User login
Diagnosing Anemia
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at crnewseditor@mdedge.com.
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at crnewseditor@mdedge.com.
This clinical puzzle is based on O’Neil. Diagnosing and classifying anemia in adult primary care. Clinician Reviews. 2017; 27(8):28-35.
For the best mobile experience, rotate screen to landscape mode.
Have feedback on our new crossword puzzle? Share your thoughts at crnewseditor@mdedge.com.
Age of migraine onset may affect stroke risk
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
FROM HEADACHE
Key clinical point: Age of migraine onset may be an important factor in assessing stroke risk.
Major finding: (multivariable adjusted hazard ratio = 2.17).
Study details: A post hoc analysis of data from more than 11,500 participants in the Atherosclerosis Risk in Communities (ARIC) study.
Disclosures: The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
Source: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
Survey: Americans support Medicare for all
A majority of Americans support the concept of Medicare for all, but “larger majorities favor more incremental changes to the health care system,” according to a new survey by the Kaiser Family Foundation.
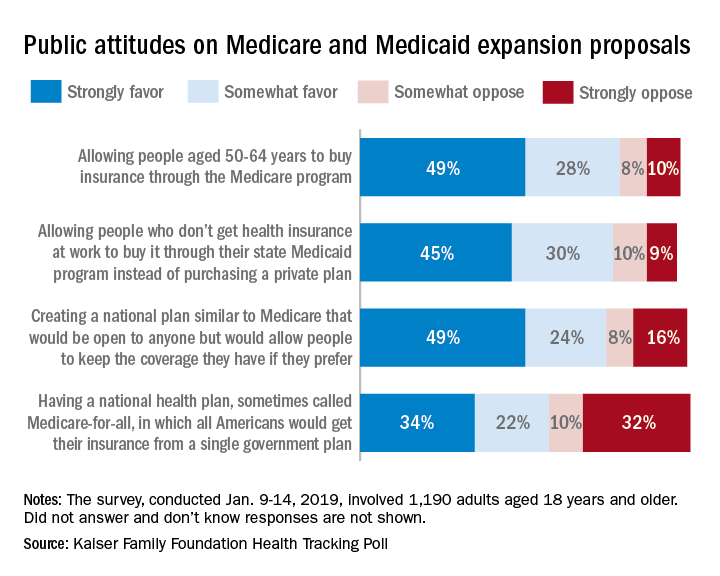
Support for a Medicare-for-all health care system came in at 56% (strongly favor, 34%; somewhat favor, 22%) among the 1,190 respondents to the latest KFF Health Tracking Poll, which was conducted Jan. 9-14, 2019. That support came largely from Democrats, 81% of whom favored the plan, compared with only 23% of Republicans, the Kaiser investigators said Jan. 23.
A Medicare buy-in plan for Americans aged 50-64 years also was highly popular, receiving support from 77% of all respondents – 85% of Democrats, 75% of Independents, and 69% of Republicans. Support by party identification was similar for a proposal to enable all those who don’t have employer-based insurance to get coverage through state Medicaid programs, which received 75% support overall, they reported.
Just behind those proposals at 74% support was a federally administered health plan that would be open to anyone but would allow people to keep the coverage they have. It was the most popular proposal among Democrats (91%) but did not garner a majority among Republicans (47%), the investigators said.
Support for the Medicare-for-all plan varied considerably, depending on number of arguments presented to respondents. When told that such a proposal would guarantee insurance as a right for all Americans, 71% favored it, and when they heard that it would eliminate health insurance premiums and reduce out-of-pockets costs, 67% of respondents expressed support. Favorable responses, however, were in the minority when people were told that Medicare-for-all would eliminate private health insurance companies (37%), threaten the current Medicare program (32%), and lead to some delayed medical tests and treatments (26%), according to the Kaiser report.
A majority of Americans support the concept of Medicare for all, but “larger majorities favor more incremental changes to the health care system,” according to a new survey by the Kaiser Family Foundation.

Support for a Medicare-for-all health care system came in at 56% (strongly favor, 34%; somewhat favor, 22%) among the 1,190 respondents to the latest KFF Health Tracking Poll, which was conducted Jan. 9-14, 2019. That support came largely from Democrats, 81% of whom favored the plan, compared with only 23% of Republicans, the Kaiser investigators said Jan. 23.
