User login
Help spark scientific breakthroughs with the AGA Research Foundation
The way we diagnose and treat patients is the result of years of research. But securing the future of the field is no small task. A donation to the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation, will help fill the funding gap and contribute to this tradition of discovery.
The foundation provides a key source of funding at a critical juncture in a young investigator’s career.
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Won Jae Huh, MD, whose research will impact the future care of patients.
“As a clinical researcher, funding for investigation is critical in scientific breakthroughs to promote more efficient and robust patient care. My project will provide novel insights into the role of distensibility in the treatment of patients with esophageal eosinophilia, potentially resulting in more efficient treatment selection and disease management.”
“This funding mechanism will secure my research time to investigate signaling pathways involved in the pathogenesis of Ménétrier’s disease, and will provide resources and support to launch my research career. The results from my research project will be helpful for treating Ménétrier’s disease patients.”
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Dr. Won Jae Huh, whose research will impact the future care of patients.
Donate on the foundation’s website at www.gastro.org/donateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
The way we diagnose and treat patients is the result of years of research. But securing the future of the field is no small task. A donation to the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation, will help fill the funding gap and contribute to this tradition of discovery.
The foundation provides a key source of funding at a critical juncture in a young investigator’s career.
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Won Jae Huh, MD, whose research will impact the future care of patients.
“As a clinical researcher, funding for investigation is critical in scientific breakthroughs to promote more efficient and robust patient care. My project will provide novel insights into the role of distensibility in the treatment of patients with esophageal eosinophilia, potentially resulting in more efficient treatment selection and disease management.”
“This funding mechanism will secure my research time to investigate signaling pathways involved in the pathogenesis of Ménétrier’s disease, and will provide resources and support to launch my research career. The results from my research project will be helpful for treating Ménétrier’s disease patients.”
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Dr. Won Jae Huh, whose research will impact the future care of patients.
Donate on the foundation’s website at www.gastro.org/donateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
The way we diagnose and treat patients is the result of years of research. But securing the future of the field is no small task. A donation to the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation, will help fill the funding gap and contribute to this tradition of discovery.
The foundation provides a key source of funding at a critical juncture in a young investigator’s career.
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Won Jae Huh, MD, whose research will impact the future care of patients.
“As a clinical researcher, funding for investigation is critical in scientific breakthroughs to promote more efficient and robust patient care. My project will provide novel insights into the role of distensibility in the treatment of patients with esophageal eosinophilia, potentially resulting in more efficient treatment selection and disease management.”
“This funding mechanism will secure my research time to investigate signaling pathways involved in the pathogenesis of Ménétrier’s disease, and will provide resources and support to launch my research career. The results from my research project will be helpful for treating Ménétrier’s disease patients.”
Help spark the scientific breakthroughs of today, so clinicians will have the tools to improve care tomorrow. Your tax-deductible donation will make a critical difference in retaining talented GI scientists, like Dr. Won Jae Huh, whose research will impact the future care of patients.
Donate on the foundation’s website at www.gastro.org/donateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
Trump zeroes in on surprise medical bills in White House chat with patients, experts
President Trump on Jan. 23 instructed administration officials to investigate how to prevent surprise medical bills, broadening his focus on drug prices to include other issues of price transparency in health care.

several attendees said.
“The pricing is hurting patients, and we’ve stopped a lot of it, but we’re going to stop all of it,” Mr. Trump said during a roundtable discussion when reporters were briefly allowed into the otherwise closed-door meeting.
David Silverstein, the founder of a Colorado-based nonprofit called Broken Healthcare who attended, said Mr. Trump struck an aggressive tone, calling for a solution with “the biggest teeth you can find.”
“Reading the tea leaves, I think there’s big change coming,” Mr. Silverstein said.
Surprise billing, or the practice of charging patients for care that is more expensive than anticipated or not covered by their insurance, has received a flood of attention in the past year, particularly as Kaiser Health News and other news organizations have undertaken investigations into patients’ most outrageous medical bills.
Attendees said each of 10 invited guests – among them patients as well as doctors with their own stories of unexpected bills – was given an opportunity to talk, though Mr. Trump did not stay to hear all of their stories during the roughly hour-long gathering.
