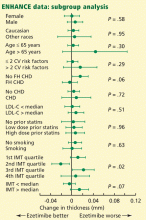
User login
Quality Summit Produces Plan
Somebody oughta fix that. I’m sure you’ve heard that phrase from friends or relatives lamenting their recent visit to a hospital.
My grandmother will use just about any opportunity to inform me that hospitals make people sicker. I’ve tried to explain that her perspective is skewed. After all, a lot of her friends were pretty sick before they entered the hospital. But “it’s that place” she vows. “They oughta change it.” Fortunately for me, my grandmother still hasn’t figured out what I do professionally, so I’m not considered part of “they.” I let her rant to my husband, since he has the letters MD after his name.
The truth, as you know, is many hospital medicine physicians and their teams are working their tails off trying to improve inpatient care. Much like my grandmother, hospital administrators haven’t identified who the “they” (change agents) are or what exactly the “it” (practices and systems that lead to suboptimal care) is that needs to be changed. It often is unclear how quality improvement initiatives affect the bottom line or which initiatives will ultimately improve outcomes. Today, a considerable amount of improvement efforts depend on the good will and perseverance of a few champions working with minimal institutional support.
The Hospital Quality and Patient Safety Committee (HQPSC) and SHM leadership recently convened a summit to define a vision for the optimal hospital stay and determine how to best train and support hospitalists as leaders and change agents.
The HQPSC and summit participants concluded SHM is, and should be, a national leader in quality improvement efforts including aspects of education, clinical care, and political advocacy for the hospital setting. To that end, the following strategy recently was submitted by the HQPSC and approved by the SHM Board of Directors to promote development of local, regional, and national infrastructures that support quality and patient safety:
Advance a national quality agenda for hospitals and hospitalists.
- Create a task force reporting to the HQPSC that partners with stakeholders to define the “ideal hospital stay” and promote quality improvement;
- Inform federal accrediting and policy-making groups about the effect of current quality measures and changes required to better support the “ideal hospital stay”;
- Advocate for the alignment of reimbursement practices that reward providers and institutions that demonstrate value and translate these practices into improved quality and patient safety;
- Establish an Acute Care Collaborative (ACC) comprising national organizations representing nurses, pharmacists, case managers, social workers, and other allied medical professionals. The ACC might be expanded to include other key physician groups (e.g., emergency physicians, geriatricians, intensivists); and
- Determine what other key national organizations are doing in quality improvement (QI) and look for opportunities for SHM to partner in these efforts.
Develop educational programs and technical support tools for all practicing hospitalists (entry level to QI leaders) engaged in quality improvement efforts.
- Delineate entry-level and advanced quality improvement offerings. Develop offerings specifically for advanced level participants;
- Expand mentored implementation programs to accommodate more participants and assess the need for other types of programs that provide longitudinal support or coaching;
- Expand current offerings, including resource rooms, mentored implementation, and expert training sessions, to other disease states, system processes, and special populations with attention to coordinating this with SHM’s The Core Competencies in Hospital Medicine: A Framework for Curriculum Development;
- Assess the need for new instructional modalities to reach a broader audience (e.g., Web based self-study modules); and
- Promote QI training in medical school, residency, and fellowship programs. Promote systems-based practice and QI throughout the continuum of education. This would include programs that engage medical students, residents, and fellows as well as the development of performance improvement modules (PIMs) for the American Board of Internal Medicine.
Improves the perceived value of implementing and sustaining QI efforts, and hospitalist leadership of those efforts.
- Advocate directly to the C-suites of hospitals to facilitate alignment of incentives that support hospitalists leading quality initiatives;
- Conduct a survey to quantify resources needed for hospitalists to successfully lead quality initiatives and develop safety programs. Develop a white paper based on survey results and distribute it to the C-suite;
- Encourage QI research that creates evidence and outcomes that can influence C-suites to commit adequate resources to QI activities;
- Explore opportunities to use existing local and national infrastructures to promote a more proactive and evidence-based approach to quality and safety rather than reactive and compliance-oriented quality projects; and
- Create a monthly column in The Hospitalist spotlighting QI efforts and assign staff to recruit submissions of “improvement stories” for the Web site.
Evaluate effectiveness of SHM’s current QI resources, educational offerings. SHM needs to assess its current offerings to understand and improve their effectiveness. Process and impact data are needed to obtain external money to create or sustain QI offerings.
- Create robust evaluations and collect better data to assess use and impact of resource rooms, quality precourses, and other SHM offerings.
Promote and support hospitalists as quality improvement experts. Contribute to the “new science” of quality improvement.
- Partner with the Research Committee to define and publish key areas in need of future research related to quality improvement. Advocate to granting agencies to put out RFPs that will help define the ideal hospital stay and support SHM’s research agenda;
- Partner with federal agencies to assess the value of current performance measures and facilitate development of more reliable and meaningful measures;
- Develop trainings for hospitalists on the methods and science of quality improvement research;
- Partner with the Research Committee to develop a research network; and
- Seek money to support demonstration projects that support our quality agenda.
Another goal, to promote development and adoption of health information technology and decision support tools that advance quality and patient safety, recently was discussed by the HQPSC and will be integrated into the next stage of planning.
Next Steps
SHM has an impressive history of working with its members to develop and implement quality initiatives. Our programs have helped reduce rates of venous thromboembolisms, improve glycemic control, and improve the discharge process. Our highly praised online resource rooms provide free tutorials in QI and implementation guides for specific interventions.
More than 250 healthcare professionals have completed our QI pre-course and more than one thousand hospitalists have completed our leadership programs. This momentum, combined with the acceleration of national interest in quality and patient safety, brings an unprecedented opportunity for SHM to advance hospital medicine, promote the highest quality care for our patients, and position hospitalists to be leaders in transforming hospital care.
During the next year, HQPSC will be translating this strategy into specific activities. SHM staff, HQPSC, and other members are developing additional training programs and technical tools.
If you are interested in becoming more involved in SHM’s quality initiatives, please contact me at tbudnitz@hospitalmedicine.org.
If you have a QI success story to share, please consider submission to the Improvement Stories section of the online Resource Rooms or The Hospitalist.
I thank all of you who are part of the “they” who are working tirelessly with SHM to fix “it.” Together we can move mountains, or something more impervious like healthcare systems and performance measures.
Somebody oughta fix that. I’m sure you’ve heard that phrase from friends or relatives lamenting their recent visit to a hospital.
My grandmother will use just about any opportunity to inform me that hospitals make people sicker. I’ve tried to explain that her perspective is skewed. After all, a lot of her friends were pretty sick before they entered the hospital. But “it’s that place” she vows. “They oughta change it.” Fortunately for me, my grandmother still hasn’t figured out what I do professionally, so I’m not considered part of “they.” I let her rant to my husband, since he has the letters MD after his name.
The truth, as you know, is many hospital medicine physicians and their teams are working their tails off trying to improve inpatient care. Much like my grandmother, hospital administrators haven’t identified who the “they” (change agents) are or what exactly the “it” (practices and systems that lead to suboptimal care) is that needs to be changed. It often is unclear how quality improvement initiatives affect the bottom line or which initiatives will ultimately improve outcomes. Today, a considerable amount of improvement efforts depend on the good will and perseverance of a few champions working with minimal institutional support.
The Hospital Quality and Patient Safety Committee (HQPSC) and SHM leadership recently convened a summit to define a vision for the optimal hospital stay and determine how to best train and support hospitalists as leaders and change agents.
The HQPSC and summit participants concluded SHM is, and should be, a national leader in quality improvement efforts including aspects of education, clinical care, and political advocacy for the hospital setting. To that end, the following strategy recently was submitted by the HQPSC and approved by the SHM Board of Directors to promote development of local, regional, and national infrastructures that support quality and patient safety:
Advance a national quality agenda for hospitals and hospitalists.
- Create a task force reporting to the HQPSC that partners with stakeholders to define the “ideal hospital stay” and promote quality improvement;
- Inform federal accrediting and policy-making groups about the effect of current quality measures and changes required to better support the “ideal hospital stay”;
- Advocate for the alignment of reimbursement practices that reward providers and institutions that demonstrate value and translate these practices into improved quality and patient safety;
- Establish an Acute Care Collaborative (ACC) comprising national organizations representing nurses, pharmacists, case managers, social workers, and other allied medical professionals. The ACC might be expanded to include other key physician groups (e.g., emergency physicians, geriatricians, intensivists); and
- Determine what other key national organizations are doing in quality improvement (QI) and look for opportunities for SHM to partner in these efforts.
Develop educational programs and technical support tools for all practicing hospitalists (entry level to QI leaders) engaged in quality improvement efforts.
- Delineate entry-level and advanced quality improvement offerings. Develop offerings specifically for advanced level participants;
- Expand mentored implementation programs to accommodate more participants and assess the need for other types of programs that provide longitudinal support or coaching;
- Expand current offerings, including resource rooms, mentored implementation, and expert training sessions, to other disease states, system processes, and special populations with attention to coordinating this with SHM’s The Core Competencies in Hospital Medicine: A Framework for Curriculum Development;
- Assess the need for new instructional modalities to reach a broader audience (e.g., Web based self-study modules); and
- Promote QI training in medical school, residency, and fellowship programs. Promote systems-based practice and QI throughout the continuum of education. This would include programs that engage medical students, residents, and fellows as well as the development of performance improvement modules (PIMs) for the American Board of Internal Medicine.
Improves the perceived value of implementing and sustaining QI efforts, and hospitalist leadership of those efforts.
- Advocate directly to the C-suites of hospitals to facilitate alignment of incentives that support hospitalists leading quality initiatives;
- Conduct a survey to quantify resources needed for hospitalists to successfully lead quality initiatives and develop safety programs. Develop a white paper based on survey results and distribute it to the C-suite;
- Encourage QI research that creates evidence and outcomes that can influence C-suites to commit adequate resources to QI activities;
- Explore opportunities to use existing local and national infrastructures to promote a more proactive and evidence-based approach to quality and safety rather than reactive and compliance-oriented quality projects; and
- Create a monthly column in The Hospitalist spotlighting QI efforts and assign staff to recruit submissions of “improvement stories” for the Web site.
Evaluate effectiveness of SHM’s current QI resources, educational offerings. SHM needs to assess its current offerings to understand and improve their effectiveness. Process and impact data are needed to obtain external money to create or sustain QI offerings.
- Create robust evaluations and collect better data to assess use and impact of resource rooms, quality precourses, and other SHM offerings.
Promote and support hospitalists as quality improvement experts. Contribute to the “new science” of quality improvement.
- Partner with the Research Committee to define and publish key areas in need of future research related to quality improvement. Advocate to granting agencies to put out RFPs that will help define the ideal hospital stay and support SHM’s research agenda;
- Partner with federal agencies to assess the value of current performance measures and facilitate development of more reliable and meaningful measures;
- Develop trainings for hospitalists on the methods and science of quality improvement research;
- Partner with the Research Committee to develop a research network; and
- Seek money to support demonstration projects that support our quality agenda.
Another goal, to promote development and adoption of health information technology and decision support tools that advance quality and patient safety, recently was discussed by the HQPSC and will be integrated into the next stage of planning.
Next Steps
SHM has an impressive history of working with its members to develop and implement quality initiatives. Our programs have helped reduce rates of venous thromboembolisms, improve glycemic control, and improve the discharge process. Our highly praised online resource rooms provide free tutorials in QI and implementation guides for specific interventions.
More than 250 healthcare professionals have completed our QI pre-course and more than one thousand hospitalists have completed our leadership programs. This momentum, combined with the acceleration of national interest in quality and patient safety, brings an unprecedented opportunity for SHM to advance hospital medicine, promote the highest quality care for our patients, and position hospitalists to be leaders in transforming hospital care.
During the next year, HQPSC will be translating this strategy into specific activities. SHM staff, HQPSC, and other members are developing additional training programs and technical tools.
If you are interested in becoming more involved in SHM’s quality initiatives, please contact me at tbudnitz@hospitalmedicine.org.
If you have a QI success story to share, please consider submission to the Improvement Stories section of the online Resource Rooms or The Hospitalist.
I thank all of you who are part of the “they” who are working tirelessly with SHM to fix “it.” Together we can move mountains, or something more impervious like healthcare systems and performance measures.
Somebody oughta fix that. I’m sure you’ve heard that phrase from friends or relatives lamenting their recent visit to a hospital.
My grandmother will use just about any opportunity to inform me that hospitals make people sicker. I’ve tried to explain that her perspective is skewed. After all, a lot of her friends were pretty sick before they entered the hospital. But “it’s that place” she vows. “They oughta change it.” Fortunately for me, my grandmother still hasn’t figured out what I do professionally, so I’m not considered part of “they.” I let her rant to my husband, since he has the letters MD after his name.
The truth, as you know, is many hospital medicine physicians and their teams are working their tails off trying to improve inpatient care. Much like my grandmother, hospital administrators haven’t identified who the “they” (change agents) are or what exactly the “it” (practices and systems that lead to suboptimal care) is that needs to be changed. It often is unclear how quality improvement initiatives affect the bottom line or which initiatives will ultimately improve outcomes. Today, a considerable amount of improvement efforts depend on the good will and perseverance of a few champions working with minimal institutional support.
The Hospital Quality and Patient Safety Committee (HQPSC) and SHM leadership recently convened a summit to define a vision for the optimal hospital stay and determine how to best train and support hospitalists as leaders and change agents.
The HQPSC and summit participants concluded SHM is, and should be, a national leader in quality improvement efforts including aspects of education, clinical care, and political advocacy for the hospital setting. To that end, the following strategy recently was submitted by the HQPSC and approved by the SHM Board of Directors to promote development of local, regional, and national infrastructures that support quality and patient safety:
Advance a national quality agenda for hospitals and hospitalists.
- Create a task force reporting to the HQPSC that partners with stakeholders to define the “ideal hospital stay” and promote quality improvement;
- Inform federal accrediting and policy-making groups about the effect of current quality measures and changes required to better support the “ideal hospital stay”;
- Advocate for the alignment of reimbursement practices that reward providers and institutions that demonstrate value and translate these practices into improved quality and patient safety;
- Establish an Acute Care Collaborative (ACC) comprising national organizations representing nurses, pharmacists, case managers, social workers, and other allied medical professionals. The ACC might be expanded to include other key physician groups (e.g., emergency physicians, geriatricians, intensivists); and
- Determine what other key national organizations are doing in quality improvement (QI) and look for opportunities for SHM to partner in these efforts.
Develop educational programs and technical support tools for all practicing hospitalists (entry level to QI leaders) engaged in quality improvement efforts.
- Delineate entry-level and advanced quality improvement offerings. Develop offerings specifically for advanced level participants;
- Expand mentored implementation programs to accommodate more participants and assess the need for other types of programs that provide longitudinal support or coaching;
- Expand current offerings, including resource rooms, mentored implementation, and expert training sessions, to other disease states, system processes, and special populations with attention to coordinating this with SHM’s The Core Competencies in Hospital Medicine: A Framework for Curriculum Development;
- Assess the need for new instructional modalities to reach a broader audience (e.g., Web based self-study modules); and
- Promote QI training in medical school, residency, and fellowship programs. Promote systems-based practice and QI throughout the continuum of education. This would include programs that engage medical students, residents, and fellows as well as the development of performance improvement modules (PIMs) for the American Board of Internal Medicine.
Improves the perceived value of implementing and sustaining QI efforts, and hospitalist leadership of those efforts.
- Advocate directly to the C-suites of hospitals to facilitate alignment of incentives that support hospitalists leading quality initiatives;
- Conduct a survey to quantify resources needed for hospitalists to successfully lead quality initiatives and develop safety programs. Develop a white paper based on survey results and distribute it to the C-suite;
- Encourage QI research that creates evidence and outcomes that can influence C-suites to commit adequate resources to QI activities;
- Explore opportunities to use existing local and national infrastructures to promote a more proactive and evidence-based approach to quality and safety rather than reactive and compliance-oriented quality projects; and
- Create a monthly column in The Hospitalist spotlighting QI efforts and assign staff to recruit submissions of “improvement stories” for the Web site.
Evaluate effectiveness of SHM’s current QI resources, educational offerings. SHM needs to assess its current offerings to understand and improve their effectiveness. Process and impact data are needed to obtain external money to create or sustain QI offerings.
- Create robust evaluations and collect better data to assess use and impact of resource rooms, quality precourses, and other SHM offerings.
Promote and support hospitalists as quality improvement experts. Contribute to the “new science” of quality improvement.
- Partner with the Research Committee to define and publish key areas in need of future research related to quality improvement. Advocate to granting agencies to put out RFPs that will help define the ideal hospital stay and support SHM’s research agenda;
- Partner with federal agencies to assess the value of current performance measures and facilitate development of more reliable and meaningful measures;
- Develop trainings for hospitalists on the methods and science of quality improvement research;
- Partner with the Research Committee to develop a research network; and
- Seek money to support demonstration projects that support our quality agenda.