A Medicare buy-in plan for Americans aged 50-64 years also was highly popular, receiving support from 77% of all respondents – 85% of Democrats, 75% of Independents, and 69% of Republicans. Support by party identification was similar for a proposal to enable all those who don’t have employer-based insurance to get coverage through state Medicaid programs, which received 75% support overall, they reported.
Just behind those proposals at 74% support was a federally administered health plan that would be open to anyone but would allow people to keep the coverage they have. It was the most popular proposal among Democrats (91%) but did not garner a majority among Republicans (47%), the investigators said.
Support for the Medicare-for-all plan varied considerably, depending on number of arguments presented to respondents. When told that such a proposal would guarantee insurance as a right for all Americans, 71% favored it, and when they heard that it would eliminate health insurance premiums and reduce out-of-pockets costs, 67% of respondents expressed support. Favorable responses, however, were in the minority when people were told that Medicare-for-all would eliminate private health insurance companies (37%), threaten the current Medicare program (32%), and lead to some delayed medical tests and treatments (26%), according to the Kaiser report.
A majority of Americans support the concept of Medicare for all, but “larger majorities favor more incremental changes to the health care system,” according to a new survey by the Kaiser Family Foundation.

Support for a Medicare-for-all health care system came in at 56% (strongly favor, 34%; somewhat favor, 22%) among the 1,190 respondents to the latest KFF Health Tracking Poll, which was conducted Jan. 9-14, 2019. That support came largely from Democrats, 81% of whom favored the plan, compared with only 23% of Republicans, the Kaiser investigators said Jan. 23.
A Medicare buy-in plan for Americans aged 50-64 years also was highly popular, receiving support from 77% of all respondents – 85% of Democrats, 75% of Independents, and 69% of Republicans. Support by party identification was similar for a proposal to enable all those who don’t have employer-based insurance to get coverage through state Medicaid programs, which received 75% support overall, they reported.
Just behind those proposals at 74% support was a federally administered health plan that would be open to anyone but would allow people to keep the coverage they have. It was the most popular proposal among Democrats (91%) but did not garner a majority among Republicans (47%), the investigators said.
Support for the Medicare-for-all plan varied considerably, depending on number of arguments presented to respondents. When told that such a proposal would guarantee insurance as a right for all Americans, 71% favored it, and when they heard that it would eliminate health insurance premiums and reduce out-of-pockets costs, 67% of respondents expressed support. Favorable responses, however, were in the minority when people were told that Medicare-for-all would eliminate private health insurance companies (37%), threaten the current Medicare program (32%), and lead to some delayed medical tests and treatments (26%), according to the Kaiser report.
Rheumatology News Best of 2018 – The RA Report: Top News Highlights
The ideas and opinions expressed in Best of 2018 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
The ideas and opinions expressed in Best of 2018 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
The ideas and opinions expressed in Best of 2018 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
FDA: Benefits still outweigh risks from paclitaxel-coated devices for PAD
This week, the FDA weighs in on concerning reports about paclitaxel-coated stents, and it approves a device to treat patent ductus arteriosus in infants weighing as little as 2 pounds. Also, a treat-to-target approach for CVD risk factors decreased atherosclerosis in rheumatoid arthritis patients, and ezetimibe was effective for primary prevention in elderly patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week, the FDA weighs in on concerning reports about paclitaxel-coated stents, and it approves a device to treat patent ductus arteriosus in infants weighing as little as 2 pounds. Also, a treat-to-target approach for CVD risk factors decreased atherosclerosis in rheumatoid arthritis patients, and ezetimibe was effective for primary prevention in elderly patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week, the FDA weighs in on concerning reports about paclitaxel-coated stents, and it approves a device to treat patent ductus arteriosus in infants weighing as little as 2 pounds. Also, a treat-to-target approach for CVD risk factors decreased atherosclerosis in rheumatoid arthritis patients, and ezetimibe was effective for primary prevention in elderly patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
Meta-analysis supports aspirin to reduce cardiovascular events
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
FROM JAMA
Key clinical point:
Major finding: The absolute risk reduction was 0.38% for a composite of cardiovascular events among aspirin users versus nonusers.
