User login
How to best advocate for HPV vaccination
Also today, FDA labeling templates smooth the way for over-the-counter naloxone, a revised Affordable Care Act premium calculator could increase the rate of uninsured, and confidential parent-free discussion should occur by age 13.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, FDA labeling templates smooth the way for over-the-counter naloxone, a revised Affordable Care Act premium calculator could increase the rate of uninsured, and confidential parent-free discussion should occur by age 13.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, FDA labeling templates smooth the way for over-the-counter naloxone, a revised Affordable Care Act premium calculator could increase the rate of uninsured, and confidential parent-free discussion should occur by age 13.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Cognition and MS
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Aplastic Anemia: Current Treatment
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
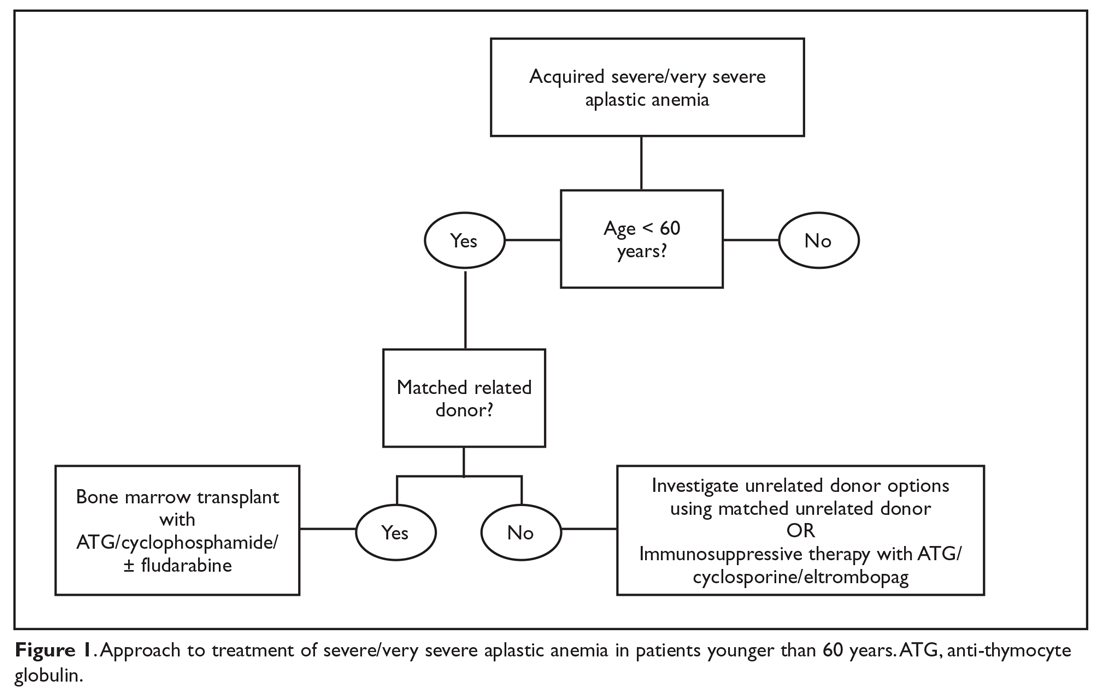
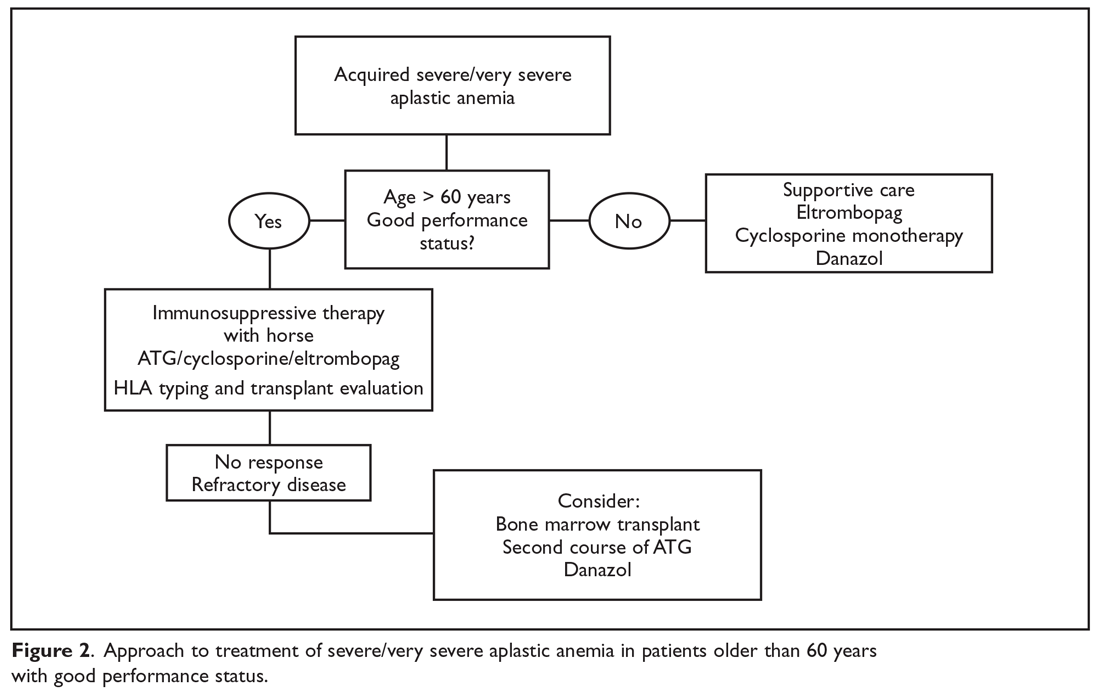
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.


Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Aplastic anemia is a rare hematologic disorder marked by pancytopenia and a hypocellular marrow. Aplastic anemia results from either inherited or acquired causes, and the treatment approach varies significantly between the 2 causes. This article reviews the treatment of inherited and acquired forms of aplastic anemia. The approach to evaluation and diagnosis of aplastic anemia is reviewed in a separate article.
Inherited Aplastic Anemia
First-line treatment options for patients with inherited marrow failure syndromes (IMFS) are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect.1 Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with bone marrow demonstrating lower rates of acute GVHD than a peripheral blood stem cell source.2-4 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.5,6 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival2,5,7 compared to cyclophosphamide conditioning, which was historically used in matched related donors.6,8 The addition of fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.7,9 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data is limited.5
For patients presenting with acute myeloid leukemia (AML) or a high-risk myelodysplastic syndrome (MDS) who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggests that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased overall survival (OS) despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.10,11 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan is being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.12
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.13,14 Transfusion support is often required to prevent complications associated with thrombocytopenia and anemia; all blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.15 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an absolute neutrophil count [ANC] < 500 cells/µL).16-19 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study by Tichelli and colleagues evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.20 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006).This difference was largely driven by a decrease in infectious episodes in patients with very severe aplastic anemia (VSAA) treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).20
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.21 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.19,22
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.18,19 This appears to be changing with time. Valdez and colleagues demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.22 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.19 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with severe aplastic anema (SAA) or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).17,23 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.17 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues demonstrated that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level: 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).24 Approximately 25% of patients in this trial demonstrated an increase in creatinine, with patients taking concomitant cyclosporine more affected than those on chelation therapy alone.24 For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.15
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.