Another goal, to promote development and adoption of health information technology and decision support tools that advance quality and patient safety, recently was discussed by the HQPSC and will be integrated into the next stage of planning.
Next Steps
SHM has an impressive history of working with its members to develop and implement quality initiatives. Our programs have helped reduce rates of venous thromboembolisms, improve glycemic control, and improve the discharge process. Our highly praised online resource rooms provide free tutorials in QI and implementation guides for specific interventions.
More than 250 healthcare professionals have completed our QI pre-course and more than one thousand hospitalists have completed our leadership programs. This momentum, combined with the acceleration of national interest in quality and patient safety, brings an unprecedented opportunity for SHM to advance hospital medicine, promote the highest quality care for our patients, and position hospitalists to be leaders in transforming hospital care.
During the next year, HQPSC will be translating this strategy into specific activities. SHM staff, HQPSC, and other members are developing additional training programs and technical tools.
If you are interested in becoming more involved in SHM’s quality initiatives, please contact me at tbudnitz@hospitalmedicine.org.
If you have a QI success story to share, please consider submission to the Improvement Stories section of the online Resource Rooms or The Hospitalist.
I thank all of you who are part of the “they” who are working tirelessly with SHM to fix “it.” Together we can move mountains, or something more impervious like healthcare systems and performance measures.
Growing Number of Textbooks Dedicated to HM
The rapidly expanding field of hospital medicine has spurred a growing number of textbooks devoted to the specialty. Textbooks by some of the specialty’s leading voices are available to those keen on honing their knowledge.
Ranging in scope from practice management issues to clinical synopses, titles include:
- “Hospitalists: A Guide to Building and Sustaining a Successful Program” by SHM founders John Nelson, MD, and Win Whitcomb, MD, and Joe Miller, SHM’s executive adviser to the CEO. (Health Administration Press, 2007, $72);
- “Comprehensive Hospital Medicine,” by Mark Williams, MD, chief, division of hospital medicine, Feinberg School of Medicine, Chicago (Elsevier, 2007, $109);
- “Hospital Medicine Secrets,” by The Hospitalist physician editor Jeff Glasheen, MD (Mosby/Elsevier, 2007, $39);
- “Understanding Patient Safety” by Robert Wachter, MD, chief of the Division of Hospital Medicine, and chief of the Medical Service at the University of California, at San Francisco Medical Center, and author of “Wachter’s World,” a blog featured on The Hospitalist Web site (McGraw-Hill, 2007, $35);
- “Hospital Medicine: Just the Facts,” by Sylvia McKean, MD, director, hospitalist service, Brigham and Women’s Hospital, Boston (McGraw-Hill, 2008, $50);
- “First Exposure. Internal Medicine: Hospital Medicine” by Charles Griffith, MD, inpatient internal medicine clerkship director, and Andrew R. Hoellein, MD, outpatient internal medicine clerkship director, Department of Internal Medicine, University of Kentucky, Lexington (McGraw-Hill, 2007, $34); and
- “Tools and Strategies for an Effective Hospitalist Program” by Jeffrey R. Dichter, MD, and Kenneth G. Simone, MD (HCPro, 2008, $299).
SHM’s offering in the arena reinforces the ideas of “the critical need for leadership of HMGs and the need to create an ownership mentality for hospitalists within an HMG,” Miller says. “The book is filled with examples, tools, and checklists” and has sold approximately 500 copies so far.
The newest text, just off the press in May, is Dr. McKean’s. “This book provides concise, templated information designed to save the clinician valuable time,” she says. It also has a variety of uses, including exam review, clinical reference, point-of-care lookup, [and] quick updates in hospital medicine for those attending on the wards. It covers vital information on issues in administration and management.”
Dr. Wachter wrote his text “because I didn’t see any book for those seeking to learn the key clinical, organizational, and systems issues in patient safety,” he says. “I tried to write it in a lively and accessible style and fill it with illustrative cases and analyses, as well as up-to-date tables, graphics, references, and tools. My goal was to introduce the patient safety field to physicians—particularly hospitalists—nurses, pharmacists, and hospital administrators, as well as to trainees in these fields. [I hope it’s a] go-to book for experienced clinicians and nonclinicians alike.”
Already in its second printing, Dr. Wachter estimates it has sold between 7,500 and 10,000 copies. He plans to update the book every two years and is working on producing some Web-based learning modules. TH
The rapidly expanding field of hospital medicine has spurred a growing number of textbooks devoted to the specialty. Textbooks by some of the specialty’s leading voices are available to those keen on honing their knowledge.
Ranging in scope from practice management issues to clinical synopses, titles include:
- “Hospitalists: A Guide to Building and Sustaining a Successful Program” by SHM founders John Nelson, MD, and Win Whitcomb, MD, and Joe Miller, SHM’s executive adviser to the CEO. (Health Administration Press, 2007, $72);
- “Comprehensive Hospital Medicine,” by Mark Williams, MD, chief, division of hospital medicine, Feinberg School of Medicine, Chicago (Elsevier, 2007, $109);
- “Hospital Medicine Secrets,” by The Hospitalist physician editor Jeff Glasheen, MD (Mosby/Elsevier, 2007, $39);
- “Understanding Patient Safety” by Robert Wachter, MD, chief of the Division of Hospital Medicine, and chief of the Medical Service at the University of California, at San Francisco Medical Center, and author of “Wachter’s World,” a blog featured on The Hospitalist Web site (McGraw-Hill, 2007, $35);
- “Hospital Medicine: Just the Facts,” by Sylvia McKean, MD, director, hospitalist service, Brigham and Women’s Hospital, Boston (McGraw-Hill, 2008, $50);
- “First Exposure. Internal Medicine: Hospital Medicine” by Charles Griffith, MD, inpatient internal medicine clerkship director, and Andrew R. Hoellein, MD, outpatient internal medicine clerkship director, Department of Internal Medicine, University of Kentucky, Lexington (McGraw-Hill, 2007, $34); and
- “Tools and Strategies for an Effective Hospitalist Program” by Jeffrey R. Dichter, MD, and Kenneth G. Simone, MD (HCPro, 2008, $299).
SHM’s offering in the arena reinforces the ideas of “the critical need for leadership of HMGs and the need to create an ownership mentality for hospitalists within an HMG,” Miller says. “The book is filled with examples, tools, and checklists” and has sold approximately 500 copies so far.
The newest text, just off the press in May, is Dr. McKean’s. “This book provides concise, templated information designed to save the clinician valuable time,” she says. It also has a variety of uses, including exam review, clinical reference, point-of-care lookup, [and] quick updates in hospital medicine for those attending on the wards. It covers vital information on issues in administration and management.”
Dr. Wachter wrote his text “because I didn’t see any book for those seeking to learn the key clinical, organizational, and systems issues in patient safety,” he says. “I tried to write it in a lively and accessible style and fill it with illustrative cases and analyses, as well as up-to-date tables, graphics, references, and tools. My goal was to introduce the patient safety field to physicians—particularly hospitalists—nurses, pharmacists, and hospital administrators, as well as to trainees in these fields. [I hope it’s a] go-to book for experienced clinicians and nonclinicians alike.”
Already in its second printing, Dr. Wachter estimates it has sold between 7,500 and 10,000 copies. He plans to update the book every two years and is working on producing some Web-based learning modules. TH
The rapidly expanding field of hospital medicine has spurred a growing number of textbooks devoted to the specialty. Textbooks by some of the specialty’s leading voices are available to those keen on honing their knowledge.
Ranging in scope from practice management issues to clinical synopses, titles include:
- “Hospitalists: A Guide to Building and Sustaining a Successful Program” by SHM founders John Nelson, MD, and Win Whitcomb, MD, and Joe Miller, SHM’s executive adviser to the CEO. (Health Administration Press, 2007, $72);
- “Comprehensive Hospital Medicine,” by Mark Williams, MD, chief, division of hospital medicine, Feinberg School of Medicine, Chicago (Elsevier, 2007, $109);
- “Hospital Medicine Secrets,” by The Hospitalist physician editor Jeff Glasheen, MD (Mosby/Elsevier, 2007, $39);
- “Understanding Patient Safety” by Robert Wachter, MD, chief of the Division of Hospital Medicine, and chief of the Medical Service at the University of California, at San Francisco Medical Center, and author of “Wachter’s World,” a blog featured on The Hospitalist Web site (McGraw-Hill, 2007, $35);
- “Hospital Medicine: Just the Facts,” by Sylvia McKean, MD, director, hospitalist service, Brigham and Women’s Hospital, Boston (McGraw-Hill, 2008, $50);
- “First Exposure. Internal Medicine: Hospital Medicine” by Charles Griffith, MD, inpatient internal medicine clerkship director, and Andrew R. Hoellein, MD, outpatient internal medicine clerkship director, Department of Internal Medicine, University of Kentucky, Lexington (McGraw-Hill, 2007, $34); and
- “Tools and Strategies for an Effective Hospitalist Program” by Jeffrey R. Dichter, MD, and Kenneth G. Simone, MD (HCPro, 2008, $299).
SHM’s offering in the arena reinforces the ideas of “the critical need for leadership of HMGs and the need to create an ownership mentality for hospitalists within an HMG,” Miller says. “The book is filled with examples, tools, and checklists” and has sold approximately 500 copies so far.
The newest text, just off the press in May, is Dr. McKean’s. “This book provides concise, templated information designed to save the clinician valuable time,” she says. It also has a variety of uses, including exam review, clinical reference, point-of-care lookup, [and] quick updates in hospital medicine for those attending on the wards. It covers vital information on issues in administration and management.”
Dr. Wachter wrote his text “because I didn’t see any book for those seeking to learn the key clinical, organizational, and systems issues in patient safety,” he says. “I tried to write it in a lively and accessible style and fill it with illustrative cases and analyses, as well as up-to-date tables, graphics, references, and tools. My goal was to introduce the patient safety field to physicians—particularly hospitalists—nurses, pharmacists, and hospital administrators, as well as to trainees in these fields. [I hope it’s a] go-to book for experienced clinicians and nonclinicians alike.”
Already in its second printing, Dr. Wachter estimates it has sold between 7,500 and 10,000 copies. He plans to update the book every two years and is working on producing some Web-based learning modules. TH
A More Perfect Union
It used to be so simple. The relationship between doctors and hospitals was a straightforward quid pro quo.
Hospitals granted privileges to physicians to admit and treat their patients, and the physicians returned the favor by assuming unpaid responsibilities like taking call, providing care to uninsured or emergency patients, and serving on administrative committees.
The hospital was like a friendly club whose members exchanged benefits for duties—a win-win situation. No more.
“You used to be part of a fraternity,” explains Win Whitcomb, MD, director of performance improvement at Mercy Medical Center in Springfield, Mass., and a co-founder of SHM. “There were social rewards. There was opportunity for collegial interchange.”
Economic pressure has taken that all away. “The pace of care has greatly intensified, and the financial reward system has deteriorated significantly,” Dr. Whitcomb continues. “We treat larger numbers of uninsured patients with chronic unmanaged illnesses that require intervention. The reward system for physicians to take call and fulfill their obligation to the hospital no longer matches the responsibility.”
To illustrate the change, Dr. Whitcomb offers an example: “We have some days of the month where the call roster for general surgeries has vacancies. A month ago we had to send a patient to another hospital for an appendectomy.”
It is not an isolated instance. “Every hospital is struggling with the fact that many physicians don’t view unassigned call as a part of membership on the staff; they want to be paid for it,” says SHM President Patrick Cawley, MD, executive medical director of the Medical University of South Carolina (MUSC). And extra “pay” for services that used to be rendered gratis is one thing today’s strapped hospitals can little afford.
Committee staffing is another area undergoing change. Attending physicians are simply declining the duty. Neal Axon, MD, a hospitalist and assistant professor of medicine and pediatrics at MUSC, has seen the transformation firsthand. At one hospital his service covered, he saw the following: “At the first staff meeting there were 50 people; there was food, liquor. It was social and attendance was mandatory. You had to make three or four meetings a year to be on medical staff at this hospital.” But then, he says, attendance waned, and in the last year “dropped off precipitously.”
The old ways don’t work so what will replace them? “The point is that both physicians and hospitals need to put something on the table to collaborate,” Dr. Cawley says. “Many are saying that the hospital-physician relationship needs to change, but everyone is still feeling their way through it. What does it mean?”
SHM’s CEO Larry Wellikson, MD, sees a layered structure ahead. “Clearly the system is evolving into three kinds of physicians who use the hospital,” he says. “We are not advocating for it—just saying what it is. This is what is evolving, and hospital staffs need to see this is coming.” His three kinds of physicians are categorized by their relationship to the hospital.
The home team: “The first group is those physicians who work only at the hospital,” he says. “Their professional life is with the hospital as an institution: hospitalists, ER doctors, critical care physicians, and sometimes the anesthesiologists and radiologists. The hospital is the location of their work and provides the tools to do their job. If the hospital works well, they can do their job well. If hospital is dysfunctional, they can’t work well.”
He describes their relationship to the hospital with an anecdote: “When I was regular physician who came to the hospital just to see my patient, if they couldn’t find the chart I would scream and yell about that one patient.” Every physician faced with a missing chart thinks of it as an individual problem. “But now as a hospitalist, I try to fix the system, because all my patients are affected,” he says. “Hospitalists are on the inside trying to make it work.”
Important visitors: The second group, whom Dr. Wellikson calls important visitors, has a totally different relationship with the hospital. These are the cardiologists, orthopedic surgeons, and other medical specialties. “They are very important,” he says. “But they use the hospital intermittently and are not as tightly connected to it.”
Even so, they desire a high-quality hospital for their patients and will be willing to help set performance standards to achieve it. But their interest may not extend to patients who are not their own. “If the hospital says you have to also take care of free patients, they may choose not to,” Dr. Wellikson notes. “In fact, sometimes they have their own outpatient centers,” making them direct competitors as their competing practices sap revenue from money-making patients and procedures—all the while sending the sickest and costliest patients to the hospital.
Office-based physicians: The last of Dr. Wellikson’s groups is office-based physicians. These are the doctors who once made daily morning and evening rounds of their hospitalized patients but are now infrequently found at the bedside. “They are the physicians who don’t come to the hospital anymore: primary care physicians, endocrinologists, rheumatologists, neurologists, physicians who do all their surgery as outpatient procedures,” Dr. Wellikson explains.
—Larry Wellikson, MD, CEO of SHM
This upheaval is due to tectonic shifts in both medical economics and lifestyle preferences. “Because the reimbursement for care has gone down, physicians have to see more patients to make the same amount of money,” explains Dr. Wellikson. Turning their hospitalized patients over to hospitalists allows office-based physicians to maximize their income and optimize their time.
“It increases satisfaction, limits the hours you spend in the hospital, and puts some boundaries on your work day,” Dr. Cawley says.
“Doctors want more a predictable lifestyle,” Dr. Whitcomb says. In fact, their absence is already a fait accompli in many community hospitals.
As Dr. Axon succinctly puts it: “The primary care doc has left the building.”
Dr. Cawley believes the new system is a relief to many office-based physicians. “Some do miss going to the hospital and seeing other physicians to network with them. Some miss taking care of their acute in-care patients. But I think most are relieved to not have to go to hospital. They say, ‘No, things are better this way.’”
With so many other physicians withdrawing from hospitals to their offices and clinics, Dr. Wellikson believes hospitalists will become increasingly crucial to the institution’s operation and governance. “Now the home team is going to be more active; how you staff, how you make the hospital more efficient,” he says. “The inside physicians will be much more interactive. That’s why hospital medicine has grown so rapidly.”
The explosive expansion of hospital medicine as a specialty is a direct result of the need to increase efficiency and quality standards in this new hospital atmosphere.
In addition, good home teams create a milieu in which other physicians—the important visitors (cardiologists, surgeons, orthopedists)—will want to work. “My job (as a hospitalist) is to create an environment where you can come in and do your surgery,” Dr. Wellikson points out.
The home team offers something else too: medical expertise. Providing post-operative care is not cost-effective for many surgeons. “The surgical specialists are not paid to manage medical issues,” Dr. Cawley says. “It takes time and if somebody else can manage it, that’s great.” That somebody is often a hospitalist. “There is a quality-control aspect as well,” he adds. “With hospitalists focusing on medical issues, the result is better patient care.”
Melding these groups of physicians with disparate interests and responsibilities is the next challenge for hospital leadership. It is a challenge fraught with potential pitfalls. As Dr. Wellikson explains, “The biggest obstacle is that physicians don’t do change very well.”
Administrators will turn to their institution’s hospitalists (both hospital-employed and contracted) to effect these changes and ensure overall standards and efficiency.
“I think hospitalists are in a position to bridge the gap between administrators and medical staff,” says David Yu, MD, medical director of hospitalist services at Decatur Memorial Hospital in Illinois. “I think that’s why there will be more and more hospitalists in leadership positions. That’s why hospitalists are unique: they have their feet in both worlds.”
Dr. Wellikson believes the home team will step up to the plate and take over many of the leadership duties of the new hospital.
