or placebo during a 24-week period. After that time, patients who received dapagliflozin had significant reductions in glycosylated hemoglobin, compared with placebo. The safety profile was similar to that in other studies with dapagliflozin.
The most common adverse events associated with Farxiga are female genital mycotic infections, nasopharyngitis, and urinary tract infections. For Xigduo XR, the most common adverse events are female genital mycotic infection, nasopharyngitis, urinary tract infection, diarrhea, and headache.
“The DERIVE study, which further confirmed the well-established efficacy and safety profile for Farxiga and Xigduo XR, has resulted in important label changes for patients with type 2 diabetes that enable a broader population with impaired renal function to potentially benefit from these important treatment options,” Jim McDermott, PhD, vice president, U.S. medical affairs, diabetes, at AstraZeneca, said in the press release.
Find the full press release on the AstraZeneca website.
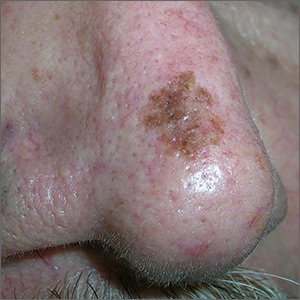
Growing spot on nose
The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Will having fewer primary care physicians shorten Americans’ lifespans?
Opportunities are being missed for advance care planning for elderly ICU patients. Insulin-treated diabetes in pregnancy carries a strong preterm risk. And U.S. measles cases are up to 159 for the year.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Opportunities are being missed for advance care planning for elderly ICU patients. Insulin-treated diabetes in pregnancy carries a strong preterm risk. And U.S. measles cases are up to 159 for the year.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Opportunities are being missed for advance care planning for elderly ICU patients. Insulin-treated diabetes in pregnancy carries a strong preterm risk. And U.S. measles cases are up to 159 for the year.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
KATHERINE trial and breast cancer
In this episode, Charles E. Geyer, MD, of Virginia Commonweath University joins guest host Jame Abraham, MD, of the Cleveland Clinic to discuss the KATHERINE trial and its impact on the treatment of HER2-positive breast cancer.
And Ilana Yurkiewicz, MD, talks about whether it’s better for physicians to be vague about prognosis. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple PodcastsGoogle Podcasts
In this episode, Charles E. Geyer, MD, of Virginia Commonweath University joins guest host Jame Abraham, MD, of the Cleveland Clinic to discuss the KATHERINE trial and its impact on the treatment of HER2-positive breast cancer.
And Ilana Yurkiewicz, MD, talks about whether it’s better for physicians to be vague about prognosis. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple PodcastsGoogle Podcasts
In this episode, Charles E. Geyer, MD, of Virginia Commonweath University joins guest host Jame Abraham, MD, of the Cleveland Clinic to discuss the KATHERINE trial and its impact on the treatment of HER2-positive breast cancer.
And Ilana Yurkiewicz, MD, talks about whether it’s better for physicians to be vague about prognosis. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple PodcastsGoogle Podcasts
Over 20 Years, Pain Is on the Rise
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”