Matched Sibling Donor Transplant
Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years of age, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).25 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and matched unrelated donor HSCT (38% and 65%).25 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.26
Current conditioning regimens typically use a combination of cyclophosphamide and ATG27,28 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.9 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (RR, 0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.29 In addition, Kim et al evaluated their experience with patients older than 40 years of age receiving matched related donors, finding comparable outcomes in those aged 41 to 50 years compared to younger patients. Outcomes did decline in those over the age of 50 years.30 Long-term data for matched related donor transplant for aplastic anemia shows excellent long-term outcomes, with minimal chronic GVHD and good performance status.31 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and matched unrelated donor (MUD) transplants for pediatric patients32,33 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.34,35
Immunosuppressive Therapy
For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.36 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.16,36,37
Anti-thymocyte globulin. Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.38 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).16 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS: 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Cyclosporine A. Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.39 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.39
The combination of the ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.37 In this study patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.40 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.41 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.42
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.42,43 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.42 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Immunosuppressive therapy plus eltrombopag. Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.44 When given at a dose of 150 mg daily in patients aged 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.44 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.45 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.44 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.46 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.4,22 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant
For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-V GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Additional benefits seen in this analysis included improved survival when transplanted under age 20 years (84% versus 72%), when transplanted within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and when the donor and recipient were cytomegalovirus-negative compared to other combinations (82% versus 76%).47 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.4,43 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).33 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients included underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.4 A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.33 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical paroxysmal nocturnal hemoglobinuria, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.48
With continued improvement of less toxic and more immunomodulating conditioning regimens, utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.49 However, there is still a large population of patients without matched sibling or unrelated donor options. In an effort to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant paroxysmal nocturnal hemoglobinuria, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.50
Summary
Aplastic anemia is a rare but potentially life-threatening disorder characterized by pancytopenia and a marked reduction in the hematopoietic stem cell compartment. Treatment should be instituted as soon as the dignosis of aplastic anemia is established. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
1. Peffault De Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience.” Blood. 2013;122:4279-4286.
2. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
3. Eapen M, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
4. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
5. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes.” Bone Marrow Transplant. 2015;50:1168-1172.
6. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
7. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
8 Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
9. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
10. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-200.
11. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
12. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
13. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
14. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
15. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
16. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Eng J Med. 2011;365:430-438.
17. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
18. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Sem Hematol. 2009;46:269-276.
19. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
20. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
21. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
22. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
23. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
24. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2554.
25. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-8.
26. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
27. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
28. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. ASH Education Program Book. 2013;2013:82-86.
29. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
30. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
31. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
32. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
33. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br. J Haematol. 2015;151:585-594.
34. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
35. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
36. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
37. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al, German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
38. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
39. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
40. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
41. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
42. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
43. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
44. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
45. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
46. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018 Oct 11. doi: 10.1002/cncr.31658.
47. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
48. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. 2018Oct 17. doi: 10.1002/ajh.25314.
49. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
50. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
Study shows evidence of herd immunity with HPV vaccine
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
FROM PEDIATRICS
Key clinical point:
Major finding: Infection rates for quadrivalent vaccine-covered HPV strains declined by 81% among vaccinated women.
Study details: Surveillance studies in 1,580 vaccinated and unvaccinated young women.
Disclosures: The study was funded by the National Institutes of Health. One author declared shares of Merck, but no other conflicts of interest were declared.
Source: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
HBV, HCV, HIV testing of new cancer patients advised
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
Oncologists should consider testing all patients with newly diagnosed cancers for infection with the hepatitis B and C viruses, a multicenter team has recommended.
A prospective study of hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV infections among 3,051 patients with newly diagnosed cancers showed that 6.5% of patients tested positive for previous HBV and 0.6% had chronic HBV infection. In addition, 2.4% of patients were positive for HCV, and 1.1% for HIV infections, reported Scott D. Ramsey, MD, PhD, from the Fred Hutchinson Cancer Research Center in Seattle, and colleagues.
“Many patients had no known risk factors for infection, suggesting that current risk-based models for screening may be insufficient. Thus, we believe our results warrant consideration of universal testing of patients with newly diagnosed cancer for HBV and HCV infection, particularly if such an approach is shown to be cost effective,” they wrote in JAMA Oncology.
The investigators noted that patients with undiagnosed hepatitis and/or HIV infections could transmit them to unsuspecting caregivers, adding that “with effective treatments available, not screening for these viruses misses an opportunity to reduce future morbidity associated with these infections and to avoid viral reactivation during treatment, with resulting morbidity and mortality.”