Kenneth Patrick, MD, the ICU director of Chestnut Hill Hospital in Philadelphia, sounds a more cautionary note. Dr. Patrick, a trained hospitalist and intensivist, believes the demise of the old “hospital privilege” model is dissolving ties between physicians and their workplace. “I think younger physicians will be much more transient and more concerned with their position, work hours, and pay,” he says.
He sees a young workforce—whether hospital or office-based—as more disengaged than physicians used to be. “They will meet hospital standards, but not be actively involved in developing them,” he believes. That will be left to a small group of hospital-based physicians “who will voluntarily come forward because it is their civic responsibility. It would be nice if more physicians would work on committees, but they look at them like jury duty and they don’t want to serve.”
“The question everyone asks is ‘What’s in it for me?’” Dr. Yu says. He notes a common sticking point: the requirement for increased documentation, which often means more work for doctors. “I think administrators are going to be in shock if they think practitioners are going to line up and say, ‘Well that’s great for the hospital.’”
The key to cooperation, says Dr. Yu, is the linking of changes to mutual benefit and patient welfare: “The administrators have to communicate that in the long run everyone will gain and it will ultimately lead to better patient care. You have to share your vision, inspire, motivate, and develop a culture of providing quality care. It’s easier said than done, but it’s the essence of medical care.”
What about patients? How do they react when a group of strangers takes over their hospital care rather than the primary care physician they often have gotten to know and trust for years? “Wanting your doctor present is counterbalanced by not having your doctor in the house,” Dr. Axon says. “Now you can see a physician anytime during the day.” And most patients are glad for the tradeoff. Dr. Yu has found the same dynamic with his patients at Decatur Memorial Hospital. “I can just count on one hand patients who were not happy the primary care physician wasn’t there,” he says. “Patients are more concerned with having their problems solved than with who is solving them.” And he makes sure his hospitalist staff never undermines the office based physicians. “We always say we are not better physicians, we are just more available.”
While they may have left the hospital, office-based physicians still will be a large presence in it by advocating for their patients. “If my whole currency is, ‘Do I have hospital privileges?’ then all my decisions are based on that,” Dr. Wellikson says.
Armed with the power of their patient referrals, office-based physicians will be able to demand that hospitals show proof of performance—thus becoming their patients’ ombudsmen. “I’m your shopper for the best healthcare, so the hospital has to step up to the plate and make sure it gets the business,” Dr. Wellikson explains. “They want standards because their patients need the best treatment, and they will have a choice of which hospital to put their patients into. If I now have a choice of three hospitals, I am looking to see that you are the Lexus of healthcare for my patients.”
Looking out for their patients’ interests is not the only way office-based physicians will continue to affect hospitals. As in-patient revenue declines, hospitals must look to the outpatient side to make up the difference. “The hospital is lucky if they break even on the inpatient side; they get the vast majority of money on the outpatient side: testing and procedures that private attendings are sending to the hospital,” Dr. Yu says.
He cautions against alienating those private practitioners by forcing change that is not mutually beneficial. “If you alienate them, you might lose money because they can send their patients to a different institution,” he warns. “These are the same doctors that never admit patients but do order the outpatient ultrasounds, blood tests, and therapies that are all money makers for the hospital. Why would you want to alienate these physicians?”
Dr. Patrick agrees: office-based physicians and hospitalists need each other. “I have to work with the primaries,” he says. “They are my source of referrals.”
There is another group that hospitals must learn to court, according to Dr. Axon: its own hospitalists. “I think you will see more innovative solutions to problems of recruiting hospital-based physicians to perform these functions,” he says. “For that to happen, the doctors will need to get more out of it. Many hospitalist groups are in a quandary; they are expected to do all these extra things, but pay is closely liked to clinical production and the number of patients they see. Those incentives will have to be aligned.”
All of which increases the reliance on—and importance of—those physicians who do work in the hospital—the home team. As Dr. Yu puts it: “I think the hospitalist model, whether you like or hate it, is the wave of the future.” TH
Carol Berczuk is a journalist based in New York.
It used to be so simple. The relationship between doctors and hospitals was a straightforward quid pro quo.
Hospitals granted privileges to physicians to admit and treat their patients, and the physicians returned the favor by assuming unpaid responsibilities like taking call, providing care to uninsured or emergency patients, and serving on administrative committees.
The hospital was like a friendly club whose members exchanged benefits for duties—a win-win situation. No more.
“You used to be part of a fraternity,” explains Win Whitcomb, MD, director of performance improvement at Mercy Medical Center in Springfield, Mass., and a co-founder of SHM. “There were social rewards. There was opportunity for collegial interchange.”
Economic pressure has taken that all away. “The pace of care has greatly intensified, and the financial reward system has deteriorated significantly,” Dr. Whitcomb continues. “We treat larger numbers of uninsured patients with chronic unmanaged illnesses that require intervention. The reward system for physicians to take call and fulfill their obligation to the hospital no longer matches the responsibility.”
To illustrate the change, Dr. Whitcomb offers an example: “We have some days of the month where the call roster for general surgeries has vacancies. A month ago we had to send a patient to another hospital for an appendectomy.”
It is not an isolated instance. “Every hospital is struggling with the fact that many physicians don’t view unassigned call as a part of membership on the staff; they want to be paid for it,” says SHM President Patrick Cawley, MD, executive medical director of the Medical University of South Carolina (MUSC). And extra “pay” for services that used to be rendered gratis is one thing today’s strapped hospitals can little afford.
Committee staffing is another area undergoing change. Attending physicians are simply declining the duty. Neal Axon, MD, a hospitalist and assistant professor of medicine and pediatrics at MUSC, has seen the transformation firsthand. At one hospital his service covered, he saw the following: “At the first staff meeting there were 50 people; there was food, liquor. It was social and attendance was mandatory. You had to make three or four meetings a year to be on medical staff at this hospital.” But then, he says, attendance waned, and in the last year “dropped off precipitously.”
The old ways don’t work so what will replace them? “The point is that both physicians and hospitals need to put something on the table to collaborate,” Dr. Cawley says. “Many are saying that the hospital-physician relationship needs to change, but everyone is still feeling their way through it. What does it mean?”
SHM’s CEO Larry Wellikson, MD, sees a layered structure ahead. “Clearly the system is evolving into three kinds of physicians who use the hospital,” he says. “We are not advocating for it—just saying what it is. This is what is evolving, and hospital staffs need to see this is coming.” His three kinds of physicians are categorized by their relationship to the hospital.
The home team: “The first group is those physicians who work only at the hospital,” he says. “Their professional life is with the hospital as an institution: hospitalists, ER doctors, critical care physicians, and sometimes the anesthesiologists and radiologists. The hospital is the location of their work and provides the tools to do their job. If the hospital works well, they can do their job well. If hospital is dysfunctional, they can’t work well.”
He describes their relationship to the hospital with an anecdote: “When I was regular physician who came to the hospital just to see my patient, if they couldn’t find the chart I would scream and yell about that one patient.” Every physician faced with a missing chart thinks of it as an individual problem. “But now as a hospitalist, I try to fix the system, because all my patients are affected,” he says. “Hospitalists are on the inside trying to make it work.”
Important visitors: The second group, whom Dr. Wellikson calls important visitors, has a totally different relationship with the hospital. These are the cardiologists, orthopedic surgeons, and other medical specialties. “They are very important,” he says. “But they use the hospital intermittently and are not as tightly connected to it.”
Even so, they desire a high-quality hospital for their patients and will be willing to help set performance standards to achieve it. But their interest may not extend to patients who are not their own. “If the hospital says you have to also take care of free patients, they may choose not to,” Dr. Wellikson notes. “In fact, sometimes they have their own outpatient centers,” making them direct competitors as their competing practices sap revenue from money-making patients and procedures—all the while sending the sickest and costliest patients to the hospital.
Office-based physicians: The last of Dr. Wellikson’s groups is office-based physicians. These are the doctors who once made daily morning and evening rounds of their hospitalized patients but are now infrequently found at the bedside. “They are the physicians who don’t come to the hospital anymore: primary care physicians, endocrinologists, rheumatologists, neurologists, physicians who do all their surgery as outpatient procedures,” Dr. Wellikson explains.
—Larry Wellikson, MD, CEO of SHM
This upheaval is due to tectonic shifts in both medical economics and lifestyle preferences. “Because the reimbursement for care has gone down, physicians have to see more patients to make the same amount of money,” explains Dr. Wellikson. Turning their hospitalized patients over to hospitalists allows office-based physicians to maximize their income and optimize their time.
“It increases satisfaction, limits the hours you spend in the hospital, and puts some boundaries on your work day,” Dr. Cawley says.
“Doctors want more a predictable lifestyle,” Dr. Whitcomb says. In fact, their absence is already a fait accompli in many community hospitals.
As Dr. Axon succinctly puts it: “The primary care doc has left the building.”
Dr. Cawley believes the new system is a relief to many office-based physicians. “Some do miss going to the hospital and seeing other physicians to network with them. Some miss taking care of their acute in-care patients. But I think most are relieved to not have to go to hospital. They say, ‘No, things are better this way.’”
With so many other physicians withdrawing from hospitals to their offices and clinics, Dr. Wellikson believes hospitalists will become increasingly crucial to the institution’s operation and governance. “Now the home team is going to be more active; how you staff, how you make the hospital more efficient,” he says. “The inside physicians will be much more interactive. That’s why hospital medicine has grown so rapidly.”
The explosive expansion of hospital medicine as a specialty is a direct result of the need to increase efficiency and quality standards in this new hospital atmosphere.
In addition, good home teams create a milieu in which other physicians—the important visitors (cardiologists, surgeons, orthopedists)—will want to work. “My job (as a hospitalist) is to create an environment where you can come in and do your surgery,” Dr. Wellikson points out.
The home team offers something else too: medical expertise. Providing post-operative care is not cost-effective for many surgeons. “The surgical specialists are not paid to manage medical issues,” Dr. Cawley says. “It takes time and if somebody else can manage it, that’s great.” That somebody is often a hospitalist. “There is a quality-control aspect as well,” he adds. “With hospitalists focusing on medical issues, the result is better patient care.”
Melding these groups of physicians with disparate interests and responsibilities is the next challenge for hospital leadership. It is a challenge fraught with potential pitfalls. As Dr. Wellikson explains, “The biggest obstacle is that physicians don’t do change very well.”
Administrators will turn to their institution’s hospitalists (both hospital-employed and contracted) to effect these changes and ensure overall standards and efficiency.
“I think hospitalists are in a position to bridge the gap between administrators and medical staff,” says David Yu, MD, medical director of hospitalist services at Decatur Memorial Hospital in Illinois. “I think that’s why there will be more and more hospitalists in leadership positions. That’s why hospitalists are unique: they have their feet in both worlds.”
Dr. Wellikson believes the home team will step up to the plate and take over many of the leadership duties of the new hospital.
Kenneth Patrick, MD, the ICU director of Chestnut Hill Hospital in Philadelphia, sounds a more cautionary note. Dr. Patrick, a trained hospitalist and intensivist, believes the demise of the old “hospital privilege” model is dissolving ties between physicians and their workplace. “I think younger physicians will be much more transient and more concerned with their position, work hours, and pay,” he says.
He sees a young workforce—whether hospital or office-based—as more disengaged than physicians used to be. “They will meet hospital standards, but not be actively involved in developing them,” he believes. That will be left to a small group of hospital-based physicians “who will voluntarily come forward because it is their civic responsibility. It would be nice if more physicians would work on committees, but they look at them like jury duty and they don’t want to serve.”
“The question everyone asks is ‘What’s in it for me?’” Dr. Yu says. He notes a common sticking point: the requirement for increased documentation, which often means more work for doctors. “I think administrators are going to be in shock if they think practitioners are going to line up and say, ‘Well that’s great for the hospital.’”
The key to cooperation, says Dr. Yu, is the linking of changes to mutual benefit and patient welfare: “The administrators have to communicate that in the long run everyone will gain and it will ultimately lead to better patient care. You have to share your vision, inspire, motivate, and develop a culture of providing quality care. It’s easier said than done, but it’s the essence of medical care.”
What about patients? How do they react when a group of strangers takes over their hospital care rather than the primary care physician they often have gotten to know and trust for years? “Wanting your doctor present is counterbalanced by not having your doctor in the house,” Dr. Axon says. “Now you can see a physician anytime during the day.” And most patients are glad for the tradeoff. Dr. Yu has found the same dynamic with his patients at Decatur Memorial Hospital. “I can just count on one hand patients who were not happy the primary care physician wasn’t there,” he says. “Patients are more concerned with having their problems solved than with who is solving them.” And he makes sure his hospitalist staff never undermines the office based physicians. “We always say we are not better physicians, we are just more available.”
While they may have left the hospital, office-based physicians still will be a large presence in it by advocating for their patients. “If my whole currency is, ‘Do I have hospital privileges?’ then all my decisions are based on that,” Dr. Wellikson says.
Armed with the power of their patient referrals, office-based physicians will be able to demand that hospitals show proof of performance—thus becoming their patients’ ombudsmen. “I’m your shopper for the best healthcare, so the hospital has to step up to the plate and make sure it gets the business,” Dr. Wellikson explains. “They want standards because their patients need the best treatment, and they will have a choice of which hospital to put their patients into. If I now have a choice of three hospitals, I am looking to see that you are the Lexus of healthcare for my patients.”
Looking out for their patients’ interests is not the only way office-based physicians will continue to affect hospitals. As in-patient revenue declines, hospitals must look to the outpatient side to make up the difference. “The hospital is lucky if they break even on the inpatient side; they get the vast majority of money on the outpatient side: testing and procedures that private attendings are sending to the hospital,” Dr. Yu says.
He cautions against alienating those private practitioners by forcing change that is not mutually beneficial. “If you alienate them, you might lose money because they can send their patients to a different institution,” he warns. “These are the same doctors that never admit patients but do order the outpatient ultrasounds, blood tests, and therapies that are all money makers for the hospital. Why would you want to alienate these physicians?”
Dr. Patrick agrees: office-based physicians and hospitalists need each other. “I have to work with the primaries,” he says. “They are my source of referrals.”
There is another group that hospitals must learn to court, according to Dr. Axon: its own hospitalists. “I think you will see more innovative solutions to problems of recruiting hospital-based physicians to perform these functions,” he says. “For that to happen, the doctors will need to get more out of it. Many hospitalist groups are in a quandary; they are expected to do all these extra things, but pay is closely liked to clinical production and the number of patients they see. Those incentives will have to be aligned.”
All of which increases the reliance on—and importance of—those physicians who do work in the hospital—the home team. As Dr. Yu puts it: “I think the hospitalist model, whether you like or hate it, is the wave of the future.” TH
Carol Berczuk is a journalist based in New York.
It used to be so simple. The relationship between doctors and hospitals was a straightforward quid pro quo.
Hospitals granted privileges to physicians to admit and treat their patients, and the physicians returned the favor by assuming unpaid responsibilities like taking call, providing care to uninsured or emergency patients, and serving on administrative committees.
The hospital was like a friendly club whose members exchanged benefits for duties—a win-win situation. No more.
“You used to be part of a fraternity,” explains Win Whitcomb, MD, director of performance improvement at Mercy Medical Center in Springfield, Mass., and a co-founder of SHM. “There were social rewards. There was opportunity for collegial interchange.”
Economic pressure has taken that all away. “The pace of care has greatly intensified, and the financial reward system has deteriorated significantly,” Dr. Whitcomb continues. “We treat larger numbers of uninsured patients with chronic unmanaged illnesses that require intervention. The reward system for physicians to take call and fulfill their obligation to the hospital no longer matches the responsibility.”
To illustrate the change, Dr. Whitcomb offers an example: “We have some days of the month where the call roster for general surgeries has vacancies. A month ago we had to send a patient to another hospital for an appendectomy.”
It is not an isolated instance. “Every hospital is struggling with the fact that many physicians don’t view unassigned call as a part of membership on the staff; they want to be paid for it,” says SHM President Patrick Cawley, MD, executive medical director of the Medical University of South Carolina (MUSC). And extra “pay” for services that used to be rendered gratis is one thing today’s strapped hospitals can little afford.
Committee staffing is another area undergoing change. Attending physicians are simply declining the duty. Neal Axon, MD, a hospitalist and assistant professor of medicine and pediatrics at MUSC, has seen the transformation firsthand. At one hospital his service covered, he saw the following: “At the first staff meeting there were 50 people; there was food, liquor. It was social and attendance was mandatory. You had to make three or four meetings a year to be on medical staff at this hospital.” But then, he says, attendance waned, and in the last year “dropped off precipitously.”
The old ways don’t work so what will replace them? “The point is that both physicians and hospitals need to put something on the table to collaborate,” Dr. Cawley says. “Many are saying that the hospital-physician relationship needs to change, but everyone is still feeling their way through it. What does it mean?”
