User login
Time for Health Education of Hospitalized Patients
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
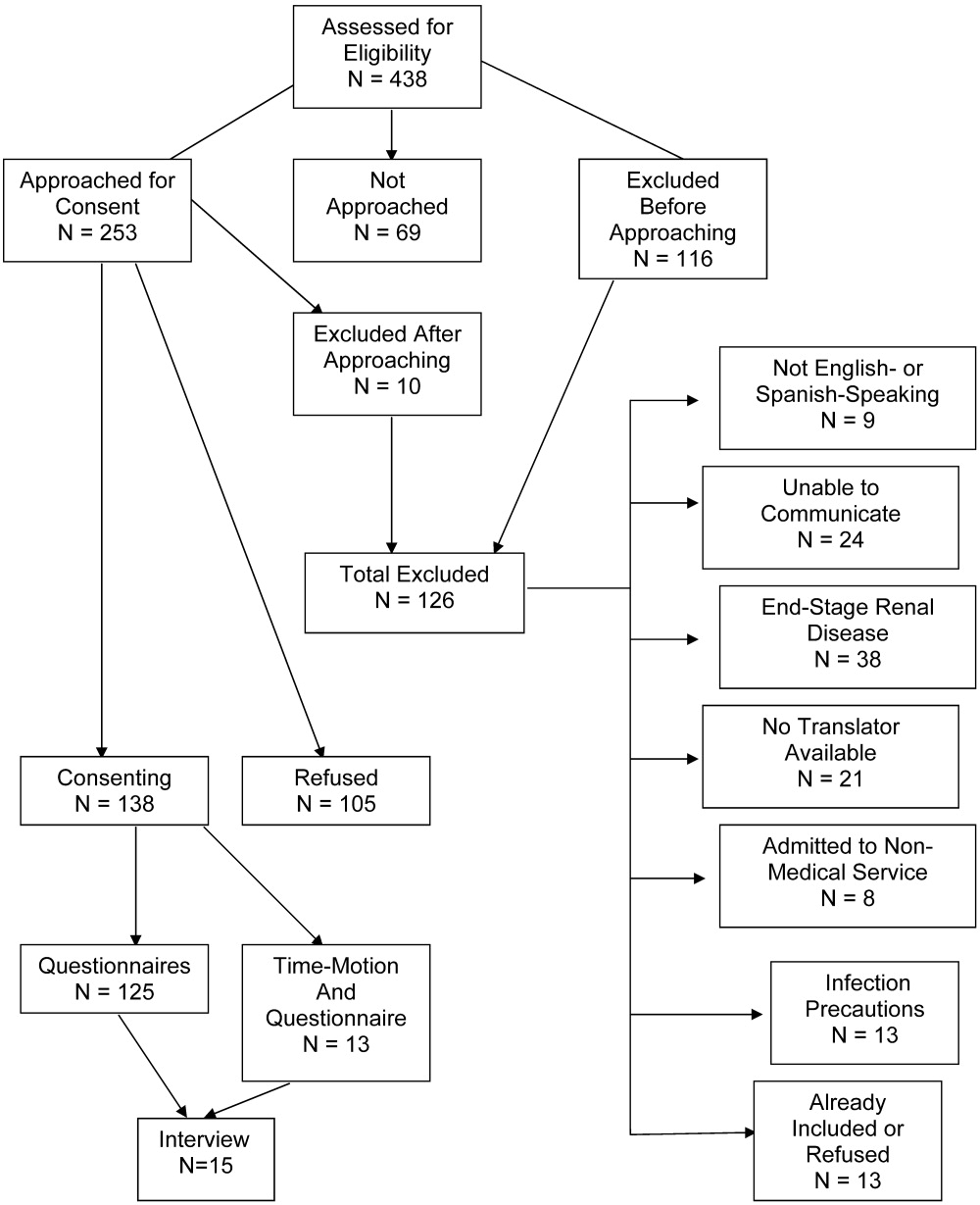
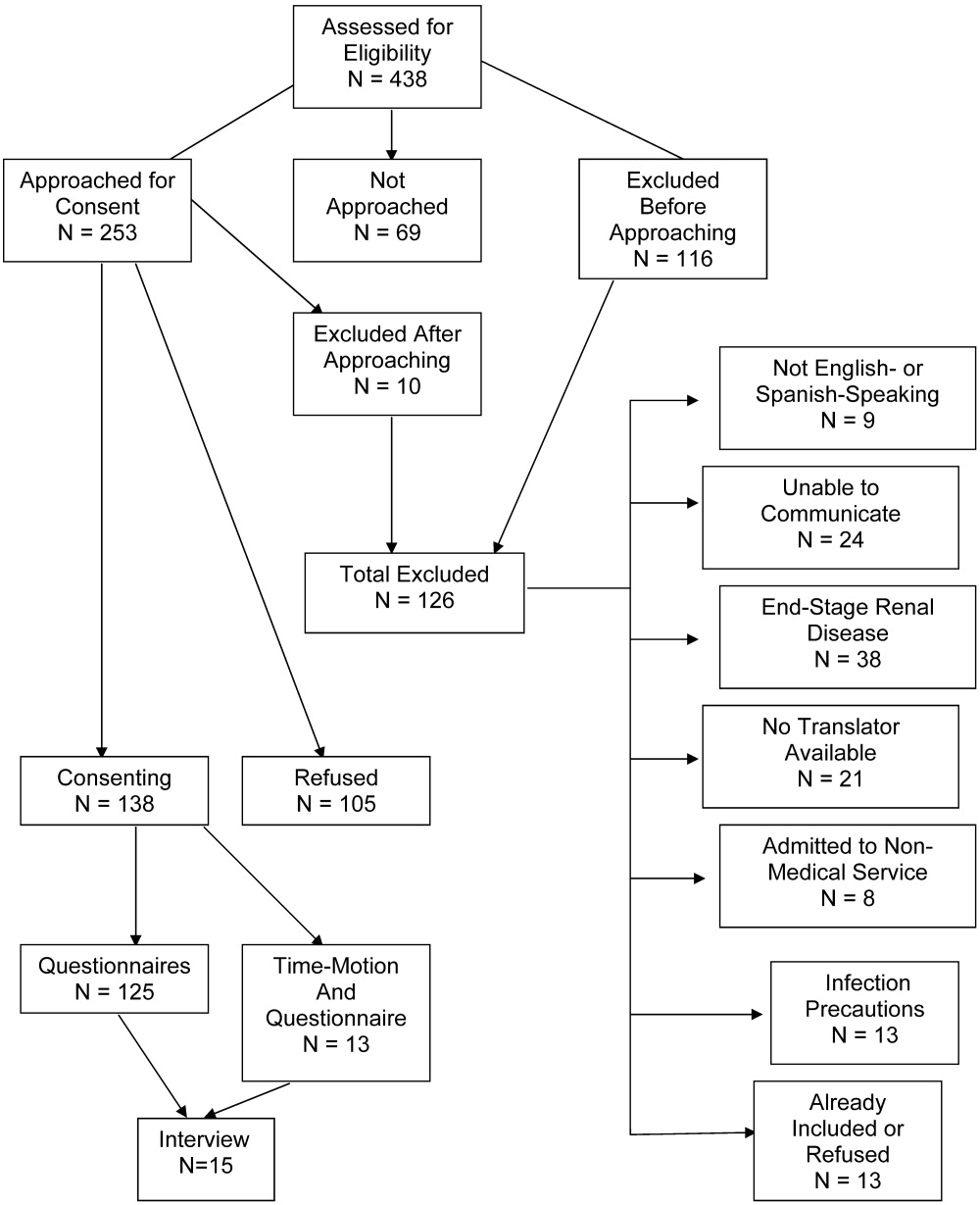
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.
| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
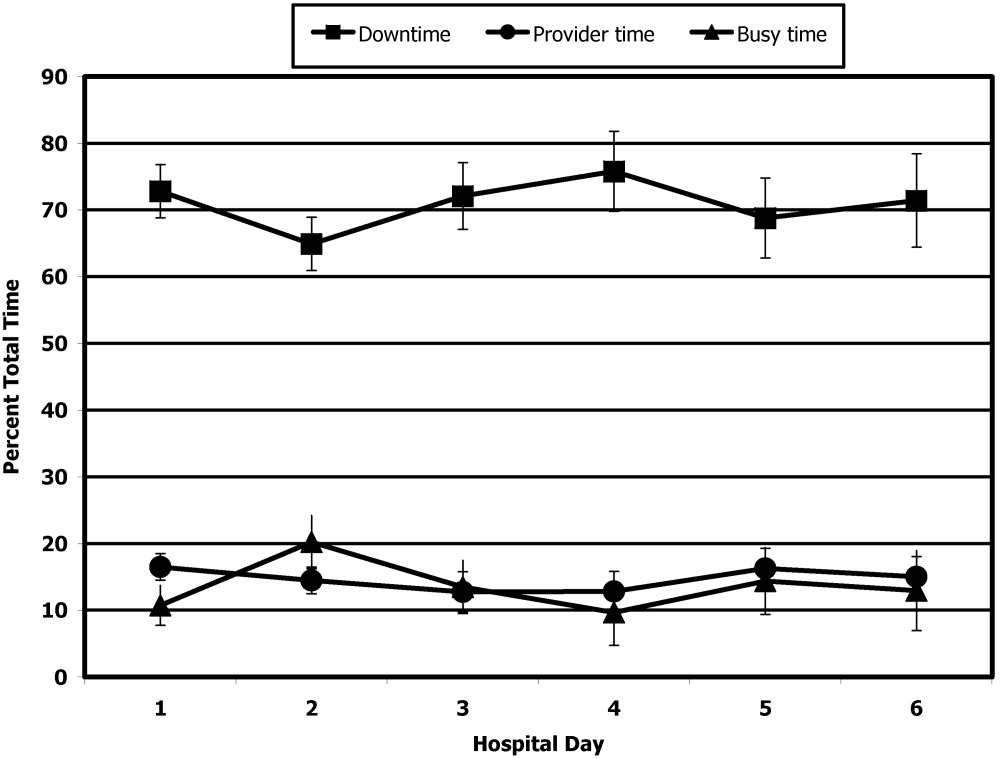
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.
Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.

| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.

Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
Educating patients about smoking cessation when they are hospitalized for acute coronary syndrome results in a 57% 1‐year quit rate.1 This rate is far higher than the typical 15%‐30% 1‐year quit rates observed with smoking cessation programs administered in the outpatient setting24 and suggests that hospitalized patients may be uniquely motivated to respond to health education.
Kerzman and colleagues5 found that 42% of hospitalized patients expressed a wish to receive more comprehensive counseling about their medications before being discharged from the hospital. And although the Joint Commission on Hospital Accreditation and the Centers for Medicare and Medicaid Services have established core quality measures mandating that patients hospitalized with congestive heart failure receive education as one component of a high‐quality discharge process,6, 7 approximately one third of patients nationally do not receive adequate patient education.8
Barber‐Parker9 suggested that because patient acuity in hospitals was so high and patients were so commonly absent from their nursing units for testing and treatment, there was little time available for health education during their hospitalization. Anecdotal observations in our institution suggested, however, that adult patients hospitalized on the general Internal Medicine service spent much of their day doing little more than lying in bed watching television. Accordingly, we hypothesized that considerable time might be available for patient education during a hospitalization. We therefore sought to quantify the fraction of time patients were not involved in treatment activities, diagnostic testing, or other evaluations and to determine whether during these times they wanted and were feeling well enough to participate in educational activities. We also sought to determine what patients wanted to know about their health problems and what types of educational activities they most preferred.
MATERIALS AND METHODS
We conducted a time‐motion and survey study from June 25, 2005, to August 15, 2005, at Denver Health Medical Center, an academic public safety‐net hospital affiliated with the University of Colorado School of Medicine. All patients older than 18 years of age who spoke English or Spanish and were admitted to the general Internal Medicine service were candidates for enrollment. Exclusion criteria were being admitted to the intensive care unit, having an inability to communicate, being in contact precautions, and being previously enrolled. The study was approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained for all study participants.
At 8:00 AM, all patients admitted during the previous 15 hours were assigned a random number from a random number table and were approached for consent in numeric order. With 2 data collectors working daily, a maximum of 12 patients could be enrolled each day. Consenting subjects who passed a vision test were given the Test of Functional Health Literacy in Adults at the time of enrollment and a written questionnaire (in either English or Spanish) on a daily basis for a maximum of 6 days. Some of these patients also participated in a structured interview that was designed to elicit their views on health education topics and formats for education of hospitalized patients. Others, again determined by random number, were subjects of a time‐motion study.
Demographic data collected included age, sex, language, race, comorbidities, insurance status, and discharge diagnosis.
