User login
Rheumatologist involvement often reclassifies interstitial lung disease
MAUI, HAWAII – The latest practice guidelines on the diagnosis of interstitial lung disease issued by the American Thoracic Society and allied organizations recommend as the standard of care a review of all cases by a multidisciplinary team consisting of a pulmonologist, radiologist, and pathologist to ensure accurate diagnosis and classification.
That’s not good enough. A rheumatologist needs to routinely be involved in those multidisciplinary discussions as well, Aryeh Fischer, MD, asserted at the 2019 Rheumatology Winter Clinical Symposium.
Why? Because a rheumatologist’s input often leads to a change in diagnosis. And that change can have important prognostic and therapeutic implications.
“We want to distinguish the IPF [idiopathic pulmonary fibrosis] patients from everybody else. The most important thing with regards to therapy is to identify the IPF patient. The IPF patients are the only ones who are able to be treated with antifibrotic agents: pirfenidone or nintedanib. And we know that immunosuppression can make patients with IPF worse; their risks of hospitalization and mortality are higher on immunosuppression. But everybody else, including anyone with any of the autoimmune diseases along with ILD [interstitial lung disease] or any other causes of ILD, gets treated with immunosuppression,” explained Dr. Fischer, a rheumatologist with a special interest in autoimmune lung disease at the University of Colorado, Denver.
He cited a recent prospective blinded study in which 60 newly diagnosed ILD patients were evaluated separately by a multidisciplinary team comprising a pulmonologist, radiologist, and pathologist and once again with the involvement of a rheumatologist. The rheumatologic assessment reclassified 21% of patients from IPF – that is, lung disease unrelated to connective tissue disease (CTD) or exposure to asbestos, bird droppings, or other triggers – to ILD with connective tissue disease (CTD-ILD). And the number of patients classified as having ILD with autoimmune features without meeting full diagnostic criteria for a major CTD, a category that includes antisynthetase syndrome and IgG4-related ILD, jumped by 77%. Also, the investigators determined that adding a rheumatologist to the multidisciplinary team would have resulted in seven fewer bronchoscopies and one less surgical biopsy among this 60-patient cohort (J Rheumatol. 2018 Nov;45[11]:1509-14).
It’s not at all uncommon to identify a new occult CTD in patients presenting with ILD. Dr. Fischer noted that in a series of 114 consecutive patients evaluated at the interdisciplinary ILD program at Johns Hopkins University, Baltimore, 30% of them had a well-defined CTD, half of whom, or 15% of the total, were newly diagnosed with CTD by virtue of their ILD evaluation (Respir Med. 2009 Aug;103[8]:1152-8).
A CTD-ILD diagnosis has prognostic implications: Survival is significantly better than with IPF, with the exception of ILD with rheumatoid arthritis (RA-ILD), where the prognosis seems to be worse than for other forms of CTD-ILD and is more akin to that of IPF. Indeed, the risk of death is threefold higher in patients with RA-ILD than in those with RA without ILD. While the predominant pattern of lung injury in RA-ILD is usual interstitial pneumonia, marked by fibrosis and honeycombing, the predominant pattern in all other forms of CTD-ILD is nonspecific interstitial pneumonia.
A French study of 778 consecutive patients with ILD highlighted how common autoimmune disease is in the ILD population. Nearly one-third of the ILD patients had autoimmune disease: 22% had CTD-ILD and another 7% had interstitial pneumonia with autoimmune features, or IPAF (Respir Med. 2017 Feb;123:56-62).
Dr. Fischer was lead author of a report by a European Respiratory Society/American Thoracic Society task force that first put forth the term IPAF to describe ILD patients with subtle serologic, clinical, and/or morphologic findings that don’t rise to the level required for formal diagnosis of a defined CTD (Eur Respir J. 2015 Oct;46[4]:976-87).
IPAF is a research construct. Patients who fall into this category are the focus of ongoing prospective studies aimed at better understanding their prognosis and appropriate treatment.
The big three autoimmune diseases comorbid with ILD as reported by Dr. Fischer and coinvestigators in a study of 237 patients with an autoimmune phenotype in a Denver ILD clinic were scleroderma, present in 37%; rheumatoid arthritis, present in 18%; and myositis, with a prevalence of 11%. Another 24% had IPAF.
“There was surprisingly little SLE [3%], and not much Sjögren’s [6%],” he observed.
When pulmonologists come knocking on Dr. Fischer’s door asking if their patients with ILD have occult CTD, he finds it helpful to look for quantifiable specific extrathoracic features suggestive of rheumatologic disease, such as Raynaud’s, sclerodactyly, mechanic hands, and Gottron’s papules.
The ILD practice guidelines recently issued jointly by the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society recommend that serologic evaluation for a long list of autoantibodies “should be performed even in the absence of signs or symptoms of connective tissue disease” (Am J Respir Crit Care Med. 2018 Sep 1;198[5]:e44-e68). Dr. Fischer indicated he has a problem with that recommendation since nonrheumatologists aren’t typically adept in interpreting the significance of autoantibodies.
“I will just tell you, I see a lot of patients for ILD evaluation, and autoimmune serologies often lead to more questions than answers. They always need to be considered within the clinical context,” the rheumatologist said.
High-resolution CT (HRCT) is the imaging method of choice for detecting ILD and classifying its severity. HRCT also holds clues for detection of CTD-ILD.
“There are no upper lung–predominant ILDs that really make you think CTD. Our diseases like the lower lung zones. We like to see multicompartment involvement. When you hear ‘airways and parenchymal,’ ‘pleural,’ ‘pericardial thickening,’ that sounds a lot like autoimmune disease,” according to Dr. Fischer.
Also, the presence of a dilated esophagus on HRCT is strongly suggestive of scleroderma, he added.
In the event a pathology report is available, it’s important to read the fine print. Secondary histopathologic features of CTD-ILD include extensive pleuritis, lymphoid aggregates with germinal center formation, dense perivascular collagen, and/or prominent plasmacytic infiltration.
While ILD can be the first manifestation of an underlying occult CTD, it’s also common for a patient with an established CTD to subsequently develop ILD.
Rheumatologists are no strangers to ambiguity, so it should come as no surprise that while audible bibasilar crackles on physical examination are strongly predictive of ILD, their absence doesn’t indicate absence of ILD. Similarly, dyspnea in a patient with established CTD can be tough to interpret, and absence of dyspnea doesn’t imply absence of ILD. If a patient with CTD is on immunosuppressive therapy, bronchoalveolar lavage is of value in ruling out infection.
When to order a surgical lung biopsy
“If you’re pretty sure your patient had a CTD and then you get a characteristic HRCT and there are no exposures to account for the imaging findings – if we have a scenario where it all fits – we often don’t biopsy. Biopsy usually means a 2-night hospital stay, and it’s reportedly associated with about a 1% mortality risk. And the clinical reality is the biopsy may not impact treatment. They’re going to give you azathioprine, mycophenolate, cyclophosphamide, or prednisone for the ILD and the extrathoracic disease irrespective of the ILD pattern,” the rheumatologist said.
He reserves surgical biopsy for patients with an atypical HRCT, those with known CTD and a possible alternative etiology for the ILD, such as exposure to asbestos or owning pet birds, and patients where he’s just not sure a CTD is actually present.
