User login
Your 15-year-old patient requests an IUD without parental knowledge
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
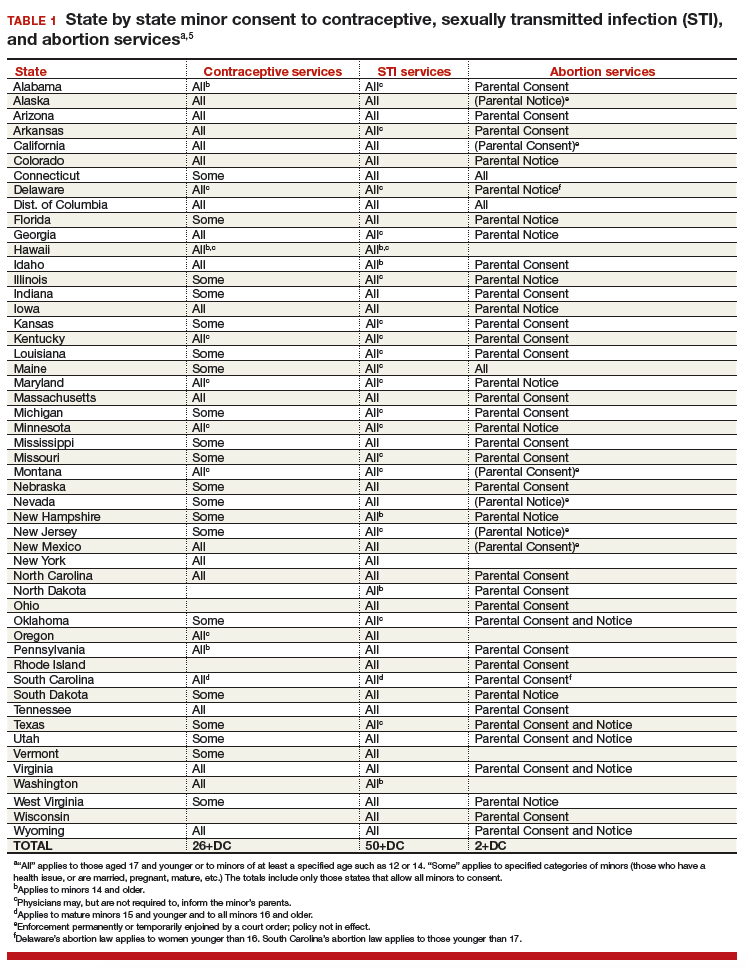
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6
Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
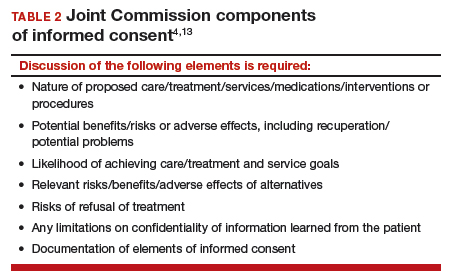
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13
Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or info@abog.org.
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6

Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13

Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or info@abog.org.
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6

Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13

Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or info@abog.org.
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
Is flu season winding down?
Flu season shows signs of peaking. Watch for depression symptom trajectory in high-risk young adults. ACOG: Avoid inductions before 39 weeks unless medically necessary. And malpractice suits are less frequent – but more costly.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Flu season shows signs of peaking. Watch for depression symptom trajectory in high-risk young adults. ACOG: Avoid inductions before 39 weeks unless medically necessary. And malpractice suits are less frequent – but more costly.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Flu season shows signs of peaking. Watch for depression symptom trajectory in high-risk young adults. ACOG: Avoid inductions before 39 weeks unless medically necessary. And malpractice suits are less frequent – but more costly.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Intervention raises pediatric patient awareness of teratogenic rheumatology medications
While many medications in rheumatology are known or suspected to be teratogenic, there are no standards on educating rheumatology patients about this risk or on screening for pregnancy, wrote Ashley M. Cooper, MD, and her colleagues at Children’s Mercy Kansas City, Mo. Their report is in the April issue of Pediatrics.
“This is problematic in pediatric rheumatology because nearly half of adolescents report a history of sexual intercourse, but only 57% report condom use and 27% another form of contraception,” they wrote, pointing out that the United States also has one of the highest rates of teen pregnancy among industrialized nations; 80% are unplanned.