SHM’s CEO Larry Wellikson, MD, sees a layered structure ahead. “Clearly the system is evolving into three kinds of physicians who use the hospital,” he says. “We are not advocating for it—just saying what it is. This is what is evolving, and hospital staffs need to see this is coming.” His three kinds of physicians are categorized by their relationship to the hospital.
The home team: “The first group is those physicians who work only at the hospital,” he says. “Their professional life is with the hospital as an institution: hospitalists, ER doctors, critical care physicians, and sometimes the anesthesiologists and radiologists. The hospital is the location of their work and provides the tools to do their job. If the hospital works well, they can do their job well. If hospital is dysfunctional, they can’t work well.”
He describes their relationship to the hospital with an anecdote: “When I was regular physician who came to the hospital just to see my patient, if they couldn’t find the chart I would scream and yell about that one patient.” Every physician faced with a missing chart thinks of it as an individual problem. “But now as a hospitalist, I try to fix the system, because all my patients are affected,” he says. “Hospitalists are on the inside trying to make it work.”
Important visitors: The second group, whom Dr. Wellikson calls important visitors, has a totally different relationship with the hospital. These are the cardiologists, orthopedic surgeons, and other medical specialties. “They are very important,” he says. “But they use the hospital intermittently and are not as tightly connected to it.”
Even so, they desire a high-quality hospital for their patients and will be willing to help set performance standards to achieve it. But their interest may not extend to patients who are not their own. “If the hospital says you have to also take care of free patients, they may choose not to,” Dr. Wellikson notes. “In fact, sometimes they have their own outpatient centers,” making them direct competitors as their competing practices sap revenue from money-making patients and procedures—all the while sending the sickest and costliest patients to the hospital.
Office-based physicians: The last of Dr. Wellikson’s groups is office-based physicians. These are the doctors who once made daily morning and evening rounds of their hospitalized patients but are now infrequently found at the bedside. “They are the physicians who don’t come to the hospital anymore: primary care physicians, endocrinologists, rheumatologists, neurologists, physicians who do all their surgery as outpatient procedures,” Dr. Wellikson explains.
—Larry Wellikson, MD, CEO of SHM
This upheaval is due to tectonic shifts in both medical economics and lifestyle preferences. “Because the reimbursement for care has gone down, physicians have to see more patients to make the same amount of money,” explains Dr. Wellikson. Turning their hospitalized patients over to hospitalists allows office-based physicians to maximize their income and optimize their time.
“It increases satisfaction, limits the hours you spend in the hospital, and puts some boundaries on your work day,” Dr. Cawley says.
“Doctors want more a predictable lifestyle,” Dr. Whitcomb says. In fact, their absence is already a fait accompli in many community hospitals.
As Dr. Axon succinctly puts it: “The primary care doc has left the building.”
Dr. Cawley believes the new system is a relief to many office-based physicians. “Some do miss going to the hospital and seeing other physicians to network with them. Some miss taking care of their acute in-care patients. But I think most are relieved to not have to go to hospital. They say, ‘No, things are better this way.’”
With so many other physicians withdrawing from hospitals to their offices and clinics, Dr. Wellikson believes hospitalists will become increasingly crucial to the institution’s operation and governance. “Now the home team is going to be more active; how you staff, how you make the hospital more efficient,” he says. “The inside physicians will be much more interactive. That’s why hospital medicine has grown so rapidly.”
The explosive expansion of hospital medicine as a specialty is a direct result of the need to increase efficiency and quality standards in this new hospital atmosphere.
In addition, good home teams create a milieu in which other physicians—the important visitors (cardiologists, surgeons, orthopedists)—will want to work. “My job (as a hospitalist) is to create an environment where you can come in and do your surgery,” Dr. Wellikson points out.
The home team offers something else too: medical expertise. Providing post-operative care is not cost-effective for many surgeons. “The surgical specialists are not paid to manage medical issues,” Dr. Cawley says. “It takes time and if somebody else can manage it, that’s great.” That somebody is often a hospitalist. “There is a quality-control aspect as well,” he adds. “With hospitalists focusing on medical issues, the result is better patient care.”
Melding these groups of physicians with disparate interests and responsibilities is the next challenge for hospital leadership. It is a challenge fraught with potential pitfalls. As Dr. Wellikson explains, “The biggest obstacle is that physicians don’t do change very well.”
Administrators will turn to their institution’s hospitalists (both hospital-employed and contracted) to effect these changes and ensure overall standards and efficiency.
“I think hospitalists are in a position to bridge the gap between administrators and medical staff,” says David Yu, MD, medical director of hospitalist services at Decatur Memorial Hospital in Illinois. “I think that’s why there will be more and more hospitalists in leadership positions. That’s why hospitalists are unique: they have their feet in both worlds.”
Dr. Wellikson believes the home team will step up to the plate and take over many of the leadership duties of the new hospital.
Kenneth Patrick, MD, the ICU director of Chestnut Hill Hospital in Philadelphia, sounds a more cautionary note. Dr. Patrick, a trained hospitalist and intensivist, believes the demise of the old “hospital privilege” model is dissolving ties between physicians and their workplace. “I think younger physicians will be much more transient and more concerned with their position, work hours, and pay,” he says.
He sees a young workforce—whether hospital or office-based—as more disengaged than physicians used to be. “They will meet hospital standards, but not be actively involved in developing them,” he believes. That will be left to a small group of hospital-based physicians “who will voluntarily come forward because it is their civic responsibility. It would be nice if more physicians would work on committees, but they look at them like jury duty and they don’t want to serve.”
“The question everyone asks is ‘What’s in it for me?’” Dr. Yu says. He notes a common sticking point: the requirement for increased documentation, which often means more work for doctors. “I think administrators are going to be in shock if they think practitioners are going to line up and say, ‘Well that’s great for the hospital.’”
The key to cooperation, says Dr. Yu, is the linking of changes to mutual benefit and patient welfare: “The administrators have to communicate that in the long run everyone will gain and it will ultimately lead to better patient care. You have to share your vision, inspire, motivate, and develop a culture of providing quality care. It’s easier said than done, but it’s the essence of medical care.”
What about patients? How do they react when a group of strangers takes over their hospital care rather than the primary care physician they often have gotten to know and trust for years? “Wanting your doctor present is counterbalanced by not having your doctor in the house,” Dr. Axon says. “Now you can see a physician anytime during the day.” And most patients are glad for the tradeoff. Dr. Yu has found the same dynamic with his patients at Decatur Memorial Hospital. “I can just count on one hand patients who were not happy the primary care physician wasn’t there,” he says. “Patients are more concerned with having their problems solved than with who is solving them.” And he makes sure his hospitalist staff never undermines the office based physicians. “We always say we are not better physicians, we are just more available.”
While they may have left the hospital, office-based physicians still will be a large presence in it by advocating for their patients. “If my whole currency is, ‘Do I have hospital privileges?’ then all my decisions are based on that,” Dr. Wellikson says.
Armed with the power of their patient referrals, office-based physicians will be able to demand that hospitals show proof of performance—thus becoming their patients’ ombudsmen. “I’m your shopper for the best healthcare, so the hospital has to step up to the plate and make sure it gets the business,” Dr. Wellikson explains. “They want standards because their patients need the best treatment, and they will have a choice of which hospital to put their patients into. If I now have a choice of three hospitals, I am looking to see that you are the Lexus of healthcare for my patients.”
Looking out for their patients’ interests is not the only way office-based physicians will continue to affect hospitals. As in-patient revenue declines, hospitals must look to the outpatient side to make up the difference. “The hospital is lucky if they break even on the inpatient side; they get the vast majority of money on the outpatient side: testing and procedures that private attendings are sending to the hospital,” Dr. Yu says.
He cautions against alienating those private practitioners by forcing change that is not mutually beneficial. “If you alienate them, you might lose money because they can send their patients to a different institution,” he warns. “These are the same doctors that never admit patients but do order the outpatient ultrasounds, blood tests, and therapies that are all money makers for the hospital. Why would you want to alienate these physicians?”
Dr. Patrick agrees: office-based physicians and hospitalists need each other. “I have to work with the primaries,” he says. “They are my source of referrals.”
There is another group that hospitals must learn to court, according to Dr. Axon: its own hospitalists. “I think you will see more innovative solutions to problems of recruiting hospital-based physicians to perform these functions,” he says. “For that to happen, the doctors will need to get more out of it. Many hospitalist groups are in a quandary; they are expected to do all these extra things, but pay is closely liked to clinical production and the number of patients they see. Those incentives will have to be aligned.”
All of which increases the reliance on—and importance of—those physicians who do work in the hospital—the home team. As Dr. Yu puts it: “I think the hospitalist model, whether you like or hate it, is the wave of the future.” TH
Carol Berczuk is a journalist based in New York.
In Demand
Hospitalist Nhi Lan Pham, MD, accepted a signing/starting bonus to relocate to Texas after finishing her residency in internal medicine in the Detroit area in 2007. The accompanying relocation expenses helped Dr. Pham begin her career near her family in Austin, and the flexible work schedule she negotiated allowed her to spend time with her family.
Another hospitalist willing to relocate for the right job used the relocation budget to his advantage. Moving to take the right job cost $2,000. The employer had budgeted $5,000 for relocation expenses, and the physician was able to arrange to have the $3,000 difference added to his signing bonus.
Yet another physician, already established as a hospitalist in an underserved area of his state, was attracted to a new position. However, his original acceptance of a loan repayment from the state as an inducement to work in the underserved region precluded his applying for the new position. His arrangement with the state did not prevent his approaching the new hospital to see what might be possible. It was a wise move; the new hospital agreed to increase the hospitalist’s signing bonus by enough to reimburse the state for the loan repayment.
One foreign-born physician secured a commitment from his recruiter that his employer would sponsor him and his family for green cards. Another candidate agreed to a reduced starting salary in return for help in securing a visa.
With a projected need for 30,000 hospitalists by 2010, hospitalists find themselves in the driver’s seat when it comes to weighing offers. Incentives are increasingly enticing as hospitalist recruiters nationwide struggle to lure top talent.
Incentives, Perks
What does this mean in practical terms? It means not only rising salaries but also incentives and extra perks to attract candidates to this fast-growing specialty.
Financial benefits are widespread, including signing and performance bonuses. Many hospitalists can plan on a guaranteed income. Employers may agree to pay off student loans or reimburse tuition. Malpractice insurance and tail coverage are commonly covered. Some employers also allow part-time or temporary employment to give a new hospitalist an opportunity to decide about the future or to accommodate a personal schedule.
“There is often a laundry list of incentives from which to choose, as well as more of a cafeteria plan that a doctor and employer can customize to meet specific needs,” according to Mark Dotson, MD, senior director of recruitment at Brentwood, Tenn.-based Cogent Healthcare. Cogent is a recruiting firm dedicated to building and managing hospitalist programs.
By far, the most appealing incentives are flexibility of scheduling and workload that allow physicians to coordinate their work schedule with their lifestyle. In fact, Dan Polk, MD, chief of the division of hospital-based medicine at Children’s Memorial Hospital in Chicago, considers flexible scheduling the basis of his plan to retain staff and build job satisfaction.
“We support lifestyle choices and respect life situations,” Dr. Polk says. “We foster the idea of joining a great team, and we make the environment attractive enough to encourage people to stay. We try to work within the team to cover those who need help, such as maternity or family leave, and we compensate for extra time at a different rate. We embrace people who want to work part time or share a job. Our goals are to support people and to make them want to get up in the morning to come to work.”
Part of the attractiveness of schedule flexibility is fewer weekend and night hours. In addition, employers may allow hospitalists to limit their caseload. Some hospitalists, for example, request a cap of 15 to 18 patients a day.
While retaining experienced, motivated staff is a goal of hospitals, lower caseloads mean “more doctors to do the work if doctors work fewer hours,” says Rusty Holman, MD, Cogent’s chief operating officer and SHM’s immediate past president. To meet that need, hospitals are turning to community-based physicians, fellows, and residents to work weekends and evenings. This, in turn, offers the perk of part-time work for those who want more personal time in their schedule.
The demand for nocturnists also is growing (The Hospitalist, January 2008, p. 22). Nocturnists work at night and on weekends and usually work shorter hours. These physicians prefer this schedule so they can have their days free for family or other pursuits. They also enjoy higher compensation, fewer workdays per month, and lower productivity expectations.
In addition to the having the options of part-time hours, temporary work, or job sharing, hospitalists also can negotiate other schedule perks. Some request and receive a two-week-on, two-week-off schedule. Many ask about the shift model, which demands nothing beyond the full eight or 12 hours of work. Still other applicants find a swing shift fits their lifestyle. There are even short-term choices: the hospitalist program at the University of California at Irvine offers recent residents the opportunity to work for one year while deciding about their career. With scheduling choices as part of an incentive package, many hospitalists achieve Dr. Polk’s goal of being eager to come to work each morning.
Physicians are not the only beneficiaries of these perks. Cogent, for example, recruits physician assistants and nurse practitioners when forming hospitalist groups. These employees also enjoy incentives, including tuition for continuing education and the same schedule flexibility as hospitalists.
Seller’s Market
Hospital medicine faces a shortage of qualified applicants. The need for hospitalists far surpasses the supply of physicians, who are in the enviable position of sifting through incentives and perks when selecting a hospitalist job.
This has become a national concern, according to Vikas Parekh, MD, assistant director of the hospitalist program and assistant professor of medicine at the University of Michigan Health Center in Ann Arbor. “We’re not seeing a pool of applicants because top residents are not pursuing hospitalist careers,” Dr. Parekh says.
Alpesh Amin, MD, MBA, a member of SHM’s Board of Directors, concurs but also points out that the number of hospitalist jobs is growing. “The need for a few hundred hospitalists 10 years ago has grown to 20,000 to 30,000 today, thus creating a need much greater than the supply,” says Dr. Amin, professor and chief, general internal medicine, executive director and founder of the hospitalist program at the University of California Irvine.
Why the shortage? First, fewer physicians are choosing to practice general medicine, either as an internist, family practitioner, or hospitalist. A recent study found fewer medical students were planning to concentrate on internal and family medicine, and that those who did planned to go into a subspecialty later.1 Dr. Parekh attributes this to a combination of reasons. “Most internal medicine residents are subspecialty oriented and may have decided their specialty early on,” Dr. Parekh says. “They may choose a subspecialty for financial reasons or prestige,” he continues, “but they may also be unclear about what a hospitalist career really is.”
Second, hospitalist programs have begun to expand from large metropolitan regions to smaller and rural areas. The result is an even greater demand for hospitalists.
Meet the Need
“There are no saturated markets within hospital medicine,” Dr. Holman says. “That is, most groups are always actively recruiting. [Cogent develops] full hospitalist programs, including recruiting, employing, managing, and training for new and existing hospitalist groups.”
Who is being recruited? Many recruiters approach residents who have not chosen a subspecialty to offer a staff position after they finish the residency. Although a recruiting firm may not offer financial aid during the residency, an employer may provide some sort of stipend if the candidate commits to remain on staff for a specified time after residency. “Recruit and retain” is the operative phrase in these cases.
Recruiters also are approaching generalists just entering the market to point out the advantages of avoiding the startup costs of establishing an outpatient practice. Further, many hospitalists are emerging from the ranks of solo practitioners interested in the financial and personal advantages of belonging to hospitalist groups. Not only does that eliminate the practice overhead (including the burden of regulatory compliance), but it also may offer additional administrative and academic opportunities. As Dr. Amin says, “There are more MD-MBA combos out there.”
Are incentives the answer to the shortage? Perhaps for now. With time, hospital medicine’s built-in perks may end the shortage and the need for added incentives. TH
Ann Kepler is a medical writer based in Chicago.
Reference
- Croasdale M. Primary care doctors in demand; signing bonuses and higher pay for some. American Medical News. June 19, 2006. Available at www.ama-assn.org/amednews/site/free/prl10619.htm. Last accessed March 19, 2008.
Hospitalist Nhi Lan Pham, MD, accepted a signing/starting bonus to relocate to Texas after finishing her residency in internal medicine in the Detroit area in 2007. The accompanying relocation expenses helped Dr. Pham begin her career near her family in Austin, and the flexible work schedule she negotiated allowed her to spend time with her family.
Another hospitalist willing to relocate for the right job used the relocation budget to his advantage. Moving to take the right job cost $2,000. The employer had budgeted $5,000 for relocation expenses, and the physician was able to arrange to have the $3,000 difference added to his signing bonus.
Yet another physician, already established as a hospitalist in an underserved area of his state, was attracted to a new position. However, his original acceptance of a loan repayment from the state as an inducement to work in the underserved region precluded his applying for the new position. His arrangement with the state did not prevent his approaching the new hospital to see what might be possible. It was a wise move; the new hospital agreed to increase the hospitalist’s signing bonus by enough to reimburse the state for the loan repayment.
One foreign-born physician secured a commitment from his recruiter that his employer would sponsor him and his family for green cards. Another candidate agreed to a reduced starting salary in return for help in securing a visa.
