User login
Combo bests standard care in younger CLL patients
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
SAN DIEGO—In a phase 3 trial, ibrutinib plus rituximab (IR) improved survival when compared with standard chemoimmunotherapy in patients younger than 70 with untreated chronic lymphocytic leukemia (CLL).
Patients who received IR had superior progression-free survival (PFS) and overall survival compared to patients who received fludarabine, cyclophosphamide, and rituximab (FCR).
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” said Tait D. Shanafelt, MD, of Stanford University in California.
In fact, the study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
He presented the results during the late-breaking abstract session at the 2018 ASH Annual Meeting (abstract LBA-4*).
The trial (NCT02048813) included 529 patients age 70 or younger with previously untreated CLL. They were randomized on a 2:1 basis to either six cycles of FCR according to standard protocols (n=175) or IR (n=354).
IR consisted of ibrutinib given at 420 mg daily for each 28-day cycle and rituximab given at 50 mg/m2 on day 1 of cycle 2, at 325 mg/m2 on day 2 of cycle 2, and at 500 mg/m2 on day 1 for cycles 3 to 7.
From cycle 8 on, patients in the IR arm received daily ibrutinib at 420 mg until disease progression.
Dr. Shanafelt said patient characteristics were well-balanced between the treatment arms.
He presented results from both an intent-to-treat (ITT) analysis and a per-protocol analysis excluding 22 patients in the IR arm and nine patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
PFS
In the ITT analysis, there were 37 cases of progression or death in the IR arm and 40 cases in the FCR arm. This difference translated into a hazard ratio (HR) for progression or death of 0.35 with IR (P<0.00001).
In the per-protocol analysis, there were 33 cases of progression or death in the IR arm and 39 cases in the FCR arm. The HR was 0.32 favoring IR (P<0.00001).
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was significantly better with IR in patients with unmutated IGHV (HR= 0.26, P<0.00001) but not in patients with mutated IGHV (HR=0.44, P=0.07).
Overall survival
In the ITT analysis, there were four deaths in the IR arm and 10 in the FCR arm (HR=0.17, P<0.0003).
In the per-protocol analysis, there were three deaths in the IR arm and 10 deaths in the FCR arm (HR=0.13, P<0.0001).
Dr. Shanafelt noted that, although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning the rate of death in the FCR arm was five-fold higher than in the IR arm.
Safety and cost
Grade 3 or greater treatment-related adverse events (AEs) occurred in 58.5% of patients in the IR arm and 72.1% of patients in the FCR arm (P=0.004).
Specific AEs that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P<0.001 for all comparisons).
AEs that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P=0.04) and hypertension (7.4% vs. 1.9%, P=0.01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost. The monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, AbbVie, Pharmacyclics, and Janssen.
*Data in the abstract differ from the presentation.
Preliminary data suggest UCART19 is safe, effective
SAN DIEGO—Preliminary data on UCART19—the first off-the-shelf, anti-CD19, allogeneic chimeric antigen receptor (CAR) T-cell therapy—suggest it can produce complete responses (CRs) and minimal residual disease (MRD) negativity, and side effects are manageable.
Investigators pooled data from the phase 1 pediatric (PALL) and adult (CALM) trials of UCART19 in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) and observed a 67% CR rate in the overall population and an 82% CR rate in patients who received a three-drug lymphodepleting regimen.
Additionally, investigators reported no instance of moderate or severe acute graft-versus-host disease (GVHD) with UCART19.
“We’ve been blessed with the new treatments that have emerged in recent years,” said Reuben Benjamin, MD, PhD, “that include BiTEs, antibody-drug conjugates, and most excitingly, the autologous CAR T-cell therapies.”
Nevertheless, some logistical issues with the autologous CAR T cells leave an unmet need in this group of patients, he noted.
“So an off-the-shelf approach using a product like UCART19 may potentially overcome some of these hurdles that we see in the autologous CAR T-cell therapy field,” he said.
Dr. Benjamin, of King’s College Hospital in London, U.K., presented the analysis of PALL and CALM data at the 2018 ASH Annual Meeting as abstract 896.*
UCART19 product
UCART19 is an allogeneic, genetically modified, CAR T-cell product (anti-CD19 scFv- 41BB-CD3ζ) manufactured from healthy donor T cells.
It has a safety switch—RQR8, which is a CD20 mimotope—that allows the CAR T cells to be targeted by rituximab.
“And importantly,” Dr. Benjamin explained, “the T-cell alpha gene has been knocked out using TALEN® gene-editing technology to prevent T-cell receptor-mediated graft-versus-host disease.”
The CD52 gene is also knocked out, which permits an anti-CD52 monoclonal antibody, such as alemtuzumab, to be used in lymphodepletion.
Study design
The primary objective of both the adult (NCT02746952) and pediatric (NCT02808442) studies was to determine the safety and tolerability of UCART19. Also, the adult study was to determine the maximum tolerated dose of UCART19 and the optimal lymphodepleting regimen.
A secondary objective of both studies was to determine the remission rate at day 28.
Eligible patients received a lymphodepleting regimen for 7 days, followed by a single infusion of UCART19.
Lymphodepletion in the pediatric trial consisted of fludarabine (F) at 150 mg/m2 and cyclophosphamide (C) at 120 mg/kg, with or without alemtuzumab (A) at 1 mg/kg capped at 40 mg.
Adults received lower doses of each agent—90 mg/m2, 1,500 mg/m2, and (optionally) 1 mg/kg or 40 mg, respectively.
Investigators included alemtuzumab in the regimen to minimize viral infections.
The UCART19 dose was weight-banded in the pediatric trial and ranged from 1.1 to 2.3 x 106 cells/kg.
The adult trial included three UCART19 dose levels:
- 6 x 106 cells (≈1 x 105 cells/kg)
- 6 or 8 x 107 cells (≈1 x 106 cells/kg)
- 8 or 2.4 x 108 cells (≈3 x 106 cells/kg).
Patients were assessed for safety and response at day 28 and regularly thereafter for up to 12 months. Patients had the option during the follow-up period to receive a second dose if they did not respond or lost their response.
Patient characteristics/status
Twenty-one patients were enrolled in the trials—seven children and 14 adults. Median ages were 2.7 years (PALL; range, 0.8–16.4) and 29.5 years (CALM; range, 18–62).
Both studies included high-risk, heavily pretreated populations, Dr. Benjamin noted.
The pooled population had a median of 4 prior lines of therapy (range, 1–6), and nine patients had a high-risk cytogenetics, including complex karyotypes, MLL rearrangements, and Ph+ disease.
Thirteen patients had prior allogeneic stem cell transplants.
Nine patients had a bone marrow tumor burden of more than 25% blasts prior to lymphodepletion.
As of the cutoff date of October 23, all patients had been treated with UCART19.
Four of the pediatric patients are still on the trial. Two are in remission, one has relapsed, and one is refractory.
Eight adult patients are still on trial. Three are in remission, three are relapsed, and two are refractory.
Safety
“UCART19 appears to show an acceptable safety profile based on the adverse events reported so far,” Dr. Benjamin said.
Nineteen patients experienced cytokine release syndrome (CRS), primarily grades 1 and 2. Eight patients had grade 1 and 2 neurotoxicity events, and two patients had grade 1 acute skin GVHD.
“In keeping with what is seen in some of the autologous CAR T-cell trials,” Dr. Benjamin explained, “prolonged cytopenias were seen, which we defined in these studies as grade 4 neutropenia or thrombocytopenia occurring at 42 days post-UCART infusion.”
Six of 21 patients developed prolonged cytopenia.
There was also an increased incidence of viral infections occurring in eight patients, including cytomegalovirus, adenovirus, BK virus, and metapneumovirus.
“Most of these infections, however, were manageable,” Dr. Benjamin said.
Two patients developed neutropenic sepsis, one grade 5, which was one of the treatment-related deaths in the CALM trial.
No treatment-related deaths occurred in the PALL study, but there were two in the CALM study—one from pulmonary hemorrhage and the other from neutropenic sepsis and grade 4 CRS.
Twelve patients are still alive, five of whom are in CR.
Efficacy
Of the patients who received FCA lymphodepletion, 82% (14/17) achieved CR/CR with incomplete hematologic recovery (CRi), and 71% (10/14) achieved MRD negativity.
An additional patient gained MRD-negative status after the second dose of UCART19.
Of the 14 patients who achieved a CR/CRi, 78% (n=11) went on to receive an allogeneic transplant.
In the entire pooled population, 67% (14/21) achieved CR/CRi.
Three patients received a second UCART19 dose, and five patients remain in CR/CRi.
UCART19 expansion
UCART19 expansion, as measured by quantitative polymerase chain reaction in PALL and flow-based methods in CALM, occurred primarily in the first 28 days in the FCA-treated population.
Investigators observed expansion in 15 of 17 patients treated with FCA. None of the patients who received FC alone (n=4) had expansion detectable in blood or bone marrow, Dr. Benjamin noted.
“The response we’ve seen in the study so far,” Dr. Benjamin clarified, “is linked to the expansion observed within the first 28-day period.”
UCART cells persisted in three patients beyond day 42. In one patient, they persisted up to day 120.
“Of interest is the T-cell recovery seen in the study,” Dr. Benjamin elaborated. “We only have data from the adult study here—14 patients. And you’ll see that, in the FCA-treated arm (n=11), you have a deeper and more sustained lymphodepletion compared to the FC-treated patients (n=3). And this may play a role in the subsequent UCART19 expansion and disease response.”
Re-dosing
Of the three patients who were re-dosed, two achieved MRD negativity.
One patient achieved MRD-negative status at day 28 but relapsed and received a second infusion 3 months after the first dose. The second expansion was not as deep as the first, but the patient nevertheless achieved MRD negativity after the second dose.
The second patient received FC lymphodepletion and was refractory at day 28.
“The second time around, he received FCA, had a slightly better expansion, and achieved molecular remission,” Dr. Benjamin said.
And the third patient had FCA lymphodepletion but was refractory at day 28.
“We elected to give a second dose at 2.4 months later, but unfortunately, there wasn’t very much expansion, even the second time around, and the patient progressed,” Dr. Benjamin said.
FCA lymphodepletion appears to be required for UCART19 expansion. There was no UCART19 expansion and no response in all four patients lymphodepleted with FC.
The evaluation of UCART19 is ongoing in pediatric and adult B-cell ALL, and “there is a plan for moving into the lymphoma space as well,” Dr. Benjamin added.
Dr. Benjamin disclosed honoraria from Amgen, Takeda, Novartis, Gilead, and Celgene, and research funding from Servier and Pfizer.
Servier and Allogene are supporting the UCART19 trials.
*Data in the abstract differ from the presentation.
SAN DIEGO—Preliminary data on UCART19—the first off-the-shelf, anti-CD19, allogeneic chimeric antigen receptor (CAR) T-cell therapy—suggest it can produce complete responses (CRs) and minimal residual disease (MRD) negativity, and side effects are manageable.
Investigators pooled data from the phase 1 pediatric (PALL) and adult (CALM) trials of UCART19 in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) and observed a 67% CR rate in the overall population and an 82% CR rate in patients who received a three-drug lymphodepleting regimen.
Additionally, investigators reported no instance of moderate or severe acute graft-versus-host disease (GVHD) with UCART19.
“We’ve been blessed with the new treatments that have emerged in recent years,” said Reuben Benjamin, MD, PhD, “that include BiTEs, antibody-drug conjugates, and most excitingly, the autologous CAR T-cell therapies.”
Nevertheless, some logistical issues with the autologous CAR T cells leave an unmet need in this group of patients, he noted.
“So an off-the-shelf approach using a product like UCART19 may potentially overcome some of these hurdles that we see in the autologous CAR T-cell therapy field,” he said.
Dr. Benjamin, of King’s College Hospital in London, U.K., presented the analysis of PALL and CALM data at the 2018 ASH Annual Meeting as abstract 896.*
UCART19 product
UCART19 is an allogeneic, genetically modified, CAR T-cell product (anti-CD19 scFv- 41BB-CD3ζ) manufactured from healthy donor T cells.
It has a safety switch—RQR8, which is a CD20 mimotope—that allows the CAR T cells to be targeted by rituximab.
“And importantly,” Dr. Benjamin explained, “the T-cell alpha gene has been knocked out using TALEN® gene-editing technology to prevent T-cell receptor-mediated graft-versus-host disease.”
The CD52 gene is also knocked out, which permits an anti-CD52 monoclonal antibody, such as alemtuzumab, to be used in lymphodepletion.
Study design
The primary objective of both the adult (NCT02746952) and pediatric (NCT02808442) studies was to determine the safety and tolerability of UCART19. Also, the adult study was to determine the maximum tolerated dose of UCART19 and the optimal lymphodepleting regimen.
A secondary objective of both studies was to determine the remission rate at day 28.
Eligible patients received a lymphodepleting regimen for 7 days, followed by a single infusion of UCART19.
Lymphodepletion in the pediatric trial consisted of fludarabine (F) at 150 mg/m2 and cyclophosphamide (C) at 120 mg/kg, with or without alemtuzumab (A) at 1 mg/kg capped at 40 mg.
Adults received lower doses of each agent—90 mg/m2, 1,500 mg/m2, and (optionally) 1 mg/kg or 40 mg, respectively.
Investigators included alemtuzumab in the regimen to minimize viral infections.
The UCART19 dose was weight-banded in the pediatric trial and ranged from 1.1 to 2.3 x 106 cells/kg.
The adult trial included three UCART19 dose levels:
- 6 x 106 cells (≈1 x 105 cells/kg)
- 6 or 8 x 107 cells (≈1 x 106 cells/kg)
- 8 or 2.4 x 108 cells (≈3 x 106 cells/kg).
Patients were assessed for safety and response at day 28 and regularly thereafter for up to 12 months. Patients had the option during the follow-up period to receive a second dose if they did not respond or lost their response.
Patient characteristics/status
Twenty-one patients were enrolled in the trials—seven children and 14 adults. Median ages were 2.7 years (PALL; range, 0.8–16.4) and 29.5 years (CALM; range, 18–62).
Both studies included high-risk, heavily pretreated populations, Dr. Benjamin noted.
The pooled population had a median of 4 prior lines of therapy (range, 1–6), and nine patients had a high-risk cytogenetics, including complex karyotypes, MLL rearrangements, and Ph+ disease.
Thirteen patients had prior allogeneic stem cell transplants.
Nine patients had a bone marrow tumor burden of more than 25% blasts prior to lymphodepletion.
As of the cutoff date of October 23, all patients had been treated with UCART19.
Four of the pediatric patients are still on the trial. Two are in remission, one has relapsed, and one is refractory.
Eight adult patients are still on trial. Three are in remission, three are relapsed, and two are refractory.
Safety
“UCART19 appears to show an acceptable safety profile based on the adverse events reported so far,” Dr. Benjamin said.
Nineteen patients experienced cytokine release syndrome (CRS), primarily grades 1 and 2. Eight patients had grade 1 and 2 neurotoxicity events, and two patients had grade 1 acute skin GVHD.
“In keeping with what is seen in some of the autologous CAR T-cell trials,” Dr. Benjamin explained, “prolonged cytopenias were seen, which we defined in these studies as grade 4 neutropenia or thrombocytopenia occurring at 42 days post-UCART infusion.”
Six of 21 patients developed prolonged cytopenia.
There was also an increased incidence of viral infections occurring in eight patients, including cytomegalovirus, adenovirus, BK virus, and metapneumovirus.
“Most of these infections, however, were manageable,” Dr. Benjamin said.
Two patients developed neutropenic sepsis, one grade 5, which was one of the treatment-related deaths in the CALM trial.
No treatment-related deaths occurred in the PALL study, but there were two in the CALM study—one from pulmonary hemorrhage and the other from neutropenic sepsis and grade 4 CRS.
Twelve patients are still alive, five of whom are in CR.
Efficacy
Of the patients who received FCA lymphodepletion, 82% (14/17) achieved CR/CR with incomplete hematologic recovery (CRi), and 71% (10/14) achieved MRD negativity.
An additional patient gained MRD-negative status after the second dose of UCART19.
Of the 14 patients who achieved a CR/CRi, 78% (n=11) went on to receive an allogeneic transplant.
In the entire pooled population, 67% (14/21) achieved CR/CRi.
Three patients received a second UCART19 dose, and five patients remain in CR/CRi.
UCART19 expansion
UCART19 expansion, as measured by quantitative polymerase chain reaction in PALL and flow-based methods in CALM, occurred primarily in the first 28 days in the FCA-treated population.
Investigators observed expansion in 15 of 17 patients treated with FCA. None of the patients who received FC alone (n=4) had expansion detectable in blood or bone marrow, Dr. Benjamin noted.
“The response we’ve seen in the study so far,” Dr. Benjamin clarified, “is linked to the expansion observed within the first 28-day period.”
UCART cells persisted in three patients beyond day 42. In one patient, they persisted up to day 120.
“Of interest is the T-cell recovery seen in the study,” Dr. Benjamin elaborated. “We only have data from the adult study here—14 patients. And you’ll see that, in the FCA-treated arm (n=11), you have a deeper and more sustained lymphodepletion compared to the FC-treated patients (n=3). And this may play a role in the subsequent UCART19 expansion and disease response.”
Re-dosing
Of the three patients who were re-dosed, two achieved MRD negativity.
One patient achieved MRD-negative status at day 28 but relapsed and received a second infusion 3 months after the first dose. The second expansion was not as deep as the first, but the patient nevertheless achieved MRD negativity after the second dose.
The second patient received FC lymphodepletion and was refractory at day 28.
“The second time around, he received FCA, had a slightly better expansion, and achieved molecular remission,” Dr. Benjamin said.
And the third patient had FCA lymphodepletion but was refractory at day 28.
“We elected to give a second dose at 2.4 months later, but unfortunately, there wasn’t very much expansion, even the second time around, and the patient progressed,” Dr. Benjamin said.
FCA lymphodepletion appears to be required for UCART19 expansion. There was no UCART19 expansion and no response in all four patients lymphodepleted with FC.
The evaluation of UCART19 is ongoing in pediatric and adult B-cell ALL, and “there is a plan for moving into the lymphoma space as well,” Dr. Benjamin added.
Dr. Benjamin disclosed honoraria from Amgen, Takeda, Novartis, Gilead, and Celgene, and research funding from Servier and Pfizer.
Servier and Allogene are supporting the UCART19 trials.
*Data in the abstract differ from the presentation.
SAN DIEGO—Preliminary data on UCART19—the first off-the-shelf, anti-CD19, allogeneic chimeric antigen receptor (CAR) T-cell therapy—suggest it can produce complete responses (CRs) and minimal residual disease (MRD) negativity, and side effects are manageable.
Investigators pooled data from the phase 1 pediatric (PALL) and adult (CALM) trials of UCART19 in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) and observed a 67% CR rate in the overall population and an 82% CR rate in patients who received a three-drug lymphodepleting regimen.
Additionally, investigators reported no instance of moderate or severe acute graft-versus-host disease (GVHD) with UCART19.
“We’ve been blessed with the new treatments that have emerged in recent years,” said Reuben Benjamin, MD, PhD, “that include BiTEs, antibody-drug conjugates, and most excitingly, the autologous CAR T-cell therapies.”
Nevertheless, some logistical issues with the autologous CAR T cells leave an unmet need in this group of patients, he noted.
“So an off-the-shelf approach using a product like UCART19 may potentially overcome some of these hurdles that we see in the autologous CAR T-cell therapy field,” he said.
Dr. Benjamin, of King’s College Hospital in London, U.K., presented the analysis of PALL and CALM data at the 2018 ASH Annual Meeting as abstract 896.*
UCART19 product
UCART19 is an allogeneic, genetically modified, CAR T-cell product (anti-CD19 scFv- 41BB-CD3ζ) manufactured from healthy donor T cells.