The intervention consisted of a number of approaches. Posters listing teratogenic medications were put in exam rooms, physicians received education about the Food and Drug Administration–mandated mycophenolate Risk Evaluation and Mitigation Strategy (REMS), and scripts were developed for staff to inform patients of teratogenic risks with medications, as well as prompts for referral to birth control clinics.
Researchers also implemented a standardized EHR template to allow clinical staff to review medication-specific teaching points and document the discussion with the patient. There also was a previsit planning process to review everything before the clinic day began, and identify patients for education or pregnancy screening.
After 8 months of the intervention – during which 1,366 eligible patient encounters occurred in rheumatology patients aged 10 years and older– researchers saw a significant increase in those encounters where teratogen education was recorded, from 25% before the intervention to 80% at 23 months after it. Pregnancy screening also increased among eligible patients, from 20% preintervention to 83% at 23 months after the first intervention.
Three patients became pregnant during the intervention period; all of the pregnancies were picked up by the screening process. In all cases, the teratogenic medication was immediately stopped; one patient underwent elective termination, one delivered a healthy term infant, and one patient was lost to follow-up. Two of the three had received education during the previous year.
“The strategies used in this project have implications that reach beyond rheumatology because teratogenic medications are commonly prescribed by other subspecialties,” the authors wrote.
Dr. Cooper and her associates noted that an original previsit checklist was later abandoned because it was viewed as being too much of a time imposition on staff at the beginning of a busy clinic day.
“The new process had higher staff buy-in because it was used to address multiple quality improvement projects and hospital requirements, some linked to provider remuneration,” they wrote.
No funding or conflicts of interest were declared.
SOURCE: Cooper AM et al. Pediatrics 2019; 143(4):e20180803. doi: 10.1542/peds.2018-0803.
While many medications in rheumatology are known or suspected to be teratogenic, there are no standards on educating rheumatology patients about this risk or on screening for pregnancy, wrote Ashley M. Cooper, MD, and her colleagues at Children’s Mercy Kansas City, Mo. Their report is in the April issue of Pediatrics.
“This is problematic in pediatric rheumatology because nearly half of adolescents report a history of sexual intercourse, but only 57% report condom use and 27% another form of contraception,” they wrote, pointing out that the United States also has one of the highest rates of teen pregnancy among industrialized nations; 80% are unplanned.
The intervention consisted of a number of approaches. Posters listing teratogenic medications were put in exam rooms, physicians received education about the Food and Drug Administration–mandated mycophenolate Risk Evaluation and Mitigation Strategy (REMS), and scripts were developed for staff to inform patients of teratogenic risks with medications, as well as prompts for referral to birth control clinics.
Researchers also implemented a standardized EHR template to allow clinical staff to review medication-specific teaching points and document the discussion with the patient. There also was a previsit planning process to review everything before the clinic day began, and identify patients for education or pregnancy screening.
After 8 months of the intervention – during which 1,366 eligible patient encounters occurred in rheumatology patients aged 10 years and older– researchers saw a significant increase in those encounters where teratogen education was recorded, from 25% before the intervention to 80% at 23 months after it. Pregnancy screening also increased among eligible patients, from 20% preintervention to 83% at 23 months after the first intervention.
Three patients became pregnant during the intervention period; all of the pregnancies were picked up by the screening process. In all cases, the teratogenic medication was immediately stopped; one patient underwent elective termination, one delivered a healthy term infant, and one patient was lost to follow-up. Two of the three had received education during the previous year.
“The strategies used in this project have implications that reach beyond rheumatology because teratogenic medications are commonly prescribed by other subspecialties,” the authors wrote.
Dr. Cooper and her associates noted that an original previsit checklist was later abandoned because it was viewed as being too much of a time imposition on staff at the beginning of a busy clinic day.
“The new process had higher staff buy-in because it was used to address multiple quality improvement projects and hospital requirements, some linked to provider remuneration,” they wrote.
No funding or conflicts of interest were declared.
SOURCE: Cooper AM et al. Pediatrics 2019; 143(4):e20180803. doi: 10.1542/peds.2018-0803.
While many medications in rheumatology are known or suspected to be teratogenic, there are no standards on educating rheumatology patients about this risk or on screening for pregnancy, wrote Ashley M. Cooper, MD, and her colleagues at Children’s Mercy Kansas City, Mo. Their report is in the April issue of Pediatrics.
