User login
Genitourinary endometriosis: Diagnosis and management
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
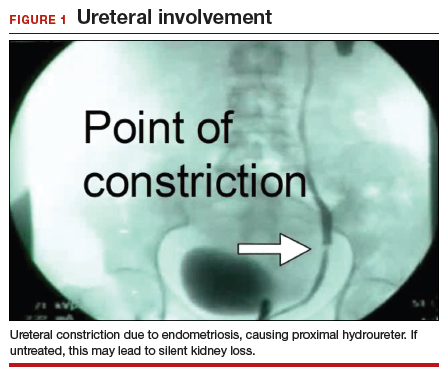
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18
Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
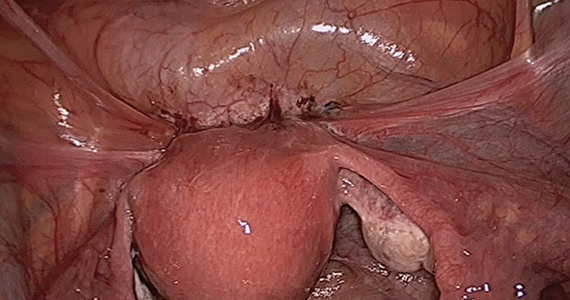
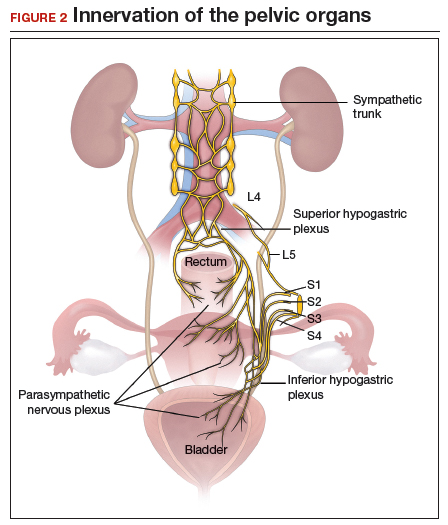
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36
Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
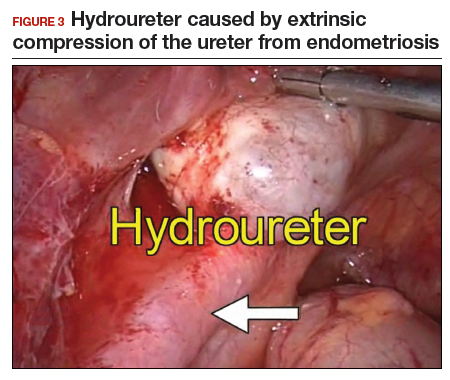
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43
Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
Pregnancy after ventral hernia repair increased the risk for recurrence
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
FROM THE AMERICAN JOURNAL OF SURGERY
Measles cases jumped 30% last week
according to the Centers for Disease Control and Prevention.

Those new cases represent a 30% increase in measles cases for the year, bringing the total to 206 reported to the CDC through Feb. 28. After just 2 months, 2019 has had more cases than all but 3 other years over the last decade, CDC data show.
The 11th state to report a case of measles this year is New Jersey, which joins California, Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New York (three outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC said.
The outbreak in Washington (4 new cases/70 for the year) had been the largest, but the majority of the new cases over the last 2 weeks have occurred in New York City, specifically Brooklyn, which reported 30 cases last week and 17 of the 32 new U.S. cases the week before.
Most of the 120 cases reported in the borough since the beginning of its outbreak in October of 2018 “have involved members of the Jewish Orthodox community. The initial child with measles was unvaccinated and acquired measles on a visit to Israel, where a large outbreak of the disease is occurring. Since then, there have been additional people from Brooklyn and Queens who were unvaccinated and acquired measles while in Israel. People who did not travel were also infected in Brooklyn or Rockland County,” the CDC said.
according to the Centers for Disease Control and Prevention.

Those new cases represent a 30% increase in measles cases for the year, bringing the total to 206 reported to the CDC through Feb. 28. After just 2 months, 2019 has had more cases than all but 3 other years over the last decade, CDC data show.
The 11th state to report a case of measles this year is New Jersey, which joins California, Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New York (three outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC said.
The outbreak in Washington (4 new cases/70 for the year) had been the largest, but the majority of the new cases over the last 2 weeks have occurred in New York City, specifically Brooklyn, which reported 30 cases last week and 17 of the 32 new U.S. cases the week before.
Most of the 120 cases reported in the borough since the beginning of its outbreak in October of 2018 “have involved members of the Jewish Orthodox community. The initial child with measles was unvaccinated and acquired measles on a visit to Israel, where a large outbreak of the disease is occurring. Since then, there have been additional people from Brooklyn and Queens who were unvaccinated and acquired measles while in Israel. People who did not travel were also infected in Brooklyn or Rockland County,” the CDC said.
according to the Centers for Disease Control and Prevention.

Those new cases represent a 30% increase in measles cases for the year, bringing the total to 206 reported to the CDC through Feb. 28. After just 2 months, 2019 has had more cases than all but 3 other years over the last decade, CDC data show.