With a projected need for 30,000 hospitalists by 2010, hospitalists find themselves in the driver’s seat when it comes to weighing offers. Incentives are increasingly enticing as hospitalist recruiters nationwide struggle to lure top talent.
Incentives, Perks
What does this mean in practical terms? It means not only rising salaries but also incentives and extra perks to attract candidates to this fast-growing specialty.
Financial benefits are widespread, including signing and performance bonuses. Many hospitalists can plan on a guaranteed income. Employers may agree to pay off student loans or reimburse tuition. Malpractice insurance and tail coverage are commonly covered. Some employers also allow part-time or temporary employment to give a new hospitalist an opportunity to decide about the future or to accommodate a personal schedule.
“There is often a laundry list of incentives from which to choose, as well as more of a cafeteria plan that a doctor and employer can customize to meet specific needs,” according to Mark Dotson, MD, senior director of recruitment at Brentwood, Tenn.-based Cogent Healthcare. Cogent is a recruiting firm dedicated to building and managing hospitalist programs.
By far, the most appealing incentives are flexibility of scheduling and workload that allow physicians to coordinate their work schedule with their lifestyle. In fact, Dan Polk, MD, chief of the division of hospital-based medicine at Children’s Memorial Hospital in Chicago, considers flexible scheduling the basis of his plan to retain staff and build job satisfaction.
“We support lifestyle choices and respect life situations,” Dr. Polk says. “We foster the idea of joining a great team, and we make the environment attractive enough to encourage people to stay. We try to work within the team to cover those who need help, such as maternity or family leave, and we compensate for extra time at a different rate. We embrace people who want to work part time or share a job. Our goals are to support people and to make them want to get up in the morning to come to work.”
Part of the attractiveness of schedule flexibility is fewer weekend and night hours. In addition, employers may allow hospitalists to limit their caseload. Some hospitalists, for example, request a cap of 15 to 18 patients a day.
While retaining experienced, motivated staff is a goal of hospitals, lower caseloads mean “more doctors to do the work if doctors work fewer hours,” says Rusty Holman, MD, Cogent’s chief operating officer and SHM’s immediate past president. To meet that need, hospitals are turning to community-based physicians, fellows, and residents to work weekends and evenings. This, in turn, offers the perk of part-time work for those who want more personal time in their schedule.
The demand for nocturnists also is growing (The Hospitalist, January 2008, p. 22). Nocturnists work at night and on weekends and usually work shorter hours. These physicians prefer this schedule so they can have their days free for family or other pursuits. They also enjoy higher compensation, fewer workdays per month, and lower productivity expectations.
In addition to the having the options of part-time hours, temporary work, or job sharing, hospitalists also can negotiate other schedule perks. Some request and receive a two-week-on, two-week-off schedule. Many ask about the shift model, which demands nothing beyond the full eight or 12 hours of work. Still other applicants find a swing shift fits their lifestyle. There are even short-term choices: the hospitalist program at the University of California at Irvine offers recent residents the opportunity to work for one year while deciding about their career. With scheduling choices as part of an incentive package, many hospitalists achieve Dr. Polk’s goal of being eager to come to work each morning.
Physicians are not the only beneficiaries of these perks. Cogent, for example, recruits physician assistants and nurse practitioners when forming hospitalist groups. These employees also enjoy incentives, including tuition for continuing education and the same schedule flexibility as hospitalists.
Seller’s Market
Hospital medicine faces a shortage of qualified applicants. The need for hospitalists far surpasses the supply of physicians, who are in the enviable position of sifting through incentives and perks when selecting a hospitalist job.
This has become a national concern, according to Vikas Parekh, MD, assistant director of the hospitalist program and assistant professor of medicine at the University of Michigan Health Center in Ann Arbor. “We’re not seeing a pool of applicants because top residents are not pursuing hospitalist careers,” Dr. Parekh says.
Alpesh Amin, MD, MBA, a member of SHM’s Board of Directors, concurs but also points out that the number of hospitalist jobs is growing. “The need for a few hundred hospitalists 10 years ago has grown to 20,000 to 30,000 today, thus creating a need much greater than the supply,” says Dr. Amin, professor and chief, general internal medicine, executive director and founder of the hospitalist program at the University of California Irvine.
Why the shortage? First, fewer physicians are choosing to practice general medicine, either as an internist, family practitioner, or hospitalist. A recent study found fewer medical students were planning to concentrate on internal and family medicine, and that those who did planned to go into a subspecialty later.1 Dr. Parekh attributes this to a combination of reasons. “Most internal medicine residents are subspecialty oriented and may have decided their specialty early on,” Dr. Parekh says. “They may choose a subspecialty for financial reasons or prestige,” he continues, “but they may also be unclear about what a hospitalist career really is.”
Second, hospitalist programs have begun to expand from large metropolitan regions to smaller and rural areas. The result is an even greater demand for hospitalists.
Meet the Need
“There are no saturated markets within hospital medicine,” Dr. Holman says. “That is, most groups are always actively recruiting. [Cogent develops] full hospitalist programs, including recruiting, employing, managing, and training for new and existing hospitalist groups.”
Who is being recruited? Many recruiters approach residents who have not chosen a subspecialty to offer a staff position after they finish the residency. Although a recruiting firm may not offer financial aid during the residency, an employer may provide some sort of stipend if the candidate commits to remain on staff for a specified time after residency. “Recruit and retain” is the operative phrase in these cases.
Recruiters also are approaching generalists just entering the market to point out the advantages of avoiding the startup costs of establishing an outpatient practice. Further, many hospitalists are emerging from the ranks of solo practitioners interested in the financial and personal advantages of belonging to hospitalist groups. Not only does that eliminate the practice overhead (including the burden of regulatory compliance), but it also may offer additional administrative and academic opportunities. As Dr. Amin says, “There are more MD-MBA combos out there.”
Are incentives the answer to the shortage? Perhaps for now. With time, hospital medicine’s built-in perks may end the shortage and the need for added incentives. TH
Ann Kepler is a medical writer based in Chicago.
Reference
- Croasdale M. Primary care doctors in demand; signing bonuses and higher pay for some. American Medical News. June 19, 2006. Available at www.ama-assn.org/amednews/site/free/prl10619.htm. Last accessed March 19, 2008.
Hospitalist Nhi Lan Pham, MD, accepted a signing/starting bonus to relocate to Texas after finishing her residency in internal medicine in the Detroit area in 2007. The accompanying relocation expenses helped Dr. Pham begin her career near her family in Austin, and the flexible work schedule she negotiated allowed her to spend time with her family.
Another hospitalist willing to relocate for the right job used the relocation budget to his advantage. Moving to take the right job cost $2,000. The employer had budgeted $5,000 for relocation expenses, and the physician was able to arrange to have the $3,000 difference added to his signing bonus.
Yet another physician, already established as a hospitalist in an underserved area of his state, was attracted to a new position. However, his original acceptance of a loan repayment from the state as an inducement to work in the underserved region precluded his applying for the new position. His arrangement with the state did not prevent his approaching the new hospital to see what might be possible. It was a wise move; the new hospital agreed to increase the hospitalist’s signing bonus by enough to reimburse the state for the loan repayment.
One foreign-born physician secured a commitment from his recruiter that his employer would sponsor him and his family for green cards. Another candidate agreed to a reduced starting salary in return for help in securing a visa.
With a projected need for 30,000 hospitalists by 2010, hospitalists find themselves in the driver’s seat when it comes to weighing offers. Incentives are increasingly enticing as hospitalist recruiters nationwide struggle to lure top talent.
Incentives, Perks
What does this mean in practical terms? It means not only rising salaries but also incentives and extra perks to attract candidates to this fast-growing specialty.
Financial benefits are widespread, including signing and performance bonuses. Many hospitalists can plan on a guaranteed income. Employers may agree to pay off student loans or reimburse tuition. Malpractice insurance and tail coverage are commonly covered. Some employers also allow part-time or temporary employment to give a new hospitalist an opportunity to decide about the future or to accommodate a personal schedule.
“There is often a laundry list of incentives from which to choose, as well as more of a cafeteria plan that a doctor and employer can customize to meet specific needs,” according to Mark Dotson, MD, senior director of recruitment at Brentwood, Tenn.-based Cogent Healthcare. Cogent is a recruiting firm dedicated to building and managing hospitalist programs.
By far, the most appealing incentives are flexibility of scheduling and workload that allow physicians to coordinate their work schedule with their lifestyle. In fact, Dan Polk, MD, chief of the division of hospital-based medicine at Children’s Memorial Hospital in Chicago, considers flexible scheduling the basis of his plan to retain staff and build job satisfaction.
“We support lifestyle choices and respect life situations,” Dr. Polk says. “We foster the idea of joining a great team, and we make the environment attractive enough to encourage people to stay. We try to work within the team to cover those who need help, such as maternity or family leave, and we compensate for extra time at a different rate. We embrace people who want to work part time or share a job. Our goals are to support people and to make them want to get up in the morning to come to work.”
Part of the attractiveness of schedule flexibility is fewer weekend and night hours. In addition, employers may allow hospitalists to limit their caseload. Some hospitalists, for example, request a cap of 15 to 18 patients a day.
While retaining experienced, motivated staff is a goal of hospitals, lower caseloads mean “more doctors to do the work if doctors work fewer hours,” says Rusty Holman, MD, Cogent’s chief operating officer and SHM’s immediate past president. To meet that need, hospitals are turning to community-based physicians, fellows, and residents to work weekends and evenings. This, in turn, offers the perk of part-time work for those who want more personal time in their schedule.
The demand for nocturnists also is growing (The Hospitalist, January 2008, p. 22). Nocturnists work at night and on weekends and usually work shorter hours. These physicians prefer this schedule so they can have their days free for family or other pursuits. They also enjoy higher compensation, fewer workdays per month, and lower productivity expectations.
In addition to the having the options of part-time hours, temporary work, or job sharing, hospitalists also can negotiate other schedule perks. Some request and receive a two-week-on, two-week-off schedule. Many ask about the shift model, which demands nothing beyond the full eight or 12 hours of work. Still other applicants find a swing shift fits their lifestyle. There are even short-term choices: the hospitalist program at the University of California at Irvine offers recent residents the opportunity to work for one year while deciding about their career. With scheduling choices as part of an incentive package, many hospitalists achieve Dr. Polk’s goal of being eager to come to work each morning.
Physicians are not the only beneficiaries of these perks. Cogent, for example, recruits physician assistants and nurse practitioners when forming hospitalist groups. These employees also enjoy incentives, including tuition for continuing education and the same schedule flexibility as hospitalists.
Seller’s Market
Hospital medicine faces a shortage of qualified applicants. The need for hospitalists far surpasses the supply of physicians, who are in the enviable position of sifting through incentives and perks when selecting a hospitalist job.
This has become a national concern, according to Vikas Parekh, MD, assistant director of the hospitalist program and assistant professor of medicine at the University of Michigan Health Center in Ann Arbor. “We’re not seeing a pool of applicants because top residents are not pursuing hospitalist careers,” Dr. Parekh says.
Alpesh Amin, MD, MBA, a member of SHM’s Board of Directors, concurs but also points out that the number of hospitalist jobs is growing. “The need for a few hundred hospitalists 10 years ago has grown to 20,000 to 30,000 today, thus creating a need much greater than the supply,” says Dr. Amin, professor and chief, general internal medicine, executive director and founder of the hospitalist program at the University of California Irvine.
Why the shortage? First, fewer physicians are choosing to practice general medicine, either as an internist, family practitioner, or hospitalist. A recent study found fewer medical students were planning to concentrate on internal and family medicine, and that those who did planned to go into a subspecialty later.1 Dr. Parekh attributes this to a combination of reasons. “Most internal medicine residents are subspecialty oriented and may have decided their specialty early on,” Dr. Parekh says. “They may choose a subspecialty for financial reasons or prestige,” he continues, “but they may also be unclear about what a hospitalist career really is.”
Second, hospitalist programs have begun to expand from large metropolitan regions to smaller and rural areas. The result is an even greater demand for hospitalists.
Meet the Need
“There are no saturated markets within hospital medicine,” Dr. Holman says. “That is, most groups are always actively recruiting. [Cogent develops] full hospitalist programs, including recruiting, employing, managing, and training for new and existing hospitalist groups.”
Who is being recruited? Many recruiters approach residents who have not chosen a subspecialty to offer a staff position after they finish the residency. Although a recruiting firm may not offer financial aid during the residency, an employer may provide some sort of stipend if the candidate commits to remain on staff for a specified time after residency. “Recruit and retain” is the operative phrase in these cases.
Recruiters also are approaching generalists just entering the market to point out the advantages of avoiding the startup costs of establishing an outpatient practice. Further, many hospitalists are emerging from the ranks of solo practitioners interested in the financial and personal advantages of belonging to hospitalist groups. Not only does that eliminate the practice overhead (including the burden of regulatory compliance), but it also may offer additional administrative and academic opportunities. As Dr. Amin says, “There are more MD-MBA combos out there.”
Are incentives the answer to the shortage? Perhaps for now. With time, hospital medicine’s built-in perks may end the shortage and the need for added incentives. TH
Ann Kepler is a medical writer based in Chicago.
Reference
- Croasdale M. Primary care doctors in demand; signing bonuses and higher pay for some. American Medical News. June 19, 2006. Available at www.ama-assn.org/amednews/site/free/prl10619.htm. Last accessed March 19, 2008.
Perioperative beta-blockers in noncardiac surgery: Evolution of the evidence
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
KEY POINTS
- Beta-blockers reduce perioperative ischemia, but the benefit may be only in high-risk patients undergoing high-risk surgery. Currently, the best evidence supports their use in two groups: patients undergoing vascular surgery who have known ischemic heart disease or multiple risk factors for it, and patients who are already on beta-blockers.
- The Perioperative Ischemic Evaluation (POISE) findings suggest that beta-blockers should be used in the immediate preoperative period only with great caution, after ensuring that the patient is clinically stable and without evidence of infection, hypovolemia, anemia, or other conditions that could make heart-rate titration misleading or use of the drug dangerous.
- When feasible, beta-blockers should be started a month before surgery, titrated to a heart rate of 60 beats per minute, and continued for approximately a month. If the drug is then to be discontinued, it should be tapered slowly.
Given the ENHANCE trial results, ezetimibe is still unproven
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
Is ezetimibe/simvastatin no better than simvastatin alone? Lessons learned and clinical implications
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
What's Eating You? Io Moth (Automeris io)
How to avoid injury to bowel during laparoscopy
The author reports no financial relationships relevant to this article.
CASE Postoperative abdominal pain. Is it gastroenteritis?
R.B., 35 years old, undergoes laparoscopic adhesiolysis for abdominal pain. Previously, she underwent exploratory laparotomy for a ruptured tubal pregnancy and, in separate operations, right oophorectomy via laparotomy for a ruptured corpus luteum cyst and diagnostic laparoscopy.
During the current surgery, extensive adhesions are observed, including interloop intestinal adhesions. The adhesions are lysed using monopolar scissors and a needle electrode, and R.B. is discharged home the same day.
Later that day and the next day, R.B. complains of abdominal pain that does not respond to prescribed analgesics, as well as nausea and vomiting. A nurse practitioner takes her call and prescribes a stronger analgesic, an antiemetic, and an antibiotic.
The following day, the patient’s husband telephones the treating gynecologist to report that his wife is still experiencing severe pain and nausea. He is told to bring her to the office, where she is described as having mild lower abdominal tenderness and mild rebound. An abdominal radiograph shows air-fluid levels and distended bowel. The gynecologist determines that the patient is experiencing gastroenteritis.
On postop day 3, R.B. continues to suffer from severe abdominal pain, nausea, and vomiting, and is unable to get out of bed. Her husband takes her to the emergency room at another hospital, where she is found to have diffuse peritonitis, absent bowel sounds, and:
- temperature, 101.8°F
- heart rate, 130/min
- respiratory rate, 24/min
- blood pressure, 90/60 mm Hg
- white blood cell (WBC) count, 21.5 × 103/μL
- x-ray showing free air.
A general surgeon performs an exploratory laparotomy and finds foul-smelling abdominal fluid, 200 to 300 mL of pus, and a 1-cm perforation of the sigmoid colon. He performs sigmoid colon resection and a left-colon colostomy. A second laparotomy is necessary to drain a subphrenic abscess.
Four months later, the colostomy is taken down and bowel continuity is established.
Subsequently, the patient experiences episodes of gaseous and fecal incontinence, which are thought to be secondary to nerve damage. A ventral hernia is also diagnosed.
Could this outcome have been avoided?
No physician would wish a major complication of surgery upon any patient. Yet, sometimes, preventive efforts fall short of the goal or the physician is slow to suspect injury when the patient experiences postoperative abdominal pain and other symptoms. Intestinal injury may not be common during laparoscopy, but it is certainly not rare. And the longer diagnosis is delayed, the greater the risk of sepsis, even death.
Recognizing the limitations of laparoscopic surgery is a first step toward reducing the complication rate.1,2 The ability to determine when laparotomy would better serve the patient’s interests is also critical, and prompt diagnosis and repair of any complication that does occur will ensure and speed the patient’s recovery.