To estimate the prevalence of the infections in patients with newly diagnosed cancers, investigators looked at a cohort of 3,051 patients with a cancer diagnosis made within the previous 120 days at nine academic medical centers and nine community oncology centers representing a total of 41 cancer clinics affiliated with the SWOG Cancer Research Network (formerly the Southwest Oncology Group).
The median patient age was 60.6 years. Female patients constitute 60.4% of the sample; 18.1% were black, and 18.3% were of Hispanic heritage.
Of 3,050 patients for whom HBV testing results were available, 6.5% (197) were positive for previous HBV infection, compared with an estimated U.S. population prevalence of 4.7%. In addition, 0.6% (19 patients) were found to have chronic HBV, compared with an estimated 0.3% US population prevalence.
HCV infections were detected in 2.4% (71 of 2990 patients), compared with an estimated population prevalence of 1.3%, and HIV infections were detected in 1.1%, compared with a background estimated population prevalence of 0.3%.
In all, 32 patients were diagnosed with viral infections by testing performed for the study, including 8 patients with chronic HBV, 22 with HCV, and 2 with HIV.
Additionally, the authors found that 4 patients with chronic HBV, 23 with HCV, and 7 with HIV had no identifiable risk factors.
The highest prevalence of infections occurred among patients with liver cancer, nonliver and noncolorectal cancers of the gastrointestinal tract, head and neck cancers, lung cancers, and prostate cancer. A finding of viral positivity changed the treatment plan in only 8% of all infected patients, however.
“Given that most HIV-infected patients in our study knew their viral status, the yield of universal HIV testing among patients with newly diagnosed cancer may likely be low. Although age-directed screening is recommended for HIV and HCV, uptake rates in primary care are variable and low overall,” Dr. Ramsey and his colleagues wrote.
The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several co-authors reported receiving NCI grants, and multiple co-authors reported grants and/or consulting fees from various companies.
SOURCE: Ramsey SD et al. JAMA Oncol. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
FROM JAMA ONCOLOGY
Key clinical point: Patients with newly diagnosed cancers should be screened for viral infections that may pose a transmission risk or could be reactivated by cancer therapies.
Major finding: Infection rates of HBV, HCV, and HIV in patients with newly diagnosed cancers were 6.5%, 2.4%, and 1.1%, respectively.
Study details: Prospective study of viral infections in 3,051 patients with a diagnosis of cancer within the previous 120 days.
Disclosures: The study was supported by grants from the National Cancer Institute. Dr. Ramsey and several coauthors reported receiving NCI grants, and multiple coauthors reported grants and/or consulting fees from various companies.
Source: Ramsey SD et al. JAMA Oncology. 2019 Jan 17. doi: 10.1001/jamaoncol.2018.6437.
This month in the journal CHEST®
Editor’s Picks
Giants in Chest Medicine – Atul C. Mehta, MBBS, FCCP
By Dr. J. K. Stoller
Screening Heroin Smokers Attending Community Drug Services for COPD.
By Dr. H. Burhan, et al.
The NHLBI LAM Registry: Prognostic Physiologic and Radiologic Biomarkers Emerge
From a 15-Year Prospective Longitudinal Analysis.
By Dr. N. Gupta, et al.
Indwelling Pleural Catheters in Hepatic Hydrothorax: A Single-Center Series of Outcomes and Complications.
By Dr. C. Kniese, et al.
Implications of the Revised Common Rule for Human Participant Research.
By Dr. E. G. DeRenzo, et al.
Editor’s Picks
Giants in Chest Medicine – Atul C. Mehta, MBBS, FCCP
By Dr. J. K. Stoller
Screening Heroin Smokers Attending Community Drug Services for COPD.
By Dr. H. Burhan, et al.
The NHLBI LAM Registry: Prognostic Physiologic and Radiologic Biomarkers Emerge
From a 15-Year Prospective Longitudinal Analysis.
By Dr. N. Gupta, et al.
Indwelling Pleural Catheters in Hepatic Hydrothorax: A Single-Center Series of Outcomes and Complications.
By Dr. C. Kniese, et al.
Implications of the Revised Common Rule for Human Participant Research.