The 11th state to report a case of measles this year is New Jersey, which joins California, Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New York (three outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC said.
The outbreak in Washington (4 new cases/70 for the year) had been the largest, but the majority of the new cases over the last 2 weeks have occurred in New York City, specifically Brooklyn, which reported 30 cases last week and 17 of the 32 new U.S. cases the week before.
Most of the 120 cases reported in the borough since the beginning of its outbreak in October of 2018 “have involved members of the Jewish Orthodox community. The initial child with measles was unvaccinated and acquired measles on a visit to Israel, where a large outbreak of the disease is occurring. Since then, there have been additional people from Brooklyn and Queens who were unvaccinated and acquired measles while in Israel. People who did not travel were also infected in Brooklyn or Rockland County,” the CDC said.
Point-of-care VL testing improves HIV suppression
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
SEATTLE – Point-of-care viral load testing improves HIV viral suppression and retention in care, according to a randomized trial of 390 subjects in South Africa.
Point-of-care (POC) testing delivers viral load results in about 2-3 hours, as opposed to the month or so patients wait to get results from a laboratory. The nearly instant turnaround gives clinicians the ability to identify patients who aren’t doing well – as indicated by high viral loads despite antiretroviral therapy (ART) – before they walk out the door, so immediate steps can be taken to address adherence or resistance problems.
However, POC viral load testing hasn’t really caught on in the United States, at least not yet, according to study leader Paul Drain, MD, assistant professor of global health at the University of Washington, Seattle.
To see if it would help, the team turned to a large public clinic in the city of Durban, and focused on adults who had been on ART for 6 months following HIV diagnosis. They randomized 195 to standard laboratory testing at study entrance, with a repeat at 6 months, at which point subjects had been on ART for 12 months; 195 others were randomized to POC testing with the Xpert HIV-1 Viral Load machine, from Cepheid, on the same schedule and with same-day counseling for those with high loads.
Treatment was otherwise similar between the groups, with clinic visits every 2 months and other measures as per South African HIV treatment guidelines.
POC testing made a difference. At study month 12,175 participants (89.7%) in the POC arm, but only 148 (75.9%) in the laboratory testing group, had reached the study’s primary endpoint: viral suppression with less than 200 copies/mL plus retention in care, meaning that subjects were still picking up their ART prescriptions.
Overall, POC testing increased viral load suppression by 10.3%, from 83.1% to 93.3% (P = .003) and retention by 7.7% from 84.6% to 92.3% (P = .03).
The investigators would like to evaluate the approach in the United States. Potentially, “POC testing will have a very important role in U.S. health care,” Dr. Drain said at the Conference on Retroviruses and Opportunistic Infections.
It “helps us identify those who are having problems right away, before they leave the clinic, because whether it’s in South Africa or Seattle, as soon as they leave, it’s very hard to get them back. The more you can do POC testing, the better we can intervene and help these people,” he said.
“You don’t need POC testing for everybody; a lot of people do just fine. They take their medications reliably. They don’t need to get their results back right away ... But there are people who have challenges and would benefit from additional adherence counseling” or who might need help overcoming drug resistance. “We want to identify” them quickly; POC testing may be the answer, he said.
The mean age in the study was 33 years, and 60% of the subjects were women. The median CD4 count at baseline was 468 cells/mm3. POC was $22 per test, versus $25 for lab testing.
The National Institutes of Health funded the work. Dr. Drain had no disclosures. Cepheid donated the POC testing machines.
SOURCE: Drain PK et al. CROI 2019, Abstract 53LB.
REPORTING FROM CROI 2019
A couple of little known side effects of medications
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
SPD president discusses pediatric research, and more
WASHINGTON – in an interview at the annual meeting of the American Academy of Dermatology.
Dr. Davis, a pediatric dermatologist at the Mayo Clinic, Rochester, Minn., said that the SPD had a strategic retreat prior to the AAD annual meeting to look at goals attained over the last 3 years “and where we’re going for the next 3 years.” Goals that have been accomplished “move us forward as a society for patient advocacy, specialty advocacy, workforce strengthening, research advancement, patient care, and education,” she noted. The education arena, for example, includes not only educating patients and their families, so they can get the best health care possible, “but we want to educate the pipeline of trainees coming through medical school so ... they have exposure to pediatric dermatology and they can hopefully develop an interest in pediatric dermatology as a future career.”
The SPD now has more than 1,200 members, and has a research arm, the Pediatric Dermatology Research Alliance (PeDRA), which has multiple projects, the largest of which is looking at the stigma of skin diseases, a study that involves patients with severe skin diseases and their families, said Dr. Davis. Watch the video above for more on PeDRA and SPD.
Dr. Davis disclosed that she is involved in a study for Regeneron but has not received any personal financial compensation.
WASHINGTON – in an interview at the annual meeting of the American Academy of Dermatology.