“This is problematic in pediatric rheumatology because nearly half of adolescents report a history of sexual intercourse, but only 57% report condom use and 27% another form of contraception,” they wrote, pointing out that the United States also has one of the highest rates of teen pregnancy among industrialized nations; 80% are unplanned.
The intervention consisted of a number of approaches. Posters listing teratogenic medications were put in exam rooms, physicians received education about the Food and Drug Administration–mandated mycophenolate Risk Evaluation and Mitigation Strategy (REMS), and scripts were developed for staff to inform patients of teratogenic risks with medications, as well as prompts for referral to birth control clinics.
Researchers also implemented a standardized EHR template to allow clinical staff to review medication-specific teaching points and document the discussion with the patient. There also was a previsit planning process to review everything before the clinic day began, and identify patients for education or pregnancy screening.
After 8 months of the intervention – during which 1,366 eligible patient encounters occurred in rheumatology patients aged 10 years and older– researchers saw a significant increase in those encounters where teratogen education was recorded, from 25% before the intervention to 80% at 23 months after it. Pregnancy screening also increased among eligible patients, from 20% preintervention to 83% at 23 months after the first intervention.
Three patients became pregnant during the intervention period; all of the pregnancies were picked up by the screening process. In all cases, the teratogenic medication was immediately stopped; one patient underwent elective termination, one delivered a healthy term infant, and one patient was lost to follow-up. Two of the three had received education during the previous year.
“The strategies used in this project have implications that reach beyond rheumatology because teratogenic medications are commonly prescribed by other subspecialties,” the authors wrote.
Dr. Cooper and her associates noted that an original previsit checklist was later abandoned because it was viewed as being too much of a time imposition on staff at the beginning of a busy clinic day.
“The new process had higher staff buy-in because it was used to address multiple quality improvement projects and hospital requirements, some linked to provider remuneration,” they wrote.
No funding or conflicts of interest were declared.
SOURCE: Cooper AM et al. Pediatrics 2019; 143(4):e20180803. doi: 10.1542/peds.2018-0803.
FROM PEDIATRICS
Key clinical point: Intervention improves awareness of teratogenic rheumatology medications among pediatric patients, as well as pregnancy screening.
Major finding: Teratogen education increased from 25% before the intervention to 80% at 23 months after it. Pregnancy screening also increased among eligible patients, from 20% preintervention to 83% at 23 months after the first intervention.
Study details: Single-center trial of 1,366 patient encounters.
Disclosures: No funding or conflicts of interest were declared.
Source: Cooper AM et al. Pediatrics 2019;143(4):e20180803. doi: 10.1542/peds.2018-0803.
Is oral or IV iron therapy more beneficial for postpartum anemia?
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
What is your approach to the persistent occiput posterior malposition?
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.
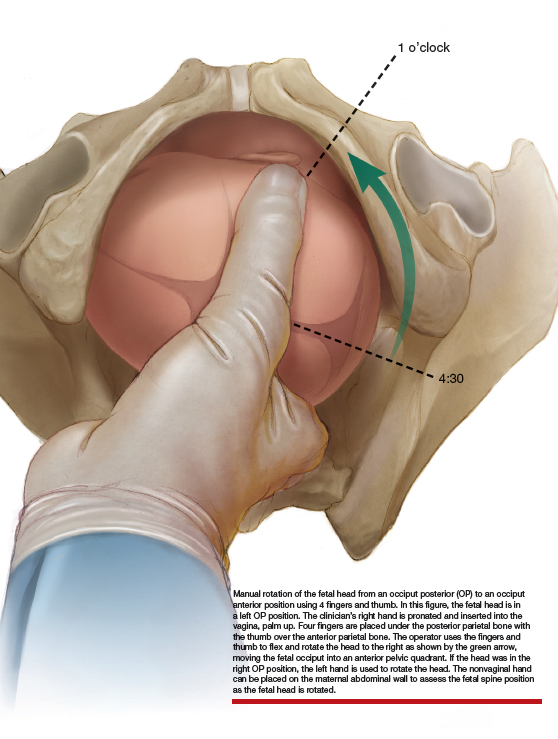
Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
MS prevalence estimates reach highest point to date
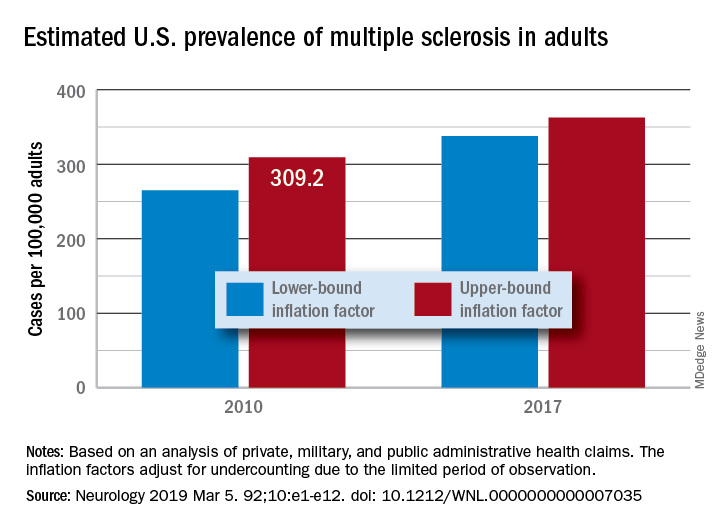
“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.