By Dr. E. G. DeRenzo, et al.
Editor’s Picks
Giants in Chest Medicine – Atul C. Mehta, MBBS, FCCP
By Dr. J. K. Stoller
Screening Heroin Smokers Attending Community Drug Services for COPD.
By Dr. H. Burhan, et al.
The NHLBI LAM Registry: Prognostic Physiologic and Radiologic Biomarkers Emerge
From a 15-Year Prospective Longitudinal Analysis.
By Dr. N. Gupta, et al.
Indwelling Pleural Catheters in Hepatic Hydrothorax: A Single-Center Series of Outcomes and Complications.
By Dr. C. Kniese, et al.
Implications of the Revised Common Rule for Human Participant Research.
By Dr. E. G. DeRenzo, et al.
Age may dictate benefit of andecaliximab in gastric cancer
SAN FRANCISCO – according to findings of the GAMMA-1 trial reported at the 2019 GI Cancers Symposium.
“Increased MMP9 expression is associated with poor prognosis across many malignancies and particularly in gastric cancer. All gastric cancers tested have MMP9 expression,” said lead investigator Manish A. Shah, MD, director of the gastrointestinal oncology program at Weill Cornell Medicine, New York, and chief of the solid tumor service and codirector of the Center for Advanced Digestive Disease at New York–Presbyterian. By activating and inactivating extracellular matrix proteins, MMP9 alters the tumor microenvironment, promoting angiogenesis, invasion, and metastases, and blunting the immune response, he said.
Main results of the phase 3, randomized, controlled GAMMA-1 trial showed that adding andecaliximab to the modified FOLFOX-6 regimen did not significantly improve outcomes among the entire population of 432 patients with untreated locally advanced or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma. However, exploratory analyses suggested that it significantly halved the risk of progression-free survival events and reduced by more than a third the risk of death among those aged 65 years or older.
“The apparent increased activity of the combination of mFOLFOX-6 and andecaliximab in patients aged 65 or older needs further study and correlative analyses,” Dr. Shah commented. “Our GAMMA-1 study was done in a nonselected population. We really don’t have a great biomarker for this [antibody]. … This is the question that we’re going to try to address over the next few months as we look at all the data.”
Because andecaliximab is expected to favorably alter the tumor immune microenvironment, it is also being tested in combination with immunotherapies, according to Dr. Shah. Results of some of those studies were also reported at the symposium.
Biological plausibility
A comprehensive view of precision oncology requires consideration not only of the tumor, but also of the microenvironment and the macroenvironment, agreed invited discussant Martine Extermann, MD, PhD, leader of the senior adult oncology program at the Moffitt Cancer Center in Tampa.
“A treatment which works better in older patients – I don’t hear that very frequently,” she commented. But data provide a biological rationale for selective benefit of andecaliximab in older adults. Specifically, this population may have higher MMP9 levels either from simple aging or from comorbidities that become more common as one grows older, such as atrial fibrillation and obstructive sleep apnea.
“My question to the investigators will be, are there serum samples that are available for MMP9 testing and could we check that to see whether these patients are the ones who had the best response to andecaliximab treatment?” Dr. Extermann said. Also, “it would be very interesting to know more about the comorbidities of the study patients and again correlate that with outcome.
“There is an intriguing potential role of andecaliximab in older gastric cancer patients, but it’s not ready for clinic yet,” she concluded. “It is certainly worth exploring because biologically, it would make sense.”
Study details
Patients in GAMMA-1 were randomized evenly to receive the mFOLFOX-6 regimen plus either placebo or andecaliximab given intravenously every 2 weeks.
Median overall survival, the primary outcome, was 12.5 months with the antibody and 11.8 months with placebo, a nonsignificant difference (hazard ratio, 0.93; P = .56), Dr. Shah reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
However, exploratory analyses showed benefit increased with quartile of age, and the difference in overall survival was significant for those 65 years and older at 13.9 months versus 10.5 months (HR, 0.64; P = .029).
The pattern was similar for progression-free survival, with only a trend among all patients (7.5 vs. 7.1 months; HR, 0.84; P = .10) but a significant reduction in risk for the older subgroup (8.7 vs 5.6 months; HR, 0.5; P less than .001).
Overall response rate was better with andecaliximab than with placebo in the entire trial population (51% vs. 41%; odds ratio, 1.47; P = .049). The rate of complete response was 8.3% and 4.7%, respectively.
“There were no meaningful differences in the safety profile of andecaliximab versus placebo in the groups treated,” Dr. Shah said. Rates and types of treatment-emergent adverse events of any grade and of grade 3 or higher were similar, with gastrointestinal and hematologic events predominating.
“We were intrigued with this phenomenon by age,” he said, and the investigators therefore assessed factors differing by age. “We looked at chemotherapy treatment and toxicity, things like that, and it didn’t appear that we could find any factor that was really associated. We did find that older patients actually received more treatment, but that’s likely because they had somewhat of a benefit with andecaliximab.”
Dr. Shah reported receiving research funding from Boston Biomedical, Gilead Sciences, Merck, and Oncolys BioPharma. The trial was sponsored by Gilead Sciences.
SOURCE: Shah MA et al. GI Cancers Symposium 2019, Abstract 4.
This article was updated 1/24/19.
SAN FRANCISCO – according to findings of the GAMMA-1 trial reported at the 2019 GI Cancers Symposium.
“Increased MMP9 expression is associated with poor prognosis across many malignancies and particularly in gastric cancer. All gastric cancers tested have MMP9 expression,” said lead investigator Manish A. Shah, MD, director of the gastrointestinal oncology program at Weill Cornell Medicine, New York, and chief of the solid tumor service and codirector of the Center for Advanced Digestive Disease at New York–Presbyterian. By activating and inactivating extracellular matrix proteins, MMP9 alters the tumor microenvironment, promoting angiogenesis, invasion, and metastases, and blunting the immune response, he said.
Main results of the phase 3, randomized, controlled GAMMA-1 trial showed that adding andecaliximab to the modified FOLFOX-6 regimen did not significantly improve outcomes among the entire population of 432 patients with untreated locally advanced or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma. However, exploratory analyses suggested that it significantly halved the risk of progression-free survival events and reduced by more than a third the risk of death among those aged 65 years or older.
“The apparent increased activity of the combination of mFOLFOX-6 and andecaliximab in patients aged 65 or older needs further study and correlative analyses,” Dr. Shah commented. “Our GAMMA-1 study was done in a nonselected population. We really don’t have a great biomarker for this [antibody]. … This is the question that we’re going to try to address over the next few months as we look at all the data.”
Because andecaliximab is expected to favorably alter the tumor immune microenvironment, it is also being tested in combination with immunotherapies, according to Dr. Shah. Results of some of those studies were also reported at the symposium.
Biological plausibility
A comprehensive view of precision oncology requires consideration not only of the tumor, but also of the microenvironment and the macroenvironment, agreed invited discussant Martine Extermann, MD, PhD, leader of the senior adult oncology program at the Moffitt Cancer Center in Tampa.
“A treatment which works better in older patients – I don’t hear that very frequently,” she commented. But data provide a biological rationale for selective benefit of andecaliximab in older adults. Specifically, this population may have higher MMP9 levels either from simple aging or from comorbidities that become more common as one grows older, such as atrial fibrillation and obstructive sleep apnea.
“My question to the investigators will be, are there serum samples that are available for MMP9 testing and could we check that to see whether these patients are the ones who had the best response to andecaliximab treatment?” Dr. Extermann said. Also, “it would be very interesting to know more about the comorbidities of the study patients and again correlate that with outcome.
“There is an intriguing potential role of andecaliximab in older gastric cancer patients, but it’s not ready for clinic yet,” she concluded. “It is certainly worth exploring because biologically, it would make sense.”
Study details
Patients in GAMMA-1 were randomized evenly to receive the mFOLFOX-6 regimen plus either placebo or andecaliximab given intravenously every 2 weeks.
Median overall survival, the primary outcome, was 12.5 months with the antibody and 11.8 months with placebo, a nonsignificant difference (hazard ratio, 0.93; P = .56), Dr. Shah reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
However, exploratory analyses showed benefit increased with quartile of age, and the difference in overall survival was significant for those 65 years and older at 13.9 months versus 10.5 months (HR, 0.64; P = .029).
The pattern was similar for progression-free survival, with only a trend among all patients (7.5 vs. 7.1 months; HR, 0.84; P = .10) but a significant reduction in risk for the older subgroup (8.7 vs 5.6 months; HR, 0.5; P less than .001).
Overall response rate was better with andecaliximab than with placebo in the entire trial population (51% vs. 41%; odds ratio, 1.47; P = .049). The rate of complete response was 8.3% and 4.7%, respectively.
“There were no meaningful differences in the safety profile of andecaliximab versus placebo in the groups treated,” Dr. Shah said. Rates and types of treatment-emergent adverse events of any grade and of grade 3 or higher were similar, with gastrointestinal and hematologic events predominating.
“We were intrigued with this phenomenon by age,” he said, and the investigators therefore assessed factors differing by age. “We looked at chemotherapy treatment and toxicity, things like that, and it didn’t appear that we could find any factor that was really associated. We did find that older patients actually received more treatment, but that’s likely because they had somewhat of a benefit with andecaliximab.”
Dr. Shah reported receiving research funding from Boston Biomedical, Gilead Sciences, Merck, and Oncolys BioPharma. The trial was sponsored by Gilead Sciences.
SOURCE: Shah MA et al. GI Cancers Symposium 2019, Abstract 4.
This article was updated 1/24/19.
SAN FRANCISCO – according to findings of the GAMMA-1 trial reported at the 2019 GI Cancers Symposium.
“Increased MMP9 expression is associated with poor prognosis across many malignancies and particularly in gastric cancer. All gastric cancers tested have MMP9 expression,” said lead investigator Manish A. Shah, MD, director of the gastrointestinal oncology program at Weill Cornell Medicine, New York, and chief of the solid tumor service and codirector of the Center for Advanced Digestive Disease at New York–Presbyterian. By activating and inactivating extracellular matrix proteins, MMP9 alters the tumor microenvironment, promoting angiogenesis, invasion, and metastases, and blunting the immune response, he said.
Main results of the phase 3, randomized, controlled GAMMA-1 trial showed that adding andecaliximab to the modified FOLFOX-6 regimen did not significantly improve outcomes among the entire population of 432 patients with untreated locally advanced or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma. However, exploratory analyses suggested that it significantly halved the risk of progression-free survival events and reduced by more than a third the risk of death among those aged 65 years or older.
“The apparent increased activity of the combination of mFOLFOX-6 and andecaliximab in patients aged 65 or older needs further study and correlative analyses,” Dr. Shah commented. “Our GAMMA-1 study was done in a nonselected population. We really don’t have a great biomarker for this [antibody]. … This is the question that we’re going to try to address over the next few months as we look at all the data.”
Because andecaliximab is expected to favorably alter the tumor immune microenvironment, it is also being tested in combination with immunotherapies, according to Dr. Shah. Results of some of those studies were also reported at the symposium.
Biological plausibility
A comprehensive view of precision oncology requires consideration not only of the tumor, but also of the microenvironment and the macroenvironment, agreed invited discussant Martine Extermann, MD, PhD, leader of the senior adult oncology program at the Moffitt Cancer Center in Tampa.
“A treatment which works better in older patients – I don’t hear that very frequently,” she commented. But data provide a biological rationale for selective benefit of andecaliximab in older adults. Specifically, this population may have higher MMP9 levels either from simple aging or from comorbidities that become more common as one grows older, such as atrial fibrillation and obstructive sleep apnea.
“My question to the investigators will be, are there serum samples that are available for MMP9 testing and could we check that to see whether these patients are the ones who had the best response to andecaliximab treatment?” Dr. Extermann said. Also, “it would be very interesting to know more about the comorbidities of the study patients and again correlate that with outcome.