The group included Paul Davis, a retired doctor from Findlay, Ohio, whose family’s experience with a $17,850 bill for a simple urine test was detailed in a KHN-NPR “Bill of the Month” feature last year.
Mr. Davis’ daughter, Elizabeth Moreno, was a college student in Texas when she had spinal surgery to remedy debilitating back pain. After the surgery, she was asked to provide a urine sample and later received a bill from an out-of-network lab in Houston that tested it. Experts said such tests rarely cost more than $200, not nearly what the lab charged Ms. Moreno and her insurance company. But fearing damage to his daughter’s credit, Mr. Davis paid the lab $5,000 and filed a complaint with the Texas attorney general’s office, alleging “price gouging of staggering proportions.”
Mr. Davis said White House officials made it clear that price transparency is a “high priority” for Trump, and while they did not see eye to eye on every subject, he said he was struck by their sincerity.
“These people seemed earnest in wanting to do something constructive to fix this,” Mr. Davis said.
Dr. Martin Makary, a surgeon and health policy expert at Johns Hopkins University who has written about transparency in health care and attended the meeting, said it was a good opportunity for the White House to hear firsthand about a serious and widespread issue.
“This is how most of America lives, and [Americans are] getting hammered,” he said.
Mr. Trump has often railed against high prescription drug prices but has said less about other problems with the nation’s health care system. In October, shortly before the midterm elections, he unveiled a proposal to tie the price Medicare pays for some drugs to the prices paid for the same drugs overseas, for example.
Mr. Trump, Mr. Azar, and Mr. Acosta said efforts to control costs in health care were yielding positive results, discussing in particular the expansion of association health plans and the new requirement that hospitals post their list prices online. The president also took credit for the recent increase in generic drug approvals, which he said would help lower drug prices.
Discussing the partial government shutdown, Mr. Trump said Americans “want to see what we’re doing, like today we lowered prescription drug prices, first time in 50 years,” according to a White House pool report.
Mr. Trump appeared to be referring to a recent claim by the White House Council of Economic Advisers that prescription drug prices fell last year.
However, as STAT pointed out in a recent fact check, the report from which that claim was gleaned said “growth in relative drug prices has slowed since January 2017,” not that there was an overall decrease in prices.
Annual increases in overall drug spending have leveled off as pharmaceutical companies have released fewer blockbuster drugs; patents have expired on brand-name drugs; and the waning effect of a spike driven by the release of astronomically expensive drugs to treat hepatitis C. Drugmakers are also wary of increasing their prices in the midst of growing political pressure.
Since Democrats seized control of the House of Representatives this month, party leaders have rushed to announce investigations and schedule hearings dealing with health care, focusing in particular on drug costs and protections for those with preexisting conditions.
Recently, the House Oversight Committee announced a “sweeping” investigation into drug prices, pointing to an AARP report saying the vast majority of brand-name drugs had more than doubled in price between 2005 and 2017.
KHN correspondents Shefali Luthra and Jay Hancock contributed to this report. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
President Trump on Jan. 23 instructed administration officials to investigate how to prevent surprise medical bills, broadening his focus on drug prices to include other issues of price transparency in health care.

several attendees said.
“The pricing is hurting patients, and we’ve stopped a lot of it, but we’re going to stop all of it,” Mr. Trump said during a roundtable discussion when reporters were briefly allowed into the otherwise closed-door meeting.
David Silverstein, the founder of a Colorado-based nonprofit called Broken Healthcare who attended, said Mr. Trump struck an aggressive tone, calling for a solution with “the biggest teeth you can find.”
“Reading the tea leaves, I think there’s big change coming,” Mr. Silverstein said.
Surprise billing, or the practice of charging patients for care that is more expensive than anticipated or not covered by their insurance, has received a flood of attention in the past year, particularly as Kaiser Health News and other news organizations have undertaken investigations into patients’ most outrageous medical bills.
Attendees said each of 10 invited guests – among them patients as well as doctors with their own stories of unexpected bills – was given an opportunity to talk, though Mr. Trump did not stay to hear all of their stories during the roughly hour-long gathering.