Treatment of CTD-ILD
“The available controlled efficacy data are limited to scleroderma-ILD, where cyclophosphamide and mycophenolate work a little bit. And that’s it. We don’t have good data to guide us on which agent for which CTD or ILD pattern, for how long, or what dose,” Dr. Fischer said. “We have good drugs for the joints, nothing for the lungs. Treatment is not evidence based. We initiate with high-dose steroids, switch to a steroid-sparing agent, we evaluate the response to treatment with surveillance every 3-6 months by 6-minute walking, lung function tests, and sometimes imaging, and we treat for a long time. Oftentimes stability equals success.”
That being said, it must be emphasized that not all CTD-ILD warrants treatment of the pulmonary disease. If the patient’s ILD is mild and indolent, clinical surveillance is often appropriate, he continued.
More important than the pharmacotherapy available at present are adjunctive nonpharmacologic approaches: supplemental oxygen, pulmonary rehabilitation, treatment of comorbid GERD, immunizations, and addressing mental health issues related to these devastating diseases.
“We really don’t do these things well,” Dr. Fischer said.
On the horizon
An investigator-driven phase 2 clinical trial of the antifibrotic drug pirfenidone (Esbriet), now approved for IPF, is underway in patients with RA-ILD. Results of a trial of antifibrotic therapy in patients with scleroderma-ILD are due to be presented this year at EULAR. And a small study of antifibrotic therapy in patients with myositis-ILD is ongoing.
As for the biologics, there is a signal in the literature that tumor necrosis factor inhibitors may be associated with increased risk of rapidly progressive lung disease in patients with RA-ILD. An influential report from the British Society for Rheumatology Biologics Register on 299 patients with preexisting RA-ILD treated with anti-TNF therapy and 68 who received traditional disease-modifying antirrheumatic drugs showed that while mortality during follow-up was similar in the two groups, the proportion of deaths attributed to RA-ILD was 21% in the group on anti-TNF therapy, compared with only 7% in those on traditional DMARDs (Ann Rheum Dis. 2010 Jun;69[6]:1086-91).
Paul Emery, MD, rose from the audience at Dr. Fischer’s request to share his extensive experience with anti-TNF therapy and rituximab (Rituxan) in the setting of RA-ILD. When he was involved in several of the pivotal clinical trials of anti-TNF agents for RA he encountered a couple of cases of rapidly progressive ILD in patients on treatment.
“We had never before seen rapidly progressive ILD that didn’t respond to cyclophosphamide. Both patients died,” recalled Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
“Our experience is if you’ve got mild ILD – and if you look hard enough you can find it in many rheumatoids – TNF inhibitor therapy doesn’t affect it. But if there’s any hint of deterioration we move away from anti-TNF therapy. Our preference has been for rituximab,” he said.
Dr. Emery was senior author of the largest study to date of rituximab in patients with RA-ILD (Rheumatology [Oxford]. 2017 Aug 1;56[8]:1348-57). While he and his coinvestigators concluded that rituximab “appears to be an acceptable therapeutic choice for patients with RA-ILD,” Dr. Fischer didn’t find persuasive evidence from this relatively small retrospective observational study in which only 44 patients had lung data available.
“My own conclusion is evidence is lacking to support a role of rituximab for treating ILD in RA,” Dr. Fischer said.
He reported receiving research grants from Boehringer-Ingelheim and Corbus and serving as a consultant to a handful of other pharmaceutical companies.
MAUI, HAWAII – The latest practice guidelines on the diagnosis of interstitial lung disease issued by the American Thoracic Society and allied organizations recommend as the standard of care a review of all cases by a multidisciplinary team consisting of a pulmonologist, radiologist, and pathologist to ensure accurate diagnosis and classification.
That’s not good enough. A rheumatologist needs to routinely be involved in those multidisciplinary discussions as well, Aryeh Fischer, MD, asserted at the 2019 Rheumatology Winter Clinical Symposium.
Why? Because a rheumatologist’s input often leads to a change in diagnosis. And that change can have important prognostic and therapeutic implications.
“We want to distinguish the IPF [idiopathic pulmonary fibrosis] patients from everybody else. The most important thing with regards to therapy is to identify the IPF patient. The IPF patients are the only ones who are able to be treated with antifibrotic agents: pirfenidone or nintedanib. And we know that immunosuppression can make patients with IPF worse; their risks of hospitalization and mortality are higher on immunosuppression. But everybody else, including anyone with any of the autoimmune diseases along with ILD [interstitial lung disease] or any other causes of ILD, gets treated with immunosuppression,” explained Dr. Fischer, a rheumatologist with a special interest in autoimmune lung disease at the University of Colorado, Denver.
He cited a recent prospective blinded study in which 60 newly diagnosed ILD patients were evaluated separately by a multidisciplinary team comprising a pulmonologist, radiologist, and pathologist and once again with the involvement of a rheumatologist. The rheumatologic assessment reclassified 21% of patients from IPF – that is, lung disease unrelated to connective tissue disease (CTD) or exposure to asbestos, bird droppings, or other triggers – to ILD with connective tissue disease (CTD-ILD). And the number of patients classified as having ILD with autoimmune features without meeting full diagnostic criteria for a major CTD, a category that includes antisynthetase syndrome and IgG4-related ILD, jumped by 77%. Also, the investigators determined that adding a rheumatologist to the multidisciplinary team would have resulted in seven fewer bronchoscopies and one less surgical biopsy among this 60-patient cohort (J Rheumatol. 2018 Nov;45[11]:1509-14).
It’s not at all uncommon to identify a new occult CTD in patients presenting with ILD. Dr. Fischer noted that in a series of 114 consecutive patients evaluated at the interdisciplinary ILD program at Johns Hopkins University, Baltimore, 30% of them had a well-defined CTD, half of whom, or 15% of the total, were newly diagnosed with CTD by virtue of their ILD evaluation (Respir Med. 2009 Aug;103[8]:1152-8).
A CTD-ILD diagnosis has prognostic implications: Survival is significantly better than with IPF, with the exception of ILD with rheumatoid arthritis (RA-ILD), where the prognosis seems to be worse than for other forms of CTD-ILD and is more akin to that of IPF. Indeed, the risk of death is threefold higher in patients with RA-ILD than in those with RA without ILD. While the predominant pattern of lung injury in RA-ILD is usual interstitial pneumonia, marked by fibrosis and honeycombing, the predominant pattern in all other forms of CTD-ILD is nonspecific interstitial pneumonia.
A French study of 778 consecutive patients with ILD highlighted how common autoimmune disease is in the ILD population. Nearly one-third of the ILD patients had autoimmune disease: 22% had CTD-ILD and another 7% had interstitial pneumonia with autoimmune features, or IPAF (Respir Med. 2017 Feb;123:56-62).
Dr. Fischer was lead author of a report by a European Respiratory Society/American Thoracic Society task force that first put forth the term IPAF to describe ILD patients with subtle serologic, clinical, and/or morphologic findings that don’t rise to the level required for formal diagnosis of a defined CTD (Eur Respir J. 2015 Oct;46[4]:976-87).
IPAF is a research construct. Patients who fall into this category are the focus of ongoing prospective studies aimed at better understanding their prognosis and appropriate treatment.
The big three autoimmune diseases comorbid with ILD as reported by Dr. Fischer and coinvestigators in a study of 237 patients with an autoimmune phenotype in a Denver ILD clinic were scleroderma, present in 37%; rheumatoid arthritis, present in 18%; and myositis, with a prevalence of 11%. Another 24% had IPAF.
“There was surprisingly little SLE [3%], and not much Sjögren’s [6%],” he observed.