“There is an intriguing potential role of andecaliximab in older gastric cancer patients, but it’s not ready for clinic yet,” she concluded. “It is certainly worth exploring because biologically, it would make sense.”
Study details
Patients in GAMMA-1 were randomized evenly to receive the mFOLFOX-6 regimen plus either placebo or andecaliximab given intravenously every 2 weeks.
Median overall survival, the primary outcome, was 12.5 months with the antibody and 11.8 months with placebo, a nonsignificant difference (hazard ratio, 0.93; P = .56), Dr. Shah reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
However, exploratory analyses showed benefit increased with quartile of age, and the difference in overall survival was significant for those 65 years and older at 13.9 months versus 10.5 months (HR, 0.64; P = .029).
The pattern was similar for progression-free survival, with only a trend among all patients (7.5 vs. 7.1 months; HR, 0.84; P = .10) but a significant reduction in risk for the older subgroup (8.7 vs 5.6 months; HR, 0.5; P less than .001).
Overall response rate was better with andecaliximab than with placebo in the entire trial population (51% vs. 41%; odds ratio, 1.47; P = .049). The rate of complete response was 8.3% and 4.7%, respectively.
“There were no meaningful differences in the safety profile of andecaliximab versus placebo in the groups treated,” Dr. Shah said. Rates and types of treatment-emergent adverse events of any grade and of grade 3 or higher were similar, with gastrointestinal and hematologic events predominating.
“We were intrigued with this phenomenon by age,” he said, and the investigators therefore assessed factors differing by age. “We looked at chemotherapy treatment and toxicity, things like that, and it didn’t appear that we could find any factor that was really associated. We did find that older patients actually received more treatment, but that’s likely because they had somewhat of a benefit with andecaliximab.”
Dr. Shah reported receiving research funding from Boston Biomedical, Gilead Sciences, Merck, and Oncolys BioPharma. The trial was sponsored by Gilead Sciences.
SOURCE: Shah MA et al. GI Cancers Symposium 2019, Abstract 4.
This article was updated 1/24/19.
REPORTING FROM THE 2019 GI CANCERS SYMPOSIUM
Key clinical point: Benefit of first-line andecaliximab in advanced gastric/gastroesophageal junction cancer may be age dependent.
Major finding: Compared with placebo plus mFOLFOX-6, andecaliximab plus mFOLFOX-6 did not improve overall survival among all patients, but it did among those aged 65 years and older in exploratory analyses (hazard ratio, 0.64; P = .029).
Study details: A phase 3, randomized, controlled trial among 432 patients with untreated locally advanced or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma (GAMMA-1 trial).
Disclosures: Dr. Shah reported receiving research funding from Boston Biomedical, Gilead Sciences, Merck (institutional), and Oncolys BioPharma. The trial was sponsored by Gilead Sciences.
Source: Shah MA et al. GI Cancers Symposium 2019, Abstract 4.
Intimate partner violence, guns, and the ObGyn
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
COPD linked to higher in-hospital death rates in patients with PAD
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
FROM RESPIRATORY MEDICINE
Key clinical point:
Major finding: All-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without (6.5% vs. 4.7%; P less than 0.001).
Study details: Database analysis of 5.6 million German PAD inpatients stratified for COPD.
Disclosures: The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
Source: Keller K et al. Respir Med. 2019 Feb;147:1-6.
AML, myeloma risk higher for breast cancer survivors
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
FROM JAMA NETWORK OPEN
Key clinical point: Breast cancer survivors should be monitored for hematologic malignancies.
Major finding: The standardized incidence ratio for AML was 2.8 and the SIR for multiple myeloma was 5.0 among French breast cancer survivors compared with women in the general French population.
Study details: Retrospective analysis of data on 439,704 women aged 20-85 years with a breast cancer diagnosis.
Disclosures: The authors did not report a study funding source. Coauthor Anthony Goncalves, MD, reported nonfinancial support from Roche, Novartis, Pfizer, Celgene, MSD, Lilly, and Astra Zeneca outside of the submitted work. No other disclosures were reported.
Source: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.