User login
Weekend Effect Persists
Recent research published in the Journal of the American Medical Association (JAMA) highlights a continued hospital care issue identified in previous studies: worse patient outcomes from in-hospital cardiac arrests occur at night or on the weekend.1
An analysis of 86,748 adult cardiac events between January 2000 and February 2007 in 507 hospitals participating in the National Registry of Cardiopulmonary Resuscitation (NRCPR) compared outcomes at night (from 11 p.m. to 6:59 a.m.) and weekends (from 11 p.m. Friday to 6:59 a.m. Monday) with day/evening shifts. The primary measure of survival to discharge and secondary outcomes from in-hospital cardiac arrests were significantly worse during nights and weekends. In essence, heart attack patients were 41% more likely to survive if treated during daytime weekday hours.
“This was the first comprehensive, large-scale study in a cross section of hospitals across the country of heart attack survival differences between shifts,” says lead author Mary Ann Peberdy, MD, of Virginia Commonwealth University in Richmond. “We adjusted for a variety of potentially confounding factors and patient characteristics, none of which explained the worse outcomes nights and weekends.”
The national database was not designed to provide an explanation for its findings, which may be due to multiple patient, event or hospital factors. “We can’t exclude physiological factors of patients or of staff working on the night shift,” Dr. Peberdy explains. “But I think we need to focus on process issues. We know that hospitals simply do not run the same way at night. Things are different—more errors, more accidents, more needle sticks, less people around. Those who work the night shift may also be less experienced,” and early identification of heart attacks is critical to positive outcome.
The JAMA results confirm previous research documenting worse outcomes on nights and weekends. Single-site studies and a smaller study of heart attack survival in New Jersey hospitals for weekend versus weekday admissions found similar trends.2
Stroke patients who enter the hospital at night or over the weekend are more likely to die in the hospital than those admitted during daytime hours (7 a.m. to 6 p.m.) on weekdays, according to two studies presented at the American Heart Association’s International Stroke Conference in New Orleans in February 2008.3 Those differences were particularly striking for hemorrhagic strokes. Similar outcomes also have been reported for pulmonary embolisms.
Shift Differences
Different studies have approached this issue in different ways, comparing business hours (e.g., 7 a.m. to 6 p.m.) with evenings, nights, and weekends, or days and evenings up to 11 p.m. with nights. Weekends are compared to weekdays but also to weekend nights.
The size of the hospital did not explain the shift differences found in Dr. Peberdy’s study. For hospitalists trying to address the underlying problems of after-hours quality, the size of the hospital is relevant. Only larger hospitals can afford hospitalist groups large enough to cover night and weekend shifts. For those that can, are all members of the group taking their turns at night, does this duty fall to the junior members, or is the group lucky enough to employ nocturnists who want to work at night? (See The Hospitalist, May 2006, p. 27, for an article on nocturnists in hospital medicine.) Whether the hospital has an academic emphasis also can influence who responds to crises after hours—attendings or house staff (in other words, sleep-deprived residents).
If hospitalists work nights, they are more likely to notice what isn’t available or what doesn’t work as well as what contributes to nocturnal quality problems and what might help to compensate for these differences. Even if hospitalists are not present in the facility at night, technology can help guide appropriate response to cardiac crises, suggests David Grace, MD, area medical officer for the Schumacher Group’s Hospital Medicine Division and a hospitalist at Southwest Medical Center in Lafayette, La.
“Several weeks ago a patient in the hospital was having chest pains,” he recalls. “A nurse called me at home and I ordered an electrocardiogram.” The electrocardiogram’s (EKG) computer program indicated “nonspecific changes” in the patient’s cardiac function, but Dr. Grace asked the nurse to scan the printout and send it to his Web-based fax number.
“I looked at the EKG on my PDA,” he continues. “It was subtle, but it seemed to me that this patient was having a myocardial infarction[MI]. I told the nurse to do the blood work for a suspected MI, give the patient an aspirin and take another EKG, which more clearly showed the MI. If I had not had the ability to look at the printout, I would have had to trust the nurse’s observation or the EKG computer program. As it was, we caught it early and the patient did well.”
Cooperation and Staffing
“I have lived it. I certainly understand the research showing different outcomes from MIs at night,” Dr. Grace observes. “At night, patients are usually asleep, so processes that begin with early warning signals, such as chest pains, may go further down the path before they are identified, especially if the patient has taken a sleeping pill. Often, nurse-to-patient staffing ratios are dramatically different at night—and somewhat reduced on weekends. I’ve also worked in hospitals where on weekends, unless it was a true life-threatening emergency, you could not get an MRI. So if you ordered one on a Saturday, it wouldn’t happen until Monday. There are things you pick up on the MRI that you miss on the CT scan; for example, bleeding, which can affect your management of the patient.”
“I would not be surprised to hear of worse survival for any of these acute decompensations—it goes for acute GI bleeds, stroke, and hemorrhage, as well as MIs,” adds Steven Liu, MD, of Emory University Eastside Medical Center in Atlanta. “We dealt with the problem in this hospital five years ago and addressed a lot of these quality issues by partnering with hospital administration and specialists.”
On weekends, many services may be less available or not available at all in the hospital, from interventional radiology to physical therapy. If patients have fewer contacts with different hospital personnel, it is more likely that subtle early signs of acute problems will go unnoticed until later.
“At my hospital, the interventional radiology [IR] department is not open 24 hours,” says Erica Grabscheid, MD, associate director of the hospitalist program at Beth Israel Hospital in New York City. If a peripherally inserted central catheter needs to be placed on the weekend but IR is not available, the patient may have to wait until Monday morning. Alternatives for the hospitalist, Dr. Grabscheid says, are to become skilled at line placement or to collect data for the hospital’s administrators on the costs of not having 24-hour IR.
Jeffrey Robinson, MD, hospitalist group leader for Intermountain Medical Center in Salt Lake City, Utah, believes staffing is an essential part of the equation. He says Intermountain Health has made a commitment to do what’s necessary for quality patient care—including adequate staffing. “Every time we feel we’re stretched, we add more hospitalist shifts, including weekends and holidays. I feel we give good patient care from the physician side. Obstacles have more to do with ancillaries, but we’ve made great progress in addressing these, as well.”
At one time, interventional radiology only was available in the hospital five days a week, Dr. Robinson notes. “You couldn’t get feeding tubes placed or certain other interventional procedures. But with the commitment of radiology and the hiring power of Intermountain Health, we now have 24-hour availability. MRIs have been harder to cover after hours, but there is now an on-call team for MRIs.”
In each case, hospitalists were important advocates for expanding the hours of availability, Dr. Robinson says: “We needed to gather data and look at results for patients staying over the weekend. We also got expanded case management, so that nursing home placements could happen on Saturdays and Sundays.” TH
Larry Beresford is a medical writer based in California.
References
- Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008; 99(7):785-792.
- Kostis WS, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007; 356(11):1099-1109.
- American Heart Association. Deaths higher in stroke patients who enter hospital at night, weekends. Available at www.sciencedaily.com/releases/2008/02/080220161720/htm. Accessed March 13, 2008.
Recent research published in the Journal of the American Medical Association (JAMA) highlights a continued hospital care issue identified in previous studies: worse patient outcomes from in-hospital cardiac arrests occur at night or on the weekend.1
An analysis of 86,748 adult cardiac events between January 2000 and February 2007 in 507 hospitals participating in the National Registry of Cardiopulmonary Resuscitation (NRCPR) compared outcomes at night (from 11 p.m. to 6:59 a.m.) and weekends (from 11 p.m. Friday to 6:59 a.m. Monday) with day/evening shifts. The primary measure of survival to discharge and secondary outcomes from in-hospital cardiac arrests were significantly worse during nights and weekends. In essence, heart attack patients were 41% more likely to survive if treated during daytime weekday hours.
“This was the first comprehensive, large-scale study in a cross section of hospitals across the country of heart attack survival differences between shifts,” says lead author Mary Ann Peberdy, MD, of Virginia Commonwealth University in Richmond. “We adjusted for a variety of potentially confounding factors and patient characteristics, none of which explained the worse outcomes nights and weekends.”
The national database was not designed to provide an explanation for its findings, which may be due to multiple patient, event or hospital factors. “We can’t exclude physiological factors of patients or of staff working on the night shift,” Dr. Peberdy explains. “But I think we need to focus on process issues. We know that hospitals simply do not run the same way at night. Things are different—more errors, more accidents, more needle sticks, less people around. Those who work the night shift may also be less experienced,” and early identification of heart attacks is critical to positive outcome.
The JAMA results confirm previous research documenting worse outcomes on nights and weekends. Single-site studies and a smaller study of heart attack survival in New Jersey hospitals for weekend versus weekday admissions found similar trends.2
Stroke patients who enter the hospital at night or over the weekend are more likely to die in the hospital than those admitted during daytime hours (7 a.m. to 6 p.m.) on weekdays, according to two studies presented at the American Heart Association’s International Stroke Conference in New Orleans in February 2008.3 Those differences were particularly striking for hemorrhagic strokes. Similar outcomes also have been reported for pulmonary embolisms.
Shift Differences
Different studies have approached this issue in different ways, comparing business hours (e.g., 7 a.m. to 6 p.m.) with evenings, nights, and weekends, or days and evenings up to 11 p.m. with nights. Weekends are compared to weekdays but also to weekend nights.
The size of the hospital did not explain the shift differences found in Dr. Peberdy’s study. For hospitalists trying to address the underlying problems of after-hours quality, the size of the hospital is relevant. Only larger hospitals can afford hospitalist groups large enough to cover night and weekend shifts. For those that can, are all members of the group taking their turns at night, does this duty fall to the junior members, or is the group lucky enough to employ nocturnists who want to work at night? (See The Hospitalist, May 2006, p. 27, for an article on nocturnists in hospital medicine.) Whether the hospital has an academic emphasis also can influence who responds to crises after hours—attendings or house staff (in other words, sleep-deprived residents).
If hospitalists work nights, they are more likely to notice what isn’t available or what doesn’t work as well as what contributes to nocturnal quality problems and what might help to compensate for these differences. Even if hospitalists are not present in the facility at night, technology can help guide appropriate response to cardiac crises, suggests David Grace, MD, area medical officer for the Schumacher Group’s Hospital Medicine Division and a hospitalist at Southwest Medical Center in Lafayette, La.
“Several weeks ago a patient in the hospital was having chest pains,” he recalls. “A nurse called me at home and I ordered an electrocardiogram.” The electrocardiogram’s (EKG) computer program indicated “nonspecific changes” in the patient’s cardiac function, but Dr. Grace asked the nurse to scan the printout and send it to his Web-based fax number.
“I looked at the EKG on my PDA,” he continues. “It was subtle, but it seemed to me that this patient was having a myocardial infarction[MI]. I told the nurse to do the blood work for a suspected MI, give the patient an aspirin and take another EKG, which more clearly showed the MI. If I had not had the ability to look at the printout, I would have had to trust the nurse’s observation or the EKG computer program. As it was, we caught it early and the patient did well.”
Cooperation and Staffing
“I have lived it. I certainly understand the research showing different outcomes from MIs at night,” Dr. Grace observes. “At night, patients are usually asleep, so processes that begin with early warning signals, such as chest pains, may go further down the path before they are identified, especially if the patient has taken a sleeping pill. Often, nurse-to-patient staffing ratios are dramatically different at night—and somewhat reduced on weekends. I’ve also worked in hospitals where on weekends, unless it was a true life-threatening emergency, you could not get an MRI. So if you ordered one on a Saturday, it wouldn’t happen until Monday. There are things you pick up on the MRI that you miss on the CT scan; for example, bleeding, which can affect your management of the patient.”
“I would not be surprised to hear of worse survival for any of these acute decompensations—it goes for acute GI bleeds, stroke, and hemorrhage, as well as MIs,” adds Steven Liu, MD, of Emory University Eastside Medical Center in Atlanta. “We dealt with the problem in this hospital five years ago and addressed a lot of these quality issues by partnering with hospital administration and specialists.”
On weekends, many services may be less available or not available at all in the hospital, from interventional radiology to physical therapy. If patients have fewer contacts with different hospital personnel, it is more likely that subtle early signs of acute problems will go unnoticed until later.
“At my hospital, the interventional radiology [IR] department is not open 24 hours,” says Erica Grabscheid, MD, associate director of the hospitalist program at Beth Israel Hospital in New York City. If a peripherally inserted central catheter needs to be placed on the weekend but IR is not available, the patient may have to wait until Monday morning. Alternatives for the hospitalist, Dr. Grabscheid says, are to become skilled at line placement or to collect data for the hospital’s administrators on the costs of not having 24-hour IR.
Jeffrey Robinson, MD, hospitalist group leader for Intermountain Medical Center in Salt Lake City, Utah, believes staffing is an essential part of the equation. He says Intermountain Health has made a commitment to do what’s necessary for quality patient care—including adequate staffing. “Every time we feel we’re stretched, we add more hospitalist shifts, including weekends and holidays. I feel we give good patient care from the physician side. Obstacles have more to do with ancillaries, but we’ve made great progress in addressing these, as well.”
At one time, interventional radiology only was available in the hospital five days a week, Dr. Robinson notes. “You couldn’t get feeding tubes placed or certain other interventional procedures. But with the commitment of radiology and the hiring power of Intermountain Health, we now have 24-hour availability. MRIs have been harder to cover after hours, but there is now an on-call team for MRIs.”
In each case, hospitalists were important advocates for expanding the hours of availability, Dr. Robinson says: “We needed to gather data and look at results for patients staying over the weekend. We also got expanded case management, so that nursing home placements could happen on Saturdays and Sundays.” TH
Larry Beresford is a medical writer based in California.
References
- Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008; 99(7):785-792.
- Kostis WS, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007; 356(11):1099-1109.
- American Heart Association. Deaths higher in stroke patients who enter hospital at night, weekends. Available at www.sciencedaily.com/releases/2008/02/080220161720/htm. Accessed March 13, 2008.
Recent research published in the Journal of the American Medical Association (JAMA) highlights a continued hospital care issue identified in previous studies: worse patient outcomes from in-hospital cardiac arrests occur at night or on the weekend.1
An analysis of 86,748 adult cardiac events between January 2000 and February 2007 in 507 hospitals participating in the National Registry of Cardiopulmonary Resuscitation (NRCPR) compared outcomes at night (from 11 p.m. to 6:59 a.m.) and weekends (from 11 p.m. Friday to 6:59 a.m. Monday) with day/evening shifts. The primary measure of survival to discharge and secondary outcomes from in-hospital cardiac arrests were significantly worse during nights and weekends. In essence, heart attack patients were 41% more likely to survive if treated during daytime weekday hours.
“This was the first comprehensive, large-scale study in a cross section of hospitals across the country of heart attack survival differences between shifts,” says lead author Mary Ann Peberdy, MD, of Virginia Commonwealth University in Richmond. “We adjusted for a variety of potentially confounding factors and patient characteristics, none of which explained the worse outcomes nights and weekends.”
The national database was not designed to provide an explanation for its findings, which may be due to multiple patient, event or hospital factors. “We can’t exclude physiological factors of patients or of staff working on the night shift,” Dr. Peberdy explains. “But I think we need to focus on process issues. We know that hospitals simply do not run the same way at night. Things are different—more errors, more accidents, more needle sticks, less people around. Those who work the night shift may also be less experienced,” and early identification of heart attacks is critical to positive outcome.
The JAMA results confirm previous research documenting worse outcomes on nights and weekends. Single-site studies and a smaller study of heart attack survival in New Jersey hospitals for weekend versus weekday admissions found similar trends.2
Stroke patients who enter the hospital at night or over the weekend are more likely to die in the hospital than those admitted during daytime hours (7 a.m. to 6 p.m.) on weekdays, according to two studies presented at the American Heart Association’s International Stroke Conference in New Orleans in February 2008.3 Those differences were particularly striking for hemorrhagic strokes. Similar outcomes also have been reported for pulmonary embolisms.
Shift Differences
Different studies have approached this issue in different ways, comparing business hours (e.g., 7 a.m. to 6 p.m.) with evenings, nights, and weekends, or days and evenings up to 11 p.m. with nights. Weekends are compared to weekdays but also to weekend nights.
The size of the hospital did not explain the shift differences found in Dr. Peberdy’s study. For hospitalists trying to address the underlying problems of after-hours quality, the size of the hospital is relevant. Only larger hospitals can afford hospitalist groups large enough to cover night and weekend shifts. For those that can, are all members of the group taking their turns at night, does this duty fall to the junior members, or is the group lucky enough to employ nocturnists who want to work at night? (See The Hospitalist, May 2006, p. 27, for an article on nocturnists in hospital medicine.) Whether the hospital has an academic emphasis also can influence who responds to crises after hours—attendings or house staff (in other words, sleep-deprived residents).
If hospitalists work nights, they are more likely to notice what isn’t available or what doesn’t work as well as what contributes to nocturnal quality problems and what might help to compensate for these differences. Even if hospitalists are not present in the facility at night, technology can help guide appropriate response to cardiac crises, suggests David Grace, MD, area medical officer for the Schumacher Group’s Hospital Medicine Division and a hospitalist at Southwest Medical Center in Lafayette, La.
“Several weeks ago a patient in the hospital was having chest pains,” he recalls. “A nurse called me at home and I ordered an electrocardiogram.” The electrocardiogram’s (EKG) computer program indicated “nonspecific changes” in the patient’s cardiac function, but Dr. Grace asked the nurse to scan the printout and send it to his Web-based fax number.
“I looked at the EKG on my PDA,” he continues. “It was subtle, but it seemed to me that this patient was having a myocardial infarction[MI]. I told the nurse to do the blood work for a suspected MI, give the patient an aspirin and take another EKG, which more clearly showed the MI. If I had not had the ability to look at the printout, I would have had to trust the nurse’s observation or the EKG computer program. As it was, we caught it early and the patient did well.”
Cooperation and Staffing
“I have lived it. I certainly understand the research showing different outcomes from MIs at night,” Dr. Grace observes. “At night, patients are usually asleep, so processes that begin with early warning signals, such as chest pains, may go further down the path before they are identified, especially if the patient has taken a sleeping pill. Often, nurse-to-patient staffing ratios are dramatically different at night—and somewhat reduced on weekends. I’ve also worked in hospitals where on weekends, unless it was a true life-threatening emergency, you could not get an MRI. So if you ordered one on a Saturday, it wouldn’t happen until Monday. There are things you pick up on the MRI that you miss on the CT scan; for example, bleeding, which can affect your management of the patient.”
“I would not be surprised to hear of worse survival for any of these acute decompensations—it goes for acute GI bleeds, stroke, and hemorrhage, as well as MIs,” adds Steven Liu, MD, of Emory University Eastside Medical Center in Atlanta. “We dealt with the problem in this hospital five years ago and addressed a lot of these quality issues by partnering with hospital administration and specialists.”
On weekends, many services may be less available or not available at all in the hospital, from interventional radiology to physical therapy. If patients have fewer contacts with different hospital personnel, it is more likely that subtle early signs of acute problems will go unnoticed until later.
“At my hospital, the interventional radiology [IR] department is not open 24 hours,” says Erica Grabscheid, MD, associate director of the hospitalist program at Beth Israel Hospital in New York City. If a peripherally inserted central catheter needs to be placed on the weekend but IR is not available, the patient may have to wait until Monday morning. Alternatives for the hospitalist, Dr. Grabscheid says, are to become skilled at line placement or to collect data for the hospital’s administrators on the costs of not having 24-hour IR.
Jeffrey Robinson, MD, hospitalist group leader for Intermountain Medical Center in Salt Lake City, Utah, believes staffing is an essential part of the equation. He says Intermountain Health has made a commitment to do what’s necessary for quality patient care—including adequate staffing. “Every time we feel we’re stretched, we add more hospitalist shifts, including weekends and holidays. I feel we give good patient care from the physician side. Obstacles have more to do with ancillaries, but we’ve made great progress in addressing these, as well.”
At one time, interventional radiology only was available in the hospital five days a week, Dr. Robinson notes. “You couldn’t get feeding tubes placed or certain other interventional procedures. But with the commitment of radiology and the hiring power of Intermountain Health, we now have 24-hour availability. MRIs have been harder to cover after hours, but there is now an on-call team for MRIs.”
In each case, hospitalists were important advocates for expanding the hours of availability, Dr. Robinson says: “We needed to gather data and look at results for patients staying over the weekend. We also got expanded case management, so that nursing home placements could happen on Saturdays and Sundays.” TH
Larry Beresford is a medical writer based in California.
References
- Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008; 99(7):785-792.