It has a safety switch—RQR8, which is a CD20 mimotope—that allows the CAR T cells to be targeted by rituximab.
“And importantly,” Dr. Benjamin explained, “the T-cell alpha gene has been knocked out using TALEN® gene-editing technology to prevent T-cell receptor-mediated graft-versus-host disease.”
The CD52 gene is also knocked out, which permits an anti-CD52 monoclonal antibody, such as alemtuzumab, to be used in lymphodepletion.
Study design
The primary objective of both the adult (NCT02746952) and pediatric (NCT02808442) studies was to determine the safety and tolerability of UCART19. Also, the adult study was to determine the maximum tolerated dose of UCART19 and the optimal lymphodepleting regimen.
A secondary objective of both studies was to determine the remission rate at day 28.
Eligible patients received a lymphodepleting regimen for 7 days, followed by a single infusion of UCART19.
Lymphodepletion in the pediatric trial consisted of fludarabine (F) at 150 mg/m2 and cyclophosphamide (C) at 120 mg/kg, with or without alemtuzumab (A) at 1 mg/kg capped at 40 mg.
Adults received lower doses of each agent—90 mg/m2, 1,500 mg/m2, and (optionally) 1 mg/kg or 40 mg, respectively.
Investigators included alemtuzumab in the regimen to minimize viral infections.
The UCART19 dose was weight-banded in the pediatric trial and ranged from 1.1 to 2.3 x 106 cells/kg.
The adult trial included three UCART19 dose levels:
- 6 x 106 cells (≈1 x 105 cells/kg)
- 6 or 8 x 107 cells (≈1 x 106 cells/kg)
- 8 or 2.4 x 108 cells (≈3 x 106 cells/kg).
Patients were assessed for safety and response at day 28 and regularly thereafter for up to 12 months. Patients had the option during the follow-up period to receive a second dose if they did not respond or lost their response.
Patient characteristics/status
Twenty-one patients were enrolled in the trials—seven children and 14 adults. Median ages were 2.7 years (PALL; range, 0.8–16.4) and 29.5 years (CALM; range, 18–62).
Both studies included high-risk, heavily pretreated populations, Dr. Benjamin noted.
The pooled population had a median of 4 prior lines of therapy (range, 1–6), and nine patients had a high-risk cytogenetics, including complex karyotypes, MLL rearrangements, and Ph+ disease.
Thirteen patients had prior allogeneic stem cell transplants.
Nine patients had a bone marrow tumor burden of more than 25% blasts prior to lymphodepletion.
As of the cutoff date of October 23, all patients had been treated with UCART19.
Four of the pediatric patients are still on the trial. Two are in remission, one has relapsed, and one is refractory.
Eight adult patients are still on trial. Three are in remission, three are relapsed, and two are refractory.
Safety
“UCART19 appears to show an acceptable safety profile based on the adverse events reported so far,” Dr. Benjamin said.
Nineteen patients experienced cytokine release syndrome (CRS), primarily grades 1 and 2. Eight patients had grade 1 and 2 neurotoxicity events, and two patients had grade 1 acute skin GVHD.
“In keeping with what is seen in some of the autologous CAR T-cell trials,” Dr. Benjamin explained, “prolonged cytopenias were seen, which we defined in these studies as grade 4 neutropenia or thrombocytopenia occurring at 42 days post-UCART infusion.”
Six of 21 patients developed prolonged cytopenia.
There was also an increased incidence of viral infections occurring in eight patients, including cytomegalovirus, adenovirus, BK virus, and metapneumovirus.
“Most of these infections, however, were manageable,” Dr. Benjamin said.
Two patients developed neutropenic sepsis, one grade 5, which was one of the treatment-related deaths in the CALM trial.
No treatment-related deaths occurred in the PALL study, but there were two in the CALM study—one from pulmonary hemorrhage and the other from neutropenic sepsis and grade 4 CRS.
Twelve patients are still alive, five of whom are in CR.
Efficacy
Of the patients who received FCA lymphodepletion, 82% (14/17) achieved CR/CR with incomplete hematologic recovery (CRi), and 71% (10/14) achieved MRD negativity.
An additional patient gained MRD-negative status after the second dose of UCART19.
Of the 14 patients who achieved a CR/CRi, 78% (n=11) went on to receive an allogeneic transplant.
In the entire pooled population, 67% (14/21) achieved CR/CRi.
Three patients received a second UCART19 dose, and five patients remain in CR/CRi.
UCART19 expansion
UCART19 expansion, as measured by quantitative polymerase chain reaction in PALL and flow-based methods in CALM, occurred primarily in the first 28 days in the FCA-treated population.
Investigators observed expansion in 15 of 17 patients treated with FCA. None of the patients who received FC alone (n=4) had expansion detectable in blood or bone marrow, Dr. Benjamin noted.
“The response we’ve seen in the study so far,” Dr. Benjamin clarified, “is linked to the expansion observed within the first 28-day period.”
UCART cells persisted in three patients beyond day 42. In one patient, they persisted up to day 120.
“Of interest is the T-cell recovery seen in the study,” Dr. Benjamin elaborated. “We only have data from the adult study here—14 patients. And you’ll see that, in the FCA-treated arm (n=11), you have a deeper and more sustained lymphodepletion compared to the FC-treated patients (n=3). And this may play a role in the subsequent UCART19 expansion and disease response.”
Re-dosing
Of the three patients who were re-dosed, two achieved MRD negativity.
One patient achieved MRD-negative status at day 28 but relapsed and received a second infusion 3 months after the first dose. The second expansion was not as deep as the first, but the patient nevertheless achieved MRD negativity after the second dose.
The second patient received FC lymphodepletion and was refractory at day 28.
“The second time around, he received FCA, had a slightly better expansion, and achieved molecular remission,” Dr. Benjamin said.
And the third patient had FCA lymphodepletion but was refractory at day 28.
“We elected to give a second dose at 2.4 months later, but unfortunately, there wasn’t very much expansion, even the second time around, and the patient progressed,” Dr. Benjamin said.
FCA lymphodepletion appears to be required for UCART19 expansion. There was no UCART19 expansion and no response in all four patients lymphodepleted with FC.
The evaluation of UCART19 is ongoing in pediatric and adult B-cell ALL, and “there is a plan for moving into the lymphoma space as well,” Dr. Benjamin added.
Dr. Benjamin disclosed honoraria from Amgen, Takeda, Novartis, Gilead, and Celgene, and research funding from Servier and Pfizer.
Servier and Allogene are supporting the UCART19 trials.
*Data in the abstract differ from the presentation.
Melflufen-dex proves active in multi-resistant MM
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
SAN DIEGO—The combination of melflufen and dexamethasone demonstrated activity in patients with multi-resistant multiple myeloma (MM) in a phase 2 trial.
Melflufen-dexamethasone produced an overall response rate of 33% in patients who had quad- or penta-refractory MM.
The combination was considered well tolerated, although 13% of patients discontinued treatment due to adverse events (AEs).
Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, presented these results, from the HORIZON trial (NCT02963493), at the 2018 ASH Annual Meeting (abstract 600*).
Dr. Richardson reported results with melflufen-dexamethasone in 83 MM patients. They had a median age of 63 (range, 35-86), and 59% were male. Their median time since diagnosis was 6.5 years (range, 0.7-25) at baseline.
The patients had received a median of 5 prior lines of therapy (range, 2-13). All patients were refractory to pomalidomide or daratumumab, and 60% were refractory to both drugs. Eighty percent of patients were refractory to a monoclonal antibody, and 55% were refractory to an alkylator.
Eighty-six percent of patients were refractory to both a proteasome inhibitor and an immunomodulatory drug. Sixty percent of patients were refractory to a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 therapy.
Ninety-three percent of patients were refractory to their last line of therapy. Sixty-nine percent had received a transplant, and 25% had received two transplants.
“[I]f you look at the refractoriness of these patients, 46% had used three or more prior regimens within the last 12 months before entering the trial, and I think that reflects a real challenge in these patients,” Dr. Richardson said.
Results
The patients received melflufen at 40 mg on day 1 of each 28-day cycle and dexamethasone at 40 mg weekly (20 mg for patients age 75 and older). Patients were treated until progression, consent withdrawal, or unacceptable toxicity.
The overall response rate (partial response or better) was 33%, the clinical benefit rate (minimal response or better) was 39%, and 84% of patients had stable disease or better.
Twenty seven patients responded, including one stringent complete response, nine very good partial responses, and 17 partial responses.
Five patients had a minimal response, 37 had stable disease, and 12 progressed. One patient was not evaluable, and one had data pending.
Dr. Richardson noted that melflufen-dexamethasone demonstrated activity regardless of a patient’s underlying refractory status, but serum albumin was a strong predictor of response. Specifically, patients with higher albumin levels were more likely to respond.
“We do not think it’s a mechanism-of-action issue with [melflufen], but we will be evaluating that,” Dr. Richardson said.
He went on to say that the median progression-free survival was 4.0 months overall, 6.4 months among patients with a partial response or better, and 1 month in patients with progressive disease.
Treatment-related grade 3/4 AEs occurred in 75% of patients. This included neutropenia (61%), thrombocytopenia (59%), anemia (25%), febrile neutropenia (6%), leukopenia (5%), lymphopenia (5%), infections and infestations (7%), and pneumonia (2%).
There were no treatment-related deaths. Thirteen percent of patients discontinued treatment due to AEs, most due to thrombocytopenia (8/11).
In closing, Dr. Richardson said toxicity was “generally manageable” with this treatment, which “has promising activity in multi-resistant, relapsed/refractory myeloma.”
He added that, in the phase 3 OCEAN trial (NCT03151811), researchers are comparing melflufen-dexamethasone to pomalidomide-dexamethasone in a less heavily pretreated MM population.
In the phase 1/2 ANCHOR trial (NCT03481556), researchers are testing melflufen-dexamethasone in combination with daratumumab or bortezomib.
Dr. Richardson disclosed relationships with Karyopharm Pharmaceuticals, Bristol-Myers Squibb, Janssen, Amgen, Jazz Pharmaceuticals, Takeda, Celgene, and Oncopeptides AB. The HORIZON trial is supported by Oncopeptides AB in collaboration with Precision Oncology.
*Data in the abstract differ from the presentation.
Study: Few physicians use telemedicine
Just 15% of U.S. physician practices report using telemedicine for patient care, with use of the technology varying widely by specialty.
Carol Kane, director of economic and health policy research, and Kurt Gillis, principal economist, both of the American Medical Association, evaluated the responses of 3,500 physicians about their telemedicine usage through data from the AMA’s 2016 Physician Practice Benchmark Survey. They took into account physicians’ specialty, age, sex, practice setting, and region, as well as the type of telemedicine services employed, if any.
In a research article published in Health Affairs, they found that in 2016, 15% of medical practices used telemedicine for patient interactions – including e-visits and store and forward services – while 11% used the technology to communicate with other health professionals.
Of the primary three telemedicine modalities, physicians used videoconferencing most often (13%), followed by store and forward of data (9%), and remote patient monitoring (7%).
Of specialists, 40% of radiologists, 28% of psychiatrists, and 24% of cardiologists used telemedicine for patient interactions, Ms. Kane and Mr. Gillis found. Emergency physicians were most likely to use telemedicine for interactions with other health professionals (39%), followed by pathologists (30%), and radiologists 26%). On the lower end of the spectrum, 6% of immunologists, 9% of ob.gyns., and 10% of general surgeons used telemedicine for patient care.
Remote patient monitoring was the least used telemedicine modality, with less than 10% of physicians in every broad specialty group using the service, with the exception of internal medicine subspecialties. Cardiologists reported the highest use of remote patient monitoring, followed by nephrologists.
Practice size and setting markedly influenced the practice of telemedicine. Use for patient interactions ranged from 8% among physicians in practices with one to four doctors to 27% among physician practices with at least 50 physicians. Similarly, telemedicine use between physicians and other health care professionals ranged from 4% among doctors in the smallest practice category to 23% in the largest practice category. Physicians in solo practice were less likely to use telemedicine for patient interactions than physicians in single- or multispecialty group practices.
Unsurprisingly, rural physicians were more likely to use telemedicine to consult with other doctors and to use video conferencing with patients than were physicians in metropolitan areas. No significant differences in telemedicine use were observed between physicians in states with parity laws. Such laws generally require that commercial insurers cover and reimburse for telemedicine services as they would for in-person services.
SOURCE: Kane et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05077.
Just 15% of U.S. physician practices report using telemedicine for patient care, with use of the technology varying widely by specialty.
Carol Kane, director of economic and health policy research, and Kurt Gillis, principal economist, both of the American Medical Association, evaluated the responses of 3,500 physicians about their telemedicine usage through data from the AMA’s 2016 Physician Practice Benchmark Survey. They took into account physicians’ specialty, age, sex, practice setting, and region, as well as the type of telemedicine services employed, if any.
In a research article published in Health Affairs, they found that in 2016, 15% of medical practices used telemedicine for patient interactions – including e-visits and store and forward services – while 11% used the technology to communicate with other health professionals.
Of the primary three telemedicine modalities, physicians used videoconferencing most often (13%), followed by store and forward of data (9%), and remote patient monitoring (7%).
Of specialists, 40% of radiologists, 28% of psychiatrists, and 24% of cardiologists used telemedicine for patient interactions, Ms. Kane and Mr. Gillis found. Emergency physicians were most likely to use telemedicine for interactions with other health professionals (39%), followed by pathologists (30%), and radiologists 26%). On the lower end of the spectrum, 6% of immunologists, 9% of ob.gyns., and 10% of general surgeons used telemedicine for patient care.
Remote patient monitoring was the least used telemedicine modality, with less than 10% of physicians in every broad specialty group using the service, with the exception of internal medicine subspecialties. Cardiologists reported the highest use of remote patient monitoring, followed by nephrologists.
Practice size and setting markedly influenced the practice of telemedicine. Use for patient interactions ranged from 8% among physicians in practices with one to four doctors to 27% among physician practices with at least 50 physicians. Similarly, telemedicine use between physicians and other health care professionals ranged from 4% among doctors in the smallest practice category to 23% in the largest practice category. Physicians in solo practice were less likely to use telemedicine for patient interactions than physicians in single- or multispecialty group practices.
Unsurprisingly, rural physicians were more likely to use telemedicine to consult with other doctors and to use video conferencing with patients than were physicians in metropolitan areas. No significant differences in telemedicine use were observed between physicians in states with parity laws. Such laws generally require that commercial insurers cover and reimburse for telemedicine services as they would for in-person services.
SOURCE: Kane et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05077.
Just 15% of U.S. physician practices report using telemedicine for patient care, with use of the technology varying widely by specialty.
Carol Kane, director of economic and health policy research, and Kurt Gillis, principal economist, both of the American Medical Association, evaluated the responses of 3,500 physicians about their telemedicine usage through data from the AMA’s 2016 Physician Practice Benchmark Survey. They took into account physicians’ specialty, age, sex, practice setting, and region, as well as the type of telemedicine services employed, if any.
In a research article published in Health Affairs, they found that in 2016, 15% of medical practices used telemedicine for patient interactions – including e-visits and store and forward services – while 11% used the technology to communicate with other health professionals.
Of the primary three telemedicine modalities, physicians used videoconferencing most often (13%), followed by store and forward of data (9%), and remote patient monitoring (7%).
Of specialists, 40% of radiologists, 28% of psychiatrists, and 24% of cardiologists used telemedicine for patient interactions, Ms. Kane and Mr. Gillis found. Emergency physicians were most likely to use telemedicine for interactions with other health professionals (39%), followed by pathologists (30%), and radiologists 26%). On the lower end of the spectrum, 6% of immunologists, 9% of ob.gyns., and 10% of general surgeons used telemedicine for patient care.
Remote patient monitoring was the least used telemedicine modality, with less than 10% of physicians in every broad specialty group using the service, with the exception of internal medicine subspecialties. Cardiologists reported the highest use of remote patient monitoring, followed by nephrologists.
Practice size and setting markedly influenced the practice of telemedicine. Use for patient interactions ranged from 8% among physicians in practices with one to four doctors to 27% among physician practices with at least 50 physicians. Similarly, telemedicine use between physicians and other health care professionals ranged from 4% among doctors in the smallest practice category to 23% in the largest practice category. Physicians in solo practice were less likely to use telemedicine for patient interactions than physicians in single- or multispecialty group practices.
Unsurprisingly, rural physicians were more likely to use telemedicine to consult with other doctors and to use video conferencing with patients than were physicians in metropolitan areas. No significant differences in telemedicine use were observed between physicians in states with parity laws. Such laws generally require that commercial insurers cover and reimburse for telemedicine services as they would for in-person services.
SOURCE: Kane et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05077.
FROM HEALTH AFFAIRS
Key clinical point: Most U.S. physicians do not use telemedicine for patient care.
Major finding: In 2016, 15% of medical practices used telemedicine to treat patients, while 11% of practices used it to consult with other health professionals.
Study details: A study of 3,500 physicians and their responses to telemedicine questions through data from the American Medical Association’s 2016 Physician Practice Benchmark Survey
Disclosures: The researchers reported no relevant conflicts of interest.
Source: Kane C et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05077
Review of Common Clinical Conditions of the Proximal Tibiofibular Joint
ABSTRACT
Current literature is limited with respect to the proximal tibiofibular joint (PTFJ) and clinical conditions relating to the PTFJ. Diagnosis and treatment of conditions that affect the PTFJ are not well described and are a topic of debate among many physicians. This manuscript aims to review and summarize the most recent literature that relates to traumatic dislocations, fractures, chronic instability, and osteoarthritis, with a focus on both diagnostic and treatment strategies of these conditions. We also review PTFJ anatomy, biomechanics, and the clinical presentation of some common PTFJ conditions.
Continue to: Clinical conditions...
Clinical conditions of the proximal tibiofibular joint (PTFJ) are an uncommon source of lateral knee complaints and are often overlooked in the differential diagnosis as a source of the knee complaint. The most common conditions of the PTFJ include traumatic dislocations, fractures, chronic instability, and osteoarthritis. This article reviews the most common diseases affecting this joint and discusses both diagnostic and treatment strategies in an attempt to raise awareness of this joint as a source of lateral knee complaints.
ANATOMY
The PTFJ is an arthrodial synovial joint between the posterolateral surface of the tibia and the proximal fibular head.1 Surrounding the synovial membrane of the articulation is a fibrous joint capsule with distinct anterior and posterior tibiofibular ligaments.2,3 The anterior tibiofibular ligament has been described as 1 or 2 bands whereas the posterior ligament consists of 1 band.3 The anterior ligament attaches anteroinferiorly to the fibular styloid and posteriorly to Gerdy’s tubercle on the tibia. It runs linearly from posterior to anterior, and the fibular footprint is immediately anterior to the insertion of the biceps femoris. The posterior ligament is located inferior to the lateral joint space, and the fibular footprint is posterior to the insertion of the biceps femoris.3 Anatomy of the PTFJ is shown schematically in Figure 1.
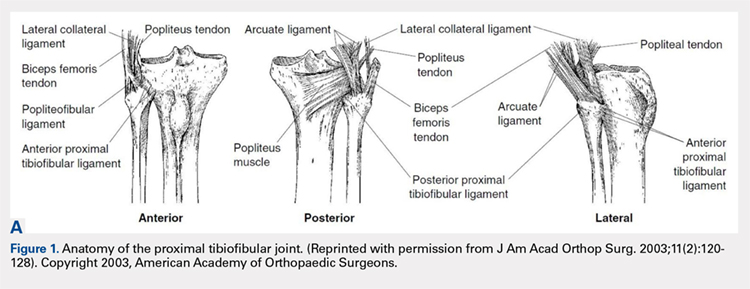
Both the lateral collateral ligament (LCL) and the tibiofibular interosseous membrane add stability to the PTFJ. The LCL travels from the lateral femoral epicondyle to the lateral side of fibula head, anterior to the fibular styloid. The interosseous membrane extends obliquely between the borders of the tibia and fibula. Additionally, the short head of the biceps femoris, fibular collateral ligament, fabellofibular ligament, popliteofibular ligament, and popliteus muscle all attach to the PTFJ and provide additional stability to the joint.
