User login
AAP policy promotes school attendance
Promoting school attendance can have positive effects on children’s health, according to a new policy statement from the American Academy of Pediatrics’ Council on School Health.
School absence can affect not only children’s academic achievement but also their health, and the AAP advises health care providers to promote regular school attendance as preventive medicine, wrote Mandy Allison, MD, of the University of Colorado and Children’s Hospital Colorado, both in Aurora, and Elliott Attisha, DO, FAAP, of the Detroit Public Schools Community District.
In the statement, published in Pediatrics, the authors detailed factors associated with chronic absenteeism and provided guidelines for how clinicians can help reduce and prevent the problem. “Regardless of whether absences are unexcused or excused, chronic absenteeism typically results in poor academic outcomes and is linked to poor health outcomes,” they noted.
Factors linked with chronic absenteeism, defined by the U.S. Department of Education as missing 15 or more days of school in a year, include socioeconomic factors such as poverty, domestic violence, and foster care, as well as poorly controlled health conditions, such as asthma and diabetes. Approximately 13% of all students meet criteria for chronic absenteeism, the researchers noted.
Chronic absenteeism has been linked to an increased risk of unhealthy behaviors, including mental health problems in teens and poor health in adulthood, and students who miss school often struggle academically and may be more likely to drop out, they noted.
The AAP statement emphasizes school strategies to improve attendance, including education on hand washing and other infection prevention measures, use of school-based flu vaccination programs, availability of school nurses and counselors, and other school-based health and nutrition services.
The policy statement encourages pediatricians and other health care providers to promote school attendance in the office setting and the community.
The AAP encourages pediatricians and their colleagues caring for children to promote school attendance. In the office setting, the AAP recommends the clinicians stress the importance of school attendance, ask whether children have been absent from school and how often, encourage families to share any health concerns with the school nurse, and provide firm and specific guidance on when children should go to school or stay home. The AAP also recommends encouraging well children to return to school after routine appointments rather than miss a whole day and documenting medical needs for an Individualized Education Program or 504 Plan to maximize learning and promote attendance.
For students who are chronically absent from school (missing 2-3 days/month), the AAP encourages clinicians to identify physical health issues and psychosocial factors that may be contributing to absenteeism and to communicate with school health providers. In rare cases, out-of-school educational services may be justified, but with an established time line for returning to school, according to the statement.
In addition, the AAP encourages clinicians to advocate in the community in support of school attendance by sharing relevant data on chronic absences, working with community leaders to send a consistent message about the value of school attendance, and serving as a school physician or on a school board or wellness committee to promote attendance.
The full statement is available online and includes links to parent handouts, a waiting room video, and a mobile-friendly website for preteens, teens, and parents.
The researchers had no financial conflicts to disclose.
SOURCE: Allison MA et al. Pediatrics. 2019. doi: 10.1542/peds.2018-3648.
“American pediatrics is somewhat unique in that we focus on promoting optimal development in addition to health as our primary mission. Pediatricians and their staffs have a role in promoting both school readiness and diminishing school absenteeism,” Francis Rushton Jr., MD, said in an interview to comment on the AAP statement.
“Sure, pediatric offices already face a tremendous amount of issues to cover at well child visits, but promoting school attendance overlaps with other discussions we already have with families. Sharing care plans with school nurses for asthmatics and medically complex children helps pediatric offices work synergistically with school health staff. Working with schools to identify social factors that contribute to poor school success and screening for social environmental or mental health issues are other ways in which we support attendance at school. These are just some examples of ideas that AAP shares with us in their recent statement, ideas we can work on, ideas that will help us enhance optimal development in our children,” Dr. Rushton added.
Dr. Rushton is affiliated with Beaufort (S.C.) Memorial Hospital, and he serves on the Pediatric News Editorial Advisory Board. He had no relevant financial conflicts to disclose.
“American pediatrics is somewhat unique in that we focus on promoting optimal development in addition to health as our primary mission. Pediatricians and their staffs have a role in promoting both school readiness and diminishing school absenteeism,” Francis Rushton Jr., MD, said in an interview to comment on the AAP statement.
“Sure, pediatric offices already face a tremendous amount of issues to cover at well child visits, but promoting school attendance overlaps with other discussions we already have with families. Sharing care plans with school nurses for asthmatics and medically complex children helps pediatric offices work synergistically with school health staff. Working with schools to identify social factors that contribute to poor school success and screening for social environmental or mental health issues are other ways in which we support attendance at school. These are just some examples of ideas that AAP shares with us in their recent statement, ideas we can work on, ideas that will help us enhance optimal development in our children,” Dr. Rushton added.
Dr. Rushton is affiliated with Beaufort (S.C.) Memorial Hospital, and he serves on the Pediatric News Editorial Advisory Board. He had no relevant financial conflicts to disclose.
“American pediatrics is somewhat unique in that we focus on promoting optimal development in addition to health as our primary mission. Pediatricians and their staffs have a role in promoting both school readiness and diminishing school absenteeism,” Francis Rushton Jr., MD, said in an interview to comment on the AAP statement.
“Sure, pediatric offices already face a tremendous amount of issues to cover at well child visits, but promoting school attendance overlaps with other discussions we already have with families. Sharing care plans with school nurses for asthmatics and medically complex children helps pediatric offices work synergistically with school health staff. Working with schools to identify social factors that contribute to poor school success and screening for social environmental or mental health issues are other ways in which we support attendance at school. These are just some examples of ideas that AAP shares with us in their recent statement, ideas we can work on, ideas that will help us enhance optimal development in our children,” Dr. Rushton added.
Dr. Rushton is affiliated with Beaufort (S.C.) Memorial Hospital, and he serves on the Pediatric News Editorial Advisory Board. He had no relevant financial conflicts to disclose.
Promoting school attendance can have positive effects on children’s health, according to a new policy statement from the American Academy of Pediatrics’ Council on School Health.
School absence can affect not only children’s academic achievement but also their health, and the AAP advises health care providers to promote regular school attendance as preventive medicine, wrote Mandy Allison, MD, of the University of Colorado and Children’s Hospital Colorado, both in Aurora, and Elliott Attisha, DO, FAAP, of the Detroit Public Schools Community District.
In the statement, published in Pediatrics, the authors detailed factors associated with chronic absenteeism and provided guidelines for how clinicians can help reduce and prevent the problem. “Regardless of whether absences are unexcused or excused, chronic absenteeism typically results in poor academic outcomes and is linked to poor health outcomes,” they noted.
Factors linked with chronic absenteeism, defined by the U.S. Department of Education as missing 15 or more days of school in a year, include socioeconomic factors such as poverty, domestic violence, and foster care, as well as poorly controlled health conditions, such as asthma and diabetes. Approximately 13% of all students meet criteria for chronic absenteeism, the researchers noted.
Chronic absenteeism has been linked to an increased risk of unhealthy behaviors, including mental health problems in teens and poor health in adulthood, and students who miss school often struggle academically and may be more likely to drop out, they noted.
The AAP statement emphasizes school strategies to improve attendance, including education on hand washing and other infection prevention measures, use of school-based flu vaccination programs, availability of school nurses and counselors, and other school-based health and nutrition services.
The policy statement encourages pediatricians and other health care providers to promote school attendance in the office setting and the community.