When pulmonologists come knocking on Dr. Fischer’s door asking if their patients with ILD have occult CTD, he finds it helpful to look for quantifiable specific extrathoracic features suggestive of rheumatologic disease, such as Raynaud’s, sclerodactyly, mechanic hands, and Gottron’s papules.
The ILD practice guidelines recently issued jointly by the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society recommend that serologic evaluation for a long list of autoantibodies “should be performed even in the absence of signs or symptoms of connective tissue disease” (Am J Respir Crit Care Med. 2018 Sep 1;198[5]:e44-e68). Dr. Fischer indicated he has a problem with that recommendation since nonrheumatologists aren’t typically adept in interpreting the significance of autoantibodies.
“I will just tell you, I see a lot of patients for ILD evaluation, and autoimmune serologies often lead to more questions than answers. They always need to be considered within the clinical context,” the rheumatologist said.
High-resolution CT (HRCT) is the imaging method of choice for detecting ILD and classifying its severity. HRCT also holds clues for detection of CTD-ILD.
“There are no upper lung–predominant ILDs that really make you think CTD. Our diseases like the lower lung zones. We like to see multicompartment involvement. When you hear ‘airways and parenchymal,’ ‘pleural,’ ‘pericardial thickening,’ that sounds a lot like autoimmune disease,” according to Dr. Fischer.
Also, the presence of a dilated esophagus on HRCT is strongly suggestive of scleroderma, he added.
In the event a pathology report is available, it’s important to read the fine print. Secondary histopathologic features of CTD-ILD include extensive pleuritis, lymphoid aggregates with germinal center formation, dense perivascular collagen, and/or prominent plasmacytic infiltration.
While ILD can be the first manifestation of an underlying occult CTD, it’s also common for a patient with an established CTD to subsequently develop ILD.
Rheumatologists are no strangers to ambiguity, so it should come as no surprise that while audible bibasilar crackles on physical examination are strongly predictive of ILD, their absence doesn’t indicate absence of ILD. Similarly, dyspnea in a patient with established CTD can be tough to interpret, and absence of dyspnea doesn’t imply absence of ILD. If a patient with CTD is on immunosuppressive therapy, bronchoalveolar lavage is of value in ruling out infection.
When to order a surgical lung biopsy
“If you’re pretty sure your patient had a CTD and then you get a characteristic HRCT and there are no exposures to account for the imaging findings – if we have a scenario where it all fits – we often don’t biopsy. Biopsy usually means a 2-night hospital stay, and it’s reportedly associated with about a 1% mortality risk. And the clinical reality is the biopsy may not impact treatment. They’re going to give you azathioprine, mycophenolate, cyclophosphamide, or prednisone for the ILD and the extrathoracic disease irrespective of the ILD pattern,” the rheumatologist said.
He reserves surgical biopsy for patients with an atypical HRCT, those with known CTD and a possible alternative etiology for the ILD, such as exposure to asbestos or owning pet birds, and patients where he’s just not sure a CTD is actually present.
Treatment of CTD-ILD
“The available controlled efficacy data are limited to scleroderma-ILD, where cyclophosphamide and mycophenolate work a little bit. And that’s it. We don’t have good data to guide us on which agent for which CTD or ILD pattern, for how long, or what dose,” Dr. Fischer said. “We have good drugs for the joints, nothing for the lungs. Treatment is not evidence based. We initiate with high-dose steroids, switch to a steroid-sparing agent, we evaluate the response to treatment with surveillance every 3-6 months by 6-minute walking, lung function tests, and sometimes imaging, and we treat for a long time. Oftentimes stability equals success.”
That being said, it must be emphasized that not all CTD-ILD warrants treatment of the pulmonary disease. If the patient’s ILD is mild and indolent, clinical surveillance is often appropriate, he continued.
More important than the pharmacotherapy available at present are adjunctive nonpharmacologic approaches: supplemental oxygen, pulmonary rehabilitation, treatment of comorbid GERD, immunizations, and addressing mental health issues related to these devastating diseases.
“We really don’t do these things well,” Dr. Fischer said.
On the horizon
An investigator-driven phase 2 clinical trial of the antifibrotic drug pirfenidone (Esbriet), now approved for IPF, is underway in patients with RA-ILD. Results of a trial of antifibrotic therapy in patients with scleroderma-ILD are due to be presented this year at EULAR. And a small study of antifibrotic therapy in patients with myositis-ILD is ongoing.
As for the biologics, there is a signal in the literature that tumor necrosis factor inhibitors may be associated with increased risk of rapidly progressive lung disease in patients with RA-ILD. An influential report from the British Society for Rheumatology Biologics Register on 299 patients with preexisting RA-ILD treated with anti-TNF therapy and 68 who received traditional disease-modifying antirrheumatic drugs showed that while mortality during follow-up was similar in the two groups, the proportion of deaths attributed to RA-ILD was 21% in the group on anti-TNF therapy, compared with only 7% in those on traditional DMARDs (Ann Rheum Dis. 2010 Jun;69[6]:1086-91).
Paul Emery, MD, rose from the audience at Dr. Fischer’s request to share his extensive experience with anti-TNF therapy and rituximab (Rituxan) in the setting of RA-ILD. When he was involved in several of the pivotal clinical trials of anti-TNF agents for RA he encountered a couple of cases of rapidly progressive ILD in patients on treatment.
“We had never before seen rapidly progressive ILD that didn’t respond to cyclophosphamide. Both patients died,” recalled Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
“Our experience is if you’ve got mild ILD – and if you look hard enough you can find it in many rheumatoids – TNF inhibitor therapy doesn’t affect it. But if there’s any hint of deterioration we move away from anti-TNF therapy. Our preference has been for rituximab,” he said.
Dr. Emery was senior author of the largest study to date of rituximab in patients with RA-ILD (Rheumatology [Oxford]. 2017 Aug 1;56[8]:1348-57). While he and his coinvestigators concluded that rituximab “appears to be an acceptable therapeutic choice for patients with RA-ILD,” Dr. Fischer didn’t find persuasive evidence from this relatively small retrospective observational study in which only 44 patients had lung data available.
“My own conclusion is evidence is lacking to support a role of rituximab for treating ILD in RA,” Dr. Fischer said.
He reported receiving research grants from Boehringer-Ingelheim and Corbus and serving as a consultant to a handful of other pharmaceutical companies.
MAUI, HAWAII – The latest practice guidelines on the diagnosis of interstitial lung disease issued by the American Thoracic Society and allied organizations recommend as the standard of care a review of all cases by a multidisciplinary team consisting of a pulmonologist, radiologist, and pathologist to ensure accurate diagnosis and classification.
That’s not good enough. A rheumatologist needs to routinely be involved in those multidisciplinary discussions as well, Aryeh Fischer, MD, asserted at the 2019 Rheumatology Winter Clinical Symposium.
Why? Because a rheumatologist’s input often leads to a change in diagnosis. And that change can have important prognostic and therapeutic implications.
“We want to distinguish the IPF [idiopathic pulmonary fibrosis] patients from everybody else. The most important thing with regards to therapy is to identify the IPF patient. The IPF patients are the only ones who are able to be treated with antifibrotic agents: pirfenidone or nintedanib. And we know that immunosuppression can make patients with IPF worse; their risks of hospitalization and mortality are higher on immunosuppression. But everybody else, including anyone with any of the autoimmune diseases along with ILD [interstitial lung disease] or any other causes of ILD, gets treated with immunosuppression,” explained Dr. Fischer, a rheumatologist with a special interest in autoimmune lung disease at the University of Colorado, Denver.