Dr. Davis, a pediatric dermatologist at the Mayo Clinic, Rochester, Minn., said that the SPD had a strategic retreat prior to the AAD annual meeting to look at goals attained over the last 3 years “and where we’re going for the next 3 years.” Goals that have been accomplished “move us forward as a society for patient advocacy, specialty advocacy, workforce strengthening, research advancement, patient care, and education,” she noted. The education arena, for example, includes not only educating patients and their families, so they can get the best health care possible, “but we want to educate the pipeline of trainees coming through medical school so ... they have exposure to pediatric dermatology and they can hopefully develop an interest in pediatric dermatology as a future career.”
The SPD now has more than 1,200 members, and has a research arm, the Pediatric Dermatology Research Alliance (PeDRA), which has multiple projects, the largest of which is looking at the stigma of skin diseases, a study that involves patients with severe skin diseases and their families, said Dr. Davis. Watch the video above for more on PeDRA and SPD.
Dr. Davis disclosed that she is involved in a study for Regeneron but has not received any personal financial compensation.
WASHINGTON – in an interview at the annual meeting of the American Academy of Dermatology.
Dr. Davis, a pediatric dermatologist at the Mayo Clinic, Rochester, Minn., said that the SPD had a strategic retreat prior to the AAD annual meeting to look at goals attained over the last 3 years “and where we’re going for the next 3 years.” Goals that have been accomplished “move us forward as a society for patient advocacy, specialty advocacy, workforce strengthening, research advancement, patient care, and education,” she noted. The education arena, for example, includes not only educating patients and their families, so they can get the best health care possible, “but we want to educate the pipeline of trainees coming through medical school so ... they have exposure to pediatric dermatology and they can hopefully develop an interest in pediatric dermatology as a future career.”
The SPD now has more than 1,200 members, and has a research arm, the Pediatric Dermatology Research Alliance (PeDRA), which has multiple projects, the largest of which is looking at the stigma of skin diseases, a study that involves patients with severe skin diseases and their families, said Dr. Davis. Watch the video above for more on PeDRA and SPD.
Dr. Davis disclosed that she is involved in a study for Regeneron but has not received any personal financial compensation.
REPORTING FROM AAD 2019
Don’t discount sleep disturbance for children with atopic dermatitis
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
FROM JAMA PEDIATRICS
Genomics have changed how researchers view trauma’s immunologic impact
SAN DIEGO – Severely injured patients often experience a massive inflammatory and immunomodulatory response that can lead to multiple organ failure, nosocomial infections, long ICU stays, and poor outcomes. But not all of them do. Some patients recover relatively rapidly, achieve earlier release, and have a faster immunologic recovery trajectory.
The longstanding theory, according to Ronald Maier, MD, a professor of surgery at the University of Washington, is that a trauma-related stimulus leads to an aggressive inflammatory response that can lead to multiple organ failure and death. In patients who recover from this early challenge, the theory goes, a counterregulatory response may overexuberantly down-regulate the inflammatory storm, which leaves the patient vulnerable to infections and poor wound healing. Then a later infection, sepsis, endotoxemia, or some other stimulus may ramp up the inflammatory system again, which leads to a crisis that can trigger mortality well after the initial trauma. Patients who recover from this challenge then return to homeostasis.
It’s a neat theory, but it’s wrong, said Dr. Maier in a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine. It has been undercut by genomic technology and high-throughput methods that have provided a new approach to investigating the underlying biology, as well as the ability to test circulating white blood cells to measure a patient’s immune response to traumatic injury.
“If you look at the underlying biology by looking at the genomic response, you can see that it’s not a sequential process. There is simultaneous up-regulation and down-regulation,” said Dr. Maier.
A study of 35 trauma patients using a gene chip found a measurable change in expression of over 17,000 genes; 5,136 genes had at least a twofold change in expression. “Eighty percent of the human genome changes measurably when you are hit by a cement truck,” said Dr. Maier.
To researchers’ surprise, more genes were found to be down-regulated in the immediate aftermath of the injury, and most of those down-regulated genes are involved in adaptive immunity. Up-regulated pathways were included in the innate and proinflammatory response. “The simultaneous down-regulation of the adaptive arm explains why the patients in the ICU with severe injuries are very susceptible to nosocomial infections, poor wound healing, and multiple complications,” said Dr. Maier.
Genomic studies also show down-regulation of genes associated with phagocytosis even out to 45 days. “Sometimes I wonder why every patient doesn’t evolve a nosocomial infection as a consequence of this impact on the immune system. In fact, I think it’s a great testimony to the countermeasures we’ve taken as far as sterility, hand washing – we’ve been able to prevent this dysfunction from being expressed as a nosocomial infection,” said Dr. Maier.
The genomic analysis can also be used to discriminate patients who regain homeostasis relatively quickly after severe trauma. There seems to be an inflection point between patients who resolve by 5 days and those who go on to experience prolonged ICU stays.
Perhaps surprisingly, the researchers found little difference between the two groups in terms of specific genes that were up- or down-regulated. Instead, the “uncomplicated” group saw their altered gene expression patterns return more quickly to baseline levels, whereas “complicated” patients lingered in the dysregulated state. “We’ve been chasing biomarkers for 35 years, and this explains why it’s very difficult. Those who do well return toward normal quickly. Those who have complications stay abnormal,” said Dr. Maier.