Study details: The data come from a meta-analysis of 13 randomized trials altogether including 1,050,511 participants-years of follow-up.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Zheng SL et al. JAMA. 2019;321(3):277-87.
Dual BRAF, MEK inhibition proves highly active in biliary tract cancer
SAN FRANCISCO – Simultaneously targeting two components of the same signaling pathway in BRAF V600E–mutated biliary tract cancer is a winning strategy, suggests an analysis of the multicenter phase 2 ROAR basket trial.
“Recently, a number of genetic targets have been identified with distinct rates of frequency in intrahepatic, extrahepatic, and gallbladder cancer that are the subject of a number of ongoing clinical trials. In retrospective studies, mutations in BRAF have been identified in about 5%-7% of patients, predominantly in the intrahepatic cohort,” lead investigator Zev A. Wainberg, MD, noted at the 2019 GI Cancers Symposium. Previous research has shown combined inhibition of BRAF and MEK – two sequential proteins on the MAP kinase signaling pathway – to be efficacious in BRAF V600E–mutated melanoma, non–small cell lung cancer, and anaplastic thyroid cancer.
In the ROAR trial, 178 patients with advanced or metastatic BRAF V600E–mutated rare cancers who had exhausted standard treatment options were treated with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist). Dr. Wainberg reported results for the 35 patients having biliary tract cancer, most of whom were heavily pretreated.
With a median duration of follow-up of 8 months, the overall response rate was 42% as assessed by investigators and 36% as assessed by independent reviewers. More than half of patients had a grade 3 or 4 adverse event, but just one had to permanently stop treatment because of toxicity.
“These results represent the first prospectively analyzed cohort of patients with BRAF V600E–mutated biliary tract cancers treated with the combination of BRAF and MEK inhibition. Efficacy in this patient population with advanced disease was comparable to that of first-line chemotherapy with gemcitabine and cisplatin,” pointed out Dr. Wainberg, codirector of the GI oncology program and an assistant professor of medicine at the University of California, Los Angeles.
“Dabrafenib and trametinib demonstrated clinical benefit in patients with BRAF-mutant biliary tract cancers and should be considered a meaningful therapeutic option for these patients,” he contended. “BRAF V600E is one of several actionable driver mutations in this disease and should be considered for routine testing in patients with biliary tract cancers. In addition, among all the other data [that are] emerging in molecular analysis of this malignancy, perhaps among all the GI malignancies, this has the potential to undergo multiple studies of other targeted therapies.”
Why stop there?
“I don’t think there is anything needed to convince you that this treatment is very effective. It’s incredibly impressive,” commented invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the gastrointestinal cancers program, University of Southern California Norris Comprehensive Cancer Center in Los Angeles.
But experience with other BRAF-mutant malignancies, such as BRAF-mutant colorectal cancer, suggests that further benefit can accrue from hitting additional molecular targets, he said. For example, rationally selected triplet combinations can suppress emergence of resistant clones up front.
“How can we do even better? I think we need to be smart in how we inhibit downstream signaling of BRAF-mutant disease,” Dr. Lenz maintained, as downstream targeting is potentially more effective. Therefore, a logical candidate for improving on the combination of BRAF and MEK inhibitors in biliary tract cancer is an ERK inhibitor. Alternatively, the combination may be synergistic when used with immune checkpoint inhibitors that target programmed death-1 or programmed death–ligand 1.
Oncologists should perform next-generation sequencing for all patients with biliary tract cancer, in Dr. Lenz’s opinion. “Biliary cancer is one of these cancers where we have many potentially actionable mutations. I know there is no drug approved, but many trials are ongoing,” he elaborated. “So I just encourage you to do that in order to identify potential trials or treatment options.”
Study details
In the ROAR trial, patients received open-label dabrafenib (150 mg, twice daily) plus trametinib (2 mg, once daily) until unacceptable toxicity, disease progression, or death.
All 35 patients with biliary cancer had received gemcitabine, and 80% had received at least two lines of prior systemic therapy.
