User login
Chemo-resistant reserve HSCs maintained in bone marrow
A small subpopulation of quiescent hematopoietic stem cells (HSCs) that restores the HSC pool and supports hematopoietic regeneration after chemotherapy has been identified, researchers reported.
These “reserve” HSCs were resistant to chemotherapy, whereas “primed” HSCs were sensitive to DNA damage from chemotherapy, the researchers said.
Both reserve and active stem cells were maintained in the bone marrow by specific niches, researchers demonstrated in experiments described in detail in Cell Reports.
Of note, primitive reserve HSCs were maintained in a bone marrow region that enriches N-cadherin expressing (N-cad+) bone and marrow stromal progenitor cells. According to investigators, those N-cad+ cells protected primitive reserve HSCs from chemotherapy-related stress, which survived to support hematopoietic regeneration after myeloablation.
These findings advance understanding of HSC biology, and could open new avenues for treating blood diseases and autoimmune disorders.
“In effect, we showed that hematopoietic stem cells have functionally distinct subpopulations: one that acts under normal conditions, and the other that acts under times of stress,” study senior author Linheng Li, PhD, a researcher at Stowers Institute for Medical Research in Kansas City, Mo., said in a statement.
Hematopoietic stem cells are heterogeneous, with some maintained in an active state, and others in a quiescent state that is related to their self-renewal capacity and linked to low metabolic activity, sometimes called HSC dormancy or hibernation, Dr. Li and his coinvestigators explained in their report.
However, despite their quiescence, the majority of those HSCs will not survive chemotherapeutic stress, they added.
A small subpopulation of cells termed reserve HSCs can survive stress related to 5-fluorouracil chemotherapy in mice and following transplantation, Dr. Li and his colleagues demonstrated in experiments described in the paper. By contrast, a much larger population of quiescent cells, primed HSCs, were chemotherapy sensitive.
“Most likely, primed HSCs reflect a transitional state between [reserve HSCs] and proliferating [short-term HSCs],” Dr. Li and his colleagues said in their report.
In one key experiment, Dr. Li and his coinvestigators used a cell surface marker to isolate reserve HSCs and primed HSCs, which were transplanted into mice. Following engraftment, the mice were treated with 5-fluorouracil. They found that reserve HSCs, reflected by their derived blood cells, were unaffected by treatment, whereas derivatives of primed HSCs declined.
In another experiment, the researchers labeled HSCs with fluorescent tags to identify their location in the bone marrow. They found reserve cells concentrated in a specific bone marrow niche adjacent to N-cad+ cells.
The N-cad+ cells were bone and marrow stromal progenitor cells (BMSPCs), giving rise to osteoblasts, adipocytes, and chondrocytes, according to investigators.
Ablating the N-cad+ niche cells impaired reserve HSC maintenance in both homeostasis and regeneration, investigators added.
The work was supported by the Stowers Institute for Medical Research, the National Cancer Institute, and other grant support. The researchers reported that they had no competing interests related to the study.
SOURCE: Zhao M et al. Cell Rep. 2019 Jan 15;26(3):652-69.e6.
A small subpopulation of quiescent hematopoietic stem cells (HSCs) that restores the HSC pool and supports hematopoietic regeneration after chemotherapy has been identified, researchers reported.
These “reserve” HSCs were resistant to chemotherapy, whereas “primed” HSCs were sensitive to DNA damage from chemotherapy, the researchers said.
Both reserve and active stem cells were maintained in the bone marrow by specific niches, researchers demonstrated in experiments described in detail in Cell Reports.
Of note, primitive reserve HSCs were maintained in a bone marrow region that enriches N-cadherin expressing (N-cad+) bone and marrow stromal progenitor cells. According to investigators, those N-cad+ cells protected primitive reserve HSCs from chemotherapy-related stress, which survived to support hematopoietic regeneration after myeloablation.
These findings advance understanding of HSC biology, and could open new avenues for treating blood diseases and autoimmune disorders.
“In effect, we showed that hematopoietic stem cells have functionally distinct subpopulations: one that acts under normal conditions, and the other that acts under times of stress,” study senior author Linheng Li, PhD, a researcher at Stowers Institute for Medical Research in Kansas City, Mo., said in a statement.