The AAP encourages pediatricians and their colleagues caring for children to promote school attendance. In the office setting, the AAP recommends the clinicians stress the importance of school attendance, ask whether children have been absent from school and how often, encourage families to share any health concerns with the school nurse, and provide firm and specific guidance on when children should go to school or stay home. The AAP also recommends encouraging well children to return to school after routine appointments rather than miss a whole day and documenting medical needs for an Individualized Education Program or 504 Plan to maximize learning and promote attendance.
For students who are chronically absent from school (missing 2-3 days/month), the AAP encourages clinicians to identify physical health issues and psychosocial factors that may be contributing to absenteeism and to communicate with school health providers. In rare cases, out-of-school educational services may be justified, but with an established time line for returning to school, according to the statement.
In addition, the AAP encourages clinicians to advocate in the community in support of school attendance by sharing relevant data on chronic absences, working with community leaders to send a consistent message about the value of school attendance, and serving as a school physician or on a school board or wellness committee to promote attendance.
The full statement is available online and includes links to parent handouts, a waiting room video, and a mobile-friendly website for preteens, teens, and parents.
The researchers had no financial conflicts to disclose.
SOURCE: Allison MA et al. Pediatrics. 2019. doi: 10.1542/peds.2018-3648.
Promoting school attendance can have positive effects on children’s health, according to a new policy statement from the American Academy of Pediatrics’ Council on School Health.
School absence can affect not only children’s academic achievement but also their health, and the AAP advises health care providers to promote regular school attendance as preventive medicine, wrote Mandy Allison, MD, of the University of Colorado and Children’s Hospital Colorado, both in Aurora, and Elliott Attisha, DO, FAAP, of the Detroit Public Schools Community District.
In the statement, published in Pediatrics, the authors detailed factors associated with chronic absenteeism and provided guidelines for how clinicians can help reduce and prevent the problem. “Regardless of whether absences are unexcused or excused, chronic absenteeism typically results in poor academic outcomes and is linked to poor health outcomes,” they noted.
Factors linked with chronic absenteeism, defined by the U.S. Department of Education as missing 15 or more days of school in a year, include socioeconomic factors such as poverty, domestic violence, and foster care, as well as poorly controlled health conditions, such as asthma and diabetes. Approximately 13% of all students meet criteria for chronic absenteeism, the researchers noted.
Chronic absenteeism has been linked to an increased risk of unhealthy behaviors, including mental health problems in teens and poor health in adulthood, and students who miss school often struggle academically and may be more likely to drop out, they noted.
The AAP statement emphasizes school strategies to improve attendance, including education on hand washing and other infection prevention measures, use of school-based flu vaccination programs, availability of school nurses and counselors, and other school-based health and nutrition services.
The policy statement encourages pediatricians and other health care providers to promote school attendance in the office setting and the community.
The AAP encourages pediatricians and their colleagues caring for children to promote school attendance. In the office setting, the AAP recommends the clinicians stress the importance of school attendance, ask whether children have been absent from school and how often, encourage families to share any health concerns with the school nurse, and provide firm and specific guidance on when children should go to school or stay home. The AAP also recommends encouraging well children to return to school after routine appointments rather than miss a whole day and documenting medical needs for an Individualized Education Program or 504 Plan to maximize learning and promote attendance.
For students who are chronically absent from school (missing 2-3 days/month), the AAP encourages clinicians to identify physical health issues and psychosocial factors that may be contributing to absenteeism and to communicate with school health providers. In rare cases, out-of-school educational services may be justified, but with an established time line for returning to school, according to the statement.
In addition, the AAP encourages clinicians to advocate in the community in support of school attendance by sharing relevant data on chronic absences, working with community leaders to send a consistent message about the value of school attendance, and serving as a school physician or on a school board or wellness committee to promote attendance.
The full statement is available online and includes links to parent handouts, a waiting room video, and a mobile-friendly website for preteens, teens, and parents.
The researchers had no financial conflicts to disclose.
SOURCE: Allison MA et al. Pediatrics. 2019. doi: 10.1542/peds.2018-3648.
FROM PEDIATRICS
Key clinical point: Clinicians can promote school attendance in the office and in the community as part of a preventive health strategy.
Major finding: Approximately 13% of all school age students in the United States miss 15 or more days of school each year, according to the American Academy of Pediatrics.
Study details: Statement by the American Academy of Pediatrics.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Allison MA et al. Pediatrics. 2019; doi: 10.1542/peds.2018-3648.
Family handgun ownership linked to young children’s gun deaths
A recent increase in U.S. handgun ownership among white families tracks with a similar trend of recently rising gun deaths among young white children, a new study found. This association held even after adjustments for multiple sociodemographic variables that research previously had linked to higher gun ownership and higher firearm mortality.
“Indeed, firearm ownership, generally, was positively associated with firearm-related mortality among 1- to 5-year-old white children, but this correlation was primarily driven by changes in the proportion of families who owned handguns: firearms more often stored unsecured and loaded,” wrote Kate C. Prickett, PhD, of the Victoria University of Wellington (New Zealand) and her associates in Pediatrics.
“These findings suggest that ease of access and use may be an important consideration when examining firearm-related fatality risk among young children,” they continued. Given the lack of attenuation in the relationship from controlling for sociodemographic variables, they add, “this finding is in line with research documenting that the presence of a firearm in the home matters above and beyond other risk factors associated with child injury.”
Even though U.S. gun ownership and pediatric firearm mortality overall have been dropping over the past several decades, the latter has stagnated recently, and gun deaths among children aged 1-4 years nearly doubled between 2006-2016, the researchers noted.
Given the counterintuitive increase in young children’s gun deaths while overall gun ownership kept dropping, the researchers took a closer look at the relationship between gun deaths among children aged 1-5 years and specific types of firearm ownership among families with children under age 5 years in the home. They relied on household data from the nationally representative General Social Survey and on fatality statistics from the National Vital Statistics System from 1976-2016.
Over those 4 decades, gun ownership in white families with small children decreased from 50% to 45% and in black families with small children from 38% to 6%.
Simultaneously, however, handgun ownership increased from 25% to 32% among white families with young children. In fact, most firearm-owning white families (72%) owned a handgun in 2016 while rifle ownership had declined substantially.
Meanwhile, “firearm-related mortality rate among young white children declined from historic highs in the late 1970s to early 1980s until 2001,” the authors reported. “After 2004, however, the mortality rate began to rise, reaching mid-1980s levels.” Further, gun deaths constituted 2% of young children’s injury deaths in 1976 but nearly 5% in 2016.
When the researchers compared these findings, they found a positive, significant association between white child firearm mortality and the proportion of white families who owned a handgun but not a rifle or shotgun.
The association remained after the researchers adjusted for several covariates already established in the evidence base to have associations with firearm ownership, child injury risk and/or firearm mortality: living in a rural area, living in the South, neither parent having a college degree, and a household income in the bottom quartile nationally. In addition, “the annual national unemployment rate by race was included as an indicator of the broader economic context,” the authors wrote.
Although young black children die from guns nearly three times more frequently than white children, the authors were unable to present detailed findings on associations with gun ownership because of small sample sizes. They noted, however, that handgun ownership actually declined during the study period from 15% to 6% in black families with young children.