He cited a recent prospective blinded study in which 60 newly diagnosed ILD patients were evaluated separately by a multidisciplinary team comprising a pulmonologist, radiologist, and pathologist and once again with the involvement of a rheumatologist. The rheumatologic assessment reclassified 21% of patients from IPF – that is, lung disease unrelated to connective tissue disease (CTD) or exposure to asbestos, bird droppings, or other triggers – to ILD with connective tissue disease (CTD-ILD). And the number of patients classified as having ILD with autoimmune features without meeting full diagnostic criteria for a major CTD, a category that includes antisynthetase syndrome and IgG4-related ILD, jumped by 77%. Also, the investigators determined that adding a rheumatologist to the multidisciplinary team would have resulted in seven fewer bronchoscopies and one less surgical biopsy among this 60-patient cohort (J Rheumatol. 2018 Nov;45[11]:1509-14).
It’s not at all uncommon to identify a new occult CTD in patients presenting with ILD. Dr. Fischer noted that in a series of 114 consecutive patients evaluated at the interdisciplinary ILD program at Johns Hopkins University, Baltimore, 30% of them had a well-defined CTD, half of whom, or 15% of the total, were newly diagnosed with CTD by virtue of their ILD evaluation (Respir Med. 2009 Aug;103[8]:1152-8).
A CTD-ILD diagnosis has prognostic implications: Survival is significantly better than with IPF, with the exception of ILD with rheumatoid arthritis (RA-ILD), where the prognosis seems to be worse than for other forms of CTD-ILD and is more akin to that of IPF. Indeed, the risk of death is threefold higher in patients with RA-ILD than in those with RA without ILD. While the predominant pattern of lung injury in RA-ILD is usual interstitial pneumonia, marked by fibrosis and honeycombing, the predominant pattern in all other forms of CTD-ILD is nonspecific interstitial pneumonia.
A French study of 778 consecutive patients with ILD highlighted how common autoimmune disease is in the ILD population. Nearly one-third of the ILD patients had autoimmune disease: 22% had CTD-ILD and another 7% had interstitial pneumonia with autoimmune features, or IPAF (Respir Med. 2017 Feb;123:56-62).
Dr. Fischer was lead author of a report by a European Respiratory Society/American Thoracic Society task force that first put forth the term IPAF to describe ILD patients with subtle serologic, clinical, and/or morphologic findings that don’t rise to the level required for formal diagnosis of a defined CTD (Eur Respir J. 2015 Oct;46[4]:976-87).
IPAF is a research construct. Patients who fall into this category are the focus of ongoing prospective studies aimed at better understanding their prognosis and appropriate treatment.
The big three autoimmune diseases comorbid with ILD as reported by Dr. Fischer and coinvestigators in a study of 237 patients with an autoimmune phenotype in a Denver ILD clinic were scleroderma, present in 37%; rheumatoid arthritis, present in 18%; and myositis, with a prevalence of 11%. Another 24% had IPAF.
“There was surprisingly little SLE [3%], and not much Sjögren’s [6%],” he observed.
When pulmonologists come knocking on Dr. Fischer’s door asking if their patients with ILD have occult CTD, he finds it helpful to look for quantifiable specific extrathoracic features suggestive of rheumatologic disease, such as Raynaud’s, sclerodactyly, mechanic hands, and Gottron’s papules.
The ILD practice guidelines recently issued jointly by the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society recommend that serologic evaluation for a long list of autoantibodies “should be performed even in the absence of signs or symptoms of connective tissue disease” (Am J Respir Crit Care Med. 2018 Sep 1;198[5]:e44-e68). Dr. Fischer indicated he has a problem with that recommendation since nonrheumatologists aren’t typically adept in interpreting the significance of autoantibodies.
“I will just tell you, I see a lot of patients for ILD evaluation, and autoimmune serologies often lead to more questions than answers. They always need to be considered within the clinical context,” the rheumatologist said.
High-resolution CT (HRCT) is the imaging method of choice for detecting ILD and classifying its severity. HRCT also holds clues for detection of CTD-ILD.
“There are no upper lung–predominant ILDs that really make you think CTD. Our diseases like the lower lung zones. We like to see multicompartment involvement. When you hear ‘airways and parenchymal,’ ‘pleural,’ ‘pericardial thickening,’ that sounds a lot like autoimmune disease,” according to Dr. Fischer.
Also, the presence of a dilated esophagus on HRCT is strongly suggestive of scleroderma, he added.
In the event a pathology report is available, it’s important to read the fine print. Secondary histopathologic features of CTD-ILD include extensive pleuritis, lymphoid aggregates with germinal center formation, dense perivascular collagen, and/or prominent plasmacytic infiltration.
While ILD can be the first manifestation of an underlying occult CTD, it’s also common for a patient with an established CTD to subsequently develop ILD.
Rheumatologists are no strangers to ambiguity, so it should come as no surprise that while audible bibasilar crackles on physical examination are strongly predictive of ILD, their absence doesn’t indicate absence of ILD. Similarly, dyspnea in a patient with established CTD can be tough to interpret, and absence of dyspnea doesn’t imply absence of ILD. If a patient with CTD is on immunosuppressive therapy, bronchoalveolar lavage is of value in ruling out infection.
When to order a surgical lung biopsy
“If you’re pretty sure your patient had a CTD and then you get a characteristic HRCT and there are no exposures to account for the imaging findings – if we have a scenario where it all fits – we often don’t biopsy. Biopsy usually means a 2-night hospital stay, and it’s reportedly associated with about a 1% mortality risk. And the clinical reality is the biopsy may not impact treatment. They’re going to give you azathioprine, mycophenolate, cyclophosphamide, or prednisone for the ILD and the extrathoracic disease irrespective of the ILD pattern,” the rheumatologist said.
He reserves surgical biopsy for patients with an atypical HRCT, those with known CTD and a possible alternative etiology for the ILD, such as exposure to asbestos or owning pet birds, and patients where he’s just not sure a CTD is actually present.
Treatment of CTD-ILD
“The available controlled efficacy data are limited to scleroderma-ILD, where cyclophosphamide and mycophenolate work a little bit. And that’s it. We don’t have good data to guide us on which agent for which CTD or ILD pattern, for how long, or what dose,” Dr. Fischer said. “We have good drugs for the joints, nothing for the lungs. Treatment is not evidence based. We initiate with high-dose steroids, switch to a steroid-sparing agent, we evaluate the response to treatment with surveillance every 3-6 months by 6-minute walking, lung function tests, and sometimes imaging, and we treat for a long time. Oftentimes stability equals success.”
That being said, it must be emphasized that not all CTD-ILD warrants treatment of the pulmonary disease. If the patient’s ILD is mild and indolent, clinical surveillance is often appropriate, he continued.
More important than the pharmacotherapy available at present are adjunctive nonpharmacologic approaches: supplemental oxygen, pulmonary rehabilitation, treatment of comorbid GERD, immunizations, and addressing mental health issues related to these devastating diseases.
“We really don’t do these things well,” Dr. Fischer said.
On the horizon
An investigator-driven phase 2 clinical trial of the antifibrotic drug pirfenidone (Esbriet), now approved for IPF, is underway in patients with RA-ILD. Results of a trial of antifibrotic therapy in patients with scleroderma-ILD are due to be presented this year at EULAR. And a small study of antifibrotic therapy in patients with myositis-ILD is ongoing.