The group included Paul Davis, a retired doctor from Findlay, Ohio, whose family’s experience with a $17,850 bill for a simple urine test was detailed in a KHN-NPR “Bill of the Month” feature last year.
Mr. Davis’ daughter, Elizabeth Moreno, was a college student in Texas when she had spinal surgery to remedy debilitating back pain. After the surgery, she was asked to provide a urine sample and later received a bill from an out-of-network lab in Houston that tested it. Experts said such tests rarely cost more than $200, not nearly what the lab charged Ms. Moreno and her insurance company. But fearing damage to his daughter’s credit, Mr. Davis paid the lab $5,000 and filed a complaint with the Texas attorney general’s office, alleging “price gouging of staggering proportions.”
Mr. Davis said White House officials made it clear that price transparency is a “high priority” for Trump, and while they did not see eye to eye on every subject, he said he was struck by their sincerity.
“These people seemed earnest in wanting to do something constructive to fix this,” Mr. Davis said.
Dr. Martin Makary, a surgeon and health policy expert at Johns Hopkins University who has written about transparency in health care and attended the meeting, said it was a good opportunity for the White House to hear firsthand about a serious and widespread issue.
“This is how most of America lives, and [Americans are] getting hammered,” he said.
Mr. Trump has often railed against high prescription drug prices but has said less about other problems with the nation’s health care system. In October, shortly before the midterm elections, he unveiled a proposal to tie the price Medicare pays for some drugs to the prices paid for the same drugs overseas, for example.
Mr. Trump, Mr. Azar, and Mr. Acosta said efforts to control costs in health care were yielding positive results, discussing in particular the expansion of association health plans and the new requirement that hospitals post their list prices online. The president also took credit for the recent increase in generic drug approvals, which he said would help lower drug prices.
Discussing the partial government shutdown, Mr. Trump said Americans “want to see what we’re doing, like today we lowered prescription drug prices, first time in 50 years,” according to a White House pool report.
Mr. Trump appeared to be referring to a recent claim by the White House Council of Economic Advisers that prescription drug prices fell last year.
However, as STAT pointed out in a recent fact check, the report from which that claim was gleaned said “growth in relative drug prices has slowed since January 2017,” not that there was an overall decrease in prices.
Annual increases in overall drug spending have leveled off as pharmaceutical companies have released fewer blockbuster drugs; patents have expired on brand-name drugs; and the waning effect of a spike driven by the release of astronomically expensive drugs to treat hepatitis C. Drugmakers are also wary of increasing their prices in the midst of growing political pressure.
Since Democrats seized control of the House of Representatives this month, party leaders have rushed to announce investigations and schedule hearings dealing with health care, focusing in particular on drug costs and protections for those with preexisting conditions.
Recently, the House Oversight Committee announced a “sweeping” investigation into drug prices, pointing to an AARP report saying the vast majority of brand-name drugs had more than doubled in price between 2005 and 2017.
KHN correspondents Shefali Luthra and Jay Hancock contributed to this report. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
President Trump on Jan. 23 instructed administration officials to investigate how to prevent surprise medical bills, broadening his focus on drug prices to include other issues of price transparency in health care.

several attendees said.
“The pricing is hurting patients, and we’ve stopped a lot of it, but we’re going to stop all of it,” Mr. Trump said during a roundtable discussion when reporters were briefly allowed into the otherwise closed-door meeting.
David Silverstein, the founder of a Colorado-based nonprofit called Broken Healthcare who attended, said Mr. Trump struck an aggressive tone, calling for a solution with “the biggest teeth you can find.”
“Reading the tea leaves, I think there’s big change coming,” Mr. Silverstein said.
Surprise billing, or the practice of charging patients for care that is more expensive than anticipated or not covered by their insurance, has received a flood of attention in the past year, particularly as Kaiser Health News and other news organizations have undertaken investigations into patients’ most outrageous medical bills.
Attendees said each of 10 invited guests – among them patients as well as doctors with their own stories of unexpected bills – was given an opportunity to talk, though Mr. Trump did not stay to hear all of their stories during the roughly hour-long gathering.
The group included Paul Davis, a retired doctor from Findlay, Ohio, whose family’s experience with a $17,850 bill for a simple urine test was detailed in a KHN-NPR “Bill of the Month” feature last year.