The researchers concluded that the recent increase in young children’s gun deaths may be partly driven by an increase in handgun ownership, even as overall gun ownership (primarily rifles and shotguns) has continued dropping.
“For young children, shootings are more likely to be unintentional, making the ease at which firearms can be accessed and used a more important determinant of mortality than perhaps for older children,” the authors wrote. “Moreover, relative to other firearms like hunting rifles, handguns, because they are more likely to be purchased for personal protection, are more likely to be stored loaded with ammunition, unlocked, and in a more easily accessible place, such as a bedroom drawer.”
The research was funded by the National Institute of Child Health and Human Development. The authors reported having no conflicts of interest.
SOURCE: Prickett KC et al. Pediatrics. 2019;143(2):e20181171.
The “unique and important approach” used by Prickett et al. to investigate an association between gun ownership and children’s gun deaths is “novel” because of their focus on firearm types and the youngest children, wrote Shilpa J. Patel, MD; Monika K. Goyal, MD; and Kavita Parikh, MD, all with the Children’s National Health System in Washington, DC, in an editorial published with the study (Pediatrics. 2018 Jan 28. doi: 10.1542/peds.2018-3611).
The findings are particularly relevant to pediatricians’ conversations with families about safe firearm storage practices. The American Academy of Pediatrics recommends all firearms are stored locked and unloaded with ammunition stored separately.
For families who find these guidelines difficult because they keep handguns at the ready for protection, “it is important to note that the risk of unintentional or intentional injury from a household firearm is much greater than the likelihood of providing protection for self-defense,” the editorial’s authors wrote. But they advocate for personalized safe storage strategies and shared decision making based on families’ needs and values.
“This study is a loud and compelling call to action for all pediatricians to start open discussions around firearm ownership with all families and to share data on the significant risks associated with unsafe storage,” they wrote. “It is an even louder call to firearm manufacturers to step up and innovate, test, and design smart handguns that are inoperable by young children to prevent unintentional injury.”
Although having no firearms in the home is the most effective way to reduce children’s risk of gun-related injuries and deaths, developing effective safety controls on guns could also substantially curtail young children’s gun deaths. “We as a society should be advocating for continued research to childproof firearms so that if families choose to have firearms in the home, the safety of their children is not compromised,” they wrote.
Dr. Parikh is a hospitalist, Dr. Goyal is assistant division chief or emergency medicine, and Dr. Patel is an emergency medicine specialist, all with Children’s National Health System in Washington, DC. They reported no funding and no disclosures.
The “unique and important approach” used by Prickett et al. to investigate an association between gun ownership and children’s gun deaths is “novel” because of their focus on firearm types and the youngest children, wrote Shilpa J. Patel, MD; Monika K. Goyal, MD; and Kavita Parikh, MD, all with the Children’s National Health System in Washington, DC, in an editorial published with the study (Pediatrics. 2018 Jan 28. doi: 10.1542/peds.2018-3611).
The findings are particularly relevant to pediatricians’ conversations with families about safe firearm storage practices. The American Academy of Pediatrics recommends all firearms are stored locked and unloaded with ammunition stored separately.
For families who find these guidelines difficult because they keep handguns at the ready for protection, “it is important to note that the risk of unintentional or intentional injury from a household firearm is much greater than the likelihood of providing protection for self-defense,” the editorial’s authors wrote. But they advocate for personalized safe storage strategies and shared decision making based on families’ needs and values.
“This study is a loud and compelling call to action for all pediatricians to start open discussions around firearm ownership with all families and to share data on the significant risks associated with unsafe storage,” they wrote. “It is an even louder call to firearm manufacturers to step up and innovate, test, and design smart handguns that are inoperable by young children to prevent unintentional injury.”
Although having no firearms in the home is the most effective way to reduce children’s risk of gun-related injuries and deaths, developing effective safety controls on guns could also substantially curtail young children’s gun deaths. “We as a society should be advocating for continued research to childproof firearms so that if families choose to have firearms in the home, the safety of their children is not compromised,” they wrote.
Dr. Parikh is a hospitalist, Dr. Goyal is assistant division chief or emergency medicine, and Dr. Patel is an emergency medicine specialist, all with Children’s National Health System in Washington, DC. They reported no funding and no disclosures.
The “unique and important approach” used by Prickett et al. to investigate an association between gun ownership and children’s gun deaths is “novel” because of their focus on firearm types and the youngest children, wrote Shilpa J. Patel, MD; Monika K. Goyal, MD; and Kavita Parikh, MD, all with the Children’s National Health System in Washington, DC, in an editorial published with the study (Pediatrics. 2018 Jan 28. doi: 10.1542/peds.2018-3611).
The findings are particularly relevant to pediatricians’ conversations with families about safe firearm storage practices. The American Academy of Pediatrics recommends all firearms are stored locked and unloaded with ammunition stored separately.
For families who find these guidelines difficult because they keep handguns at the ready for protection, “it is important to note that the risk of unintentional or intentional injury from a household firearm is much greater than the likelihood of providing protection for self-defense,” the editorial’s authors wrote. But they advocate for personalized safe storage strategies and shared decision making based on families’ needs and values.
“This study is a loud and compelling call to action for all pediatricians to start open discussions around firearm ownership with all families and to share data on the significant risks associated with unsafe storage,” they wrote. “It is an even louder call to firearm manufacturers to step up and innovate, test, and design smart handguns that are inoperable by young children to prevent unintentional injury.”
Although having no firearms in the home is the most effective way to reduce children’s risk of gun-related injuries and deaths, developing effective safety controls on guns could also substantially curtail young children’s gun deaths. “We as a society should be advocating for continued research to childproof firearms so that if families choose to have firearms in the home, the safety of their children is not compromised,” they wrote.
Dr. Parikh is a hospitalist, Dr. Goyal is assistant division chief or emergency medicine, and Dr. Patel is an emergency medicine specialist, all with Children’s National Health System in Washington, DC. They reported no funding and no disclosures.
A recent increase in U.S. handgun ownership among white families tracks with a similar trend of recently rising gun deaths among young white children, a new study found. This association held even after adjustments for multiple sociodemographic variables that research previously had linked to higher gun ownership and higher firearm mortality.
“Indeed, firearm ownership, generally, was positively associated with firearm-related mortality among 1- to 5-year-old white children, but this correlation was primarily driven by changes in the proportion of families who owned handguns: firearms more often stored unsecured and loaded,” wrote Kate C. Prickett, PhD, of the Victoria University of Wellington (New Zealand) and her associates in Pediatrics.
“These findings suggest that ease of access and use may be an important consideration when examining firearm-related fatality risk among young children,” they continued. Given the lack of attenuation in the relationship from controlling for sociodemographic variables, they add, “this finding is in line with research documenting that the presence of a firearm in the home matters above and beyond other risk factors associated with child injury.”
Even though U.S. gun ownership and pediatric firearm mortality overall have been dropping over the past several decades, the latter has stagnated recently, and gun deaths among children aged 1-4 years nearly doubled between 2006-2016, the researchers noted.
Given the counterintuitive increase in young children’s gun deaths while overall gun ownership kept dropping, the researchers took a closer look at the relationship between gun deaths among children aged 1-5 years and specific types of firearm ownership among families with children under age 5 years in the home. They relied on household data from the nationally representative General Social Survey and on fatality statistics from the National Vital Statistics System from 1976-2016.