- Kostis WS, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007; 356(11):1099-1109.
- American Heart Association. Deaths higher in stroke patients who enter hospital at night, weekends. Available at www.sciencedaily.com/releases/2008/02/080220161720/htm. Accessed March 13, 2008.
Delirium Dilemma
Delirium Dilemma
I heard that Medicare is thinking about not paying the hospital if a hospitalized patient develops delirium. I am a geriatric hospitalist and unfortunately, this is a common problem in my patient population. This doesn’t sound reasonable. Is this really true?
Delirious in Denver
Dr. Hospitalist responds: For those of you who read this column regularly, you have seen me comment in the past about hospital acquired conditions.
In 2005, Congress authorized the Center for Medicare and Medicaid Services (CMS) to adjust hospital payments to encourage prevention of hospital acquired conditions. This was “part of an array of Medicare value-based purchasing tools that CMS is using to promote increased quality and efficiency of care.”
In August 2007, CMS announced that starting Oct. 1, 2007, hospitals were required to submit information on Medicare claims regarding whether a list of specific diagnoses were present on admission (POA). CMS was trying to determine how often patients were developing specific complications while they were hospitalized. This list of conditions included a foreign object retained after surgery, air embolism, blood incompatibility, stage three and four pressure ulcers, injuries due to falls/trauma, catheter-associated urinary tract infections, vascular catheter-associated infection and mediastinitis after coronary artery bypass graft.
They also announced that beginning Oct. 1, CMS will pay hospitals as though that complication did not occur (i.e., not pay for the additional costs associated with managing these complications). These policy changes sent hospital administrators scrambling to develop and implement plans to prevent these types of incidents from occurring in hospitalized patients.
CMS has continued to work with the Centers for Disease Control and Prevention (CDC) and other stakeholders to identify additional hospital acquired conditions. In April, CMS proposed to make the following list of conditions subject to the POA payment provision in fiscal year 2009. These include:
- Legionnaires’ disease;
- Iatrogenic pneumothorax;
- Ventilator-associated pneumonia;
- Deep-vein thrombosis (DVT/ pulmonary embolism(PE);
- Staphylococcus aureus septicemia;
- Clostridium Difficile-associated disease;
- Surgical site infections following several elective surgeries (total knee replacement, laparoscopic gastric bypass and gastroenerostomy, ligation, and stripping of varicose veins);
- Several conditions regarding glycemic control in hospitalized patients (diabetic ketoacidosis, nonketotic hyperosmolar coma, diabetic coma, and hypoglycemic coma); and
- Delirium.
Last year, when I heard CMS was going to stop paying for the cost of care associated with errors like transfusing the wrong type of blood and leaving foreign objects in patients during surgery, I cheered. The policy was long overdue. At present, I share your concerns when I look at this list of proposed hospital acquired conditions for fiscal year 2009.
I have no problems with CMS not paying for the costs of caring for a patient if a patient slips into a diabetic coma during his or her hospital stay. Careful monitoring and adherence to evidence based guidelines should prevent that. But I agree with you, can we really prevent delirium in all our hospitalized patients?
I took care of a patient recently with known dementia who developed delirium after her hip surgery. Dementia, surgery, and medications are known risk factors for delirium. Despite careful monitoring and thoughtful care, she was delirious after the surgery. Was I surprised? No. Could I have done more to prevent delirium? No. Why should the hospital be punished in this case? It shouldn’t. The patient needed the surgery, and I wasn’t going to withhold the narcotics just to minimize the risk of delirium. I suspect there is room for improvement in our hospitals when it comes to caring for vulnerable populations of patients. We can likely reduce the rates of delirium in our hospitalized patients, but I doubt we can truly prevent all delirium.
CMS published its proposal in April, well in advance of the new fiscal year that begins in October, because CMS is interested in seeking public comments on “the degree to which (each condition) is reasonably preventable through the application of evidence-based guidelines.”
Based on public comment, CMS will choose to select or not select each condition listed as a hospital-acquired condition. CMS is expected to make its decision known by the end of this month. Let it be known that this hospitalist is opposed to the delirium measure. I am not alone. A number of professional societies, including SHM, American College of Chest Physicians, Society of Critical Care Medicine, American Thoracic Society, among others, have expressed reservations to CMS about several of the proposed hospital-acquired conditions.
These societies believe ventilator-associated pneumonia, DVT/PE, and iatrogenic pneumothorax also should not be included as hospital-acquired conditions because they, like delirium, are not entirely preventable. Further, they believe the incidence of these four conditions can be reduced by adherence to evidence based guidelines but there is insufficient evidence to guide prevention of these conditions.
In addition, SHM raised concern that listing Legionnaires’ disease as a hospital-acquired condition may lead to unintended consequences, such as routine testing for Legionnaires’ in all patients presenting with community acquired pneumonia.
We won’t know until the end of this month whether CMS will make any or all of these conditions subject to the POA payment provision in fiscal year 2009. But kudos to the professional societies and all who have helped CMS think about these important issues. TH
Delirium Dilemma
I heard that Medicare is thinking about not paying the hospital if a hospitalized patient develops delirium. I am a geriatric hospitalist and unfortunately, this is a common problem in my patient population. This doesn’t sound reasonable. Is this really true?
Delirious in Denver
Dr. Hospitalist responds: For those of you who read this column regularly, you have seen me comment in the past about hospital acquired conditions.
In 2005, Congress authorized the Center for Medicare and Medicaid Services (CMS) to adjust hospital payments to encourage prevention of hospital acquired conditions. This was “part of an array of Medicare value-based purchasing tools that CMS is using to promote increased quality and efficiency of care.”
In August 2007, CMS announced that starting Oct. 1, 2007, hospitals were required to submit information on Medicare claims regarding whether a list of specific diagnoses were present on admission (POA). CMS was trying to determine how often patients were developing specific complications while they were hospitalized. This list of conditions included a foreign object retained after surgery, air embolism, blood incompatibility, stage three and four pressure ulcers, injuries due to falls/trauma, catheter-associated urinary tract infections, vascular catheter-associated infection and mediastinitis after coronary artery bypass graft.
They also announced that beginning Oct. 1, CMS will pay hospitals as though that complication did not occur (i.e., not pay for the additional costs associated with managing these complications). These policy changes sent hospital administrators scrambling to develop and implement plans to prevent these types of incidents from occurring in hospitalized patients.
CMS has continued to work with the Centers for Disease Control and Prevention (CDC) and other stakeholders to identify additional hospital acquired conditions. In April, CMS proposed to make the following list of conditions subject to the POA payment provision in fiscal year 2009. These include:
- Legionnaires’ disease;
- Iatrogenic pneumothorax;
- Ventilator-associated pneumonia;
- Deep-vein thrombosis (DVT/ pulmonary embolism(PE);
- Staphylococcus aureus septicemia;
- Clostridium Difficile-associated disease;
- Surgical site infections following several elective surgeries (total knee replacement, laparoscopic gastric bypass and gastroenerostomy, ligation, and stripping of varicose veins);
- Several conditions regarding glycemic control in hospitalized patients (diabetic ketoacidosis, nonketotic hyperosmolar coma, diabetic coma, and hypoglycemic coma); and
- Delirium.
Last year, when I heard CMS was going to stop paying for the cost of care associated with errors like transfusing the wrong type of blood and leaving foreign objects in patients during surgery, I cheered. The policy was long overdue. At present, I share your concerns when I look at this list of proposed hospital acquired conditions for fiscal year 2009.
I have no problems with CMS not paying for the costs of caring for a patient if a patient slips into a diabetic coma during his or her hospital stay. Careful monitoring and adherence to evidence based guidelines should prevent that. But I agree with you, can we really prevent delirium in all our hospitalized patients?
I took care of a patient recently with known dementia who developed delirium after her hip surgery. Dementia, surgery, and medications are known risk factors for delirium. Despite careful monitoring and thoughtful care, she was delirious after the surgery. Was I surprised? No. Could I have done more to prevent delirium? No. Why should the hospital be punished in this case? It shouldn’t. The patient needed the surgery, and I wasn’t going to withhold the narcotics just to minimize the risk of delirium. I suspect there is room for improvement in our hospitals when it comes to caring for vulnerable populations of patients. We can likely reduce the rates of delirium in our hospitalized patients, but I doubt we can truly prevent all delirium.
CMS published its proposal in April, well in advance of the new fiscal year that begins in October, because CMS is interested in seeking public comments on “the degree to which (each condition) is reasonably preventable through the application of evidence-based guidelines.”
Based on public comment, CMS will choose to select or not select each condition listed as a hospital-acquired condition. CMS is expected to make its decision known by the end of this month. Let it be known that this hospitalist is opposed to the delirium measure. I am not alone. A number of professional societies, including SHM, American College of Chest Physicians, Society of Critical Care Medicine, American Thoracic Society, among others, have expressed reservations to CMS about several of the proposed hospital-acquired conditions.
These societies believe ventilator-associated pneumonia, DVT/PE, and iatrogenic pneumothorax also should not be included as hospital-acquired conditions because they, like delirium, are not entirely preventable. Further, they believe the incidence of these four conditions can be reduced by adherence to evidence based guidelines but there is insufficient evidence to guide prevention of these conditions.
In addition, SHM raised concern that listing Legionnaires’ disease as a hospital-acquired condition may lead to unintended consequences, such as routine testing for Legionnaires’ in all patients presenting with community acquired pneumonia.
We won’t know until the end of this month whether CMS will make any or all of these conditions subject to the POA payment provision in fiscal year 2009. But kudos to the professional societies and all who have helped CMS think about these important issues. TH
Delirium Dilemma
I heard that Medicare is thinking about not paying the hospital if a hospitalized patient develops delirium. I am a geriatric hospitalist and unfortunately, this is a common problem in my patient population. This doesn’t sound reasonable. Is this really true?
Delirious in Denver
Dr. Hospitalist responds: For those of you who read this column regularly, you have seen me comment in the past about hospital acquired conditions.
In 2005, Congress authorized the Center for Medicare and Medicaid Services (CMS) to adjust hospital payments to encourage prevention of hospital acquired conditions. This was “part of an array of Medicare value-based purchasing tools that CMS is using to promote increased quality and efficiency of care.”
In August 2007, CMS announced that starting Oct. 1, 2007, hospitals were required to submit information on Medicare claims regarding whether a list of specific diagnoses were present on admission (POA). CMS was trying to determine how often patients were developing specific complications while they were hospitalized. This list of conditions included a foreign object retained after surgery, air embolism, blood incompatibility, stage three and four pressure ulcers, injuries due to falls/trauma, catheter-associated urinary tract infections, vascular catheter-associated infection and mediastinitis after coronary artery bypass graft.
They also announced that beginning Oct. 1, CMS will pay hospitals as though that complication did not occur (i.e., not pay for the additional costs associated with managing these complications). These policy changes sent hospital administrators scrambling to develop and implement plans to prevent these types of incidents from occurring in hospitalized patients.
CMS has continued to work with the Centers for Disease Control and Prevention (CDC) and other stakeholders to identify additional hospital acquired conditions. In April, CMS proposed to make the following list of conditions subject to the POA payment provision in fiscal year 2009. These include:
- Legionnaires’ disease;
- Iatrogenic pneumothorax;
- Ventilator-associated pneumonia;
- Deep-vein thrombosis (DVT/ pulmonary embolism(PE);
- Staphylococcus aureus septicemia;
- Clostridium Difficile-associated disease;
- Surgical site infections following several elective surgeries (total knee replacement, laparoscopic gastric bypass and gastroenerostomy, ligation, and stripping of varicose veins);
- Several conditions regarding glycemic control in hospitalized patients (diabetic ketoacidosis, nonketotic hyperosmolar coma, diabetic coma, and hypoglycemic coma); and
- Delirium.
Last year, when I heard CMS was going to stop paying for the cost of care associated with errors like transfusing the wrong type of blood and leaving foreign objects in patients during surgery, I cheered. The policy was long overdue. At present, I share your concerns when I look at this list of proposed hospital acquired conditions for fiscal year 2009.
I have no problems with CMS not paying for the costs of caring for a patient if a patient slips into a diabetic coma during his or her hospital stay. Careful monitoring and adherence to evidence based guidelines should prevent that. But I agree with you, can we really prevent delirium in all our hospitalized patients?
I took care of a patient recently with known dementia who developed delirium after her hip surgery. Dementia, surgery, and medications are known risk factors for delirium. Despite careful monitoring and thoughtful care, she was delirious after the surgery. Was I surprised? No. Could I have done more to prevent delirium? No. Why should the hospital be punished in this case? It shouldn’t. The patient needed the surgery, and I wasn’t going to withhold the narcotics just to minimize the risk of delirium. I suspect there is room for improvement in our hospitals when it comes to caring for vulnerable populations of patients. We can likely reduce the rates of delirium in our hospitalized patients, but I doubt we can truly prevent all delirium.
CMS published its proposal in April, well in advance of the new fiscal year that begins in October, because CMS is interested in seeking public comments on “the degree to which (each condition) is reasonably preventable through the application of evidence-based guidelines.”
Based on public comment, CMS will choose to select or not select each condition listed as a hospital-acquired condition. CMS is expected to make its decision known by the end of this month. Let it be known that this hospitalist is opposed to the delirium measure. I am not alone. A number of professional societies, including SHM, American College of Chest Physicians, Society of Critical Care Medicine, American Thoracic Society, among others, have expressed reservations to CMS about several of the proposed hospital-acquired conditions.
These societies believe ventilator-associated pneumonia, DVT/PE, and iatrogenic pneumothorax also should not be included as hospital-acquired conditions because they, like delirium, are not entirely preventable. Further, they believe the incidence of these four conditions can be reduced by adherence to evidence based guidelines but there is insufficient evidence to guide prevention of these conditions.
In addition, SHM raised concern that listing Legionnaires’ disease as a hospital-acquired condition may lead to unintended consequences, such as routine testing for Legionnaires’ in all patients presenting with community acquired pneumonia.
We won’t know until the end of this month whether CMS will make any or all of these conditions subject to the POA payment provision in fiscal year 2009. But kudos to the professional societies and all who have helped CMS think about these important issues. TH
Foster Ownership Culture
In my June column (“Follow the Money,” p. 61), I wrote about my concern that SHM’s “2007-2008 SHM Survey: State of the Hospital Medicine Movement” showed that more than one-third of hospitalist group leaders reported they did not know their groups’ annual professional fee revenues or expenses.
This is consistent with my experience working as a consultant with many other practices, and is one of many common findings in a struggling practice.
What about the opposite side of the coin? What are the common attributes of a healthy, successful practice? I talk about this all the time with my consulting colleague, Leslie Flores (director of practice management for SHM). We’ve become convinced that while the attributes to ensure success vary a little from one practice to the next, they can be rolled into the global heading of a “culture of ownership.” That is, the practices in which hospitalists think of themselves as owners of the practice (even if they are, in fact, employees of the hospital or some other organization) are most likely to be successful.
What is it?
Ownership culture is a mind-set, not a legal description of who has contractual ownership of the practice. I learned the hard way that not everyone knows what I mean when I talk about an ownership culture.
During the course of a conference a few years ago, I had several conversations with a sharp hospitalist practice leader about the problems his group faced. Apparently, many other doctors at the hospital treated them like residents. As I learned more it sounded as though this largely was the fault of the hospitalists themselves. It seemed clear to me the underlying theme was each doctor in the practice felt little connection to his/her hospitalist colleagues and the hospital in which they worked.
I began talking with the practice leader about how things could be different if the hospitalists would think of themselves as business owners and act accordingly. Yet, he couldn’t make sense of what I was saying since he thought I was suggesting that all the hospitalists resign from employment by the hospital (and presumably give up the financial support it provided) and form their own corporation. That isn’t necessary. It is possible to maintain an ownership culture even if the hospitalists are employees of a larger organization like a hospital (and not owners of their practice in the contractual sense).
Recognize it
A Web search on “ownership culture” returns a number of interesting sites. In fact, there is a National Center for Employee Ownership, which has an interesting Web site geared toward employees who own a significant portion of their company’s stock.
You also might want to look at their article titled “What Is an Ownership Culture?” (www.nceo.org/library/ownership_culture.html) and think about how your practice fits into that description.
Leslie and I have developed an informal quiz to help hospitalist practices think about whether they are supporting an ownership mindset. While we haven’t done research to validate these measures, we have considerable anecdotal experience supporting the idea that a high score on the questionnaire (i.e., lots of answers in the “pretty much” or “100%” columns) correlates well with an ownership mentality on the part of the doctors in the practice.
We’ve found such practices usually function more effectively and have happier hospitalists and customers (e.g., hospital personnel, other doctors, and patients). If you have an idea for valuable additions, deletions, or modifications to the questionnaire I’d love to hear from you.
Does it Matter?
While there are lots of other components to a good practice, I believe an ownership culture is one of the most important features leading to a successful and thriving practice. It is difficult to maintain a successful practice for very long without it.
You don’t have to take my word for it. Writing in The Baptist Health Care Journey to Excellence, Al Stubblefield says:
“Because it is so rare, an organization that is able to create this culture of ownership within its workforce has a high probability of creating a sustainable competitive advantage … The second advantage, which came as an unexpected bonus for us, is that creating a strong, attractive culture results in incredible recruiting power.” TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
In my June column (“Follow the Money,” p. 61), I wrote about my concern that SHM’s “2007-2008 SHM Survey: State of the Hospital Medicine Movement” showed that more than one-third of hospitalist group leaders reported they did not know their groups’ annual professional fee revenues or expenses.
This is consistent with my experience working as a consultant with many other practices, and is one of many common findings in a struggling practice.
What about the opposite side of the coin? What are the common attributes of a healthy, successful practice? I talk about this all the time with my consulting colleague, Leslie Flores (director of practice management for SHM). We’ve become convinced that while the attributes to ensure success vary a little from one practice to the next, they can be rolled into the global heading of a “culture of ownership.” That is, the practices in which hospitalists think of themselves as owners of the practice (even if they are, in fact, employees of the hospital or some other organization) are most likely to be successful.
What is it?
Ownership culture is a mind-set, not a legal description of who has contractual ownership of the practice. I learned the hard way that not everyone knows what I mean when I talk about an ownership culture.
During the course of a conference a few years ago, I had several conversations with a sharp hospitalist practice leader about the problems his group faced. Apparently, many other doctors at the hospital treated them like residents. As I learned more it sounded as though this largely was the fault of the hospitalists themselves. It seemed clear to me the underlying theme was each doctor in the practice felt little connection to his/her hospitalist colleagues and the hospital in which they worked.
I began talking with the practice leader about how things could be different if the hospitalists would think of themselves as business owners and act accordingly. Yet, he couldn’t make sense of what I was saying since he thought I was suggesting that all the hospitalists resign from employment by the hospital (and presumably give up the financial support it provided) and form their own corporation. That isn’t necessary. It is possible to maintain an ownership culture even if the hospitalists are employees of a larger organization like a hospital (and not owners of their practice in the contractual sense).
Recognize it
A Web search on “ownership culture” returns a number of interesting sites. In fact, there is a National Center for Employee Ownership, which has an interesting Web site geared toward employees who own a significant portion of their company’s stock.
You also might want to look at their article titled “What Is an Ownership Culture?” (www.nceo.org/library/ownership_culture.html) and think about how your practice fits into that description.
Leslie and I have developed an informal quiz to help hospitalist practices think about whether they are supporting an ownership mindset. While we haven’t done research to validate these measures, we have considerable anecdotal experience supporting the idea that a high score on the questionnaire (i.e., lots of answers in the “pretty much” or “100%” columns) correlates well with an ownership mentality on the part of the doctors in the practice.
We’ve found such practices usually function more effectively and have happier hospitalists and customers (e.g., hospital personnel, other doctors, and patients). If you have an idea for valuable additions, deletions, or modifications to the questionnaire I’d love to hear from you.
Does it Matter?
While there are lots of other components to a good practice, I believe an ownership culture is one of the most important features leading to a successful and thriving practice. It is difficult to maintain a successful practice for very long without it.
You don’t have to take my word for it. Writing in The Baptist Health Care Journey to Excellence, Al Stubblefield says:
“Because it is so rare, an organization that is able to create this culture of ownership within its workforce has a high probability of creating a sustainable competitive advantage … The second advantage, which came as an unexpected bonus for us, is that creating a strong, attractive culture results in incredible recruiting power.” TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
In my June column (“Follow the Money,” p. 61), I wrote about my concern that SHM’s “2007-2008 SHM Survey: State of the Hospital Medicine Movement” showed that more than one-third of hospitalist group leaders reported they did not know their groups’ annual professional fee revenues or expenses.