It is important to note that the common peroneal nerve passes posteriorly over the fibula neck, can be involved in the clinical presentation, and is a potential source of concern with any injury to or surgery on the joint.4
Many studies have demonstrated that a communication with the tibiofemoral joint exists through the subpopliteal recess, but the rate of communication has varied widely.5-8 Most recently, Bozkurt and colleagues5 found the rate of communication between the PTFJ and lateral femorotibial space to be 57.9%. When distinct communication exists, the PTFJ must be considered as a fourth compartment of the knee and is subject to any process that affects the knee joint proper.
Continue to: Ogden described 2 types...
Ogden1 described 2 types of PTFJs, horizontal and oblique, with the latter being considered less stable because of less rotational mobility. The horizontal configuration is defined as <20° of inclination of joint surface in relation to the horizontal plane, and the oblique variation is defined as >20° of inclination of the joint surface in relation to the horizontal plane.1
BIOMECHANICS AND FUNCTION
The primary function of the PTFJ is to dissipate torsional loads applied to the ankle, attenuate lateral tibial bending moments, and transmit axial loads from weight bearing on the extremity.1 The degree of knee flexion, ankle dorsiflexion, and tibial rotation all play an important role in PTFJ biomechanics. In knee flexion, the proximal fibula moves anteriorly because of the relative laxity of the LCL and biceps femoris tendons. In knee extension, the LCL and biceps femoris tighten, pulling the proximal fibula posteriorly.1 Because the LCL and biceps tendon are both relaxed and less supportive during knee flexion, the PTFJ is more prone to injury with a flexed knee. The ankle plays an important role in the biomechanics of the PTFJ because it contains the distal syndesmosis, where both the tibia and fibula are firmly attached distally. During ankle dorsiflexion, the fibula must externally rotate to accommodate a wider anterior talus.1 In regard to tibia rotation, Scott and colleagues9 demonstrated the relationship between tibial rotation and fibular translation. With internal tibial rotation, the fibular head translated posteriorly and with external tibial rotation, the fibula translated anteriorly. The greatest translational motion was seen during loading of the knee into varus and during external tibial rotation at all flexion angles.
CLINICAL CONDITIONS
Ogden10 classified instability of the PTFJ into 4 main groups: anterolateral dislocation, posteromedial dislocation, superior dislocation, and atraumatic subluxation. Injury to the PTFJ usually occurs in younger, athletic patients during sports that require violent twisting motions such as soccer, basketball, dance, skiing, horseback riding, parachute jumping, jet skiing, and judo. Patients with generalized ligamentous laxity have been described as at increased risk for joint instability.10,11
ACUTE DISLOCATION
The most common injury to the PTFJ is an anterolateral dislocation and involves injury to both the anterior and posterior capsular ligaments, and occasionally the LCL.10 Anterolateral dislocation is usually the result of a fall on a hyperflexed knee with the foot inverted and plantarflexed.11 While most anterolateral dislocations are the result of indirect sports trauma, several have been associated with other types of skeletal injuries such as fracture-dislocation of the hip, crush injury of the proximal and distal ends of the tibia, fracture-dislocation of the ankle, proximal tibial fracture, and fracture-dislocation of the distal femoral epiphysis.10 Ogden10 described the mechanism as follows: (1) sudden inversion and plantar flexion of the foot causing tension in the peroneal muscle group, extensor digitorum longus, and extensor halluces longus, which applies a forward dislocating force to the proximal end of the fibula; (2) simultaneous flexion of the knee, relaxing the biceps tendon and LCL; and (3) twisting of the body over the knee, transmitting energy along the femur to the tibia, exerting a relative external rotatory torque of the tibia on the foot, which is already fixed in inversion. Steps (2) and (3) spring the proximal end of the fibula laterally while the contracting muscles of (1) pull the fibula anteriorly.
Posteromedial dislocation is the second most common type of acute PTFJ dislocation. Posteromedial dislocations usually involve direct trauma and are associated with peroneal nerve injuries.1,2,10 The mechanism of dislocation results in tearing of the anterior and posterior PTFJ capsular ligaments, followed by injury to the LCL and other surrounding ligaments. This allows the biceps femoris to draw the unsupported proximal part of the fibula posteromedially along the posterolateral tibial metaphysis.7
Continue to: Superior dislocations are...
Superior dislocations are the least frequent form of acute PTFJ dislocations and are associated with high-energy ankle injuries.2,10,12 Superior dislocation results in injury to the interosseous membrane between the tibia and fibula and is frequently associated with tibial shaft fracture.10,11 Atraumatic, acquired superior dislocation of the PTFJ has also been associated with congenital dislocation of the knee.10,11
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ classically involves excessive and symptomatic anterior-posterior motion without actual dislocation of the joint.11 Subluxations of the PTFJ typically occur without any known trauma or injury and are most frequently associated with benign hyperlaxity syndrome, Ehlers-Danlos syndrome, or muscular dystrophy.4,10
Semonian and colleagues13 suggest that subluxation of the PTFJ is not given enough recognition in the literature and that instability should not be considered a rare condition. They hypothesize that many patients have joints that do not tolerate increases in fibula rotation secondary to subclinical trauma, repetitive overuse, or biomechanical variation of the joint. Semonian and associates13 state that the condition begins with the anterior capsule and anterior tibiofibular ligament attenuation as a result of excessive fibular rotation. Once stretched, the functional pull of the biceps femoris and soleus maintain the fibula in a relatively posterior and externally rotated position. Furthermore, Ogden10 found that 70% of the dislocations and subluxations he studied were of the oblique variant compared with that of the horizontal variant. Many authors suggest that the oblique variant is more at risk for injury because of its decreased joint surface area causing decreased rotational mobility.1,2,10
Early recognition is extremely important in dislocations and subluxations of the PTFJ as undiagnosed acute trauma can turn into chronic subluxation, and chronic subluxation may lead to dislocation.13 Additionally, chronic subluxation or dislocation are thought result in osteoarthritis of the PTFJ.14
OSTEOARTHRITIS
The literature on osteoarthritis of the PTFJ is limited. Eichenblat and Nathan7 studied the PTFJ in cadavers and dry bones and found that 28% had evidence of osteoarthritis. Clinically, however, osteoarthritis of the PTFJ is a rare primary diagnosis, suggesting that involvement of the PTFJ is either asymptomatic or that symptoms are associated with osteoarthritis of the knee joint. Boya and colleagues15 and Eichenblat and Nathan7 both found a high correlation between the presence of osteoarthritis of the PTFJ and osteoarthritis of the tibiofemoral joint in cadavers. The authors suggest this correlation may be related to the presence of anatomical communication between the 2 joints. Theoretically, inflammatory mediators flow freely between the joint spaces and contribute to arthritis in both joints. The possibility of degenerative arthritis of the PTFJ accompanying degenerative arthritis of the knee warrants evaluation, especially in patients considering total knee arthroplasty. Unrecognized arthritis of the PTFJ might influence outcome scores and be an unsolved source of lateral knee pain post-knee replacement.16
Continue to: CLINICAL PRESENTATION
CLINCIAL PRESENTATION
ACUTE DISLOCATION
Patients with acute PTFJ dislocation present with pain, tenderness, swelling, and asymmetry of the lateral side of the knee, while the knee joint is not swollen and range of knee motion is not limited.17 A bony prominence might be felt, and the biceps femoris tendon can often appear to be tense.13 Active or passive ankle movements often exacerbate the lateral knee pain.11 It is also important to examine the peroneal nerve, as transient peroneal palsy has been described in all types of PTFJ dislocations but most often with posteromedial dislocations. Sensory disturbance in the peroneal nerve distribution is more common than motor loss, but foot drop is also a potential presenting sign.11 On examination, palpation of the fibular head illustrates tenderness and aggravates the pain.
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ can be difficult to recognize because the history, signs, and symptoms of lateral knee pain can be subtle and sometimes misleading. In addition, current literature provides little information on specific tests, measurements, signs, or subjective information regarding subluxation. Patients rarely reveal a history of trauma or mechanism of injury. Subluxations are often associated with patients participating in repetitive sports requiring running, jumping, or twisting movements, and can be present bilateral. Instability has also been described in patients with osteomyelitis, rheumatoid arthritis, septic arthritis, pigmented villonodular synovitis, below-knee amputations, osteochondroma, and in runners who recently increased mileage (especially during the first 2-3 miles and during downhill running).11,13 Patients normally do not have difficulty with activities of daily living, but symptoms may arise when making movements with a sudden change of direction.11
These patients usually complain of instability of the knee and pain along the lateral aspect of the knee. Pain radiating proximally into the region of the iliotibial band and medially into the patellofemoral joint can be seen.13 Patients may also report clicking, popping, or catching of the lateral knee; while others will report a sense of giving way of the knee joint.11,13 Progressive peroneal nerve symptoms are usually seen in older patients; however, they are more common with acute PTFJ dislocations as discussed.13
A clinical method for examining a PTFJ with possible subluxation or chronic instability has been described by Sijbrandij.18 With the patient in the supine position, the knee is flexed to 90° to relax the LCL and biceps femoris tendon. The fibular head is then held between the thumb and index finger, and moved anteriorly and laterally. Dislocation or subluxation will be felt and visualized as the fibular head translates, and should be compared with the uninjured PTFJ. On release, the fibular head will return to its normal position, often with a click. Asking the patient if this subluxation/reduction maneuver reproduces the symptoms or causes apprehension or pain may also be helpful.18 Another method for examination is eliciting the Radulescu sign.11,13 While the patient lies prone, the examiner stabilizes the thigh with 1 hand while the knee is flexed to 90°. The examiner then applies an internal rotation force on the lower leg. Observing an abnormal excursion of the fibular head in an anterior and lateral direction represents a positive test.11,13
OSTEOARTHRITIS
Clinical evaluation for osteoarthritis in the PTFJ is not well described in the literature. A single report describes applying manual pressure over the fibular head during active ankle motion.16 A test known as the grinding test is used as a sign to detect the involvement of the PTFJ as a component of osteoarthritis of the knee. A positive test will elicit pain and/or tenderness of the joint.16
Continue to: DIAGNOSTIC IMAGING
DIAGNOSTIC IMAGING
Plain radiographs in the anteroposterior (AP) and true lateral views are useful as first-line investigations in suspected PTFJ dislocation. Comparable AP and lateral radiographs of each knee are highly recommended to detect findings that suggest dislocation.2 Abnormal findings include increased interosseous space, medial or lateral displacement of the fibula on the anteroposterior view, and anterior or posterior displacement of the fibula head on lateral view as shown in Figure 2.19,20
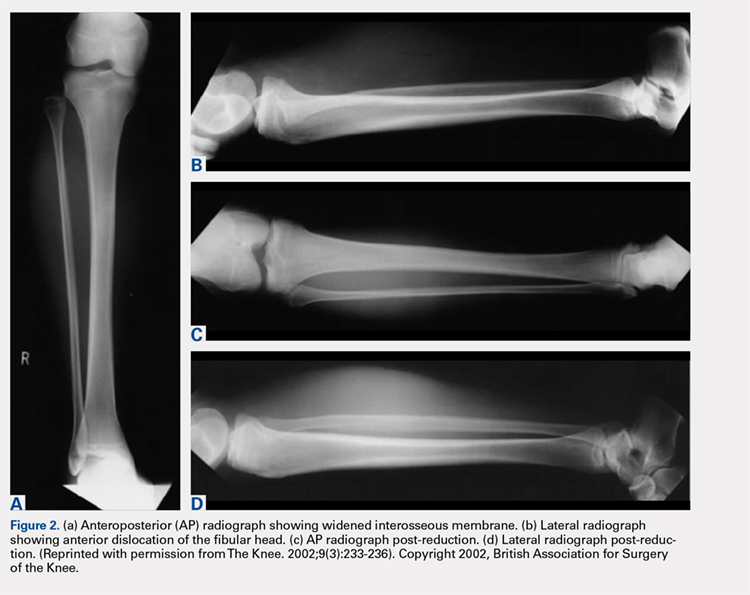
Resnick and colleagues2 proposed the use of the linear sloping radiodensity that defines the posteromedial corner of the lateral tibial condyle as an indicator of anterolateral or posteromedial PTFJ dislocation. However, this application is limited because of the PTFJ’s highly variable morphology.8 In a recent study conducted by Hey and colleagues,21 5968 (2984 patients) knee radiographs were retrospectively collected and subjected to radiographical measurements and statistical analysis. The tibiofibular overlap method had a specificity of 94.1% and 84.5% when diagnosing PTFJ dislocations on the AP and lateral views, respectively.21
If a diagnosis of PTFJ is suspected but not clearly established based on radiography, computed tomography with comparison views of the contralateral knee are recommended to confirm the diagnosis.17,22 This becomes more critical in cases of suspected subluxation/chronic PTFJ instability. Additionally, magnetic resonance imaging (MRI) can be used to assess chronic PTFJ instability. Recently, Burke and colleagues23 performed a 10-year retrospective case series that included 7 patients with chronic PTFJ instability and included MRI as part of their evaluation. The MRI abnormalities in these patients included periarticular soft tissue edema, including in the proximal soleus muscle (n = 5), periarticular ganglion or ganglia (n = 4), tibiofibular ligament edema (n = 4), subchondral marrow edema (n = 3), posterior tibiofibular ligament thickening (n = 2), subcortical cyst at a ligament insertion (n = 2), partial-thickness tear of the anterior tibiofibular ligament (n = 1), and tibiofibular joint effusion (n = 1).
OSTEOARTHRITIS
Routine knee radiographs can show PTFJ joint space narrowing, sclerosis, marginal osteophytes, and local osteopenia as conventional components of osteoarthritis of any joint. Serial radiographs have also been described as effective in evaluating progressive degenerative changes of the PTFJ.14 An MRI will show osteophyte formation, subchondral cysts, subchondral sclerosis, joint effusion, joint space narrowing, and is highly sensitive for detecting degenerative changes in cartilage, as well as identifying other possible pathologies such as synovial cysts or pigmented villonodular synovitis. Chronic PTFJ instability appears to predispose to tibiofibular osteoarthritis as reported by Burke and colleagues,23 who found a particularly high incidence (42.9%) of osteoarthritis in patients with chronic PTFJ instability. Additionally, Veth and colleagues14 found degenerative changes in 8 of 19 patients presenting with PTFJ dislocations.
TREATMENT
ACUTE DISLOCATION
Prompt recognition and treatment of any acute PTFJ dislocation are necessary to avoid long-term instability and other possible sequelae.11 Treatment consists of reduction followed by restriction of weight-bearing.11 Traditionally, the knee is immobilized with a cast in extension for 3 to 4 weeks followed by knee mobilization and progressive range of motion exercises,24 but there is some controversy regarding complete immobilization.11,25,26
Continue to: Initially, closed reduction...
Initially, closed reduction is advised as the treatment for acute PTFJ dislocation.11,19,24 It involves placing the knee in 80°to 110° of flexion to relax the biceps femoris and LCL, then applying an appropriate force to the fibular head in a direction opposite the displacement.11,24 An audible pop is often heard as the fibula reduces back into normal alignment. Stability of the reduction and stability of the knee should be determined with respect to both posterolateral structures and the LCL after reduction.11 Anterolateral dislocations are usually easier to reduce, as a posteromedial or superior dislocation can result in the fibular head being perched on the lateral tibial ridge, and held by the LCL.11
Calabró and colleagues27 described a new, simple, and safe alternative technique of closed reduction of an anterior dislocation if the classical method fails. This technique relies on ligamentotaxis and a dynamic counteraction between muscles and ligaments to reduce the joint. The patient flexes the knee >90° while the physician applies a counterforce to the heel with the palm. Simultaneously, gentle direct pressure should be applied to the fibular head to move it toward the lateral tibial ridge. With a relaxed LCL, the biceps femoris tendon will actively reduce the proximal fibular head back into its correct anatomic orientation.27
When a closed reduction in an awake patient has failed, a reduction under sedation or anesthesia should be performed. If that fails, an open reduction should be performed. Following an open reduction, the joint is stabilized with Kirschner wires, bioabsorbable pins, or cortical screws.24-26,28 The torn capsule and any injured ligaments should also be primarily repaired. After approximately 6 weeks, the stabilization hardware may be removed.24,25
Acute posteromedial dislocations are treated similarly to anterolateral dislocations; however, open reduction and repair of the capsule and ligaments are more frequently required.10 Superior dislocations are also more frequently reduced by open methods, sometimes dictated by open treatment of associated tibia or ankle fracture.10 Damage to any structure of the posterolateral knee as a result of acute PTFJ injury should be repaired, as this has been associated with better outcomes.14
PTFJ dislocation and tibia fracture can occur together, and in a retrospective study conducted by Herzog and colleagues29 the authors recorded the incidence of PTFJ dislocation as 1.5% of operative tibial shaft fractures and 1.9% of operative tibial plateau fractures. Haupt and colleagues30 also conducted a retrospective study in which the authors recorded the incidence of PTFJ dislocations in 1.06% of all tibial shaft fractures in their series. In both studies combined, all except 1 PTFJ dislocation had been caused by high-energy trauma.29,30 In the case of PTFJ dislocation with tibial shaft fracture, intramedullary nailing of the tibial shaft fracture followed by open reduction of the PTFJ with 1 or 2 positioning screws just below the PTFJ has yielded satisfactory results.30 The positioning screw should be removed 6 weeks post-operation to prevent PTFJ arthrodesis and patients should be supported in full weight-bearing.30 An illustrative case report from our institution is shown in Figure 3.
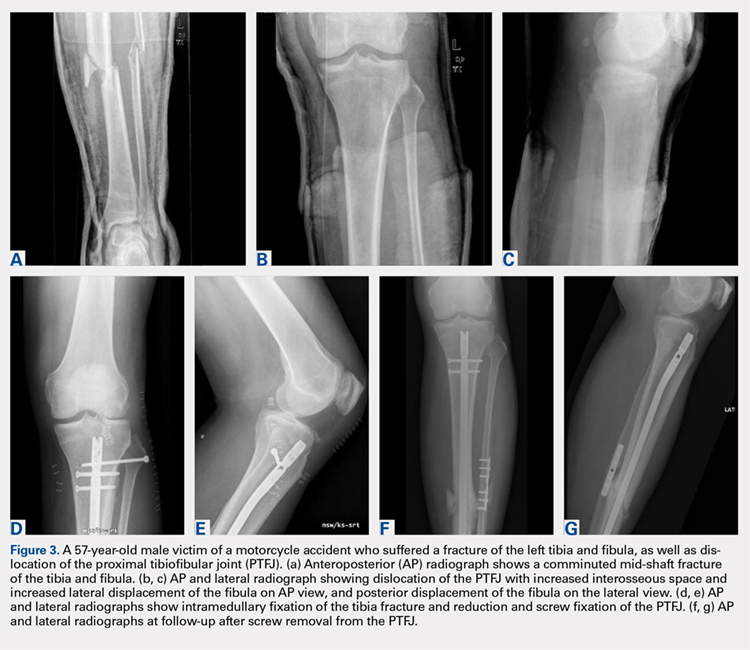
Continue to: CHRONIC INSTABILITY
CHRONIC INSTABILITY
Chronic instability is commonly the result of untreated or misdiagnosed subluxation of the PTFJ. Ogden10 reported that 57% of patients with acute proximal tibiofibular dislocations required surgery for ongoing symptoms after treatment failure with closed reduction and 3 weeks of immobilization. The first step in the management of chronic instability of the PTFJ is usually a nonoperative approach. Immobilization, activity level modification, utilization of a supportive strap placed 1 centimeter below the fibular head for pain relief, and participation in a strength-training program of the lower leg are initial treatment recommendations.10,11,13 Many patients with chronic PTFJ instability do not respond to conservative treatment and may pursue surgical intervention. Surgical treatment options include permanent arthrodesis, resection of the fibular head, soft tissue reconstruction, and temporary fixation.10,13,26,31 Given the rare nature of the injury and lack of data on varying treatments, there is no clear consensus on the optimal surgical procedure.