As for the biologics, there is a signal in the literature that tumor necrosis factor inhibitors may be associated with increased risk of rapidly progressive lung disease in patients with RA-ILD. An influential report from the British Society for Rheumatology Biologics Register on 299 patients with preexisting RA-ILD treated with anti-TNF therapy and 68 who received traditional disease-modifying antirrheumatic drugs showed that while mortality during follow-up was similar in the two groups, the proportion of deaths attributed to RA-ILD was 21% in the group on anti-TNF therapy, compared with only 7% in those on traditional DMARDs (Ann Rheum Dis. 2010 Jun;69[6]:1086-91).
Paul Emery, MD, rose from the audience at Dr. Fischer’s request to share his extensive experience with anti-TNF therapy and rituximab (Rituxan) in the setting of RA-ILD. When he was involved in several of the pivotal clinical trials of anti-TNF agents for RA he encountered a couple of cases of rapidly progressive ILD in patients on treatment.
“We had never before seen rapidly progressive ILD that didn’t respond to cyclophosphamide. Both patients died,” recalled Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
“Our experience is if you’ve got mild ILD – and if you look hard enough you can find it in many rheumatoids – TNF inhibitor therapy doesn’t affect it. But if there’s any hint of deterioration we move away from anti-TNF therapy. Our preference has been for rituximab,” he said.
Dr. Emery was senior author of the largest study to date of rituximab in patients with RA-ILD (Rheumatology [Oxford]. 2017 Aug 1;56[8]:1348-57). While he and his coinvestigators concluded that rituximab “appears to be an acceptable therapeutic choice for patients with RA-ILD,” Dr. Fischer didn’t find persuasive evidence from this relatively small retrospective observational study in which only 44 patients had lung data available.
“My own conclusion is evidence is lacking to support a role of rituximab for treating ILD in RA,” Dr. Fischer said.
He reported receiving research grants from Boehringer-Ingelheim and Corbus and serving as a consultant to a handful of other pharmaceutical companies.
REPORTING FROM RWCS 2019
Hospitalists on the Hill
Advocating for HM in DC
Another Hill Day is coming – the all-day advocacy event on Capitol Hill is scheduled in conjunction with the Society of Hospital Medicine’s Annual Conference whenever it is held in Washington, DC. In 2019, Hill Day will take place on March 27, the final day of HM19.
This will be the fourth Hill Day, and the last for some time, said Ron Greeno, MD, FCCP, MHM, senior advisor for government affairs at SHM and the society’s immediate past president. For at least the next 5 years, SHM’s annual conferences won’t be held in Washington, so there will not be any opportunities to plan a Hill Day during that time. “Members may want to take advantage of this opportunity,” Dr. Greeno said. “The people who do this never forget it.”
How Hill Day works
Sign up for Hill Day and you’ll spend a day visiting legislators and their health care staffers to educate them on what hospital medicine is, what a hospitalist does, and some of the pressing issues that affect the profession, said Joshua Lenchus, DO, RPh, FACP, SFHM, chair of the SHM Public Policy Committee. “We try to leverage participants’ work and home addresses to pair them up with legislators from that area. Some hospitalists have personal or professional relationships with some of the legislators, and even if they’re not in their area, we’ll try to leverage that. And for people who have expertise in a particular topic, we try to arrange an audience with a member of Congress who may be promoting or sponsoring a bill related to that.”
Hill Day volunteers will attend an orientation to learn more about what the day will look like and what they’ll be talking about in their meetings. “We’ll only have time to cover one or two issues, and we’re in the process now of choosing the issues we want to address. We orient participants on those subjects so everybody is kind of saying the same thing,” Dr. Greeno said. “People shouldn’t be afraid of not being conversant with the issues because we do sufficient orientation that everybody gets comfortable enough to do a good job.”
Registration for Hill Day is happening online now. HM19 attendees can register at https://s1.goeshow.com/shm/annual/2019/registration_form.cfm.
“We beg people: If you sign up, show up, because we have many more people trying to participate than we can accommodate,” Dr. Greeno said. “If you change your mind, that’s fine because we have a waiting list, but please let us know because somebody else wants to take your place.”
The purpose of Hill Day
Educating legislators and their health care staff is the goal of the day, and it’s an important job. “Hospital medicine is still a relatively new field,” Dr. Lenchus said. “There are a fair number of legislators who still don’t know what a hospitalist is or what hospital medicine is. Part of our visits is always to educate them about what we do and what our impact is on the health care landscape of the country.” He added that educating Hill staff about the most pressing issues is another primary goal.
“Finally, and this is what separates us from other organizations that do legislative advocacy, we try to leave them with the idea that we’re here to help,” Dr. Lenchus said. “If there’s an issue or a particular bill that we’re asking them to sponsor or cosponsor, that’s one part of a visit. But by and large, we are trying to leave them with the sense that SHM is a resource when it comes to health care–related issues. We want to be there for legislators so that they can understand our position accurately from the outset.”
In short, Hill Day offers a rare opportunity to have direct access to the people who are voting on new legislation affecting hospitalists and affecting the implementation of existing legislation. “This is where the rubber meets the road,” Dr. Lenchus said. Each time a Hill Day is held, he noted, attendance increases. “That’s a true testament to the level of involvement and the interest that hospitalists have across the country. If you’re at all interested, you should absolutely sign up. This will be an amazing experience.”
The lasting impact
Though it’s just one day, Hill Day’s effects are significant.
“Before I started doing this work, I often thought, ‘What impact could someone have going into a legislator’s office?’ ” Dr. Greeno said. “But the answer is ‘A lot.’ The members and staff really do listen – especially if an advocate is highly educated and represent what legislators consider an important constituency, like health care providers. Health care is a hot topic, and it’s probably going to be one of the hot topics in the next election. Hospitalists have good ideas, and as a result these meetings are extremely influential; we wouldn’t do it otherwise. It is fun, but we’re not doing it for fun. We’re doing it because we know we can make a difference.”
In fact, in terms of impact on Capitol Hill, SHM punches above its weight, he added.
“We’re a relatively new society; we’re not huge. There are lots of societies that are much bigger than us and have many more resources, but people on the Hill have told us they like talking with us because they know we’re not looking at things the same way,” Dr. Greeno revealed. “We’re trying to help, and the issues that we’re addressing are not necessarily self-serving. We’re not saying, ‘You need to do this because it will make more money for our doctors.’ Instead, we’re saying, ‘You need to do this because the way it’s being done now is hurting patients. It’s hurting the health care system, and we have ideas about how to make that better.’ ”
SHM’s impressive track record has earned the society a positive reputation that will underlie the Hill Day meetings. “When we first set up the policy shop at SHM, we wanted to be seen as providers who cared about the American health care system and our patients,” Dr. Greeno said. “We have established that reputation, and that has led members on Capitol Hill to recognize us as being well intentioned and knowledgeable. So we have an outsize influence in Congress for our age and our size. When 200 hospitalists go to Capitol Hill, it’s an important thing.”
For more information about Hill Day, including details about participation, visit shmannualconference.org/hill-day/.
Advocating for HM in DC
Advocating for HM in DC
Another Hill Day is coming – the all-day advocacy event on Capitol Hill is scheduled in conjunction with the Society of Hospital Medicine’s Annual Conference whenever it is held in Washington, DC. In 2019, Hill Day will take place on March 27, the final day of HM19.
This will be the fourth Hill Day, and the last for some time, said Ron Greeno, MD, FCCP, MHM, senior advisor for government affairs at SHM and the society’s immediate past president. For at least the next 5 years, SHM’s annual conferences won’t be held in Washington, so there will not be any opportunities to plan a Hill Day during that time. “Members may want to take advantage of this opportunity,” Dr. Greeno said. “The people who do this never forget it.”