Mr. Davis’ daughter, Elizabeth Moreno, was a college student in Texas when she had spinal surgery to remedy debilitating back pain. After the surgery, she was asked to provide a urine sample and later received a bill from an out-of-network lab in Houston that tested it. Experts said such tests rarely cost more than $200, not nearly what the lab charged Ms. Moreno and her insurance company. But fearing damage to his daughter’s credit, Mr. Davis paid the lab $5,000 and filed a complaint with the Texas attorney general’s office, alleging “price gouging of staggering proportions.”
Mr. Davis said White House officials made it clear that price transparency is a “high priority” for Trump, and while they did not see eye to eye on every subject, he said he was struck by their sincerity.
“These people seemed earnest in wanting to do something constructive to fix this,” Mr. Davis said.
Dr. Martin Makary, a surgeon and health policy expert at Johns Hopkins University who has written about transparency in health care and attended the meeting, said it was a good opportunity for the White House to hear firsthand about a serious and widespread issue.
“This is how most of America lives, and [Americans are] getting hammered,” he said.
Mr. Trump has often railed against high prescription drug prices but has said less about other problems with the nation’s health care system. In October, shortly before the midterm elections, he unveiled a proposal to tie the price Medicare pays for some drugs to the prices paid for the same drugs overseas, for example.
Mr. Trump, Mr. Azar, and Mr. Acosta said efforts to control costs in health care were yielding positive results, discussing in particular the expansion of association health plans and the new requirement that hospitals post their list prices online. The president also took credit for the recent increase in generic drug approvals, which he said would help lower drug prices.
Discussing the partial government shutdown, Mr. Trump said Americans “want to see what we’re doing, like today we lowered prescription drug prices, first time in 50 years,” according to a White House pool report.
Mr. Trump appeared to be referring to a recent claim by the White House Council of Economic Advisers that prescription drug prices fell last year.
However, as STAT pointed out in a recent fact check, the report from which that claim was gleaned said “growth in relative drug prices has slowed since January 2017,” not that there was an overall decrease in prices.
Annual increases in overall drug spending have leveled off as pharmaceutical companies have released fewer blockbuster drugs; patents have expired on brand-name drugs; and the waning effect of a spike driven by the release of astronomically expensive drugs to treat hepatitis C. Drugmakers are also wary of increasing their prices in the midst of growing political pressure.
Since Democrats seized control of the House of Representatives this month, party leaders have rushed to announce investigations and schedule hearings dealing with health care, focusing in particular on drug costs and protections for those with preexisting conditions.
Recently, the House Oversight Committee announced a “sweeping” investigation into drug prices, pointing to an AARP report saying the vast majority of brand-name drugs had more than doubled in price between 2005 and 2017.
KHN correspondents Shefali Luthra and Jay Hancock contributed to this report. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Half of parents unaware of teens’ suicidal thoughts
Most parents are unaware their teenager has been having suicidal thoughts or thinking about death, according to a study published in Pediatrics.
Jason D. Jones, PhD, from the Children’s Hospital of Philadelphia, and his coauthors wrote that more than two-thirds of adolescents who experience suicidal thoughts do not get medical help, and this may be because their parents – the gatekeepers for mental health services – are unaware of what their teen is going through.
In this study, researchers recruited 5,137 adolescents aged 11-17 years and either a parent or step-parent, and interviewed both about the adolescent’s lifetime suicidal thoughts.
While 413 (8%) of the adolescents surveyed said they had had thoughts about killing themselves, 50% of those adolescents’ parents said their teen hadn’t experienced suicidal thoughts. Similarly, 786 (15%) of adolescents surveyed said they had had thoughts about death and dying, but three-quarters of their parents were unaware.
A significant number of parents – 8% – said their teenager had had suicidal thoughts, but in 48% of these cases, the teenager said they had not thought about killing themselves.
Researchers saw more agreement between parents and adolescents when the adolescents were older: The parents were less likely to be unaware that their older teen had had suicidal thoughts, and older adolescents were less likely to deny it.