Arthrodesis and fibular head resection are 2 traditional methods of surgically addressing the PTFJ, but both have limitations that need to be recognized. Arthrodesis involves clearing the PTFJ of all articular cartilage, bone grafting, and then reducing the joint using screw fixation.11 Rigid fixation prevents rotation of the fibula which puts additional stress on the ankle, frequently causing pain and instability of the ankle joint.10,11 The other traditional surgical option, fibular head resection, involves excision of the head and neck of the fibula while preserving the fibular styloid and LCL.4 Fibular head resection is indicated when peroneal nerve symptoms or palsy occur in PTFJ injuries.4,10 Unfortunately, resection may disrupt the posterolateral corner structures of the knee and result in pain and instability.3,13 As a general rule, it is advisable to avoid arthrodesis and fibular head resection in children and athletes because of the length of time during which complications can develop.11
Van den Bekerom and colleagues26 suggest that their technique of temporary fixation of the PTFJ using a cancellous screw yields satisfactory outcomes in treating chronic PTFJ instability. The method entails having the ankle dorsiflexed and the head of the fibula slightly externally rotated and reduced into the most stable position. A hole is drilled in the anteromedial direction from posterior fibula head into the tibia. A non-tapped cortical screw is used to fix the fibula head in the reduced position.26 The screw is removed after 3 to 6 months. Seven of 8 patients treated with this technique by Van den Bekerom and colleagues26 have had alleviation of symptoms, although the screws broke in 2 cases before their planned removal.
Soft tissue reconstruction of the PTFJ has been an evolving area of treatment. Several techniques using a variety of tissue grafts and fixation methods have been described. Giachino32 proposed a reconstruction in which the fibular head is stabilized with a strip of ipsilateral biceps femoris tendon still attached distally to the fibular head and deep fascia of the leg. Drill holes are made in the tibia, and the tissue is secured anteriorly to the fascia with a suture. Shapiro and colleagues33 performed a stabilization using a strip of the iliotibial band still connected to its insertion on Gerdy’s tubercle. The graft was tunneled through a tibial drill hole from anterior to posterior and through the fibular head from posterior to anterior before suturing back onto itself. Another technique described by Mena and colleagues34 uses a split biceps tendon autograft, harnessing the strong attachments of the tendon to the fibular head to stabilize the reduced PTFJ.
More recently, Miller35 employed elements of the techniques described by Gianchino,32 Shapiro and colleagues,33 and Mena and colleagues34 to develop a method involving the biceps femoris tendon and iliotibial band in a soft tissue reconstruction. The technique involves harvesting the biceps femoris tendon while preserving the distal insertion and harvesting the iliotibial band while leaving the distal insertion at the Gerdy’s tubercle intact.35 The biceps tendon is passed from posterior to anterior through a tibial bone tunnel, and the iliotibial band graft is passed through the same tunnel but in the anterior to posterior direction. A bioabsorbable interference screw is placed into the tibial tunnel from anterior to posterior. The remaining biceps tendon graft is sewn into the soft tissue of the anterior tibia. The tail of the iliotibial band graft is passed through the fibular bone tunnel in the posterior to anterior direction and looped around the fibular head and under the LCL before being sewn back onto itself.35 Kobbe and colleagues36 described a surgical technique using the ipsilateral semitendinosis tendon while Maffulli and colleagues37 and Morrison and colleagues38 presented techniques using the ipsilateral gracilis tendon. These techniques do not jeopardize lateral knee or fibular head stability by avoiding the use of the biceps femoris and iliotibial band, as well as local knee stabilizers. Warner and colleagues31 proposed a similar technique to the aforementioned using a semitendinosus tendon; however, they do not reconstruct the anterior ligamentous structures, as seen in Figures 4A and 4B.39 The authors have concerns that a 2-limb reconstruction as described by Kobbe and colleagues36 and Morrison and colleagues38 may be prone to overconstraint or other errors in tensioning because it does not allow for differential tension to be applied to each limb.31 According to Warner and colleagues,31 the anterior structures of their patients with chronic PTFJ instability have appeared normal, and isolated posterior ligamentous reconstruction has been adequate for the restoration of stability. Camarda and colleagues28 proposed a technique very similar to that of Warner and colleagues;31 however, the tibial tunnel is reamed from posterolateral to the anteromedial aspect of the tibia, exploiting the skin incision previously used for tendon harvest.
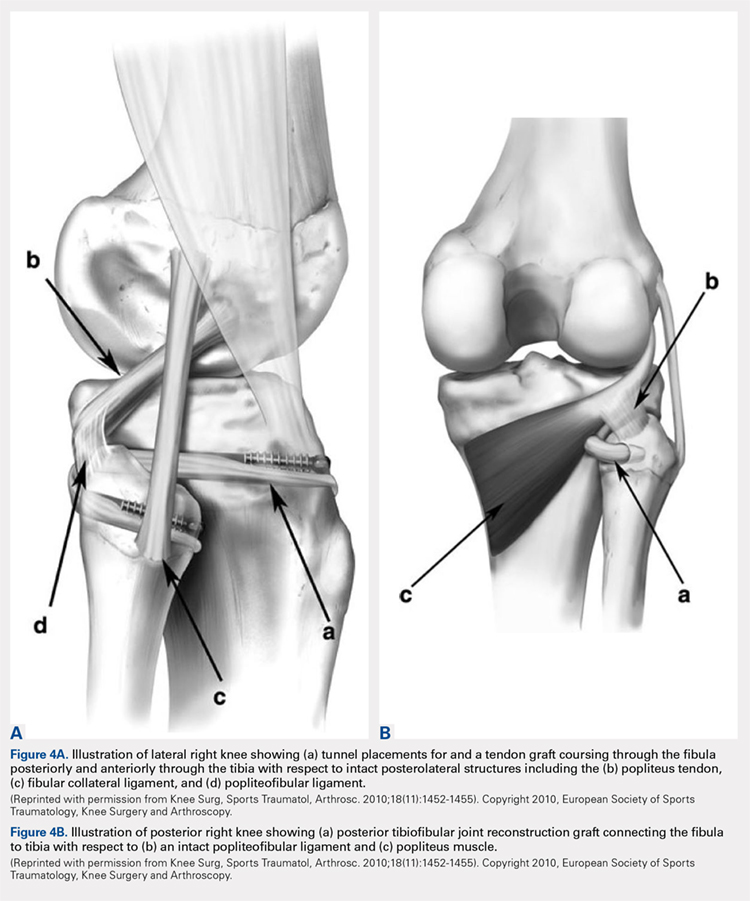
Continue to: ARTHRITIS
ARTHRITIS
The management options for secondary arthritis due to chronic PTFJ instability have rarely been discussed in the literature. Arthrodesis or fibular head resection are options for the treatment of arthritis, and the above discussion applies here as well. Yaniv and colleagues40 describe a technical procedure for addressing both instability and secondary joint arthritis. The authors performed a ligament reconstruction of the PTFJ using the anterior part of the biceps femoris combined with interpositional joint arthroplasty using a vascularized fascia lata strip. Two weeks post-operation, the patient was walking with full weight-bearing and 6 weeks post-operation the first sports activities were allowed.40
CONCLUSION
Conditions affecting the PTFJ, their diagnosis, and treatment are infrequent topics of discussion in the literature. While PTFJ injury, instability, and other disease states are admittedly rare, clinicians need to include them in the differential diagnosis of patients presenting with lateral knee complaints. Diagnostic imaging is a critical component in early identification of PTFJ conditions to prevent long-term complications. Most injuries are treated first with conservative methods, reserving surgery as an option when first-line measures are unsuccessful. Advancements in surgical options for dislocation and subluxation/chronic instability of the joint have been made, but further research on their effectiveness and long-term outcomes is needed before a gold-standard treatment can be determined.
- Ogden JA. The anatomy and function of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):186-191.
- Resnick D, Newell JD, Guerra J, Jr., Danzig LA, Niwayama G, Goergen TG. Proximal tibiofibular joint: anatomic-pathologic-radiographic correlation. AJR Am J Rroentgenol. 1978;131(1):133-138. doi: 10.2214/ajr.131.1.133.
- See A, Bear RR, Owens BD. Anatomic mapping for surgical reconstruction of the proximal tibiofibular ligaments. Orthopedics. 2013;36(1):e58-63. doi: 10.3928/01477447-20121217-19.
- Ogden JA. Subluxation of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):192-197. doi: 10.2106/00004623-197456010-00015
- Bozkurt M, Yilmaz E, Akseki D, Havitcioglu H, Gunal I. The evaluation of the proximal tibiofibular joint for patients with lateral knee pain. Knee. 2004;11(4):307-312. doi: 10.1016/j.knee.2003.08.006
- Dirim B, Wangwinyuvirat M, Frank A, et al. Communication between the proximal tibiofibular joint and knee via the subpopliteal recess: MR arthrography with histologic correlation and stratigraphic dissection. AJR Am J Roentgenol. 2008;191(2):W44-W51. doi: 10.2214/AJR.07.3406.
- Eichenblat M, Nathan H. The proximal tibio fibular joint. An anatomical study with clinical and pathological considerations. Int Orthop. 1983;7(1):31-39. doi: 10.1007/bf00267557
- Espregueira-Mendes JD, da Silva MV. Anatomy of the proximal tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):241-249. doi: 10.1007/s00167-005-0684-z.
- Scott J, Lee H, Barsoum W, van den Bogert AJ. The effect of tibiofemoral loading on proximal tibiofibular joint motion. J Anat. 2007;211(5):647-653. doi: 10.1111/j.1469-7580.2007.00803.x.
- Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154. doi: 10.2106/00004623-197456010-00015
- Sekiya JK, Kuhn JE. Instability of the proximal tibiofibular joint. J Am Acad Orthop Surg. 2003;11(2):120-128. doi: 10.5435/00124635-200303000-00006
- Horan J, Quin G. Proximal tibiofibular dislocation. Emerg Med Jl : EMJ. 2006;23(5):e33. doi: 10.1136/emj.2005.032144.
- Semonian RH, Denlinger PM, Duggan RJ. Proximal tibiofibular subluxation relationship to lateral knee pain: a review of proximal tibiofibular joint pathologies. J Orthop Sports Phys Ther. 1995;21(5):248-257. doi: 10.2519/jospt.1995.21.5.248.
- Veth RP, Kingma LM, Nielsen HK. The abnormal proximal tibiofibular joint. Arch Orthop Trauma Surg. 1984;102(3):167-171. doi: 10.1007/bf00575227
- Boya H, Ozcan O, Oztekin HH. Radiological evaluation of the proximal tibiofibular joint in knees with severe primary osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2008;16(2):157-159. doi: 10.1007/s00167-007-0442-5.
- Öztuna V, Yildiz A, Özer C, Milcan A, Kuyurtar F, Turgut A. Involvement of the proximal tibiofibular joint in osteoarthritis of the knee. The Knee. 2003;10(4):347-349. doi: 10.1016/s0968-0160(03)00004-8
- Milankov M, Kecojević V, Gvozdenović N, Obradović M. Dislocation of the proximal tibiofibular joint. Med Pregl. 2013;66(9-10):387-391. doi: 10.2298/mpns1310387m
- Sijbrandij S. Instability of the proximal tibio-fibular joint. Acta Orthop Scand. 1978;49(6):621-626. doi: 10.3109/17453677808993250
- Aladin A, Lam KS, Szypryt EP. The importance of early diagnosis in the management of proximal tibiofibular dislocation: a 9- and 5-year follow-up of a bilateral case. Knee. 2002;9(3):233-236. doi: 10.1016/S0968-0160(02)00012-1
- Turco VJ, Spinella AJ. Anterolateral dislocation of the head of the fibula in sports. Am J Sports Med. 1985;13(4):209-215. doi: 10.1177/036354658501300401.
- Hey HW, Ng LW, Ng YH, Sng WZ, Manohara R, Thambiah JS. Radiographical definition of the proximal tibiofibular joint - A cross-sectional study of 2984 knees and literature review. Injury. 2016;47(6):1276-1281. doi: 10.1016/j.injury.2016.01.035.
- Voglino JA, Denton JR. Acute traumatic proximal tibiofibular joint dislocation confirmed by computed tomography. Orthopedics. 1999;22(2):255-258.
- Burke CJ, Grimm LJ, Boyle MJ, Moorman CT, 3rd, Hash TW, 2nd. Imaging of Proximal Tibiofibular Joint Instability: A 10 year retrospective case series. Clin Imaging. 2016;40(3):470-476. doi: 10.1016/j.clinimag.2015.12.011.
- Parkes JC II, Zelko RR. Isolated acute dislocation of the proximal tibiofibular joint. Case report. J Bone Joint Surg Am. 1973;55(1):177-183. Doi: 10.2106/00004623-197355010-00019
- Gvozdenović N, Gvozdenović K, Obradović M, Stanković M. Modified technique of the treatment for proximal tibiofibular joint dislocation. Vojnosanitetski Pregled. 2017;74(3):282-286. doi: 10.2298/VSP150318177G
- van den Bekerom MP, Weir A, van der Flier RE. Surgical stabilisation of the proximal tibiofibular joint using temporary fixation: a technical note. Acta Orthop Belg. 2004;70(6):604-608.
- Calabró T, Cevolani L, Chehrassan M, Gasbarrini A. A new technique of reduction for isolated proximal tibiofibular joint dislocation: a case report. Eur Rev Med Pharmacol Sci. 2014;18(1):93-95.
- Camarda L, Abruzzese A, D'Arienzo M. Proximal tibiofibular joint reconstruction with autogenous semitendinosus tendon graft. Tech Orthop. 2013;28(3):269-272. doi. 10.1097/BTO.0b013e31827b7182
- Herzog GA, Serrano-Riera R, Sagi HC. Traumatic Proximal Tibiofibular Dislocation: A Marker of Severely Traumatized Extremities. J Orthop Trauma. 2015;29(10):456-459. doi: 10.1097/BOT.0000000000000348.
- Haupt S, Frima H, Sommer C. Proximal tibiofibular joint dislocation associated with tibial shaft fractures - 7 cases. Injury. 2016;47(4):950-953. doi: 10.1016/j.injury.2016.01.037.
- Warner BT, Moulton SG, Cram TR, LaPrade RF. Anatomic Reconstruction of the Proximal Tibiofibular Joint. Arthrosc Tech. 2016;5(1):e207-e210. doi: 10.1016/j.eats.2015.11.004
- Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106. doi: 10.2106/00004623-198668070-00023
- Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
- Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671. doi: 10.1053/jars.2001.22359.
- Miller T. New technique of soft tissue reconstruction for proximal tibiofibular joint instability using iliotibial band and biceps femoris longhead autograft. Tech Orthop. 2014;29(4):243-247. doi: 10.1097/BTO.0000000000000046
- Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
- Maffulli N, Spiezia F, Oliva F, Testa V, Capasso G, Denaro V. Gracilis autograft for recurrent posttraumatic instability of the superior tibiofibular joint. Am J Sports Med. 2010;38(11):2294-2298. doi: 10.1177/0363546510373472.
- Morrison TD, Shaer JA, Little JE. Bilateral, atraumatic, proximal tibiofibular joint instability. Orthopedics. 2011;34(2):133. doi: 10.3928/01477447-20101221-28.
- Horst PK, LaPrade RF. Anatomic reconstruction of chronic symptomatic anterolateral proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1452-1455. doi: 10.1007/s00167-010-1049-9.
- Yaniv M, Koenig U, Imhoff AB. A technical solution for secondary arthritis due to chronic proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):334-336. doi: 10.1007/s001670050173.
ABSTRACT
Current literature is limited with respect to the proximal tibiofibular joint (PTFJ) and clinical conditions relating to the PTFJ. Diagnosis and treatment of conditions that affect the PTFJ are not well described and are a topic of debate among many physicians. This manuscript aims to review and summarize the most recent literature that relates to traumatic dislocations, fractures, chronic instability, and osteoarthritis, with a focus on both diagnostic and treatment strategies of these conditions. We also review PTFJ anatomy, biomechanics, and the clinical presentation of some common PTFJ conditions.
Continue to: Clinical conditions...
Clinical conditions of the proximal tibiofibular joint (PTFJ) are an uncommon source of lateral knee complaints and are often overlooked in the differential diagnosis as a source of the knee complaint. The most common conditions of the PTFJ include traumatic dislocations, fractures, chronic instability, and osteoarthritis. This article reviews the most common diseases affecting this joint and discusses both diagnostic and treatment strategies in an attempt to raise awareness of this joint as a source of lateral knee complaints.
ANATOMY
The PTFJ is an arthrodial synovial joint between the posterolateral surface of the tibia and the proximal fibular head.1 Surrounding the synovial membrane of the articulation is a fibrous joint capsule with distinct anterior and posterior tibiofibular ligaments.2,3 The anterior tibiofibular ligament has been described as 1 or 2 bands whereas the posterior ligament consists of 1 band.3 The anterior ligament attaches anteroinferiorly to the fibular styloid and posteriorly to Gerdy’s tubercle on the tibia. It runs linearly from posterior to anterior, and the fibular footprint is immediately anterior to the insertion of the biceps femoris. The posterior ligament is located inferior to the lateral joint space, and the fibular footprint is posterior to the insertion of the biceps femoris.3 Anatomy of the PTFJ is shown schematically in Figure 1.

Both the lateral collateral ligament (LCL) and the tibiofibular interosseous membrane add stability to the PTFJ. The LCL travels from the lateral femoral epicondyle to the lateral side of fibula head, anterior to the fibular styloid. The interosseous membrane extends obliquely between the borders of the tibia and fibula. Additionally, the short head of the biceps femoris, fibular collateral ligament, fabellofibular ligament, popliteofibular ligament, and popliteus muscle all attach to the PTFJ and provide additional stability to the joint.
It is important to note that the common peroneal nerve passes posteriorly over the fibula neck, can be involved in the clinical presentation, and is a potential source of concern with any injury to or surgery on the joint.4
Many studies have demonstrated that a communication with the tibiofemoral joint exists through the subpopliteal recess, but the rate of communication has varied widely.5-8 Most recently, Bozkurt and colleagues5 found the rate of communication between the PTFJ and lateral femorotibial space to be 57.9%. When distinct communication exists, the PTFJ must be considered as a fourth compartment of the knee and is subject to any process that affects the knee joint proper.
Continue to: Ogden described 2 types...
Ogden1 described 2 types of PTFJs, horizontal and oblique, with the latter being considered less stable because of less rotational mobility. The horizontal configuration is defined as <20° of inclination of joint surface in relation to the horizontal plane, and the oblique variation is defined as >20° of inclination of the joint surface in relation to the horizontal plane.1
BIOMECHANICS AND FUNCTION
The primary function of the PTFJ is to dissipate torsional loads applied to the ankle, attenuate lateral tibial bending moments, and transmit axial loads from weight bearing on the extremity.1 The degree of knee flexion, ankle dorsiflexion, and tibial rotation all play an important role in PTFJ biomechanics. In knee flexion, the proximal fibula moves anteriorly because of the relative laxity of the LCL and biceps femoris tendons. In knee extension, the LCL and biceps femoris tighten, pulling the proximal fibula posteriorly.1 Because the LCL and biceps tendon are both relaxed and less supportive during knee flexion, the PTFJ is more prone to injury with a flexed knee. The ankle plays an important role in the biomechanics of the PTFJ because it contains the distal syndesmosis, where both the tibia and fibula are firmly attached distally. During ankle dorsiflexion, the fibula must externally rotate to accommodate a wider anterior talus.1 In regard to tibia rotation, Scott and colleagues9 demonstrated the relationship between tibial rotation and fibular translation. With internal tibial rotation, the fibular head translated posteriorly and with external tibial rotation, the fibula translated anteriorly. The greatest translational motion was seen during loading of the knee into varus and during external tibial rotation at all flexion angles.