The most serious complications associated with diagnostic and operative laparoscopy are major vessel and intestinal injuries. Both types of injury significantly raise the risk of mortality, which ranges from 2% to 23%.3,4 The overall risk of injury to the gastrointestinal tract averages 1.6 to 2.0 for every 1,000 cases. The risk of major vessel injury averages 0.5 for every 1,000 cases.5-9
In an earlier article for OBG Management, I reviewed vascular injury during laparoscopy.10 In Part 1 of this article, I focus on ways to avoid intestinal injury.In Part 2 , I outline strategies to identify it in a timely manner when it does occur.
- Avoid laparoscopy when severe adhesions are anticipated—such as when the patient has a history of multiple laparotomies, or when significant adhesions have been documented.
- Be aware that laparoscopy carries additional risks beyond those of the primary surgical procedure, owing to factors peculiar to endoscopic technique and instrumentation.
- Consider open laparoscopy or insert the primary trocar at an alternative location, such as the left upper quadrant, when the patient has a history of laparotomy.
- Avoid blunt dissection for anything other than mild (filmy) adhesions. Sharp dissection associated with hydrodissection is the safest method of adhesiolysis. Clear visualization of the operative site is the sine qua non for precise dissection.
- Avoid monopolar electrosurgical devices for laparoscopic surgery whenever possible. Also remember that bipolar and ultrasonic devices can cause thermal injury by heat conduction as well as by direct application. Laser energy will continue beyond the target unless provision is made to absorb the residual energy.
- At the conclusion of any laparoscopic procedure, especially after adhesiolysis or bowel dissection, inspect the intestines and include the details in the operative report.
- After any laparoscopic procedure, if the patient does not improve steadily, the first presumptive diagnosis to be excluded is injury secondary to the procedure or technique.
- The major symptom of intestinal perforation is abdominal pain, which does not ease without increasing quantities of analgesics.
- Investigate any bowel injury thoroughly to determine viability at the site of injury. Whenever possible, repair all injuries intraoperatively.
- After intestinal perforation, the risk of sepsis is high. Look for early signs such as tachycardia, subnormal body temperature, depressed WBC count, and the appearance of immature white cell elements.
A thorough familiarity with pelvic anatomy is important to avoid injury at trocar entry, but it is even more critical in regard to operative injury. The small intestine spreads diffusely throughout the abdomen beneath the anterior abdominal wall. It lies beneath the umbilicus and anterior midline, whereas the large bowel is located at the periphery. The sigmoid colon swings left to right before joining the rectum anterior to the presacral space. The sigmoid junction with the descending colon lies well to the left of the midline, and the cecum lies at the pelvic brim to the right of midline.
In some women, the intestines droop into the pelvis and cover the adnexa, making adhesions between these structures highly likely following dissection in the vicinity of the tubes and ovaries.
Depending on the degree of redundancy of the mesentery of the cecum or sigmoid colon, these structures may droop into the pelvis and cover the adnexa. Therefore, adhesions are likely to develop between the large or small intestine, or both, and the adnexa following dissection in the vicinity of or immediately over the tubes and ovaries. Knowing the normal anatomic relationships is vital for restorative surgery.
When severe adhesions involve the large intestine, it is critical to know the anatomy of the retroperitoneum and be skilled enough to gain safe entry and to dissect that space to safely separate the adnexa when they are densely adhered to the pelvic sidewall in the area of the obturator fossa.
As laparoscopy evolves, the injury rate rises
Over the past 40 years, laparoscopy has evolved from an uncommonly utilized diagnostic tool to a minimally invasive alternative to laparotomy for even the most difficult and complex operations, reaching a high point with robotic laparoscopy. As this technology has developed, serious complications—to some degree, unique to laparoscopy—have increased. In the future, as less skilled surgeons perform a greater percentage of laparoscopic surgeries, a still greater number of complications will arise.
The frequency of intestinal perforation is not great relative to the total number of laparoscopic procedures performed. The TABLE lists several series totaling more than 380,000 laparoscopic operations. The risk of reported bowel perforation ranged from 0.6 to 6 for every 1,000 procedures, with a mean risk of 2.4 for every 1,000. However, these data are inconclusive because the total number of laparoscopic operations performed in the United States is not accurately known. Nor is the precise number of complications associated with these procedures known—specifically, the number of intestinal perforations—as no law requires them to be reported.
Research surveys are unreliable in many cases. In addition, the relative expertise of the surgeon is impossible to quantify. For example, although a surgeon may have many years of operative experience, it is unclear whether this always translates into skill or comfort with laparoscopic procedures. And, when a resident scrubs in with a faculty surgeon, any data collected fail to reflect which part of the surgery was performed by the resident and which by the fully trained gynecologist.
These unknown variables are important in terms of risk, surgical complications, and outcomes. Surgical skill is the greatest unknown factor in any outcome study of any surgical procedure.
TABLE
Studies of complications reveal: Gastrointestinal injury is no rare event during laparoscopic surgery
| Study (year; country) | Cases | Complications | Deaths | GI injury |
|---|---|---|---|---|
| Brown et al (1978; UK)16 | 50,247 | 345 | 4 | 117 (2.3/1,000) |
| Soderstrom (1993; US)17 | No data | No data | 3 | 66 |
| Bateman et al (1996; US)18 | 1,162 | No data | No data | 3 (2.6/1,000)* |
| Champault et al (1996; France)15 | 103,852 | 337 | 6 | 63 (0.6/1,000)† |
| Saidi et al (1996; US)19 | 452 | 47 | 0 | 0 |
| Jansen et al (1997; Netherlands)5 | 25,764 | 145 | 2 | 29 (1.13/1,000) |
| Harkki-Siren et al (1997; Finland)8 | 70,607 | 96 | 0 | 44 (0.6/1,000) |
| Harkki-Siren et al (1997; Finland)7 | 1,165 | 119 | 0 | 5 (4/1,000)‡ |
| Chapron et al (1998; France)6 | 29,996 | 96 | 1 | 48 (1.6/1,000) |
| Chapron et al (1999; France)9 | No data | No data | No data | 62 (0.6–1.6/1,000) |
| Gordts et al (2001; France)20 | 3,667 | No data | No data | 24 (6/1,000) |
| Bhoyrul et al (2001; US)13 | No data | 629 | 32 | 128§ |
| Wang et al (2001; Taiwan)21 | 6,451 | 42 | 0 | 10 (1.6/1,000) |
| Sharp et al (2002; US)14 | 185 | 84 | 2 | 24** |
| Brosens et al (2003; Belgium)22 | 85,727 | No data | No data | 195 (2.3/1,000) |
| * 80 open laparoscopy procedures; 30 closed laparoscopy procedures | ||||
| † Limited to trocar injuries | ||||
| ‡ Laparoscopic hysterectomy | ||||
| § All trocar injuries obtained through Food and Drug Administration reports | ||||
| ** Limited to optical access trocars | ||||
Classifying intestinal injuries
As in the case of major vessel injury, intestinal injury sustained during laparoscopy can be classified as either:
- Injury secondary to the approach. This category refers to entry complications associated with creation of the pneumoperitoneum and insertion of primary and secondary trocars.
- Injury secondary to the procedure or operation. This type of injury occurs as a result of manipulation with various devices during laparoscopy. The devices may include probes, forceps, scissors, or energy devices such as laser, electrosurgical, and ultrasonic instruments.
How trocar injury happens
Several studies have demonstrated that abdominal adhesions place any patient into a high-risk category for trocar injury to the intestines. Patients who have undergone multiple laparotomies, like the patient in the case that opened this article, are more likely to have severe adhesions and fall into the highest risk category for bowel perforation.11 It is impossible to predict with any degree of accuracy whether the intestine is adherent to the entry site.
Pneumoperitoneum can be protective
Creation of a pneumoperitoneum creates a cushion of gas between the intestines and the anterior abdominal wall (provided the intestines are not adherent to the abdominal wall). Manufacturers of disposable trocars with a retractable shield recommend creating an adequate pneumoperitoneum so that the “safety shield” deploys quickly and properly, unlike direct insertion, in which no gas is infused and space is insufficient for complete shield activation.
Open laparoscopy techniques, which allow the surgeon to enter the peritoneal cavity by direct vision without a sharp trocar, may diminish but not eliminate the risk of bowel injury.
What the data show
Of the 130 intestinal injuries recently reported by Baggish, 62 of 81 (77%) small bowel injuries were related to trocar insertion, as were 20 of 49 (41%) large intestinal injuries.12 In other words, 82 of 130 intestinal injuries (63%) were the direct result of trocar entry.
Bhoyrul and associates reported 629 trocar injuries, of which 182 were visceral.13 Of the 32 deaths, six were secondary to unrecognized bowel injury. Of 176 nonfatal visceral injuries, 128 (73%) involved the intestines, and 22 were unrecognized.
Optical-access and open laparoscopic systems were designed to prevent such injuries. Sharp and colleagues reported 24 intestinal injuries out of a total of 79 complications (30%) associated with optical-access trocars after reviewing data obtained from the Medical Device Reports (MDR) and Maude databases maintained by the Food and Drug Administration.14 In the Baggish series, 4.6% of injuries were associated with open laparoscopy.12
Champault and colleagues reviewed complications in a survey of 103,852 operations.15 Although they recommended use of open laparoscopy as opposed to blind insertion, they presented no data on the safety of open techniques.
How intraoperative injury happens
Operative injury of the large or small bowel often occurs during sharp or blunt dissection, performed during laparoscopy using accessory mechanical or energy devices. The latter type of device is utilized increasingly because laparoscopic knot tying and suturing are rather awkward and slow, and laparoscopic suturing to control bleeding is difficult. The size of the needle required for laparoscopic suture placement must be small enough to navigate a trocar sleeve.
Avoid blunt dissection when adhesions are present
The separation of dense adhesions between the intestines and neighboring bowel, other viscera, or abdominal wall is risky when blunt dissection is used. The tensile strength of the fibrotic connective tissue may well exceed that of the thin intestinal wall. Tearing the adhesion free may bring with it a portion of the bowel wall. Such injuries are frequently missed or described as serosal injuries and left unexplored and unrepaired.
Hydrodissection is a safer alternative. It involves the infiltration of sterile water or saline under low pressure between the parietal peritoneum and underlying retroperitoneal structures, providing a safe and natural plane for dissection. In addition, when the CO2 laser is used, the liquid acts as a heat sink to absorb any penetrating laser energy.
Energy devices create thermal effects
Energy devices used to cut tissue during operative laparoscopy coagulate blood vessels in a variety of ways, but the common pathway is thermal. Many hypotheses have evolved to explain how vessels are sealed, but none has demonstrated nonthermal activity except for cryocoagulation.
The devices most commonly used for cutting and hemostasis at laparoscopy are:
- electrosurgical (both monopolar and bipolar). Bipolar electrosurgical devices have advantages over monopolar devices when it comes to high-frequency leaks, direct coupling, and capacitive coupling.
- laser (CO2, holmium:YAG, Nd:YAG, KTP-532, argon). As I mentioned, CO2 laser devices are effectively backstopped by water, especially in strategic areas such as over and around intestines, major vessels, and the ureters.
- ultrasonic (Harmonic Scalpel, ultrasonic aspirator [CUSA]).
Laser and ultrasonic devices do not require a flow of electrons to create coagulation, but do produce heat that will spread peripherally by thermal conduction from the zone of impact (target).
The extent of energy-inflicted injury cannot be predicted
Inadvertent injury with energy devices can occur directly through contact with the bowel, indirectly by heat conduction through tissue, through capacitive coupling (monopolar electrical only), and by forward scatter (laser only).
Upon direct contact with the intestine, energy devices cut into the tissue in a manner similar to mechanical scissors or a knife but produce a larger wound. The reason? The transfer of heat to areas adjacent to the primary wound produces additional necrosis. Heat conduction, capacitive coupling, high-frequency leaks, and front scatter coagulate the intestinal wall with subsequent tissue devitalization and necrosis, the extent of which depends on the power density at contact and the duration of energy applied.
It is impossible to predict the depth or area of devitalization in energy-inflicted injury by visualization of the event.
In the Baggish review of 130 intestinal injuries, the number of injuries sustained during the operative procedure was 19 involving the small intestine and 29 involving the large bowel.12 Of this subset, 44% (21 cases) were secondary to the use of energy devices, with monopolar electrosurgical instruments alone accounting for 9 (43%) of the injuries.
Even best-laid plans can go awry
Despite our best intentions and precautions, accidents do sometimes happen, and bowel injury is no exception.In Part 2 of this article, I detail steps you can take to detect injuries in as timely a manner as possible.
1. Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10 110 hysterectomies by type of approach. Hum Reprod. 2001;16:1473-1478.
2. Fuller J, Ashar BS, Corrado-Carey J. Trocar-associated injuries and fatalities: an analysis of 1399 reports to the FDA. J Minim Invasive Gynecol. 2005;12:302-307.
3. Chapron CM, Pierre F, Lacroix S, Querleu D, Lansac J, Dubuisson JB. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
4. Baggish MS. Analysis of 31 cases of major-vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
5. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicenter observational study. Br J Obstet Gynaecol. 1997;104:595-600.
6. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
7. Härkki-Sirén P, Sjöberg J, Mäkinen J, et al. Finnish National Register of Laparoscopic Hysterectomies: a review and complications of 1165 operations. Am J Obstet Gynecol. 1997;176(1 Pt. 1):118-122.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108-112.
9. Chapron C, Pierre F, Harchaoui Y, et al. Gastrointestinal injuries during gynaecological laparoscopy. Hum Reprod. 1999;14:333-337.
10. Baggish MS. Avoiding vascular injury at laparoscopy. OBG Management. 2004;16(10):70-87.
11. Smith ARB. Postoperative complications following minimal access surgery. Baillieres Clin Obstet Gynecol. 2000;14:123.-
12. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
13. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
14. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
15. Champault G, Cazacu F, Taffi nder N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367-370.
16. Brown JA, Chamberlain GVP, Jordan JA, et al. Gynaecological laparoscopy. The Report of the Working Party of the Confidential Enquiry into Gynaecological Laparoscopy. Br J Obstet Gynaecol. 1978;85:401-403.
17. Soderstrom RM. Bowel injury ligation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74-77.
18. Bateman BG, Kolp LA, Hoeger K. Complications of laparoscopy—operative and diagnostic. Fertil Steril. 1996;66:30-35.
19. Saidi MH, Vancaillie TG, White J, Sadler RK, Akright BD, Farhart SA. Complications of major operative laparoscopy. A review of 452 cases. J Reprod Med. 1996;41:471-476.
20. Gordts S, Watrelot A, Camp R, Brosens I. Risk and outcome of bowel injury during transvaginal pelvic endoscopy. Fertil Steril. 2001;76:1238-1241.
21. Wang PH, Lee WL, Yuan CC, et al. Major complications of operative and diagnostic laparoscopy for gynecologic disease. J Am Assoc Gynecol Laparosc. 2001;8:68-73.
22. Brosens I, Gordon A, Campo R, Gordts S. Bowel injury in gynecologic laparoscopy. J Am Assoc Gynecol Laparosc. 2003;10:9-13.
The author reports no financial relationships relevant to this article.
CASE Postoperative abdominal pain. Is it gastroenteritis?
R.B., 35 years old, undergoes laparoscopic adhesiolysis for abdominal pain. Previously, she underwent exploratory laparotomy for a ruptured tubal pregnancy and, in separate operations, right oophorectomy via laparotomy for a ruptured corpus luteum cyst and diagnostic laparoscopy.
During the current surgery, extensive adhesions are observed, including interloop intestinal adhesions. The adhesions are lysed using monopolar scissors and a needle electrode, and R.B. is discharged home the same day.
Later that day and the next day, R.B. complains of abdominal pain that does not respond to prescribed analgesics, as well as nausea and vomiting. A nurse practitioner takes her call and prescribes a stronger analgesic, an antiemetic, and an antibiotic.
The following day, the patient’s husband telephones the treating gynecologist to report that his wife is still experiencing severe pain and nausea. He is told to bring her to the office, where she is described as having mild lower abdominal tenderness and mild rebound. An abdominal radiograph shows air-fluid levels and distended bowel. The gynecologist determines that the patient is experiencing gastroenteritis.
On postop day 3, R.B. continues to suffer from severe abdominal pain, nausea, and vomiting, and is unable to get out of bed. Her husband takes her to the emergency room at another hospital, where she is found to have diffuse peritonitis, absent bowel sounds, and:
- temperature, 101.8°F
- heart rate, 130/min
- respiratory rate, 24/min
- blood pressure, 90/60 mm Hg
- white blood cell (WBC) count, 21.5 × 103/μL
- x-ray showing free air.
A general surgeon performs an exploratory laparotomy and finds foul-smelling abdominal fluid, 200 to 300 mL of pus, and a 1-cm perforation of the sigmoid colon. He performs sigmoid colon resection and a left-colon colostomy. A second laparotomy is necessary to drain a subphrenic abscess.
