User login
Real-world data reveal long-lasting effects achieved with RRMS treatments
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
REPORTING FROM ECTRIMS 2018
Parental leave for residents
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Rivaroxaban can reduce VTE in cancer patients
SAN DIEGO—In the phase 3 CASSINI trial, prophylaxis with rivaroxaban reduced the rate of venous thromboembolism (VTE) and VTE-related death in high-risk ambulatory cancer patients receiving systemic therapy.
The reduction in VTE and related death was not statistically significant in the primary analysis, which included a period of time after treatment had stopped.
However, the reduction in VTE and related death was significant in a prespecified secondary analysis limited to the on-treatment period.
Alok A. Khorana, MD, of the Cleveland Clinic in Ohio, presented these results at the 2018 ASH Annual Meeting (abstract LBA-1).
The trial (NCT02555878) included 841 patients enrolled at 143 centers in 11 countries. The patients had solid tumor malignancies or lymphomas and a high risk of VTE (Khorana score of 2 or greater).
The patients were randomized to either rivaroxaban at 10 mg once daily (n=420) or placebo once daily (n=421).
In the primary analysis period of 180 days, the composite endpoint of VTE or VTE-related death occurred in 5.95% of the rivaroxaban-treated group and 8.79% of the placebo group (hazard ratio [HR]=0.66; 95% confidence interval [CI], 0.40-1.09; P=0.101).
However, 177 patients (43.7%) in the rivaroxaban group stopped treatment before the 180-day mark, as did 203 patients (50.2%) in the placebo group.
In a prespecified secondary analysis looking only at the treatment period, rivaroxaban did significantly reduce the risk of VTE and VTE-related death, Dr. Khorana said. The composite endpoint occurred in 2.62% of rivaroxaban-treated patients and 6.41% of placebo-treated patients in that analysis (HR=0.40; 95% CI, 0.20-0.80; P=0.007).
Rates of major bleeding and clinically relevant nonmajor bleeding were not significantly different between the groups.
Major bleeding occurred in 1.98% (n=8) of rivaroxaban-treated patients and 0.99% (n=4) of placebo-treated patients (HR=1.96, 0.59-6.49; P=0.265).
Clinically relevant nonmajor bleeding occurred in 2.72% (n=11) and 1.98% (n=8), respectively (HR=1.34, 95% CI, 0.54-3.32; P=0.532).
CASSINI was sponsored by Bayer and Janssen. Dr. Khorana reported relationships with Janssen, Pharmacyclics, PharmaCyte, TriSalus Life Sciences, PAREXEL, Pfizer, Sanofi, Halozyme, Seattle Genetics, AngioDynamics, LEO Pharma, Medscape/WebMD, and Bayer.
SAN DIEGO—In the phase 3 CASSINI trial, prophylaxis with rivaroxaban reduced the rate of venous thromboembolism (VTE) and VTE-related death in high-risk ambulatory cancer patients receiving systemic therapy.
The reduction in VTE and related death was not statistically significant in the primary analysis, which included a period of time after treatment had stopped.
However, the reduction in VTE and related death was significant in a prespecified secondary analysis limited to the on-treatment period.
Alok A. Khorana, MD, of the Cleveland Clinic in Ohio, presented these results at the 2018 ASH Annual Meeting (abstract LBA-1).
The trial (NCT02555878) included 841 patients enrolled at 143 centers in 11 countries. The patients had solid tumor malignancies or lymphomas and a high risk of VTE (Khorana score of 2 or greater).
The patients were randomized to either rivaroxaban at 10 mg once daily (n=420) or placebo once daily (n=421).
In the primary analysis period of 180 days, the composite endpoint of VTE or VTE-related death occurred in 5.95% of the rivaroxaban-treated group and 8.79% of the placebo group (hazard ratio [HR]=0.66; 95% confidence interval [CI], 0.40-1.09; P=0.101).
However, 177 patients (43.7%) in the rivaroxaban group stopped treatment before the 180-day mark, as did 203 patients (50.2%) in the placebo group.
In a prespecified secondary analysis looking only at the treatment period, rivaroxaban did significantly reduce the risk of VTE and VTE-related death, Dr. Khorana said. The composite endpoint occurred in 2.62% of rivaroxaban-treated patients and 6.41% of placebo-treated patients in that analysis (HR=0.40; 95% CI, 0.20-0.80; P=0.007).
Rates of major bleeding and clinically relevant nonmajor bleeding were not significantly different between the groups.
Major bleeding occurred in 1.98% (n=8) of rivaroxaban-treated patients and 0.99% (n=4) of placebo-treated patients (HR=1.96, 0.59-6.49; P=0.265).
Clinically relevant nonmajor bleeding occurred in 2.72% (n=11) and 1.98% (n=8), respectively (HR=1.34, 95% CI, 0.54-3.32; P=0.532).
CASSINI was sponsored by Bayer and Janssen. Dr. Khorana reported relationships with Janssen, Pharmacyclics, PharmaCyte, TriSalus Life Sciences, PAREXEL, Pfizer, Sanofi, Halozyme, Seattle Genetics, AngioDynamics, LEO Pharma, Medscape/WebMD, and Bayer.
SAN DIEGO—In the phase 3 CASSINI trial, prophylaxis with rivaroxaban reduced the rate of venous thromboembolism (VTE) and VTE-related death in high-risk ambulatory cancer patients receiving systemic therapy.
The reduction in VTE and related death was not statistically significant in the primary analysis, which included a period of time after treatment had stopped.
However, the reduction in VTE and related death was significant in a prespecified secondary analysis limited to the on-treatment period.
Alok A. Khorana, MD, of the Cleveland Clinic in Ohio, presented these results at the 2018 ASH Annual Meeting (abstract LBA-1).
The trial (NCT02555878) included 841 patients enrolled at 143 centers in 11 countries. The patients had solid tumor malignancies or lymphomas and a high risk of VTE (Khorana score of 2 or greater).
The patients were randomized to either rivaroxaban at 10 mg once daily (n=420) or placebo once daily (n=421).
In the primary analysis period of 180 days, the composite endpoint of VTE or VTE-related death occurred in 5.95% of the rivaroxaban-treated group and 8.79% of the placebo group (hazard ratio [HR]=0.66; 95% confidence interval [CI], 0.40-1.09; P=0.101).
However, 177 patients (43.7%) in the rivaroxaban group stopped treatment before the 180-day mark, as did 203 patients (50.2%) in the placebo group.
In a prespecified secondary analysis looking only at the treatment period, rivaroxaban did significantly reduce the risk of VTE and VTE-related death, Dr. Khorana said. The composite endpoint occurred in 2.62% of rivaroxaban-treated patients and 6.41% of placebo-treated patients in that analysis (HR=0.40; 95% CI, 0.20-0.80; P=0.007).
Rates of major bleeding and clinically relevant nonmajor bleeding were not significantly different between the groups.
Major bleeding occurred in 1.98% (n=8) of rivaroxaban-treated patients and 0.99% (n=4) of placebo-treated patients (HR=1.96, 0.59-6.49; P=0.265).
Clinically relevant nonmajor bleeding occurred in 2.72% (n=11) and 1.98% (n=8), respectively (HR=1.34, 95% CI, 0.54-3.32; P=0.532).
CASSINI was sponsored by Bayer and Janssen. Dr. Khorana reported relationships with Janssen, Pharmacyclics, PharmaCyte, TriSalus Life Sciences, PAREXEL, Pfizer, Sanofi, Halozyme, Seattle Genetics, AngioDynamics, LEO Pharma, Medscape/WebMD, and Bayer.
When Skin Damage Takes Sides
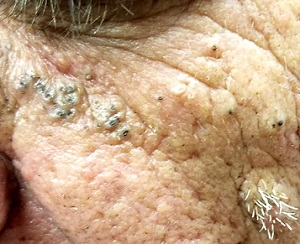
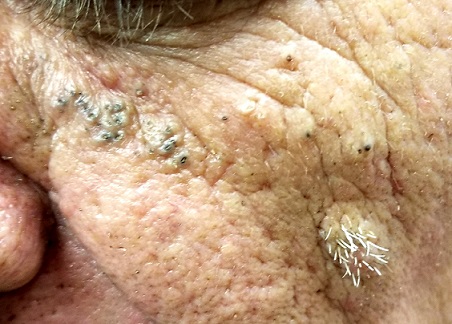
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.
EXAMINATION
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.

EXAMINATION
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.

EXAMINATION
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
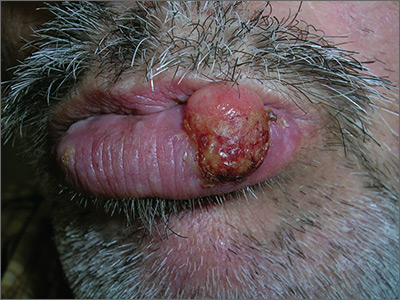
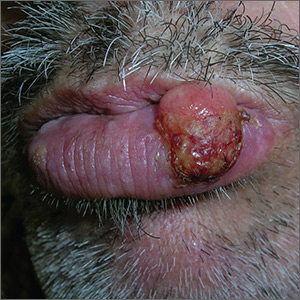
Growing lesion on lip
The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Bacterial contamination behind most cosmetics recalls
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.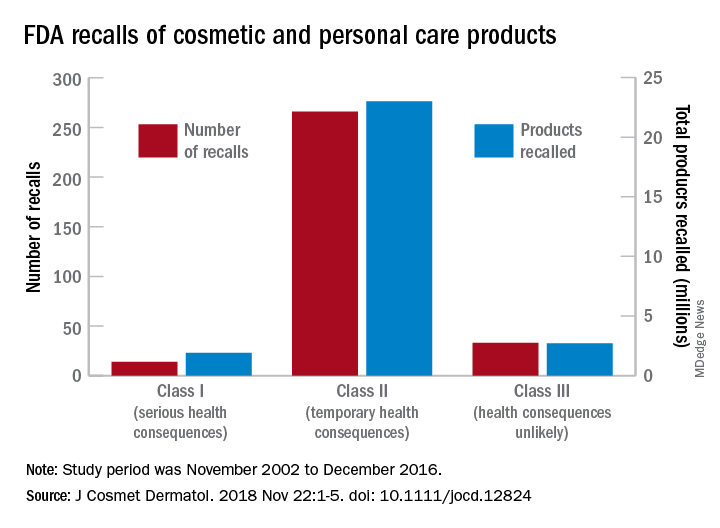
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
rfranki@mdedge.com
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
rfranki@mdedge.com
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
Most of the 313 cosmetic and personal care products recalled from 2002 to 2016 had problems with bacterial contamination, according to data obtained from the Food and Drug Administration.
, said Timothy M. Janetos, MD, and his associates at Northwestern University in Chicago. Bacterial contamination was by far the most common reason – 76% of the recalls over that period (11 class I, 217 class II, and 9 class II) – with unapproved ingredients and labeling problems well behind at 6%.
Recalls are classified by the FDA according to risk to patient safety: Class I means there is “reasonable probability of causing serious adverse health outcomes or death,” class II defines the risk as “temporary or reversible,” and class III recalls are “unlikely to cause an adverse health consequence,” they explained.
“While the number of total recalls per year was low in the context of the industry’s size and the ubiquity of cosmetic use by consumers (median: 17/year), these events involved millions of products distributed worldwide,” Dr. Janetos and his associates wrote. The class I recalls covered over 1.9 million products in distribution, the class II recalls accounted for 23 million products, and class II recalls involved over 2.7 million products.
Baby products were the category most likely to be affected, accounting for 76 (24%) of all recalls, the investigators said, with 30 involving one manufacturer of cleansing kits intended for hospital use. All but 3 of the 76 recalls resulted from bacterial contamination.
“The FDA currently has no authority to order a cosmetics manufacturer to recall a product,” they wrote, and “inspectors are only capable of inspecting 0.3% of foreign-imported products yearly,” so underreporting of such problems is likely. “Dermatologists are often the first to encounter [adverse events] related to cosmetic products and can help strengthen public safety by actively reporting these events and advocating for recalls,” said Dr. Janetos and his associates, who did not declare any conflicts of interest.
Information on reporting cosmetic-related complaints to the FDA is available on the FDA website at: https://www.fda.gov/Cosmetics/ComplianceEnforcement/AdverseEventReporting/default.htm.
rfranki@mdedge.com
SOURCE: Janetos TM et al. J Cosmet Dermatol. 2018 Nov 22:1-5. doi: 10.1111/jocd.12824.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Psilocybin yields encouraging results in addiction studies
BONITA SPRINGS, FLA. – Small but tantalizing studies are showing benefits from treatment with psilocybin, the psychedelic substance, for alcohol and cocaine addiction, according to findings presented at the annual meeting of the American Academy of Addiction Psychiatry.
Researchers emphasized that the results are in the early stages, and that treatment is administered only after careful patient screening and in controlled environments. In a proof-of-concept study of 10 patients, subjects with alcohol use disorder (AUD) underwent 12 weeks of psychosocial treatment, with two psilocybin “sessions” mixed in, at weeks 4 and 8. At baseline, 35% of the patients’ days were heavy drinking days, but by weeks 25-36, only about 12% were heavy drinking days, a significant reduction. Days with any drinking fell from just over 40% to about 15% over that period, also a significant drop, said Michael P. Bogenschutz, MD, professor of psychiatry at New York University, who is leading the research (J Psychopharmacol. 2015 Mar;29[3]:289-99).
In a current trial of more than 100 participants that he is leading, early data are available on the first 56 patients through 12 weeks. Patients have been treated with psilocybin or diphenhydramine as placebo, along with Motivational Enhancement Therapy and Taking Action therapy. They are not dependent on any other substance and are medically healthy. For psilocybin administration, patients lie on a couch with a sleep mask on, and therapists are present for all sessions.
With the study still blinded, researchers assessed Mystical Experience Questionnaire (MEQ) scores as a signal on whether patients were in the psilocybin group or not. Those with high MEQ scores had a significant reduction in percentage of heavy drinking days through week 12, Dr. Bogenschutz said.
Meanwhile, results were presented for the first 10 patients in a 40-patient study of psilocybin for cocaine treatment at the University of Alabama at Birmingham. Patients taking psilocybin have significantly more days of abstinence and significantly better scores on the Severity of Dependence Scale, compared with placebo, said Peter L. Hendricks, MD, of the department of anesthesiology at UAB. He said patients have reported no adverse effects of the treatment so far.
The mechanisms at work remain something of a puzzle, researchers said. Some patients report lower levels of depression and higher life satisfaction scores, which suggests that the treatment could lead to better coping ability.
Finally, research at Johns Hopkins University in Baltimore involving psilocybin administration to healthy volunteers has found that a high mystical experience score predicts feelings of meaningfulness, spiritual significance, and openness at 14 months and longer after it is administered, said Roland R. Griffiths, PhD, professor of psychiatry and behavioral science and of neuroscience at Johns Hopkins.
“Psilocybin, when administered to carefully screened and prepared volunteers, can occasion unique experiences that are judged to be personally meaningful and which are associated with enduring positive changes in mood, attitudes, and behavior,” he said. Neuroplasticity and changes in functional connections in the brain also could be playing a role, Dr. Bogenschutz said.
In his study of AUD, Dr. Bogenschutz said, occasionally patients do report unpleasant experiences with psilocybin administration. However, one patient called the sessions a “crash course in dealing with feelings of disappointment, regret, shame, and unworthiness.”
he said, “that for many people seem to be equally important in terms of generating change.”
Dr. Bogenshutz reported funding from several sources, including the Heffter Research Institute, the Multidisciplinary Association for Psychedelic Studies, the National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism. Dr. Griffiths reported funding from Heffter, the Fetzer Institute, the Templeton Foundation, the Riverstyx Foundation, the Council of Spiritual Practices, and NIDA. Dr. Hendricks reported no relevant disclosures.
BONITA SPRINGS, FLA. – Small but tantalizing studies are showing benefits from treatment with psilocybin, the psychedelic substance, for alcohol and cocaine addiction, according to findings presented at the annual meeting of the American Academy of Addiction Psychiatry.
Researchers emphasized that the results are in the early stages, and that treatment is administered only after careful patient screening and in controlled environments. In a proof-of-concept study of 10 patients, subjects with alcohol use disorder (AUD) underwent 12 weeks of psychosocial treatment, with two psilocybin “sessions” mixed in, at weeks 4 and 8. At baseline, 35% of the patients’ days were heavy drinking days, but by weeks 25-36, only about 12% were heavy drinking days, a significant reduction. Days with any drinking fell from just over 40% to about 15% over that period, also a significant drop, said Michael P. Bogenschutz, MD, professor of psychiatry at New York University, who is leading the research (J Psychopharmacol. 2015 Mar;29[3]:289-99).
In a current trial of more than 100 participants that he is leading, early data are available on the first 56 patients through 12 weeks. Patients have been treated with psilocybin or diphenhydramine as placebo, along with Motivational Enhancement Therapy and Taking Action therapy. They are not dependent on any other substance and are medically healthy. For psilocybin administration, patients lie on a couch with a sleep mask on, and therapists are present for all sessions.
With the study still blinded, researchers assessed Mystical Experience Questionnaire (MEQ) scores as a signal on whether patients were in the psilocybin group or not. Those with high MEQ scores had a significant reduction in percentage of heavy drinking days through week 12, Dr. Bogenschutz said.
Meanwhile, results were presented for the first 10 patients in a 40-patient study of psilocybin for cocaine treatment at the University of Alabama at Birmingham. Patients taking psilocybin have significantly more days of abstinence and significantly better scores on the Severity of Dependence Scale, compared with placebo, said Peter L. Hendricks, MD, of the department of anesthesiology at UAB. He said patients have reported no adverse effects of the treatment so far.
The mechanisms at work remain something of a puzzle, researchers said. Some patients report lower levels of depression and higher life satisfaction scores, which suggests that the treatment could lead to better coping ability.
Finally, research at Johns Hopkins University in Baltimore involving psilocybin administration to healthy volunteers has found that a high mystical experience score predicts feelings of meaningfulness, spiritual significance, and openness at 14 months and longer after it is administered, said Roland R. Griffiths, PhD, professor of psychiatry and behavioral science and of neuroscience at Johns Hopkins.
“Psilocybin, when administered to carefully screened and prepared volunteers, can occasion unique experiences that are judged to be personally meaningful and which are associated with enduring positive changes in mood, attitudes, and behavior,” he said. Neuroplasticity and changes in functional connections in the brain also could be playing a role, Dr. Bogenschutz said.
In his study of AUD, Dr. Bogenschutz said, occasionally patients do report unpleasant experiences with psilocybin administration. However, one patient called the sessions a “crash course in dealing with feelings of disappointment, regret, shame, and unworthiness.”
he said, “that for many people seem to be equally important in terms of generating change.”
Dr. Bogenshutz reported funding from several sources, including the Heffter Research Institute, the Multidisciplinary Association for Psychedelic Studies, the National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism. Dr. Griffiths reported funding from Heffter, the Fetzer Institute, the Templeton Foundation, the Riverstyx Foundation, the Council of Spiritual Practices, and NIDA. Dr. Hendricks reported no relevant disclosures.
BONITA SPRINGS, FLA. – Small but tantalizing studies are showing benefits from treatment with psilocybin, the psychedelic substance, for alcohol and cocaine addiction, according to findings presented at the annual meeting of the American Academy of Addiction Psychiatry.
Researchers emphasized that the results are in the early stages, and that treatment is administered only after careful patient screening and in controlled environments. In a proof-of-concept study of 10 patients, subjects with alcohol use disorder (AUD) underwent 12 weeks of psychosocial treatment, with two psilocybin “sessions” mixed in, at weeks 4 and 8. At baseline, 35% of the patients’ days were heavy drinking days, but by weeks 25-36, only about 12% were heavy drinking days, a significant reduction. Days with any drinking fell from just over 40% to about 15% over that period, also a significant drop, said Michael P. Bogenschutz, MD, professor of psychiatry at New York University, who is leading the research (J Psychopharmacol. 2015 Mar;29[3]:289-99).
In a current trial of more than 100 participants that he is leading, early data are available on the first 56 patients through 12 weeks. Patients have been treated with psilocybin or diphenhydramine as placebo, along with Motivational Enhancement Therapy and Taking Action therapy. They are not dependent on any other substance and are medically healthy. For psilocybin administration, patients lie on a couch with a sleep mask on, and therapists are present for all sessions.
With the study still blinded, researchers assessed Mystical Experience Questionnaire (MEQ) scores as a signal on whether patients were in the psilocybin group or not. Those with high MEQ scores had a significant reduction in percentage of heavy drinking days through week 12, Dr. Bogenschutz said.
Meanwhile, results were presented for the first 10 patients in a 40-patient study of psilocybin for cocaine treatment at the University of Alabama at Birmingham. Patients taking psilocybin have significantly more days of abstinence and significantly better scores on the Severity of Dependence Scale, compared with placebo, said Peter L. Hendricks, MD, of the department of anesthesiology at UAB. He said patients have reported no adverse effects of the treatment so far.
The mechanisms at work remain something of a puzzle, researchers said. Some patients report lower levels of depression and higher life satisfaction scores, which suggests that the treatment could lead to better coping ability.
Finally, research at Johns Hopkins University in Baltimore involving psilocybin administration to healthy volunteers has found that a high mystical experience score predicts feelings of meaningfulness, spiritual significance, and openness at 14 months and longer after it is administered, said Roland R. Griffiths, PhD, professor of psychiatry and behavioral science and of neuroscience at Johns Hopkins.
“Psilocybin, when administered to carefully screened and prepared volunteers, can occasion unique experiences that are judged to be personally meaningful and which are associated with enduring positive changes in mood, attitudes, and behavior,” he said. Neuroplasticity and changes in functional connections in the brain also could be playing a role, Dr. Bogenschutz said.
In his study of AUD, Dr. Bogenschutz said, occasionally patients do report unpleasant experiences with psilocybin administration. However, one patient called the sessions a “crash course in dealing with feelings of disappointment, regret, shame, and unworthiness.”
he said, “that for many people seem to be equally important in terms of generating change.”
Dr. Bogenshutz reported funding from several sources, including the Heffter Research Institute, the Multidisciplinary Association for Psychedelic Studies, the National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism. Dr. Griffiths reported funding from Heffter, the Fetzer Institute, the Templeton Foundation, the Riverstyx Foundation, the Council of Spiritual Practices, and NIDA. Dr. Hendricks reported no relevant disclosures.
REPORTING FROM AAAP 2018
Does education to enhance maternal awareness of fetal movements help reduce stillbirth?
WHAT DOES THIS MEAN FOR PRACTICE?
- Data indicate that fetal movement counting does not help to reduce stillbirth incidence
- Continue to use fetal movement counting to maintain patient engagement in managing her own pregnancy
- Encourage fetal movement awareness but also counsel patients that awareness does not reduce stillbirth
- This may decrease a patient’s feelings of guilt (because she did not maintain fetal kick counting) should a stillbirth occur
Neurologic Disease Eventually Affects Half of Women and One-Third of Men
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Be judicious with empiric antibiotics for febrile neutropenia
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
EXPERT ANALYSIS FROM IDWEEK 2018