“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.

“Our findings suggest that there has been a steady rise in the prevalence of MS over the past 5 decades, that the prevalence of MS remains higher for women than men, and that a north-south geographic gradient still persists,” wrote lead author Mitchell T. Wallin, MD, of Georgetown University, Washington, and his coauthors. The study was published in Neurology.
To determine adult cases of MS, Dr. Wallin and colleagues applied a validated algorithm to private, military, and public AHC datasets. Data from the 2010 U.S. Census were also used to standardize age and sex. In total, 125 million people over 18 years of age were captured in the study, nearly 45% of the U.S. population.
After adjustment, the 2010 prevalence for MS cumulated over 10 years was 309.2 per 100,000 adults (95% confidence interval, 308.1-310.1). This represented a total of 727,344 people with MS. The female to male ratio was 2.8, with a prevalence of 450.1 per 100,000 (95% CI, 448.1-451.6) for women versus a prevalence of 159.7 (95% CI, 158.7-160.6) for men. The age group with the highest estimated prevalence was 55-64 years old, and the prevalence in northern regions of the United States was statistically significantly higher than in southern regions.
The limitations of this study included not including children, the Indian Health Service, the U.S. prison system, or undocumented U.S. residents in the prevalence estimates. However, the authors did note that “these segments of the population are relatively small or, in the case of children, would contribute few cases.” In addition, they were unable to acquire more than 3 years of data for all insurance pools because of high costs.
The study was funded by a grant from the National Multiple Sclerosis Society. The authors reported numerous disclosures, including receiving consulting fees, researching funding, and grant support from various government agencies, foundations, and pharmaceutical companies.
SOURCE: Wallin MT et al. Neurology. 2019 Feb 15. doi: 10.1212/WNL.0000000000007035.
FROM NEUROLOGY
New SOFA version could streamline outcomes research
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
SAN DIEGO – The new method replaces some of SOFA’s more subjective criteria with objective measures.
eSOFA relies on electronic health records to reduce reliance on administrative records, which suffer from cross-hospital variability in diagnosis and coding practices, as well as changes in these practices over time. The diagnosis of sepsis itself is also highly subjective. Instead, eSOFA determines dysfunction in six organ systems, indicated by use of vasopressors and mechanical ventilation, and the presence of abnormal laboratory values.
“The SOFA score includes measures like the Glasgow Coma Scale, which undoubtedly at the bedside is a very important clinical sign, but when trying to implement something that is objective for purposes of retrospective case counting and standardization, it can be problematic. The measures we chose [for eSOFA] are concrete, important maneuvers that were initiated by clinicians,” Chanu Rhee, MD, said in an interview.
Dr. Rhee is assistant professor of population medicine at Harvard Medical School and Brigham and Women’s Hospital, Boston. He presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine, and the work was simultaneously published online in Critical Care Medicine.