This is consistent with my experience working as a consultant with many other practices, and is one of many common findings in a struggling practice.
What about the opposite side of the coin? What are the common attributes of a healthy, successful practice? I talk about this all the time with my consulting colleague, Leslie Flores (director of practice management for SHM). We’ve become convinced that while the attributes to ensure success vary a little from one practice to the next, they can be rolled into the global heading of a “culture of ownership.” That is, the practices in which hospitalists think of themselves as owners of the practice (even if they are, in fact, employees of the hospital or some other organization) are most likely to be successful.
What is it?
Ownership culture is a mind-set, not a legal description of who has contractual ownership of the practice. I learned the hard way that not everyone knows what I mean when I talk about an ownership culture.
During the course of a conference a few years ago, I had several conversations with a sharp hospitalist practice leader about the problems his group faced. Apparently, many other doctors at the hospital treated them like residents. As I learned more it sounded as though this largely was the fault of the hospitalists themselves. It seemed clear to me the underlying theme was each doctor in the practice felt little connection to his/her hospitalist colleagues and the hospital in which they worked.
I began talking with the practice leader about how things could be different if the hospitalists would think of themselves as business owners and act accordingly. Yet, he couldn’t make sense of what I was saying since he thought I was suggesting that all the hospitalists resign from employment by the hospital (and presumably give up the financial support it provided) and form their own corporation. That isn’t necessary. It is possible to maintain an ownership culture even if the hospitalists are employees of a larger organization like a hospital (and not owners of their practice in the contractual sense).
Recognize it
A Web search on “ownership culture” returns a number of interesting sites. In fact, there is a National Center for Employee Ownership, which has an interesting Web site geared toward employees who own a significant portion of their company’s stock.
You also might want to look at their article titled “What Is an Ownership Culture?” (www.nceo.org/library/ownership_culture.html) and think about how your practice fits into that description.
Leslie and I have developed an informal quiz to help hospitalist practices think about whether they are supporting an ownership mindset. While we haven’t done research to validate these measures, we have considerable anecdotal experience supporting the idea that a high score on the questionnaire (i.e., lots of answers in the “pretty much” or “100%” columns) correlates well with an ownership mentality on the part of the doctors in the practice.
We’ve found such practices usually function more effectively and have happier hospitalists and customers (e.g., hospital personnel, other doctors, and patients). If you have an idea for valuable additions, deletions, or modifications to the questionnaire I’d love to hear from you.
Does it Matter?
While there are lots of other components to a good practice, I believe an ownership culture is one of the most important features leading to a successful and thriving practice. It is difficult to maintain a successful practice for very long without it.
You don’t have to take my word for it. Writing in The Baptist Health Care Journey to Excellence, Al Stubblefield says:
“Because it is so rare, an organization that is able to create this culture of ownership within its workforce has a high probability of creating a sustainable competitive advantage … The second advantage, which came as an unexpected bonus for us, is that creating a strong, attractive culture results in incredible recruiting power.” TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
A 44-year-old man with hemoptysis: A review of pertinent imaging studies and radiographic interventions
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
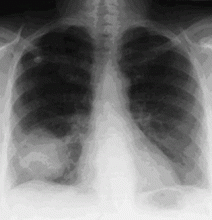
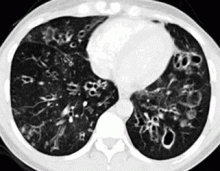
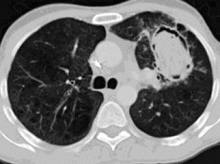
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
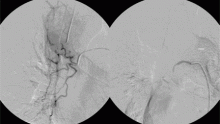
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
A 44-year-old man comes to the emergency room because of light-headedness and fatigue. He says he has had several similar but milder episodes in the last several months. He also mentions that he thinks he has been coughing up blood. He says he has no major medical or surgical problems of which he is aware, but he appears confused and unable to give an accurate history. No family members can be contacted for further history at the moment.
Physical examination reveals nothing remarkable, but the patient does cough up some blood during the examination. His hemoglobin level is 6.0 g/dL (reference range 13.5–17.5).
What imaging tests would be helpful in this patient’s evaluation?
HEMOPTYSIS HAS MANY CAUSES
Hemoptysis is defined as the expectoration of blood originating from the tracheobronchial tree or the pulmonary parenchyma.
Most cases of hemoptysis are benign and self-limited; life-threatening hemoptysis is rare.1–3 However, hemoptysis can be a sign of serious tracheopulmonary disease.
Definition of ‘massive’ hemoptysis can vary
Various definitions of the severity of hemoptysis have been proposed. The threshold of “massive” hemoptysis has been defined as as low as 100 mL/24 hours and as high as 1 L/24 hours; the most common definition is 300 mL, or about 1 cup.2,3,5–10
However, the patient’s cardiorespiratory status must also be considered.5,6,9 If the patient cannot maintain his or her airway, a small amount of bleeding could be life-threatening and should be considered significant or massive. Thus, we define massive hemoptysis as more than 300 mL of blood within 24 hours or any amount of blood with concurrent cardiorespiratory compromise.
It is important to recognize massive hemoptysis quickly, because without urgent treatment, up to 80% of patients may die.5,6,11 This can sometimes pose a challenge, as the history may not always be helpful and the patient’s perception of massive hemoptysis may differ from the clinically accepted definition. For example, in a patient without respiratory compromise, we would not consider bloodtinged sputum or small amounts of blood that add up to 1 to 2 teaspoons (5–10 mL) to be massive, although the patient might. On the other hand, hemoptysis with cardiorespiratory compromise must be considered significant (and very possibly massive) until proven otherwise, even if the amount of blood is small.
Massive hemoptysis is usually the result of erosion of systemic (rather than pulmonary) arteries by bronchial neoplasm, active tuberculosis, or aspergilloma.6,9,12,13 Arteriovenous malformations and pulmonary artery aneurysms are much less common causes.5,11,13
IMAGING AND DIAGNOSTIC OPTIONS
Chest radiography
In as many as 40% of cases of hemoptysis, however, the findings on chest radiography are normal or do not reveal the source of the bleeding.15,16 Approximately 5% to 6% of patients with hemoptysis and normal results on radiography are eventually found to have lung cancer.14 Thus, while a localizing finding on radiography is helpful, a normal or nonlocalizing finding warrants further evaluation by other means, including conventional CT, multidetector CT angiography, or bronchoscopy.
Computed tomography
CT is superior to fiberoptic bronchoscopy in finding a cause of hemoptysis, its main advantage being its ability to show distal airways beyond the reach of the bronchoscope, and the lung parenchyma surrounding these distal airways.5,15,16 In locating the site of bleeding, CT performs about as well as fiberoptic bronchoscopy.5
However, while CT imaging is extremely useful in evaluating bleeding from larger vessels, it adds little information beyond that obtained by chest radiography in cases of diffuse alveolar hemorrhage.4
Multidetector CT angiography is the optimal CT study for evaluating hemoptysis. In addition to showing the lung parenchyma and airways, it allows one to evaluate the integrity of pulmonary, bronchial, and nonbronchial systemic arteries within the chest. It is at least as good as (and, with multiplanar reformatted images, possibly even better than) conventional angiography in evaluating bronchial and nonbronchial systemic arteries. Multidetector CT angiography is recommended before bronchial artery embolization to help one plan the procedure and shorten the procedure time, if the patient is stable enough that this imaging study can be done first.6,12,13
The iodinated contrast material used in CT angiography can cause contrast nephropathy in patients with renal failure. At Cleveland Clinic, we avoid using contrast if the patient’s serum creatinine level is 2.0 mg/dL or greater or if it is rapidly rising, even if it is in the normal range or only slightly elevated; a rapid rise would indicate acute renal failure (eg, in glomerulonephritis). In these cases, we recommend CT without contrast.
CT of the chest has revealed malignancies in cases of hemoptysis in which radiography and bronchoscopy did not.15,17 Although CT is more than 90% sensitive in detecting endobronchial lesions, it has limitations: a blood clot within the bronchus can look like a tumor, and acute bleeding can obscure an endobronchial lesion.5 Thus, bronchoscopy remains an important, complementary diagnostic tool in the evaluation of acute hemoptysis.
Bronchoscopy
Bronchoscopy is overall much less sensitive than CT in detecting the cause of the bleeding,15,16,18 but, if performed early it as useful as CT in finding the site of bleeding,5,9 information that can be helpful in planning further therapy.19 It may be more useful than CT in evaluating endobronchial lesions during acute hemoptysis, as active bleeding can obscure an endobronchial lesion on CT.5 However, the distal airways are often filled with blood, making them difficult to evaluate via bronchoscopy.
In approximately 10% of cases of massive hemoptysis, rigid bronchoscopy is preferred over fiberoptic bronchoscopy, and it is often used in a perioperative setting. However, its use is not usually possible in unstable patients receiving intensive care. Instead, flexible fiberoptic bronchoscopy can be used in patients whose condition is too unstable to allow them to leave the intensive care unit to undergo CT. Flexible fiberoptic bronchoscopy does not require an operating room or anesthesia,19 and can be done in the intensive care unit itself.
Not only can bronchoscopy accurately locate the site of bleeding, it can also aid in controlling the airway in patients with catastrophic hemorrhage and temporarily control bleeding through Fogarty balloon tamponade, direct application of a mixture of epinephrine and cold saline, or topical hemostatic tamponade therapy with a solution of thrombin or fibrinogen and thrombin.2,3,19 It also provides complementary information about endobronchial lesions and is valuable in providing samples for tissue diagnosis and microbial cultures.
Diagnostic angiography has limitations
Although it is possible to bypass radiography, CT, and bronchoscopy in a case of massive hemoptysis and to rush the patient to the angiography suite for combined diagnostic angiography and therapeutic bronchial artery embolization, this approach has limitations. Diagnostic angiography does not identify the source of bleeding as well as CT does.6 It is important to locate the bleeding site first via CT, multidetector CT angiography, or bronchoscopy. Diagnostic angiography can be time-consuming. The procedure time can be significantly shorter if CT, bronchoscopy, or both are done first to ascertain the site of bleeding before bronchial artery embolization.1,6 Another reason that performing CT first is important is that it can rule out situations in which surgery would be preferred over bronchial artery embolization.6
In more than 90% of cases of hemoptysis requiring embolization or surgery, the bleeding is from the bronchial arteries.5,6,9,11–13 However, bronchoscopy before bronchial artery embolization is unnecessary in patients with hemoptysis of known cause if the site of bleeding can be determined from radiography or CT and if no bronchoscopic airway management is needed.18
BRONCHIAL ARTERY EMBOLIZATION: AN ALTERNATIVE TO SURGERY
After a cause of the hemoptysis has been established by radiography, CT, or bronchoscopy, bronchial artery embolization is an effective first-line therapy to control massive, life-threatening bleeding.6 It is an alternative in patients who cannot undergo surgery because of bilateral or extensive disease that renders them unable to tolerate life after a lobectomy.6,12,18
Indications for bronchial artery embolization include failure of conservative management, massive hemoptysis, recurrent hemoptysis, and poor surgical risk. It is also done to control bleeding temporarily before surgery.1
Another indication for this therapy is peripheral pulmonary artery pseudoaneurysm, which is found in up to 11% of patients undergoing bronchial angiography for hemoptysis. These patients typically present with recurrent hemoptysis (sometimes massive) and occasionally with both hemoptysis and clubbing. Most of these patients have either chronic active pulmonary tuberculosis or a mycetoma complicating sarcoidosis or tuberculosis. Occlusion of the pulmonary artery pseudoaneurysm may require embolization of bronchial arteries, nonbronchial systemic arteries, or pulmonary artery branches.20
Surgery, however, is still the definitive treatment of choice for thoracic vascular injury, bronchial adenoma, aspergilloma resistant to other therapies, and hydatid cyst.6 A cardiothoracic surgeon should be consulted in these cases.
Outcomes of embolization
If a patient with massive hemoptysis undergoes successful bronchial artery embolization but the bleeding recurs 1 to 6 months later, the cause is likely an undetected nonbronchial systemic arterial supply and incomplete embolization.1,22 Late rebleeding (6–12 months after the procedure) occurs in 20% to 40% of patients and is likely to be from disease progression.1,7
Common complications of bronchial artery embolization are transient chest pain and dysphagia. Very rare complications include subintimal dissection and spinal cord ischemia due to inadvertent occlusion of the spinal arteries.6 Another complication in patients with renal failure is contrast nephropathy, the risk of which must be weighed against the possible consequences—including death—of not performing bronchial artery embolization in a patient who cannot undergo surgery.
CASE REVISITED: CLINICAL COURSE
In the patient described at the beginning of this article, a chest radiograph obtained in the emergency room showed an area of nonspecific consolidation in the left upper lung. Conventional chest CT was then ordered (Figure 4), and it revealed a cavitary lesion in the left upper lobe, consistent with aspergilloma. Bronchoscopy was then performed, and it too indicated that the bleeding was coming from the left upper lobe. Samples obtained during the procedure were sent to the laboratory for bacterial and fungal cultures.
In the meantime, family members were contacted, and they revealed that the patient had a history of sarcoidosis.
The patient went on to develop massive hemoptysis. Although the treatment of choice for mycetoma is primary resection, our patient’s respiratory status was poor as a result of extensive pulmonary sarcoidosis, and he was not considered a candidate for emergency surgery at that time. He was rushed to the angiography suite and successfully underwent emergency bronchial artery embolization.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
- Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiologica 2006; 47:780–792.
- Corder R. Hemoptysis. Emerg Med Clin North Am 2003; 21:421–435.
- Valipour A, Kreuzer A, Koller H, Koessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005; 127:2113–2118.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol 2007; 80:21–25.
- Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002; 22:1395–1409.
- Johnson JL. Manifestations of hemoptysis. How to manage minor, moderate, and massive bleeding. Postgrad Med 2002; 112 4:101–113.
- Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Phys 2005; 72:1253–1260.
- Bruzzi JF, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26:3–22.
- Ozgul MA, Turna A, Yildiz P, Ertan E, Kahraman S, Yilmaz V. Risk factors and recurrence patterns in 203 patients with hemoptysis. Tuberk Toraks 2006; 54:243–248.
- Khalil A, Fartoukh M, Tassart M, Parrot A, Marsault C, Carette MF. Role of MDCT in identification of the bleeding site and the vessels causing hemoptysis. AJR Am J Roentgenol 2007; 188:W117–W125.
- Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 2004; 233:741–749.
- Yoon YC, Lee KS, Jeong YJ, Shin SW, Chung MJ, Kwon OJ. Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT. Radiology 2005; 234:292–298.
- Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest 2001; 120:1592–1594.
- Naidich DP, Funt S, Ettenger NA, Arranda C. Hemoptysis: CT-bronchoscopic correlations in 58 cases. Radiology 1990; 177:357–362.
- McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WN, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest 1994; 105:1155–1162.
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179:1217–1224.
- Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177:861–867.
- Raoof S, Mehrishi S, Prakash UB. Role of bronchoscopy in modern medical intensive care unit. Clin Chest Med 2001; 22:241–261.
- Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. AJR Am J Roentgenol 2005; 184:1253–1259.
- Swanson KL, Johnson CM, Prakash UB, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest 2002; 121:789–795.
- Yoon W, Kim YH, Kim JK, Kim YC, Park JG, Kang HK. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology 2003; 227:232–238.
KEY POINTS
- We recommend chest radiography in the initial stages of evaluation of hemoptysis, whether the hemoptysis is massive or nonmassive.
- In cases of hemoptysis that is intermittent (whether massive or nonmassive) in patients whose condition is stable, CT, multidetector CT angiography, and bronchoscopy are all useful.
- In cases of hemoptysis that is active, persistent, and massive, multidetector CT angiography, bronchoscopy, and conventional bronchial angiography are all useful, depending on the hemodynamic stability of the patient.
- Bronchial artery embolization is the preferred noninvasive first-line treatment for hemoptysis and offers an excellent alternative to surgery for patients who are poor candidates for surgery.
Update in infectious disease treatment
Studies published during the past year provide information that could influence how we treat several infectious diseases in daily practice. Here is a brief overview of these “impact” studies.
VANCOMYCIN BEATS METRONIDAZOLE FOR SEVERE C DIFFICILE DIARRHEA
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-assoicated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
Clostridium difficile is the most common infectious cause of nosocomial diarrhea. Furthermore, a unique and highly virulent strain has emerged.
Which drug should be the treatment of choice: metronidazole (Flagyl) or oral vancomycin (Vancocin)? Over time, some infectious disease practitioners have believed that oral vancomycin is superior to oral metronidazole for the treatment of severe C difficile-associated diarrhea. Indeed, in a recently published survey, more than 25% of infectious disease practitioners said they used vancomycin as initial therapy for C difficile-associated diarrhea.1 Until recently, there has been no evidence to support this preference.
Ever since the first description of C difficile-associated diarrhea in the late 1970s, only two head-to-head studies have compared the efficacy of metronidazole vs vancomycin for the treatment of this disorder. Both studies were underpowered and neither was blinded. In 1983, Teasley et al2 treated 101 patients with metronidazole or vancomycin in a non-blinded, nonrandomized study and found no difference in efficacy. In 1996, Wenisch et al,3 in a prospective, randomized, but nonblinded study in 119 patients, compared vancomycin, metronidazole, fusidic acid, and teicoplanin (Targocid) and also found no significant difference in efficacy.
The study. Zar et al,4 in a prospective, double-blind trial at a single institution over an 8-year period, randomized 172 patients with C difficile-associated diarrhea to receive either oral metronidazole 250 mg four times a day or oral vancomycin 125 mg four times a day, both for 10 days. (The appropriate dosage of vancomycin has been debated over the years. In 1989, Fekety et al5 treated patients who had antibiotic-associated C difficile colitis with either 125 or 500 mg of vancomycin, four times a day, and found that the low dosage was as effective as the high dosage.) Both groups also received an oral placebo in addition to the study drug.
In the study of Zar et al, criteria for inclusion were diarrhea (defined as having more than two nonformed stools per 24 hours) and the finding of either toxin A in the stool or pseudomembranes on endoscopic examination. Patients were excluded if they were pregnant, had suspected or proven life-threatening intra-abdominal complications, were allergic to either study drug, had taken one of the study drugs during the last 14 days, or had previously had C difficile-associated diarrhea that did not respond to either study drug.
Patients were followed for up to 21 days. The primary end points were cure, treatment failure, or relapse. Cure was defined as the resolution of diarrhea and no C difficile toxin A detected on stool assay at days 6 and 10.
Disease severity was classified as either mild or severe based on a point system: patients received a single point each for being older than 60 years, being febrile, having an albumin level of less than 2.5 mg/dL, or having a white blood cell count of more than 15 × 109/L. Patients were classified as having severe disease if they had two or more points. They received two points (ie, they were automatically classified as having severe disease) if they had pseudomembranous colitis or if they developed C difficile infection that required treatment in an intensive care unit.
Findings. The overall cure rate in patients receiving vancomycin was 97%, compared with 84% for those on metronidazole (P = .006). This difference was attributable to the group of patients with severe disease; no difference in treatment outcome was found in patients with mild disease. The relapse rates did not differ significantly between treatment groups in patients with either mild or severe disease.
Comments. The study was limited in that it was done at a single center and was done before the current highly virulent strain emerged. Whether these data can be extrapolated to today’s epidemic is unclear. Moreover, the investigators did not test for antimicrobial susceptibility (although metronidazole resistance is still uncommon). Finally, the development of colonization with vancomycin-resistant enterococci, one of the reasons that oral vancomycin is often not recommended, was not assessed.
Despite the study’s limitations, it shows that for severely ill patients with C difficile-associated diarrhea, oral vancomycin should be the treatment of choice.
IS CEFEPIME SAFE?
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338 348.
Cefepime (Maxipime) is a broad-spectrum, fourth-generation cephalosporin. It is widely used for its approved indications: pneumonia; bacteremia; urinary tract, abdominal, skin, and soft-tissue infections; and febrile neutropenia.
In 2006, Paul et al6 reviewed 33 controlled trials of empiric cefepime monotherapy for febrile neutropenia and found a higher death rate with cefepime than with other beta-lactam antibiotics. That preliminary study spawned the following more comprehensive review by the same group.
The study. Yahav et al7 performed a meta-analysis of randomized trials that compared cefepime with another beta-lactam antibiotic alone or combined with a non-beta-lactam drug given in both treatment groups. Two reviewers independently identified studies from a number of databases and extracted data.