Instead, researchers identified a panel of 63 gene probes that can track the overall progress of the “genomic storm,” as he referred to the changes that occur in the wake of trauma. “This panel of 63 genes is the best predictor of the patient’s response to injury – better than injury severity score, better than multiple organ failure scores,” he said.
Dr. Maier is confident that such panels can alter patient management, even outside of trauma. “It may allow us to show which patients are going to have risk of early recurrence because of alterations in their adaptive immunity versus those who aren’t. We’re also going to predict those who are going to have infectious complications. Hopefully we’ll soon have a handle on which patients we need to be most aggressive with, and we can use monitoring to measure our therapeutic impact,” said Dr. Maier.
email address
SOURCE: Add the first author et al., journal citation/abstract number, and hyperlink it here.
SAN DIEGO – Severely injured patients often experience a massive inflammatory and immunomodulatory response that can lead to multiple organ failure, nosocomial infections, long ICU stays, and poor outcomes. But not all of them do. Some patients recover relatively rapidly, achieve earlier release, and have a faster immunologic recovery trajectory.
The longstanding theory, according to Ronald Maier, MD, a professor of surgery at the University of Washington, is that a trauma-related stimulus leads to an aggressive inflammatory response that can lead to multiple organ failure and death. In patients who recover from this early challenge, the theory goes, a counterregulatory response may overexuberantly down-regulate the inflammatory storm, which leaves the patient vulnerable to infections and poor wound healing. Then a later infection, sepsis, endotoxemia, or some other stimulus may ramp up the inflammatory system again, which leads to a crisis that can trigger mortality well after the initial trauma. Patients who recover from this challenge then return to homeostasis.
It’s a neat theory, but it’s wrong, said Dr. Maier in a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine. It has been undercut by genomic technology and high-throughput methods that have provided a new approach to investigating the underlying biology, as well as the ability to test circulating white blood cells to measure a patient’s immune response to traumatic injury.
“If you look at the underlying biology by looking at the genomic response, you can see that it’s not a sequential process. There is simultaneous up-regulation and down-regulation,” said Dr. Maier.
A study of 35 trauma patients using a gene chip found a measurable change in expression of over 17,000 genes; 5,136 genes had at least a twofold change in expression. “Eighty percent of the human genome changes measurably when you are hit by a cement truck,” said Dr. Maier.
To researchers’ surprise, more genes were found to be down-regulated in the immediate aftermath of the injury, and most of those down-regulated genes are involved in adaptive immunity. Up-regulated pathways were included in the innate and proinflammatory response. “The simultaneous down-regulation of the adaptive arm explains why the patients in the ICU with severe injuries are very susceptible to nosocomial infections, poor wound healing, and multiple complications,” said Dr. Maier.
Genomic studies also show down-regulation of genes associated with phagocytosis even out to 45 days. “Sometimes I wonder why every patient doesn’t evolve a nosocomial infection as a consequence of this impact on the immune system. In fact, I think it’s a great testimony to the countermeasures we’ve taken as far as sterility, hand washing – we’ve been able to prevent this dysfunction from being expressed as a nosocomial infection,” said Dr. Maier.
The genomic analysis can also be used to discriminate patients who regain homeostasis relatively quickly after severe trauma. There seems to be an inflection point between patients who resolve by 5 days and those who go on to experience prolonged ICU stays.
Perhaps surprisingly, the researchers found little difference between the two groups in terms of specific genes that were up- or down-regulated. Instead, the “uncomplicated” group saw their altered gene expression patterns return more quickly to baseline levels, whereas “complicated” patients lingered in the dysregulated state. “We’ve been chasing biomarkers for 35 years, and this explains why it’s very difficult. Those who do well return toward normal quickly. Those who have complications stay abnormal,” said Dr. Maier.
Instead, researchers identified a panel of 63 gene probes that can track the overall progress of the “genomic storm,” as he referred to the changes that occur in the wake of trauma. “This panel of 63 genes is the best predictor of the patient’s response to injury – better than injury severity score, better than multiple organ failure scores,” he said.
Dr. Maier is confident that such panels can alter patient management, even outside of trauma. “It may allow us to show which patients are going to have risk of early recurrence because of alterations in their adaptive immunity versus those who aren’t. We’re also going to predict those who are going to have infectious complications. Hopefully we’ll soon have a handle on which patients we need to be most aggressive with, and we can use monitoring to measure our therapeutic impact,” said Dr. Maier.
email address
SOURCE: Add the first author et al., journal citation/abstract number, and hyperlink it here.
SAN DIEGO – Severely injured patients often experience a massive inflammatory and immunomodulatory response that can lead to multiple organ failure, nosocomial infections, long ICU stays, and poor outcomes. But not all of them do. Some patients recover relatively rapidly, achieve earlier release, and have a faster immunologic recovery trajectory.