Key elements of SOFA that pose challenges for administrative data include: PaO2/FiO2, which are not routinely measured, and can be difficult to assign to arterial or venous samples; inconsistency in blood pressure and transient increases in vasopressor dose; the subjectivity of the Glasgow Coma Scale, which is also difficult to assess in sedated patients; and inconsistent urine output.
eSOFA introduced new measures for various organ functions, including cardiovascular (vasopressor initiation), pulmonary (mechanical ventilation initiation), renal (doubling of creatinine levels or a 50% or greater decrease in estimated glomerular filtration rate, compared with baseline), hepatic (bilirubin levels greater than or equal to 2.0 mg/dL and at least doubled from baseline), coagulation (platelet count less than 100 cells/mcL and at least a 50% decrease from a baseline of at least 100 cells/mcL), and neurological (lactate greater than or equal to 2.0 mmol/L).
“[eSOFA] opens a window into inter-facility comparisons that has not been possible to do. It’s really critical to ask, ‘How am I doing compared to my peer institutions?’ If you’re doing worse, you can look at the whole spectrum of things to try to drive improvements in care,” said Dr. Rhee.
The new tool isn’t just limited to quality improvement research. Shaeesta Khan, MD, assistant professor of critical care medicine at Geisinger Medical Center,Danville, Pa., has found eSOFA to be useful in her research into how genetic polymorphisms play a role in sepsis outcomes. Geisinger has a large population of patients with completed whole genome sequencing, and Dr. Khan began by trying to glean sepsis outcomes from administrative data.
“I explained SOFA scores to our data broker, and he pulled up 3,000 patients and gave everybody a SOFA score based on the algorithm he created, and it was all over the chart. Once I started doing chart review and phenotype verification, it was just a nightmare,” Dr. Khan said in an interview.
After struggling with the project, one of her mentors put her in touch with one of Dr. Rhee’s colleagues, and she asked the data broker to modify the eSOFA algorithm to fit her specific criteria. “It was a blessing,” she said.
Now, she has data from 5,000 patients with sepsis and sequenced DNA, and can begin comparing outcomes and genetic variants. About 20 candidate genes for sepsis outcomes have been identified to date, but she has a particular interest in PCSK9, which is an innate immune system regulator. She hopes to present results at CCC49 in 2020.
Validating mortality prediction
The researchers compared eSOFA and SOFA in a sample from 111 U.S. acute care hospitals to see if eSOFA had a comparable predictive validity for mortality. The analysis included 942,360 adults seen between 2013 and 2015. A total of 11.1% (104,903) had a presumed serious infection based on a blood culture order and at least 4 consecutive days of antibiotic use.
The analysis showed that 6.1% of those with infections had a sepsis event based on at least a 2-point increase in SOFA score from baseline (Sepsis-3 criteria), compared with 4.4% identified by at least a 1-point increase in eSOFA score. A total of 34,174 patients (3.6%) overlapped between SOFA and eSOFA, which represented good agreement (Cronbach’s alpha, 0.81). Compared with SOFA/Sepsis-3, eSOFA had a sensitivity of 60%, and a positive predictive value of 82%.
Patients identified by eSOFA were slightly more ill, with more requiring ICU admission (41% vs. 35%), and a greater frequency of in-hospital mortality (17% vs. 14%). Those patients who were identified by SOFA/Sepsis-3, but missed by eSOFA, had an overall lower mortality (6%).
There was a similar risk of mortality across deciles between SOFA- and eSOFA-identified sepsis patients. In an independent analysis of four hospitals from the Emory system, the area under the receiver operating characteristics was 0.77 for eSOFA and 0.76 for SOFA (P less than .001).
The Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality funded the study. Dr. Rhee and Dr. Khan have no relevant financial conflicts.
SOURCE: Rhee C et al. Crit Care Med. 2019;47(3):307-14.
REPORTING FROM CCC48
Anthracycline-free regimen OK in HER2-negative early breast cancer
It appears to be safe to hold the anthracycline in patients with HER2-negative early breast cancer who are at intermediate-to-high genomic risk, results of a large randomized trial suggest.
Among both pre- and postmenopausal women with pathologic stage T1 to T4c with positive nodes or node-negative but high-risk early breast cancer, there were no significant differences in 5-year outcomes for patients treated with six cycles of docetaxel and cyclophosphamide (TC) or four cycles of epirubicin and cyclophosphamide followed by four cycles of docetaxel (EC-T), reported Ulrike Nitz, MD, from the West German Study Group in Mönchengladbach, Germany, and her colleagues in the West German Study PlanB Trial.
