User login
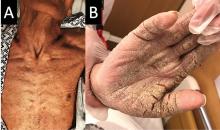
Norwegian scabies
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
Does early repolarization on ECG increase the risk of cardiac death in healthy people?
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
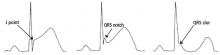
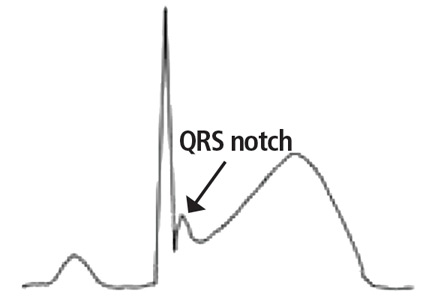
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
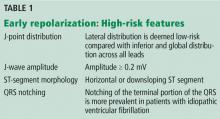
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
A paraneoplastic potassium and acid-base disturbance
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
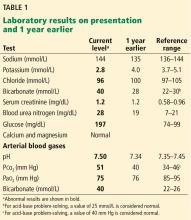
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
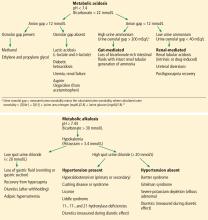
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
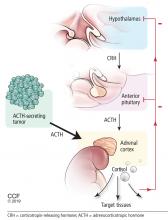
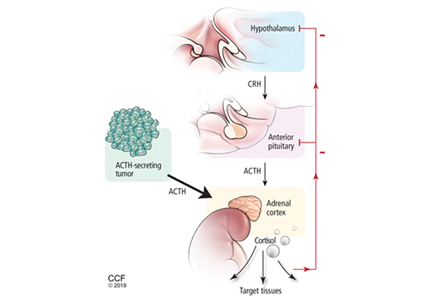
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
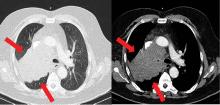
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
Cancer screening: A modest proposal for prevention
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
Correction: Hypertension guidelines
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
In Aleyadeh W, Hutt-Centeno E, Ahmed HM, Shah NP. Hypertension guidelines: treat patients, not numbers. Cleve Clin J Med 2019; 86(1):47–56. doi:10.3949/ccjm.86a.18027, on page 50, the following statement was incorrect: “In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 130 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9” In fact, the ACP and AAFP recommended a tighter goal of less than 140 mm Hg for this higher-risk group. This has been corrected online.
Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
© 2019 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
© 2019 Society of Hospital Medicine
Leadership & Professional Development: Know Your TLR
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
Things We Do For No Reason: Contact Precautions for MRSA and VRE
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest for any authors, financial or other.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
© 2019 Society of Hospital Medicine
The Complex Problem of Women Trainees in Academic Medicine
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
© 2019 Society of Hospital Medicine