CLINICAL CONDITIONS
Ogden10 classified instability of the PTFJ into 4 main groups: anterolateral dislocation, posteromedial dislocation, superior dislocation, and atraumatic subluxation. Injury to the PTFJ usually occurs in younger, athletic patients during sports that require violent twisting motions such as soccer, basketball, dance, skiing, horseback riding, parachute jumping, jet skiing, and judo. Patients with generalized ligamentous laxity have been described as at increased risk for joint instability.10,11
ACUTE DISLOCATION
The most common injury to the PTFJ is an anterolateral dislocation and involves injury to both the anterior and posterior capsular ligaments, and occasionally the LCL.10 Anterolateral dislocation is usually the result of a fall on a hyperflexed knee with the foot inverted and plantarflexed.11 While most anterolateral dislocations are the result of indirect sports trauma, several have been associated with other types of skeletal injuries such as fracture-dislocation of the hip, crush injury of the proximal and distal ends of the tibia, fracture-dislocation of the ankle, proximal tibial fracture, and fracture-dislocation of the distal femoral epiphysis.10 Ogden10 described the mechanism as follows: (1) sudden inversion and plantar flexion of the foot causing tension in the peroneal muscle group, extensor digitorum longus, and extensor halluces longus, which applies a forward dislocating force to the proximal end of the fibula; (2) simultaneous flexion of the knee, relaxing the biceps tendon and LCL; and (3) twisting of the body over the knee, transmitting energy along the femur to the tibia, exerting a relative external rotatory torque of the tibia on the foot, which is already fixed in inversion. Steps (2) and (3) spring the proximal end of the fibula laterally while the contracting muscles of (1) pull the fibula anteriorly.
Posteromedial dislocation is the second most common type of acute PTFJ dislocation. Posteromedial dislocations usually involve direct trauma and are associated with peroneal nerve injuries.1,2,10 The mechanism of dislocation results in tearing of the anterior and posterior PTFJ capsular ligaments, followed by injury to the LCL and other surrounding ligaments. This allows the biceps femoris to draw the unsupported proximal part of the fibula posteromedially along the posterolateral tibial metaphysis.7
Continue to: Superior dislocations are...
Superior dislocations are the least frequent form of acute PTFJ dislocations and are associated with high-energy ankle injuries.2,10,12 Superior dislocation results in injury to the interosseous membrane between the tibia and fibula and is frequently associated with tibial shaft fracture.10,11 Atraumatic, acquired superior dislocation of the PTFJ has also been associated with congenital dislocation of the knee.10,11
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ classically involves excessive and symptomatic anterior-posterior motion without actual dislocation of the joint.11 Subluxations of the PTFJ typically occur without any known trauma or injury and are most frequently associated with benign hyperlaxity syndrome, Ehlers-Danlos syndrome, or muscular dystrophy.4,10
Semonian and colleagues13 suggest that subluxation of the PTFJ is not given enough recognition in the literature and that instability should not be considered a rare condition. They hypothesize that many patients have joints that do not tolerate increases in fibula rotation secondary to subclinical trauma, repetitive overuse, or biomechanical variation of the joint. Semonian and associates13 state that the condition begins with the anterior capsule and anterior tibiofibular ligament attenuation as a result of excessive fibular rotation. Once stretched, the functional pull of the biceps femoris and soleus maintain the fibula in a relatively posterior and externally rotated position. Furthermore, Ogden10 found that 70% of the dislocations and subluxations he studied were of the oblique variant compared with that of the horizontal variant. Many authors suggest that the oblique variant is more at risk for injury because of its decreased joint surface area causing decreased rotational mobility.1,2,10
Early recognition is extremely important in dislocations and subluxations of the PTFJ as undiagnosed acute trauma can turn into chronic subluxation, and chronic subluxation may lead to dislocation.13 Additionally, chronic subluxation or dislocation are thought result in osteoarthritis of the PTFJ.14
OSTEOARTHRITIS
The literature on osteoarthritis of the PTFJ is limited. Eichenblat and Nathan7 studied the PTFJ in cadavers and dry bones and found that 28% had evidence of osteoarthritis. Clinically, however, osteoarthritis of the PTFJ is a rare primary diagnosis, suggesting that involvement of the PTFJ is either asymptomatic or that symptoms are associated with osteoarthritis of the knee joint. Boya and colleagues15 and Eichenblat and Nathan7 both found a high correlation between the presence of osteoarthritis of the PTFJ and osteoarthritis of the tibiofemoral joint in cadavers. The authors suggest this correlation may be related to the presence of anatomical communication between the 2 joints. Theoretically, inflammatory mediators flow freely between the joint spaces and contribute to arthritis in both joints. The possibility of degenerative arthritis of the PTFJ accompanying degenerative arthritis of the knee warrants evaluation, especially in patients considering total knee arthroplasty. Unrecognized arthritis of the PTFJ might influence outcome scores and be an unsolved source of lateral knee pain post-knee replacement.16
Continue to: CLINICAL PRESENTATION
CLINCIAL PRESENTATION
ACUTE DISLOCATION
Patients with acute PTFJ dislocation present with pain, tenderness, swelling, and asymmetry of the lateral side of the knee, while the knee joint is not swollen and range of knee motion is not limited.17 A bony prominence might be felt, and the biceps femoris tendon can often appear to be tense.13 Active or passive ankle movements often exacerbate the lateral knee pain.11 It is also important to examine the peroneal nerve, as transient peroneal palsy has been described in all types of PTFJ dislocations but most often with posteromedial dislocations. Sensory disturbance in the peroneal nerve distribution is more common than motor loss, but foot drop is also a potential presenting sign.11 On examination, palpation of the fibular head illustrates tenderness and aggravates the pain.
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ can be difficult to recognize because the history, signs, and symptoms of lateral knee pain can be subtle and sometimes misleading. In addition, current literature provides little information on specific tests, measurements, signs, or subjective information regarding subluxation. Patients rarely reveal a history of trauma or mechanism of injury. Subluxations are often associated with patients participating in repetitive sports requiring running, jumping, or twisting movements, and can be present bilateral. Instability has also been described in patients with osteomyelitis, rheumatoid arthritis, septic arthritis, pigmented villonodular synovitis, below-knee amputations, osteochondroma, and in runners who recently increased mileage (especially during the first 2-3 miles and during downhill running).11,13 Patients normally do not have difficulty with activities of daily living, but symptoms may arise when making movements with a sudden change of direction.11
These patients usually complain of instability of the knee and pain along the lateral aspect of the knee. Pain radiating proximally into the region of the iliotibial band and medially into the patellofemoral joint can be seen.13 Patients may also report clicking, popping, or catching of the lateral knee; while others will report a sense of giving way of the knee joint.11,13 Progressive peroneal nerve symptoms are usually seen in older patients; however, they are more common with acute PTFJ dislocations as discussed.13
A clinical method for examining a PTFJ with possible subluxation or chronic instability has been described by Sijbrandij.18 With the patient in the supine position, the knee is flexed to 90° to relax the LCL and biceps femoris tendon. The fibular head is then held between the thumb and index finger, and moved anteriorly and laterally. Dislocation or subluxation will be felt and visualized as the fibular head translates, and should be compared with the uninjured PTFJ. On release, the fibular head will return to its normal position, often with a click. Asking the patient if this subluxation/reduction maneuver reproduces the symptoms or causes apprehension or pain may also be helpful.18 Another method for examination is eliciting the Radulescu sign.11,13 While the patient lies prone, the examiner stabilizes the thigh with 1 hand while the knee is flexed to 90°. The examiner then applies an internal rotation force on the lower leg. Observing an abnormal excursion of the fibular head in an anterior and lateral direction represents a positive test.11,13
OSTEOARTHRITIS
Clinical evaluation for osteoarthritis in the PTFJ is not well described in the literature. A single report describes applying manual pressure over the fibular head during active ankle motion.16 A test known as the grinding test is used as a sign to detect the involvement of the PTFJ as a component of osteoarthritis of the knee. A positive test will elicit pain and/or tenderness of the joint.16
Continue to: DIAGNOSTIC IMAGING
DIAGNOSTIC IMAGING
Plain radiographs in the anteroposterior (AP) and true lateral views are useful as first-line investigations in suspected PTFJ dislocation. Comparable AP and lateral radiographs of each knee are highly recommended to detect findings that suggest dislocation.2 Abnormal findings include increased interosseous space, medial or lateral displacement of the fibula on the anteroposterior view, and anterior or posterior displacement of the fibula head on lateral view as shown in Figure 2.19,20

Resnick and colleagues2 proposed the use of the linear sloping radiodensity that defines the posteromedial corner of the lateral tibial condyle as an indicator of anterolateral or posteromedial PTFJ dislocation. However, this application is limited because of the PTFJ’s highly variable morphology.8 In a recent study conducted by Hey and colleagues,21 5968 (2984 patients) knee radiographs were retrospectively collected and subjected to radiographical measurements and statistical analysis. The tibiofibular overlap method had a specificity of 94.1% and 84.5% when diagnosing PTFJ dislocations on the AP and lateral views, respectively.21
If a diagnosis of PTFJ is suspected but not clearly established based on radiography, computed tomography with comparison views of the contralateral knee are recommended to confirm the diagnosis.17,22 This becomes more critical in cases of suspected subluxation/chronic PTFJ instability. Additionally, magnetic resonance imaging (MRI) can be used to assess chronic PTFJ instability. Recently, Burke and colleagues23 performed a 10-year retrospective case series that included 7 patients with chronic PTFJ instability and included MRI as part of their evaluation. The MRI abnormalities in these patients included periarticular soft tissue edema, including in the proximal soleus muscle (n = 5), periarticular ganglion or ganglia (n = 4), tibiofibular ligament edema (n = 4), subchondral marrow edema (n = 3), posterior tibiofibular ligament thickening (n = 2), subcortical cyst at a ligament insertion (n = 2), partial-thickness tear of the anterior tibiofibular ligament (n = 1), and tibiofibular joint effusion (n = 1).
OSTEOARTHRITIS
Routine knee radiographs can show PTFJ joint space narrowing, sclerosis, marginal osteophytes, and local osteopenia as conventional components of osteoarthritis of any joint. Serial radiographs have also been described as effective in evaluating progressive degenerative changes of the PTFJ.14 An MRI will show osteophyte formation, subchondral cysts, subchondral sclerosis, joint effusion, joint space narrowing, and is highly sensitive for detecting degenerative changes in cartilage, as well as identifying other possible pathologies such as synovial cysts or pigmented villonodular synovitis. Chronic PTFJ instability appears to predispose to tibiofibular osteoarthritis as reported by Burke and colleagues,23 who found a particularly high incidence (42.9%) of osteoarthritis in patients with chronic PTFJ instability. Additionally, Veth and colleagues14 found degenerative changes in 8 of 19 patients presenting with PTFJ dislocations.
TREATMENT
ACUTE DISLOCATION
Prompt recognition and treatment of any acute PTFJ dislocation are necessary to avoid long-term instability and other possible sequelae.11 Treatment consists of reduction followed by restriction of weight-bearing.11 Traditionally, the knee is immobilized with a cast in extension for 3 to 4 weeks followed by knee mobilization and progressive range of motion exercises,24 but there is some controversy regarding complete immobilization.11,25,26
Continue to: Initially, closed reduction...
Initially, closed reduction is advised as the treatment for acute PTFJ dislocation.11,19,24 It involves placing the knee in 80°to 110° of flexion to relax the biceps femoris and LCL, then applying an appropriate force to the fibular head in a direction opposite the displacement.11,24 An audible pop is often heard as the fibula reduces back into normal alignment. Stability of the reduction and stability of the knee should be determined with respect to both posterolateral structures and the LCL after reduction.11 Anterolateral dislocations are usually easier to reduce, as a posteromedial or superior dislocation can result in the fibular head being perched on the lateral tibial ridge, and held by the LCL.11
Calabró and colleagues27 described a new, simple, and safe alternative technique of closed reduction of an anterior dislocation if the classical method fails. This technique relies on ligamentotaxis and a dynamic counteraction between muscles and ligaments to reduce the joint. The patient flexes the knee >90° while the physician applies a counterforce to the heel with the palm. Simultaneously, gentle direct pressure should be applied to the fibular head to move it toward the lateral tibial ridge. With a relaxed LCL, the biceps femoris tendon will actively reduce the proximal fibular head back into its correct anatomic orientation.27
When a closed reduction in an awake patient has failed, a reduction under sedation or anesthesia should be performed. If that fails, an open reduction should be performed. Following an open reduction, the joint is stabilized with Kirschner wires, bioabsorbable pins, or cortical screws.24-26,28 The torn capsule and any injured ligaments should also be primarily repaired. After approximately 6 weeks, the stabilization hardware may be removed.24,25
Acute posteromedial dislocations are treated similarly to anterolateral dislocations; however, open reduction and repair of the capsule and ligaments are more frequently required.10 Superior dislocations are also more frequently reduced by open methods, sometimes dictated by open treatment of associated tibia or ankle fracture.10 Damage to any structure of the posterolateral knee as a result of acute PTFJ injury should be repaired, as this has been associated with better outcomes.14
PTFJ dislocation and tibia fracture can occur together, and in a retrospective study conducted by Herzog and colleagues29 the authors recorded the incidence of PTFJ dislocation as 1.5% of operative tibial shaft fractures and 1.9% of operative tibial plateau fractures. Haupt and colleagues30 also conducted a retrospective study in which the authors recorded the incidence of PTFJ dislocations in 1.06% of all tibial shaft fractures in their series. In both studies combined, all except 1 PTFJ dislocation had been caused by high-energy trauma.29,30 In the case of PTFJ dislocation with tibial shaft fracture, intramedullary nailing of the tibial shaft fracture followed by open reduction of the PTFJ with 1 or 2 positioning screws just below the PTFJ has yielded satisfactory results.30 The positioning screw should be removed 6 weeks post-operation to prevent PTFJ arthrodesis and patients should be supported in full weight-bearing.30 An illustrative case report from our institution is shown in Figure 3.

Continue to: CHRONIC INSTABILITY
CHRONIC INSTABILITY
Chronic instability is commonly the result of untreated or misdiagnosed subluxation of the PTFJ. Ogden10 reported that 57% of patients with acute proximal tibiofibular dislocations required surgery for ongoing symptoms after treatment failure with closed reduction and 3 weeks of immobilization. The first step in the management of chronic instability of the PTFJ is usually a nonoperative approach. Immobilization, activity level modification, utilization of a supportive strap placed 1 centimeter below the fibular head for pain relief, and participation in a strength-training program of the lower leg are initial treatment recommendations.10,11,13 Many patients with chronic PTFJ instability do not respond to conservative treatment and may pursue surgical intervention. Surgical treatment options include permanent arthrodesis, resection of the fibular head, soft tissue reconstruction, and temporary fixation.10,13,26,31 Given the rare nature of the injury and lack of data on varying treatments, there is no clear consensus on the optimal surgical procedure.
Arthrodesis and fibular head resection are 2 traditional methods of surgically addressing the PTFJ, but both have limitations that need to be recognized. Arthrodesis involves clearing the PTFJ of all articular cartilage, bone grafting, and then reducing the joint using screw fixation.11 Rigid fixation prevents rotation of the fibula which puts additional stress on the ankle, frequently causing pain and instability of the ankle joint.10,11 The other traditional surgical option, fibular head resection, involves excision of the head and neck of the fibula while preserving the fibular styloid and LCL.4 Fibular head resection is indicated when peroneal nerve symptoms or palsy occur in PTFJ injuries.4,10 Unfortunately, resection may disrupt the posterolateral corner structures of the knee and result in pain and instability.3,13 As a general rule, it is advisable to avoid arthrodesis and fibular head resection in children and athletes because of the length of time during which complications can develop.11
Van den Bekerom and colleagues26 suggest that their technique of temporary fixation of the PTFJ using a cancellous screw yields satisfactory outcomes in treating chronic PTFJ instability. The method entails having the ankle dorsiflexed and the head of the fibula slightly externally rotated and reduced into the most stable position. A hole is drilled in the anteromedial direction from posterior fibula head into the tibia. A non-tapped cortical screw is used to fix the fibula head in the reduced position.26 The screw is removed after 3 to 6 months. Seven of 8 patients treated with this technique by Van den Bekerom and colleagues26 have had alleviation of symptoms, although the screws broke in 2 cases before their planned removal.
Soft tissue reconstruction of the PTFJ has been an evolving area of treatment. Several techniques using a variety of tissue grafts and fixation methods have been described. Giachino32 proposed a reconstruction in which the fibular head is stabilized with a strip of ipsilateral biceps femoris tendon still attached distally to the fibular head and deep fascia of the leg. Drill holes are made in the tibia, and the tissue is secured anteriorly to the fascia with a suture. Shapiro and colleagues33 performed a stabilization using a strip of the iliotibial band still connected to its insertion on Gerdy’s tubercle. The graft was tunneled through a tibial drill hole from anterior to posterior and through the fibular head from posterior to anterior before suturing back onto itself. Another technique described by Mena and colleagues34 uses a split biceps tendon autograft, harnessing the strong attachments of the tendon to the fibular head to stabilize the reduced PTFJ.
More recently, Miller35 employed elements of the techniques described by Gianchino,32 Shapiro and colleagues,33 and Mena and colleagues34 to develop a method involving the biceps femoris tendon and iliotibial band in a soft tissue reconstruction. The technique involves harvesting the biceps femoris tendon while preserving the distal insertion and harvesting the iliotibial band while leaving the distal insertion at the Gerdy’s tubercle intact.35 The biceps tendon is passed from posterior to anterior through a tibial bone tunnel, and the iliotibial band graft is passed through the same tunnel but in the anterior to posterior direction. A bioabsorbable interference screw is placed into the tibial tunnel from anterior to posterior. The remaining biceps tendon graft is sewn into the soft tissue of the anterior tibia. The tail of the iliotibial band graft is passed through the fibular bone tunnel in the posterior to anterior direction and looped around the fibular head and under the LCL before being sewn back onto itself.35 Kobbe and colleagues36 described a surgical technique using the ipsilateral semitendinosis tendon while Maffulli and colleagues37 and Morrison and colleagues38 presented techniques using the ipsilateral gracilis tendon. These techniques do not jeopardize lateral knee or fibular head stability by avoiding the use of the biceps femoris and iliotibial band, as well as local knee stabilizers. Warner and colleagues31 proposed a similar technique to the aforementioned using a semitendinosus tendon; however, they do not reconstruct the anterior ligamentous structures, as seen in Figures 4A and 4B.39 The authors have concerns that a 2-limb reconstruction as described by Kobbe and colleagues36 and Morrison and colleagues38 may be prone to overconstraint or other errors in tensioning because it does not allow for differential tension to be applied to each limb.31 According to Warner and colleagues,31 the anterior structures of their patients with chronic PTFJ instability have appeared normal, and isolated posterior ligamentous reconstruction has been adequate for the restoration of stability. Camarda and colleagues28 proposed a technique very similar to that of Warner and colleagues;31 however, the tibial tunnel is reamed from posterolateral to the anteromedial aspect of the tibia, exploiting the skin incision previously used for tendon harvest.