The primary end point was the rate of death from all causes at 30 days. Secondary end points were clinical failure (defined as unresolved infection, treatment modification, or death from infection), failure to eradicate the causative pathogens, superinfection with different bacterial, fungal, or viral organisms, and adverse events.
More than 8,000 patients were involved in 57 trials: 20 trials evaluated therapy for neutropenic fever, 18 for pneumonia, 5 for urogenital infections, 2 for meningitis, and 10 for mixed infections.
Comparison drugs for febrile neutropenia were ceftazidime (Ceptaz, Fortaz, Tazicef); im-ipenem-cilastatin (Primaxin) or meropenem (Merrem); piperacillin-tazobactam (Zosyn); and ceftriaxone (Rocephin). Aminoglycosides were added to both treatment groups in six trials and vancomycin was added in one trial.
For pneumonia, comparison drugs were ceftazidime, ceftriaxone, cefotaxime (Claforan), and cefoperazone-sulbactam.
Adequate allocation concealment and allocation-sequence generation were described in 30 studies. Scores for baseline patient risk factors did not differ significantly between study populations.
Findings. The death rate from all causes was higher in patients taking cefepime than with other beta-lactam antibiotics (risk ratio [RR] 1.26, 95% confidence interval [CI] 1.08–1.49, P = .005). The rate was lower with each of the alternative antibiotics, but the difference was statistically significant only for cefepime vs piperacillin-tazobactam (RR 2.14, 95% CI 1.17–3.89, P = .05).
The rate of death from all causes was higher for cefepime in all types of infections (except urinary tract infection, in which no deaths occurred in any of the treatment arms), although the difference was statistically significant only for febrile neutropenia (RR 1.42, 95% CI 1.09–1.84, P = .009). No differences were found in secondary outcomes, either by disease or by drug used.
Comments. This meta-analysis supports previous findings that more patients die when cefepime is used. The mechanism, however, is unclear. The authors call for reconsideration of the use of cefepime for febrile neutropenia, community-acquired pneumonia, and health-care associated pneumonia. In November 2007, the US Food and Drug Administration (FDA) launched an investigation into the risk of cefepime but has not yet made recommendations. Practitioners should be aware of these data when considering antimicrobial options for treatment in these settings. Knowledge of local antimicrobial susceptibility data of key pathogens is essential in determining optimal empiric and pathogen-specific therapy.
AN ANTIBIOTIC AND A NASAL STEROID ARE INEFFECTIVE IN ACUTE SINUSITIS
Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487 2496.
In the United States and Europe 1% to 2% of all primary care office visits are for acute sinusitis. Studies indicate that 67% to nearly 100% of patients with symptoms of sinusitis receive an antibiotic for it, even though the evidence of efficacy is weak and guidelines do not support this practice. Cochrane reviews8,9 have suggested that topical corticosteroids, penicillin, and amoxicillin have marginal benefit in acute sinusitis, but the studies on which the analyses were based were flawed.
The Berg and Carenfelt criteria were developed to help diagnose bacterial sinusitis.10 At the time they were developed, computed tomography was not routinely done to search for sinusitis, so plain film diagnosis was compared with clinical criteria. The Berg and Carenfelt criteria include three symptoms and one sign: a history of purulent unilateral nasal discharge, unilateral facial pain, or bilateral purulent discharge and pus in the nares on inspection. The presence of two criteria has reasonable sensitivity (81%), specificity (89%), and positive predictive value (86%) for detecting acute bacterial or maxillary sinusitis in the office setting.
The study. Williamson et al11 conducted a double-blind, randomized, placebo-controlled trial of antibiotic and topical nasal steroid use in patients with suspected acute maxillary sinusitis. The trial included 240 patients who were seen in 58 family practices over 4 years in the United Kingdom and who had acute nonrecurrent sinusitis based on Berg and Carenfelt criteria. Patients were at least 16 years old; the average age was 44. Three-quarters were women. Few had fever, and 70% met only two Berg and Carenfelt criteria; the remaining 30% met three or all four criteria. Patients were excluded who had at least two sinusitis attacks per year, underlying nasal pathology, significant comorbidities, or a history of penicillin allergy, or if they had been treated with antibiotics or steroids during the past month.
Patients were randomized to receive one of four treatments:
- Amoxicillin 500 mg three times a day for 7 days plus budesonide (Rhinocort) 200 μg in each nostril once a day for 10 days
- Placebo amoxicillin plus real budesonide
- Amoxicillin plus placebo budesonide
- Placebo amoxicillin plus placebo budes-onide.
The groups were well matched. Outcomes were based on a questionnaire and a patient diary that assessed the duration and severity of 11 symptoms.
Findings. No difference was found between the treatment groups in overall outcome, in the proportion of those with symptoms at 10 days, or in daily symptom severity. The secondary analysis suggested that nasal steroids were marginally more effective in patients with less severe symptoms.
The authors concluded that neither an antibiotic nor a nasal steroid, alone or in combination, is effective for acute maxillary sinusitis in the primary care setting, and they recommended against their routine use.
Comments. This study had limitations. Some cases of viral disease may have been included: no objective reference standard (ie, computed tomography of the sinuses or sinus aspiration) was used, and although the Berg and Carenfelt criteria have been validated in secondary care settings, they have not been validated in primary care settings. In addition, fever was absent in most patients, and mild symptoms were poorly defined. Moreover, recruitment of patients was slow, raising questions of bias and generalizability. The study also did not address patients with comorbidities.
Nevertheless, the study shows that outpatients with symptoms of sinusitis without fever or significant comorbidities should not be treated with oral antibiotics or nasal steroids. Otherwise, antibiotic therapy may still be appropriate in certain patients at high risk and in those with fever.
PREDNISOLONE IS BENEFICIAL IN ACUTE BELL PALSY, ACYCLOVIR IS NOT
Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell palsy. N Engl J Med 2007; 357:1598 1607.
Bell palsy accounts for about two-thirds of cases of acute unilateral facial nerve palsy in the United States. Virologic studies from patients undergoing surgery for facial nerve decompression have suggested a possible association with herpes simplex virus. Other causes of acute unilateral facial nerve palsy include Lyme disease, sarcoidosis, Sjögren syndrome, trauma, carotid tumors, and diabetes. Bell palsy occurs most often during middle age, peaking between ages 30 and 45. As many as 30% of patients are left with significant neurologic residua. Corticosteroids and antiviral medications are commonly used to treat Bell palsy, but evidence for their efficacy is weak.
The study. Sullivan et al12 conducted a double-blind, placebo-controlled, randomized trial over 2 years in Scotland with 551 patients, age 16 years or older, recruited within 72 hours of the onset of symptoms. Patients who were pregnant or breastfeeding or who had uncontrolled diabetes, peptic ulcer disease, suppurative otitis, zoster, multiple sclerosis, sarcoidosis, or systemic infection were excluded. They were randomized to treatment for 10 days with either acyclovir (Zovirax) 400 mg five times daily or prednisolone 25 mg twice daily, both agents, or placebo.
The primary outcome was recovery of facial function based on the House-Brackmann grading system. Digital photographs of patients at 3 and 9 months of treatment were evaluated independently by three experts who were unaware of study group assignment or stage of assessment. These included a neurologist, an otorhinolaryn-gologist, and a plastic surgeon. The secondary outcomes were quality of life, facial appearance, and pain, as assessed by the patients.
Findings. At 3 months, 83% of the prednisolone recipients had no facial asymmetry, increasing to 94% at 9 months. In comparison, the numbers were 64% and 82% in those who did not receive prednisolone, and these differences were statistically significant. Acyclovir was found to be of no benefit at either 3 or 9 months.
The authors concluded that early treatment of Bell palsy with prednisolone improves the chance of complete recovery, and that acyclovir alone or in combination with steroids confers no benefit.
Comments. At about the same time that this study was published, Hato et al13 evaluated valacyclovir (Valtrex) plus prednisolone vs placebo plus prednisolone and found that patients with severe Bell palsy (defined as complete facial nerve paralysis) benefited from antiviral therapy.
Corticosteroids are indicated for acute Bell palsy. In patients with complete facial nerve paralysis, valacyclovir should be considered.
POSACONAZOLE AS PROPHYLAXIS IN FEBRILE NEUTROPENIA
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348 359.
For many years, amphotericin B was the only drug available for antifungal prophylaxis and therapy. Then, in the early 1990s, a number of studies suggested that the triazoles, notably fluconazole (Diflucan), were effective in a variety of clinical settings for both prophylaxis and therapy of serious fungal infections. In 1992 and 1995, two studies found that fluconazole prophylaxis was as effective as amphotericin B in preventing fungal infections in patients undergoing hematopoietic stem cell transplantation.14,15 Based on these studies, clinical practice changed, not only for patients undergoing hematopoietic stem cell transplantation, but also for empiric antifungal prophylaxis in patients receiving myeloablative chemotherapy to treat hematologic malignancies.
Fluconazole is not active against invasive molds, and newer drugs—itraconazole (Sporanox), voriconazole (Vfend), and most recently posaconazole (Noxafil)—were developed with expanded clinical activity. Studies in the 1990s found that itraconazole and voriconazole performed better than fluconazole but did not provide complete prophylaxis.
The study. Cornely et al16 compared posaconazole with fluconazole or itraconazole in 602 patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia. Although patients were randomized to either the posaconazole group or the fluconazole-or-itraconazole group, investigators could choose either fluconazole or itraconazole for patients randomized to that group. Most patients in the latter group (240 of 298) received fluconazole.
Patients were at least 13 years old, were able to take oral medications, had newly diagnosed disease or were having a first relapse, and had or were anticipated to have neutropenia for at least 7 days. The study excluded patients with invasive fungal infection within 30 days, significant liver or kidney dysfunction, an abnormal QT interval corrected for heart rate, an Eastern Cooperative Oncology Group performance status score of more than 2 (in bed more than half of the day), or allergy or a contraindication to azoles.
The trial treatment was started with each cycle of chemotherapy and was continued until recovery from neutropenia and complete remission, until invasive fungal infection developed, or for up to 12 weeks, whichever came first.
The primary end point was the incidence of proven or probable invasive fungal infection during the treatment phase. Secondary end points included death from any cause and time to death.
Findings. Posaconazole recipients fared significantly better than patients in the other treatment group with respect to the incidence of proven or probable invasive fungal infection, invasive aspergillosis, probability of death, death at 100 days, and death secondary to fungal infection. Treatment-related severe adverse events were a bit more common with posaconazole.
The authors suggest that posaconazole prophylaxis may have a place in prophylaxis in patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia.
Comments. It is not surprising that posaconazole performed better, because the standard treatment arm contained an agent (fluconazole) that did not cover Aspergillus, the most frequently identified source of invasive fungal infection during the treatment phase of the study.
In an editorial accompanying the article, De Pauw and Donnelly17 pointed out that whether posaconazole prophylaxis would be appropriate in a given case depends upon how likely infection is with Aspergillus. An institution with very few Aspergillus infections would have a much higher number needed to treat with posaconazole to prevent one case of aspergillosis than in this study, in which the number needed to treat was 16. Thus, knowledge of local epidemiology and incidence of invasive mold infections should guide selection of the optimal antifungal agent for prophylaxis in patients undergoing myeloablative chemotherapy for acute myelogenous leukemia or myelodysplasia.
ANIDULAFUNGIN VS FLUCONAZOLE FOR INVASIVE CANDIDIASIS
Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472 2482.
In 2002, caspofungin (Cancidas) was the first of a new class of drugs, the echinocandins, to be approved by the FDA. The echinocandins have been shown to be as effective as amphotericin B for the treatment of invasive candidiasis, but how they compare with azoles is an ongoing debate. Currently approved treatments for candidiasis, an important cause of disease and death in hospitalized patients, include fluconazole, voriconazole, caspofungin, and amphotericin B. Anidulafungin is the newest echinocandin and has been shown in a phase 2 study to be effective against invasive candidiasis.
The study. Reboli et al18 performed a randomized, double-blind, noninferiority trial comparing anidulafungin and fluconazole to treat candidemia and other forms of candidiasis. The trial was conducted in multiple centers over 15 months and involved 245 patients at least 16 years old who had a single blood culture or culture from a normally sterile site that was positive for Candida species, and who also had one or more of the following: fever, hypothermia, hypotension, local signs and symptoms, or radiographic findings of candidiasis. Patients were excluded if they had had more than 48 hours of systemic therapy with either of these agents or another antifungal drug, if they had had prophylaxis with an azole for more than 7 of the previous 30 days, or if they had refractory candidal infection, elevated liver function test results, Candida krusei infection, meningitis, endocarditis, or osteomyelitis. Removal of central venous catheters was recommended for all patients with candidemia.
Patients were initially stratified by severity of illness based on the Acute Physiology and Chronic Health Evaluation (APACHE II) score (= 20 or > 20, with higher scores indicating more severe disease) and the presence or absence of neutropenia at enrollment. They were then randomly assigned to receive either intravenous anidulafungin (200 mg on day 1 and then 100 mg daily) or intravenous fluconazole (800 mg on day 1 and then 400 mg daily, with the dose adjusted according to creatinine clearance) for at least 14 days after a negative blood culture and improved clinical state and for up to 42 days in total. After 10 days of intravenous therapy, all patients could receive oral fluconazole 400 mg daily at the investigators’ discretion if clinical improvement criteria were met.
The primary end point was global response at the end of intravenous therapy, defined as clinical and microbiologic improvement. A number of secondary end points were also studied. Response failure was defined as no significant clinical improvement, death due to candidiasis, persistent or recurrent candidiasis or a new Candida infection, or an indeterminate response (eg, loss to follow-up or death not attributed to candidiasis).
Of the 245 patients in the primary analysis, 89% had candidemia alone, and nearly two-thirds of those cases were caused by Candida albicans. Only 3% of patients had neutropenia at baseline. Fluconazole resistance was monitored and was rare.
Findings. Intravenous therapy was successful in 76% of patients receiving anidulafungin and in 60% of fluconazole recipients, a difference of 15.4 percentage points (95% CI 3.9–27.0). Results were similar for other efficacy end points. The rate of death from all causes was 31% in the fluconazole group and 23% in the anidulafungin group (P = .13). The frequency and types of adverse events were similar in the two groups. The authors concluded that anidulafungin was not inferior to fluconazole in the treatment of invasive candidiasis.
Comments. Does this study prove that anidulafungin is the treatment of choice for invasive candidiasis? Although the study noted trends in favor of anidulafungin, the differences did not achieve statistical significance for superiority. In addition, the study included so few patients with neutropenia that the results are not applicable to those patients. Finally, anidulafungin is several times more expensive than fluconazole.
Fluconazole has stood the test of time and is probably still the treatment of choice in patients who have suspected or proven candidemia or invasive candidiasis, unless they have already been treated with azoles or are critically ill. In those settings, echinocandins may be the preferred treatment.
- Nielsen ND, Layton BA, McDonald LC, Gerding DN, Liedtke LA, Strausbaugh LJ Infectious Diseases Society of America Emerging Infections Network. Changing epidemiology of Clostridium difficile-associated disease. Infect Dis Clin Pract 2006; 14:296–302.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 1983; 2:1043–1046.
- Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996; 22:813–818. Erratum in: Clin Infect Dis 1996; 23:423.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med 1989; 86:15–19.
- Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2006; 57:176–189.
- Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338–348.
- Williams JW, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2003; 2:CD000243.
- Zalmanovici A, Yaphe J. Steroids for acute sinusitis. Cochrane Database Syst Rev 2007; 2:CD005149.
- Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988; 105:343–349.
- Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487–2496.
- Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med 2007; 357:1598–1607.
- Hato N, Yamada H, Kohno H, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007; 28:408–413.
- Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326:845–851.
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after bone marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171:1545–1552.
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–359.
- De Pauw BE, Donnelly JP. Prophylaxis and aspergillosis—Has the principle been proven? N Engl J Med 2007; 356:409–411.
- Reboli AC, Rotstein C, Pappas PG, et al Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
Studies published during the past year provide information that could influence how we treat several infectious diseases in daily practice. Here is a brief overview of these “impact” studies.
VANCOMYCIN BEATS METRONIDAZOLE FOR SEVERE C DIFFICILE DIARRHEA
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-assoicated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
Clostridium difficile is the most common infectious cause of nosocomial diarrhea. Furthermore, a unique and highly virulent strain has emerged.
Which drug should be the treatment of choice: metronidazole (Flagyl) or oral vancomycin (Vancocin)? Over time, some infectious disease practitioners have believed that oral vancomycin is superior to oral metronidazole for the treatment of severe C difficile-associated diarrhea. Indeed, in a recently published survey, more than 25% of infectious disease practitioners said they used vancomycin as initial therapy for C difficile-associated diarrhea.1 Until recently, there has been no evidence to support this preference.
Ever since the first description of C difficile-associated diarrhea in the late 1970s, only two head-to-head studies have compared the efficacy of metronidazole vs vancomycin for the treatment of this disorder. Both studies were underpowered and neither was blinded. In 1983, Teasley et al2 treated 101 patients with metronidazole or vancomycin in a non-blinded, nonrandomized study and found no difference in efficacy. In 1996, Wenisch et al,3 in a prospective, randomized, but nonblinded study in 119 patients, compared vancomycin, metronidazole, fusidic acid, and teicoplanin (Targocid) and also found no significant difference in efficacy.
The study. Zar et al,4 in a prospective, double-blind trial at a single institution over an 8-year period, randomized 172 patients with C difficile-associated diarrhea to receive either oral metronidazole 250 mg four times a day or oral vancomycin 125 mg four times a day, both for 10 days. (The appropriate dosage of vancomycin has been debated over the years. In 1989, Fekety et al5 treated patients who had antibiotic-associated C difficile colitis with either 125 or 500 mg of vancomycin, four times a day, and found that the low dosage was as effective as the high dosage.) Both groups also received an oral placebo in addition to the study drug.
In the study of Zar et al, criteria for inclusion were diarrhea (defined as having more than two nonformed stools per 24 hours) and the finding of either toxin A in the stool or pseudomembranes on endoscopic examination. Patients were excluded if they were pregnant, had suspected or proven life-threatening intra-abdominal complications, were allergic to either study drug, had taken one of the study drugs during the last 14 days, or had previously had C difficile-associated diarrhea that did not respond to either study drug.
Patients were followed for up to 21 days. The primary end points were cure, treatment failure, or relapse. Cure was defined as the resolution of diarrhea and no C difficile toxin A detected on stool assay at days 6 and 10.
Disease severity was classified as either mild or severe based on a point system: patients received a single point each for being older than 60 years, being febrile, having an albumin level of less than 2.5 mg/dL, or having a white blood cell count of more than 15 × 109/L. Patients were classified as having severe disease if they had two or more points. They received two points (ie, they were automatically classified as having severe disease) if they had pseudomembranous colitis or if they developed C difficile infection that required treatment in an intensive care unit.
Findings. The overall cure rate in patients receiving vancomycin was 97%, compared with 84% for those on metronidazole (P = .006). This difference was attributable to the group of patients with severe disease; no difference in treatment outcome was found in patients with mild disease. The relapse rates did not differ significantly between treatment groups in patients with either mild or severe disease.
Comments. The study was limited in that it was done at a single center and was done before the current highly virulent strain emerged. Whether these data can be extrapolated to today’s epidemic is unclear. Moreover, the investigators did not test for antimicrobial susceptibility (although metronidazole resistance is still uncommon). Finally, the development of colonization with vancomycin-resistant enterococci, one of the reasons that oral vancomycin is often not recommended, was not assessed.
Despite the study’s limitations, it shows that for severely ill patients with C difficile-associated diarrhea, oral vancomycin should be the treatment of choice.
IS CEFEPIME SAFE?
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338 348.
Cefepime (Maxipime) is a broad-spectrum, fourth-generation cephalosporin. It is widely used for its approved indications: pneumonia; bacteremia; urinary tract, abdominal, skin, and soft-tissue infections; and febrile neutropenia.
In 2006, Paul et al6 reviewed 33 controlled trials of empiric cefepime monotherapy for febrile neutropenia and found a higher death rate with cefepime than with other beta-lactam antibiotics. That preliminary study spawned the following more comprehensive review by the same group.
The study. Yahav et al7 performed a meta-analysis of randomized trials that compared cefepime with another beta-lactam antibiotic alone or combined with a non-beta-lactam drug given in both treatment groups. Two reviewers independently identified studies from a number of databases and extracted data.
The primary end point was the rate of death from all causes at 30 days. Secondary end points were clinical failure (defined as unresolved infection, treatment modification, or death from infection), failure to eradicate the causative pathogens, superinfection with different bacterial, fungal, or viral organisms, and adverse events.