Over those 4 decades, gun ownership in white families with small children decreased from 50% to 45% and in black families with small children from 38% to 6%.
Simultaneously, however, handgun ownership increased from 25% to 32% among white families with young children. In fact, most firearm-owning white families (72%) owned a handgun in 2016 while rifle ownership had declined substantially.
Meanwhile, “firearm-related mortality rate among young white children declined from historic highs in the late 1970s to early 1980s until 2001,” the authors reported. “After 2004, however, the mortality rate began to rise, reaching mid-1980s levels.” Further, gun deaths constituted 2% of young children’s injury deaths in 1976 but nearly 5% in 2016.
When the researchers compared these findings, they found a positive, significant association between white child firearm mortality and the proportion of white families who owned a handgun but not a rifle or shotgun.
The association remained after the researchers adjusted for several covariates already established in the evidence base to have associations with firearm ownership, child injury risk and/or firearm mortality: living in a rural area, living in the South, neither parent having a college degree, and a household income in the bottom quartile nationally. In addition, “the annual national unemployment rate by race was included as an indicator of the broader economic context,” the authors wrote.
Although young black children die from guns nearly three times more frequently than white children, the authors were unable to present detailed findings on associations with gun ownership because of small sample sizes. They noted, however, that handgun ownership actually declined during the study period from 15% to 6% in black families with young children.
The researchers concluded that the recent increase in young children’s gun deaths may be partly driven by an increase in handgun ownership, even as overall gun ownership (primarily rifles and shotguns) has continued dropping.
“For young children, shootings are more likely to be unintentional, making the ease at which firearms can be accessed and used a more important determinant of mortality than perhaps for older children,” the authors wrote. “Moreover, relative to other firearms like hunting rifles, handguns, because they are more likely to be purchased for personal protection, are more likely to be stored loaded with ammunition, unlocked, and in a more easily accessible place, such as a bedroom drawer.”
The research was funded by the National Institute of Child Health and Human Development. The authors reported having no conflicts of interest.
SOURCE: Prickett KC et al. Pediatrics. 2019;143(2):e20181171.
A recent increase in U.S. handgun ownership among white families tracks with a similar trend of recently rising gun deaths among young white children, a new study found. This association held even after adjustments for multiple sociodemographic variables that research previously had linked to higher gun ownership and higher firearm mortality.
“Indeed, firearm ownership, generally, was positively associated with firearm-related mortality among 1- to 5-year-old white children, but this correlation was primarily driven by changes in the proportion of families who owned handguns: firearms more often stored unsecured and loaded,” wrote Kate C. Prickett, PhD, of the Victoria University of Wellington (New Zealand) and her associates in Pediatrics.
“These findings suggest that ease of access and use may be an important consideration when examining firearm-related fatality risk among young children,” they continued. Given the lack of attenuation in the relationship from controlling for sociodemographic variables, they add, “this finding is in line with research documenting that the presence of a firearm in the home matters above and beyond other risk factors associated with child injury.”
Even though U.S. gun ownership and pediatric firearm mortality overall have been dropping over the past several decades, the latter has stagnated recently, and gun deaths among children aged 1-4 years nearly doubled between 2006-2016, the researchers noted.
Given the counterintuitive increase in young children’s gun deaths while overall gun ownership kept dropping, the researchers took a closer look at the relationship between gun deaths among children aged 1-5 years and specific types of firearm ownership among families with children under age 5 years in the home. They relied on household data from the nationally representative General Social Survey and on fatality statistics from the National Vital Statistics System from 1976-2016.
Over those 4 decades, gun ownership in white families with small children decreased from 50% to 45% and in black families with small children from 38% to 6%.
Simultaneously, however, handgun ownership increased from 25% to 32% among white families with young children. In fact, most firearm-owning white families (72%) owned a handgun in 2016 while rifle ownership had declined substantially.
Meanwhile, “firearm-related mortality rate among young white children declined from historic highs in the late 1970s to early 1980s until 2001,” the authors reported. “After 2004, however, the mortality rate began to rise, reaching mid-1980s levels.” Further, gun deaths constituted 2% of young children’s injury deaths in 1976 but nearly 5% in 2016.
When the researchers compared these findings, they found a positive, significant association between white child firearm mortality and the proportion of white families who owned a handgun but not a rifle or shotgun.
The association remained after the researchers adjusted for several covariates already established in the evidence base to have associations with firearm ownership, child injury risk and/or firearm mortality: living in a rural area, living in the South, neither parent having a college degree, and a household income in the bottom quartile nationally. In addition, “the annual national unemployment rate by race was included as an indicator of the broader economic context,” the authors wrote.
Although young black children die from guns nearly three times more frequently than white children, the authors were unable to present detailed findings on associations with gun ownership because of small sample sizes. They noted, however, that handgun ownership actually declined during the study period from 15% to 6% in black families with young children.
The researchers concluded that the recent increase in young children’s gun deaths may be partly driven by an increase in handgun ownership, even as overall gun ownership (primarily rifles and shotguns) has continued dropping.
“For young children, shootings are more likely to be unintentional, making the ease at which firearms can be accessed and used a more important determinant of mortality than perhaps for older children,” the authors wrote. “Moreover, relative to other firearms like hunting rifles, handguns, because they are more likely to be purchased for personal protection, are more likely to be stored loaded with ammunition, unlocked, and in a more easily accessible place, such as a bedroom drawer.”
The research was funded by the National Institute of Child Health and Human Development. The authors reported having no conflicts of interest.
SOURCE: Prickett KC et al. Pediatrics. 2019;143(2):e20181171.
FROM PEDIATRICS
Key clinical point: Greater handgun ownership in families may increase young children’s risk of gun death.
Major finding: Handgun ownership in white families with young children rose from 25% to 32% during 1976-2016, alongside increasing rates of firearm deaths in young white children.
Study details: The findings are based on analysis of data on U.S. family firearm ownership and pediatric gun deaths in the General Social Study and National Vital Statistics System from 1976-2016.
Disclosures: The research was funded by the National Institute of Child Health and Human Development. The authors reported having no conflicts of interest.
Source: Prickett KC et al. Pediatrics. 2019 Jan 28;143(2):e20181171.
Pediatricians get more guidance to be proactive on youth e-cig use
The American Academy of Pediatrics is pushing for pediatricians to be more proactive in keeping youth from becoming addicted to nicotine through the use of electronic cigarettes. “E-cigarettes are the most common tobacco product used among youth,” Brian Jenssen, MD, policy chair of the AAP Section on Tobacco Control and Susan Walley, MD, chair of the section, wrote in recommendations for pediatricians and policy makers regarding the use of e-cigarettes and similar devices. These recommendations were published in0 Pediatrics.
“To prevent children, adolescents, and young adults from transitioning from e-cigarettes to traditional cigarettes and to minimize the potential public health harm from e-cigarette use, there is a critical need for e-cigarette legislative action, and counterpromotion to help youth live tobacco-free lives,” the authors continued.
To that end, AAP is making a series of recommended actions by pediatricians. First, they are calling for pediatricians to screen for e-cigarette use and exposure and to provide prevention counseling in clinical practice.