The longstanding theory, according to Ronald Maier, MD, a professor of surgery at the University of Washington, is that a trauma-related stimulus leads to an aggressive inflammatory response that can lead to multiple organ failure and death. In patients who recover from this early challenge, the theory goes, a counterregulatory response may overexuberantly down-regulate the inflammatory storm, which leaves the patient vulnerable to infections and poor wound healing. Then a later infection, sepsis, endotoxemia, or some other stimulus may ramp up the inflammatory system again, which leads to a crisis that can trigger mortality well after the initial trauma. Patients who recover from this challenge then return to homeostasis.
It’s a neat theory, but it’s wrong, said Dr. Maier in a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine. It has been undercut by genomic technology and high-throughput methods that have provided a new approach to investigating the underlying biology, as well as the ability to test circulating white blood cells to measure a patient’s immune response to traumatic injury.
“If you look at the underlying biology by looking at the genomic response, you can see that it’s not a sequential process. There is simultaneous up-regulation and down-regulation,” said Dr. Maier.
A study of 35 trauma patients using a gene chip found a measurable change in expression of over 17,000 genes; 5,136 genes had at least a twofold change in expression. “Eighty percent of the human genome changes measurably when you are hit by a cement truck,” said Dr. Maier.
To researchers’ surprise, more genes were found to be down-regulated in the immediate aftermath of the injury, and most of those down-regulated genes are involved in adaptive immunity. Up-regulated pathways were included in the innate and proinflammatory response. “The simultaneous down-regulation of the adaptive arm explains why the patients in the ICU with severe injuries are very susceptible to nosocomial infections, poor wound healing, and multiple complications,” said Dr. Maier.
Genomic studies also show down-regulation of genes associated with phagocytosis even out to 45 days. “Sometimes I wonder why every patient doesn’t evolve a nosocomial infection as a consequence of this impact on the immune system. In fact, I think it’s a great testimony to the countermeasures we’ve taken as far as sterility, hand washing – we’ve been able to prevent this dysfunction from being expressed as a nosocomial infection,” said Dr. Maier.
The genomic analysis can also be used to discriminate patients who regain homeostasis relatively quickly after severe trauma. There seems to be an inflection point between patients who resolve by 5 days and those who go on to experience prolonged ICU stays.
Perhaps surprisingly, the researchers found little difference between the two groups in terms of specific genes that were up- or down-regulated. Instead, the “uncomplicated” group saw their altered gene expression patterns return more quickly to baseline levels, whereas “complicated” patients lingered in the dysregulated state. “We’ve been chasing biomarkers for 35 years, and this explains why it’s very difficult. Those who do well return toward normal quickly. Those who have complications stay abnormal,” said Dr. Maier.
Instead, researchers identified a panel of 63 gene probes that can track the overall progress of the “genomic storm,” as he referred to the changes that occur in the wake of trauma. “This panel of 63 genes is the best predictor of the patient’s response to injury – better than injury severity score, better than multiple organ failure scores,” he said.
Dr. Maier is confident that such panels can alter patient management, even outside of trauma. “It may allow us to show which patients are going to have risk of early recurrence because of alterations in their adaptive immunity versus those who aren’t. We’re also going to predict those who are going to have infectious complications. Hopefully we’ll soon have a handle on which patients we need to be most aggressive with, and we can use monitoring to measure our therapeutic impact,” said Dr. Maier.
email address
SOURCE: Add the first author et al., journal citation/abstract number, and hyperlink it here.
EXPERT ANALYSIS FROM CCC48
31-GEP test predicts likelihood of metastasis for cutaneous melanoma
WASHINGTON – The for accurately predicting recurrence-free survival and distant metastasis-free survival and melanoma-specific survival, according to results presented by Bradley N. Greenhaw, MD, at a late-breaking research session at the annual meeting of the American Academy of Dermatology.
Dr. Greenhaw, a dermatologist affiliated with the North Mississippi Medical Center-Tupelo, and his colleagues pooled together 1,268 patients from the following studies that analyzed results from melanoma patients who had their disease classified with the 31-gene expression profile (31-GEP) test.
- A single-center study, conducted by Dr. Greenhaw and his associates (Greenhaw BN et al. Dermatol Surg. 2018 Dec. doi: 10.1097/DSS.0000000000001588.
- A multicenter prospective study (J Hematol Oncol. 2017 Aug. doi: 10.1186/s13045-017-0520-1.
- A retrospective archival study (J Am Acad Dermatol. 2019 Jan. doi: 10.1016/j.jaad.2018.07.028.
The 31-GEP test stratifies an individual’s likelihood of developing metastasis within 5 years as low and high risk. In the three studies, the test was used to identify tumors with low-risk (class 1A, class 1B), higher-risk (class 2A), and highest-risk (class 2B) melanoma based on tumor gene expression. In these individual studies, class 2B melanoma independently predicted recurrence-free survival (RFS), distant metastasis–free, and melanoma-specific survival.
Dr. Greenhaw and colleagues performed a meta-analysis of 1,268 patients with stage I through stage III melanoma from those three studies, using fixed and random effects weighting to account for study differences and heterogeneity, respectively. For class 2B tumors, they found a 2.96 increased risk for recurrent metastases and a 2.88 increased risk for distant metastases. The researchers also found no heterogeneity across the studies.