More than 8,000 patients were involved in 57 trials: 20 trials evaluated therapy for neutropenic fever, 18 for pneumonia, 5 for urogenital infections, 2 for meningitis, and 10 for mixed infections.
Comparison drugs for febrile neutropenia were ceftazidime (Ceptaz, Fortaz, Tazicef); im-ipenem-cilastatin (Primaxin) or meropenem (Merrem); piperacillin-tazobactam (Zosyn); and ceftriaxone (Rocephin). Aminoglycosides were added to both treatment groups in six trials and vancomycin was added in one trial.
For pneumonia, comparison drugs were ceftazidime, ceftriaxone, cefotaxime (Claforan), and cefoperazone-sulbactam.
Adequate allocation concealment and allocation-sequence generation were described in 30 studies. Scores for baseline patient risk factors did not differ significantly between study populations.
Findings. The death rate from all causes was higher in patients taking cefepime than with other beta-lactam antibiotics (risk ratio [RR] 1.26, 95% confidence interval [CI] 1.08–1.49, P = .005). The rate was lower with each of the alternative antibiotics, but the difference was statistically significant only for cefepime vs piperacillin-tazobactam (RR 2.14, 95% CI 1.17–3.89, P = .05).
The rate of death from all causes was higher for cefepime in all types of infections (except urinary tract infection, in which no deaths occurred in any of the treatment arms), although the difference was statistically significant only for febrile neutropenia (RR 1.42, 95% CI 1.09–1.84, P = .009). No differences were found in secondary outcomes, either by disease or by drug used.
Comments. This meta-analysis supports previous findings that more patients die when cefepime is used. The mechanism, however, is unclear. The authors call for reconsideration of the use of cefepime for febrile neutropenia, community-acquired pneumonia, and health-care associated pneumonia. In November 2007, the US Food and Drug Administration (FDA) launched an investigation into the risk of cefepime but has not yet made recommendations. Practitioners should be aware of these data when considering antimicrobial options for treatment in these settings. Knowledge of local antimicrobial susceptibility data of key pathogens is essential in determining optimal empiric and pathogen-specific therapy.
AN ANTIBIOTIC AND A NASAL STEROID ARE INEFFECTIVE IN ACUTE SINUSITIS
Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487 2496.
In the United States and Europe 1% to 2% of all primary care office visits are for acute sinusitis. Studies indicate that 67% to nearly 100% of patients with symptoms of sinusitis receive an antibiotic for it, even though the evidence of efficacy is weak and guidelines do not support this practice. Cochrane reviews8,9 have suggested that topical corticosteroids, penicillin, and amoxicillin have marginal benefit in acute sinusitis, but the studies on which the analyses were based were flawed.
The Berg and Carenfelt criteria were developed to help diagnose bacterial sinusitis.10 At the time they were developed, computed tomography was not routinely done to search for sinusitis, so plain film diagnosis was compared with clinical criteria. The Berg and Carenfelt criteria include three symptoms and one sign: a history of purulent unilateral nasal discharge, unilateral facial pain, or bilateral purulent discharge and pus in the nares on inspection. The presence of two criteria has reasonable sensitivity (81%), specificity (89%), and positive predictive value (86%) for detecting acute bacterial or maxillary sinusitis in the office setting.
The study. Williamson et al11 conducted a double-blind, randomized, placebo-controlled trial of antibiotic and topical nasal steroid use in patients with suspected acute maxillary sinusitis. The trial included 240 patients who were seen in 58 family practices over 4 years in the United Kingdom and who had acute nonrecurrent sinusitis based on Berg and Carenfelt criteria. Patients were at least 16 years old; the average age was 44. Three-quarters were women. Few had fever, and 70% met only two Berg and Carenfelt criteria; the remaining 30% met three or all four criteria. Patients were excluded who had at least two sinusitis attacks per year, underlying nasal pathology, significant comorbidities, or a history of penicillin allergy, or if they had been treated with antibiotics or steroids during the past month.
Patients were randomized to receive one of four treatments:
- Amoxicillin 500 mg three times a day for 7 days plus budesonide (Rhinocort) 200 μg in each nostril once a day for 10 days
- Placebo amoxicillin plus real budesonide
- Amoxicillin plus placebo budesonide
- Placebo amoxicillin plus placebo budes-onide.
The groups were well matched. Outcomes were based on a questionnaire and a patient diary that assessed the duration and severity of 11 symptoms.
Findings. No difference was found between the treatment groups in overall outcome, in the proportion of those with symptoms at 10 days, or in daily symptom severity. The secondary analysis suggested that nasal steroids were marginally more effective in patients with less severe symptoms.
The authors concluded that neither an antibiotic nor a nasal steroid, alone or in combination, is effective for acute maxillary sinusitis in the primary care setting, and they recommended against their routine use.
Comments. This study had limitations. Some cases of viral disease may have been included: no objective reference standard (ie, computed tomography of the sinuses or sinus aspiration) was used, and although the Berg and Carenfelt criteria have been validated in secondary care settings, they have not been validated in primary care settings. In addition, fever was absent in most patients, and mild symptoms were poorly defined. Moreover, recruitment of patients was slow, raising questions of bias and generalizability. The study also did not address patients with comorbidities.
Nevertheless, the study shows that outpatients with symptoms of sinusitis without fever or significant comorbidities should not be treated with oral antibiotics or nasal steroids. Otherwise, antibiotic therapy may still be appropriate in certain patients at high risk and in those with fever.
PREDNISOLONE IS BENEFICIAL IN ACUTE BELL PALSY, ACYCLOVIR IS NOT
Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell palsy. N Engl J Med 2007; 357:1598 1607.
Bell palsy accounts for about two-thirds of cases of acute unilateral facial nerve palsy in the United States. Virologic studies from patients undergoing surgery for facial nerve decompression have suggested a possible association with herpes simplex virus. Other causes of acute unilateral facial nerve palsy include Lyme disease, sarcoidosis, Sjögren syndrome, trauma, carotid tumors, and diabetes. Bell palsy occurs most often during middle age, peaking between ages 30 and 45. As many as 30% of patients are left with significant neurologic residua. Corticosteroids and antiviral medications are commonly used to treat Bell palsy, but evidence for their efficacy is weak.
The study. Sullivan et al12 conducted a double-blind, placebo-controlled, randomized trial over 2 years in Scotland with 551 patients, age 16 years or older, recruited within 72 hours of the onset of symptoms. Patients who were pregnant or breastfeeding or who had uncontrolled diabetes, peptic ulcer disease, suppurative otitis, zoster, multiple sclerosis, sarcoidosis, or systemic infection were excluded. They were randomized to treatment for 10 days with either acyclovir (Zovirax) 400 mg five times daily or prednisolone 25 mg twice daily, both agents, or placebo.
The primary outcome was recovery of facial function based on the House-Brackmann grading system. Digital photographs of patients at 3 and 9 months of treatment were evaluated independently by three experts who were unaware of study group assignment or stage of assessment. These included a neurologist, an otorhinolaryn-gologist, and a plastic surgeon. The secondary outcomes were quality of life, facial appearance, and pain, as assessed by the patients.
Findings. At 3 months, 83% of the prednisolone recipients had no facial asymmetry, increasing to 94% at 9 months. In comparison, the numbers were 64% and 82% in those who did not receive prednisolone, and these differences were statistically significant. Acyclovir was found to be of no benefit at either 3 or 9 months.
The authors concluded that early treatment of Bell palsy with prednisolone improves the chance of complete recovery, and that acyclovir alone or in combination with steroids confers no benefit.
Comments. At about the same time that this study was published, Hato et al13 evaluated valacyclovir (Valtrex) plus prednisolone vs placebo plus prednisolone and found that patients with severe Bell palsy (defined as complete facial nerve paralysis) benefited from antiviral therapy.
Corticosteroids are indicated for acute Bell palsy. In patients with complete facial nerve paralysis, valacyclovir should be considered.
POSACONAZOLE AS PROPHYLAXIS IN FEBRILE NEUTROPENIA
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348 359.
For many years, amphotericin B was the only drug available for antifungal prophylaxis and therapy. Then, in the early 1990s, a number of studies suggested that the triazoles, notably fluconazole (Diflucan), were effective in a variety of clinical settings for both prophylaxis and therapy of serious fungal infections. In 1992 and 1995, two studies found that fluconazole prophylaxis was as effective as amphotericin B in preventing fungal infections in patients undergoing hematopoietic stem cell transplantation.14,15 Based on these studies, clinical practice changed, not only for patients undergoing hematopoietic stem cell transplantation, but also for empiric antifungal prophylaxis in patients receiving myeloablative chemotherapy to treat hematologic malignancies.
Fluconazole is not active against invasive molds, and newer drugs—itraconazole (Sporanox), voriconazole (Vfend), and most recently posaconazole (Noxafil)—were developed with expanded clinical activity. Studies in the 1990s found that itraconazole and voriconazole performed better than fluconazole but did not provide complete prophylaxis.
The study. Cornely et al16 compared posaconazole with fluconazole or itraconazole in 602 patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia. Although patients were randomized to either the posaconazole group or the fluconazole-or-itraconazole group, investigators could choose either fluconazole or itraconazole for patients randomized to that group. Most patients in the latter group (240 of 298) received fluconazole.
Patients were at least 13 years old, were able to take oral medications, had newly diagnosed disease or were having a first relapse, and had or were anticipated to have neutropenia for at least 7 days. The study excluded patients with invasive fungal infection within 30 days, significant liver or kidney dysfunction, an abnormal QT interval corrected for heart rate, an Eastern Cooperative Oncology Group performance status score of more than 2 (in bed more than half of the day), or allergy or a contraindication to azoles.
The trial treatment was started with each cycle of chemotherapy and was continued until recovery from neutropenia and complete remission, until invasive fungal infection developed, or for up to 12 weeks, whichever came first.
The primary end point was the incidence of proven or probable invasive fungal infection during the treatment phase. Secondary end points included death from any cause and time to death.
Findings. Posaconazole recipients fared significantly better than patients in the other treatment group with respect to the incidence of proven or probable invasive fungal infection, invasive aspergillosis, probability of death, death at 100 days, and death secondary to fungal infection. Treatment-related severe adverse events were a bit more common with posaconazole.
The authors suggest that posaconazole prophylaxis may have a place in prophylaxis in patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia.
Comments. It is not surprising that posaconazole performed better, because the standard treatment arm contained an agent (fluconazole) that did not cover Aspergillus, the most frequently identified source of invasive fungal infection during the treatment phase of the study.
In an editorial accompanying the article, De Pauw and Donnelly17 pointed out that whether posaconazole prophylaxis would be appropriate in a given case depends upon how likely infection is with Aspergillus. An institution with very few Aspergillus infections would have a much higher number needed to treat with posaconazole to prevent one case of aspergillosis than in this study, in which the number needed to treat was 16. Thus, knowledge of local epidemiology and incidence of invasive mold infections should guide selection of the optimal antifungal agent for prophylaxis in patients undergoing myeloablative chemotherapy for acute myelogenous leukemia or myelodysplasia.
ANIDULAFUNGIN VS FLUCONAZOLE FOR INVASIVE CANDIDIASIS
Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472 2482.
In 2002, caspofungin (Cancidas) was the first of a new class of drugs, the echinocandins, to be approved by the FDA. The echinocandins have been shown to be as effective as amphotericin B for the treatment of invasive candidiasis, but how they compare with azoles is an ongoing debate. Currently approved treatments for candidiasis, an important cause of disease and death in hospitalized patients, include fluconazole, voriconazole, caspofungin, and amphotericin B. Anidulafungin is the newest echinocandin and has been shown in a phase 2 study to be effective against invasive candidiasis.
The study. Reboli et al18 performed a randomized, double-blind, noninferiority trial comparing anidulafungin and fluconazole to treat candidemia and other forms of candidiasis. The trial was conducted in multiple centers over 15 months and involved 245 patients at least 16 years old who had a single blood culture or culture from a normally sterile site that was positive for Candida species, and who also had one or more of the following: fever, hypothermia, hypotension, local signs and symptoms, or radiographic findings of candidiasis. Patients were excluded if they had had more than 48 hours of systemic therapy with either of these agents or another antifungal drug, if they had had prophylaxis with an azole for more than 7 of the previous 30 days, or if they had refractory candidal infection, elevated liver function test results, Candida krusei infection, meningitis, endocarditis, or osteomyelitis. Removal of central venous catheters was recommended for all patients with candidemia.
Patients were initially stratified by severity of illness based on the Acute Physiology and Chronic Health Evaluation (APACHE II) score (= 20 or > 20, with higher scores indicating more severe disease) and the presence or absence of neutropenia at enrollment. They were then randomly assigned to receive either intravenous anidulafungin (200 mg on day 1 and then 100 mg daily) or intravenous fluconazole (800 mg on day 1 and then 400 mg daily, with the dose adjusted according to creatinine clearance) for at least 14 days after a negative blood culture and improved clinical state and for up to 42 days in total. After 10 days of intravenous therapy, all patients could receive oral fluconazole 400 mg daily at the investigators’ discretion if clinical improvement criteria were met.
The primary end point was global response at the end of intravenous therapy, defined as clinical and microbiologic improvement. A number of secondary end points were also studied. Response failure was defined as no significant clinical improvement, death due to candidiasis, persistent or recurrent candidiasis or a new Candida infection, or an indeterminate response (eg, loss to follow-up or death not attributed to candidiasis).
Of the 245 patients in the primary analysis, 89% had candidemia alone, and nearly two-thirds of those cases were caused by Candida albicans. Only 3% of patients had neutropenia at baseline. Fluconazole resistance was monitored and was rare.
Findings. Intravenous therapy was successful in 76% of patients receiving anidulafungin and in 60% of fluconazole recipients, a difference of 15.4 percentage points (95% CI 3.9–27.0). Results were similar for other efficacy end points. The rate of death from all causes was 31% in the fluconazole group and 23% in the anidulafungin group (P = .13). The frequency and types of adverse events were similar in the two groups. The authors concluded that anidulafungin was not inferior to fluconazole in the treatment of invasive candidiasis.
Comments. Does this study prove that anidulafungin is the treatment of choice for invasive candidiasis? Although the study noted trends in favor of anidulafungin, the differences did not achieve statistical significance for superiority. In addition, the study included so few patients with neutropenia that the results are not applicable to those patients. Finally, anidulafungin is several times more expensive than fluconazole.
Fluconazole has stood the test of time and is probably still the treatment of choice in patients who have suspected or proven candidemia or invasive candidiasis, unless they have already been treated with azoles or are critically ill. In those settings, echinocandins may be the preferred treatment.
Studies published during the past year provide information that could influence how we treat several infectious diseases in daily practice. Here is a brief overview of these “impact” studies.
VANCOMYCIN BEATS METRONIDAZOLE FOR SEVERE C DIFFICILE DIARRHEA
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-assoicated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
Clostridium difficile is the most common infectious cause of nosocomial diarrhea. Furthermore, a unique and highly virulent strain has emerged.
Which drug should be the treatment of choice: metronidazole (Flagyl) or oral vancomycin (Vancocin)? Over time, some infectious disease practitioners have believed that oral vancomycin is superior to oral metronidazole for the treatment of severe C difficile-associated diarrhea. Indeed, in a recently published survey, more than 25% of infectious disease practitioners said they used vancomycin as initial therapy for C difficile-associated diarrhea.1 Until recently, there has been no evidence to support this preference.
Ever since the first description of C difficile-associated diarrhea in the late 1970s, only two head-to-head studies have compared the efficacy of metronidazole vs vancomycin for the treatment of this disorder. Both studies were underpowered and neither was blinded. In 1983, Teasley et al2 treated 101 patients with metronidazole or vancomycin in a non-blinded, nonrandomized study and found no difference in efficacy. In 1996, Wenisch et al,3 in a prospective, randomized, but nonblinded study in 119 patients, compared vancomycin, metronidazole, fusidic acid, and teicoplanin (Targocid) and also found no significant difference in efficacy.
The study. Zar et al,4 in a prospective, double-blind trial at a single institution over an 8-year period, randomized 172 patients with C difficile-associated diarrhea to receive either oral metronidazole 250 mg four times a day or oral vancomycin 125 mg four times a day, both for 10 days. (The appropriate dosage of vancomycin has been debated over the years. In 1989, Fekety et al5 treated patients who had antibiotic-associated C difficile colitis with either 125 or 500 mg of vancomycin, four times a day, and found that the low dosage was as effective as the high dosage.) Both groups also received an oral placebo in addition to the study drug.
In the study of Zar et al, criteria for inclusion were diarrhea (defined as having more than two nonformed stools per 24 hours) and the finding of either toxin A in the stool or pseudomembranes on endoscopic examination. Patients were excluded if they were pregnant, had suspected or proven life-threatening intra-abdominal complications, were allergic to either study drug, had taken one of the study drugs during the last 14 days, or had previously had C difficile-associated diarrhea that did not respond to either study drug.
Patients were followed for up to 21 days. The primary end points were cure, treatment failure, or relapse. Cure was defined as the resolution of diarrhea and no C difficile toxin A detected on stool assay at days 6 and 10.
Disease severity was classified as either mild or severe based on a point system: patients received a single point each for being older than 60 years, being febrile, having an albumin level of less than 2.5 mg/dL, or having a white blood cell count of more than 15 × 109/L. Patients were classified as having severe disease if they had two or more points. They received two points (ie, they were automatically classified as having severe disease) if they had pseudomembranous colitis or if they developed C difficile infection that required treatment in an intensive care unit.
Findings. The overall cure rate in patients receiving vancomycin was 97%, compared with 84% for those on metronidazole (P = .006). This difference was attributable to the group of patients with severe disease; no difference in treatment outcome was found in patients with mild disease. The relapse rates did not differ significantly between treatment groups in patients with either mild or severe disease.
Comments. The study was limited in that it was done at a single center and was done before the current highly virulent strain emerged. Whether these data can be extrapolated to today’s epidemic is unclear. Moreover, the investigators did not test for antimicrobial susceptibility (although metronidazole resistance is still uncommon). Finally, the development of colonization with vancomycin-resistant enterococci, one of the reasons that oral vancomycin is often not recommended, was not assessed.
Despite the study’s limitations, it shows that for severely ill patients with C difficile-associated diarrhea, oral vancomycin should be the treatment of choice.
IS CEFEPIME SAFE?
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338 348.
Cefepime (Maxipime) is a broad-spectrum, fourth-generation cephalosporin. It is widely used for its approved indications: pneumonia; bacteremia; urinary tract, abdominal, skin, and soft-tissue infections; and febrile neutropenia.
In 2006, Paul et al6 reviewed 33 controlled trials of empiric cefepime monotherapy for febrile neutropenia and found a higher death rate with cefepime than with other beta-lactam antibiotics. That preliminary study spawned the following more comprehensive review by the same group.
The study. Yahav et al7 performed a meta-analysis of randomized trials that compared cefepime with another beta-lactam antibiotic alone or combined with a non-beta-lactam drug given in both treatment groups. Two reviewers independently identified studies from a number of databases and extracted data.
The primary end point was the rate of death from all causes at 30 days. Secondary end points were clinical failure (defined as unresolved infection, treatment modification, or death from infection), failure to eradicate the causative pathogens, superinfection with different bacterial, fungal, or viral organisms, and adverse events.
More than 8,000 patients were involved in 57 trials: 20 trials evaluated therapy for neutropenic fever, 18 for pneumonia, 5 for urogenital infections, 2 for meningitis, and 10 for mixed infections.
Comparison drugs for febrile neutropenia were ceftazidime (Ceptaz, Fortaz, Tazicef); im-ipenem-cilastatin (Primaxin) or meropenem (Merrem); piperacillin-tazobactam (Zosyn); and ceftriaxone (Rocephin). Aminoglycosides were added to both treatment groups in six trials and vancomycin was added in one trial.
For pneumonia, comparison drugs were ceftazidime, ceftriaxone, cefotaxime (Claforan), and cefoperazone-sulbactam.
Adequate allocation concealment and allocation-sequence generation were described in 30 studies. Scores for baseline patient risk factors did not differ significantly between study populations.
Findings. The death rate from all causes was higher in patients taking cefepime than with other beta-lactam antibiotics (risk ratio [RR] 1.26, 95% confidence interval [CI] 1.08–1.49, P = .005). The rate was lower with each of the alternative antibiotics, but the difference was statistically significant only for cefepime vs piperacillin-tazobactam (RR 2.14, 95% CI 1.17–3.89, P = .05).