The median treatment duration was 6 months, Dr. Wainberg reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Fully 86% of patients were on treatment for longer than 3 months.
In addition to the impressive response rates, the patients had a median progression-free survival of 9.2 months, and a median overall survival of 11.7 months.
Adverse events were as expected based on previous experience with these targeted therapies, according to Dr. Wainberg. The rate of grade 3 or 4 adverse events was 57%. “These were predominantly pyrexia, a known side effect of BRAF inhibitors, which is managed with antipyretic therapy and dexamethasone,” he noted. Rash and gastrointestinal toxicity were also common.
Although adverse events often led to dose reductions and dose interruptions, only a single patient (3% of the cohort) had to stop treatment early because of an event (cholangitis).
SOURCE: Wainberg ZA et al. 2019 GI Cancers Symposium, Abstract 187.
SAN FRANCISCO – Simultaneously targeting two components of the same signaling pathway in BRAF V600E–mutated biliary tract cancer is a winning strategy, suggests an analysis of the multicenter phase 2 ROAR basket trial.
“Recently, a number of genetic targets have been identified with distinct rates of frequency in intrahepatic, extrahepatic, and gallbladder cancer that are the subject of a number of ongoing clinical trials. In retrospective studies, mutations in BRAF have been identified in about 5%-7% of patients, predominantly in the intrahepatic cohort,” lead investigator Zev A. Wainberg, MD, noted at the 2019 GI Cancers Symposium. Previous research has shown combined inhibition of BRAF and MEK – two sequential proteins on the MAP kinase signaling pathway – to be efficacious in BRAF V600E–mutated melanoma, non–small cell lung cancer, and anaplastic thyroid cancer.
In the ROAR trial, 178 patients with advanced or metastatic BRAF V600E–mutated rare cancers who had exhausted standard treatment options were treated with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist). Dr. Wainberg reported results for the 35 patients having biliary tract cancer, most of whom were heavily pretreated.
With a median duration of follow-up of 8 months, the overall response rate was 42% as assessed by investigators and 36% as assessed by independent reviewers. More than half of patients had a grade 3 or 4 adverse event, but just one had to permanently stop treatment because of toxicity.
“These results represent the first prospectively analyzed cohort of patients with BRAF V600E–mutated biliary tract cancers treated with the combination of BRAF and MEK inhibition. Efficacy in this patient population with advanced disease was comparable to that of first-line chemotherapy with gemcitabine and cisplatin,” pointed out Dr. Wainberg, codirector of the GI oncology program and an assistant professor of medicine at the University of California, Los Angeles.
“Dabrafenib and trametinib demonstrated clinical benefit in patients with BRAF-mutant biliary tract cancers and should be considered a meaningful therapeutic option for these patients,” he contended. “BRAF V600E is one of several actionable driver mutations in this disease and should be considered for routine testing in patients with biliary tract cancers. In addition, among all the other data [that are] emerging in molecular analysis of this malignancy, perhaps among all the GI malignancies, this has the potential to undergo multiple studies of other targeted therapies.”
Why stop there?
“I don’t think there is anything needed to convince you that this treatment is very effective. It’s incredibly impressive,” commented invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the gastrointestinal cancers program, University of Southern California Norris Comprehensive Cancer Center in Los Angeles.
But experience with other BRAF-mutant malignancies, such as BRAF-mutant colorectal cancer, suggests that further benefit can accrue from hitting additional molecular targets, he said. For example, rationally selected triplet combinations can suppress emergence of resistant clones up front.
“How can we do even better? I think we need to be smart in how we inhibit downstream signaling of BRAF-mutant disease,” Dr. Lenz maintained, as downstream targeting is potentially more effective. Therefore, a logical candidate for improving on the combination of BRAF and MEK inhibitors in biliary tract cancer is an ERK inhibitor. Alternatively, the combination may be synergistic when used with immune checkpoint inhibitors that target programmed death-1 or programmed death–ligand 1.
Oncologists should perform next-generation sequencing for all patients with biliary tract cancer, in Dr. Lenz’s opinion. “Biliary cancer is one of these cancers where we have many potentially actionable mutations. I know there is no drug approved, but many trials are ongoing,” he elaborated. “So I just encourage you to do that in order to identify potential trials or treatment options.”