“This indicates that younger adolescents may be more likely to go unnoticed and not receive services either because their parents are unaware of their suicidal thoughts or because they deny suicidal thoughts that their parents think they are having,” Dr. Jones and his associates wrote. They also suggested younger adolescents may have “interpretive difficulties” around questions of suicidal ideation.
“These age findings are particularly noteworthy in light of recent evidence that deaths by suicide have increased among younger adolescents,” they noted.
There also was an interaction between age and gender. For girls, parents were less likely to be aware of suicidal thoughts in their younger daughters but more likely to be aware of them in their older daughters. However the opposite was true for boys: Parental unawareness increased slightly in older boys.
Parents of Hispanic or Latino ethnicity were less likely to be aware that their offspring had had thoughts about death and dying.
Generally fathers were less likely than mothers to be aware of suicidal thoughts in their adolescents.
However, if adolescents had previously received psychiatric treatment, or there was a family history of suicide, parents were more likely to be aware of suicidal thoughts, and adolescents who had a history of psychiatric hospitalization were less likely to deny suicidal thoughts, the researchers reported.
The study was supported by grants from the National Institutes of Health, the Dowshen Program for Neuroscience, and the Lifespan Brain Institute of the Children’s Hospital of Philadelphia and University of Pennsylvania. The study was funded by NIH. One author declared a board position and stock options in Taliaz Health unrelated to the study subject; the other authors said they had no relevant financial disclosures.
SOURCE: Jones JD et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-1771.
Suicide prevention relies on identifying individuals at risk, but in the case of young people, this often relies on parents. This study, and previous research, highlights the limitations of parent report of adolescents’ suicidal thoughts, as well as the issue of adolescents’ denying suicidal thoughts when parents report them.
Given that as many as 40% of adolescents who think about suicide act on those thoughts, it is vital that we achieve more specificity in identifying young people at risk of attempting suicide. These findings have implications for screening in the primary care setting, and they suggest a need for multi-informant assessments, as well as careful exploration of disagreements between parents’ and adolescent’s reports.
Khyati Brahmbhatt, MD, and Jacqueline Grupp-Phelan, MD, MPH, are from the University of California, San Francisco, Benioff Children’s Hospitals. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-3071). No conflicts of interest were declared. The editorial was funded by the National Institutes of Health.
Suicide prevention relies on identifying individuals at risk, but in the case of young people, this often relies on parents. This study, and previous research, highlights the limitations of parent report of adolescents’ suicidal thoughts, as well as the issue of adolescents’ denying suicidal thoughts when parents report them.
Given that as many as 40% of adolescents who think about suicide act on those thoughts, it is vital that we achieve more specificity in identifying young people at risk of attempting suicide. These findings have implications for screening in the primary care setting, and they suggest a need for multi-informant assessments, as well as careful exploration of disagreements between parents’ and adolescent’s reports.
Khyati Brahmbhatt, MD, and Jacqueline Grupp-Phelan, MD, MPH, are from the University of California, San Francisco, Benioff Children’s Hospitals. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-3071). No conflicts of interest were declared. The editorial was funded by the National Institutes of Health.
Suicide prevention relies on identifying individuals at risk, but in the case of young people, this often relies on parents. This study, and previous research, highlights the limitations of parent report of adolescents’ suicidal thoughts, as well as the issue of adolescents’ denying suicidal thoughts when parents report them.
Given that as many as 40% of adolescents who think about suicide act on those thoughts, it is vital that we achieve more specificity in identifying young people at risk of attempting suicide. These findings have implications for screening in the primary care setting, and they suggest a need for multi-informant assessments, as well as careful exploration of disagreements between parents’ and adolescent’s reports.
Khyati Brahmbhatt, MD, and Jacqueline Grupp-Phelan, MD, MPH, are from the University of California, San Francisco, Benioff Children’s Hospitals. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-3071). No conflicts of interest were declared. The editorial was funded by the National Institutes of Health.
Most parents are unaware their teenager has been having suicidal thoughts or thinking about death, according to a study published in Pediatrics.
Jason D. Jones, PhD, from the Children’s Hospital of Philadelphia, and his coauthors wrote that more than two-thirds of adolescents who experience suicidal thoughts do not get medical help, and this may be because their parents – the gatekeepers for mental health services – are unaware of what their teen is going through.