The rate of death from all causes was higher for cefepime in all types of infections (except urinary tract infection, in which no deaths occurred in any of the treatment arms), although the difference was statistically significant only for febrile neutropenia (RR 1.42, 95% CI 1.09–1.84, P = .009). No differences were found in secondary outcomes, either by disease or by drug used.
Comments. This meta-analysis supports previous findings that more patients die when cefepime is used. The mechanism, however, is unclear. The authors call for reconsideration of the use of cefepime for febrile neutropenia, community-acquired pneumonia, and health-care associated pneumonia. In November 2007, the US Food and Drug Administration (FDA) launched an investigation into the risk of cefepime but has not yet made recommendations. Practitioners should be aware of these data when considering antimicrobial options for treatment in these settings. Knowledge of local antimicrobial susceptibility data of key pathogens is essential in determining optimal empiric and pathogen-specific therapy.
AN ANTIBIOTIC AND A NASAL STEROID ARE INEFFECTIVE IN ACUTE SINUSITIS
Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487 2496.
In the United States and Europe 1% to 2% of all primary care office visits are for acute sinusitis. Studies indicate that 67% to nearly 100% of patients with symptoms of sinusitis receive an antibiotic for it, even though the evidence of efficacy is weak and guidelines do not support this practice. Cochrane reviews8,9 have suggested that topical corticosteroids, penicillin, and amoxicillin have marginal benefit in acute sinusitis, but the studies on which the analyses were based were flawed.
The Berg and Carenfelt criteria were developed to help diagnose bacterial sinusitis.10 At the time they were developed, computed tomography was not routinely done to search for sinusitis, so plain film diagnosis was compared with clinical criteria. The Berg and Carenfelt criteria include three symptoms and one sign: a history of purulent unilateral nasal discharge, unilateral facial pain, or bilateral purulent discharge and pus in the nares on inspection. The presence of two criteria has reasonable sensitivity (81%), specificity (89%), and positive predictive value (86%) for detecting acute bacterial or maxillary sinusitis in the office setting.
The study. Williamson et al11 conducted a double-blind, randomized, placebo-controlled trial of antibiotic and topical nasal steroid use in patients with suspected acute maxillary sinusitis. The trial included 240 patients who were seen in 58 family practices over 4 years in the United Kingdom and who had acute nonrecurrent sinusitis based on Berg and Carenfelt criteria. Patients were at least 16 years old; the average age was 44. Three-quarters were women. Few had fever, and 70% met only two Berg and Carenfelt criteria; the remaining 30% met three or all four criteria. Patients were excluded who had at least two sinusitis attacks per year, underlying nasal pathology, significant comorbidities, or a history of penicillin allergy, or if they had been treated with antibiotics or steroids during the past month.
Patients were randomized to receive one of four treatments:
- Amoxicillin 500 mg three times a day for 7 days plus budesonide (Rhinocort) 200 μg in each nostril once a day for 10 days
- Placebo amoxicillin plus real budesonide
- Amoxicillin plus placebo budesonide
- Placebo amoxicillin plus placebo budes-onide.
The groups were well matched. Outcomes were based on a questionnaire and a patient diary that assessed the duration and severity of 11 symptoms.
Findings. No difference was found between the treatment groups in overall outcome, in the proportion of those with symptoms at 10 days, or in daily symptom severity. The secondary analysis suggested that nasal steroids were marginally more effective in patients with less severe symptoms.
The authors concluded that neither an antibiotic nor a nasal steroid, alone or in combination, is effective for acute maxillary sinusitis in the primary care setting, and they recommended against their routine use.
Comments. This study had limitations. Some cases of viral disease may have been included: no objective reference standard (ie, computed tomography of the sinuses or sinus aspiration) was used, and although the Berg and Carenfelt criteria have been validated in secondary care settings, they have not been validated in primary care settings. In addition, fever was absent in most patients, and mild symptoms were poorly defined. Moreover, recruitment of patients was slow, raising questions of bias and generalizability. The study also did not address patients with comorbidities.
Nevertheless, the study shows that outpatients with symptoms of sinusitis without fever or significant comorbidities should not be treated with oral antibiotics or nasal steroids. Otherwise, antibiotic therapy may still be appropriate in certain patients at high risk and in those with fever.
PREDNISOLONE IS BENEFICIAL IN ACUTE BELL PALSY, ACYCLOVIR IS NOT
Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell palsy. N Engl J Med 2007; 357:1598 1607.
Bell palsy accounts for about two-thirds of cases of acute unilateral facial nerve palsy in the United States. Virologic studies from patients undergoing surgery for facial nerve decompression have suggested a possible association with herpes simplex virus. Other causes of acute unilateral facial nerve palsy include Lyme disease, sarcoidosis, Sjögren syndrome, trauma, carotid tumors, and diabetes. Bell palsy occurs most often during middle age, peaking between ages 30 and 45. As many as 30% of patients are left with significant neurologic residua. Corticosteroids and antiviral medications are commonly used to treat Bell palsy, but evidence for their efficacy is weak.
The study. Sullivan et al12 conducted a double-blind, placebo-controlled, randomized trial over 2 years in Scotland with 551 patients, age 16 years or older, recruited within 72 hours of the onset of symptoms. Patients who were pregnant or breastfeeding or who had uncontrolled diabetes, peptic ulcer disease, suppurative otitis, zoster, multiple sclerosis, sarcoidosis, or systemic infection were excluded. They were randomized to treatment for 10 days with either acyclovir (Zovirax) 400 mg five times daily or prednisolone 25 mg twice daily, both agents, or placebo.
The primary outcome was recovery of facial function based on the House-Brackmann grading system. Digital photographs of patients at 3 and 9 months of treatment were evaluated independently by three experts who were unaware of study group assignment or stage of assessment. These included a neurologist, an otorhinolaryn-gologist, and a plastic surgeon. The secondary outcomes were quality of life, facial appearance, and pain, as assessed by the patients.
Findings. At 3 months, 83% of the prednisolone recipients had no facial asymmetry, increasing to 94% at 9 months. In comparison, the numbers were 64% and 82% in those who did not receive prednisolone, and these differences were statistically significant. Acyclovir was found to be of no benefit at either 3 or 9 months.
The authors concluded that early treatment of Bell palsy with prednisolone improves the chance of complete recovery, and that acyclovir alone or in combination with steroids confers no benefit.
Comments. At about the same time that this study was published, Hato et al13 evaluated valacyclovir (Valtrex) plus prednisolone vs placebo plus prednisolone and found that patients with severe Bell palsy (defined as complete facial nerve paralysis) benefited from antiviral therapy.
Corticosteroids are indicated for acute Bell palsy. In patients with complete facial nerve paralysis, valacyclovir should be considered.
POSACONAZOLE AS PROPHYLAXIS IN FEBRILE NEUTROPENIA
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348 359.
For many years, amphotericin B was the only drug available for antifungal prophylaxis and therapy. Then, in the early 1990s, a number of studies suggested that the triazoles, notably fluconazole (Diflucan), were effective in a variety of clinical settings for both prophylaxis and therapy of serious fungal infections. In 1992 and 1995, two studies found that fluconazole prophylaxis was as effective as amphotericin B in preventing fungal infections in patients undergoing hematopoietic stem cell transplantation.14,15 Based on these studies, clinical practice changed, not only for patients undergoing hematopoietic stem cell transplantation, but also for empiric antifungal prophylaxis in patients receiving myeloablative chemotherapy to treat hematologic malignancies.
Fluconazole is not active against invasive molds, and newer drugs—itraconazole (Sporanox), voriconazole (Vfend), and most recently posaconazole (Noxafil)—were developed with expanded clinical activity. Studies in the 1990s found that itraconazole and voriconazole performed better than fluconazole but did not provide complete prophylaxis.
The study. Cornely et al16 compared posaconazole with fluconazole or itraconazole in 602 patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia. Although patients were randomized to either the posaconazole group or the fluconazole-or-itraconazole group, investigators could choose either fluconazole or itraconazole for patients randomized to that group. Most patients in the latter group (240 of 298) received fluconazole.
Patients were at least 13 years old, were able to take oral medications, had newly diagnosed disease or were having a first relapse, and had or were anticipated to have neutropenia for at least 7 days. The study excluded patients with invasive fungal infection within 30 days, significant liver or kidney dysfunction, an abnormal QT interval corrected for heart rate, an Eastern Cooperative Oncology Group performance status score of more than 2 (in bed more than half of the day), or allergy or a contraindication to azoles.
The trial treatment was started with each cycle of chemotherapy and was continued until recovery from neutropenia and complete remission, until invasive fungal infection developed, or for up to 12 weeks, whichever came first.
The primary end point was the incidence of proven or probable invasive fungal infection during the treatment phase. Secondary end points included death from any cause and time to death.
Findings. Posaconazole recipients fared significantly better than patients in the other treatment group with respect to the incidence of proven or probable invasive fungal infection, invasive aspergillosis, probability of death, death at 100 days, and death secondary to fungal infection. Treatment-related severe adverse events were a bit more common with posaconazole.
The authors suggest that posaconazole prophylaxis may have a place in prophylaxis in patients undergoing chemotherapy for acute myelogenous leukemia or myelodysplasia.
Comments. It is not surprising that posaconazole performed better, because the standard treatment arm contained an agent (fluconazole) that did not cover Aspergillus, the most frequently identified source of invasive fungal infection during the treatment phase of the study.
In an editorial accompanying the article, De Pauw and Donnelly17 pointed out that whether posaconazole prophylaxis would be appropriate in a given case depends upon how likely infection is with Aspergillus. An institution with very few Aspergillus infections would have a much higher number needed to treat with posaconazole to prevent one case of aspergillosis than in this study, in which the number needed to treat was 16. Thus, knowledge of local epidemiology and incidence of invasive mold infections should guide selection of the optimal antifungal agent for prophylaxis in patients undergoing myeloablative chemotherapy for acute myelogenous leukemia or myelodysplasia.
ANIDULAFUNGIN VS FLUCONAZOLE FOR INVASIVE CANDIDIASIS
Reboli AC, Rotstein C, Pappas PG, et al; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472 2482.
In 2002, caspofungin (Cancidas) was the first of a new class of drugs, the echinocandins, to be approved by the FDA. The echinocandins have been shown to be as effective as amphotericin B for the treatment of invasive candidiasis, but how they compare with azoles is an ongoing debate. Currently approved treatments for candidiasis, an important cause of disease and death in hospitalized patients, include fluconazole, voriconazole, caspofungin, and amphotericin B. Anidulafungin is the newest echinocandin and has been shown in a phase 2 study to be effective against invasive candidiasis.
The study. Reboli et al18 performed a randomized, double-blind, noninferiority trial comparing anidulafungin and fluconazole to treat candidemia and other forms of candidiasis. The trial was conducted in multiple centers over 15 months and involved 245 patients at least 16 years old who had a single blood culture or culture from a normally sterile site that was positive for Candida species, and who also had one or more of the following: fever, hypothermia, hypotension, local signs and symptoms, or radiographic findings of candidiasis. Patients were excluded if they had had more than 48 hours of systemic therapy with either of these agents or another antifungal drug, if they had had prophylaxis with an azole for more than 7 of the previous 30 days, or if they had refractory candidal infection, elevated liver function test results, Candida krusei infection, meningitis, endocarditis, or osteomyelitis. Removal of central venous catheters was recommended for all patients with candidemia.
Patients were initially stratified by severity of illness based on the Acute Physiology and Chronic Health Evaluation (APACHE II) score (= 20 or > 20, with higher scores indicating more severe disease) and the presence or absence of neutropenia at enrollment. They were then randomly assigned to receive either intravenous anidulafungin (200 mg on day 1 and then 100 mg daily) or intravenous fluconazole (800 mg on day 1 and then 400 mg daily, with the dose adjusted according to creatinine clearance) for at least 14 days after a negative blood culture and improved clinical state and for up to 42 days in total. After 10 days of intravenous therapy, all patients could receive oral fluconazole 400 mg daily at the investigators’ discretion if clinical improvement criteria were met.
The primary end point was global response at the end of intravenous therapy, defined as clinical and microbiologic improvement. A number of secondary end points were also studied. Response failure was defined as no significant clinical improvement, death due to candidiasis, persistent or recurrent candidiasis or a new Candida infection, or an indeterminate response (eg, loss to follow-up or death not attributed to candidiasis).
Of the 245 patients in the primary analysis, 89% had candidemia alone, and nearly two-thirds of those cases were caused by Candida albicans. Only 3% of patients had neutropenia at baseline. Fluconazole resistance was monitored and was rare.
Findings. Intravenous therapy was successful in 76% of patients receiving anidulafungin and in 60% of fluconazole recipients, a difference of 15.4 percentage points (95% CI 3.9–27.0). Results were similar for other efficacy end points. The rate of death from all causes was 31% in the fluconazole group and 23% in the anidulafungin group (P = .13). The frequency and types of adverse events were similar in the two groups. The authors concluded that anidulafungin was not inferior to fluconazole in the treatment of invasive candidiasis.
Comments. Does this study prove that anidulafungin is the treatment of choice for invasive candidiasis? Although the study noted trends in favor of anidulafungin, the differences did not achieve statistical significance for superiority. In addition, the study included so few patients with neutropenia that the results are not applicable to those patients. Finally, anidulafungin is several times more expensive than fluconazole.
Fluconazole has stood the test of time and is probably still the treatment of choice in patients who have suspected or proven candidemia or invasive candidiasis, unless they have already been treated with azoles or are critically ill. In those settings, echinocandins may be the preferred treatment.
- Nielsen ND, Layton BA, McDonald LC, Gerding DN, Liedtke LA, Strausbaugh LJ Infectious Diseases Society of America Emerging Infections Network. Changing epidemiology of Clostridium difficile-associated disease. Infect Dis Clin Pract 2006; 14:296–302.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 1983; 2:1043–1046.
- Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996; 22:813–818. Erratum in: Clin Infect Dis 1996; 23:423.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med 1989; 86:15–19.
- Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2006; 57:176–189.
- Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338–348.
- Williams JW, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2003; 2:CD000243.
- Zalmanovici A, Yaphe J. Steroids for acute sinusitis. Cochrane Database Syst Rev 2007; 2:CD005149.
- Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988; 105:343–349.
- Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487–2496.
- Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med 2007; 357:1598–1607.
- Hato N, Yamada H, Kohno H, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007; 28:408–413.
- Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326:845–851.
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after bone marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171:1545–1552.
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–359.
- De Pauw BE, Donnelly JP. Prophylaxis and aspergillosis—Has the principle been proven? N Engl J Med 2007; 356:409–411.
- Reboli AC, Rotstein C, Pappas PG, et al Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
- Nielsen ND, Layton BA, McDonald LC, Gerding DN, Liedtke LA, Strausbaugh LJ Infectious Diseases Society of America Emerging Infections Network. Changing epidemiology of Clostridium difficile-associated disease. Infect Dis Clin Pract 2006; 14:296–302.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 1983; 2:1043–1046.
- Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996; 22:813–818. Erratum in: Clin Infect Dis 1996; 23:423.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med 1989; 86:15–19.
- Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2006; 57:176–189.
- Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2007; 7:338–348.
- Williams JW, Aguilar C, Cornell J, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2003; 2:CD000243.
- Zalmanovici A, Yaphe J. Steroids for acute sinusitis. Cochrane Database Syst Rev 2007; 2:CD005149.
- Berg O, Carenfelt C. Analysis of symptoms and clinical signs in the maxillary sinus empyema. Acta Otolaryngol 1988; 105:343–349.
- Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007; 298:2487–2496.
- Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med 2007; 357:1598–1607.
- Hato N, Yamada H, Kohno H, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007; 28:408–413.
- Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326:845–851.
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after bone marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171:1545–1552.
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–359.
- De Pauw BE, Donnelly JP. Prophylaxis and aspergillosis—Has the principle been proven? N Engl J Med 2007; 356:409–411.
- Reboli AC, Rotstein C, Pappas PG, et al Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356:2472–2482.
Take charge of your e-mail!
E-mail is a mixed blessing. When used appropriately, it saves time and paper and increases efficiency in the workplace. Unfortunately, as our lives get busier and our e-mail inboxes get fuller, e-mail is becoming an untamed monster.
In this article, we discuss various tools and strategies to reclaim lost e-mail productivity by reducing the volume of unsolicited e-mail (“spam”), following e-mail etiquette to eliminate unnecessary messaging, and managing and organizing our e-mail more effectively. What we present is as relevant for readers who use personal e-mail clients such as Hotmail, Gmail, YahooMail, and AOLMail as it is for those who use e-mail at work via programs such as Microsoft Outlook and IBM Lotus Notes.
FROM HUMBLE BEGINNINGS
The first e-mail messages were sent in the early 1960s, but they could be sent only to users of a single computer. In 1971, Ray Tomlinson programmed and sent the first e-mail message from one computer to another over a network. To separate the name of the user from that of the computer, he used the “@” sign. E-mail at that time could accommodate plain text only—ie, no attachments, no images.1
E-mail has come a long way from these humble beginnings to become one of the most heavily used aspects of the Internet. Its popularity stems mainly from its simplicity and efficiency in facilitating asynchronous communication. However, e-mail is fast becoming too successful, leading to overload for those who use it.
WHAT’S IN YOUR IN-BOX?
Incoming e-mail messages can be classified into the following categories:
- Messages that directly concern you or your work
- Copies of messages indirectly related to your work, sent to you to “keep you in the loop”
- Notices of events or meetings
- Messages acknowledging the receipt of e-mail messages that you sent
- Messages from organizations that you have some relationship with and that you have given your e-mail address to, such as professional organizations, mailing lists (listservs), retailers, service providers, or information providers
- Messages from friends, family, and colleagues that contain non-work-related information, such as jokes, news stories, and links to interesting Web sites
- Unsolicited messages from unknown senders (eg, online retailers, scam artists) with whom you have no relationship, often trying to sell you a product or service or to trick you into giving up information
- Unsolicited messages from senders you know but may not want to get messages from.
E-MAIL OVERLOAD CONSUMES TIME, ADDS STRESS
Each of these types of messages has to be handled in a specific manner. Thus, when a person gets dozens of these messages a day, it can significantly add to the workload. This problem was recognized as early as 1996, when Whittaker and Sidner2 coined the term “e-mail overload” and systematically studied it for the first time. Bellotti and others3 at the Palo Alto Research Lab concluded that it is not just the volume of the e-mail but the collaborative quality of e-mail task management and project management that leads to overload. Thus, the e-mail in-box requires not just a filing cabinet to sort the messages but a mechanism to support collaboration and project management.3
Studies show that e-mail overload causes people to work 1 to 2 extra hours a day, either at work or when they get home. Despite these issues, the Pew Internet and American Life Project reported in 2002 that more than half the Americans surveyed who use e-mail at work thought it was “essential to their work.”4 However, many also reported that e-mail has been distracting, has caused misunderstandings, and has added a new source of stress to their lives. Professionals have the added burden of e-mail being treated as a medicolegal document that can be discoverable and, hence, used as legal evidence.
No wonder, then, that many e-mail providers include tools to manage the flow and to organize both the wanted and unwanted e-mail messages. However, as with many tools, there are effective ways to use them.
CANNING THE SPAM: DECREASING UNWANTED MESSAGES
Spam is unsolicited e-mail, often of a commercial nature, sent indiscriminately to multiple mailing lists, individuals, or newsgroups. It is the Internet version of junk mail. An estimated 12 billion spam messages are sent every day, accounting for 40% to 60% of all e-mail messages.5
Spam takes up time and space and is an intrusion of privacy. In addition, it has financial costs to organizations. By one estimate, an organization with 1,000 employees loses over $200,000 a year in productivity due to spam.6
The reason spam is so widespread is that it is very cheap and profitable for the spammer. It costs next to nothing to send, and getting even 100 responses from 10 million messages sent is enough to turn a profit!6
Unfortunately, the Controlling the Assault of Non-Solicited Pornography and Marketing (CAN-SPAM) Act7 of 2003 had a mixed impact on limiting the volume of spam,8 and may have trumped state laws that were already in place to regulate spam.
To decrease the amount of spam you receive in your daily e-mail, it helps to understand that spam has three steps:
- Harvesting
- Confirming
- Spamming.