Study details
In the ROAR trial, patients received open-label dabrafenib (150 mg, twice daily) plus trametinib (2 mg, once daily) until unacceptable toxicity, disease progression, or death.
All 35 patients with biliary cancer had received gemcitabine, and 80% had received at least two lines of prior systemic therapy.
The median treatment duration was 6 months, Dr. Wainberg reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Fully 86% of patients were on treatment for longer than 3 months.
In addition to the impressive response rates, the patients had a median progression-free survival of 9.2 months, and a median overall survival of 11.7 months.
Adverse events were as expected based on previous experience with these targeted therapies, according to Dr. Wainberg. The rate of grade 3 or 4 adverse events was 57%. “These were predominantly pyrexia, a known side effect of BRAF inhibitors, which is managed with antipyretic therapy and dexamethasone,” he noted. Rash and gastrointestinal toxicity were also common.
Although adverse events often led to dose reductions and dose interruptions, only a single patient (3% of the cohort) had to stop treatment early because of an event (cholangitis).
SOURCE: Wainberg ZA et al. 2019 GI Cancers Symposium, Abstract 187.
SAN FRANCISCO – Simultaneously targeting two components of the same signaling pathway in BRAF V600E–mutated biliary tract cancer is a winning strategy, suggests an analysis of the multicenter phase 2 ROAR basket trial.
“Recently, a number of genetic targets have been identified with distinct rates of frequency in intrahepatic, extrahepatic, and gallbladder cancer that are the subject of a number of ongoing clinical trials. In retrospective studies, mutations in BRAF have been identified in about 5%-7% of patients, predominantly in the intrahepatic cohort,” lead investigator Zev A. Wainberg, MD, noted at the 2019 GI Cancers Symposium. Previous research has shown combined inhibition of BRAF and MEK – two sequential proteins on the MAP kinase signaling pathway – to be efficacious in BRAF V600E–mutated melanoma, non–small cell lung cancer, and anaplastic thyroid cancer.
In the ROAR trial, 178 patients with advanced or metastatic BRAF V600E–mutated rare cancers who had exhausted standard treatment options were treated with the combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist). Dr. Wainberg reported results for the 35 patients having biliary tract cancer, most of whom were heavily pretreated.
With a median duration of follow-up of 8 months, the overall response rate was 42% as assessed by investigators and 36% as assessed by independent reviewers. More than half of patients had a grade 3 or 4 adverse event, but just one had to permanently stop treatment because of toxicity.
“These results represent the first prospectively analyzed cohort of patients with BRAF V600E–mutated biliary tract cancers treated with the combination of BRAF and MEK inhibition. Efficacy in this patient population with advanced disease was comparable to that of first-line chemotherapy with gemcitabine and cisplatin,” pointed out Dr. Wainberg, codirector of the GI oncology program and an assistant professor of medicine at the University of California, Los Angeles.
“Dabrafenib and trametinib demonstrated clinical benefit in patients with BRAF-mutant biliary tract cancers and should be considered a meaningful therapeutic option for these patients,” he contended. “BRAF V600E is one of several actionable driver mutations in this disease and should be considered for routine testing in patients with biliary tract cancers. In addition, among all the other data [that are] emerging in molecular analysis of this malignancy, perhaps among all the GI malignancies, this has the potential to undergo multiple studies of other targeted therapies.”
Why stop there?
“I don’t think there is anything needed to convince you that this treatment is very effective. It’s incredibly impressive,” commented invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the gastrointestinal cancers program, University of Southern California Norris Comprehensive Cancer Center in Los Angeles.
But experience with other BRAF-mutant malignancies, such as BRAF-mutant colorectal cancer, suggests that further benefit can accrue from hitting additional molecular targets, he said. For example, rationally selected triplet combinations can suppress emergence of resistant clones up front.