Four months later, the colostomy is taken down and bowel continuity is established.
Subsequently, the patient experiences episodes of gaseous and fecal incontinence, which are thought to be secondary to nerve damage. A ventral hernia is also diagnosed.
Could this outcome have been avoided?
No physician would wish a major complication of surgery upon any patient. Yet, sometimes, preventive efforts fall short of the goal or the physician is slow to suspect injury when the patient experiences postoperative abdominal pain and other symptoms. Intestinal injury may not be common during laparoscopy, but it is certainly not rare. And the longer diagnosis is delayed, the greater the risk of sepsis, even death.
Recognizing the limitations of laparoscopic surgery is a first step toward reducing the complication rate.1,2 The ability to determine when laparotomy would better serve the patient’s interests is also critical, and prompt diagnosis and repair of any complication that does occur will ensure and speed the patient’s recovery.
The most serious complications associated with diagnostic and operative laparoscopy are major vessel and intestinal injuries. Both types of injury significantly raise the risk of mortality, which ranges from 2% to 23%.3,4 The overall risk of injury to the gastrointestinal tract averages 1.6 to 2.0 for every 1,000 cases. The risk of major vessel injury averages 0.5 for every 1,000 cases.5-9
In an earlier article for OBG Management, I reviewed vascular injury during laparoscopy.10 In Part 1 of this article, I focus on ways to avoid intestinal injury.In Part 2 , I outline strategies to identify it in a timely manner when it does occur.
- Avoid laparoscopy when severe adhesions are anticipated—such as when the patient has a history of multiple laparotomies, or when significant adhesions have been documented.
- Be aware that laparoscopy carries additional risks beyond those of the primary surgical procedure, owing to factors peculiar to endoscopic technique and instrumentation.
- Consider open laparoscopy or insert the primary trocar at an alternative location, such as the left upper quadrant, when the patient has a history of laparotomy.
- Avoid blunt dissection for anything other than mild (filmy) adhesions. Sharp dissection associated with hydrodissection is the safest method of adhesiolysis. Clear visualization of the operative site is the sine qua non for precise dissection.
- Avoid monopolar electrosurgical devices for laparoscopic surgery whenever possible. Also remember that bipolar and ultrasonic devices can cause thermal injury by heat conduction as well as by direct application. Laser energy will continue beyond the target unless provision is made to absorb the residual energy.
- At the conclusion of any laparoscopic procedure, especially after adhesiolysis or bowel dissection, inspect the intestines and include the details in the operative report.
- After any laparoscopic procedure, if the patient does not improve steadily, the first presumptive diagnosis to be excluded is injury secondary to the procedure or technique.
- The major symptom of intestinal perforation is abdominal pain, which does not ease without increasing quantities of analgesics.
- Investigate any bowel injury thoroughly to determine viability at the site of injury. Whenever possible, repair all injuries intraoperatively.
- After intestinal perforation, the risk of sepsis is high. Look for early signs such as tachycardia, subnormal body temperature, depressed WBC count, and the appearance of immature white cell elements.
A thorough familiarity with pelvic anatomy is important to avoid injury at trocar entry, but it is even more critical in regard to operative injury. The small intestine spreads diffusely throughout the abdomen beneath the anterior abdominal wall. It lies beneath the umbilicus and anterior midline, whereas the large bowel is located at the periphery. The sigmoid colon swings left to right before joining the rectum anterior to the presacral space. The sigmoid junction with the descending colon lies well to the left of the midline, and the cecum lies at the pelvic brim to the right of midline.
In some women, the intestines droop into the pelvis and cover the adnexa, making adhesions between these structures highly likely following dissection in the vicinity of the tubes and ovaries.
Depending on the degree of redundancy of the mesentery of the cecum or sigmoid colon, these structures may droop into the pelvis and cover the adnexa. Therefore, adhesions are likely to develop between the large or small intestine, or both, and the adnexa following dissection in the vicinity of or immediately over the tubes and ovaries. Knowing the normal anatomic relationships is vital for restorative surgery.
When severe adhesions involve the large intestine, it is critical to know the anatomy of the retroperitoneum and be skilled enough to gain safe entry and to dissect that space to safely separate the adnexa when they are densely adhered to the pelvic sidewall in the area of the obturator fossa.
As laparoscopy evolves, the injury rate rises
Over the past 40 years, laparoscopy has evolved from an uncommonly utilized diagnostic tool to a minimally invasive alternative to laparotomy for even the most difficult and complex operations, reaching a high point with robotic laparoscopy. As this technology has developed, serious complications—to some degree, unique to laparoscopy—have increased. In the future, as less skilled surgeons perform a greater percentage of laparoscopic surgeries, a still greater number of complications will arise.
The frequency of intestinal perforation is not great relative to the total number of laparoscopic procedures performed. The TABLE lists several series totaling more than 380,000 laparoscopic operations. The risk of reported bowel perforation ranged from 0.6 to 6 for every 1,000 procedures, with a mean risk of 2.4 for every 1,000. However, these data are inconclusive because the total number of laparoscopic operations performed in the United States is not accurately known. Nor is the precise number of complications associated with these procedures known—specifically, the number of intestinal perforations—as no law requires them to be reported.
Research surveys are unreliable in many cases. In addition, the relative expertise of the surgeon is impossible to quantify. For example, although a surgeon may have many years of operative experience, it is unclear whether this always translates into skill or comfort with laparoscopic procedures. And, when a resident scrubs in with a faculty surgeon, any data collected fail to reflect which part of the surgery was performed by the resident and which by the fully trained gynecologist.
These unknown variables are important in terms of risk, surgical complications, and outcomes. Surgical skill is the greatest unknown factor in any outcome study of any surgical procedure.
TABLE
Studies of complications reveal: Gastrointestinal injury is no rare event during laparoscopic surgery
| Study (year; country) | Cases | Complications | Deaths | GI injury |
|---|---|---|---|---|
| Brown et al (1978; UK)16 | 50,247 | 345 | 4 | 117 (2.3/1,000) |
| Soderstrom (1993; US)17 | No data | No data | 3 | 66 |
| Bateman et al (1996; US)18 | 1,162 | No data | No data | 3 (2.6/1,000)* |
| Champault et al (1996; France)15 | 103,852 | 337 | 6 | 63 (0.6/1,000)† |
| Saidi et al (1996; US)19 | 452 | 47 | 0 | 0 |
| Jansen et al (1997; Netherlands)5 | 25,764 | 145 | 2 | 29 (1.13/1,000) |
| Harkki-Siren et al (1997; Finland)8 | 70,607 | 96 | 0 | 44 (0.6/1,000) |
| Harkki-Siren et al (1997; Finland)7 | 1,165 | 119 | 0 | 5 (4/1,000)‡ |
| Chapron et al (1998; France)6 | 29,996 | 96 | 1 | 48 (1.6/1,000) |
| Chapron et al (1999; France)9 | No data | No data | No data | 62 (0.6–1.6/1,000) |
| Gordts et al (2001; France)20 | 3,667 | No data | No data | 24 (6/1,000) |
| Bhoyrul et al (2001; US)13 | No data | 629 | 32 | 128§ |
| Wang et al (2001; Taiwan)21 | 6,451 | 42 | 0 | 10 (1.6/1,000) |
| Sharp et al (2002; US)14 | 185 | 84 | 2 | 24** |
| Brosens et al (2003; Belgium)22 | 85,727 | No data | No data | 195 (2.3/1,000) |
| * 80 open laparoscopy procedures; 30 closed laparoscopy procedures | ||||
| † Limited to trocar injuries | ||||
| ‡ Laparoscopic hysterectomy | ||||
| § All trocar injuries obtained through Food and Drug Administration reports | ||||
| ** Limited to optical access trocars | ||||
Classifying intestinal injuries
As in the case of major vessel injury, intestinal injury sustained during laparoscopy can be classified as either:
- Injury secondary to the approach. This category refers to entry complications associated with creation of the pneumoperitoneum and insertion of primary and secondary trocars.
- Injury secondary to the procedure or operation. This type of injury occurs as a result of manipulation with various devices during laparoscopy. The devices may include probes, forceps, scissors, or energy devices such as laser, electrosurgical, and ultrasonic instruments.
How trocar injury happens
Several studies have demonstrated that abdominal adhesions place any patient into a high-risk category for trocar injury to the intestines. Patients who have undergone multiple laparotomies, like the patient in the case that opened this article, are more likely to have severe adhesions and fall into the highest risk category for bowel perforation.11 It is impossible to predict with any degree of accuracy whether the intestine is adherent to the entry site.
Pneumoperitoneum can be protective
Creation of a pneumoperitoneum creates a cushion of gas between the intestines and the anterior abdominal wall (provided the intestines are not adherent to the abdominal wall). Manufacturers of disposable trocars with a retractable shield recommend creating an adequate pneumoperitoneum so that the “safety shield” deploys quickly and properly, unlike direct insertion, in which no gas is infused and space is insufficient for complete shield activation.
Open laparoscopy techniques, which allow the surgeon to enter the peritoneal cavity by direct vision without a sharp trocar, may diminish but not eliminate the risk of bowel injury.
What the data show
Of the 130 intestinal injuries recently reported by Baggish, 62 of 81 (77%) small bowel injuries were related to trocar insertion, as were 20 of 49 (41%) large intestinal injuries.12 In other words, 82 of 130 intestinal injuries (63%) were the direct result of trocar entry.
Bhoyrul and associates reported 629 trocar injuries, of which 182 were visceral.13 Of the 32 deaths, six were secondary to unrecognized bowel injury. Of 176 nonfatal visceral injuries, 128 (73%) involved the intestines, and 22 were unrecognized.
Optical-access and open laparoscopic systems were designed to prevent such injuries. Sharp and colleagues reported 24 intestinal injuries out of a total of 79 complications (30%) associated with optical-access trocars after reviewing data obtained from the Medical Device Reports (MDR) and Maude databases maintained by the Food and Drug Administration.14 In the Baggish series, 4.6% of injuries were associated with open laparoscopy.12
Champault and colleagues reviewed complications in a survey of 103,852 operations.15 Although they recommended use of open laparoscopy as opposed to blind insertion, they presented no data on the safety of open techniques.
How intraoperative injury happens
Operative injury of the large or small bowel often occurs during sharp or blunt dissection, performed during laparoscopy using accessory mechanical or energy devices. The latter type of device is utilized increasingly because laparoscopic knot tying and suturing are rather awkward and slow, and laparoscopic suturing to control bleeding is difficult. The size of the needle required for laparoscopic suture placement must be small enough to navigate a trocar sleeve.
Avoid blunt dissection when adhesions are present
The separation of dense adhesions between the intestines and neighboring bowel, other viscera, or abdominal wall is risky when blunt dissection is used. The tensile strength of the fibrotic connective tissue may well exceed that of the thin intestinal wall. Tearing the adhesion free may bring with it a portion of the bowel wall. Such injuries are frequently missed or described as serosal injuries and left unexplored and unrepaired.
Hydrodissection is a safer alternative. It involves the infiltration of sterile water or saline under low pressure between the parietal peritoneum and underlying retroperitoneal structures, providing a safe and natural plane for dissection. In addition, when the CO2 laser is used, the liquid acts as a heat sink to absorb any penetrating laser energy.
Energy devices create thermal effects
Energy devices used to cut tissue during operative laparoscopy coagulate blood vessels in a variety of ways, but the common pathway is thermal. Many hypotheses have evolved to explain how vessels are sealed, but none has demonstrated nonthermal activity except for cryocoagulation.
The devices most commonly used for cutting and hemostasis at laparoscopy are:
- electrosurgical (both monopolar and bipolar). Bipolar electrosurgical devices have advantages over monopolar devices when it comes to high-frequency leaks, direct coupling, and capacitive coupling.
- laser (CO2, holmium:YAG, Nd:YAG, KTP-532, argon). As I mentioned, CO2 laser devices are effectively backstopped by water, especially in strategic areas such as over and around intestines, major vessels, and the ureters.
- ultrasonic (Harmonic Scalpel, ultrasonic aspirator [CUSA]).
Laser and ultrasonic devices do not require a flow of electrons to create coagulation, but do produce heat that will spread peripherally by thermal conduction from the zone of impact (target).
The extent of energy-inflicted injury cannot be predicted
Inadvertent injury with energy devices can occur directly through contact with the bowel, indirectly by heat conduction through tissue, through capacitive coupling (monopolar electrical only), and by forward scatter (laser only).
Upon direct contact with the intestine, energy devices cut into the tissue in a manner similar to mechanical scissors or a knife but produce a larger wound. The reason? The transfer of heat to areas adjacent to the primary wound produces additional necrosis. Heat conduction, capacitive coupling, high-frequency leaks, and front scatter coagulate the intestinal wall with subsequent tissue devitalization and necrosis, the extent of which depends on the power density at contact and the duration of energy applied.
It is impossible to predict the depth or area of devitalization in energy-inflicted injury by visualization of the event.
In the Baggish review of 130 intestinal injuries, the number of injuries sustained during the operative procedure was 19 involving the small intestine and 29 involving the large bowel.12 Of this subset, 44% (21 cases) were secondary to the use of energy devices, with monopolar electrosurgical instruments alone accounting for 9 (43%) of the injuries.
Even best-laid plans can go awry
Despite our best intentions and precautions, accidents do sometimes happen, and bowel injury is no exception.In Part 2 of this article, I detail steps you can take to detect injuries in as timely a manner as possible.
The author reports no financial relationships relevant to this article.
CASE Postoperative abdominal pain. Is it gastroenteritis?
R.B., 35 years old, undergoes laparoscopic adhesiolysis for abdominal pain. Previously, she underwent exploratory laparotomy for a ruptured tubal pregnancy and, in separate operations, right oophorectomy via laparotomy for a ruptured corpus luteum cyst and diagnostic laparoscopy.
During the current surgery, extensive adhesions are observed, including interloop intestinal adhesions. The adhesions are lysed using monopolar scissors and a needle electrode, and R.B. is discharged home the same day.
Later that day and the next day, R.B. complains of abdominal pain that does not respond to prescribed analgesics, as well as nausea and vomiting. A nurse practitioner takes her call and prescribes a stronger analgesic, an antiemetic, and an antibiotic.
The following day, the patient’s husband telephones the treating gynecologist to report that his wife is still experiencing severe pain and nausea. He is told to bring her to the office, where she is described as having mild lower abdominal tenderness and mild rebound. An abdominal radiograph shows air-fluid levels and distended bowel. The gynecologist determines that the patient is experiencing gastroenteritis.
On postop day 3, R.B. continues to suffer from severe abdominal pain, nausea, and vomiting, and is unable to get out of bed. Her husband takes her to the emergency room at another hospital, where she is found to have diffuse peritonitis, absent bowel sounds, and:
- temperature, 101.8°F
- heart rate, 130/min
- respiratory rate, 24/min
- blood pressure, 90/60 mm Hg
- white blood cell (WBC) count, 21.5 × 103/μL
- x-ray showing free air.
A general surgeon performs an exploratory laparotomy and finds foul-smelling abdominal fluid, 200 to 300 mL of pus, and a 1-cm perforation of the sigmoid colon. He performs sigmoid colon resection and a left-colon colostomy. A second laparotomy is necessary to drain a subphrenic abscess.
Four months later, the colostomy is taken down and bowel continuity is established.
Subsequently, the patient experiences episodes of gaseous and fecal incontinence, which are thought to be secondary to nerve damage. A ventral hernia is also diagnosed.
Could this outcome have been avoided?
No physician would wish a major complication of surgery upon any patient. Yet, sometimes, preventive efforts fall short of the goal or the physician is slow to suspect injury when the patient experiences postoperative abdominal pain and other symptoms. Intestinal injury may not be common during laparoscopy, but it is certainly not rare. And the longer diagnosis is delayed, the greater the risk of sepsis, even death.
Recognizing the limitations of laparoscopic surgery is a first step toward reducing the complication rate.1,2 The ability to determine when laparotomy would better serve the patient’s interests is also critical, and prompt diagnosis and repair of any complication that does occur will ensure and speed the patient’s recovery.
The most serious complications associated with diagnostic and operative laparoscopy are major vessel and intestinal injuries. Both types of injury significantly raise the risk of mortality, which ranges from 2% to 23%.3,4 The overall risk of injury to the gastrointestinal tract averages 1.6 to 2.0 for every 1,000 cases. The risk of major vessel injury averages 0.5 for every 1,000 cases.5-9
In an earlier article for OBG Management, I reviewed vascular injury during laparoscopy.10 In Part 1 of this article, I focus on ways to avoid intestinal injury.In Part 2 , I outline strategies to identify it in a timely manner when it does occur.
- Avoid laparoscopy when severe adhesions are anticipated—such as when the patient has a history of multiple laparotomies, or when significant adhesions have been documented.
- Be aware that laparoscopy carries additional risks beyond those of the primary surgical procedure, owing to factors peculiar to endoscopic technique and instrumentation.