Disease-free survival (DFS, the primary endpoint), distant recurrence-free interval (dRFI), and overall survival (OS) “were excellent and virtually identical in patients who received the anthracycline-containing or the anthracycline-free regimen. Subgroups that benefited from the anthracycline-containing regimen were not identified by interaction analysis, although a potentially clinically relevant benefit in particular (e.g., high-risk) subgroups cannot be ruled out,” they wrote. The report is in Journal of Clinical Oncology.
The investigators noted that anthracyclines are associated with increased risk for cardiac disease and hematologic malignancies, prompting investigators in other trials to consider anthracycline-free regimens.
“As the number of long-term survivors, elderly patients, and patients with preexisting cardiac risk factors increases, the toxicity profile becomes a more important discriminator in adjuvant treatment selection,” they wrote.
The investigators enrolled and randomized 2,449 women (median age 55, range 25-77 years) who had histologically confirmed, unilateral primary invasive breast cancer, adequate surgical treatment, and no evidence of metastatic disease. The patients all had HER2-negative disease, pT1 to pT4c, known hormone receptor status, and either pN+ or pN0 with one or more risk factors.
The intention-to-treat analysis included 1,227 patients assigned to EC-T and 1,222 assigned to TC in the efficacy population, and 1,167 and 1,178 patients, respectively, in the safety population.
After a median follow-up of 60 months, the 5-year DFS rate in the TC-treated group was 89.6%, compared with 89.9% in the EC-T–treated group. The estimated 5-year dRFI rates were 94.1% vs. 93.4%, and the estimated 5-year OS rates were 94.7% vs. 94.5%, respectively. None of the comparisons were statistically significant.
There were five treatment-related deaths in the TC arm, (one each from urosepsis, Streptococcus septicemia, peritonitis/diverticulitis, Staphylococcus epidermidis septicemia, and pulmonary embolism), and one in the EC-T arm (from septicemia).
In an interim safety analysis, the rate of febrile neutropenia was 6.1% in the TC arm and 3.9% in the EC-T arm, leading to a recommendation for “generous” prophylaxis with granulocyte-colony stimulating factor, and ciprofloxacine for patients with a history of diverticulitis or chronic infectious GI disease, or expected duration of neutropenia greater than 1 week.
Rates of grade 3 or 4 leukopenia, neutropenia, nausea, vomiting, peripheral polyneuropathy, hand-foot syndrome, mucositis/stomatitis, arthralgia, myalgia, and fatigue were significantly higher among patients treated with EC-T. There were numerically more grade 3-4 infections and febrile neutropenia within the TC arm, but this trend did not reach statistical significance.
The investigators noted that the results of their trial provide the strongest evidence for patients with pathologic NO or N1 disease, and that the trial did not examine the question of dose-dense chemotherapy in patients with high-risk early breast cancer.
Genomic Health, Sanofi, and Amgen supported the study. Dr. Nitz and multiple coauthors disclosed financial relationships with these companies and others.
SOURCE: Nitz U et al. J Clin Oncol. 2019 Feb 20. doi: 10.1200/JCO.18.00028.
It appears to be safe to hold the anthracycline in patients with HER2-negative early breast cancer who are at intermediate-to-high genomic risk, results of a large randomized trial suggest.
Among both pre- and postmenopausal women with pathologic stage T1 to T4c with positive nodes or node-negative but high-risk early breast cancer, there were no significant differences in 5-year outcomes for patients treated with six cycles of docetaxel and cyclophosphamide (TC) or four cycles of epirubicin and cyclophosphamide followed by four cycles of docetaxel (EC-T), reported Ulrike Nitz, MD, from the West German Study Group in Mönchengladbach, Germany, and her colleagues in the West German Study PlanB Trial.
Disease-free survival (DFS, the primary endpoint), distant recurrence-free interval (dRFI), and overall survival (OS) “were excellent and virtually identical in patients who received the anthracycline-containing or the anthracycline-free regimen. Subgroups that benefited from the anthracycline-containing regimen were not identified by interaction analysis, although a potentially clinically relevant benefit in particular (e.g., high-risk) subgroups cannot be ruled out,” they wrote. The report is in Journal of Clinical Oncology.