Continue to: ARTHRITIS
ARTHRITIS
The management options for secondary arthritis due to chronic PTFJ instability have rarely been discussed in the literature. Arthrodesis or fibular head resection are options for the treatment of arthritis, and the above discussion applies here as well. Yaniv and colleagues40 describe a technical procedure for addressing both instability and secondary joint arthritis. The authors performed a ligament reconstruction of the PTFJ using the anterior part of the biceps femoris combined with interpositional joint arthroplasty using a vascularized fascia lata strip. Two weeks post-operation, the patient was walking with full weight-bearing and 6 weeks post-operation the first sports activities were allowed.40
CONCLUSION
Conditions affecting the PTFJ, their diagnosis, and treatment are infrequent topics of discussion in the literature. While PTFJ injury, instability, and other disease states are admittedly rare, clinicians need to include them in the differential diagnosis of patients presenting with lateral knee complaints. Diagnostic imaging is a critical component in early identification of PTFJ conditions to prevent long-term complications. Most injuries are treated first with conservative methods, reserving surgery as an option when first-line measures are unsuccessful. Advancements in surgical options for dislocation and subluxation/chronic instability of the joint have been made, but further research on their effectiveness and long-term outcomes is needed before a gold-standard treatment can be determined.
ABSTRACT
Current literature is limited with respect to the proximal tibiofibular joint (PTFJ) and clinical conditions relating to the PTFJ. Diagnosis and treatment of conditions that affect the PTFJ are not well described and are a topic of debate among many physicians. This manuscript aims to review and summarize the most recent literature that relates to traumatic dislocations, fractures, chronic instability, and osteoarthritis, with a focus on both diagnostic and treatment strategies of these conditions. We also review PTFJ anatomy, biomechanics, and the clinical presentation of some common PTFJ conditions.
Continue to: Clinical conditions...
Clinical conditions of the proximal tibiofibular joint (PTFJ) are an uncommon source of lateral knee complaints and are often overlooked in the differential diagnosis as a source of the knee complaint. The most common conditions of the PTFJ include traumatic dislocations, fractures, chronic instability, and osteoarthritis. This article reviews the most common diseases affecting this joint and discusses both diagnostic and treatment strategies in an attempt to raise awareness of this joint as a source of lateral knee complaints.
ANATOMY
The PTFJ is an arthrodial synovial joint between the posterolateral surface of the tibia and the proximal fibular head.1 Surrounding the synovial membrane of the articulation is a fibrous joint capsule with distinct anterior and posterior tibiofibular ligaments.2,3 The anterior tibiofibular ligament has been described as 1 or 2 bands whereas the posterior ligament consists of 1 band.3 The anterior ligament attaches anteroinferiorly to the fibular styloid and posteriorly to Gerdy’s tubercle on the tibia. It runs linearly from posterior to anterior, and the fibular footprint is immediately anterior to the insertion of the biceps femoris. The posterior ligament is located inferior to the lateral joint space, and the fibular footprint is posterior to the insertion of the biceps femoris.3 Anatomy of the PTFJ is shown schematically in Figure 1.

Both the lateral collateral ligament (LCL) and the tibiofibular interosseous membrane add stability to the PTFJ. The LCL travels from the lateral femoral epicondyle to the lateral side of fibula head, anterior to the fibular styloid. The interosseous membrane extends obliquely between the borders of the tibia and fibula. Additionally, the short head of the biceps femoris, fibular collateral ligament, fabellofibular ligament, popliteofibular ligament, and popliteus muscle all attach to the PTFJ and provide additional stability to the joint.
It is important to note that the common peroneal nerve passes posteriorly over the fibula neck, can be involved in the clinical presentation, and is a potential source of concern with any injury to or surgery on the joint.4
Many studies have demonstrated that a communication with the tibiofemoral joint exists through the subpopliteal recess, but the rate of communication has varied widely.5-8 Most recently, Bozkurt and colleagues5 found the rate of communication between the PTFJ and lateral femorotibial space to be 57.9%. When distinct communication exists, the PTFJ must be considered as a fourth compartment of the knee and is subject to any process that affects the knee joint proper.
Continue to: Ogden described 2 types...
Ogden1 described 2 types of PTFJs, horizontal and oblique, with the latter being considered less stable because of less rotational mobility. The horizontal configuration is defined as <20° of inclination of joint surface in relation to the horizontal plane, and the oblique variation is defined as >20° of inclination of the joint surface in relation to the horizontal plane.1
BIOMECHANICS AND FUNCTION
The primary function of the PTFJ is to dissipate torsional loads applied to the ankle, attenuate lateral tibial bending moments, and transmit axial loads from weight bearing on the extremity.1 The degree of knee flexion, ankle dorsiflexion, and tibial rotation all play an important role in PTFJ biomechanics. In knee flexion, the proximal fibula moves anteriorly because of the relative laxity of the LCL and biceps femoris tendons. In knee extension, the LCL and biceps femoris tighten, pulling the proximal fibula posteriorly.1 Because the LCL and biceps tendon are both relaxed and less supportive during knee flexion, the PTFJ is more prone to injury with a flexed knee. The ankle plays an important role in the biomechanics of the PTFJ because it contains the distal syndesmosis, where both the tibia and fibula are firmly attached distally. During ankle dorsiflexion, the fibula must externally rotate to accommodate a wider anterior talus.1 In regard to tibia rotation, Scott and colleagues9 demonstrated the relationship between tibial rotation and fibular translation. With internal tibial rotation, the fibular head translated posteriorly and with external tibial rotation, the fibula translated anteriorly. The greatest translational motion was seen during loading of the knee into varus and during external tibial rotation at all flexion angles.
CLINICAL CONDITIONS
Ogden10 classified instability of the PTFJ into 4 main groups: anterolateral dislocation, posteromedial dislocation, superior dislocation, and atraumatic subluxation. Injury to the PTFJ usually occurs in younger, athletic patients during sports that require violent twisting motions such as soccer, basketball, dance, skiing, horseback riding, parachute jumping, jet skiing, and judo. Patients with generalized ligamentous laxity have been described as at increased risk for joint instability.10,11
ACUTE DISLOCATION
The most common injury to the PTFJ is an anterolateral dislocation and involves injury to both the anterior and posterior capsular ligaments, and occasionally the LCL.10 Anterolateral dislocation is usually the result of a fall on a hyperflexed knee with the foot inverted and plantarflexed.11 While most anterolateral dislocations are the result of indirect sports trauma, several have been associated with other types of skeletal injuries such as fracture-dislocation of the hip, crush injury of the proximal and distal ends of the tibia, fracture-dislocation of the ankle, proximal tibial fracture, and fracture-dislocation of the distal femoral epiphysis.10 Ogden10 described the mechanism as follows: (1) sudden inversion and plantar flexion of the foot causing tension in the peroneal muscle group, extensor digitorum longus, and extensor halluces longus, which applies a forward dislocating force to the proximal end of the fibula; (2) simultaneous flexion of the knee, relaxing the biceps tendon and LCL; and (3) twisting of the body over the knee, transmitting energy along the femur to the tibia, exerting a relative external rotatory torque of the tibia on the foot, which is already fixed in inversion. Steps (2) and (3) spring the proximal end of the fibula laterally while the contracting muscles of (1) pull the fibula anteriorly.
Posteromedial dislocation is the second most common type of acute PTFJ dislocation. Posteromedial dislocations usually involve direct trauma and are associated with peroneal nerve injuries.1,2,10 The mechanism of dislocation results in tearing of the anterior and posterior PTFJ capsular ligaments, followed by injury to the LCL and other surrounding ligaments. This allows the biceps femoris to draw the unsupported proximal part of the fibula posteromedially along the posterolateral tibial metaphysis.7
Continue to: Superior dislocations are...
Superior dislocations are the least frequent form of acute PTFJ dislocations and are associated with high-energy ankle injuries.2,10,12 Superior dislocation results in injury to the interosseous membrane between the tibia and fibula and is frequently associated with tibial shaft fracture.10,11 Atraumatic, acquired superior dislocation of the PTFJ has also been associated with congenital dislocation of the knee.10,11
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ classically involves excessive and symptomatic anterior-posterior motion without actual dislocation of the joint.11 Subluxations of the PTFJ typically occur without any known trauma or injury and are most frequently associated with benign hyperlaxity syndrome, Ehlers-Danlos syndrome, or muscular dystrophy.4,10
Semonian and colleagues13 suggest that subluxation of the PTFJ is not given enough recognition in the literature and that instability should not be considered a rare condition. They hypothesize that many patients have joints that do not tolerate increases in fibula rotation secondary to subclinical trauma, repetitive overuse, or biomechanical variation of the joint. Semonian and associates13 state that the condition begins with the anterior capsule and anterior tibiofibular ligament attenuation as a result of excessive fibular rotation. Once stretched, the functional pull of the biceps femoris and soleus maintain the fibula in a relatively posterior and externally rotated position. Furthermore, Ogden10 found that 70% of the dislocations and subluxations he studied were of the oblique variant compared with that of the horizontal variant. Many authors suggest that the oblique variant is more at risk for injury because of its decreased joint surface area causing decreased rotational mobility.1,2,10
Early recognition is extremely important in dislocations and subluxations of the PTFJ as undiagnosed acute trauma can turn into chronic subluxation, and chronic subluxation may lead to dislocation.13 Additionally, chronic subluxation or dislocation are thought result in osteoarthritis of the PTFJ.14
OSTEOARTHRITIS
The literature on osteoarthritis of the PTFJ is limited. Eichenblat and Nathan7 studied the PTFJ in cadavers and dry bones and found that 28% had evidence of osteoarthritis. Clinically, however, osteoarthritis of the PTFJ is a rare primary diagnosis, suggesting that involvement of the PTFJ is either asymptomatic or that symptoms are associated with osteoarthritis of the knee joint. Boya and colleagues15 and Eichenblat and Nathan7 both found a high correlation between the presence of osteoarthritis of the PTFJ and osteoarthritis of the tibiofemoral joint in cadavers. The authors suggest this correlation may be related to the presence of anatomical communication between the 2 joints. Theoretically, inflammatory mediators flow freely between the joint spaces and contribute to arthritis in both joints. The possibility of degenerative arthritis of the PTFJ accompanying degenerative arthritis of the knee warrants evaluation, especially in patients considering total knee arthroplasty. Unrecognized arthritis of the PTFJ might influence outcome scores and be an unsolved source of lateral knee pain post-knee replacement.16
Continue to: CLINICAL PRESENTATION
CLINCIAL PRESENTATION
ACUTE DISLOCATION
Patients with acute PTFJ dislocation present with pain, tenderness, swelling, and asymmetry of the lateral side of the knee, while the knee joint is not swollen and range of knee motion is not limited.17 A bony prominence might be felt, and the biceps femoris tendon can often appear to be tense.13 Active or passive ankle movements often exacerbate the lateral knee pain.11 It is also important to examine the peroneal nerve, as transient peroneal palsy has been described in all types of PTFJ dislocations but most often with posteromedial dislocations. Sensory disturbance in the peroneal nerve distribution is more common than motor loss, but foot drop is also a potential presenting sign.11 On examination, palpation of the fibular head illustrates tenderness and aggravates the pain.
SUBLUXATION/CHRONIC INSTABILITY
Subluxation of the PTFJ can be difficult to recognize because the history, signs, and symptoms of lateral knee pain can be subtle and sometimes misleading. In addition, current literature provides little information on specific tests, measurements, signs, or subjective information regarding subluxation. Patients rarely reveal a history of trauma or mechanism of injury. Subluxations are often associated with patients participating in repetitive sports requiring running, jumping, or twisting movements, and can be present bilateral. Instability has also been described in patients with osteomyelitis, rheumatoid arthritis, septic arthritis, pigmented villonodular synovitis, below-knee amputations, osteochondroma, and in runners who recently increased mileage (especially during the first 2-3 miles and during downhill running).11,13 Patients normally do not have difficulty with activities of daily living, but symptoms may arise when making movements with a sudden change of direction.11
These patients usually complain of instability of the knee and pain along the lateral aspect of the knee. Pain radiating proximally into the region of the iliotibial band and medially into the patellofemoral joint can be seen.13 Patients may also report clicking, popping, or catching of the lateral knee; while others will report a sense of giving way of the knee joint.11,13 Progressive peroneal nerve symptoms are usually seen in older patients; however, they are more common with acute PTFJ dislocations as discussed.13
A clinical method for examining a PTFJ with possible subluxation or chronic instability has been described by Sijbrandij.18 With the patient in the supine position, the knee is flexed to 90° to relax the LCL and biceps femoris tendon. The fibular head is then held between the thumb and index finger, and moved anteriorly and laterally. Dislocation or subluxation will be felt and visualized as the fibular head translates, and should be compared with the uninjured PTFJ. On release, the fibular head will return to its normal position, often with a click. Asking the patient if this subluxation/reduction maneuver reproduces the symptoms or causes apprehension or pain may also be helpful.18 Another method for examination is eliciting the Radulescu sign.11,13 While the patient lies prone, the examiner stabilizes the thigh with 1 hand while the knee is flexed to 90°. The examiner then applies an internal rotation force on the lower leg. Observing an abnormal excursion of the fibular head in an anterior and lateral direction represents a positive test.11,13
OSTEOARTHRITIS
Clinical evaluation for osteoarthritis in the PTFJ is not well described in the literature. A single report describes applying manual pressure over the fibular head during active ankle motion.16 A test known as the grinding test is used as a sign to detect the involvement of the PTFJ as a component of osteoarthritis of the knee. A positive test will elicit pain and/or tenderness of the joint.16
Continue to: DIAGNOSTIC IMAGING
DIAGNOSTIC IMAGING
Plain radiographs in the anteroposterior (AP) and true lateral views are useful as first-line investigations in suspected PTFJ dislocation. Comparable AP and lateral radiographs of each knee are highly recommended to detect findings that suggest dislocation.2 Abnormal findings include increased interosseous space, medial or lateral displacement of the fibula on the anteroposterior view, and anterior or posterior displacement of the fibula head on lateral view as shown in Figure 2.19,20

Resnick and colleagues2 proposed the use of the linear sloping radiodensity that defines the posteromedial corner of the lateral tibial condyle as an indicator of anterolateral or posteromedial PTFJ dislocation. However, this application is limited because of the PTFJ’s highly variable morphology.8 In a recent study conducted by Hey and colleagues,21 5968 (2984 patients) knee radiographs were retrospectively collected and subjected to radiographical measurements and statistical analysis. The tibiofibular overlap method had a specificity of 94.1% and 84.5% when diagnosing PTFJ dislocations on the AP and lateral views, respectively.21
If a diagnosis of PTFJ is suspected but not clearly established based on radiography, computed tomography with comparison views of the contralateral knee are recommended to confirm the diagnosis.17,22 This becomes more critical in cases of suspected subluxation/chronic PTFJ instability. Additionally, magnetic resonance imaging (MRI) can be used to assess chronic PTFJ instability. Recently, Burke and colleagues23 performed a 10-year retrospective case series that included 7 patients with chronic PTFJ instability and included MRI as part of their evaluation. The MRI abnormalities in these patients included periarticular soft tissue edema, including in the proximal soleus muscle (n = 5), periarticular ganglion or ganglia (n = 4), tibiofibular ligament edema (n = 4), subchondral marrow edema (n = 3), posterior tibiofibular ligament thickening (n = 2), subcortical cyst at a ligament insertion (n = 2), partial-thickness tear of the anterior tibiofibular ligament (n = 1), and tibiofibular joint effusion (n = 1).
OSTEOARTHRITIS
Routine knee radiographs can show PTFJ joint space narrowing, sclerosis, marginal osteophytes, and local osteopenia as conventional components of osteoarthritis of any joint. Serial radiographs have also been described as effective in evaluating progressive degenerative changes of the PTFJ.14 An MRI will show osteophyte formation, subchondral cysts, subchondral sclerosis, joint effusion, joint space narrowing, and is highly sensitive for detecting degenerative changes in cartilage, as well as identifying other possible pathologies such as synovial cysts or pigmented villonodular synovitis. Chronic PTFJ instability appears to predispose to tibiofibular osteoarthritis as reported by Burke and colleagues,23 who found a particularly high incidence (42.9%) of osteoarthritis in patients with chronic PTFJ instability. Additionally, Veth and colleagues14 found degenerative changes in 8 of 19 patients presenting with PTFJ dislocations.
TREATMENT
ACUTE DISLOCATION
Prompt recognition and treatment of any acute PTFJ dislocation are necessary to avoid long-term instability and other possible sequelae.11 Treatment consists of reduction followed by restriction of weight-bearing.11 Traditionally, the knee is immobilized with a cast in extension for 3 to 4 weeks followed by knee mobilization and progressive range of motion exercises,24 but there is some controversy regarding complete immobilization.11,25,26
Continue to: Initially, closed reduction...
Initially, closed reduction is advised as the treatment for acute PTFJ dislocation.11,19,24 It involves placing the knee in 80°to 110° of flexion to relax the biceps femoris and LCL, then applying an appropriate force to the fibular head in a direction opposite the displacement.11,24 An audible pop is often heard as the fibula reduces back into normal alignment. Stability of the reduction and stability of the knee should be determined with respect to both posterolateral structures and the LCL after reduction.11 Anterolateral dislocations are usually easier to reduce, as a posteromedial or superior dislocation can result in the fibular head being perched on the lateral tibial ridge, and held by the LCL.11
Calabró and colleagues27 described a new, simple, and safe alternative technique of closed reduction of an anterior dislocation if the classical method fails. This technique relies on ligamentotaxis and a dynamic counteraction between muscles and ligaments to reduce the joint. The patient flexes the knee >90° while the physician applies a counterforce to the heel with the palm. Simultaneously, gentle direct pressure should be applied to the fibular head to move it toward the lateral tibial ridge. With a relaxed LCL, the biceps femoris tendon will actively reduce the proximal fibular head back into its correct anatomic orientation.27
When a closed reduction in an awake patient has failed, a reduction under sedation or anesthesia should be performed. If that fails, an open reduction should be performed. Following an open reduction, the joint is stabilized with Kirschner wires, bioabsorbable pins, or cortical screws.24-26,28 The torn capsule and any injured ligaments should also be primarily repaired. After approximately 6 weeks, the stabilization hardware may be removed.24,25
Acute posteromedial dislocations are treated similarly to anterolateral dislocations; however, open reduction and repair of the capsule and ligaments are more frequently required.10 Superior dislocations are also more frequently reduced by open methods, sometimes dictated by open treatment of associated tibia or ankle fracture.10 Damage to any structure of the posterolateral knee as a result of acute PTFJ injury should be repaired, as this has been associated with better outcomes.14
PTFJ dislocation and tibia fracture can occur together, and in a retrospective study conducted by Herzog and colleagues29 the authors recorded the incidence of PTFJ dislocation as 1.5% of operative tibial shaft fractures and 1.9% of operative tibial plateau fractures. Haupt and colleagues30 also conducted a retrospective study in which the authors recorded the incidence of PTFJ dislocations in 1.06% of all tibial shaft fractures in their series. In both studies combined, all except 1 PTFJ dislocation had been caused by high-energy trauma.29,30 In the case of PTFJ dislocation with tibial shaft fracture, intramedullary nailing of the tibial shaft fracture followed by open reduction of the PTFJ with 1 or 2 positioning screws just below the PTFJ has yielded satisfactory results.30 The positioning screw should be removed 6 weeks post-operation to prevent PTFJ arthrodesis and patients should be supported in full weight-bearing.30 An illustrative case report from our institution is shown in Figure 3.