Second, the organization is calling on pediatricians to provide counseling that areas where youth spend time – including homes, cars, schools, and other places – should have “comprehensive tobacco-free bans that include e-cigarettes as well as combustible tobacco products.”
Finally, pediatricians should never recommend e-cigarettes as a tobacco-dependence treatment product.
AAP in the guidance document also made a series of policy recommendations, including calling on the Food and Drug Administration to regulate e-cigarettes as they do traditional tobacco products; ban the sale of e-cigarettes to anyone under 21 years of age; ban all flavored e-cigarettes, including menthol; ban advertising of e-cigarettes that is accessible to youth; tax e-cigarettes similar to traditional cigarettes; and incorporate e-cigarettes into current tobacco-free laws and ordinances.
Dr. Jenssen and Dr. Walley also call for more research to inform public policy and understand health effects.
“Additional research is needed to understand the trajectory of addiction among youth and the progression to combustible tobacco products,” they wrote. “Studies are needed to determine if and how e-cigarettes may be effective for smoking cessation; these trials must be carefully designed and adequately powered. Finally, research is needed to evaluate effective countermessaging and public health interventions.”
SOURCE: Jenssen B et al. Pediatrics. doi: 10.1542/peds.2018-3652.
The American Academy of Pediatrics is pushing for pediatricians to be more proactive in keeping youth from becoming addicted to nicotine through the use of electronic cigarettes. “E-cigarettes are the most common tobacco product used among youth,” Brian Jenssen, MD, policy chair of the AAP Section on Tobacco Control and Susan Walley, MD, chair of the section, wrote in recommendations for pediatricians and policy makers regarding the use of e-cigarettes and similar devices. These recommendations were published in0 Pediatrics.
“To prevent children, adolescents, and young adults from transitioning from e-cigarettes to traditional cigarettes and to minimize the potential public health harm from e-cigarette use, there is a critical need for e-cigarette legislative action, and counterpromotion to help youth live tobacco-free lives,” the authors continued.
To that end, AAP is making a series of recommended actions by pediatricians. First, they are calling for pediatricians to screen for e-cigarette use and exposure and to provide prevention counseling in clinical practice.
Second, the organization is calling on pediatricians to provide counseling that areas where youth spend time – including homes, cars, schools, and other places – should have “comprehensive tobacco-free bans that include e-cigarettes as well as combustible tobacco products.”
Finally, pediatricians should never recommend e-cigarettes as a tobacco-dependence treatment product.
AAP in the guidance document also made a series of policy recommendations, including calling on the Food and Drug Administration to regulate e-cigarettes as they do traditional tobacco products; ban the sale of e-cigarettes to anyone under 21 years of age; ban all flavored e-cigarettes, including menthol; ban advertising of e-cigarettes that is accessible to youth; tax e-cigarettes similar to traditional cigarettes; and incorporate e-cigarettes into current tobacco-free laws and ordinances.
Dr. Jenssen and Dr. Walley also call for more research to inform public policy and understand health effects.
“Additional research is needed to understand the trajectory of addiction among youth and the progression to combustible tobacco products,” they wrote. “Studies are needed to determine if and how e-cigarettes may be effective for smoking cessation; these trials must be carefully designed and adequately powered. Finally, research is needed to evaluate effective countermessaging and public health interventions.”
SOURCE: Jenssen B et al. Pediatrics. doi: 10.1542/peds.2018-3652.
The American Academy of Pediatrics is pushing for pediatricians to be more proactive in keeping youth from becoming addicted to nicotine through the use of electronic cigarettes. “E-cigarettes are the most common tobacco product used among youth,” Brian Jenssen, MD, policy chair of the AAP Section on Tobacco Control and Susan Walley, MD, chair of the section, wrote in recommendations for pediatricians and policy makers regarding the use of e-cigarettes and similar devices. These recommendations were published in0 Pediatrics.
“To prevent children, adolescents, and young adults from transitioning from e-cigarettes to traditional cigarettes and to minimize the potential public health harm from e-cigarette use, there is a critical need for e-cigarette legislative action, and counterpromotion to help youth live tobacco-free lives,” the authors continued.
To that end, AAP is making a series of recommended actions by pediatricians. First, they are calling for pediatricians to screen for e-cigarette use and exposure and to provide prevention counseling in clinical practice.
Second, the organization is calling on pediatricians to provide counseling that areas where youth spend time – including homes, cars, schools, and other places – should have “comprehensive tobacco-free bans that include e-cigarettes as well as combustible tobacco products.”
Finally, pediatricians should never recommend e-cigarettes as a tobacco-dependence treatment product.
AAP in the guidance document also made a series of policy recommendations, including calling on the Food and Drug Administration to regulate e-cigarettes as they do traditional tobacco products; ban the sale of e-cigarettes to anyone under 21 years of age; ban all flavored e-cigarettes, including menthol; ban advertising of e-cigarettes that is accessible to youth; tax e-cigarettes similar to traditional cigarettes; and incorporate e-cigarettes into current tobacco-free laws and ordinances.
Dr. Jenssen and Dr. Walley also call for more research to inform public policy and understand health effects.
“Additional research is needed to understand the trajectory of addiction among youth and the progression to combustible tobacco products,” they wrote. “Studies are needed to determine if and how e-cigarettes may be effective for smoking cessation; these trials must be carefully designed and adequately powered. Finally, research is needed to evaluate effective countermessaging and public health interventions.”
SOURCE: Jenssen B et al. Pediatrics. doi: 10.1542/peds.2018-3652.
FROM PEDIATRICS
Key clinical point: Pediatrics, policy makers need to be more proactive to curb e-cig use in youth
Major finding: There is a critical need for more regulation to protect youth from harmful effects of e-cigs.
Study details: Recommendations by the American Academy of Pediatrics to help minimize youth exposure to e-cigs.
Disclosures: No disclosures were reported by the authors.
Source: Jenssen B et al. Pediatrics. 2019. doi: 10.1542/peds.2018-3652.
Long-term opioid use substantial in elderly adults prior to total joint replacement
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Long-term opioid use is highly prevalent among older adults with osteoarthritis who underwent total joint replacement.
Major finding: Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama.
Study details: An observational cohort study including 358,121 Medicare enrollees with advanced osteoarthritis.
Disclosures: Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Coauthors provided disclosures related to a number of pharmaceutical companies.
Source: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
What are the best treatments for reducing osteoporotic compression fracture pain?
EVIDENCE SUMMARY
A 2015 meta-analysis of 8 RCTs compared pain reduction in adults >50 years with osteoporotic compression fractures who received either vertebral augmentation (vertebroplasty or balloon kyphoplasty; 495 patients) or conservative or sham treatment (492 patients).1 Pain was measured by the visual analog scale (VAS) periodically between 1 week and 1 year.
The study included patients of both sexes who had an acute or chronic osteoporotic vertebral compression fracture that caused pain and functional limitations in daily activities. It excluded patients with neoplasm, pre-existing chronic pain or functional disability unrelated to vertebral fractures, and vertebral fractures unaccompanied by signal changes on magnetic resonance imaging.