“How can we do even better? I think we need to be smart in how we inhibit downstream signaling of BRAF-mutant disease,” Dr. Lenz maintained, as downstream targeting is potentially more effective. Therefore, a logical candidate for improving on the combination of BRAF and MEK inhibitors in biliary tract cancer is an ERK inhibitor. Alternatively, the combination may be synergistic when used with immune checkpoint inhibitors that target programmed death-1 or programmed death–ligand 1.
Oncologists should perform next-generation sequencing for all patients with biliary tract cancer, in Dr. Lenz’s opinion. “Biliary cancer is one of these cancers where we have many potentially actionable mutations. I know there is no drug approved, but many trials are ongoing,” he elaborated. “So I just encourage you to do that in order to identify potential trials or treatment options.”
Study details
In the ROAR trial, patients received open-label dabrafenib (150 mg, twice daily) plus trametinib (2 mg, once daily) until unacceptable toxicity, disease progression, or death.
All 35 patients with biliary cancer had received gemcitabine, and 80% had received at least two lines of prior systemic therapy.
The median treatment duration was 6 months, Dr. Wainberg reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Fully 86% of patients were on treatment for longer than 3 months.
In addition to the impressive response rates, the patients had a median progression-free survival of 9.2 months, and a median overall survival of 11.7 months.
Adverse events were as expected based on previous experience with these targeted therapies, according to Dr. Wainberg. The rate of grade 3 or 4 adverse events was 57%. “These were predominantly pyrexia, a known side effect of BRAF inhibitors, which is managed with antipyretic therapy and dexamethasone,” he noted. Rash and gastrointestinal toxicity were also common.
Although adverse events often led to dose reductions and dose interruptions, only a single patient (3% of the cohort) had to stop treatment early because of an event (cholangitis).
SOURCE: Wainberg ZA et al. 2019 GI Cancers Symposium, Abstract 187.
REPORTING FROM THE 2019 GI CANCERS SYMPOSIUM
Key clinical point:
Major finding: The overall response rate was 42% as assessed by investigators and 36% as assessed by independent reviewers.
Study details: A multicenter, single-arm, open-label phase 2 basket trial with reporting of data for 35 patients having BRAF V600E–mutated biliary tract cancer (ROAR trial).
Disclosures: Dr. Wainberg disclosed that he has a consulting or advisory role with and receives research funding from numerous pharmaceutical companies including Novartis. The trial was sponsored by GlaxoSmithKline and Novartis.
Source: Wainberg ZA et al. GI Cancers Symposium, Abstract 187.
Combo treatment may improve quality of life in CTCL
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of six evaluable CTCL patients had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment.
Study details: A phase 2 study with results reported for 10 patients.
Disclosures: The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. The principal investigator reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
Are single agents better than chemo for relapsed/refractory PTCL?
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results from the COMPLETE registry suggest newer single agents may be more effective than combination chemotherapy for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
Complete response (CR) rates and median survival times were significantly better among patients who received single agents than among those who received combination therapy.
Although researchers don’t know what is driving these differences in outcomes, they did find that outcomes were best among patients who received single-agent brentuximab vedotin (BV), and a disproportionate number of patients received BV.
The researchers also found that patients who received single-agent therapy were more likely to proceed to stem cell transplant.
Therefore, it’s still unclear if single-agent treatment is superior to combination therapy for relapsed/refractory PTCL, according to Robert Stuver, MD, of Beth Israel Deaconess Medical Center in Boston.
Dr. Stuver presented data from the COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment) registry at the annual T-cell Lymphoma Forum.
The registry (NCT01110733) enrolled patients newly diagnosed with PTCL. Dr. Stuver presented results among patients who had relapsed after, or were refractory to, upfront therapy and went on to receive single-agent therapy or any combination regimen excluding those single agents. Outcome data were collected for 5 years or until death.
Patients and treatment
There were 26 patients in the combination treatment group — 10 with PTCL not otherwise specified (NOS), 6 with angioimmunoblastic T-cell lymphoma (AITL), 5 with natural killer T-cell lymphoma (NKTL), 3 with anaplastic large-cell lymphoma (ALCL), 1 with enteropathy-associated T-cell lymphoma (EATL), and 1 with hepatosplenic T-cell lymphoma (HSTCL).