The investigators noted that anthracyclines are associated with increased risk for cardiac disease and hematologic malignancies, prompting investigators in other trials to consider anthracycline-free regimens.
“As the number of long-term survivors, elderly patients, and patients with preexisting cardiac risk factors increases, the toxicity profile becomes a more important discriminator in adjuvant treatment selection,” they wrote.
The investigators enrolled and randomized 2,449 women (median age 55, range 25-77 years) who had histologically confirmed, unilateral primary invasive breast cancer, adequate surgical treatment, and no evidence of metastatic disease. The patients all had HER2-negative disease, pT1 to pT4c, known hormone receptor status, and either pN+ or pN0 with one or more risk factors.
The intention-to-treat analysis included 1,227 patients assigned to EC-T and 1,222 assigned to TC in the efficacy population, and 1,167 and 1,178 patients, respectively, in the safety population.
After a median follow-up of 60 months, the 5-year DFS rate in the TC-treated group was 89.6%, compared with 89.9% in the EC-T–treated group. The estimated 5-year dRFI rates were 94.1% vs. 93.4%, and the estimated 5-year OS rates were 94.7% vs. 94.5%, respectively. None of the comparisons were statistically significant.
There were five treatment-related deaths in the TC arm, (one each from urosepsis, Streptococcus septicemia, peritonitis/diverticulitis, Staphylococcus epidermidis septicemia, and pulmonary embolism), and one in the EC-T arm (from septicemia).
In an interim safety analysis, the rate of febrile neutropenia was 6.1% in the TC arm and 3.9% in the EC-T arm, leading to a recommendation for “generous” prophylaxis with granulocyte-colony stimulating factor, and ciprofloxacine for patients with a history of diverticulitis or chronic infectious GI disease, or expected duration of neutropenia greater than 1 week.
Rates of grade 3 or 4 leukopenia, neutropenia, nausea, vomiting, peripheral polyneuropathy, hand-foot syndrome, mucositis/stomatitis, arthralgia, myalgia, and fatigue were significantly higher among patients treated with EC-T. There were numerically more grade 3-4 infections and febrile neutropenia within the TC arm, but this trend did not reach statistical significance.
The investigators noted that the results of their trial provide the strongest evidence for patients with pathologic NO or N1 disease, and that the trial did not examine the question of dose-dense chemotherapy in patients with high-risk early breast cancer.
Genomic Health, Sanofi, and Amgen supported the study. Dr. Nitz and multiple coauthors disclosed financial relationships with these companies and others.
SOURCE: Nitz U et al. J Clin Oncol. 2019 Feb 20. doi: 10.1200/JCO.18.00028.
It appears to be safe to hold the anthracycline in patients with HER2-negative early breast cancer who are at intermediate-to-high genomic risk, results of a large randomized trial suggest.
Among both pre- and postmenopausal women with pathologic stage T1 to T4c with positive nodes or node-negative but high-risk early breast cancer, there were no significant differences in 5-year outcomes for patients treated with six cycles of docetaxel and cyclophosphamide (TC) or four cycles of epirubicin and cyclophosphamide followed by four cycles of docetaxel (EC-T), reported Ulrike Nitz, MD, from the West German Study Group in Mönchengladbach, Germany, and her colleagues in the West German Study PlanB Trial.
Disease-free survival (DFS, the primary endpoint), distant recurrence-free interval (dRFI), and overall survival (OS) “were excellent and virtually identical in patients who received the anthracycline-containing or the anthracycline-free regimen. Subgroups that benefited from the anthracycline-containing regimen were not identified by interaction analysis, although a potentially clinically relevant benefit in particular (e.g., high-risk) subgroups cannot be ruled out,” they wrote. The report is in Journal of Clinical Oncology.
The investigators noted that anthracyclines are associated with increased risk for cardiac disease and hematologic malignancies, prompting investigators in other trials to consider anthracycline-free regimens.
“As the number of long-term survivors, elderly patients, and patients with preexisting cardiac risk factors increases, the toxicity profile becomes a more important discriminator in adjuvant treatment selection,” they wrote.
The investigators enrolled and randomized 2,449 women (median age 55, range 25-77 years) who had histologically confirmed, unilateral primary invasive breast cancer, adequate surgical treatment, and no evidence of metastatic disease. The patients all had HER2-negative disease, pT1 to pT4c, known hormone receptor status, and either pN+ or pN0 with one or more risk factors.