Vertebral augmentation resulted in small to moderate reductions in pain scores compared with placebo at 1 to 4 weeks (7 trials, 938 patients; standardized mean difference [SMD]=0.3; 95% confidence interval [CI], 0.1-0.5), 2 to 3 months (7 trials, 953 patients; SMD=0.3; 95% CI, 0.1-0.4), and 1 year (5 trials, 744 patients; SMD=0.3; 95% CI, 0.1-0.4). The study is considered low-quality because of increased heterogeneity.
Calcitonin reduces pain but with some adverse effects
A 2011 meta-analysis of 10 RCTs (467 patients) examined the analgesic effectiveness of calcitonin in adults >60 years, of either sex, with osteoporotic compression fractures who received calcitonin in the acute phase (<10 days after fracture) and chronic phase (>3 months after fracture).2 For acute fractures, pain was measured at 1, 2, 3, and 4 weeks following treatment. For chronic fractures, pain was measured at 1, 3, and 6 months post-treatment.
Continue to: Calcitonin was administered...
Calcitonin was administered in varying doses by various routes (200 IU intranasal, 50-200 IU intramuscular or subcutaneous injection, or 200 IU rectal suppository) and compared with placebo, usual treatment, or other analgesia. The VAS was varied (10 cm, 100 mm, or 5-point) and assessed pain and length of time to mobilization with patients at rest, sitting, standing, and walking by using mean deviation (MD) and SMD.
In the acute phase, calcitonin resulted in greater pain relief 1 week after fracture at rest (4 trials; 260 patients; 10-cm VAS; MD=−3.4; 95% CI, −4 to −2.8) and with walking (4 trials, 228 patients; SMD=2.6; 95% CI, −4.1 to −1.1) compared with the control group. At 6 months, calcitonin had reduced pain in mobile patients more than in the control group (7 trials, 207 patients; SMD=−0.5; 95% CI, −0.9 to −0.1).
Statistically significant adverse effects of calcitonin included gastrointestinal disturbances and flushing compared with placebo. Adverse effects were more predominant in the studies that used injectable calcitonin and in the chronic pain group. The study is considered low-quality because of increased heterogeneity in the acute pain studies.
1. Li L, Ren J, Liu J, et al. Results of vertebral augmentation treatment for patients of painful osteoporotic vertebral compression fractures: a meta-analysis of eight randomized controlled trials. PLoS ONE. 2015;10:e0138126.
2. Knopp-Sihota JA, Newburn-Cook CV, Homik J, et al. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic compression fractures: a systematic review and meta-analysis. Osteoporos Intl. 2012;23:17-38.
EVIDENCE SUMMARY
A 2015 meta-analysis of 8 RCTs compared pain reduction in adults >50 years with osteoporotic compression fractures who received either vertebral augmentation (vertebroplasty or balloon kyphoplasty; 495 patients) or conservative or sham treatment (492 patients).1 Pain was measured by the visual analog scale (VAS) periodically between 1 week and 1 year.
The study included patients of both sexes who had an acute or chronic osteoporotic vertebral compression fracture that caused pain and functional limitations in daily activities. It excluded patients with neoplasm, pre-existing chronic pain or functional disability unrelated to vertebral fractures, and vertebral fractures unaccompanied by signal changes on magnetic resonance imaging.
Vertebral augmentation resulted in small to moderate reductions in pain scores compared with placebo at 1 to 4 weeks (7 trials, 938 patients; standardized mean difference [SMD]=0.3; 95% confidence interval [CI], 0.1-0.5), 2 to 3 months (7 trials, 953 patients; SMD=0.3; 95% CI, 0.1-0.4), and 1 year (5 trials, 744 patients; SMD=0.3; 95% CI, 0.1-0.4). The study is considered low-quality because of increased heterogeneity.
Calcitonin reduces pain but with some adverse effects
A 2011 meta-analysis of 10 RCTs (467 patients) examined the analgesic effectiveness of calcitonin in adults >60 years, of either sex, with osteoporotic compression fractures who received calcitonin in the acute phase (<10 days after fracture) and chronic phase (>3 months after fracture).2 For acute fractures, pain was measured at 1, 2, 3, and 4 weeks following treatment. For chronic fractures, pain was measured at 1, 3, and 6 months post-treatment.
Continue to: Calcitonin was administered...
Calcitonin was administered in varying doses by various routes (200 IU intranasal, 50-200 IU intramuscular or subcutaneous injection, or 200 IU rectal suppository) and compared with placebo, usual treatment, or other analgesia. The VAS was varied (10 cm, 100 mm, or 5-point) and assessed pain and length of time to mobilization with patients at rest, sitting, standing, and walking by using mean deviation (MD) and SMD.
In the acute phase, calcitonin resulted in greater pain relief 1 week after fracture at rest (4 trials; 260 patients; 10-cm VAS; MD=−3.4; 95% CI, −4 to −2.8) and with walking (4 trials, 228 patients; SMD=2.6; 95% CI, −4.1 to −1.1) compared with the control group. At 6 months, calcitonin had reduced pain in mobile patients more than in the control group (7 trials, 207 patients; SMD=−0.5; 95% CI, −0.9 to −0.1).
Statistically significant adverse effects of calcitonin included gastrointestinal disturbances and flushing compared with placebo. Adverse effects were more predominant in the studies that used injectable calcitonin and in the chronic pain group. The study is considered low-quality because of increased heterogeneity in the acute pain studies.
EVIDENCE SUMMARY
A 2015 meta-analysis of 8 RCTs compared pain reduction in adults >50 years with osteoporotic compression fractures who received either vertebral augmentation (vertebroplasty or balloon kyphoplasty; 495 patients) or conservative or sham treatment (492 patients).1 Pain was measured by the visual analog scale (VAS) periodically between 1 week and 1 year.
The study included patients of both sexes who had an acute or chronic osteoporotic vertebral compression fracture that caused pain and functional limitations in daily activities. It excluded patients with neoplasm, pre-existing chronic pain or functional disability unrelated to vertebral fractures, and vertebral fractures unaccompanied by signal changes on magnetic resonance imaging.
Vertebral augmentation resulted in small to moderate reductions in pain scores compared with placebo at 1 to 4 weeks (7 trials, 938 patients; standardized mean difference [SMD]=0.3; 95% confidence interval [CI], 0.1-0.5), 2 to 3 months (7 trials, 953 patients; SMD=0.3; 95% CI, 0.1-0.4), and 1 year (5 trials, 744 patients; SMD=0.3; 95% CI, 0.1-0.4). The study is considered low-quality because of increased heterogeneity.
Calcitonin reduces pain but with some adverse effects
A 2011 meta-analysis of 10 RCTs (467 patients) examined the analgesic effectiveness of calcitonin in adults >60 years, of either sex, with osteoporotic compression fractures who received calcitonin in the acute phase (<10 days after fracture) and chronic phase (>3 months after fracture).2 For acute fractures, pain was measured at 1, 2, 3, and 4 weeks following treatment. For chronic fractures, pain was measured at 1, 3, and 6 months post-treatment.
Continue to: Calcitonin was administered...
Calcitonin was administered in varying doses by various routes (200 IU intranasal, 50-200 IU intramuscular or subcutaneous injection, or 200 IU rectal suppository) and compared with placebo, usual treatment, or other analgesia. The VAS was varied (10 cm, 100 mm, or 5-point) and assessed pain and length of time to mobilization with patients at rest, sitting, standing, and walking by using mean deviation (MD) and SMD.
In the acute phase, calcitonin resulted in greater pain relief 1 week after fracture at rest (4 trials; 260 patients; 10-cm VAS; MD=−3.4; 95% CI, −4 to −2.8) and with walking (4 trials, 228 patients; SMD=2.6; 95% CI, −4.1 to −1.1) compared with the control group. At 6 months, calcitonin had reduced pain in mobile patients more than in the control group (7 trials, 207 patients; SMD=−0.5; 95% CI, −0.9 to −0.1).
Statistically significant adverse effects of calcitonin included gastrointestinal disturbances and flushing compared with placebo. Adverse effects were more predominant in the studies that used injectable calcitonin and in the chronic pain group. The study is considered low-quality because of increased heterogeneity in the acute pain studies.
1. Li L, Ren J, Liu J, et al. Results of vertebral augmentation treatment for patients of painful osteoporotic vertebral compression fractures: a meta-analysis of eight randomized controlled trials. PLoS ONE. 2015;10:e0138126.
2. Knopp-Sihota JA, Newburn-Cook CV, Homik J, et al. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic compression fractures: a systematic review and meta-analysis. Osteoporos Intl. 2012;23:17-38.
1. Li L, Ren J, Liu J, et al. Results of vertebral augmentation treatment for patients of painful osteoporotic vertebral compression fractures: a meta-analysis of eight randomized controlled trials. PLoS ONE. 2015;10:e0138126.
2. Knopp-Sihota JA, Newburn-Cook CV, Homik J, et al. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic compression fractures: a systematic review and meta-analysis. Osteoporos Intl. 2012;23:17-38.
EVIDENCE-BASED ANSWER:
Vertebral augmentation with vertebroplasty or balloon kyphoplasty yields a small reduction in both acute and chronic pain scores in adults with osteoporotic compression fractures compared with conservative therapy or sham treatment (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs]).
When compared with placebo, usual treatment, or other analgesia, calcitonin reduces the severity and duration of pain at rest and with mobility 1 week after an osteoporotic compression fracture and with mobility at 6 months postfracture (SOR: B, meta-analysis of RCTs).
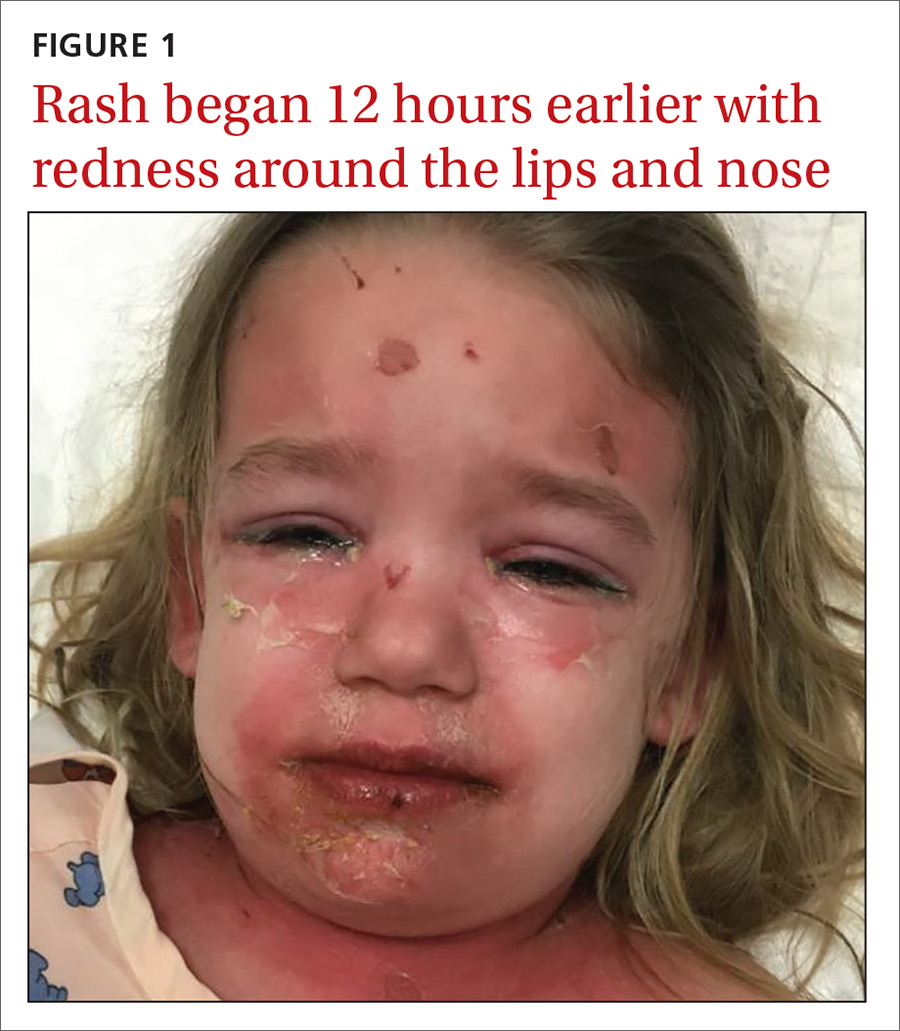
A young girl with a painful rash
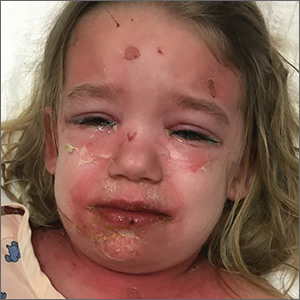
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.
On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; npotisek@wakehealth.edu
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.
On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; npotisek@wakehealth.edu
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.
On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; npotisek@wakehealth.edu
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
History of melanoma in situ • dyspnea • rib pain • Dx?
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
Leg-length discrepancy • asymmetric gluteal folds and popliteal fossae • positive Galeazzi test • Dx?
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.
Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).
DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5
What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; bethpdavis@emory.edu.
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.
Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).
DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5
What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; bethpdavis@emory.edu.
THE CASE
A healthy 6-month-old girl born via spontaneous vaginal delivery to a 33-year-old mother presented to her family physician (FP) for a routine well-child examination. The mother’s prenatal anatomy scan, delivery, and personal and family history were unremarkable. The patient was not firstborn or breech, and there was no family history of hip dysplasia. On prior infant well-child examinations, Ortolani and Barlow maneuvers were negative, and the patient demonstrated spontaneous movement of both legs. There was no evidence of hip dysplasia, lower extremity weakness, musculoskeletal abnormalities, or abnormal skin markings. The patient had normal growth and development (50th percentile for height and weight, average Ages & Stages Questionnaire scores) and no history of infection or trauma.
At the current presentation, the FP noted a leg-length discrepancy while palpating the bony (patellar and malleolar) landmarks of the lower extremities, but the right and left anterior superior iliac spine was symmetrical. The gluteal folds and popliteal fossae were asymmetric, a Galeazzi test was positive, and the right leg measured approximately 2 cm shorter than the left leg. There was no evidence of scoliosis or pelvic abnormalities. Physical examination revealed no ecchymosis or trauma. Orthopedic evaluation by the FP of the hips, knees, and ankles was normal, including negative repeat Ortolani and Barlow maneuvers and normal range of motion. We obtained x-rays of the lower extremities and ordered an orthopedic consultation.