Patients in the combination group received gemcitabine-based therapy (n = 10), ifosfamide-based therapy (n = 7), platinum-based therapy (n = 4), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like therapy (n = 1), DHAP (dexamethasone, high-dose cytarabine, and cisplatin; n = 1), and other combinations (n = 3).
There were 31 patients in the single-agent group – 13 with PTCL-NOS, 7 with ALCL, 5 with AITL, 2 with EATL, 2 with NKTL, and 2 with HSTCL.
These patients were treated with BV (n = 12), romidepsin (n = 8), pralatrexate (n = 5), alisertib (n = 3), bendamustine (n = 1), denileukin diftitox (n = 1), and lenalidomide (n = 1).
Response
The CR rates were significantly higher among patients who received single-agent treatment than among those who received combination therapy — 41.4% (12/31) and 19.2% (5/26), respectively (P = .02). The partial response rates were 17.2% (5/31) and 26.9% (7/26), respectively. Rates of stable disease were 3.4% (1/31) and 30.8% (8/26), respectively.
Complete responders in the single-agent arm were treated with BV (n = 7), romidepsin (n = 2), pralatrexate (n = 1), alisertib (n = 1), and bendamustine (n = 1). Four of the patients treated with BV had ALCL.
“We had an enrichment of patients treated with brentuximab,” Dr. Stuver said. “So the obvious question this begs is, ‘Are the favorable results that were seen for single agents over combination therapy solely due to patients treated with brentuximab?’ ”
To investigate, Dr. Stuver and his colleagues compared responses among patients who received BV with patients who received other single agents or combination therapies.
The CR rate was 58.3% (7/12) among BV recipients, 29.4% (5/17) among patients who received other single agents, and 19.2% (5/26) among patients who received combination therapy.
“The takeaway here is that, when you do divide the single-agent group into BV and other single agents, you’re seeing that BV is doing much better than every other group,” Dr. Stuver said. “And all the other single agents are doing somewhat similarly to the combination group, although there’s still a 10% difference, 29% versus 19%.”
Survival
The median progression-free survival (PFS) and overall survival (OS) were significantly better among patients who received single-agent therapy. The median PFS was 11.7 months in the single-agent group and 6.7 months in the combination group (P = .0197). The median OS was 38.9 months and 17.1 months, respectively (P = .0170).
A factor that may have affected survival is that patients were more likely to undergo stem cell transplant after single-agent therapy (25.8%, 8/31), compared with those who had received combination therapy (7.7%, 2/26).
Another factor that may have affected the survival differences is the enrichment of patients treated with BV.
The researchers found the median PFS was 11.9 months among BV recipients, 10.4 months among patients who received other single agents, and 6.7 months in the combination-therapy group. The median OS was 44.5 months, 19.1 months, and 17.1 months, respectively.
Dr. Stuver said these results suggest there is a role for single agents as first retreatment in the salvage setting. However, this analysis was limited by the small sample size and the enrichment of patients treated with BV.
Larger, randomized studies are needed to identify the “truly superior” treatment strategy for relapsed/refractory PTCL, Dr. Stuver said.
The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The complete response rate was significantly higher among patients who received single-agent treatment than it was among those who received combination therapy – 41.4% and 19.2%, respectively (P = .02).
Study details: Analysis of 57 patients with relapsed/refractory PTCL in the COMPLETE registry.
Disclosures: The COMPLETE registry is sponsored by Spectrum Pharmaceuticals. Dr. Stuver did not declare any conflicts of interest.