- Consider open laparoscopy or insert the primary trocar at an alternative location, such as the left upper quadrant, when the patient has a history of laparotomy.
- Avoid blunt dissection for anything other than mild (filmy) adhesions. Sharp dissection associated with hydrodissection is the safest method of adhesiolysis. Clear visualization of the operative site is the sine qua non for precise dissection.
- Avoid monopolar electrosurgical devices for laparoscopic surgery whenever possible. Also remember that bipolar and ultrasonic devices can cause thermal injury by heat conduction as well as by direct application. Laser energy will continue beyond the target unless provision is made to absorb the residual energy.
- At the conclusion of any laparoscopic procedure, especially after adhesiolysis or bowel dissection, inspect the intestines and include the details in the operative report.
- After any laparoscopic procedure, if the patient does not improve steadily, the first presumptive diagnosis to be excluded is injury secondary to the procedure or technique.
- The major symptom of intestinal perforation is abdominal pain, which does not ease without increasing quantities of analgesics.
- Investigate any bowel injury thoroughly to determine viability at the site of injury. Whenever possible, repair all injuries intraoperatively.
- After intestinal perforation, the risk of sepsis is high. Look for early signs such as tachycardia, subnormal body temperature, depressed WBC count, and the appearance of immature white cell elements.
A thorough familiarity with pelvic anatomy is important to avoid injury at trocar entry, but it is even more critical in regard to operative injury. The small intestine spreads diffusely throughout the abdomen beneath the anterior abdominal wall. It lies beneath the umbilicus and anterior midline, whereas the large bowel is located at the periphery. The sigmoid colon swings left to right before joining the rectum anterior to the presacral space. The sigmoid junction with the descending colon lies well to the left of the midline, and the cecum lies at the pelvic brim to the right of midline.
In some women, the intestines droop into the pelvis and cover the adnexa, making adhesions between these structures highly likely following dissection in the vicinity of the tubes and ovaries.
Depending on the degree of redundancy of the mesentery of the cecum or sigmoid colon, these structures may droop into the pelvis and cover the adnexa. Therefore, adhesions are likely to develop between the large or small intestine, or both, and the adnexa following dissection in the vicinity of or immediately over the tubes and ovaries. Knowing the normal anatomic relationships is vital for restorative surgery.
When severe adhesions involve the large intestine, it is critical to know the anatomy of the retroperitoneum and be skilled enough to gain safe entry and to dissect that space to safely separate the adnexa when they are densely adhered to the pelvic sidewall in the area of the obturator fossa.
As laparoscopy evolves, the injury rate rises
Over the past 40 years, laparoscopy has evolved from an uncommonly utilized diagnostic tool to a minimally invasive alternative to laparotomy for even the most difficult and complex operations, reaching a high point with robotic laparoscopy. As this technology has developed, serious complications—to some degree, unique to laparoscopy—have increased. In the future, as less skilled surgeons perform a greater percentage of laparoscopic surgeries, a still greater number of complications will arise.
The frequency of intestinal perforation is not great relative to the total number of laparoscopic procedures performed. The TABLE lists several series totaling more than 380,000 laparoscopic operations. The risk of reported bowel perforation ranged from 0.6 to 6 for every 1,000 procedures, with a mean risk of 2.4 for every 1,000. However, these data are inconclusive because the total number of laparoscopic operations performed in the United States is not accurately known. Nor is the precise number of complications associated with these procedures known—specifically, the number of intestinal perforations—as no law requires them to be reported.
Research surveys are unreliable in many cases. In addition, the relative expertise of the surgeon is impossible to quantify. For example, although a surgeon may have many years of operative experience, it is unclear whether this always translates into skill or comfort with laparoscopic procedures. And, when a resident scrubs in with a faculty surgeon, any data collected fail to reflect which part of the surgery was performed by the resident and which by the fully trained gynecologist.
These unknown variables are important in terms of risk, surgical complications, and outcomes. Surgical skill is the greatest unknown factor in any outcome study of any surgical procedure.
TABLE
Studies of complications reveal: Gastrointestinal injury is no rare event during laparoscopic surgery
| Study (year; country) | Cases | Complications | Deaths | GI injury |
|---|---|---|---|---|
| Brown et al (1978; UK)16 | 50,247 | 345 | 4 | 117 (2.3/1,000) |
| Soderstrom (1993; US)17 | No data | No data | 3 | 66 |
| Bateman et al (1996; US)18 | 1,162 | No data | No data | 3 (2.6/1,000)* |
| Champault et al (1996; France)15 | 103,852 | 337 | 6 | 63 (0.6/1,000)† |
| Saidi et al (1996; US)19 | 452 | 47 | 0 | 0 |
| Jansen et al (1997; Netherlands)5 | 25,764 | 145 | 2 | 29 (1.13/1,000) |
| Harkki-Siren et al (1997; Finland)8 | 70,607 | 96 | 0 | 44 (0.6/1,000) |
| Harkki-Siren et al (1997; Finland)7 | 1,165 | 119 | 0 | 5 (4/1,000)‡ |
| Chapron et al (1998; France)6 | 29,996 | 96 | 1 | 48 (1.6/1,000) |
| Chapron et al (1999; France)9 | No data | No data | No data | 62 (0.6–1.6/1,000) |
| Gordts et al (2001; France)20 | 3,667 | No data | No data | 24 (6/1,000) |
| Bhoyrul et al (2001; US)13 | No data | 629 | 32 | 128§ |
| Wang et al (2001; Taiwan)21 | 6,451 | 42 | 0 | 10 (1.6/1,000) |
| Sharp et al (2002; US)14 | 185 | 84 | 2 | 24** |
| Brosens et al (2003; Belgium)22 | 85,727 | No data | No data | 195 (2.3/1,000) |
| * 80 open laparoscopy procedures; 30 closed laparoscopy procedures | ||||
| † Limited to trocar injuries | ||||
| ‡ Laparoscopic hysterectomy | ||||
| § All trocar injuries obtained through Food and Drug Administration reports | ||||
| ** Limited to optical access trocars | ||||
Classifying intestinal injuries
As in the case of major vessel injury, intestinal injury sustained during laparoscopy can be classified as either:
- Injury secondary to the approach. This category refers to entry complications associated with creation of the pneumoperitoneum and insertion of primary and secondary trocars.
- Injury secondary to the procedure or operation. This type of injury occurs as a result of manipulation with various devices during laparoscopy. The devices may include probes, forceps, scissors, or energy devices such as laser, electrosurgical, and ultrasonic instruments.
How trocar injury happens
Several studies have demonstrated that abdominal adhesions place any patient into a high-risk category for trocar injury to the intestines. Patients who have undergone multiple laparotomies, like the patient in the case that opened this article, are more likely to have severe adhesions and fall into the highest risk category for bowel perforation.11 It is impossible to predict with any degree of accuracy whether the intestine is adherent to the entry site.
Pneumoperitoneum can be protective
Creation of a pneumoperitoneum creates a cushion of gas between the intestines and the anterior abdominal wall (provided the intestines are not adherent to the abdominal wall). Manufacturers of disposable trocars with a retractable shield recommend creating an adequate pneumoperitoneum so that the “safety shield” deploys quickly and properly, unlike direct insertion, in which no gas is infused and space is insufficient for complete shield activation.
Open laparoscopy techniques, which allow the surgeon to enter the peritoneal cavity by direct vision without a sharp trocar, may diminish but not eliminate the risk of bowel injury.
What the data show
Of the 130 intestinal injuries recently reported by Baggish, 62 of 81 (77%) small bowel injuries were related to trocar insertion, as were 20 of 49 (41%) large intestinal injuries.12 In other words, 82 of 130 intestinal injuries (63%) were the direct result of trocar entry.
Bhoyrul and associates reported 629 trocar injuries, of which 182 were visceral.13 Of the 32 deaths, six were secondary to unrecognized bowel injury. Of 176 nonfatal visceral injuries, 128 (73%) involved the intestines, and 22 were unrecognized.
Optical-access and open laparoscopic systems were designed to prevent such injuries. Sharp and colleagues reported 24 intestinal injuries out of a total of 79 complications (30%) associated with optical-access trocars after reviewing data obtained from the Medical Device Reports (MDR) and Maude databases maintained by the Food and Drug Administration.14 In the Baggish series, 4.6% of injuries were associated with open laparoscopy.12
Champault and colleagues reviewed complications in a survey of 103,852 operations.15 Although they recommended use of open laparoscopy as opposed to blind insertion, they presented no data on the safety of open techniques.
How intraoperative injury happens
Operative injury of the large or small bowel often occurs during sharp or blunt dissection, performed during laparoscopy using accessory mechanical or energy devices. The latter type of device is utilized increasingly because laparoscopic knot tying and suturing are rather awkward and slow, and laparoscopic suturing to control bleeding is difficult. The size of the needle required for laparoscopic suture placement must be small enough to navigate a trocar sleeve.
Avoid blunt dissection when adhesions are present
The separation of dense adhesions between the intestines and neighboring bowel, other viscera, or abdominal wall is risky when blunt dissection is used. The tensile strength of the fibrotic connective tissue may well exceed that of the thin intestinal wall. Tearing the adhesion free may bring with it a portion of the bowel wall. Such injuries are frequently missed or described as serosal injuries and left unexplored and unrepaired.
Hydrodissection is a safer alternative. It involves the infiltration of sterile water or saline under low pressure between the parietal peritoneum and underlying retroperitoneal structures, providing a safe and natural plane for dissection. In addition, when the CO2 laser is used, the liquid acts as a heat sink to absorb any penetrating laser energy.
Energy devices create thermal effects
Energy devices used to cut tissue during operative laparoscopy coagulate blood vessels in a variety of ways, but the common pathway is thermal. Many hypotheses have evolved to explain how vessels are sealed, but none has demonstrated nonthermal activity except for cryocoagulation.
The devices most commonly used for cutting and hemostasis at laparoscopy are:
- electrosurgical (both monopolar and bipolar). Bipolar electrosurgical devices have advantages over monopolar devices when it comes to high-frequency leaks, direct coupling, and capacitive coupling.
- laser (CO2, holmium:YAG, Nd:YAG, KTP-532, argon). As I mentioned, CO2 laser devices are effectively backstopped by water, especially in strategic areas such as over and around intestines, major vessels, and the ureters.
- ultrasonic (Harmonic Scalpel, ultrasonic aspirator [CUSA]).
Laser and ultrasonic devices do not require a flow of electrons to create coagulation, but do produce heat that will spread peripherally by thermal conduction from the zone of impact (target).
The extent of energy-inflicted injury cannot be predicted
Inadvertent injury with energy devices can occur directly through contact with the bowel, indirectly by heat conduction through tissue, through capacitive coupling (monopolar electrical only), and by forward scatter (laser only).
Upon direct contact with the intestine, energy devices cut into the tissue in a manner similar to mechanical scissors or a knife but produce a larger wound. The reason? The transfer of heat to areas adjacent to the primary wound produces additional necrosis. Heat conduction, capacitive coupling, high-frequency leaks, and front scatter coagulate the intestinal wall with subsequent tissue devitalization and necrosis, the extent of which depends on the power density at contact and the duration of energy applied.
It is impossible to predict the depth or area of devitalization in energy-inflicted injury by visualization of the event.
In the Baggish review of 130 intestinal injuries, the number of injuries sustained during the operative procedure was 19 involving the small intestine and 29 involving the large bowel.12 Of this subset, 44% (21 cases) were secondary to the use of energy devices, with monopolar electrosurgical instruments alone accounting for 9 (43%) of the injuries.
Even best-laid plans can go awry
Despite our best intentions and precautions, accidents do sometimes happen, and bowel injury is no exception.In Part 2 of this article, I detail steps you can take to detect injuries in as timely a manner as possible.
1. Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10 110 hysterectomies by type of approach. Hum Reprod. 2001;16:1473-1478.
2. Fuller J, Ashar BS, Corrado-Carey J. Trocar-associated injuries and fatalities: an analysis of 1399 reports to the FDA. J Minim Invasive Gynecol. 2005;12:302-307.
3. Chapron CM, Pierre F, Lacroix S, Querleu D, Lansac J, Dubuisson JB. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
4. Baggish MS. Analysis of 31 cases of major-vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
5. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicenter observational study. Br J Obstet Gynaecol. 1997;104:595-600.
6. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
7. Härkki-Sirén P, Sjöberg J, Mäkinen J, et al. Finnish National Register of Laparoscopic Hysterectomies: a review and complications of 1165 operations. Am J Obstet Gynecol. 1997;176(1 Pt. 1):118-122.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108-112.
9. Chapron C, Pierre F, Harchaoui Y, et al. Gastrointestinal injuries during gynaecological laparoscopy. Hum Reprod. 1999;14:333-337.
10. Baggish MS. Avoiding vascular injury at laparoscopy. OBG Management. 2004;16(10):70-87.
11. Smith ARB. Postoperative complications following minimal access surgery. Baillieres Clin Obstet Gynecol. 2000;14:123.-
12. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
13. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
14. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
15. Champault G, Cazacu F, Taffi nder N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367-370.
16. Brown JA, Chamberlain GVP, Jordan JA, et al. Gynaecological laparoscopy. The Report of the Working Party of the Confidential Enquiry into Gynaecological Laparoscopy. Br J Obstet Gynaecol. 1978;85:401-403.
17. Soderstrom RM. Bowel injury ligation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74-77.
18. Bateman BG, Kolp LA, Hoeger K. Complications of laparoscopy—operative and diagnostic. Fertil Steril. 1996;66:30-35.
19. Saidi MH, Vancaillie TG, White J, Sadler RK, Akright BD, Farhart SA. Complications of major operative laparoscopy. A review of 452 cases. J Reprod Med. 1996;41:471-476.
20. Gordts S, Watrelot A, Camp R, Brosens I. Risk and outcome of bowel injury during transvaginal pelvic endoscopy. Fertil Steril. 2001;76:1238-1241.
21. Wang PH, Lee WL, Yuan CC, et al. Major complications of operative and diagnostic laparoscopy for gynecologic disease. J Am Assoc Gynecol Laparosc. 2001;8:68-73.
22. Brosens I, Gordon A, Campo R, Gordts S. Bowel injury in gynecologic laparoscopy. J Am Assoc Gynecol Laparosc. 2003;10:9-13.
1. Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10 110 hysterectomies by type of approach. Hum Reprod. 2001;16:1473-1478.
2. Fuller J, Ashar BS, Corrado-Carey J. Trocar-associated injuries and fatalities: an analysis of 1399 reports to the FDA. J Minim Invasive Gynecol. 2005;12:302-307.
3. Chapron CM, Pierre F, Lacroix S, Querleu D, Lansac J, Dubuisson JB. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
4. Baggish MS. Analysis of 31 cases of major-vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
5. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicenter observational study. Br J Obstet Gynaecol. 1997;104:595-600.
6. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
7. Härkki-Sirén P, Sjöberg J, Mäkinen J, et al. Finnish National Register of Laparoscopic Hysterectomies: a review and complications of 1165 operations. Am J Obstet Gynecol. 1997;176(1 Pt. 1):118-122.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108-112.
9. Chapron C, Pierre F, Harchaoui Y, et al. Gastrointestinal injuries during gynaecological laparoscopy. Hum Reprod. 1999;14:333-337.
10. Baggish MS. Avoiding vascular injury at laparoscopy. OBG Management. 2004;16(10):70-87.
11. Smith ARB. Postoperative complications following minimal access surgery. Baillieres Clin Obstet Gynecol. 2000;14:123.-
12. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
13. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
14. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
15. Champault G, Cazacu F, Taffi nder N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367-370.
16. Brown JA, Chamberlain GVP, Jordan JA, et al. Gynaecological laparoscopy. The Report of the Working Party of the Confidential Enquiry into Gynaecological Laparoscopy. Br J Obstet Gynaecol. 1978;85:401-403.
17. Soderstrom RM. Bowel injury ligation after laparoscopy. J Am Assoc Gynecol Laparosc. 1993;1:74-77.
18. Bateman BG, Kolp LA, Hoeger K. Complications of laparoscopy—operative and diagnostic. Fertil Steril. 1996;66:30-35.
19. Saidi MH, Vancaillie TG, White J, Sadler RK, Akright BD, Farhart SA. Complications of major operative laparoscopy. A review of 452 cases. J Reprod Med. 1996;41:471-476.
20. Gordts S, Watrelot A, Camp R, Brosens I. Risk and outcome of bowel injury during transvaginal pelvic endoscopy. Fertil Steril. 2001;76:1238-1241.
21. Wang PH, Lee WL, Yuan CC, et al. Major complications of operative and diagnostic laparoscopy for gynecologic disease. J Am Assoc Gynecol Laparosc. 2001;8:68-73.
22. Brosens I, Gordon A, Campo R, Gordts S. Bowel injury in gynecologic laparoscopy. J Am Assoc Gynecol Laparosc. 2003;10:9-13.
Lessons in timely recognition of laparoscopy-related bowel injury
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.