Melanoma-specific survival was not included in the meta-analysis because one paper did not contain any mortality events in class 1A melanoma patients.
“The meta-analysis demonstrated that the GEP test was able to accurately identify those melanoma patients who were at higher risk of metastasis, and we saw a consistent effect across multiple studies,” Dr. Greenhaw said.
Since publication of the 2019 JAAD paper, there were an additional 211 patients who met inclusion criteria and were included in an additional meta-analysis to determine whether inclusion of these patients affected the results. Dr. Greenhaw and colleagues found a 91.4% recurrence-free survival rate and a 94.1% distant metastasis–free survival rate for class 1A melanomas, compared with 45.7% and 55.5% , respectively, for class 2B tumors.
“You can see a big divergence,” Dr. Greenhaw said at the meeting. “Just by using this one test, it’s able to separate out melanomas that otherwise may be grouped in together under current AJCC [American Joint Committee on Cancer] staging,” he added. “The class 2B designation really did confirm a higher risk for recurrence in distant metastasis.”
The researchers used the SORT method to rate the quality of the data across all three studies. Level 1 evidence under the SORT method represents a systematic review or meta-analysis of good-quality studies and/or a prospective study with good follow-up, while an A-level recommendation represents good, quality evidence. Based on the meta-analysis results, the 31-GEP test meets level 1A evidence under the SORT method, Dr. Greenhaw said.
As a prognostic tool, 31-GEP has the potential to change how dermatologists manage their patients with regard to follow-up and adjuvant therapy. “It is being used not just as this novel test that gives us more information, it’s being used clinically,” said Dr. Greenhaw, who noted he regularly uses the 31-GEP test in his practice.
This is the first time that a meta-analysis has been performed for this test, he noted.
Dr. Greenhaw reports a pending relationship with Castle Biosciences.
SOURCE: Greenhaw BN et al. AAD 19. Session F055, Abstract 11370.
WASHINGTON – The for accurately predicting recurrence-free survival and distant metastasis-free survival and melanoma-specific survival, according to results presented by Bradley N. Greenhaw, MD, at a late-breaking research session at the annual meeting of the American Academy of Dermatology.
Dr. Greenhaw, a dermatologist affiliated with the North Mississippi Medical Center-Tupelo, and his colleagues pooled together 1,268 patients from the following studies that analyzed results from melanoma patients who had their disease classified with the 31-gene expression profile (31-GEP) test.
- A single-center study, conducted by Dr. Greenhaw and his associates (Greenhaw BN et al. Dermatol Surg. 2018 Dec. doi: 10.1097/DSS.0000000000001588.
- A multicenter prospective study (J Hematol Oncol. 2017 Aug. doi: 10.1186/s13045-017-0520-1.
- A retrospective archival study (J Am Acad Dermatol. 2019 Jan. doi: 10.1016/j.jaad.2018.07.028.
The 31-GEP test stratifies an individual’s likelihood of developing metastasis within 5 years as low and high risk. In the three studies, the test was used to identify tumors with low-risk (class 1A, class 1B), higher-risk (class 2A), and highest-risk (class 2B) melanoma based on tumor gene expression. In these individual studies, class 2B melanoma independently predicted recurrence-free survival (RFS), distant metastasis–free, and melanoma-specific survival.
Dr. Greenhaw and colleagues performed a meta-analysis of 1,268 patients with stage I through stage III melanoma from those three studies, using fixed and random effects weighting to account for study differences and heterogeneity, respectively. For class 2B tumors, they found a 2.96 increased risk for recurrent metastases and a 2.88 increased risk for distant metastases. The researchers also found no heterogeneity across the studies.
Melanoma-specific survival was not included in the meta-analysis because one paper did not contain any mortality events in class 1A melanoma patients.
“The meta-analysis demonstrated that the GEP test was able to accurately identify those melanoma patients who were at higher risk of metastasis, and we saw a consistent effect across multiple studies,” Dr. Greenhaw said.
Since publication of the 2019 JAAD paper, there were an additional 211 patients who met inclusion criteria and were included in an additional meta-analysis to determine whether inclusion of these patients affected the results. Dr. Greenhaw and colleagues found a 91.4% recurrence-free survival rate and a 94.1% distant metastasis–free survival rate for class 1A melanomas, compared with 45.7% and 55.5% , respectively, for class 2B tumors.
“You can see a big divergence,” Dr. Greenhaw said at the meeting. “Just by using this one test, it’s able to separate out melanomas that otherwise may be grouped in together under current AJCC [American Joint Committee on Cancer] staging,” he added. “The class 2B designation really did confirm a higher risk for recurrence in distant metastasis.”
The researchers used the SORT method to rate the quality of the data across all three studies. Level 1 evidence under the SORT method represents a systematic review or meta-analysis of good-quality studies and/or a prospective study with good follow-up, while an A-level recommendation represents good, quality evidence. Based on the meta-analysis results, the 31-GEP test meets level 1A evidence under the SORT method, Dr. Greenhaw said.