Hematopoietic stem cells are heterogeneous, with some maintained in an active state, and others in a quiescent state that is related to their self-renewal capacity and linked to low metabolic activity, sometimes called HSC dormancy or hibernation, Dr. Li and his coinvestigators explained in their report.
However, despite their quiescence, the majority of those HSCs will not survive chemotherapeutic stress, they added.
A small subpopulation of cells termed reserve HSCs can survive stress related to 5-fluorouracil chemotherapy in mice and following transplantation, Dr. Li and his colleagues demonstrated in experiments described in the paper. By contrast, a much larger population of quiescent cells, primed HSCs, were chemotherapy sensitive.
“Most likely, primed HSCs reflect a transitional state between [reserve HSCs] and proliferating [short-term HSCs],” Dr. Li and his colleagues said in their report.
In one key experiment, Dr. Li and his coinvestigators used a cell surface marker to isolate reserve HSCs and primed HSCs, which were transplanted into mice. Following engraftment, the mice were treated with 5-fluorouracil. They found that reserve HSCs, reflected by their derived blood cells, were unaffected by treatment, whereas derivatives of primed HSCs declined.
In another experiment, the researchers labeled HSCs with fluorescent tags to identify their location in the bone marrow. They found reserve cells concentrated in a specific bone marrow niche adjacent to N-cad+ cells.
The N-cad+ cells were bone and marrow stromal progenitor cells (BMSPCs), giving rise to osteoblasts, adipocytes, and chondrocytes, according to investigators.
Ablating the N-cad+ niche cells impaired reserve HSC maintenance in both homeostasis and regeneration, investigators added.
The work was supported by the Stowers Institute for Medical Research, the National Cancer Institute, and other grant support. The researchers reported that they had no competing interests related to the study.
SOURCE: Zhao M et al. Cell Rep. 2019 Jan 15;26(3):652-69.e6.
A small subpopulation of quiescent hematopoietic stem cells (HSCs) that restores the HSC pool and supports hematopoietic regeneration after chemotherapy has been identified, researchers reported.
These “reserve” HSCs were resistant to chemotherapy, whereas “primed” HSCs were sensitive to DNA damage from chemotherapy, the researchers said.
Both reserve and active stem cells were maintained in the bone marrow by specific niches, researchers demonstrated in experiments described in detail in Cell Reports.
Of note, primitive reserve HSCs were maintained in a bone marrow region that enriches N-cadherin expressing (N-cad+) bone and marrow stromal progenitor cells. According to investigators, those N-cad+ cells protected primitive reserve HSCs from chemotherapy-related stress, which survived to support hematopoietic regeneration after myeloablation.
These findings advance understanding of HSC biology, and could open new avenues for treating blood diseases and autoimmune disorders.
“In effect, we showed that hematopoietic stem cells have functionally distinct subpopulations: one that acts under normal conditions, and the other that acts under times of stress,” study senior author Linheng Li, PhD, a researcher at Stowers Institute for Medical Research in Kansas City, Mo., said in a statement.
Hematopoietic stem cells are heterogeneous, with some maintained in an active state, and others in a quiescent state that is related to their self-renewal capacity and linked to low metabolic activity, sometimes called HSC dormancy or hibernation, Dr. Li and his coinvestigators explained in their report.
However, despite their quiescence, the majority of those HSCs will not survive chemotherapeutic stress, they added.
A small subpopulation of cells termed reserve HSCs can survive stress related to 5-fluorouracil chemotherapy in mice and following transplantation, Dr. Li and his colleagues demonstrated in experiments described in the paper. By contrast, a much larger population of quiescent cells, primed HSCs, were chemotherapy sensitive.
“Most likely, primed HSCs reflect a transitional state between [reserve HSCs] and proliferating [short-term HSCs],” Dr. Li and his colleagues said in their report.
In one key experiment, Dr. Li and his coinvestigators used a cell surface marker to isolate reserve HSCs and primed HSCs, which were transplanted into mice. Following engraftment, the mice were treated with 5-fluorouracil. They found that reserve HSCs, reflected by their derived blood cells, were unaffected by treatment, whereas derivatives of primed HSCs declined.
In another experiment, the researchers labeled HSCs with fluorescent tags to identify their location in the bone marrow. They found reserve cells concentrated in a specific bone marrow niche adjacent to N-cad+ cells.