In this study, researchers recruited 5,137 adolescents aged 11-17 years and either a parent or step-parent, and interviewed both about the adolescent’s lifetime suicidal thoughts.
While 413 (8%) of the adolescents surveyed said they had had thoughts about killing themselves, 50% of those adolescents’ parents said their teen hadn’t experienced suicidal thoughts. Similarly, 786 (15%) of adolescents surveyed said they had had thoughts about death and dying, but three-quarters of their parents were unaware.
A significant number of parents – 8% – said their teenager had had suicidal thoughts, but in 48% of these cases, the teenager said they had not thought about killing themselves.
Researchers saw more agreement between parents and adolescents when the adolescents were older: The parents were less likely to be unaware that their older teen had had suicidal thoughts, and older adolescents were less likely to deny it.
“This indicates that younger adolescents may be more likely to go unnoticed and not receive services either because their parents are unaware of their suicidal thoughts or because they deny suicidal thoughts that their parents think they are having,” Dr. Jones and his associates wrote. They also suggested younger adolescents may have “interpretive difficulties” around questions of suicidal ideation.
“These age findings are particularly noteworthy in light of recent evidence that deaths by suicide have increased among younger adolescents,” they noted.
There also was an interaction between age and gender. For girls, parents were less likely to be aware of suicidal thoughts in their younger daughters but more likely to be aware of them in their older daughters. However the opposite was true for boys: Parental unawareness increased slightly in older boys.
Parents of Hispanic or Latino ethnicity were less likely to be aware that their offspring had had thoughts about death and dying.
Generally fathers were less likely than mothers to be aware of suicidal thoughts in their adolescents.
However, if adolescents had previously received psychiatric treatment, or there was a family history of suicide, parents were more likely to be aware of suicidal thoughts, and adolescents who had a history of psychiatric hospitalization were less likely to deny suicidal thoughts, the researchers reported.
The study was supported by grants from the National Institutes of Health, the Dowshen Program for Neuroscience, and the Lifespan Brain Institute of the Children’s Hospital of Philadelphia and University of Pennsylvania. The study was funded by NIH. One author declared a board position and stock options in Taliaz Health unrelated to the study subject; the other authors said they had no relevant financial disclosures.
SOURCE: Jones JD et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-1771.
Most parents are unaware their teenager has been having suicidal thoughts or thinking about death, according to a study published in Pediatrics.
Jason D. Jones, PhD, from the Children’s Hospital of Philadelphia, and his coauthors wrote that more than two-thirds of adolescents who experience suicidal thoughts do not get medical help, and this may be because their parents – the gatekeepers for mental health services – are unaware of what their teen is going through.
In this study, researchers recruited 5,137 adolescents aged 11-17 years and either a parent or step-parent, and interviewed both about the adolescent’s lifetime suicidal thoughts.
While 413 (8%) of the adolescents surveyed said they had had thoughts about killing themselves, 50% of those adolescents’ parents said their teen hadn’t experienced suicidal thoughts. Similarly, 786 (15%) of adolescents surveyed said they had had thoughts about death and dying, but three-quarters of their parents were unaware.
A significant number of parents – 8% – said their teenager had had suicidal thoughts, but in 48% of these cases, the teenager said they had not thought about killing themselves.
Researchers saw more agreement between parents and adolescents when the adolescents were older: The parents were less likely to be unaware that their older teen had had suicidal thoughts, and older adolescents were less likely to deny it.
“This indicates that younger adolescents may be more likely to go unnoticed and not receive services either because their parents are unaware of their suicidal thoughts or because they deny suicidal thoughts that their parents think they are having,” Dr. Jones and his associates wrote. They also suggested younger adolescents may have “interpretive difficulties” around questions of suicidal ideation.
“These age findings are particularly noteworthy in light of recent evidence that deaths by suicide have increased among younger adolescents,” they noted.
There also was an interaction between age and gender. For girls, parents were less likely to be aware of suicidal thoughts in their younger daughters but more likely to be aware of them in their older daughters. However the opposite was true for boys: Parental unawareness increased slightly in older boys.
Parents of Hispanic or Latino ethnicity were less likely to be aware that their offspring had had thoughts about death and dying.