Prevent spammers from harvesting your e-mail address
Since a spammer first has to harvest your e-mail address, you should try not to give it away. Spammers use programs that troll the Internet looking for e-mail addresses. Tips for guarding your address:
- Try not to display it in public, eg, in chat rooms, message boards, listservs
- If you have to post your e-mail address in public, reformat it so it cannot be easily recognized as an e-mail address by the trolling software (see sidebar, “More ways to outsmart the spammer”)
- Check a Web site’s privacy policy before submitting your information
- Take the time to review “opt-out” options on Web sites you use, to prevent your e-mail address from being used by a third party
- Consider using separate e-mail addresses for your personal business and your work to limit spam in your workplace: to prevent unintended disclosure of your work address, give your family and friends only your personal e-mail address, and ask them to use “blind copy” so that other recipients don’t have access to it
- Consider using “disposable” e-mail addresses, especially when making on-line purchases or when requesting services.
Prevent spammers from confirming your e-mail address
If spammers do obtain your e-mail address, you can prevent them from verifying it:
- Do not reply to or click on any links in a spam or other unsolicited e-mail message, including links to “unsubscribe” from an unsolicited newsletter, chain letter, or special offer
- Set your e-mail application to display messages in plain text rather than HTML, or turn off the automatic images in your e-mail application: the opening of the e-mail and subsequent displaying of the images can automatically verify your address to the sender!
- Check your e-mail application to ensure that it sends automatic “out of office” or “vacation” replies only to your contacts or ask your e-mail administrator for help with this at your workplace.
Keep spam out of your in-box
Finally, if spam is sent, there are several ways to keep it from reaching your in-box:
- Sophisticated users can use e-mail rules to direct spam-marked e-mail to a separate junk folder
- Add frequent unsolicited e-mail senders to a “blocked sender” list (Table 1).
A WORD ABOUT ‘PHISHING’
Like spam, “phishing” is a form of unsolicited e-mail, but its intent is much more malignant. The perpetrator sends out legitimate-looking e-mail in an attempt to gather personal and financial information from recipients. Typically, the messages appear to come from well-known and trustworthy Web sites such as banks, Pay-Pal, eBay, MSN, or Yahoo, and they ask the recipient for an account number and the related password. For more on this topic, see http://en.wikipedia.org/wiki/Phishing.
GOOD MANNERS IN THE E-WORKPLACE
Even if you could block all spam messages from reaching your inbox, you still might receive unwanted messages from colleagues or friends. The only way to reduce unnecessary e-mail in the workplace is to ask your colleagues and employees to use e-mail appropriately. Here are some rules that could help decrease e-mail overload at your work:
Do not overuse “reply to all.” This is one of the most common reasons for e-mail overload in the workplace. Only use “reply to all” if you really need your message to be seen by each person who received the original message.
Use “cc:” sparingly. Try not to use the “cc:” field unless the recipients in that field know they are receiving a copy of the message. It also exposes the e-mail identity of other users and can promote spam, especially if you are sending e-mail to an external client.
Do not forward chain letters. It is safe to say that all chain letters are hoaxes. Just delete them as soon as you receive them.
Use alternate means of communication (eg, phone, meetings) if you really need to have a discussion. E-mail is not the richest medium for discussion, and sometimes it is better to talk to a person or group face to face or on the phone rather than to bounce e-mail back and forth multiple times between multiple users.
Use appropriate subject headings like “no response needed” or “FYI-Reference” when you don’t need a reply. For example, someone asks you to send a file by e-mail; you send it as an attachment, and in the subject line you say, “Here is the file you wanted. NRN.” The recipient will know not to respond with “Got it, thanks” or something like that. He can thank you when you next see him.
Avoid sending unnecessary attachments. Suppose you want to inform some colleagues about a visiting professor giving a grand rounds talk. Instead of creating a Word document and attaching it to the e-mail message, you can include the information in the body of the message itself. Thus, the recipients will not have to go through the additional step of opening the attachment. Also, the plain text message in the body of the message will be a smaller file than an e-mail message with an attached Word document.
Do not request delivery notification. This is irritating to most users, requiring an extra click before reading the message. It may not work with all e-mail clients, and a user can elect not to send the notification. And what would you do with such information anyway (especially if it is incomplete)?
Use a meaningful subject header. This will allow the user to decide whether and when to open the message.
Do not abuse the “urgent” or “high priority” flag. This is like crying wolf and diminishes the user’s ability to effectively manage his or her e-mail.
Anticipate and preempt e-mail volleys. Suppose a journal editor e-mails you to ask if you can review an article that was submitted to the journal, and says that your review would need to be completed by next month. You respond that you cannot do it within that time frame, but that you could do it the month after that. Anticipating the next question, you also provide the names of two colleagues who might be able to do the review. This will prevent any further e-mail on this topic unless the editor is willing to wait longer to get your review.
Maintain e-mail threads if possible. When responding to an e-mail message, use “reply” instead of starting a new message. This will maintain the thread of the discussion and make it easier to follow.
Do not send an e-mail message when you are angry or upset. It is better to wait until you calm down (sleep on it if possible) and then write the message or have a conversation on the phone or face to face. Nothing good comes out of writing an e-mail message in the heat of the moment, and it only leads to bad feelings and miscommunication. Along the same line, avoid sarcasm or humor in professional e-mail, as it can be misconstrued.
Avoid putting information in e-mail that you do not want unintended parties to read
It is only too easy to forward or share the e-mail message with others.
Avoid sending confidential patient information in e-mail unless your institution has set up some ground rules. The American Medical Informatics Association has issued guidelines on this topic.9
ORGANIZE YOUR E-MAIL AND RELATED INFORMATION
Once you have limited the amount of unwanted e-mail, the next step is to deal with the remaining messages.
The task is difficult, for several reasons. Some people feel they will need the information in their e-mail at a later date and thus don’t want to remove it from the in-box. Some messages may require a significant amount of thought or time to respond to and cannot be dealt with immediately. Having limits on the amount of e-mail that can be stored in the in-box adds to the problem, as older messages have to be stored outside the in-box, creating one more place you will have to search for them. Having multiple computers and locations where you check your e-mail also adds to the problem. In the workplace, e-mail is used increasingly as a task- and project-management tool—something it was not designed to be.4 This leads to procrastination in dealing with e-mail and a fear of not being able to find information when needed or not remembering to follow up on items in the e-mail.
Be methodical
First, decide on when and how often you will work on your in-box. Depending on your preference and work schedule, you may check e-mail several times a day or just once. Reserving a dedicated time in your daily schedule (either early in the morning or later in the evening) is probably the best way to deal with e-mail that you cannot readily reply to because it requires some thinking or action on your part.
Analyze your activities and create an organized list of folders and subfolders for each activity. An additional strategy is to prefix important folders with a number so that they appear near the top of an alphabetized list in order of importance that you assign.
Create an identical folder structure in your in-box, on your primary computer, and in a safe location that is backed up regularly, eg, a network drive or Web storage service. As activities change, update this folder structure on a regular basis.
Create rules to allow easier scanning and sorting of e-mail messages. For example, messages from mailing lists can automatically be moved to a specific folder, or messages from your boss can be color-coded in red. These features depend on your e-mail client.
Deal with your legitimate e-mail by type
Regardless of the folder structure and rules that you apply to organize your e-mail, you have to go through all your messages regularly, say, at the end of the day. One of the best strategies is to never open a message and then close it without doing anything with it. At the minimum, try to categorize each message when you first open it. Messages can be classified by content and by the type of action required.
Reference items have information that you might need later and that needs to be saved in an appropriate location. Depending on when and where you might need the information again, you need to save it in the appropriate folder in your in-box, on your hard drive, or in a network or web drive. Remember that the folders in your in-box count towards the total limit of your e-mail storage. The benefit of storing it in your in-box is that you can access it from any computer from which you can access your e-mail.
Notices about events or meetings. Decide if you may want to attend or not. In the latter case, delete the message. If you do think you may want to attend, you can move the information to your calendar and create an appointment, possibly with a reminder. This will let you view the information at the time it is needed. One point to keep in mind is that if you move the item to your calendar, it will disappear from your mailbox. In some cases, appointment request messages (Lotus Notes, Outlook) will automatically move from your in-box to your calendar once you “accept” the event (meeting) notice.
Action items need some type of action on your part. There are four actions you can take with the message: delete it, do it, delegate it, or defer it.10 You can do several things to remind yourself to deal with them later. In some e-mail programs you can:
- Mark them as unread
- Flag them for follow-up with reminders (in some cases with different-colored flags)
- Change the e-mail messages to tasks with reminders: marking or flagging the items will then allow you to easily fllter or sort the items that you had deferred initially and will provide you flexibility to take appropriate actions at a convenient time.
- Tomlinson R. Email home. http://openmap.bbn.com/~tomlinso/ray/home.html. Accessed 7/14/2008.
- Whittaker S, Sidner C. Email overload: exploring personal information management of email. Conference on Human Factors in Computing Systems, Vancouver, British Columbia, 1996. New York, ACM, 1996:276–283.
- Bellotti V, Ducheneau TN, Howard MA, Smith IE, Grinter RE. Quality versus quantity: e-mail-centric task management and its relation with overload. Human-computer Interaction 2005; 20:89–138.
- Fallows D. Email at work. Few feel overwhelmed and most are pleased with the way email helps them do their jobs. Pew Internet and American Life Project. www.pewinternet.org/pdfs/PIP_Work_Email_Report.pdf. Accessed 7/14/2008.
- Evett D. Spam statistics 2006. Top Ten Reviews. spam-filter-review.toptenreviews.com/spam-statistics.html. Accessed 7/14/2008.
- Can spam today! Forever. A multilayered approach to the spam problem. Messaging Architects, Novell. www.novell.com/info/collateral/docs/4820891.01/4820891.pdf. Accessed 7/14/2008.
- Public Law 108-187. 108th Congress. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=108_cong_public_laws&docid=f:publ187.108.pdf. Accessed 7/14/2008.
- Keizer G. CAN-SPAM act fails to slow junk mail. ChannelWeb. www.crn.com/security/18831412. Accessed 7/14/2008.
- Kane B, Sands DZ, for the AMIA Internet Work Group, Task force on Guidelines for the Use of Clinic-Patient Electronic Mail. Guidelines for the clinical use of electronic mail with patients. J Am Med Informatics Assoc 1998; 5:104–111. www.amia.org/mbrcenter/pubs/email_guidelines.asp. Accessed 7/14/2008.
- Mcghee S. 4 Ways to take control of your e-mail inbox. Microsoft at Work. www.microsoft.com/atwork/manage-info/email.mspx. Accessed 7/14/2008.
E-mail is a mixed blessing. When used appropriately, it saves time and paper and increases efficiency in the workplace. Unfortunately, as our lives get busier and our e-mail inboxes get fuller, e-mail is becoming an untamed monster.
In this article, we discuss various tools and strategies to reclaim lost e-mail productivity by reducing the volume of unsolicited e-mail (“spam”), following e-mail etiquette to eliminate unnecessary messaging, and managing and organizing our e-mail more effectively. What we present is as relevant for readers who use personal e-mail clients such as Hotmail, Gmail, YahooMail, and AOLMail as it is for those who use e-mail at work via programs such as Microsoft Outlook and IBM Lotus Notes.
FROM HUMBLE BEGINNINGS
The first e-mail messages were sent in the early 1960s, but they could be sent only to users of a single computer. In 1971, Ray Tomlinson programmed and sent the first e-mail message from one computer to another over a network. To separate the name of the user from that of the computer, he used the “@” sign. E-mail at that time could accommodate plain text only—ie, no attachments, no images.1
E-mail has come a long way from these humble beginnings to become one of the most heavily used aspects of the Internet. Its popularity stems mainly from its simplicity and efficiency in facilitating asynchronous communication. However, e-mail is fast becoming too successful, leading to overload for those who use it.
WHAT’S IN YOUR IN-BOX?
Incoming e-mail messages can be classified into the following categories:
- Messages that directly concern you or your work
- Copies of messages indirectly related to your work, sent to you to “keep you in the loop”
- Notices of events or meetings
- Messages acknowledging the receipt of e-mail messages that you sent
- Messages from organizations that you have some relationship with and that you have given your e-mail address to, such as professional organizations, mailing lists (listservs), retailers, service providers, or information providers
- Messages from friends, family, and colleagues that contain non-work-related information, such as jokes, news stories, and links to interesting Web sites
- Unsolicited messages from unknown senders (eg, online retailers, scam artists) with whom you have no relationship, often trying to sell you a product or service or to trick you into giving up information
- Unsolicited messages from senders you know but may not want to get messages from.
E-MAIL OVERLOAD CONSUMES TIME, ADDS STRESS
Each of these types of messages has to be handled in a specific manner. Thus, when a person gets dozens of these messages a day, it can significantly add to the workload. This problem was recognized as early as 1996, when Whittaker and Sidner2 coined the term “e-mail overload” and systematically studied it for the first time. Bellotti and others3 at the Palo Alto Research Lab concluded that it is not just the volume of the e-mail but the collaborative quality of e-mail task management and project management that leads to overload. Thus, the e-mail in-box requires not just a filing cabinet to sort the messages but a mechanism to support collaboration and project management.3
Studies show that e-mail overload causes people to work 1 to 2 extra hours a day, either at work or when they get home. Despite these issues, the Pew Internet and American Life Project reported in 2002 that more than half the Americans surveyed who use e-mail at work thought it was “essential to their work.”4 However, many also reported that e-mail has been distracting, has caused misunderstandings, and has added a new source of stress to their lives. Professionals have the added burden of e-mail being treated as a medicolegal document that can be discoverable and, hence, used as legal evidence.
No wonder, then, that many e-mail providers include tools to manage the flow and to organize both the wanted and unwanted e-mail messages. However, as with many tools, there are effective ways to use them.
CANNING THE SPAM: DECREASING UNWANTED MESSAGES
Spam is unsolicited e-mail, often of a commercial nature, sent indiscriminately to multiple mailing lists, individuals, or newsgroups. It is the Internet version of junk mail. An estimated 12 billion spam messages are sent every day, accounting for 40% to 60% of all e-mail messages.5
Spam takes up time and space and is an intrusion of privacy. In addition, it has financial costs to organizations. By one estimate, an organization with 1,000 employees loses over $200,000 a year in productivity due to spam.6
The reason spam is so widespread is that it is very cheap and profitable for the spammer. It costs next to nothing to send, and getting even 100 responses from 10 million messages sent is enough to turn a profit!6
Unfortunately, the Controlling the Assault of Non-Solicited Pornography and Marketing (CAN-SPAM) Act7 of 2003 had a mixed impact on limiting the volume of spam,8 and may have trumped state laws that were already in place to regulate spam.
To decrease the amount of spam you receive in your daily e-mail, it helps to understand that spam has three steps:
- Harvesting
- Confirming
- Spamming.

Prevent spammers from harvesting your e-mail address
Since a spammer first has to harvest your e-mail address, you should try not to give it away. Spammers use programs that troll the Internet looking for e-mail addresses. Tips for guarding your address:
- Try not to display it in public, eg, in chat rooms, message boards, listservs
- If you have to post your e-mail address in public, reformat it so it cannot be easily recognized as an e-mail address by the trolling software (see sidebar, “More ways to outsmart the spammer”)
- Check a Web site’s privacy policy before submitting your information
- Take the time to review “opt-out” options on Web sites you use, to prevent your e-mail address from being used by a third party
- Consider using separate e-mail addresses for your personal business and your work to limit spam in your workplace: to prevent unintended disclosure of your work address, give your family and friends only your personal e-mail address, and ask them to use “blind copy” so that other recipients don’t have access to it
- Consider using “disposable” e-mail addresses, especially when making on-line purchases or when requesting services.
Prevent spammers from confirming your e-mail address
If spammers do obtain your e-mail address, you can prevent them from verifying it:
- Do not reply to or click on any links in a spam or other unsolicited e-mail message, including links to “unsubscribe” from an unsolicited newsletter, chain letter, or special offer
- Set your e-mail application to display messages in plain text rather than HTML, or turn off the automatic images in your e-mail application: the opening of the e-mail and subsequent displaying of the images can automatically verify your address to the sender!
- Check your e-mail application to ensure that it sends automatic “out of office” or “vacation” replies only to your contacts or ask your e-mail administrator for help with this at your workplace.
Keep spam out of your in-box
Finally, if spam is sent, there are several ways to keep it from reaching your in-box:
- Sophisticated users can use e-mail rules to direct spam-marked e-mail to a separate junk folder
- Add frequent unsolicited e-mail senders to a “blocked sender” list (Table 1).
A WORD ABOUT ‘PHISHING’
Like spam, “phishing” is a form of unsolicited e-mail, but its intent is much more malignant. The perpetrator sends out legitimate-looking e-mail in an attempt to gather personal and financial information from recipients. Typically, the messages appear to come from well-known and trustworthy Web sites such as banks, Pay-Pal, eBay, MSN, or Yahoo, and they ask the recipient for an account number and the related password. For more on this topic, see http://en.wikipedia.org/wiki/Phishing.
GOOD MANNERS IN THE E-WORKPLACE
Even if you could block all spam messages from reaching your inbox, you still might receive unwanted messages from colleagues or friends. The only way to reduce unnecessary e-mail in the workplace is to ask your colleagues and employees to use e-mail appropriately. Here are some rules that could help decrease e-mail overload at your work:
Do not overuse “reply to all.” This is one of the most common reasons for e-mail overload in the workplace. Only use “reply to all” if you really need your message to be seen by each person who received the original message.
Use “cc:” sparingly. Try not to use the “cc:” field unless the recipients in that field know they are receiving a copy of the message. It also exposes the e-mail identity of other users and can promote spam, especially if you are sending e-mail to an external client.
Do not forward chain letters. It is safe to say that all chain letters are hoaxes. Just delete them as soon as you receive them.
Use alternate means of communication (eg, phone, meetings) if you really need to have a discussion. E-mail is not the richest medium for discussion, and sometimes it is better to talk to a person or group face to face or on the phone rather than to bounce e-mail back and forth multiple times between multiple users.
Use appropriate subject headings like “no response needed” or “FYI-Reference” when you don’t need a reply. For example, someone asks you to send a file by e-mail; you send it as an attachment, and in the subject line you say, “Here is the file you wanted. NRN.” The recipient will know not to respond with “Got it, thanks” or something like that. He can thank you when you next see him.
Avoid sending unnecessary attachments. Suppose you want to inform some colleagues about a visiting professor giving a grand rounds talk. Instead of creating a Word document and attaching it to the e-mail message, you can include the information in the body of the message itself. Thus, the recipients will not have to go through the additional step of opening the attachment. Also, the plain text message in the body of the message will be a smaller file than an e-mail message with an attached Word document.
Do not request delivery notification. This is irritating to most users, requiring an extra click before reading the message. It may not work with all e-mail clients, and a user can elect not to send the notification. And what would you do with such information anyway (especially if it is incomplete)?
Use a meaningful subject header. This will allow the user to decide whether and when to open the message.
Do not abuse the “urgent” or “high priority” flag. This is like crying wolf and diminishes the user’s ability to effectively manage his or her e-mail.
Anticipate and preempt e-mail volleys. Suppose a journal editor e-mails you to ask if you can review an article that was submitted to the journal, and says that your review would need to be completed by next month. You respond that you cannot do it within that time frame, but that you could do it the month after that. Anticipating the next question, you also provide the names of two colleagues who might be able to do the review. This will prevent any further e-mail on this topic unless the editor is willing to wait longer to get your review.
Maintain e-mail threads if possible. When responding to an e-mail message, use “reply” instead of starting a new message. This will maintain the thread of the discussion and make it easier to follow.
Do not send an e-mail message when you are angry or upset. It is better to wait until you calm down (sleep on it if possible) and then write the message or have a conversation on the phone or face to face. Nothing good comes out of writing an e-mail message in the heat of the moment, and it only leads to bad feelings and miscommunication. Along the same line, avoid sarcasm or humor in professional e-mail, as it can be misconstrued.
Avoid putting information in e-mail that you do not want unintended parties to read
It is only too easy to forward or share the e-mail message with others.
Avoid sending confidential patient information in e-mail unless your institution has set up some ground rules. The American Medical Informatics Association has issued guidelines on this topic.9
ORGANIZE YOUR E-MAIL AND RELATED INFORMATION
Once you have limited the amount of unwanted e-mail, the next step is to deal with the remaining messages.
The task is difficult, for several reasons. Some people feel they will need the information in their e-mail at a later date and thus don’t want to remove it from the in-box. Some messages may require a significant amount of thought or time to respond to and cannot be dealt with immediately. Having limits on the amount of e-mail that can be stored in the in-box adds to the problem, as older messages have to be stored outside the in-box, creating one more place you will have to search for them. Having multiple computers and locations where you check your e-mail also adds to the problem. In the workplace, e-mail is used increasingly as a task- and project-management tool—something it was not designed to be.4 This leads to procrastination in dealing with e-mail and a fear of not being able to find information when needed or not remembering to follow up on items in the e-mail.