Continue to: CHRONIC INSTABILITY
CHRONIC INSTABILITY
Chronic instability is commonly the result of untreated or misdiagnosed subluxation of the PTFJ. Ogden10 reported that 57% of patients with acute proximal tibiofibular dislocations required surgery for ongoing symptoms after treatment failure with closed reduction and 3 weeks of immobilization. The first step in the management of chronic instability of the PTFJ is usually a nonoperative approach. Immobilization, activity level modification, utilization of a supportive strap placed 1 centimeter below the fibular head for pain relief, and participation in a strength-training program of the lower leg are initial treatment recommendations.10,11,13 Many patients with chronic PTFJ instability do not respond to conservative treatment and may pursue surgical intervention. Surgical treatment options include permanent arthrodesis, resection of the fibular head, soft tissue reconstruction, and temporary fixation.10,13,26,31 Given the rare nature of the injury and lack of data on varying treatments, there is no clear consensus on the optimal surgical procedure.
Arthrodesis and fibular head resection are 2 traditional methods of surgically addressing the PTFJ, but both have limitations that need to be recognized. Arthrodesis involves clearing the PTFJ of all articular cartilage, bone grafting, and then reducing the joint using screw fixation.11 Rigid fixation prevents rotation of the fibula which puts additional stress on the ankle, frequently causing pain and instability of the ankle joint.10,11 The other traditional surgical option, fibular head resection, involves excision of the head and neck of the fibula while preserving the fibular styloid and LCL.4 Fibular head resection is indicated when peroneal nerve symptoms or palsy occur in PTFJ injuries.4,10 Unfortunately, resection may disrupt the posterolateral corner structures of the knee and result in pain and instability.3,13 As a general rule, it is advisable to avoid arthrodesis and fibular head resection in children and athletes because of the length of time during which complications can develop.11
Van den Bekerom and colleagues26 suggest that their technique of temporary fixation of the PTFJ using a cancellous screw yields satisfactory outcomes in treating chronic PTFJ instability. The method entails having the ankle dorsiflexed and the head of the fibula slightly externally rotated and reduced into the most stable position. A hole is drilled in the anteromedial direction from posterior fibula head into the tibia. A non-tapped cortical screw is used to fix the fibula head in the reduced position.26 The screw is removed after 3 to 6 months. Seven of 8 patients treated with this technique by Van den Bekerom and colleagues26 have had alleviation of symptoms, although the screws broke in 2 cases before their planned removal.
Soft tissue reconstruction of the PTFJ has been an evolving area of treatment. Several techniques using a variety of tissue grafts and fixation methods have been described. Giachino32 proposed a reconstruction in which the fibular head is stabilized with a strip of ipsilateral biceps femoris tendon still attached distally to the fibular head and deep fascia of the leg. Drill holes are made in the tibia, and the tissue is secured anteriorly to the fascia with a suture. Shapiro and colleagues33 performed a stabilization using a strip of the iliotibial band still connected to its insertion on Gerdy’s tubercle. The graft was tunneled through a tibial drill hole from anterior to posterior and through the fibular head from posterior to anterior before suturing back onto itself. Another technique described by Mena and colleagues34 uses a split biceps tendon autograft, harnessing the strong attachments of the tendon to the fibular head to stabilize the reduced PTFJ.
More recently, Miller35 employed elements of the techniques described by Gianchino,32 Shapiro and colleagues,33 and Mena and colleagues34 to develop a method involving the biceps femoris tendon and iliotibial band in a soft tissue reconstruction. The technique involves harvesting the biceps femoris tendon while preserving the distal insertion and harvesting the iliotibial band while leaving the distal insertion at the Gerdy’s tubercle intact.35 The biceps tendon is passed from posterior to anterior through a tibial bone tunnel, and the iliotibial band graft is passed through the same tunnel but in the anterior to posterior direction. A bioabsorbable interference screw is placed into the tibial tunnel from anterior to posterior. The remaining biceps tendon graft is sewn into the soft tissue of the anterior tibia. The tail of the iliotibial band graft is passed through the fibular bone tunnel in the posterior to anterior direction and looped around the fibular head and under the LCL before being sewn back onto itself.35 Kobbe and colleagues36 described a surgical technique using the ipsilateral semitendinosis tendon while Maffulli and colleagues37 and Morrison and colleagues38 presented techniques using the ipsilateral gracilis tendon. These techniques do not jeopardize lateral knee or fibular head stability by avoiding the use of the biceps femoris and iliotibial band, as well as local knee stabilizers. Warner and colleagues31 proposed a similar technique to the aforementioned using a semitendinosus tendon; however, they do not reconstruct the anterior ligamentous structures, as seen in Figures 4A and 4B.39 The authors have concerns that a 2-limb reconstruction as described by Kobbe and colleagues36 and Morrison and colleagues38 may be prone to overconstraint or other errors in tensioning because it does not allow for differential tension to be applied to each limb.31 According to Warner and colleagues,31 the anterior structures of their patients with chronic PTFJ instability have appeared normal, and isolated posterior ligamentous reconstruction has been adequate for the restoration of stability. Camarda and colleagues28 proposed a technique very similar to that of Warner and colleagues;31 however, the tibial tunnel is reamed from posterolateral to the anteromedial aspect of the tibia, exploiting the skin incision previously used for tendon harvest.

Continue to: ARTHRITIS
ARTHRITIS
The management options for secondary arthritis due to chronic PTFJ instability have rarely been discussed in the literature. Arthrodesis or fibular head resection are options for the treatment of arthritis, and the above discussion applies here as well. Yaniv and colleagues40 describe a technical procedure for addressing both instability and secondary joint arthritis. The authors performed a ligament reconstruction of the PTFJ using the anterior part of the biceps femoris combined with interpositional joint arthroplasty using a vascularized fascia lata strip. Two weeks post-operation, the patient was walking with full weight-bearing and 6 weeks post-operation the first sports activities were allowed.40
CONCLUSION
Conditions affecting the PTFJ, their diagnosis, and treatment are infrequent topics of discussion in the literature. While PTFJ injury, instability, and other disease states are admittedly rare, clinicians need to include them in the differential diagnosis of patients presenting with lateral knee complaints. Diagnostic imaging is a critical component in early identification of PTFJ conditions to prevent long-term complications. Most injuries are treated first with conservative methods, reserving surgery as an option when first-line measures are unsuccessful. Advancements in surgical options for dislocation and subluxation/chronic instability of the joint have been made, but further research on their effectiveness and long-term outcomes is needed before a gold-standard treatment can be determined.
- Ogden JA. The anatomy and function of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):186-191.
- Resnick D, Newell JD, Guerra J, Jr., Danzig LA, Niwayama G, Goergen TG. Proximal tibiofibular joint: anatomic-pathologic-radiographic correlation. AJR Am J Rroentgenol. 1978;131(1):133-138. doi: 10.2214/ajr.131.1.133.
- See A, Bear RR, Owens BD. Anatomic mapping for surgical reconstruction of the proximal tibiofibular ligaments. Orthopedics. 2013;36(1):e58-63. doi: 10.3928/01477447-20121217-19.
- Ogden JA. Subluxation of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):192-197. doi: 10.2106/00004623-197456010-00015
- Bozkurt M, Yilmaz E, Akseki D, Havitcioglu H, Gunal I. The evaluation of the proximal tibiofibular joint for patients with lateral knee pain. Knee. 2004;11(4):307-312. doi: 10.1016/j.knee.2003.08.006
- Dirim B, Wangwinyuvirat M, Frank A, et al. Communication between the proximal tibiofibular joint and knee via the subpopliteal recess: MR arthrography with histologic correlation and stratigraphic dissection. AJR Am J Roentgenol. 2008;191(2):W44-W51. doi: 10.2214/AJR.07.3406.
- Eichenblat M, Nathan H. The proximal tibio fibular joint. An anatomical study with clinical and pathological considerations. Int Orthop. 1983;7(1):31-39. doi: 10.1007/bf00267557
- Espregueira-Mendes JD, da Silva MV. Anatomy of the proximal tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):241-249. doi: 10.1007/s00167-005-0684-z.
- Scott J, Lee H, Barsoum W, van den Bogert AJ. The effect of tibiofemoral loading on proximal tibiofibular joint motion. J Anat. 2007;211(5):647-653. doi: 10.1111/j.1469-7580.2007.00803.x.
- Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154. doi: 10.2106/00004623-197456010-00015
- Sekiya JK, Kuhn JE. Instability of the proximal tibiofibular joint. J Am Acad Orthop Surg. 2003;11(2):120-128. doi: 10.5435/00124635-200303000-00006
- Horan J, Quin G. Proximal tibiofibular dislocation. Emerg Med Jl : EMJ. 2006;23(5):e33. doi: 10.1136/emj.2005.032144.
- Semonian RH, Denlinger PM, Duggan RJ. Proximal tibiofibular subluxation relationship to lateral knee pain: a review of proximal tibiofibular joint pathologies. J Orthop Sports Phys Ther. 1995;21(5):248-257. doi: 10.2519/jospt.1995.21.5.248.
- Veth RP, Kingma LM, Nielsen HK. The abnormal proximal tibiofibular joint. Arch Orthop Trauma Surg. 1984;102(3):167-171. doi: 10.1007/bf00575227
- Boya H, Ozcan O, Oztekin HH. Radiological evaluation of the proximal tibiofibular joint in knees with severe primary osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2008;16(2):157-159. doi: 10.1007/s00167-007-0442-5.
- Öztuna V, Yildiz A, Özer C, Milcan A, Kuyurtar F, Turgut A. Involvement of the proximal tibiofibular joint in osteoarthritis of the knee. The Knee. 2003;10(4):347-349. doi: 10.1016/s0968-0160(03)00004-8
- Milankov M, Kecojević V, Gvozdenović N, Obradović M. Dislocation of the proximal tibiofibular joint. Med Pregl. 2013;66(9-10):387-391. doi: 10.2298/mpns1310387m
- Sijbrandij S. Instability of the proximal tibio-fibular joint. Acta Orthop Scand. 1978;49(6):621-626. doi: 10.3109/17453677808993250
- Aladin A, Lam KS, Szypryt EP. The importance of early diagnosis in the management of proximal tibiofibular dislocation: a 9- and 5-year follow-up of a bilateral case. Knee. 2002;9(3):233-236. doi: 10.1016/S0968-0160(02)00012-1
- Turco VJ, Spinella AJ. Anterolateral dislocation of the head of the fibula in sports. Am J Sports Med. 1985;13(4):209-215. doi: 10.1177/036354658501300401.
- Hey HW, Ng LW, Ng YH, Sng WZ, Manohara R, Thambiah JS. Radiographical definition of the proximal tibiofibular joint - A cross-sectional study of 2984 knees and literature review. Injury. 2016;47(6):1276-1281. doi: 10.1016/j.injury.2016.01.035.
- Voglino JA, Denton JR. Acute traumatic proximal tibiofibular joint dislocation confirmed by computed tomography. Orthopedics. 1999;22(2):255-258.
- Burke CJ, Grimm LJ, Boyle MJ, Moorman CT, 3rd, Hash TW, 2nd. Imaging of Proximal Tibiofibular Joint Instability: A 10 year retrospective case series. Clin Imaging. 2016;40(3):470-476. doi: 10.1016/j.clinimag.2015.12.011.
- Parkes JC II, Zelko RR. Isolated acute dislocation of the proximal tibiofibular joint. Case report. J Bone Joint Surg Am. 1973;55(1):177-183. Doi: 10.2106/00004623-197355010-00019
- Gvozdenović N, Gvozdenović K, Obradović M, Stanković M. Modified technique of the treatment for proximal tibiofibular joint dislocation. Vojnosanitetski Pregled. 2017;74(3):282-286. doi: 10.2298/VSP150318177G
- van den Bekerom MP, Weir A, van der Flier RE. Surgical stabilisation of the proximal tibiofibular joint using temporary fixation: a technical note. Acta Orthop Belg. 2004;70(6):604-608.
- Calabró T, Cevolani L, Chehrassan M, Gasbarrini A. A new technique of reduction for isolated proximal tibiofibular joint dislocation: a case report. Eur Rev Med Pharmacol Sci. 2014;18(1):93-95.
- Camarda L, Abruzzese A, D'Arienzo M. Proximal tibiofibular joint reconstruction with autogenous semitendinosus tendon graft. Tech Orthop. 2013;28(3):269-272. doi. 10.1097/BTO.0b013e31827b7182
- Herzog GA, Serrano-Riera R, Sagi HC. Traumatic Proximal Tibiofibular Dislocation: A Marker of Severely Traumatized Extremities. J Orthop Trauma. 2015;29(10):456-459. doi: 10.1097/BOT.0000000000000348.
- Haupt S, Frima H, Sommer C. Proximal tibiofibular joint dislocation associated with tibial shaft fractures - 7 cases. Injury. 2016;47(4):950-953. doi: 10.1016/j.injury.2016.01.037.
- Warner BT, Moulton SG, Cram TR, LaPrade RF. Anatomic Reconstruction of the Proximal Tibiofibular Joint. Arthrosc Tech. 2016;5(1):e207-e210. doi: 10.1016/j.eats.2015.11.004
- Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106. doi: 10.2106/00004623-198668070-00023
- Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
- Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671. doi: 10.1053/jars.2001.22359.
- Miller T. New technique of soft tissue reconstruction for proximal tibiofibular joint instability using iliotibial band and biceps femoris longhead autograft. Tech Orthop. 2014;29(4):243-247. doi: 10.1097/BTO.0000000000000046
- Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
- Maffulli N, Spiezia F, Oliva F, Testa V, Capasso G, Denaro V. Gracilis autograft for recurrent posttraumatic instability of the superior tibiofibular joint. Am J Sports Med. 2010;38(11):2294-2298. doi: 10.1177/0363546510373472.
- Morrison TD, Shaer JA, Little JE. Bilateral, atraumatic, proximal tibiofibular joint instability. Orthopedics. 2011;34(2):133. doi: 10.3928/01477447-20101221-28.
- Horst PK, LaPrade RF. Anatomic reconstruction of chronic symptomatic anterolateral proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1452-1455. doi: 10.1007/s00167-010-1049-9.
- Yaniv M, Koenig U, Imhoff AB. A technical solution for secondary arthritis due to chronic proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):334-336. doi: 10.1007/s001670050173.
- Ogden JA. The anatomy and function of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):186-191.
- Resnick D, Newell JD, Guerra J, Jr., Danzig LA, Niwayama G, Goergen TG. Proximal tibiofibular joint: anatomic-pathologic-radiographic correlation. AJR Am J Rroentgenol. 1978;131(1):133-138. doi: 10.2214/ajr.131.1.133.
- See A, Bear RR, Owens BD. Anatomic mapping for surgical reconstruction of the proximal tibiofibular ligaments. Orthopedics. 2013;36(1):e58-63. doi: 10.3928/01477447-20121217-19.
- Ogden JA. Subluxation of the proximal tibiofibular joint. Clin Orthop Relat Res. 1974(101):192-197. doi: 10.2106/00004623-197456010-00015
- Bozkurt M, Yilmaz E, Akseki D, Havitcioglu H, Gunal I. The evaluation of the proximal tibiofibular joint for patients with lateral knee pain. Knee. 2004;11(4):307-312. doi: 10.1016/j.knee.2003.08.006
- Dirim B, Wangwinyuvirat M, Frank A, et al. Communication between the proximal tibiofibular joint and knee via the subpopliteal recess: MR arthrography with histologic correlation and stratigraphic dissection. AJR Am J Roentgenol. 2008;191(2):W44-W51. doi: 10.2214/AJR.07.3406.
- Eichenblat M, Nathan H. The proximal tibio fibular joint. An anatomical study with clinical and pathological considerations. Int Orthop. 1983;7(1):31-39. doi: 10.1007/bf00267557
- Espregueira-Mendes JD, da Silva MV. Anatomy of the proximal tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):241-249. doi: 10.1007/s00167-005-0684-z.
- Scott J, Lee H, Barsoum W, van den Bogert AJ. The effect of tibiofemoral loading on proximal tibiofibular joint motion. J Anat. 2007;211(5):647-653. doi: 10.1111/j.1469-7580.2007.00803.x.
- Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154. doi: 10.2106/00004623-197456010-00015
- Sekiya JK, Kuhn JE. Instability of the proximal tibiofibular joint. J Am Acad Orthop Surg. 2003;11(2):120-128. doi: 10.5435/00124635-200303000-00006
- Horan J, Quin G. Proximal tibiofibular dislocation. Emerg Med Jl : EMJ. 2006;23(5):e33. doi: 10.1136/emj.2005.032144.
- Semonian RH, Denlinger PM, Duggan RJ. Proximal tibiofibular subluxation relationship to lateral knee pain: a review of proximal tibiofibular joint pathologies. J Orthop Sports Phys Ther. 1995;21(5):248-257. doi: 10.2519/jospt.1995.21.5.248.
- Veth RP, Kingma LM, Nielsen HK. The abnormal proximal tibiofibular joint. Arch Orthop Trauma Surg. 1984;102(3):167-171. doi: 10.1007/bf00575227
- Boya H, Ozcan O, Oztekin HH. Radiological evaluation of the proximal tibiofibular joint in knees with severe primary osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2008;16(2):157-159. doi: 10.1007/s00167-007-0442-5.
- Öztuna V, Yildiz A, Özer C, Milcan A, Kuyurtar F, Turgut A. Involvement of the proximal tibiofibular joint in osteoarthritis of the knee. The Knee. 2003;10(4):347-349. doi: 10.1016/s0968-0160(03)00004-8
- Milankov M, Kecojević V, Gvozdenović N, Obradović M. Dislocation of the proximal tibiofibular joint. Med Pregl. 2013;66(9-10):387-391. doi: 10.2298/mpns1310387m
- Sijbrandij S. Instability of the proximal tibio-fibular joint. Acta Orthop Scand. 1978;49(6):621-626. doi: 10.3109/17453677808993250
- Aladin A, Lam KS, Szypryt EP. The importance of early diagnosis in the management of proximal tibiofibular dislocation: a 9- and 5-year follow-up of a bilateral case. Knee. 2002;9(3):233-236. doi: 10.1016/S0968-0160(02)00012-1
- Turco VJ, Spinella AJ. Anterolateral dislocation of the head of the fibula in sports. Am J Sports Med. 1985;13(4):209-215. doi: 10.1177/036354658501300401.
- Hey HW, Ng LW, Ng YH, Sng WZ, Manohara R, Thambiah JS. Radiographical definition of the proximal tibiofibular joint - A cross-sectional study of 2984 knees and literature review. Injury. 2016;47(6):1276-1281. doi: 10.1016/j.injury.2016.01.035.
- Voglino JA, Denton JR. Acute traumatic proximal tibiofibular joint dislocation confirmed by computed tomography. Orthopedics. 1999;22(2):255-258.
- Burke CJ, Grimm LJ, Boyle MJ, Moorman CT, 3rd, Hash TW, 2nd. Imaging of Proximal Tibiofibular Joint Instability: A 10 year retrospective case series. Clin Imaging. 2016;40(3):470-476. doi: 10.1016/j.clinimag.2015.12.011.
- Parkes JC II, Zelko RR. Isolated acute dislocation of the proximal tibiofibular joint. Case report. J Bone Joint Surg Am. 1973;55(1):177-183. Doi: 10.2106/00004623-197355010-00019
- Gvozdenović N, Gvozdenović K, Obradović M, Stanković M. Modified technique of the treatment for proximal tibiofibular joint dislocation. Vojnosanitetski Pregled. 2017;74(3):282-286. doi: 10.2298/VSP150318177G
- van den Bekerom MP, Weir A, van der Flier RE. Surgical stabilisation of the proximal tibiofibular joint using temporary fixation: a technical note. Acta Orthop Belg. 2004;70(6):604-608.
- Calabró T, Cevolani L, Chehrassan M, Gasbarrini A. A new technique of reduction for isolated proximal tibiofibular joint dislocation: a case report. Eur Rev Med Pharmacol Sci. 2014;18(1):93-95.