Generally fathers were less likely than mothers to be aware of suicidal thoughts in their adolescents.
However, if adolescents had previously received psychiatric treatment, or there was a family history of suicide, parents were more likely to be aware of suicidal thoughts, and adolescents who had a history of psychiatric hospitalization were less likely to deny suicidal thoughts, the researchers reported.
The study was supported by grants from the National Institutes of Health, the Dowshen Program for Neuroscience, and the Lifespan Brain Institute of the Children’s Hospital of Philadelphia and University of Pennsylvania. The study was funded by NIH. One author declared a board position and stock options in Taliaz Health unrelated to the study subject; the other authors said they had no relevant financial disclosures.
SOURCE: Jones JD et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-1771.
FROM PEDIATRICS
Key clinical point: Many parents are unaware of their teenager’s suicidal thoughts.
Major finding: Half of parents are unaware that their adolescent child has had suicidal thoughts.
Study details: Survey of 5,137 adolescents and their parents or step-parents.
Disclosures: The study was supported by grants from the National Institutes of Health, the Dowshen Program for Neuroscience, and the Lifespan Brain Institute of the Children’s Hospital of Philadelphia and University of Pennsylvania. The study was funded by NIH. One author declared a board position and stock options in Taliaz Health unrelated to the study subject; the other authors said they had no relevant financial disclosures.
Source: Jones JD et al. Pediatrics. 2019, Jan 14. doi: 10.1542/peds.2018-1771.
No evidence for disease-modifying effect of levodopa in Parkinson’s disease
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Levodopa did not have any significant disease-modifying effects in patients with early Parkinson’s disease.
Major finding: Change in the Unified Parkinson’s Disease Rating Scale (UPDRS) was –1.0 after 80 weeks of levodopa/carbodopa versus –2.0 for 40 weeks of placebo followed by 40 weeks of treatment (P = .44).
Study details: A delayed-start trial including 445 patients with early Parkinson’s disease randomized to 80 weeks of treatment or 40 weeks of placebo plus 40 weeks of treatment.
Disclosures: Study authors reported disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
Source: Verschuur CVM et al. N Engl J Med. 2019;380:315-324.
FDA approves 0.5-mL Fluzone Quadrivalent vaccine in young children
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
Study hints at lacosamide’s efficacy for small fiber neuropathy
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
FROM BRAIN
Key clinical point:
Major finding: In the lacosamide group, 50.0% of patients reported mean average pain decreasing by at least 1 point, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213).
Study details: A randomized, placebo-controlled, double-blind, crossover-design study of 25 patients with Nav1.7-related small fiber neuropathy who received lacosamide followed by placebo, or vice versa.
Disclosures: The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
Source: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
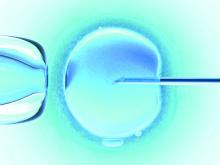
Endometrial scratching doesn’t lead to higher live birth rate for IVF patients
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The live birth rate for women in the endometrial scratch group was 26% of 690 participants, compared with 26% of 674 women in the control group, a nonsignificant difference.
Study details: A multicenter, open-label, randomized controlled trial of 1,364 women undergoing IVF at 13 different sites in 5 countries.
Disclosures: This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
Source: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
Avelumab active in recurrent or refractory ovarian cancer
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
FROM JAMA ONCOLOGY
Key clinical point: Single-agent treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients.
Major finding: Treatment yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months.
Study details: Phase 1b results from the JAVELIN solid tumor trial, which included 125 women with recurrent or refractory ovarian cancer.
Disclosures: The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Study authors reported disclosures related to Pfizer, Merck, Celgene, EMD Serono, Epithany, Janssen, and Seattle Genetics, among others.
Source: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Buprenorphine for NAS shows promise in reducing length of stay
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
FROM JAMA PEDIATRICS
Key clinical point: A larger study comparing buprenorphine and morphine is needed to confirm study findings.
Major finding: Although morphine and phenobarbital are prescribed most frequently in the United States, they were found to be the least effective treatments available.
Study details: Systematic review and network meta-analysis.
Disclosures: The authors had no financial relationships relevant to this article to disclose.
Source: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
Small White Spots on the Lips
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.