How Hill Day works
Sign up for Hill Day and you’ll spend a day visiting legislators and their health care staffers to educate them on what hospital medicine is, what a hospitalist does, and some of the pressing issues that affect the profession, said Joshua Lenchus, DO, RPh, FACP, SFHM, chair of the SHM Public Policy Committee. “We try to leverage participants’ work and home addresses to pair them up with legislators from that area. Some hospitalists have personal or professional relationships with some of the legislators, and even if they’re not in their area, we’ll try to leverage that. And for people who have expertise in a particular topic, we try to arrange an audience with a member of Congress who may be promoting or sponsoring a bill related to that.”
Hill Day volunteers will attend an orientation to learn more about what the day will look like and what they’ll be talking about in their meetings. “We’ll only have time to cover one or two issues, and we’re in the process now of choosing the issues we want to address. We orient participants on those subjects so everybody is kind of saying the same thing,” Dr. Greeno said. “People shouldn’t be afraid of not being conversant with the issues because we do sufficient orientation that everybody gets comfortable enough to do a good job.”
Registration for Hill Day is happening online now. HM19 attendees can register at https://s1.goeshow.com/shm/annual/2019/registration_form.cfm.
“We beg people: If you sign up, show up, because we have many more people trying to participate than we can accommodate,” Dr. Greeno said. “If you change your mind, that’s fine because we have a waiting list, but please let us know because somebody else wants to take your place.”
The purpose of Hill Day
Educating legislators and their health care staff is the goal of the day, and it’s an important job. “Hospital medicine is still a relatively new field,” Dr. Lenchus said. “There are a fair number of legislators who still don’t know what a hospitalist is or what hospital medicine is. Part of our visits is always to educate them about what we do and what our impact is on the health care landscape of the country.” He added that educating Hill staff about the most pressing issues is another primary goal.
“Finally, and this is what separates us from other organizations that do legislative advocacy, we try to leave them with the idea that we’re here to help,” Dr. Lenchus said. “If there’s an issue or a particular bill that we’re asking them to sponsor or cosponsor, that’s one part of a visit. But by and large, we are trying to leave them with the sense that SHM is a resource when it comes to health care–related issues. We want to be there for legislators so that they can understand our position accurately from the outset.”
In short, Hill Day offers a rare opportunity to have direct access to the people who are voting on new legislation affecting hospitalists and affecting the implementation of existing legislation. “This is where the rubber meets the road,” Dr. Lenchus said. Each time a Hill Day is held, he noted, attendance increases. “That’s a true testament to the level of involvement and the interest that hospitalists have across the country. If you’re at all interested, you should absolutely sign up. This will be an amazing experience.”
The lasting impact
Though it’s just one day, Hill Day’s effects are significant.
“Before I started doing this work, I often thought, ‘What impact could someone have going into a legislator’s office?’ ” Dr. Greeno said. “But the answer is ‘A lot.’ The members and staff really do listen – especially if an advocate is highly educated and represent what legislators consider an important constituency, like health care providers. Health care is a hot topic, and it’s probably going to be one of the hot topics in the next election. Hospitalists have good ideas, and as a result these meetings are extremely influential; we wouldn’t do it otherwise. It is fun, but we’re not doing it for fun. We’re doing it because we know we can make a difference.”
In fact, in terms of impact on Capitol Hill, SHM punches above its weight, he added.
“We’re a relatively new society; we’re not huge. There are lots of societies that are much bigger than us and have many more resources, but people on the Hill have told us they like talking with us because they know we’re not looking at things the same way,” Dr. Greeno revealed. “We’re trying to help, and the issues that we’re addressing are not necessarily self-serving. We’re not saying, ‘You need to do this because it will make more money for our doctors.’ Instead, we’re saying, ‘You need to do this because the way it’s being done now is hurting patients. It’s hurting the health care system, and we have ideas about how to make that better.’ ”
SHM’s impressive track record has earned the society a positive reputation that will underlie the Hill Day meetings. “When we first set up the policy shop at SHM, we wanted to be seen as providers who cared about the American health care system and our patients,” Dr. Greeno said. “We have established that reputation, and that has led members on Capitol Hill to recognize us as being well intentioned and knowledgeable. So we have an outsize influence in Congress for our age and our size. When 200 hospitalists go to Capitol Hill, it’s an important thing.”
For more information about Hill Day, including details about participation, visit shmannualconference.org/hill-day/.
Another Hill Day is coming – the all-day advocacy event on Capitol Hill is scheduled in conjunction with the Society of Hospital Medicine’s Annual Conference whenever it is held in Washington, DC. In 2019, Hill Day will take place on March 27, the final day of HM19.
This will be the fourth Hill Day, and the last for some time, said Ron Greeno, MD, FCCP, MHM, senior advisor for government affairs at SHM and the society’s immediate past president. For at least the next 5 years, SHM’s annual conferences won’t be held in Washington, so there will not be any opportunities to plan a Hill Day during that time. “Members may want to take advantage of this opportunity,” Dr. Greeno said. “The people who do this never forget it.”
How Hill Day works
Sign up for Hill Day and you’ll spend a day visiting legislators and their health care staffers to educate them on what hospital medicine is, what a hospitalist does, and some of the pressing issues that affect the profession, said Joshua Lenchus, DO, RPh, FACP, SFHM, chair of the SHM Public Policy Committee. “We try to leverage participants’ work and home addresses to pair them up with legislators from that area. Some hospitalists have personal or professional relationships with some of the legislators, and even if they’re not in their area, we’ll try to leverage that. And for people who have expertise in a particular topic, we try to arrange an audience with a member of Congress who may be promoting or sponsoring a bill related to that.”
Hill Day volunteers will attend an orientation to learn more about what the day will look like and what they’ll be talking about in their meetings. “We’ll only have time to cover one or two issues, and we’re in the process now of choosing the issues we want to address. We orient participants on those subjects so everybody is kind of saying the same thing,” Dr. Greeno said. “People shouldn’t be afraid of not being conversant with the issues because we do sufficient orientation that everybody gets comfortable enough to do a good job.”
Registration for Hill Day is happening online now. HM19 attendees can register at https://s1.goeshow.com/shm/annual/2019/registration_form.cfm.
“We beg people: If you sign up, show up, because we have many more people trying to participate than we can accommodate,” Dr. Greeno said. “If you change your mind, that’s fine because we have a waiting list, but please let us know because somebody else wants to take your place.”
The purpose of Hill Day
Educating legislators and their health care staff is the goal of the day, and it’s an important job. “Hospital medicine is still a relatively new field,” Dr. Lenchus said. “There are a fair number of legislators who still don’t know what a hospitalist is or what hospital medicine is. Part of our visits is always to educate them about what we do and what our impact is on the health care landscape of the country.” He added that educating Hill staff about the most pressing issues is another primary goal.
“Finally, and this is what separates us from other organizations that do legislative advocacy, we try to leave them with the idea that we’re here to help,” Dr. Lenchus said. “If there’s an issue or a particular bill that we’re asking them to sponsor or cosponsor, that’s one part of a visit. But by and large, we are trying to leave them with the sense that SHM is a resource when it comes to health care–related issues. We want to be there for legislators so that they can understand our position accurately from the outset.”
In short, Hill Day offers a rare opportunity to have direct access to the people who are voting on new legislation affecting hospitalists and affecting the implementation of existing legislation. “This is where the rubber meets the road,” Dr. Lenchus said. Each time a Hill Day is held, he noted, attendance increases. “That’s a true testament to the level of involvement and the interest that hospitalists have across the country. If you’re at all interested, you should absolutely sign up. This will be an amazing experience.”