THE DIAGNOSIS
The differential diagnosis included congenital, traumatic, infectious, inflammatory, idiopathic, and neurologic causes.1-3 The most common etiologies of leg-length discrepancies are summarized in TABLE 1.1-3 Radiographic imaging showed a femur length discrepancy, which was determined to be congenital without indication of trauma or disease; therefore, a diagnosis of congenital femoral bowing was made.
Initial orthopedic evaluation revealed a femur length discrepancy of approximately 2 cm. Plain films showed lateral femoral bowing (FIGURE 1A).
DISCUSSION
Congenital femoral bowing, which can present as a leg-length discrepancy in infants, is a relatively rare finding with an incidence of 1 per 52,000 births.4 Our patient presented with an isolated limb deformity, but congenital femoral bowing is recognized as a clinical feature of several skeletal dysplasias (TABLE 2).5
What’s recommended
The American Academy of Pediatrics recommends routine age-appropriate physical examination without specifying leg-length assessment.6 There is insufficient evidence, according to
Congenital femoral bowing requires plain film diagnosis
Following physical examination, diagnosis of congenital femoral bowing should be confirmed by plain films. Plain radiography remains the main imaging modality for proximal focal femoral deficiency and fibular hemimelia, and appropriate identification of the osseous abnormalities seen on radiographs allows for accurate classification of congenital femoral bowing, prognosis, and surgical planning.
Continue to: Early diagnosis can improve treatment outcome
Early diagnosis can improve treatment outcome
Both early diagnosis of congenital femoral bowing and prediction of leg-length discrepancy at skeletal maturity can influence potential treatment options, which range from conservative management (eg, watchful waiting, physical therapy, shoe lifts, orthotics, bracing) to surgical intervention. Several models have been used to predict skeletal growth, including the Moseley straight line graph, Green and Anderson growth curve, Amstutz method, and Paley’s multiplier method.4,9-14
Intervention for leg-length discrepancy generally is dictated by the magnitude of the inequality and the presence of functional deficits and/or pain.2 If the degree of femur angulation begins to affect structural development, surgical intervention should be considered to align development and/or correct the discrepancy. Physical therapy, shoe lifts, orthotics, and bracing are treatment options for managing smaller discrepancies.2,15
Our patient. The physician (CP) reviewed treatment options with the family that included watchful waiting, use of a shoe lift and/or orthotics, and bracing. The family chose watchful waiting due to the structural integrity of the patient’s other major joints and her relatively preserved function.
Surgery. Ultimately the patient underwent medial distal femoral hemiepiphysiodesis of the right lower extremity at 6 years of age due to increasing leg-length discrepancy and lateralization of the patella from the valgus deformity. The patient’s mother reported that she did well postoperatively, with increased range of motion, improved physical capabilities, and reduced discomfort in the right leg.
A second surgery. At approximately 8 years and 9 months of age, the orthopedist noted that the patient’s leg-length discrepancy had increased, and she had right extensor mechanism malalignment and severe patellar subluxation. The patient subsequently underwent surgery to remove the existing hardware, including right extensor mechanism realignment via a Roux-Goldthwait procedure (with reconstruction of the medial patellofemoral ligament and anterior cruciate ligament), as well as left distal femoral epiphysiodesis. She did very well postoperatively and continues to participate in physical therapy approximately once weekly. She has had an improvement in her gait and stability using shoe lifts.
Continue to: THE TAKEAWAY
THE TAKEAWAY
A routine well-child examination can be an opportunity to identify congenital musculoskeletal problems. Congenital femoral bowing is a relatively rare finding4 that may present as a leg-length discrepancy. With proper evaluation, including visual inspection, palpation, range-of-motion testing, and special tests as needed (eg, Galeazzi test, Ortolani and Barlow maneuvers), early intervention is possible if a leg-length discrepancy is noted. Close monitoring of gait abnormalities at routine well-child visits is essential.
Physical therapy, shoe lift therapy, and surgical approaches are treatment options for leg-length discrepancy,2 and early intervention can improve treatment outcomes.14 Understanding how to manage congenital femoral bowing over time is important in providing options and counselling patients and their families.15
Treatment of leg-length discrepancy in pediatric patients requires long-term management with a team approach that includes patients and their families. The goal of intervention is to reduce physical and emotional trauma, while addressing complications and maintaining function of the affected limb, as well as the whole body.15
CORRESPONDENCE
Beth P. Davis, DPT, MBA, FNAP, Emory University School of Medicine, Department of Rehabilitation Medicine, Division of Physical Therapy, 1462 Clifton Road NE, Suite 312, Atlanta, GA 30342; bethpdavis@emory.edu.
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
1. Shailam R, Jaramillo D, Kan JH. Growth arrest and leg-length discrepancy. Pediatr Radiol. 2013:43(suppl 1):S155-S165.
2. Brady RJ, Dean JB, Skinner TM, et al. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33:221-234.
3. Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143-153.
4. Bedoya MA, Chauvin NA, Jaramillo D, et al. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35:1191-1207.
5. Alanay Y, Krakow D, Rimoin DL, et al. Angulated femurs and the skeletal dysplasias: experience of the International Skeletal Dysplasia Registry (1988-2006). Am J Med Genet. 2007;143A:1159-1168.
6. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017.
7. Shipman SA, Hefland M, Moyer VA, et al. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:557-576.
8. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
9. Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432-1446.
10. Castaneda P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correcting of angular deformity about the knee. J Pediatr Orthop. 2008;28:188-191.
11. Mosley CF. A straight-line graph for leg-length discrepancies. J Bone Joint Surg Am. 1977; 59:174-179.
12. Anderson M, Messner MB, Green WT. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg Am. 1964;46:1197-1202.
13. Amstutz HC. The morphology, natural history, and treatment of proximal femoral focal deficiency. In: GT Aitken, ed. Proximal Femoral Focal Deficiency: A Congenital Anomaly. Washington, DC: National Academy of Sciences; 1969: 50-76.
14. Amstutz HC. Natural history and treatment of congenital absence of the fibula. J Bone and Joint Surg Am. 1972;54(A):1349.
15. Guidera KJ, Helal AA, Zuern KA. Management of pediatric limb length inequality. Adv Pediatr. 1995;42:501-543.
Neonatal hyperbilirubinemia: An evidence-based approach
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; kd6fp@virginia.edu.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; kd6fp@virginia.edu.
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; kd6fp@virginia.edu.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
PRACTICE RECOMMENDATIONS
› Diagnose hyperbilirubinemia in infants with bilirubin measured at >95th percentile for age in hours. Do not use visual assessment of jaundice for diagnosis as it may lead to errors. C
› Determine the threshold for initiation of phototherapy by applying serum bilirubin and age in hours to the American Academy of Pediatrics phototherapy nomogram along a risk curve assigned based on gestational age and neurotoxicity risk factors (not major and minor risk factors for severe hyperbilirubinemia). C
› Make arrangements to ensure that all infants are seen by a health care provider within 2 days of discharge (within 1 day if significant risk factors for development of severe hyperbilirubinemia are present). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We must counsel against heat-not-burn cigarettes
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.