Management of hypertensive disorders in pregnancy
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 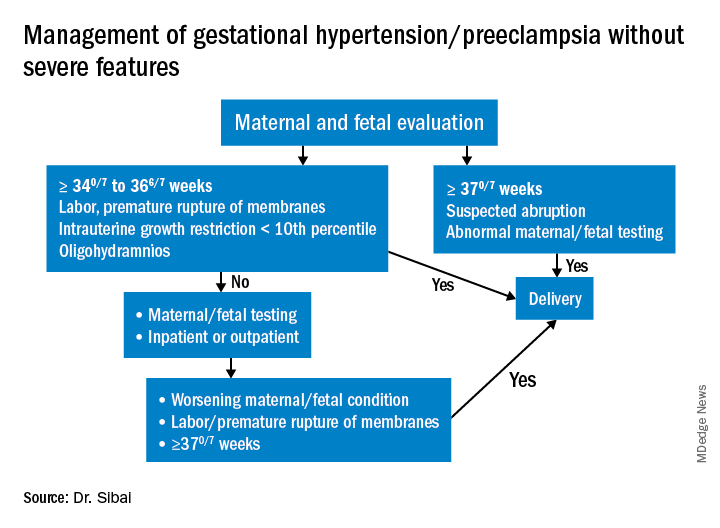
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 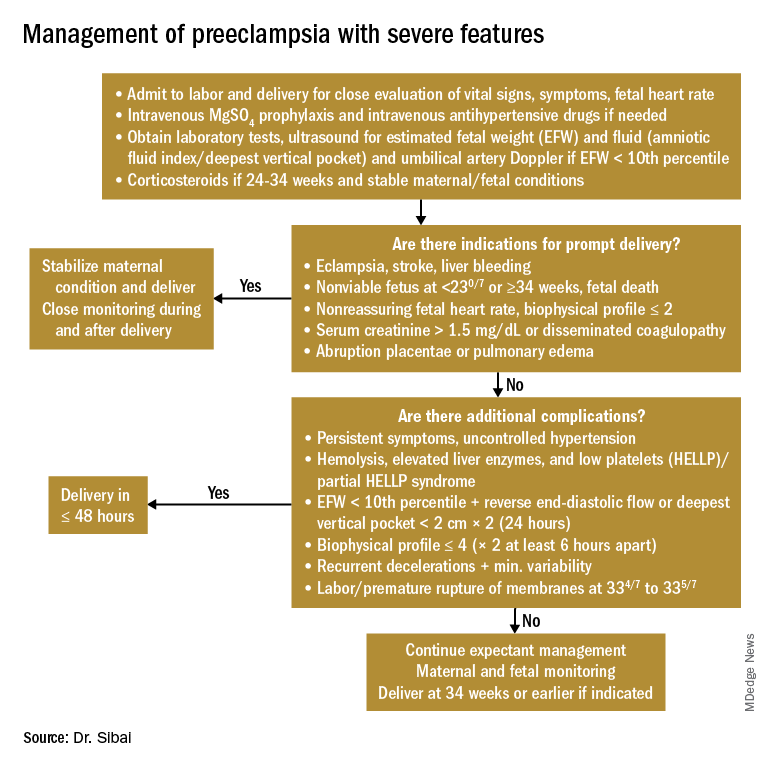
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.
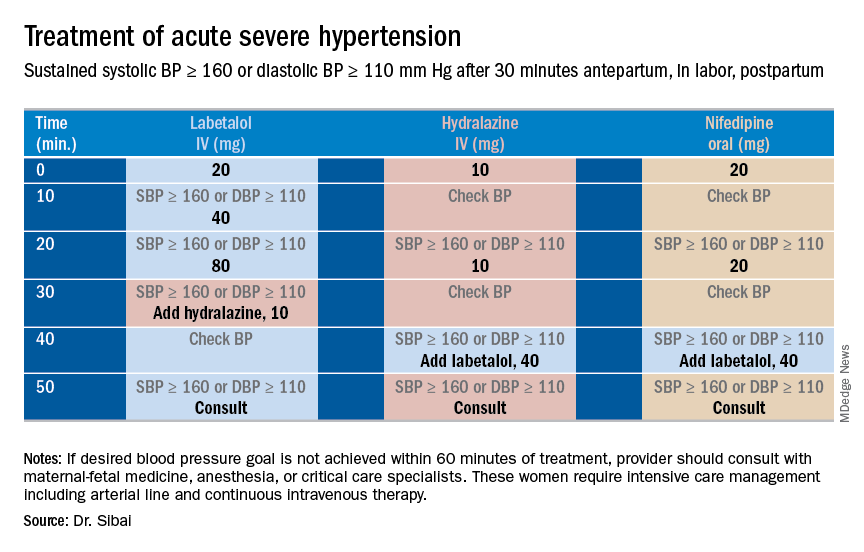
Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.