The lasting impact
Though it’s just one day, Hill Day’s effects are significant.
“Before I started doing this work, I often thought, ‘What impact could someone have going into a legislator’s office?’ ” Dr. Greeno said. “But the answer is ‘A lot.’ The members and staff really do listen – especially if an advocate is highly educated and represent what legislators consider an important constituency, like health care providers. Health care is a hot topic, and it’s probably going to be one of the hot topics in the next election. Hospitalists have good ideas, and as a result these meetings are extremely influential; we wouldn’t do it otherwise. It is fun, but we’re not doing it for fun. We’re doing it because we know we can make a difference.”
In fact, in terms of impact on Capitol Hill, SHM punches above its weight, he added.
“We’re a relatively new society; we’re not huge. There are lots of societies that are much bigger than us and have many more resources, but people on the Hill have told us they like talking with us because they know we’re not looking at things the same way,” Dr. Greeno revealed. “We’re trying to help, and the issues that we’re addressing are not necessarily self-serving. We’re not saying, ‘You need to do this because it will make more money for our doctors.’ Instead, we’re saying, ‘You need to do this because the way it’s being done now is hurting patients. It’s hurting the health care system, and we have ideas about how to make that better.’ ”
SHM’s impressive track record has earned the society a positive reputation that will underlie the Hill Day meetings. “When we first set up the policy shop at SHM, we wanted to be seen as providers who cared about the American health care system and our patients,” Dr. Greeno said. “We have established that reputation, and that has led members on Capitol Hill to recognize us as being well intentioned and knowledgeable. So we have an outsize influence in Congress for our age and our size. When 200 hospitalists go to Capitol Hill, it’s an important thing.”
For more information about Hill Day, including details about participation, visit shmannualconference.org/hill-day/.
Recurrence of Elevated Intracranial Pressure Following Tetracycline Antibiotic Use
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
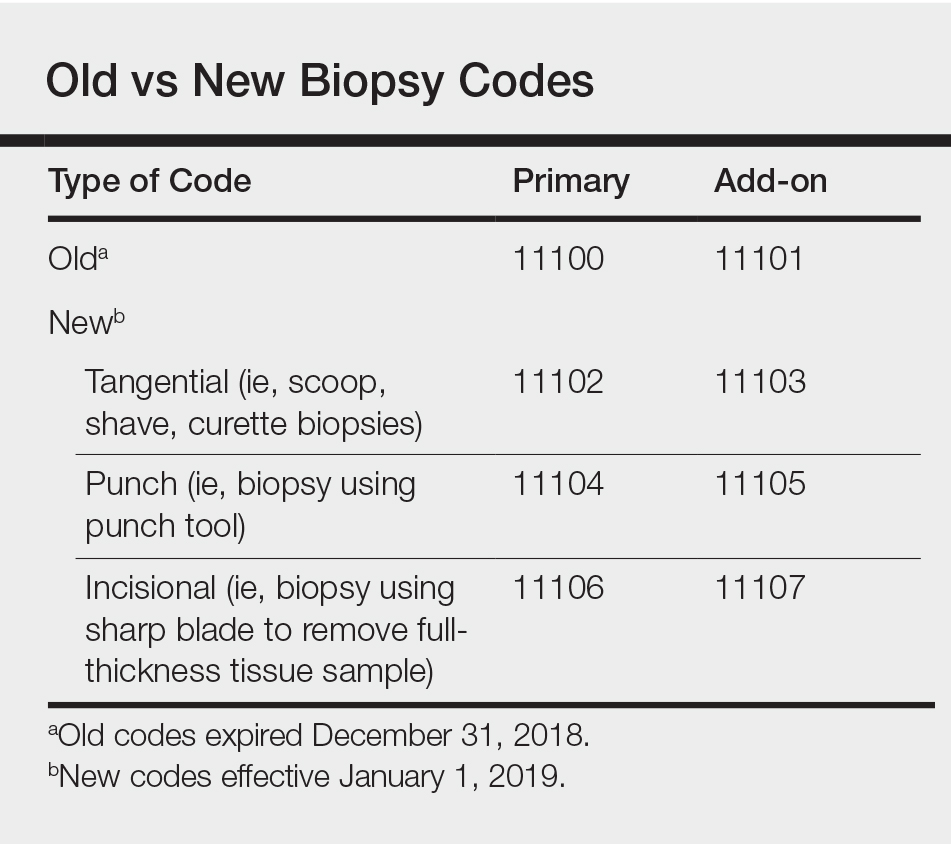
A Closer Look at the New Biopsy Codes
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.
Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.
Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.
Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.
Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.
Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32
Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.
Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.
Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32
Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.
Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.
Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32
Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Practice Points
- Diffuse dermal angiomatosis is commonly reported in patients with hypoxic comorbidities such as smoking or vascular disease as well as in women with large pendulous breasts.
- Effective treatments include control of comorbidities, revascularization, withdrawal of the offending agent, steroids, and isotretinoin.
Better epilepsy outcomes with stereoelectroencephalography versus subdural electrode
Robotic stereoelectroencephalography for the localization of seizures in epilepsy is associated with less procedural morbidity, such as infection and hemorrhage, and better outcomes, compared with subdural electrode implantation.
A paper published in JAMA Neurology detailed the results of a retrospective cohort study of the outcomes of 239 patients with medically intractable epilepsy, 121 of whom underwent stereoelectroencephalography (SEEG) and 139 underwent subdural electrode (SDE) implantation.
The authors noted a significant difference between the two groups in complication rates. There were seven symptomatic hemorrhagic sequelae and three infections related to SDE implantation, and in one of these cases the patient suffered long-term neurologic consequences.
In contrast, there were no symptomatic complications in the SEEG cohort, although two patients were found to have small asymptomatic subdural hematomas that were spotted on CT scans.
Patients in the implantation group also received significantly more narcotics and had a much higher rate of perioperative blood transfusions (13.7% vs. 0.8%, P less than .001), compared with those in the SEEG group.
The study also looked at epilepsy outcomes in the two groups. Significantly more of the patients who had subdural electrodes underwent resection or ablative surgery with laser interstitial thermal therapy, compared with the patients who had SEEG (91.4% vs. 74.4%, P less than .001).
“Thus, the SEEG and SDE cohorts were significantly different in the proportions of cases that were lesional, suggesting that these modalities were used to evaluate somewhat different populations, although the same group of physicians at the same center managed and referred these cases,” wrote Nitin Tandon, MD, from the University of Texas, Houston, and his coauthors. “However, this shift in the patient pool would be expected to bias outcomes against SEEG, because these patients generally have less favorable outcomes.”
Yet the authors saw that a significantly greater proportion of the SEEG patients were free of disabling seizures, compared with the SDE implant group at 6 months (83.9% vs. 66.1%) and 1 year (76% vs. 54.6%) after resection.
To account for the difference between the two groups in the proportion of cases that were lesional, the authors conducted a subgroup analysis of patients with abnormalities on imaging. Again, they saw that a significantly greater proportion of the SEEG patients achieved good outcomes at 6 months and 1 year, compared with the electrode group.
While subdural electrodes have long been the standard approach for delineating epileptogenic zones, the authors wrote that SEEG offers improved coverage and precise targeting of deeper structures. “In addition, the ability of the SEEG method to map distributed epileptic networks involved in epileptic activity has been hypothesized to be responsible for improved outcomes in patients with epilepsy that is difficult to localize.”