As a prognostic tool, 31-GEP has the potential to change how dermatologists manage their patients with regard to follow-up and adjuvant therapy. “It is being used not just as this novel test that gives us more information, it’s being used clinically,” said Dr. Greenhaw, who noted he regularly uses the 31-GEP test in his practice.
This is the first time that a meta-analysis has been performed for this test, he noted.
Dr. Greenhaw reports a pending relationship with Castle Biosciences.
SOURCE: Greenhaw BN et al. AAD 19. Session F055, Abstract 11370.
WASHINGTON – The for accurately predicting recurrence-free survival and distant metastasis-free survival and melanoma-specific survival, according to results presented by Bradley N. Greenhaw, MD, at a late-breaking research session at the annual meeting of the American Academy of Dermatology.
Dr. Greenhaw, a dermatologist affiliated with the North Mississippi Medical Center-Tupelo, and his colleagues pooled together 1,268 patients from the following studies that analyzed results from melanoma patients who had their disease classified with the 31-gene expression profile (31-GEP) test.
- A single-center study, conducted by Dr. Greenhaw and his associates (Greenhaw BN et al. Dermatol Surg. 2018 Dec. doi: 10.1097/DSS.0000000000001588.
- A multicenter prospective study (J Hematol Oncol. 2017 Aug. doi: 10.1186/s13045-017-0520-1.
- A retrospective archival study (J Am Acad Dermatol. 2019 Jan. doi: 10.1016/j.jaad.2018.07.028.
The 31-GEP test stratifies an individual’s likelihood of developing metastasis within 5 years as low and high risk. In the three studies, the test was used to identify tumors with low-risk (class 1A, class 1B), higher-risk (class 2A), and highest-risk (class 2B) melanoma based on tumor gene expression. In these individual studies, class 2B melanoma independently predicted recurrence-free survival (RFS), distant metastasis–free, and melanoma-specific survival.
Dr. Greenhaw and colleagues performed a meta-analysis of 1,268 patients with stage I through stage III melanoma from those three studies, using fixed and random effects weighting to account for study differences and heterogeneity, respectively. For class 2B tumors, they found a 2.96 increased risk for recurrent metastases and a 2.88 increased risk for distant metastases. The researchers also found no heterogeneity across the studies.
Melanoma-specific survival was not included in the meta-analysis because one paper did not contain any mortality events in class 1A melanoma patients.
“The meta-analysis demonstrated that the GEP test was able to accurately identify those melanoma patients who were at higher risk of metastasis, and we saw a consistent effect across multiple studies,” Dr. Greenhaw said.
Since publication of the 2019 JAAD paper, there were an additional 211 patients who met inclusion criteria and were included in an additional meta-analysis to determine whether inclusion of these patients affected the results. Dr. Greenhaw and colleagues found a 91.4% recurrence-free survival rate and a 94.1% distant metastasis–free survival rate for class 1A melanomas, compared with 45.7% and 55.5% , respectively, for class 2B tumors.
“You can see a big divergence,” Dr. Greenhaw said at the meeting. “Just by using this one test, it’s able to separate out melanomas that otherwise may be grouped in together under current AJCC [American Joint Committee on Cancer] staging,” he added. “The class 2B designation really did confirm a higher risk for recurrence in distant metastasis.”
The researchers used the SORT method to rate the quality of the data across all three studies. Level 1 evidence under the SORT method represents a systematic review or meta-analysis of good-quality studies and/or a prospective study with good follow-up, while an A-level recommendation represents good, quality evidence. Based on the meta-analysis results, the 31-GEP test meets level 1A evidence under the SORT method, Dr. Greenhaw said.
As a prognostic tool, 31-GEP has the potential to change how dermatologists manage their patients with regard to follow-up and adjuvant therapy. “It is being used not just as this novel test that gives us more information, it’s being used clinically,” said Dr. Greenhaw, who noted he regularly uses the 31-GEP test in his practice.
This is the first time that a meta-analysis has been performed for this test, he noted.
Dr. Greenhaw reports a pending relationship with Castle Biosciences.
SOURCE: Greenhaw BN et al. AAD 19. Session F055, Abstract 11370.
REPORTING FROM AAD 19
Belimumab data out to 13 years show continued safety, efficacy
New data from the longest continuous belimumab treatment in a clinical trial of patients with systemic lupus erythematosus (SLE) indicate similar or lower adverse events each year and maintenance of efficacy for up to 13 years in those who initially respond to and stay on treatment.
First author Daniel J. Wallace, MD, and his colleagues reported in Arthritis & Rheumatology on 298 patients who continued from a phase 2 trial of 476 patients and its extension phase to a continuation study with belimumab (Benlysta) plus standard of care. These patients entered the continuation study having gone from placebo to 10 mg/kg belimumab or continued on 1, 4, or 10 mg/kg belimumab or escalated treatment up to 10 mg/kg. They needed to have an improvement in Physician’s Global Assessment (PGA) score, compared with baseline, and had no severe flare in the last 30 days of the extension study.