The N-cad+ cells were bone and marrow stromal progenitor cells (BMSPCs), giving rise to osteoblasts, adipocytes, and chondrocytes, according to investigators.
Ablating the N-cad+ niche cells impaired reserve HSC maintenance in both homeostasis and regeneration, investigators added.
The work was supported by the Stowers Institute for Medical Research, the National Cancer Institute, and other grant support. The researchers reported that they had no competing interests related to the study.
SOURCE: Zhao M et al. Cell Rep. 2019 Jan 15;26(3):652-69.e6.
FROM CELL REPORTS
Key clinical point:
Major finding: Reserve HSCs were resistant to chemotherapy, whereas by contrast, primed HSCs were chemosensitive.
Study details: A series of preclinical experiments designed to characterize HSCs and document the effects of chemotherapy-related stress.
Disclosures: The work was supported by the Stowers Institute for Medical Research, the National Cancer Institute, and other grant support. The researchers reported that they had no competing interests related to the study.
Source: Zhao M et al. Cell Rep. 2019 Jan 15;26(3):652-69.e6.
PCSK9 inhibition isn’t the answer for high Lp(a)
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Evolocumab has no effect on arterial wall inflammation in patients with severely elevated Lp(a).
Major finding: Median Lp(a) declined modestly from 202 nmol/L to 188 nmol/L in response to evolocumab.
Study details: This multicenter, 16-week, double-blind, placebo-controlled study included 128 patients with both elevated LDL and Lp(a).
Disclosures: The ANITSCHKOW study was sponsored by Amgen. The presenter reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
Flu activity increases after 2 weeks of declines
according to the Centers for Disease Control and Prevention.
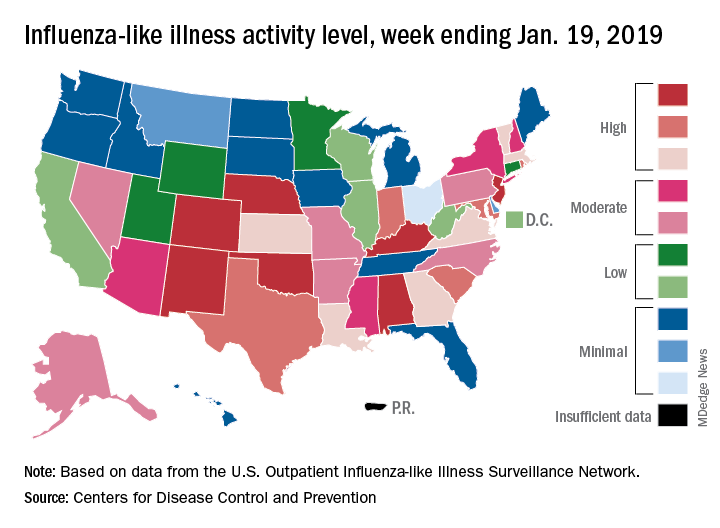
The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
Women with RA have reduced chance of live birth after assisted reproduction treatment
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Women with rheumatoid arthritis (RA) who undergo assisted reproduction treatment have decreased chances of live births, compared with women without RA.
Major finding: The odds ratio for live births per embryo transfer in women with RA, as compared to women without RA, was 0.78 (95% confidence interval, 0.65-0.92).
Study details: A nationwide cohort study including 1,149 embryo transfers in women with rheumatoid arthritis and 198,941 without rheumatoid arthritis
Disclosures: Study authors had no disclosures. Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense (Denmark) University Hospital.
Source: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Impaired clot lysis associated with mild bleeding symptoms
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
FROM THROMBOSIS RESEARCH
Key clinical point: Patients with self-reported mild bleeding symptoms may have impaired clot lysis, in contrast with known bleeding disorders.
Major finding: Patients with mild bleeding had longer whole blood tissue plasminogen activator-rotational thromboelastometry lysis times (P = .022) than did patients without symptoms.
Study details: An observational study of 335 adult patients undergoing elective surgery.
Disclosures: This study was funded by the Sint Annadal Foundation, Maastricht University Medical Center, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
Source: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Join the Conversation on SVSConnect
Transitioning toward retirement or looking for postgrad training opportunities? Or maybe you need some advice on good “power meals” to have during your busy days? These are only a few of the many threads attracting comments on SVSConnect, your online community. Members can discuss, collaborate, network and even share documents with one another. Log in with your SVS credentials and add your thoughts. If you’re not signed up, do so today.
Transitioning toward retirement or looking for postgrad training opportunities? Or maybe you need some advice on good “power meals” to have during your busy days? These are only a few of the many threads attracting comments on SVSConnect, your online community. Members can discuss, collaborate, network and even share documents with one another. Log in with your SVS credentials and add your thoughts. If you’re not signed up, do so today.
Transitioning toward retirement or looking for postgrad training opportunities? Or maybe you need some advice on good “power meals” to have during your busy days? These are only a few of the many threads attracting comments on SVSConnect, your online community. Members can discuss, collaborate, network and even share documents with one another. Log in with your SVS credentials and add your thoughts. If you’re not signed up, do so today.
Asthma patients with sinusitis, polyps fare poorly after sinus surgery
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
CORONADO, CALIF. – Eosinophilic chronic rhinosinusitis with nasal polyposis decreases quality of life improvement after sinus surgery in patients with concurrent asthma, results from a retrospective study demonstrated.
They also have significantly higher Lund-Kennedy endoscopy and Lund-McKay CT scores, compared with control groups.
“Patients with concurrent asthma and chronic sinusitis require more aggressive management than nonasthmatics,” one of the study authors, Aykut A. Unsal, DO, said in an interview in advance of the Triological Society’s Combined Sections Meeting. “Additionally, the degree of improvement of not only their sinusitis but possibly their asthma following medical/surgical treatment will also be limited if that patient also suffers from nasal polyps and/or eosinophilia. These patients will ultimately become more difficult to manage.”
In order to examine the relationship of eosinophilia and nasal polyps on quality of life (QOL) in patients with asthma who have chronic rhinosinusitis (CRS) who were treated with surgery, Dr. Unsal and his associates reviewed the records of 457 patients with a diagnosis of CRS who underwent sinus surgery in the department of otolaryngology at the Medical College of Georgia, Augusta. The researchers subdivided patients based on the presence or absence of an asthma diagnosis and further subdivided them based on tissue eosinophilia and nasal polyposis. Next, they compared the Sinonasal Outcome Test (SNOT-22), Lund-Kennedy endoscopy scores, and Lund-McKay CT scores preoperatively and postoperatively at 6 months – 1 year and at 2, 3, 4, and 5 years. They performed a T-test analysis to determine statistical significance.
Of the 457 patients included in the analysis, 92 had asthma and eosinophilic CRS with nasal polyps (eCRScNP), 20 had asthma and eosinophilic CRS without nasal polyps (eCRSsNP), 8 had asthma and noneosinophilic CRS with nasal polyps (neCRScNP), and 16 had asthma and noneosinophilic CRS without nasal polyps (neCRSsNP). The researchers observed that patients in the eCRScNP group showed no difference in QOL preoperatively, but their QOL declined significantly at the 1- and 2-year analysis (P less than .03). No significant QOL improvement appeared in the eCRSsNP group until 4 years (P less than .008), and there was no significant QOL difference among the neCRS groups regardless of nasal polyposis. A statistical difference in endoscopy scores was seen among patients in the preoperative neCRScNP group (P less than .001) and in the eCRScNP group from preoperatively until 5 years postoperatively (P less than .03). Finally, statistical significance appeared in preoperative CT scores analysis among patients in the eCRScNP group (P less than .001).
Dr. Unsal and his associates launched the study expecting that all patients with asthma were not only going to have worse symptoms scores, but also more recalcitrant disease. “This is based on our clinical experience, as well as previous literature that has shown that patients with exacerbations of asthma or sinusitis can worsen the symptoms of the other comorbid disease,” he said. “The opposite is also true; effective treatment of chronic sinusitis has been shown to also improve asthma symptoms. Our findings partially validated what we expected, as asthma patients were typically worse by symptom, endoscopy, and CT scores across the board.
“What we discovered, however, was there was one population of patients where no differences demonstrated between the two groups preoperatively and postoperatively: Patients who were negative for both polyp disease and eosinophilia, considered the least severe sinus disease. Additionally, generally no statistical differences in disease and symptom severity were identified following surgery between the two groups if they had a moderately severe form of chronic sinusitis [patients who were either positive for polyps or positive for eosinophilia],” Dr. Unsal said.
He and his colleagues also found that the group with the most severe form (positive eosinophila and positive polyps) fared worse symptomatically and objectively both preoperatively and postoperatively, compared with the other groups.
Dr. Unsal acknowledged certain limitations of the study, including that the type of asthma each patient had (whether they were controlled intermittent or whether they had moderate or persistent asthma) was not recorded, “so we don’t actually know to what degree asthma severity played a role in sinus disease, nor the improvement in asthma severity following sinus surgery/medical therapy,” he said. “Lastly, we did lose several patients to follow-up in the later years so the data is not as robust in the very long term.”
The researchers reported having no financial disclosures.
The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
REPORTING FROM THE TRIOLOGICAL CSWM
Key clinical point: Patients with asthma and the most severe form of chronic rhinosinusitis fare poorly on quality of life measures following sinus surgery.
Major finding: QOL in patients who had asthma and eosinophilic CRS with nasal polyps declined significantly at the 1- and 2-year analysis (P less than .03).
Study details: A single-center review of 457 patients with CRS who underwent sinus surgery.
Disclosures: The researchers reported having no financial disclosures.
Early parent-child psychotherapy is effective for childhood depression
BROOKLYN, N.Y. – There are studies demonstrating depression is more likely to resolve if depressed parents are also treated dating back more than 10 years, but new evidence suggests this effect may extend to children as young as 3 years of age, according to an update on current strategies for early intervention presented at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Of multiple triggers that can be caught and treated early to prevent children from progressing to chronic depression, addressing parental depression is an important target, according to Karen Dineen Wagner, MD, Director of the Division of Child and Adolescent Psychiatry at the University of Texas Medical Branch in Galveston.
In an overview of strategies for intervening early in children who have or are at risk for depression, Dr. Wagner looked at several targets. The purpose of recognizing and addressing such targets as parental depression is to get children well faster and avoid disease chronicity.
“If we want to intervene and potentially prevent the occurrence of depression, we need to look at disorders or triggers that may precede the depression and that, had they been treated, might have eliminated the stressors that tip the child into depression,” Dr. Wagner said.
Parental depression was just one of these factors, but along with others, such as child abuse of any kind and bullying, each poses a threat for chronic mood disorders, according to Dr. Wagner.
In the case of parental depression, Dr. Wagner cited numerous studies demonstrating a close correlation between remission in the parent and remission in the child. These trajectories interact, so children are less likely to get well if an affected parent does not get well.
“Make sure you consider depression in the parent when you are doing an evaluation, and it is not just depression in the parent who is there. Ask about the other partner who is not there,” Dr. Wagner advised. Parents reluctant to address their own depression should be informed that the mental health of their child is at risk.
The most recent evidence to show benefit from treating both child and parent was drawn from a controlled study that enrolled young children (Luby JL et al. Am J Psychiatry. 2018 Jun 20. doi: 10.1176/appi.ajp.2018.18030321).
In this study, 229 parent-child dyads were randomized to receive parent-child psychotherapy for early childhood depression or to a wait-list. The age range for the children was 3-6.2 years. The therapy was specifically designed to improve the parents’ ability to help young children cope with their feelings.
The parent-child interaction therapy “focused on emotional development which was designed to train parents to work with the child on developing emotional competence in which the child understands their emotions, understands how events affect how they are feeling, and controls emotional reactivity,” Dr. Wagner explained.
For the primary outcome of depression at the end of 18 weeks, the rates were significantly lower in those who participated in the interaction therapy than they were in those on the wait-list. Measures of parent depression and stress were also lower in the therapy group.
Currently, the U. S. Preventive Task Force recommends screening all children at age 12 for depression with the adolescent version of the Patient Health Questionnaire (PHQ-A), according to Dr. Wagner. Given the rising prevalence of depression in adolescents, which climbed 46% from 2005 (8.7%) to 2015 (12.7%) according to a published national survey, this screening is prudent, Dr. Wagner indicated. However, she further suggested that it is reasonable to screen children with risk factors, such as learning disorders or anxiety disorders, even earlier.
The reason is that there are effective therapies. Early treatment may prevent chronic and more severe forms of depression, according to Dr. Wagner. She suggested that there is a growing emphasis on not just treating depression at its early stages but also in recognizing the child at risk, identifying subsyndromal symptoms leading toward depression, and preventing children from ever reaching diagnostic criteria.
Indeed, an initiative for better and earlier detection and treatment of depression in children was begun by the AACAP when Dr. Wagner served recently as its president. Several parts of that program have now been launched. Dr. Wagner encouraged those with an interest to visit the AACAP website, where more information about this initiative can be accessed.
Dr. Wagner reported no potential conflicts of interest.
BROOKLYN, N.Y. – There are studies demonstrating depression is more likely to resolve if depressed parents are also treated dating back more than 10 years, but new evidence suggests this effect may extend to children as young as 3 years of age, according to an update on current strategies for early intervention presented at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Of multiple triggers that can be caught and treated early to prevent children from progressing to chronic depression, addressing parental depression is an important target, according to Karen Dineen Wagner, MD, Director of the Division of Child and Adolescent Psychiatry at the University of Texas Medical Branch in Galveston.
In an overview of strategies for intervening early in children who have or are at risk for depression, Dr. Wagner looked at several targets. The purpose of recognizing and addressing such targets as parental depression is to get children well faster and avoid disease chronicity.
“If we want to intervene and potentially prevent the occurrence of depression, we need to look at disorders or triggers that may precede the depression and that, had they been treated, might have eliminated the stressors that tip the child into depression,” Dr. Wagner said.
Parental depression was just one of these factors, but along with others, such as child abuse of any kind and bullying, each poses a threat for chronic mood disorders, according to Dr. Wagner.
In the case of parental depression, Dr. Wagner cited numerous studies demonstrating a close correlation between remission in the parent and remission in the child. These trajectories interact, so children are less likely to get well if an affected parent does not get well.
“Make sure you consider depression in the parent when you are doing an evaluation, and it is not just depression in the parent who is there. Ask about the other partner who is not there,” Dr. Wagner advised. Parents reluctant to address their own depression should be informed that the mental health of their child is at risk.
The most recent evidence to show benefit from treating both child and parent was drawn from a controlled study that enrolled young children (Luby JL et al. Am J Psychiatry. 2018 Jun 20. doi: 10.1176/appi.ajp.2018.18030321).
In this study, 229 parent-child dyads were randomized to receive parent-child psychotherapy for early childhood depression or to a wait-list. The age range for the children was 3-6.2 years. The therapy was specifically designed to improve the parents’ ability to help young children cope with their feelings.
The parent-child interaction therapy “focused on emotional development which was designed to train parents to work with the child on developing emotional competence in which the child understands their emotions, understands how events affect how they are feeling, and controls emotional reactivity,” Dr. Wagner explained.
For the primary outcome of depression at the end of 18 weeks, the rates were significantly lower in those who participated in the interaction therapy than they were in those on the wait-list. Measures of parent depression and stress were also lower in the therapy group.
Currently, the U. S. Preventive Task Force recommends screening all children at age 12 for depression with the adolescent version of the Patient Health Questionnaire (PHQ-A), according to Dr. Wagner. Given the rising prevalence of depression in adolescents, which climbed 46% from 2005 (8.7%) to 2015 (12.7%) according to a published national survey, this screening is prudent, Dr. Wagner indicated. However, she further suggested that it is reasonable to screen children with risk factors, such as learning disorders or anxiety disorders, even earlier.
The reason is that there are effective therapies. Early treatment may prevent chronic and more severe forms of depression, according to Dr. Wagner. She suggested that there is a growing emphasis on not just treating depression at its early stages but also in recognizing the child at risk, identifying subsyndromal symptoms leading toward depression, and preventing children from ever reaching diagnostic criteria.
Indeed, an initiative for better and earlier detection and treatment of depression in children was begun by the AACAP when Dr. Wagner served recently as its president. Several parts of that program have now been launched. Dr. Wagner encouraged those with an interest to visit the AACAP website, where more information about this initiative can be accessed.
Dr. Wagner reported no potential conflicts of interest.
BROOKLYN, N.Y. – There are studies demonstrating depression is more likely to resolve if depressed parents are also treated dating back more than 10 years, but new evidence suggests this effect may extend to children as young as 3 years of age, according to an update on current strategies for early intervention presented at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Of multiple triggers that can be caught and treated early to prevent children from progressing to chronic depression, addressing parental depression is an important target, according to Karen Dineen Wagner, MD, Director of the Division of Child and Adolescent Psychiatry at the University of Texas Medical Branch in Galveston.