- Camarda L, Abruzzese A, D'Arienzo M. Proximal tibiofibular joint reconstruction with autogenous semitendinosus tendon graft. Tech Orthop. 2013;28(3):269-272. doi. 10.1097/BTO.0b013e31827b7182
- Herzog GA, Serrano-Riera R, Sagi HC. Traumatic Proximal Tibiofibular Dislocation: A Marker of Severely Traumatized Extremities. J Orthop Trauma. 2015;29(10):456-459. doi: 10.1097/BOT.0000000000000348.
- Haupt S, Frima H, Sommer C. Proximal tibiofibular joint dislocation associated with tibial shaft fractures - 7 cases. Injury. 2016;47(4):950-953. doi: 10.1016/j.injury.2016.01.037.
- Warner BT, Moulton SG, Cram TR, LaPrade RF. Anatomic Reconstruction of the Proximal Tibiofibular Joint. Arthrosc Tech. 2016;5(1):e207-e210. doi: 10.1016/j.eats.2015.11.004
- Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106. doi: 10.2106/00004623-198668070-00023
- Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
- Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671. doi: 10.1053/jars.2001.22359.
- Miller T. New technique of soft tissue reconstruction for proximal tibiofibular joint instability using iliotibial band and biceps femoris longhead autograft. Tech Orthop. 2014;29(4):243-247. doi: 10.1097/BTO.0000000000000046
- Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
- Maffulli N, Spiezia F, Oliva F, Testa V, Capasso G, Denaro V. Gracilis autograft for recurrent posttraumatic instability of the superior tibiofibular joint. Am J Sports Med. 2010;38(11):2294-2298. doi: 10.1177/0363546510373472.
- Morrison TD, Shaer JA, Little JE. Bilateral, atraumatic, proximal tibiofibular joint instability. Orthopedics. 2011;34(2):133. doi: 10.3928/01477447-20101221-28.
- Horst PK, LaPrade RF. Anatomic reconstruction of chronic symptomatic anterolateral proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1452-1455. doi: 10.1007/s00167-010-1049-9.
- Yaniv M, Koenig U, Imhoff AB. A technical solution for secondary arthritis due to chronic proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):334-336. doi: 10.1007/s001670050173.
TAKE-HOME POINTS
- Problems of the proximal tibiofibular joint (PFTJ) should be considered in the differential diagnosis when a patient presents with complaints in the lateral aspect of the knee.
- The primary function of the PTFJ is to transmit and absorb axial loads from weight bearing on the extremity, and to dissipate torsional loads applied to the leg and ankle.
- The most common instability pattern is anterolateral fibular displacement.
- Most proximal tibiofibular joint instabilities can be treated with closed reduction and conservative care, but some require internal fixation or soft-tissue reconstruction.
- Arthritic conditions of the PTFJ are treated similar to those of any diarthrodial joint, with additional option of surgical arthrodesis or resection arthroplasty.
Obesity meds used by just over half of pediatric obesity programs
NASHVILLE, TENN. –
Programs that didn’t offer pharmacotherapy for children and adolescents with obesity cited a variety of reasons in responses to a survey of 33 multicomponent pediatric weight management programs (PWMPs).
Simply not being in favor of using pharmacotherapy for obesity treatment was the most frequently cited reason, named by seven PWMPs that didn’t prescribe obesity medications.
The second most common response to the survey, cited by six programs, was a lack of knowledge about prescribing medications for obesity, and concerns about insurance coverage were noted by five programs, said Claudia Fox, MD, and her colleagues in a poster presentation at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. “Despite recommendations, few youth with severe obesity are treated with medications.”
Of the programs that did offer pharmacotherapy, 14 prescribed topiramate, and 13 prescribed phentermine. Metformin was used by 11 programs, and orlistat by eight. Six programs prescribed the fixed-dose combination of topiramate and phentermine.
Lorcaserin, naltrexone/bupropion, liraglutide, phendimetrazine, and naltrexone alone all were used by fewer than five programs each.
The national Pediatric Obesity Weight Evaluation Registry (POWER) “was established in 2013 to identify and promote effective intervention strategies for pediatric obesity,” wrote Dr. Fox and her colleagues
Of the 33 POWER PWMPs who were invited to participate, 30 completed a program profile survey. Of these, 16 programs (53%) offered pharmacotherapy, wrote Dr. Fox, the codirector of the University of Minnesota’s Center for Pediatric Obesity Medicine, Minneapolis, and her colleagues in the POWER work group.
In addition to not being in favor of prescribing obesity medication for pediatric patients, lack of knowledge, and insurance concerns, one program cited limited outcome studies for pediatric obesity pharmacotherapy. One other program’s response noted that patients couldn’t be seen frequently enough to assess the safety of obesity medications.
Taken together, the POWER sites had 7,880 patients. Just 5% were aged 2- 5 years, 48% were aged 6-11 years, and 47% were aged 12-18 years. Just over half (53%) were female.
At baseline, about a quarter of patients (26.4%) had class 1 obesity, defined as a body mass index of at least the 95th age- and sex-adjusted percentile. Children and adolescents with class 2 obesity (BMI of at least 1.2-1.4 times the 95th percentile) made up 35.3% of patients; 38.3% had class 3 obesity, with BMIs greater than 1.4 times the 95th percentile.
In 2017, the Endocrine Society published updated clinical practice guidelines for the assessment, treatment, and prevention of pediatric obesity (J Clin Endocrin Metab. 2017 Mar;102:3;709-57). The guidelines for pediatric obesity treatment recommend intensive lifestyle modifications including dietary, physical activity, and behavioral interventions. Pharmacotherapy is suggested “only after a formal program of intensive lifestyle modification has failed to limit weight gain or to ameliorate comorbidities.” Additionally, say the guidelines, Food and Drug Administration–approved pharmacotherapy should be used only “with a concomitant lifestyle modification program of the highest intensity available and only by clinicians who are experienced in the use of anti-obesity agents and are aware of the potential for adverse reactions.”
“Most commonly prescribed medications are not FDA approved for indication of obesity in pediatrics,” noted Dr. Fox and her coauthors. “Further research is needed to evaluate efficacy of pharmacotherapy in the pediatric population and to understand factors impacting prescribing practices.”
Dr. Fox reported no outside sources of funding and had no relevant financial disclosures.
NASHVILLE, TENN. –
Programs that didn’t offer pharmacotherapy for children and adolescents with obesity cited a variety of reasons in responses to a survey of 33 multicomponent pediatric weight management programs (PWMPs).
Simply not being in favor of using pharmacotherapy for obesity treatment was the most frequently cited reason, named by seven PWMPs that didn’t prescribe obesity medications.
The second most common response to the survey, cited by six programs, was a lack of knowledge about prescribing medications for obesity, and concerns about insurance coverage were noted by five programs, said Claudia Fox, MD, and her colleagues in a poster presentation at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. “Despite recommendations, few youth with severe obesity are treated with medications.”
Of the programs that did offer pharmacotherapy, 14 prescribed topiramate, and 13 prescribed phentermine. Metformin was used by 11 programs, and orlistat by eight. Six programs prescribed the fixed-dose combination of topiramate and phentermine.
Lorcaserin, naltrexone/bupropion, liraglutide, phendimetrazine, and naltrexone alone all were used by fewer than five programs each.
The national Pediatric Obesity Weight Evaluation Registry (POWER) “was established in 2013 to identify and promote effective intervention strategies for pediatric obesity,” wrote Dr. Fox and her colleagues
Of the 33 POWER PWMPs who were invited to participate, 30 completed a program profile survey. Of these, 16 programs (53%) offered pharmacotherapy, wrote Dr. Fox, the codirector of the University of Minnesota’s Center for Pediatric Obesity Medicine, Minneapolis, and her colleagues in the POWER work group.
In addition to not being in favor of prescribing obesity medication for pediatric patients, lack of knowledge, and insurance concerns, one program cited limited outcome studies for pediatric obesity pharmacotherapy. One other program’s response noted that patients couldn’t be seen frequently enough to assess the safety of obesity medications.
Taken together, the POWER sites had 7,880 patients. Just 5% were aged 2- 5 years, 48% were aged 6-11 years, and 47% were aged 12-18 years. Just over half (53%) were female.
At baseline, about a quarter of patients (26.4%) had class 1 obesity, defined as a body mass index of at least the 95th age- and sex-adjusted percentile. Children and adolescents with class 2 obesity (BMI of at least 1.2-1.4 times the 95th percentile) made up 35.3% of patients; 38.3% had class 3 obesity, with BMIs greater than 1.4 times the 95th percentile.
In 2017, the Endocrine Society published updated clinical practice guidelines for the assessment, treatment, and prevention of pediatric obesity (J Clin Endocrin Metab. 2017 Mar;102:3;709-57). The guidelines for pediatric obesity treatment recommend intensive lifestyle modifications including dietary, physical activity, and behavioral interventions. Pharmacotherapy is suggested “only after a formal program of intensive lifestyle modification has failed to limit weight gain or to ameliorate comorbidities.” Additionally, say the guidelines, Food and Drug Administration–approved pharmacotherapy should be used only “with a concomitant lifestyle modification program of the highest intensity available and only by clinicians who are experienced in the use of anti-obesity agents and are aware of the potential for adverse reactions.”
“Most commonly prescribed medications are not FDA approved for indication of obesity in pediatrics,” noted Dr. Fox and her coauthors. “Further research is needed to evaluate efficacy of pharmacotherapy in the pediatric population and to understand factors impacting prescribing practices.”
Dr. Fox reported no outside sources of funding and had no relevant financial disclosures.
NASHVILLE, TENN. –
Programs that didn’t offer pharmacotherapy for children and adolescents with obesity cited a variety of reasons in responses to a survey of 33 multicomponent pediatric weight management programs (PWMPs).
Simply not being in favor of using pharmacotherapy for obesity treatment was the most frequently cited reason, named by seven PWMPs that didn’t prescribe obesity medications.
The second most common response to the survey, cited by six programs, was a lack of knowledge about prescribing medications for obesity, and concerns about insurance coverage were noted by five programs, said Claudia Fox, MD, and her colleagues in a poster presentation at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. “Despite recommendations, few youth with severe obesity are treated with medications.”
Of the programs that did offer pharmacotherapy, 14 prescribed topiramate, and 13 prescribed phentermine. Metformin was used by 11 programs, and orlistat by eight. Six programs prescribed the fixed-dose combination of topiramate and phentermine.
Lorcaserin, naltrexone/bupropion, liraglutide, phendimetrazine, and naltrexone alone all were used by fewer than five programs each.
The national Pediatric Obesity Weight Evaluation Registry (POWER) “was established in 2013 to identify and promote effective intervention strategies for pediatric obesity,” wrote Dr. Fox and her colleagues
Of the 33 POWER PWMPs who were invited to participate, 30 completed a program profile survey. Of these, 16 programs (53%) offered pharmacotherapy, wrote Dr. Fox, the codirector of the University of Minnesota’s Center for Pediatric Obesity Medicine, Minneapolis, and her colleagues in the POWER work group.
In addition to not being in favor of prescribing obesity medication for pediatric patients, lack of knowledge, and insurance concerns, one program cited limited outcome studies for pediatric obesity pharmacotherapy. One other program’s response noted that patients couldn’t be seen frequently enough to assess the safety of obesity medications.
Taken together, the POWER sites had 7,880 patients. Just 5% were aged 2- 5 years, 48% were aged 6-11 years, and 47% were aged 12-18 years. Just over half (53%) were female.
At baseline, about a quarter of patients (26.4%) had class 1 obesity, defined as a body mass index of at least the 95th age- and sex-adjusted percentile. Children and adolescents with class 2 obesity (BMI of at least 1.2-1.4 times the 95th percentile) made up 35.3% of patients; 38.3% had class 3 obesity, with BMIs greater than 1.4 times the 95th percentile.
In 2017, the Endocrine Society published updated clinical practice guidelines for the assessment, treatment, and prevention of pediatric obesity (J Clin Endocrin Metab. 2017 Mar;102:3;709-57). The guidelines for pediatric obesity treatment recommend intensive lifestyle modifications including dietary, physical activity, and behavioral interventions. Pharmacotherapy is suggested “only after a formal program of intensive lifestyle modification has failed to limit weight gain or to ameliorate comorbidities.” Additionally, say the guidelines, Food and Drug Administration–approved pharmacotherapy should be used only “with a concomitant lifestyle modification program of the highest intensity available and only by clinicians who are experienced in the use of anti-obesity agents and are aware of the potential for adverse reactions.”
“Most commonly prescribed medications are not FDA approved for indication of obesity in pediatrics,” noted Dr. Fox and her coauthors. “Further research is needed to evaluate efficacy of pharmacotherapy in the pediatric population and to understand factors impacting prescribing practices.”
Dr. Fox reported no outside sources of funding and had no relevant financial disclosures.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Just over half of pediatric weight management programs prescribed obesity medications.
Major finding: Of 30 programs responding, 16 (53%) prescribed obesity medication.
Study details: Survey of 33 programs in the Pediatric Obesity Weight Evaluation Registry (POWER).
Disclosures: Dr. Fox reported no outside sources of funding and no conflicts of interest.
Higher Rate of Loss in Unplanned Pregnancies for Women With Epilepsy
The risk of fetal loss is greater if the interval between pregnancies is under one year.
Unplanned pregnancy among women with epilepsy is associated with twice the risk of spontaneous fetal loss (SFL) when compared with women with epilepsy who planned their pregnancy, according to results from a retrospective study published online ahead of print October 15 in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of SFL in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” said Andrew G. Herzog, MD, a neurologist at the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Massachusetts, and colleagues.
The Epilepsy Birth Control Registry
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014. Respondents provided data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were between ages 18 and 47 (mean, 28.5). Approximately 8.7% of the cohort were minorities, and 39.8% had household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, and pregnancy outcomes such as live birth, induced abortion, and SFL. Patients were categorized as receiving no therapy, monotherapy, or polytherapy. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed.
Most Pregnancies Were Unplanned
Of 794 pregnancies, 530 (66.8%) were unplanned and 264 (33.2%) were planned. Outcomes included 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFLs (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with planned (43 patients, 16.4%) The risk ratio (RR) of SFL was 2.14. According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio [OR], 2.75), as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR, 3.57).
There was an association between maternal age (OR, 0.957) and risk of SFL. Risk was lower in the 18- to 27-year-olds (118 patients; 29.5%; RR, 0.57) and 28- to 37-year-olds (44 patients; 20.8%; RR, 0.40), compared with the under-18 group (15 patients, 51.7%). Risk of SFL was related to interpregnancy interval (OR, 2.878). This risk was greater if the interpregnancy interval was under one year (56 patients, 45.9%), compared with one year (56 patients, 22.8%) or higher (RR, 2.02).
The Epilepsy Foun-dation and Lundbeck funded the study. Dr. Herzog reports grants, and two coauthors received salary support from grants, from the two organizations.
—Jeff Craven
Suggested Reading
Herzog AG, Mandle HB, MacEachern DB. Association of unintended pregnancy with spontaneous fetal loss in women with epilepsy: findings of the Epilepsy Birth Control Registry. JAMA Neurol. 2018 Oct 15 [Epub ahead of print].
The risk of fetal loss is greater if the interval between pregnancies is under one year.
The risk of fetal loss is greater if the interval between pregnancies is under one year.
Unplanned pregnancy among women with epilepsy is associated with twice the risk of spontaneous fetal loss (SFL) when compared with women with epilepsy who planned their pregnancy, according to results from a retrospective study published online ahead of print October 15 in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of SFL in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” said Andrew G. Herzog, MD, a neurologist at the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Massachusetts, and colleagues.
The Epilepsy Birth Control Registry
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014. Respondents provided data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were between ages 18 and 47 (mean, 28.5). Approximately 8.7% of the cohort were minorities, and 39.8% had household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, and pregnancy outcomes such as live birth, induced abortion, and SFL. Patients were categorized as receiving no therapy, monotherapy, or polytherapy. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed.
Most Pregnancies Were Unplanned
Of 794 pregnancies, 530 (66.8%) were unplanned and 264 (33.2%) were planned. Outcomes included 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFLs (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with planned (43 patients, 16.4%) The risk ratio (RR) of SFL was 2.14. According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio [OR], 2.75), as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR, 3.57).
There was an association between maternal age (OR, 0.957) and risk of SFL. Risk was lower in the 18- to 27-year-olds (118 patients; 29.5%; RR, 0.57) and 28- to 37-year-olds (44 patients; 20.8%; RR, 0.40), compared with the under-18 group (15 patients, 51.7%). Risk of SFL was related to interpregnancy interval (OR, 2.878). This risk was greater if the interpregnancy interval was under one year (56 patients, 45.9%), compared with one year (56 patients, 22.8%) or higher (RR, 2.02).
The Epilepsy Foun-dation and Lundbeck funded the study. Dr. Herzog reports grants, and two coauthors received salary support from grants, from the two organizations.
—Jeff Craven
Suggested Reading
Herzog AG, Mandle HB, MacEachern DB. Association of unintended pregnancy with spontaneous fetal loss in women with epilepsy: findings of the Epilepsy Birth Control Registry. JAMA Neurol. 2018 Oct 15 [Epub ahead of print].
Unplanned pregnancy among women with epilepsy is associated with twice the risk of spontaneous fetal loss (SFL) when compared with women with epilepsy who planned their pregnancy, according to results from a retrospective study published online ahead of print October 15 in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of SFL in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” said Andrew G. Herzog, MD, a neurologist at the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Massachusetts, and colleagues.
The Epilepsy Birth Control Registry
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014. Respondents provided data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were between ages 18 and 47 (mean, 28.5). Approximately 8.7% of the cohort were minorities, and 39.8% had household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, and pregnancy outcomes such as live birth, induced abortion, and SFL. Patients were categorized as receiving no therapy, monotherapy, or polytherapy. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed.
Most Pregnancies Were Unplanned
Of 794 pregnancies, 530 (66.8%) were unplanned and 264 (33.2%) were planned. Outcomes included 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFLs (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with planned (43 patients, 16.4%) The risk ratio (RR) of SFL was 2.14. According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio [OR], 2.75), as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR, 3.57).
There was an association between maternal age (OR, 0.957) and risk of SFL. Risk was lower in the 18- to 27-year-olds (118 patients; 29.5%; RR, 0.57) and 28- to 37-year-olds (44 patients; 20.8%; RR, 0.40), compared with the under-18 group (15 patients, 51.7%). Risk of SFL was related to interpregnancy interval (OR, 2.878). This risk was greater if the interpregnancy interval was under one year (56 patients, 45.9%), compared with one year (56 patients, 22.8%) or higher (RR, 2.02).
The Epilepsy Foun-dation and Lundbeck funded the study. Dr. Herzog reports grants, and two coauthors received salary support from grants, from the two organizations.
—Jeff Craven
Suggested Reading
Herzog AG, Mandle HB, MacEachern DB. Association of unintended pregnancy with spontaneous fetal loss in women with epilepsy: findings of the Epilepsy Birth Control Registry. JAMA Neurol. 2018 Oct 15 [Epub ahead of print].
Virus-Specific T-Cell Infusions May Resolve Progressive Multifocal Leukoencephalopathy
Two of three patients cleared JC virus from CSF after infusion.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Two of three patients cleared JC virus from CSF after infusion.
Two of three patients cleared JC virus from CSF after infusion.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Methotrexate relieves pain of Chikungunya-associated arthritis
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: On a 10-point visual analog scale, the pain reduction from baseline on methotrexate at 8 weeks was 4.5 (P less than .0001).
Study details: Retrospective observational study.
Disclosures: The authors declared no potential conflicts of interest.
Source: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943
Debunking Psoriasis Myths: Psoriasis Is More Than Skin Deep
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.