Be methodical
First, decide on when and how often you will work on your in-box. Depending on your preference and work schedule, you may check e-mail several times a day or just once. Reserving a dedicated time in your daily schedule (either early in the morning or later in the evening) is probably the best way to deal with e-mail that you cannot readily reply to because it requires some thinking or action on your part.
Analyze your activities and create an organized list of folders and subfolders for each activity. An additional strategy is to prefix important folders with a number so that they appear near the top of an alphabetized list in order of importance that you assign.
Create an identical folder structure in your in-box, on your primary computer, and in a safe location that is backed up regularly, eg, a network drive or Web storage service. As activities change, update this folder structure on a regular basis.
Create rules to allow easier scanning and sorting of e-mail messages. For example, messages from mailing lists can automatically be moved to a specific folder, or messages from your boss can be color-coded in red. These features depend on your e-mail client.
Deal with your legitimate e-mail by type
Regardless of the folder structure and rules that you apply to organize your e-mail, you have to go through all your messages regularly, say, at the end of the day. One of the best strategies is to never open a message and then close it without doing anything with it. At the minimum, try to categorize each message when you first open it. Messages can be classified by content and by the type of action required.
Reference items have information that you might need later and that needs to be saved in an appropriate location. Depending on when and where you might need the information again, you need to save it in the appropriate folder in your in-box, on your hard drive, or in a network or web drive. Remember that the folders in your in-box count towards the total limit of your e-mail storage. The benefit of storing it in your in-box is that you can access it from any computer from which you can access your e-mail.
Notices about events or meetings. Decide if you may want to attend or not. In the latter case, delete the message. If you do think you may want to attend, you can move the information to your calendar and create an appointment, possibly with a reminder. This will let you view the information at the time it is needed. One point to keep in mind is that if you move the item to your calendar, it will disappear from your mailbox. In some cases, appointment request messages (Lotus Notes, Outlook) will automatically move from your in-box to your calendar once you “accept” the event (meeting) notice.
Action items need some type of action on your part. There are four actions you can take with the message: delete it, do it, delegate it, or defer it.10 You can do several things to remind yourself to deal with them later. In some e-mail programs you can:
- Mark them as unread
- Flag them for follow-up with reminders (in some cases with different-colored flags)
- Change the e-mail messages to tasks with reminders: marking or flagging the items will then allow you to easily fllter or sort the items that you had deferred initially and will provide you flexibility to take appropriate actions at a convenient time.
E-mail is a mixed blessing. When used appropriately, it saves time and paper and increases efficiency in the workplace. Unfortunately, as our lives get busier and our e-mail inboxes get fuller, e-mail is becoming an untamed monster.
In this article, we discuss various tools and strategies to reclaim lost e-mail productivity by reducing the volume of unsolicited e-mail (“spam”), following e-mail etiquette to eliminate unnecessary messaging, and managing and organizing our e-mail more effectively. What we present is as relevant for readers who use personal e-mail clients such as Hotmail, Gmail, YahooMail, and AOLMail as it is for those who use e-mail at work via programs such as Microsoft Outlook and IBM Lotus Notes.
FROM HUMBLE BEGINNINGS
The first e-mail messages were sent in the early 1960s, but they could be sent only to users of a single computer. In 1971, Ray Tomlinson programmed and sent the first e-mail message from one computer to another over a network. To separate the name of the user from that of the computer, he used the “@” sign. E-mail at that time could accommodate plain text only—ie, no attachments, no images.1
E-mail has come a long way from these humble beginnings to become one of the most heavily used aspects of the Internet. Its popularity stems mainly from its simplicity and efficiency in facilitating asynchronous communication. However, e-mail is fast becoming too successful, leading to overload for those who use it.
WHAT’S IN YOUR IN-BOX?
Incoming e-mail messages can be classified into the following categories:
- Messages that directly concern you or your work
- Copies of messages indirectly related to your work, sent to you to “keep you in the loop”
- Notices of events or meetings
- Messages acknowledging the receipt of e-mail messages that you sent
- Messages from organizations that you have some relationship with and that you have given your e-mail address to, such as professional organizations, mailing lists (listservs), retailers, service providers, or information providers
- Messages from friends, family, and colleagues that contain non-work-related information, such as jokes, news stories, and links to interesting Web sites
- Unsolicited messages from unknown senders (eg, online retailers, scam artists) with whom you have no relationship, often trying to sell you a product or service or to trick you into giving up information
- Unsolicited messages from senders you know but may not want to get messages from.
E-MAIL OVERLOAD CONSUMES TIME, ADDS STRESS
Each of these types of messages has to be handled in a specific manner. Thus, when a person gets dozens of these messages a day, it can significantly add to the workload. This problem was recognized as early as 1996, when Whittaker and Sidner2 coined the term “e-mail overload” and systematically studied it for the first time. Bellotti and others3 at the Palo Alto Research Lab concluded that it is not just the volume of the e-mail but the collaborative quality of e-mail task management and project management that leads to overload. Thus, the e-mail in-box requires not just a filing cabinet to sort the messages but a mechanism to support collaboration and project management.3
Studies show that e-mail overload causes people to work 1 to 2 extra hours a day, either at work or when they get home. Despite these issues, the Pew Internet and American Life Project reported in 2002 that more than half the Americans surveyed who use e-mail at work thought it was “essential to their work.”4 However, many also reported that e-mail has been distracting, has caused misunderstandings, and has added a new source of stress to their lives. Professionals have the added burden of e-mail being treated as a medicolegal document that can be discoverable and, hence, used as legal evidence.
No wonder, then, that many e-mail providers include tools to manage the flow and to organize both the wanted and unwanted e-mail messages. However, as with many tools, there are effective ways to use them.
CANNING THE SPAM: DECREASING UNWANTED MESSAGES
Spam is unsolicited e-mail, often of a commercial nature, sent indiscriminately to multiple mailing lists, individuals, or newsgroups. It is the Internet version of junk mail. An estimated 12 billion spam messages are sent every day, accounting for 40% to 60% of all e-mail messages.5
Spam takes up time and space and is an intrusion of privacy. In addition, it has financial costs to organizations. By one estimate, an organization with 1,000 employees loses over $200,000 a year in productivity due to spam.6
The reason spam is so widespread is that it is very cheap and profitable for the spammer. It costs next to nothing to send, and getting even 100 responses from 10 million messages sent is enough to turn a profit!6
Unfortunately, the Controlling the Assault of Non-Solicited Pornography and Marketing (CAN-SPAM) Act7 of 2003 had a mixed impact on limiting the volume of spam,8 and may have trumped state laws that were already in place to regulate spam.
To decrease the amount of spam you receive in your daily e-mail, it helps to understand that spam has three steps:
- Harvesting
- Confirming
- Spamming.

Prevent spammers from harvesting your e-mail address
Since a spammer first has to harvest your e-mail address, you should try not to give it away. Spammers use programs that troll the Internet looking for e-mail addresses. Tips for guarding your address:
- Try not to display it in public, eg, in chat rooms, message boards, listservs
- If you have to post your e-mail address in public, reformat it so it cannot be easily recognized as an e-mail address by the trolling software (see sidebar, “More ways to outsmart the spammer”)
- Check a Web site’s privacy policy before submitting your information
- Take the time to review “opt-out” options on Web sites you use, to prevent your e-mail address from being used by a third party
- Consider using separate e-mail addresses for your personal business and your work to limit spam in your workplace: to prevent unintended disclosure of your work address, give your family and friends only your personal e-mail address, and ask them to use “blind copy” so that other recipients don’t have access to it
- Consider using “disposable” e-mail addresses, especially when making on-line purchases or when requesting services.
Prevent spammers from confirming your e-mail address
If spammers do obtain your e-mail address, you can prevent them from verifying it:
- Do not reply to or click on any links in a spam or other unsolicited e-mail message, including links to “unsubscribe” from an unsolicited newsletter, chain letter, or special offer
- Set your e-mail application to display messages in plain text rather than HTML, or turn off the automatic images in your e-mail application: the opening of the e-mail and subsequent displaying of the images can automatically verify your address to the sender!
- Check your e-mail application to ensure that it sends automatic “out of office” or “vacation” replies only to your contacts or ask your e-mail administrator for help with this at your workplace.
Keep spam out of your in-box
Finally, if spam is sent, there are several ways to keep it from reaching your in-box:
- Sophisticated users can use e-mail rules to direct spam-marked e-mail to a separate junk folder
- Add frequent unsolicited e-mail senders to a “blocked sender” list (Table 1).
A WORD ABOUT ‘PHISHING’
Like spam, “phishing” is a form of unsolicited e-mail, but its intent is much more malignant. The perpetrator sends out legitimate-looking e-mail in an attempt to gather personal and financial information from recipients. Typically, the messages appear to come from well-known and trustworthy Web sites such as banks, Pay-Pal, eBay, MSN, or Yahoo, and they ask the recipient for an account number and the related password. For more on this topic, see http://en.wikipedia.org/wiki/Phishing.
GOOD MANNERS IN THE E-WORKPLACE
Even if you could block all spam messages from reaching your inbox, you still might receive unwanted messages from colleagues or friends. The only way to reduce unnecessary e-mail in the workplace is to ask your colleagues and employees to use e-mail appropriately. Here are some rules that could help decrease e-mail overload at your work:
Do not overuse “reply to all.” This is one of the most common reasons for e-mail overload in the workplace. Only use “reply to all” if you really need your message to be seen by each person who received the original message.
Use “cc:” sparingly. Try not to use the “cc:” field unless the recipients in that field know they are receiving a copy of the message. It also exposes the e-mail identity of other users and can promote spam, especially if you are sending e-mail to an external client.
Do not forward chain letters. It is safe to say that all chain letters are hoaxes. Just delete them as soon as you receive them.
Use alternate means of communication (eg, phone, meetings) if you really need to have a discussion. E-mail is not the richest medium for discussion, and sometimes it is better to talk to a person or group face to face or on the phone rather than to bounce e-mail back and forth multiple times between multiple users.
Use appropriate subject headings like “no response needed” or “FYI-Reference” when you don’t need a reply. For example, someone asks you to send a file by e-mail; you send it as an attachment, and in the subject line you say, “Here is the file you wanted. NRN.” The recipient will know not to respond with “Got it, thanks” or something like that. He can thank you when you next see him.
Avoid sending unnecessary attachments. Suppose you want to inform some colleagues about a visiting professor giving a grand rounds talk. Instead of creating a Word document and attaching it to the e-mail message, you can include the information in the body of the message itself. Thus, the recipients will not have to go through the additional step of opening the attachment. Also, the plain text message in the body of the message will be a smaller file than an e-mail message with an attached Word document.
Do not request delivery notification. This is irritating to most users, requiring an extra click before reading the message. It may not work with all e-mail clients, and a user can elect not to send the notification. And what would you do with such information anyway (especially if it is incomplete)?
Use a meaningful subject header. This will allow the user to decide whether and when to open the message.
Do not abuse the “urgent” or “high priority” flag. This is like crying wolf and diminishes the user’s ability to effectively manage his or her e-mail.
Anticipate and preempt e-mail volleys. Suppose a journal editor e-mails you to ask if you can review an article that was submitted to the journal, and says that your review would need to be completed by next month. You respond that you cannot do it within that time frame, but that you could do it the month after that. Anticipating the next question, you also provide the names of two colleagues who might be able to do the review. This will prevent any further e-mail on this topic unless the editor is willing to wait longer to get your review.
Maintain e-mail threads if possible. When responding to an e-mail message, use “reply” instead of starting a new message. This will maintain the thread of the discussion and make it easier to follow.
Do not send an e-mail message when you are angry or upset. It is better to wait until you calm down (sleep on it if possible) and then write the message or have a conversation on the phone or face to face. Nothing good comes out of writing an e-mail message in the heat of the moment, and it only leads to bad feelings and miscommunication. Along the same line, avoid sarcasm or humor in professional e-mail, as it can be misconstrued.
Avoid putting information in e-mail that you do not want unintended parties to read
It is only too easy to forward or share the e-mail message with others.
Avoid sending confidential patient information in e-mail unless your institution has set up some ground rules. The American Medical Informatics Association has issued guidelines on this topic.9
ORGANIZE YOUR E-MAIL AND RELATED INFORMATION
Once you have limited the amount of unwanted e-mail, the next step is to deal with the remaining messages.
The task is difficult, for several reasons. Some people feel they will need the information in their e-mail at a later date and thus don’t want to remove it from the in-box. Some messages may require a significant amount of thought or time to respond to and cannot be dealt with immediately. Having limits on the amount of e-mail that can be stored in the in-box adds to the problem, as older messages have to be stored outside the in-box, creating one more place you will have to search for them. Having multiple computers and locations where you check your e-mail also adds to the problem. In the workplace, e-mail is used increasingly as a task- and project-management tool—something it was not designed to be.4 This leads to procrastination in dealing with e-mail and a fear of not being able to find information when needed or not remembering to follow up on items in the e-mail.
Be methodical
First, decide on when and how often you will work on your in-box. Depending on your preference and work schedule, you may check e-mail several times a day or just once. Reserving a dedicated time in your daily schedule (either early in the morning or later in the evening) is probably the best way to deal with e-mail that you cannot readily reply to because it requires some thinking or action on your part.
Analyze your activities and create an organized list of folders and subfolders for each activity. An additional strategy is to prefix important folders with a number so that they appear near the top of an alphabetized list in order of importance that you assign.
Create an identical folder structure in your in-box, on your primary computer, and in a safe location that is backed up regularly, eg, a network drive or Web storage service. As activities change, update this folder structure on a regular basis.
Create rules to allow easier scanning and sorting of e-mail messages. For example, messages from mailing lists can automatically be moved to a specific folder, or messages from your boss can be color-coded in red. These features depend on your e-mail client.
Deal with your legitimate e-mail by type
Regardless of the folder structure and rules that you apply to organize your e-mail, you have to go through all your messages regularly, say, at the end of the day. One of the best strategies is to never open a message and then close it without doing anything with it. At the minimum, try to categorize each message when you first open it. Messages can be classified by content and by the type of action required.
Reference items have information that you might need later and that needs to be saved in an appropriate location. Depending on when and where you might need the information again, you need to save it in the appropriate folder in your in-box, on your hard drive, or in a network or web drive. Remember that the folders in your in-box count towards the total limit of your e-mail storage. The benefit of storing it in your in-box is that you can access it from any computer from which you can access your e-mail.
Notices about events or meetings. Decide if you may want to attend or not. In the latter case, delete the message. If you do think you may want to attend, you can move the information to your calendar and create an appointment, possibly with a reminder. This will let you view the information at the time it is needed. One point to keep in mind is that if you move the item to your calendar, it will disappear from your mailbox. In some cases, appointment request messages (Lotus Notes, Outlook) will automatically move from your in-box to your calendar once you “accept” the event (meeting) notice.
Action items need some type of action on your part. There are four actions you can take with the message: delete it, do it, delegate it, or defer it.10 You can do several things to remind yourself to deal with them later. In some e-mail programs you can:
- Mark them as unread
- Flag them for follow-up with reminders (in some cases with different-colored flags)
- Change the e-mail messages to tasks with reminders: marking or flagging the items will then allow you to easily fllter or sort the items that you had deferred initially and will provide you flexibility to take appropriate actions at a convenient time.
- Tomlinson R. Email home. http://openmap.bbn.com/~tomlinso/ray/home.html. Accessed 7/14/2008.
- Whittaker S, Sidner C. Email overload: exploring personal information management of email. Conference on Human Factors in Computing Systems, Vancouver, British Columbia, 1996. New York, ACM, 1996:276–283.
- Bellotti V, Ducheneau TN, Howard MA, Smith IE, Grinter RE. Quality versus quantity: e-mail-centric task management and its relation with overload. Human-computer Interaction 2005; 20:89–138.
- Fallows D. Email at work. Few feel overwhelmed and most are pleased with the way email helps them do their jobs. Pew Internet and American Life Project. www.pewinternet.org/pdfs/PIP_Work_Email_Report.pdf. Accessed 7/14/2008.
- Evett D. Spam statistics 2006. Top Ten Reviews. spam-filter-review.toptenreviews.com/spam-statistics.html. Accessed 7/14/2008.
- Can spam today! Forever. A multilayered approach to the spam problem. Messaging Architects, Novell. www.novell.com/info/collateral/docs/4820891.01/4820891.pdf. Accessed 7/14/2008.
- Public Law 108-187. 108th Congress. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=108_cong_public_laws&docid=f:publ187.108.pdf. Accessed 7/14/2008.
- Keizer G. CAN-SPAM act fails to slow junk mail. ChannelWeb. www.crn.com/security/18831412. Accessed 7/14/2008.
- Kane B, Sands DZ, for the AMIA Internet Work Group, Task force on Guidelines for the Use of Clinic-Patient Electronic Mail. Guidelines for the clinical use of electronic mail with patients. J Am Med Informatics Assoc 1998; 5:104–111. www.amia.org/mbrcenter/pubs/email_guidelines.asp. Accessed 7/14/2008.
- Mcghee S. 4 Ways to take control of your e-mail inbox. Microsoft at Work. www.microsoft.com/atwork/manage-info/email.mspx. Accessed 7/14/2008.
- Tomlinson R. Email home. http://openmap.bbn.com/~tomlinso/ray/home.html. Accessed 7/14/2008.
- Whittaker S, Sidner C. Email overload: exploring personal information management of email. Conference on Human Factors in Computing Systems, Vancouver, British Columbia, 1996. New York, ACM, 1996:276–283.
- Bellotti V, Ducheneau TN, Howard MA, Smith IE, Grinter RE. Quality versus quantity: e-mail-centric task management and its relation with overload. Human-computer Interaction 2005; 20:89–138.
- Fallows D. Email at work. Few feel overwhelmed and most are pleased with the way email helps them do their jobs. Pew Internet and American Life Project. www.pewinternet.org/pdfs/PIP_Work_Email_Report.pdf. Accessed 7/14/2008.
- Evett D. Spam statistics 2006. Top Ten Reviews. spam-filter-review.toptenreviews.com/spam-statistics.html. Accessed 7/14/2008.
- Can spam today! Forever. A multilayered approach to the spam problem. Messaging Architects, Novell. www.novell.com/info/collateral/docs/4820891.01/4820891.pdf. Accessed 7/14/2008.
- Public Law 108-187. 108th Congress. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=108_cong_public_laws&docid=f:publ187.108.pdf. Accessed 7/14/2008.
- Keizer G. CAN-SPAM act fails to slow junk mail. ChannelWeb. www.crn.com/security/18831412. Accessed 7/14/2008.
- Kane B, Sands DZ, for the AMIA Internet Work Group, Task force on Guidelines for the Use of Clinic-Patient Electronic Mail. Guidelines for the clinical use of electronic mail with patients. J Am Med Informatics Assoc 1998; 5:104–111. www.amia.org/mbrcenter/pubs/email_guidelines.asp. Accessed 7/14/2008.
- Mcghee S. 4 Ways to take control of your e-mail inbox. Microsoft at Work. www.microsoft.com/atwork/manage-info/email.mspx. Accessed 7/14/2008.
KEY POINTS
- Decrease the amount of unwanted e-mail by zealously guarding your e-mail address, separating work e-mail from personal e-mail, and encouraging coworkers to follow appropriate e-mail etiquette.
- Handle the messages you receive in a disciplined and consistent manner. Schedule regular times to deal with e-mail.
- Delete spam messages without viewing images and without clicking on links. File any information that may be needed later. Messages that need action require one of the “four Ds”: delete it, do it, delegate it, or defer it.
- Never open a message and then close it without doing anything about it.
Man, 48, With Excruciating Leg Pain
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
Erratum (2008;81:421-426)
Malpractice Minute
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
August 2008 Instant Poll Results
MARCH 2008
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?
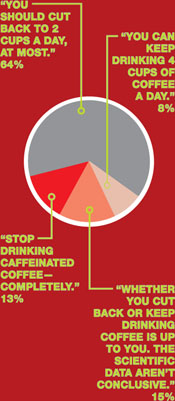
APRIL 2008
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours:
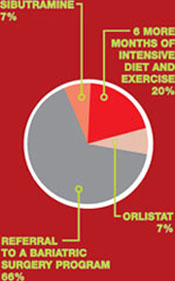
MARCH 2008
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?

APRIL 2008
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours:

MARCH 2008
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?

APRIL 2008
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours: