User login
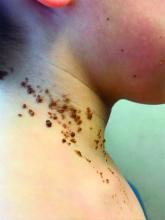
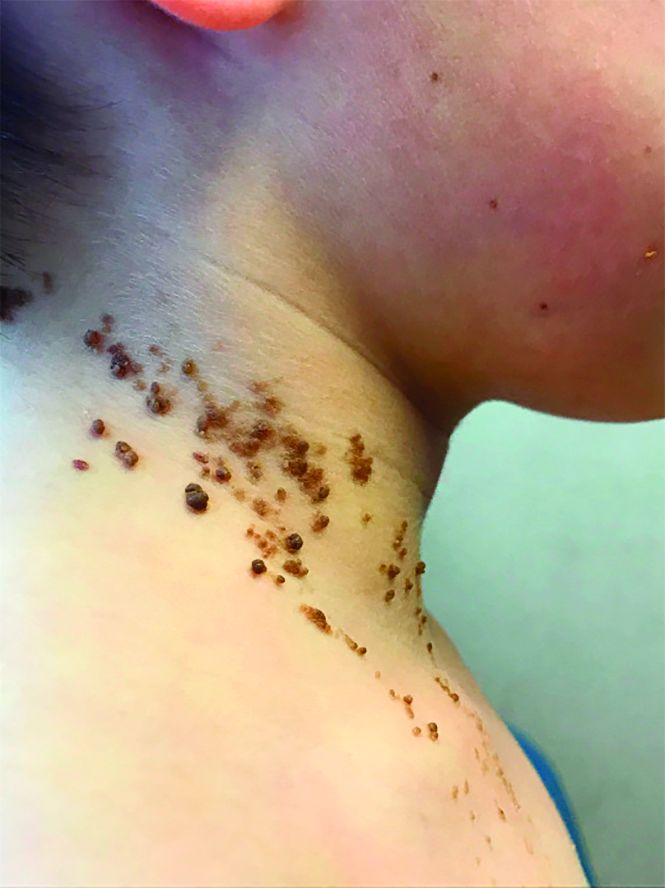
How to Identify Frontal Fibrosing Alopecia
Home-based exercise for PAD tops supervised treadmill exercise
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
REPORTING FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CABG surpasses PCI for diabetics out to 7.5 years
CHICAGO – Patients with diabetes who underwent coronary artery bypass grafting had significantly better survival than patients with diabetes who underwent percutaneous coronary intervention after a median 7.5 years of follow-up.

Those patients comprised about half the patients enrolled in the FREEDOM randomized trial.
Long-term follow-up was only possible for just under half the 1,900 patients with diabetes and multivessel coronary disease originally enrolled in FREEDOM, but when researchers combined the long-term results with the data collected in the original study that had a median 3.8-year follow-up, they found all-cause mortality occurred in 18.3% of the patients who underwent coronary artery bypass grafting (CABG) and in 24.3% of patients treated with percutaneous coronary intervention (PCI), a 6% absolute between-group difference that was statistically significant, Valentin Fuster, MD, said at the American Heart Association scientific sessions. This fully jibed with the primary FREEDOM results, which found after 5 years a statistically significant reduction in all-cause death with CABG, compared with PCI, and also a significant reduction in the study’s primary endpoint (a combination of all-cause death, MI, and stroke), which occurred in 18.7% of patients randomized to CABG and in 26.6% of those randomized to PCI (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
The extended follow-up finding lent additional support to existing society recommendations that CABG is the preferred revascularization strategy for patients with diabetes and multivessel coronary disease, most recently from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394), said Dr. Fuster, professor of medicine at the Icahn School of Medicine at Mount Sinai and director of Mount Sinai Heart in New York. A subgroup analysis of the extended follow-up also suggested that the survival benefit from CABG, compared with PCI, was especially strong among patients at or below the study’s median age of 63 years. In the younger subgroup survival among patients treated with CABG was twice as good as it was among patients treated with PCI.
Dr. Fuster noted that few data have been previously reported for survival rates beyond 5 years after revascularization. “This was a difficult study. Following patients for more than 5 years is hard,” he said. Concurrently with his report at the meeting the results also appeared online (J Am Coll Cardiol. 2018 Nov 11. doi: 10.1016/j.jacc.2018.11.001).
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial enrolled patients at 140 participating centers during 2005-2010. A total of 25 sites agreed to participate in the extended follow-up and could track 943 patients, 50% of the starting cohort of 1,900 and 89% of the patients originally enrolled at these 25 centers. Dr. Fuster stressed that the 957 patients not included in the follow-up had not been lost, but rather had been managed at sites that declined to participate in this additional study.
Dr. Fuster acknowledged that methods and hardware for PCI have changed since the study ran a decade ago, as have options for medical management. He also highlighted that the long-term follow-up results had no data on rates of MIs and strokes.
FREEDOM had no commercial funding. Dr. Fuster reported no relevant disclosures.
SOURCE: Fuster V et al. AHA 2018, Abstract 18609.
These extended results from the FREEDOM trial that followed many patients for 10 years or longer add to the consistent evidence base that supports coronary artery bypass grafting (CABG) as the preferred revascularization strategy for patients with diabetes and multivessel coronary disease. The new findings support existing society guidelines that recommend CABG over percutaneous coronary intervention in these patients, most recently in the revascularization guidelines from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394). An update to the U.S. guidelines should appear in 2019.
Continued improvement of revascularization techniques, hardware, and medical management of patients with diabetes and multivessel coronary artery disease makes it challenging to apply the results of studies run in earlier eras to today’s practice. It is possible that continued evolution of coronary stent technology may reduce the differences in outcomes between bypass surgery and percutaneous coronary interventions, although this is less likely if much of CABG’s success relates to the protection it gives against new disease. Future comparisons of different approaches with revascularization will need to take into account the potential contribution of other procedures, other adverse outcomes aside from mortality during long-term follow-up, the consequences of incomplete revascularization, and the impact of new medications for treating diabetes that have been recently shown to also have cardiovascular disease effects. All these factors in concert will define the optimal approach to managing these patients.
Alice K. Jacobs, MD , is director of the cardiac catheterization laboratory at Boston Medical Center and a professor of medicine at Boston University. She has received research support from Abbott Vascular. She made these comments as designated discussant for the study.
These extended results from the FREEDOM trial that followed many patients for 10 years or longer add to the consistent evidence base that supports coronary artery bypass grafting (CABG) as the preferred revascularization strategy for patients with diabetes and multivessel coronary disease. The new findings support existing society guidelines that recommend CABG over percutaneous coronary intervention in these patients, most recently in the revascularization guidelines from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394). An update to the U.S. guidelines should appear in 2019.
Continued improvement of revascularization techniques, hardware, and medical management of patients with diabetes and multivessel coronary artery disease makes it challenging to apply the results of studies run in earlier eras to today’s practice. It is possible that continued evolution of coronary stent technology may reduce the differences in outcomes between bypass surgery and percutaneous coronary interventions, although this is less likely if much of CABG’s success relates to the protection it gives against new disease. Future comparisons of different approaches with revascularization will need to take into account the potential contribution of other procedures, other adverse outcomes aside from mortality during long-term follow-up, the consequences of incomplete revascularization, and the impact of new medications for treating diabetes that have been recently shown to also have cardiovascular disease effects. All these factors in concert will define the optimal approach to managing these patients.
Alice K. Jacobs, MD , is director of the cardiac catheterization laboratory at Boston Medical Center and a professor of medicine at Boston University. She has received research support from Abbott Vascular. She made these comments as designated discussant for the study.
These extended results from the FREEDOM trial that followed many patients for 10 years or longer add to the consistent evidence base that supports coronary artery bypass grafting (CABG) as the preferred revascularization strategy for patients with diabetes and multivessel coronary disease. The new findings support existing society guidelines that recommend CABG over percutaneous coronary intervention in these patients, most recently in the revascularization guidelines from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394). An update to the U.S. guidelines should appear in 2019.
Continued improvement of revascularization techniques, hardware, and medical management of patients with diabetes and multivessel coronary artery disease makes it challenging to apply the results of studies run in earlier eras to today’s practice. It is possible that continued evolution of coronary stent technology may reduce the differences in outcomes between bypass surgery and percutaneous coronary interventions, although this is less likely if much of CABG’s success relates to the protection it gives against new disease. Future comparisons of different approaches with revascularization will need to take into account the potential contribution of other procedures, other adverse outcomes aside from mortality during long-term follow-up, the consequences of incomplete revascularization, and the impact of new medications for treating diabetes that have been recently shown to also have cardiovascular disease effects. All these factors in concert will define the optimal approach to managing these patients.
Alice K. Jacobs, MD , is director of the cardiac catheterization laboratory at Boston Medical Center and a professor of medicine at Boston University. She has received research support from Abbott Vascular. She made these comments as designated discussant for the study.
CHICAGO – Patients with diabetes who underwent coronary artery bypass grafting had significantly better survival than patients with diabetes who underwent percutaneous coronary intervention after a median 7.5 years of follow-up.

Those patients comprised about half the patients enrolled in the FREEDOM randomized trial.
Long-term follow-up was only possible for just under half the 1,900 patients with diabetes and multivessel coronary disease originally enrolled in FREEDOM, but when researchers combined the long-term results with the data collected in the original study that had a median 3.8-year follow-up, they found all-cause mortality occurred in 18.3% of the patients who underwent coronary artery bypass grafting (CABG) and in 24.3% of patients treated with percutaneous coronary intervention (PCI), a 6% absolute between-group difference that was statistically significant, Valentin Fuster, MD, said at the American Heart Association scientific sessions. This fully jibed with the primary FREEDOM results, which found after 5 years a statistically significant reduction in all-cause death with CABG, compared with PCI, and also a significant reduction in the study’s primary endpoint (a combination of all-cause death, MI, and stroke), which occurred in 18.7% of patients randomized to CABG and in 26.6% of those randomized to PCI (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
The extended follow-up finding lent additional support to existing society recommendations that CABG is the preferred revascularization strategy for patients with diabetes and multivessel coronary disease, most recently from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394), said Dr. Fuster, professor of medicine at the Icahn School of Medicine at Mount Sinai and director of Mount Sinai Heart in New York. A subgroup analysis of the extended follow-up also suggested that the survival benefit from CABG, compared with PCI, was especially strong among patients at or below the study’s median age of 63 years. In the younger subgroup survival among patients treated with CABG was twice as good as it was among patients treated with PCI.
Dr. Fuster noted that few data have been previously reported for survival rates beyond 5 years after revascularization. “This was a difficult study. Following patients for more than 5 years is hard,” he said. Concurrently with his report at the meeting the results also appeared online (J Am Coll Cardiol. 2018 Nov 11. doi: 10.1016/j.jacc.2018.11.001).
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial enrolled patients at 140 participating centers during 2005-2010. A total of 25 sites agreed to participate in the extended follow-up and could track 943 patients, 50% of the starting cohort of 1,900 and 89% of the patients originally enrolled at these 25 centers. Dr. Fuster stressed that the 957 patients not included in the follow-up had not been lost, but rather had been managed at sites that declined to participate in this additional study.
Dr. Fuster acknowledged that methods and hardware for PCI have changed since the study ran a decade ago, as have options for medical management. He also highlighted that the long-term follow-up results had no data on rates of MIs and strokes.
FREEDOM had no commercial funding. Dr. Fuster reported no relevant disclosures.
SOURCE: Fuster V et al. AHA 2018, Abstract 18609.
CHICAGO – Patients with diabetes who underwent coronary artery bypass grafting had significantly better survival than patients with diabetes who underwent percutaneous coronary intervention after a median 7.5 years of follow-up.

Those patients comprised about half the patients enrolled in the FREEDOM randomized trial.
Long-term follow-up was only possible for just under half the 1,900 patients with diabetes and multivessel coronary disease originally enrolled in FREEDOM, but when researchers combined the long-term results with the data collected in the original study that had a median 3.8-year follow-up, they found all-cause mortality occurred in 18.3% of the patients who underwent coronary artery bypass grafting (CABG) and in 24.3% of patients treated with percutaneous coronary intervention (PCI), a 6% absolute between-group difference that was statistically significant, Valentin Fuster, MD, said at the American Heart Association scientific sessions. This fully jibed with the primary FREEDOM results, which found after 5 years a statistically significant reduction in all-cause death with CABG, compared with PCI, and also a significant reduction in the study’s primary endpoint (a combination of all-cause death, MI, and stroke), which occurred in 18.7% of patients randomized to CABG and in 26.6% of those randomized to PCI (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
The extended follow-up finding lent additional support to existing society recommendations that CABG is the preferred revascularization strategy for patients with diabetes and multivessel coronary disease, most recently from the European Society of Cardiology (Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394), said Dr. Fuster, professor of medicine at the Icahn School of Medicine at Mount Sinai and director of Mount Sinai Heart in New York. A subgroup analysis of the extended follow-up also suggested that the survival benefit from CABG, compared with PCI, was especially strong among patients at or below the study’s median age of 63 years. In the younger subgroup survival among patients treated with CABG was twice as good as it was among patients treated with PCI.
Dr. Fuster noted that few data have been previously reported for survival rates beyond 5 years after revascularization. “This was a difficult study. Following patients for more than 5 years is hard,” he said. Concurrently with his report at the meeting the results also appeared online (J Am Coll Cardiol. 2018 Nov 11. doi: 10.1016/j.jacc.2018.11.001).
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial enrolled patients at 140 participating centers during 2005-2010. A total of 25 sites agreed to participate in the extended follow-up and could track 943 patients, 50% of the starting cohort of 1,900 and 89% of the patients originally enrolled at these 25 centers. Dr. Fuster stressed that the 957 patients not included in the follow-up had not been lost, but rather had been managed at sites that declined to participate in this additional study.
Dr. Fuster acknowledged that methods and hardware for PCI have changed since the study ran a decade ago, as have options for medical management. He also highlighted that the long-term follow-up results had no data on rates of MIs and strokes.
FREEDOM had no commercial funding. Dr. Fuster reported no relevant disclosures.
SOURCE: Fuster V et al. AHA 2018, Abstract 18609.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After 7.5 years, mortality in the full FREEDOM cohort was 18% after coronary artery bypass grafting and 24% after percutaneous coronary intervention.
Study details: An extended follow-up of 943 of patients enrolled in FREEDOM, a randomized, multicenter trial.
Disclosures: FREEDOM had no commercial funding. Dr. Fuster reported no relevant disclosures.
Source: Fuster V et al. AHA 2018, Abstract 18609.
Partial- and whole-breast irradiation very close in efficacy
SAN ANTONIO – , suggests a phase 3, randomized, controlled trial conducted by NRG Oncology.
At a median follow-up of 10.2 years, the trial was unable to refute the hypothesis that the partial technique was inferior in terms of ipsilateral breast tumor recurrences; however, the difference between techniques in this outcome was an absolute 0.7%, lead investigator Frank Vicini, MD, principal investigator at the MHP Radiation Oncology Institute/21st Century Oncology in Pontiac, Mich., reported in a session and press conference at the San Antonio Breast Cancer Symposium. The difference in recurrence-free interval was significant but likewise small, at 1.6%, and other efficacy outcomes were similar.
Meanwhile, the groups had low, statistically indistinguishable rates of grade 3-5 toxicities and second cancers. The investigators are still analyzing quality of life and cosmesis outcomes.
“This was the largest trial ever looking at partial-breast [irradiation] in a very diverse group of patients. Even though we weren’t able to demonstrate equivalence, it’s nice to see that in this large population of patients with extended follow-up, the differences are quite small,” Dr. Vicini said. “Because the differences for both ipsilateral breast tumor recurrence and recurrence-free interval were very small, partial-breast irradiation may be an acceptable alternative to whole-breast irradiation for a proportion of women who are undergoing breast-conserving surgery.”
Implications for practice and research
SABCS codirector and press conference moderator Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, asked how the findings have influenced his practice.
“This trial is over 15 years old now, and a lot of these techniques have been refined. But we are offering partial-breast irradiation to our patients,” Dr. Vicini replied. “There are a lot of competing ways to do radiation now; probably the biggest competing way is to do 3 weeks of whole-breast irradiation. But for those patients who have transportation issues and more elderly patients, we try to offer partial-breast irradiation, within the guidelines of ASTRO [American Society for Therapeutic Radiology and Oncology].”
Some of the women enrolled had risk factors that fall outside those guidelines, for example, higher-grade tumors or axillary node involvement, he acknowledged. “We tried to do exploratory analyses to look at whether certain patients did better with whole-breast irradiation or not, and we weren’t able to really pick out any group of patients that had better or worse outcome based on those criteria,” he said. “We have yet to look at the quality indices for radiation therapy, in other words, how much the breast actually needs to be treated. But at the present time, I would just suggest sticking with the ASTRO guidelines.”
It is noteworthy that likely the most important endpoints, disease-free and overall survival, did not differ between groups, according to Dr. Vicini. “Certainly, a recurrence is still an important event for a patient, so our goal is to always limit that as much as possible,” he said. “But putting it into perspective, does a 0.7% higher risk of recurrence [matter] when you know the survival rates are the same? That’s what patients and doctors need to take into consideration. This is a pretty dramatic difference in treatment [duration], 6 weeks, down to 1 week or less. There have been many studies looking at quality of life and, as you can imagine, quality of life is better” with the shorter therapy.
The trial’s results can also inform statistical planning of future trials, according to Dr. Kaklamani. “It’s important when we design the trials to look at clinically meaningful differences because we don’t want to harm our patients, but at the same time, we are also harming them by giving them more treatment. So if you are designing a trial where a 0.7% difference is statistically significant, you probably would have been able to get away with many fewer patients and a difference of 1.5% or 2% not being significant, and I think everybody would be happy with that.”
Study details
Dr. Vicini and colleagues enrolled in their trial 4,216 women with stage 0-II breast cancer who had undergone lumpectomy. They were randomized to whole-breast irradiation (5-6 weeks of radiation therapy at that time) or partial-breast irradiation using one of three techniques (3D conformal external beam radiation completed in 5 days, interstitial brachytherapy completed in 5 days, or device-based brachytherapy).
The hazard ratio for ipsilateral breast tumor recurrence (invasive or DCIS) as a first recurrence with partial-breast irradiation versus whole-breast irradiation was 1.22, with the upper bound of the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5), Dr. Vicini reported. However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was merely 0.7% (4.6% vs. 3.9%).
The 10-year recurrence-free interval was inferior with partial-breast irradiation (hazard ratio, 1.33; P = .02), but the absolute difference was again small at 1.6% (91.8% vs 93.4%). The partial- and whole-breast irradiation groups were statistically indistinguishable on distant disease-free interval (96.7% vs 97.1%; HR, 1.31; P = .15) and overall survival (90.6% vs. 91.3%; HR, 1.10; P = .35).
Dr. Vicini disclosed that he is a research advisor for ImpediMed. The study was sponsored by the National Cancer Institute.
SOURCE: Vicini FA et al. SABCS 2018, Abstract GS4-04,
SAN ANTONIO – , suggests a phase 3, randomized, controlled trial conducted by NRG Oncology.
At a median follow-up of 10.2 years, the trial was unable to refute the hypothesis that the partial technique was inferior in terms of ipsilateral breast tumor recurrences; however, the difference between techniques in this outcome was an absolute 0.7%, lead investigator Frank Vicini, MD, principal investigator at the MHP Radiation Oncology Institute/21st Century Oncology in Pontiac, Mich., reported in a session and press conference at the San Antonio Breast Cancer Symposium. The difference in recurrence-free interval was significant but likewise small, at 1.6%, and other efficacy outcomes were similar.
Meanwhile, the groups had low, statistically indistinguishable rates of grade 3-5 toxicities and second cancers. The investigators are still analyzing quality of life and cosmesis outcomes.
“This was the largest trial ever looking at partial-breast [irradiation] in a very diverse group of patients. Even though we weren’t able to demonstrate equivalence, it’s nice to see that in this large population of patients with extended follow-up, the differences are quite small,” Dr. Vicini said. “Because the differences for both ipsilateral breast tumor recurrence and recurrence-free interval were very small, partial-breast irradiation may be an acceptable alternative to whole-breast irradiation for a proportion of women who are undergoing breast-conserving surgery.”
Implications for practice and research
SABCS codirector and press conference moderator Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, asked how the findings have influenced his practice.
“This trial is over 15 years old now, and a lot of these techniques have been refined. But we are offering partial-breast irradiation to our patients,” Dr. Vicini replied. “There are a lot of competing ways to do radiation now; probably the biggest competing way is to do 3 weeks of whole-breast irradiation. But for those patients who have transportation issues and more elderly patients, we try to offer partial-breast irradiation, within the guidelines of ASTRO [American Society for Therapeutic Radiology and Oncology].”
Some of the women enrolled had risk factors that fall outside those guidelines, for example, higher-grade tumors or axillary node involvement, he acknowledged. “We tried to do exploratory analyses to look at whether certain patients did better with whole-breast irradiation or not, and we weren’t able to really pick out any group of patients that had better or worse outcome based on those criteria,” he said. “We have yet to look at the quality indices for radiation therapy, in other words, how much the breast actually needs to be treated. But at the present time, I would just suggest sticking with the ASTRO guidelines.”
It is noteworthy that likely the most important endpoints, disease-free and overall survival, did not differ between groups, according to Dr. Vicini. “Certainly, a recurrence is still an important event for a patient, so our goal is to always limit that as much as possible,” he said. “But putting it into perspective, does a 0.7% higher risk of recurrence [matter] when you know the survival rates are the same? That’s what patients and doctors need to take into consideration. This is a pretty dramatic difference in treatment [duration], 6 weeks, down to 1 week or less. There have been many studies looking at quality of life and, as you can imagine, quality of life is better” with the shorter therapy.
The trial’s results can also inform statistical planning of future trials, according to Dr. Kaklamani. “It’s important when we design the trials to look at clinically meaningful differences because we don’t want to harm our patients, but at the same time, we are also harming them by giving them more treatment. So if you are designing a trial where a 0.7% difference is statistically significant, you probably would have been able to get away with many fewer patients and a difference of 1.5% or 2% not being significant, and I think everybody would be happy with that.”
Study details
Dr. Vicini and colleagues enrolled in their trial 4,216 women with stage 0-II breast cancer who had undergone lumpectomy. They were randomized to whole-breast irradiation (5-6 weeks of radiation therapy at that time) or partial-breast irradiation using one of three techniques (3D conformal external beam radiation completed in 5 days, interstitial brachytherapy completed in 5 days, or device-based brachytherapy).
The hazard ratio for ipsilateral breast tumor recurrence (invasive or DCIS) as a first recurrence with partial-breast irradiation versus whole-breast irradiation was 1.22, with the upper bound of the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5), Dr. Vicini reported. However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was merely 0.7% (4.6% vs. 3.9%).
The 10-year recurrence-free interval was inferior with partial-breast irradiation (hazard ratio, 1.33; P = .02), but the absolute difference was again small at 1.6% (91.8% vs 93.4%). The partial- and whole-breast irradiation groups were statistically indistinguishable on distant disease-free interval (96.7% vs 97.1%; HR, 1.31; P = .15) and overall survival (90.6% vs. 91.3%; HR, 1.10; P = .35).
Dr. Vicini disclosed that he is a research advisor for ImpediMed. The study was sponsored by the National Cancer Institute.
SOURCE: Vicini FA et al. SABCS 2018, Abstract GS4-04,
SAN ANTONIO – , suggests a phase 3, randomized, controlled trial conducted by NRG Oncology.
At a median follow-up of 10.2 years, the trial was unable to refute the hypothesis that the partial technique was inferior in terms of ipsilateral breast tumor recurrences; however, the difference between techniques in this outcome was an absolute 0.7%, lead investigator Frank Vicini, MD, principal investigator at the MHP Radiation Oncology Institute/21st Century Oncology in Pontiac, Mich., reported in a session and press conference at the San Antonio Breast Cancer Symposium. The difference in recurrence-free interval was significant but likewise small, at 1.6%, and other efficacy outcomes were similar.
Meanwhile, the groups had low, statistically indistinguishable rates of grade 3-5 toxicities and second cancers. The investigators are still analyzing quality of life and cosmesis outcomes.
“This was the largest trial ever looking at partial-breast [irradiation] in a very diverse group of patients. Even though we weren’t able to demonstrate equivalence, it’s nice to see that in this large population of patients with extended follow-up, the differences are quite small,” Dr. Vicini said. “Because the differences for both ipsilateral breast tumor recurrence and recurrence-free interval were very small, partial-breast irradiation may be an acceptable alternative to whole-breast irradiation for a proportion of women who are undergoing breast-conserving surgery.”
Implications for practice and research
SABCS codirector and press conference moderator Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, asked how the findings have influenced his practice.
“This trial is over 15 years old now, and a lot of these techniques have been refined. But we are offering partial-breast irradiation to our patients,” Dr. Vicini replied. “There are a lot of competing ways to do radiation now; probably the biggest competing way is to do 3 weeks of whole-breast irradiation. But for those patients who have transportation issues and more elderly patients, we try to offer partial-breast irradiation, within the guidelines of ASTRO [American Society for Therapeutic Radiology and Oncology].”
Some of the women enrolled had risk factors that fall outside those guidelines, for example, higher-grade tumors or axillary node involvement, he acknowledged. “We tried to do exploratory analyses to look at whether certain patients did better with whole-breast irradiation or not, and we weren’t able to really pick out any group of patients that had better or worse outcome based on those criteria,” he said. “We have yet to look at the quality indices for radiation therapy, in other words, how much the breast actually needs to be treated. But at the present time, I would just suggest sticking with the ASTRO guidelines.”
It is noteworthy that likely the most important endpoints, disease-free and overall survival, did not differ between groups, according to Dr. Vicini. “Certainly, a recurrence is still an important event for a patient, so our goal is to always limit that as much as possible,” he said. “But putting it into perspective, does a 0.7% higher risk of recurrence [matter] when you know the survival rates are the same? That’s what patients and doctors need to take into consideration. This is a pretty dramatic difference in treatment [duration], 6 weeks, down to 1 week or less. There have been many studies looking at quality of life and, as you can imagine, quality of life is better” with the shorter therapy.
The trial’s results can also inform statistical planning of future trials, according to Dr. Kaklamani. “It’s important when we design the trials to look at clinically meaningful differences because we don’t want to harm our patients, but at the same time, we are also harming them by giving them more treatment. So if you are designing a trial where a 0.7% difference is statistically significant, you probably would have been able to get away with many fewer patients and a difference of 1.5% or 2% not being significant, and I think everybody would be happy with that.”
Study details
Dr. Vicini and colleagues enrolled in their trial 4,216 women with stage 0-II breast cancer who had undergone lumpectomy. They were randomized to whole-breast irradiation (5-6 weeks of radiation therapy at that time) or partial-breast irradiation using one of three techniques (3D conformal external beam radiation completed in 5 days, interstitial brachytherapy completed in 5 days, or device-based brachytherapy).
The hazard ratio for ipsilateral breast tumor recurrence (invasive or DCIS) as a first recurrence with partial-breast irradiation versus whole-breast irradiation was 1.22, with the upper bound of the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5), Dr. Vicini reported. However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was merely 0.7% (4.6% vs. 3.9%).
The 10-year recurrence-free interval was inferior with partial-breast irradiation (hazard ratio, 1.33; P = .02), but the absolute difference was again small at 1.6% (91.8% vs 93.4%). The partial- and whole-breast irradiation groups were statistically indistinguishable on distant disease-free interval (96.7% vs 97.1%; HR, 1.31; P = .15) and overall survival (90.6% vs. 91.3%; HR, 1.10; P = .35).
Dr. Vicini disclosed that he is a research advisor for ImpediMed. The study was sponsored by the National Cancer Institute.
SOURCE: Vicini FA et al. SABCS 2018, Abstract GS4-04,
REPORTING FROM SABCS 2018
Key clinical point: Partial- and whole-breast irradiation yield outcomes that are statistically nonequivalent but very similar.
Major finding: The hazard ratio for ipsilateral recurrences with partial- vs. whole-breast irradiation was 1.22, with the confidence interval falling just outside the range for equivalence, but the absolute difference in 10-year rate was just 0.7% (4.6% vs. 3.9%).
Study details: A phase 3, randomized, controlled trial among women who underwent lumpectomy for stage 0-II breast cancer, conducted by NRG Oncology (NSABP B-39/RTOG 0413).
Disclosures: Dr. Vicini disclosed that he is a research advisor for ImpediMed. The study was sponsored by the National Cancer Institute.
Source: Vicini FA et al. SABCS 2018, Abstract GS4-04.
Brain injury in sickle cell merits more attention
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
EXPERT ANALYSIS FROM SICKLE CELL IN FOCUS
No matter the valve, protective device cuts post-TAVR stroke risk
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
REPORTING FROM TCT 2018
What is your diagnosis?
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
A 6-year-old, otherwise-healthy male is brought into clinic for evaluation of papules on his neck. The rash has been present since 1 year of age and has been growing in size proportionately. He claims there is occasional itching but no pain or redness. He does not seem to be disturbed by his rash. He has two siblings, aged 2 and 4 years, without lesions.
On physical exam, he is noted to have a linear plaque of hyperpigmented verrucous papules on his neck.
Challenges in managing chronic pelvic pain in women
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
Antipsychotic use in young people tied to 80% increased risk of death
Children and young people who received antipsychotic doses higher than 50-mg chlorpromazine equivalents had an 80% increased risk of death at follow-up, compared with a control group, according to a study of young Medicaid enrollees who recently had begun medication.
“The study findings seem to reinforce existing guidelines for improving the outcomes of antipsychotic therapy in children and youths,” wrote lead author Wayne A. Ray, PhD, of the department of health policy at the Vanderbilt University in Nashville, Tenn., and his coauthors. Those guidelines include using “psychosocial interventions when possible, cardiometabolic assessment before treatment and monitoring after treatment, and limiting therapy to the lowest dose and shortest duration possible,” they wrote.
The study, published online in JAMA Psychiatry, analyzed children and young adults from Tennessee, aged 5-24 years, who were new medication users, and had been enrolled in Medicaid between 1999 and 2014.
They were split into three groups: a control group (189,361) with users primarily taking attention-deficit/hyperactivity disorder medications and antidepressants; a group (28,377) with users who received antipsychotic doses of 50 mg or less chlorpromazine equivalents; and a group (30,120) with users who received doses higher than 50-mg chlorpromazine equivalents.
At follow-up, the incidence of death in the higher-dose group was 146.2 per 100,000 person-years (95% confidence interval, 107.3-199.4 per 100,000 person-years), compared with 49.5 in the lower-dose group (95% CI, 24.8-99.0) and 54.5 in the control group (95% CI, 42.9-69.2). This difference was attributed to unexpected deaths, which accounted for 52.5% of deaths in the higher-dose group. No increased risk of death was noted for injuries or suicides. “The elevated risk persisted for unexpected deaths not due to overdose, with a 4.3-fold increased risk of death from cardiovascular or metabolic causes,” Dr. Ray and his coauthors wrote.
The authors shared potential limitations of their study, including a relatively small number of deaths during follow-up and subsequent statistical adjustment during analysis. They also recognized that their data did not factor in important characteristics such as body mass index and family history, and that a “single-state Medicaid cohort may limit the study’s generalizability.”
Nonetheless, they emphasized Medicaid’s relevance as coverage provider for an estimated 39% of U.S. children, along with noting that
“Further studies are needed that compare antipsychotic users and controls within more narrow comorbidity ranges or in analyses that include richer clinical data,” they wrote.
The study was supported by grants from the National Heart, Lung, and Blood Institute, and the National Institute for Child Health and Human Development. No conflicts of interest were reported.
SOURCE: Ray WA et al. JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3421.
This study by Wayne A. Ray, PhD, and his colleagues addresses the risks of antipsychotic use in childhood while highlighting the contradictions in how psychiatrically ill children are treated and medicated, according to Barbara Geller, MD, of the department of psychiatry at Washington University in St. Louis.
Before commenting on the study itself, Dr. Geller noted that child psychiatry is not a subspecialty that deals with “little patients and little problems,” despite that lingering perception among some. “Fifty percent of psychiatry disorders begin by age 14 years,” she wrote, “and childhood age at onset is a risk factor for a more severe longitudinal course in mood and other disorders.”
In addition, though it seems instinctually that antipsychotic medications would have lesser side effects on healthy children, that is not always the case. “The opposite is true for certain metabolic and endocrine effects,” she explained, “such as relatively greater weight gain and prolactin level elevation than adults and the onset of type 2 diabetes within the first year of treatment.”
When it came to the study, Dr. Geller posed questions about the findings, including whether an increase in unexpected deaths among the higher-dose group could be attributed to suicide. She also recommended that future investigations “examine outcomes within child, adolescent, and young adult age subgroups, as opposed to combining all youth 6 to 24 years old.”
That said, this research does probe depths that require continued exploration. “Results in the study by Ray et al. heighten the already increased caution about prescribing antipsychotics to children and adolescents,” she wrote, “and emphasize the need to consider situational triggers of psychopathology to avoid medicating the environment.”
These comments are adapted from an accompanying editorial (JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3409). No conflicts of interest were reported.
This study by Wayne A. Ray, PhD, and his colleagues addresses the risks of antipsychotic use in childhood while highlighting the contradictions in how psychiatrically ill children are treated and medicated, according to Barbara Geller, MD, of the department of psychiatry at Washington University in St. Louis.
Before commenting on the study itself, Dr. Geller noted that child psychiatry is not a subspecialty that deals with “little patients and little problems,” despite that lingering perception among some. “Fifty percent of psychiatry disorders begin by age 14 years,” she wrote, “and childhood age at onset is a risk factor for a more severe longitudinal course in mood and other disorders.”
In addition, though it seems instinctually that antipsychotic medications would have lesser side effects on healthy children, that is not always the case. “The opposite is true for certain metabolic and endocrine effects,” she explained, “such as relatively greater weight gain and prolactin level elevation than adults and the onset of type 2 diabetes within the first year of treatment.”
When it came to the study, Dr. Geller posed questions about the findings, including whether an increase in unexpected deaths among the higher-dose group could be attributed to suicide. She also recommended that future investigations “examine outcomes within child, adolescent, and young adult age subgroups, as opposed to combining all youth 6 to 24 years old.”
That said, this research does probe depths that require continued exploration. “Results in the study by Ray et al. heighten the already increased caution about prescribing antipsychotics to children and adolescents,” she wrote, “and emphasize the need to consider situational triggers of psychopathology to avoid medicating the environment.”
These comments are adapted from an accompanying editorial (JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3409). No conflicts of interest were reported.
This study by Wayne A. Ray, PhD, and his colleagues addresses the risks of antipsychotic use in childhood while highlighting the contradictions in how psychiatrically ill children are treated and medicated, according to Barbara Geller, MD, of the department of psychiatry at Washington University in St. Louis.
Before commenting on the study itself, Dr. Geller noted that child psychiatry is not a subspecialty that deals with “little patients and little problems,” despite that lingering perception among some. “Fifty percent of psychiatry disorders begin by age 14 years,” she wrote, “and childhood age at onset is a risk factor for a more severe longitudinal course in mood and other disorders.”
In addition, though it seems instinctually that antipsychotic medications would have lesser side effects on healthy children, that is not always the case. “The opposite is true for certain metabolic and endocrine effects,” she explained, “such as relatively greater weight gain and prolactin level elevation than adults and the onset of type 2 diabetes within the first year of treatment.”
When it came to the study, Dr. Geller posed questions about the findings, including whether an increase in unexpected deaths among the higher-dose group could be attributed to suicide. She also recommended that future investigations “examine outcomes within child, adolescent, and young adult age subgroups, as opposed to combining all youth 6 to 24 years old.”
That said, this research does probe depths that require continued exploration. “Results in the study by Ray et al. heighten the already increased caution about prescribing antipsychotics to children and adolescents,” she wrote, “and emphasize the need to consider situational triggers of psychopathology to avoid medicating the environment.”
These comments are adapted from an accompanying editorial (JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3409). No conflicts of interest were reported.
Children and young people who received antipsychotic doses higher than 50-mg chlorpromazine equivalents had an 80% increased risk of death at follow-up, compared with a control group, according to a study of young Medicaid enrollees who recently had begun medication.
“The study findings seem to reinforce existing guidelines for improving the outcomes of antipsychotic therapy in children and youths,” wrote lead author Wayne A. Ray, PhD, of the department of health policy at the Vanderbilt University in Nashville, Tenn., and his coauthors. Those guidelines include using “psychosocial interventions when possible, cardiometabolic assessment before treatment and monitoring after treatment, and limiting therapy to the lowest dose and shortest duration possible,” they wrote.
The study, published online in JAMA Psychiatry, analyzed children and young adults from Tennessee, aged 5-24 years, who were new medication users, and had been enrolled in Medicaid between 1999 and 2014.
They were split into three groups: a control group (189,361) with users primarily taking attention-deficit/hyperactivity disorder medications and antidepressants; a group (28,377) with users who received antipsychotic doses of 50 mg or less chlorpromazine equivalents; and a group (30,120) with users who received doses higher than 50-mg chlorpromazine equivalents.
At follow-up, the incidence of death in the higher-dose group was 146.2 per 100,000 person-years (95% confidence interval, 107.3-199.4 per 100,000 person-years), compared with 49.5 in the lower-dose group (95% CI, 24.8-99.0) and 54.5 in the control group (95% CI, 42.9-69.2). This difference was attributed to unexpected deaths, which accounted for 52.5% of deaths in the higher-dose group. No increased risk of death was noted for injuries or suicides. “The elevated risk persisted for unexpected deaths not due to overdose, with a 4.3-fold increased risk of death from cardiovascular or metabolic causes,” Dr. Ray and his coauthors wrote.
The authors shared potential limitations of their study, including a relatively small number of deaths during follow-up and subsequent statistical adjustment during analysis. They also recognized that their data did not factor in important characteristics such as body mass index and family history, and that a “single-state Medicaid cohort may limit the study’s generalizability.”
Nonetheless, they emphasized Medicaid’s relevance as coverage provider for an estimated 39% of U.S. children, along with noting that
“Further studies are needed that compare antipsychotic users and controls within more narrow comorbidity ranges or in analyses that include richer clinical data,” they wrote.
The study was supported by grants from the National Heart, Lung, and Blood Institute, and the National Institute for Child Health and Human Development. No conflicts of interest were reported.
SOURCE: Ray WA et al. JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3421.
Children and young people who received antipsychotic doses higher than 50-mg chlorpromazine equivalents had an 80% increased risk of death at follow-up, compared with a control group, according to a study of young Medicaid enrollees who recently had begun medication.
“The study findings seem to reinforce existing guidelines for improving the outcomes of antipsychotic therapy in children and youths,” wrote lead author Wayne A. Ray, PhD, of the department of health policy at the Vanderbilt University in Nashville, Tenn., and his coauthors. Those guidelines include using “psychosocial interventions when possible, cardiometabolic assessment before treatment and monitoring after treatment, and limiting therapy to the lowest dose and shortest duration possible,” they wrote.
The study, published online in JAMA Psychiatry, analyzed children and young adults from Tennessee, aged 5-24 years, who were new medication users, and had been enrolled in Medicaid between 1999 and 2014.
They were split into three groups: a control group (189,361) with users primarily taking attention-deficit/hyperactivity disorder medications and antidepressants; a group (28,377) with users who received antipsychotic doses of 50 mg or less chlorpromazine equivalents; and a group (30,120) with users who received doses higher than 50-mg chlorpromazine equivalents.
At follow-up, the incidence of death in the higher-dose group was 146.2 per 100,000 person-years (95% confidence interval, 107.3-199.4 per 100,000 person-years), compared with 49.5 in the lower-dose group (95% CI, 24.8-99.0) and 54.5 in the control group (95% CI, 42.9-69.2). This difference was attributed to unexpected deaths, which accounted for 52.5% of deaths in the higher-dose group. No increased risk of death was noted for injuries or suicides. “The elevated risk persisted for unexpected deaths not due to overdose, with a 4.3-fold increased risk of death from cardiovascular or metabolic causes,” Dr. Ray and his coauthors wrote.
The authors shared potential limitations of their study, including a relatively small number of deaths during follow-up and subsequent statistical adjustment during analysis. They also recognized that their data did not factor in important characteristics such as body mass index and family history, and that a “single-state Medicaid cohort may limit the study’s generalizability.”
Nonetheless, they emphasized Medicaid’s relevance as coverage provider for an estimated 39% of U.S. children, along with noting that
“Further studies are needed that compare antipsychotic users and controls within more narrow comorbidity ranges or in analyses that include richer clinical data,” they wrote.
The study was supported by grants from the National Heart, Lung, and Blood Institute, and the National Institute for Child Health and Human Development. No conflicts of interest were reported.
SOURCE: Ray WA et al. JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3421.
FROM JAMA PSYCHIATRY
Key clinical point: Children and youths who received higher doses of antipsychotic medication had an 80% increased risk of death, compared with those in a control group.
Major finding: The incidence of unexpected death was 76.8 per 100,000 person-years in the higher-dose group, compared with 17.9 per 100,000 person-years in the control group.
Study details: A retrospective cohort study of Medicaid-enrolled children and young adults from Tennessee, aged 5-24 years, who were new users of antipsychotic or control medications.
Disclosures: The study was supported by grants from the National Heart, Lung, and Blood Institute, and the National Institute for Child Health and Human Development. No conflicts of interest were reported.
Source: Ray WA et al. JAMA Psychiatry. 2018 Dec 12. doi: 10.1001/jamapsychiatry.2018.3421.
More data link severe sleep apnea and aggressive melanoma
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
FROM CHEST®
Key clinical point: Severe sleep disordered breathing is independently associated with more severe melanoma.
Major finding: Patients in the upper tertiles of AHI or DI4% were 1.94 and 1.93 times more likely, respectively, to have aggressive melanoma compared with patients with less severe sleep apnea.
Study details: The data come from an observational study of 443 adults diagnosed with melanoma.
Disclosures: The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose.
Source: Martinez-Garcia M et al. Chest. 2018. doi: 10.1016/j.chest.2018.07.015.