At year 5, 70% of patients were still in the study, and this declined to 44% at year 10 and 32% (96 patients) at the end of the study. There were stable or declining rates of the most common adverse events from year 1 to year 11 or later, and serious infections and infestations occurred at a stable rate, from 3.7 per 100 patient-years in year 1 to 6.7 per 100 patients-years through year 11, despite a reduction in immunoglobulin G levels during the study. A total of 15% of patients overall withdrew because of adverse events.
The overall SLE Responder Index response rate rose as the number of participants declined, going from 33% at 1 year and 16 weeks to 76% at 12 years and 32 weeks.
In addition to consistently low flare rates starting at year 5, “those patients remaining had reduced requirements for corticosteroids, and the percentage achieving low disease activity increased. Furthermore, patients continued to have serological improvements. ” Dr. Wallace and his coauthors wrote.
“Patients who remained in the study were likely to be those who responded better or tolerated belimumab better than patients who withdrew; hence, the findings may not be representative of all patients with SLE,” they said.
GlaxoSmithKline and Human Genome Sciences funded the study. Most of the investigators received grants, research support, or consulting fees; held shares in; or were employees of GlaxoSmithKline.
New data from the longest continuous belimumab treatment in a clinical trial of patients with systemic lupus erythematosus (SLE) indicate similar or lower adverse events each year and maintenance of efficacy for up to 13 years in those who initially respond to and stay on treatment.
First author Daniel J. Wallace, MD, and his colleagues reported in Arthritis & Rheumatology on 298 patients who continued from a phase 2 trial of 476 patients and its extension phase to a continuation study with belimumab (Benlysta) plus standard of care. These patients entered the continuation study having gone from placebo to 10 mg/kg belimumab or continued on 1, 4, or 10 mg/kg belimumab or escalated treatment up to 10 mg/kg. They needed to have an improvement in Physician’s Global Assessment (PGA) score, compared with baseline, and had no severe flare in the last 30 days of the extension study.
At year 5, 70% of patients were still in the study, and this declined to 44% at year 10 and 32% (96 patients) at the end of the study. There were stable or declining rates of the most common adverse events from year 1 to year 11 or later, and serious infections and infestations occurred at a stable rate, from 3.7 per 100 patient-years in year 1 to 6.7 per 100 patients-years through year 11, despite a reduction in immunoglobulin G levels during the study. A total of 15% of patients overall withdrew because of adverse events.
The overall SLE Responder Index response rate rose as the number of participants declined, going from 33% at 1 year and 16 weeks to 76% at 12 years and 32 weeks.
In addition to consistently low flare rates starting at year 5, “those patients remaining had reduced requirements for corticosteroids, and the percentage achieving low disease activity increased. Furthermore, patients continued to have serological improvements. ” Dr. Wallace and his coauthors wrote.
“Patients who remained in the study were likely to be those who responded better or tolerated belimumab better than patients who withdrew; hence, the findings may not be representative of all patients with SLE,” they said.
GlaxoSmithKline and Human Genome Sciences funded the study. Most of the investigators received grants, research support, or consulting fees; held shares in; or were employees of GlaxoSmithKline.
New data from the longest continuous belimumab treatment in a clinical trial of patients with systemic lupus erythematosus (SLE) indicate similar or lower adverse events each year and maintenance of efficacy for up to 13 years in those who initially respond to and stay on treatment.
First author Daniel J. Wallace, MD, and his colleagues reported in Arthritis & Rheumatology on 298 patients who continued from a phase 2 trial of 476 patients and its extension phase to a continuation study with belimumab (Benlysta) plus standard of care. These patients entered the continuation study having gone from placebo to 10 mg/kg belimumab or continued on 1, 4, or 10 mg/kg belimumab or escalated treatment up to 10 mg/kg. They needed to have an improvement in Physician’s Global Assessment (PGA) score, compared with baseline, and had no severe flare in the last 30 days of the extension study.
At year 5, 70% of patients were still in the study, and this declined to 44% at year 10 and 32% (96 patients) at the end of the study. There were stable or declining rates of the most common adverse events from year 1 to year 11 or later, and serious infections and infestations occurred at a stable rate, from 3.7 per 100 patient-years in year 1 to 6.7 per 100 patients-years through year 11, despite a reduction in immunoglobulin G levels during the study. A total of 15% of patients overall withdrew because of adverse events.
The overall SLE Responder Index response rate rose as the number of participants declined, going from 33% at 1 year and 16 weeks to 76% at 12 years and 32 weeks.
In addition to consistently low flare rates starting at year 5, “those patients remaining had reduced requirements for corticosteroids, and the percentage achieving low disease activity increased. Furthermore, patients continued to have serological improvements. ” Dr. Wallace and his coauthors wrote.
“Patients who remained in the study were likely to be those who responded better or tolerated belimumab better than patients who withdrew; hence, the findings may not be representative of all patients with SLE,” they said.
GlaxoSmithKline and Human Genome Sciences funded the study. Most of the investigators received grants, research support, or consulting fees; held shares in; or were employees of GlaxoSmithKline.
FROM ARTHRITIS & RHEUMATOLOGY