The intention-to-treat analysis included 1,227 patients assigned to EC-T and 1,222 assigned to TC in the efficacy population, and 1,167 and 1,178 patients, respectively, in the safety population.
After a median follow-up of 60 months, the 5-year DFS rate in the TC-treated group was 89.6%, compared with 89.9% in the EC-T–treated group. The estimated 5-year dRFI rates were 94.1% vs. 93.4%, and the estimated 5-year OS rates were 94.7% vs. 94.5%, respectively. None of the comparisons were statistically significant.
There were five treatment-related deaths in the TC arm, (one each from urosepsis, Streptococcus septicemia, peritonitis/diverticulitis, Staphylococcus epidermidis septicemia, and pulmonary embolism), and one in the EC-T arm (from septicemia).
In an interim safety analysis, the rate of febrile neutropenia was 6.1% in the TC arm and 3.9% in the EC-T arm, leading to a recommendation for “generous” prophylaxis with granulocyte-colony stimulating factor, and ciprofloxacine for patients with a history of diverticulitis or chronic infectious GI disease, or expected duration of neutropenia greater than 1 week.
Rates of grade 3 or 4 leukopenia, neutropenia, nausea, vomiting, peripheral polyneuropathy, hand-foot syndrome, mucositis/stomatitis, arthralgia, myalgia, and fatigue were significantly higher among patients treated with EC-T. There were numerically more grade 3-4 infections and febrile neutropenia within the TC arm, but this trend did not reach statistical significance.
The investigators noted that the results of their trial provide the strongest evidence for patients with pathologic NO or N1 disease, and that the trial did not examine the question of dose-dense chemotherapy in patients with high-risk early breast cancer.
Genomic Health, Sanofi, and Amgen supported the study. Dr. Nitz and multiple coauthors disclosed financial relationships with these companies and others.
SOURCE: Nitz U et al. J Clin Oncol. 2019 Feb 20. doi: 10.1200/JCO.18.00028.
FROM JOURNAL of CLINICAL ONCOLOGY
ASCO issues guideline for early detection, management of colorectal cancer
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flexible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding, abdominal pain, and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
This story was updated on March 4, 2019.
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flexible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding, abdominal pain, and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
This story was updated on March 4, 2019.
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flexible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding, abdominal pain, and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
This story was updated on March 4, 2019.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Age 1 food allergies often disappear by age 6
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
SAN FRANCISCO –
Among 131 infants diagnosed with a peanut allergy when they were 1 year old and then followed with repeat testing 5 years later, 41 (31%) had complete resolution of their peanut allergy, while the allergy persisted in the other 90 children, Rachel L. Peters, PhD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The study also followed 404 infants diagnosed with an egg allergy at 1 year of age and found that by age 6 the allergy had resolved in 368 (91%), while persisting in 36 children, said Dr. Peters, an epidemiologist at Murdoch Children’s Research Institute in Parkville, Australia.
The analysis also identified risk factors that linked with an increased rate of allergy persistence. For peanut allergy persistence beyond the first year, the correlating factors were early-onset eczema, tree nut allergy, and a stronger peanut allergy identified by a greater than 4-mm reaction to a peanut skin-prick test. Factors that linked with an increased rate of persistent egg allergy were eczema, peanut allergy, gastrointestinal or respiratory reaction symptoms to milk, and reaction on an oral food challenge elicited by a low dose (less than 0.5 mL) of milk.
A consequence of the frequent resolution of these food allergies was that a positive skin-prick test reaction to either peanut or egg at 1 year old was poorly predictive of allergy status at age 6, while skin-prick tests at age 6 worked well for identifying a persistent food allergy at that age.
The analyses that Dr. Peters and her associates ran used data collected in the HealthNuts study, a comprehensive, prospective, population-based study of food allergies in children that enrolled 5,276 infants at 1 year old. The HealthNuts researchers enrolled infants at immunization clinics in the Melbourne area, with enrollment stratified to represent the people who live in that region (Clin Exp Allergy. 2010 Oct;40[10]:1516-22).
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Peters R et al. J Allergy Clin Immunol. 2019 Feb;143[2]:AB421.
REPORTING FROM AAAAI