They also commented that, in their institution, they saw much greater patient tolerance for SEEG and slightly better outcomes in those patients who underwent resection or ablation.
One author reported a position with a company specializing in outpatient clinical neurophysiological testing services. No other conflicts of interest were reported.
SOURCE: Tandon N et al. JAMA Neurol. 2019 Mar 4. doi: 10.1001/jamaneurol.2019.0098.
Robotic stereoelectroencephalography for the localization of seizures in epilepsy is associated with less procedural morbidity, such as infection and hemorrhage, and better outcomes, compared with subdural electrode implantation.
A paper published in JAMA Neurology detailed the results of a retrospective cohort study of the outcomes of 239 patients with medically intractable epilepsy, 121 of whom underwent stereoelectroencephalography (SEEG) and 139 underwent subdural electrode (SDE) implantation.
The authors noted a significant difference between the two groups in complication rates. There were seven symptomatic hemorrhagic sequelae and three infections related to SDE implantation, and in one of these cases the patient suffered long-term neurologic consequences.
In contrast, there were no symptomatic complications in the SEEG cohort, although two patients were found to have small asymptomatic subdural hematomas that were spotted on CT scans.
Patients in the implantation group also received significantly more narcotics and had a much higher rate of perioperative blood transfusions (13.7% vs. 0.8%, P less than .001), compared with those in the SEEG group.
The study also looked at epilepsy outcomes in the two groups. Significantly more of the patients who had subdural electrodes underwent resection or ablative surgery with laser interstitial thermal therapy, compared with the patients who had SEEG (91.4% vs. 74.4%, P less than .001).
“Thus, the SEEG and SDE cohorts were significantly different in the proportions of cases that were lesional, suggesting that these modalities were used to evaluate somewhat different populations, although the same group of physicians at the same center managed and referred these cases,” wrote Nitin Tandon, MD, from the University of Texas, Houston, and his coauthors. “However, this shift in the patient pool would be expected to bias outcomes against SEEG, because these patients generally have less favorable outcomes.”
Yet the authors saw that a significantly greater proportion of the SEEG patients were free of disabling seizures, compared with the SDE implant group at 6 months (83.9% vs. 66.1%) and 1 year (76% vs. 54.6%) after resection.
To account for the difference between the two groups in the proportion of cases that were lesional, the authors conducted a subgroup analysis of patients with abnormalities on imaging. Again, they saw that a significantly greater proportion of the SEEG patients achieved good outcomes at 6 months and 1 year, compared with the electrode group.
While subdural electrodes have long been the standard approach for delineating epileptogenic zones, the authors wrote that SEEG offers improved coverage and precise targeting of deeper structures. “In addition, the ability of the SEEG method to map distributed epileptic networks involved in epileptic activity has been hypothesized to be responsible for improved outcomes in patients with epilepsy that is difficult to localize.”
They also commented that, in their institution, they saw much greater patient tolerance for SEEG and slightly better outcomes in those patients who underwent resection or ablation.
One author reported a position with a company specializing in outpatient clinical neurophysiological testing services. No other conflicts of interest were reported.
SOURCE: Tandon N et al. JAMA Neurol. 2019 Mar 4. doi: 10.1001/jamaneurol.2019.0098.
Robotic stereoelectroencephalography for the localization of seizures in epilepsy is associated with less procedural morbidity, such as infection and hemorrhage, and better outcomes, compared with subdural electrode implantation.
A paper published in JAMA Neurology detailed the results of a retrospective cohort study of the outcomes of 239 patients with medically intractable epilepsy, 121 of whom underwent stereoelectroencephalography (SEEG) and 139 underwent subdural electrode (SDE) implantation.
The authors noted a significant difference between the two groups in complication rates. There were seven symptomatic hemorrhagic sequelae and three infections related to SDE implantation, and in one of these cases the patient suffered long-term neurologic consequences.
In contrast, there were no symptomatic complications in the SEEG cohort, although two patients were found to have small asymptomatic subdural hematomas that were spotted on CT scans.
Patients in the implantation group also received significantly more narcotics and had a much higher rate of perioperative blood transfusions (13.7% vs. 0.8%, P less than .001), compared with those in the SEEG group.
The study also looked at epilepsy outcomes in the two groups. Significantly more of the patients who had subdural electrodes underwent resection or ablative surgery with laser interstitial thermal therapy, compared with the patients who had SEEG (91.4% vs. 74.4%, P less than .001).
“Thus, the SEEG and SDE cohorts were significantly different in the proportions of cases that were lesional, suggesting that these modalities were used to evaluate somewhat different populations, although the same group of physicians at the same center managed and referred these cases,” wrote Nitin Tandon, MD, from the University of Texas, Houston, and his coauthors. “However, this shift in the patient pool would be expected to bias outcomes against SEEG, because these patients generally have less favorable outcomes.”
Yet the authors saw that a significantly greater proportion of the SEEG patients were free of disabling seizures, compared with the SDE implant group at 6 months (83.9% vs. 66.1%) and 1 year (76% vs. 54.6%) after resection.
To account for the difference between the two groups in the proportion of cases that were lesional, the authors conducted a subgroup analysis of patients with abnormalities on imaging. Again, they saw that a significantly greater proportion of the SEEG patients achieved good outcomes at 6 months and 1 year, compared with the electrode group.
While subdural electrodes have long been the standard approach for delineating epileptogenic zones, the authors wrote that SEEG offers improved coverage and precise targeting of deeper structures. “In addition, the ability of the SEEG method to map distributed epileptic networks involved in epileptic activity has been hypothesized to be responsible for improved outcomes in patients with epilepsy that is difficult to localize.”
They also commented that, in their institution, they saw much greater patient tolerance for SEEG and slightly better outcomes in those patients who underwent resection or ablation.
One author reported a position with a company specializing in outpatient clinical neurophysiological testing services. No other conflicts of interest were reported.
SOURCE: Tandon N et al. JAMA Neurol. 2019 Mar 4. doi: 10.1001/jamaneurol.2019.0098.
FROM JAMA NEUROLOGY
Key clinical point: Stereoelectroencephalography in epilepsy associated with fewer complications than subdural electrode implantation
Major finding: Subdural electrode implantation is associated with significantly more hemorrhagic complications and infections than stereoelectroencephalography.
Study details: A retrospective cohort study in 239 patients with medically intractable epilepsy.
Disclosures: One author reported a position with a company specializing in outpatient clinical neurophysiological testing services. No other conflicts of interest were reported.
Source: Tandon N et al. JAMA Neurol. 2019 Mar 4. doi: 10.1001/jamaneurol.2019.0098.
Achieving recovery not a one-size-fits-all proposition
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
No increased pregnancy loss risk for women conceiving soon after stillbirth
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
FROM THE LANCET
Subscribe to SVS Student Newsletters
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
More Items Added to ‘Spectacular’ Auction
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.
We’re still a few months away from the Vascular Spectacular Gala at VAM, but excitement is growing concurrently with the list of items contributed to the event's live and silent auctions. You’ll definitely leave VAM with new professional connections and inspiration, but perhaps you’ll also take home a one-week stay in Camden, Maine. Or maybe you’ll be anticipating a new charbroiled oyster kit, vouchers to one of the nation's best botanical gardens, jewelry or free registration for next year’s VAM. These are just a few of the items our generous donors have added to our growing list. If you can't make it to the gala or VAM, you can still join in on the fun by donating an item or bidding online. All gala proceeds benefit the SVS Foundation.