In an overview of strategies for intervening early in children who have or are at risk for depression, Dr. Wagner looked at several targets. The purpose of recognizing and addressing such targets as parental depression is to get children well faster and avoid disease chronicity.
“If we want to intervene and potentially prevent the occurrence of depression, we need to look at disorders or triggers that may precede the depression and that, had they been treated, might have eliminated the stressors that tip the child into depression,” Dr. Wagner said.
Parental depression was just one of these factors, but along with others, such as child abuse of any kind and bullying, each poses a threat for chronic mood disorders, according to Dr. Wagner.
In the case of parental depression, Dr. Wagner cited numerous studies demonstrating a close correlation between remission in the parent and remission in the child. These trajectories interact, so children are less likely to get well if an affected parent does not get well.
“Make sure you consider depression in the parent when you are doing an evaluation, and it is not just depression in the parent who is there. Ask about the other partner who is not there,” Dr. Wagner advised. Parents reluctant to address their own depression should be informed that the mental health of their child is at risk.
The most recent evidence to show benefit from treating both child and parent was drawn from a controlled study that enrolled young children (Luby JL et al. Am J Psychiatry. 2018 Jun 20. doi: 10.1176/appi.ajp.2018.18030321).
In this study, 229 parent-child dyads were randomized to receive parent-child psychotherapy for early childhood depression or to a wait-list. The age range for the children was 3-6.2 years. The therapy was specifically designed to improve the parents’ ability to help young children cope with their feelings.
The parent-child interaction therapy “focused on emotional development which was designed to train parents to work with the child on developing emotional competence in which the child understands their emotions, understands how events affect how they are feeling, and controls emotional reactivity,” Dr. Wagner explained.
For the primary outcome of depression at the end of 18 weeks, the rates were significantly lower in those who participated in the interaction therapy than they were in those on the wait-list. Measures of parent depression and stress were also lower in the therapy group.
Currently, the U. S. Preventive Task Force recommends screening all children at age 12 for depression with the adolescent version of the Patient Health Questionnaire (PHQ-A), according to Dr. Wagner. Given the rising prevalence of depression in adolescents, which climbed 46% from 2005 (8.7%) to 2015 (12.7%) according to a published national survey, this screening is prudent, Dr. Wagner indicated. However, she further suggested that it is reasonable to screen children with risk factors, such as learning disorders or anxiety disorders, even earlier.
The reason is that there are effective therapies. Early treatment may prevent chronic and more severe forms of depression, according to Dr. Wagner. She suggested that there is a growing emphasis on not just treating depression at its early stages but also in recognizing the child at risk, identifying subsyndromal symptoms leading toward depression, and preventing children from ever reaching diagnostic criteria.
Indeed, an initiative for better and earlier detection and treatment of depression in children was begun by the AACAP when Dr. Wagner served recently as its president. Several parts of that program have now been launched. Dr. Wagner encouraged those with an interest to visit the AACAP website, where more information about this initiative can be accessed.
Dr. Wagner reported no potential conflicts of interest.
REPORTING FROM AACAP PEDIATRIC PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Key clinical point: New data expand evidence that treating depression in parents treats depression in children.
Major finding: Interactive psychotherapy was associated with improved outcomes in children as young as 3 years.
Study details: Expert review.
Disclosures: Dr. Wagner reported no potential conflicts of interest.
LAA closure safely treated AF patients with prior ICH
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point: Tailoring the anticlotting regimen atrial fibrillation (AF) patients received after left atrial appendage (LAA) closure led to good outcomes despite prior intracranial hemorrhages.
Major finding: The 6-month rate of death, stroke, or major bleed was about 5% in AF patients who underwent LAA closure after intracranial hemorrhage.
Study details: Retrospective review of 158 atrial fibrillation patients who underwent LAA closure at any of three U.S. centers.
Disclosures: The study received no commercial funding. Dr. Mansour has been a consultant to Abbott, Biosense Webster, Boston Scientific, and Medtronic; he has received research funding from Abbott, Biosense Webster, Boehringer Ingelheim, Boston Scientific, and Pfizer; and he has an equity interest in EPD Solutions and NewPace.
FDA: Nitrosamine-contaminated ARBs marketed for 4 years
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.