Time‐Motion Study
Patients were observed from 8:00 AM to noon and from 1:00 to 5:00 PM7 days a week. Data were collected using TimerPro on a Dell Axim A5 pocket PC and imported daily into an Excel spreadsheet. Observations were categorized as downtime, busy time, or provider time and subcategorized as summarized in Table 1.
| First level | Second level | Third level |
|---|---|---|
| Downtime | Alone | TV |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone | ||
| Other | ||
| Friends/family | TV | |
| Resting | ||
| Sleeping | ||
| Reading | ||
| Telephone/talk | ||
| Other | ||
| Provider | Physician | |
| Nurse | ||
| Physician and nurse | ||
| Physician and other | ||
| Other | ||
| Busy | ADL | |
| Meal | ||
| Out of room | ||
| Other |
Questionnaire
We were unable to find a validated questionnaire in the literature that was designed to assess patient opinion or level of interest in educational activities during a hospitalization. Accordingly, we developed our own using a 5‐point Likert scale (Box 1). Two outcomes researchers with expertise in using questionnaires for clinical research independently reviewed the questionnaire to establish face validity.
Box 1. Daily Questionnaire on In‐Hospital Health Education
The following statements were read to the patients on a daily basis and answered using the following scale: 1 = Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree
I feel well enough today to learn about my illness or my health.
I want to learn more about my illness or my health today.
I have time to learn about my health today.
It is important to me to learn more about my illness or health while in the hospital.
Interview
All patients were asked the open‐ended questions listed in Box 2, and the entire interview was recorded on audiotape for subsequent analysis.
Box 2. Interview Questions with Probes for Educational Preferences
What things related to your health would you like to learn more about while you are in the hospital? (list up to three topics in order of impotance to you).
How can we help you learn more about your illness or health while in the hospital?
Who should do the teaching (eg, an MD, a nurse, a dietician, a medical student, peers, physical therapists, respiratory therapists)?
Who else should be present (eg, patients with similar illness, family, no one)?
How should this teaching be done (eg, didactic sessions, hands‐on, video tape, pre‐ and post‐testing)?
Data Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered significant.
Time‐Motion Data
Mixed‐effects general linear models (growth curve or repeated measures), using SAS Proc Mixed, were used to test whether the proportions of downtime, busy time, and provider time differed by day of hospitalization. Linear growth curve models were used to test whether a linear trend was present. If not, repeated‐measures models were used to obtain estimates by day of hospitalization.
Questionnaire Data
Mixed‐effects general linear models (growth curve or repeated measures) were used to determine whether patient responses differed by day of hospitalization, as described above.
Interview Data
Tape recordings were reviewed in depth to code participant responses to the structured questions. We utilized the template style of analysis, coding segments of the interviews and identifying illustrative quotes whenever possible. Key patterns and themes were summarized along with specific patient preferences regarding topics of interest and learning opportunities while in the hospital and after discharge.
RESULTS
Patient selection is described in Figure 1, and patient demographics are summarized in Tables 2 and 3.

| Demographic | Time‐motion | Interview | Questionnaire |
|---|---|---|---|
| |||
| Study subjects | 13 | 15 | 125 |
| Sex | |||
| Male | 6 (46) | 7 (47) | 61 (49) |
| Female | 7 (54) | 8 (53) | 64 (51) |
| Age (years), median (IQR) | 47 (20) | 51 (20.5) | 51 (18) |
| Race/ethnicity | |||
| White, non‐Hispanic | 6 (46) | 5 (33) | 46 (37) |
| Black/African American | 3 (23) | 4 (27) | 27 (22) |
| American Indian | 0 (0) | 0 (0) | 1 (1) |
| Hispanic | 4 (31) | 6 (40) | 51 (41) |
| Primary language | |||
| English | 12 (92) | 14 (93) | 109 (87) |
| Spanish | 1 (8) | 1 (7) | 16 (13) |
| Health literacy* | |||
| Adequate | 3 (75) | 9 (82) | 60 (71) |
| Marginal | 1 (25) | 0 (0) | 6 (7) |
| Inadequate | 0 (0) | 2 (18) | 18 (22) |
| Insurance status | |||
| Self‐pay | 3 (23) | 1 (7) | 24 (19) |
| Medicaid | 1 (8) | 4 (27) | 19 (15) |
| Medicare | 3 (23) | 0 (0) | 2 (2) |
| Colorado Indigent Care Program | 3 (23) | 7 (47) | 51 (41) |
| Private | 2 (15) | 1 (7) | 5 (4) |
| Other | 1 (8) | 2 (14) | 24 (18) |
| Time‐motion | Interview | Questionnaire | |
|---|---|---|---|
| Study subjects (n) | 13 | 15 | 125 |
| Discharge diagnoses (selected) | |||
| Coronary artery disease (including angina) | 1 (8) | 2 (13) | 24 (19) |
| Congestive heart failure | 1 (8) | 1 (7) | 4 (3) |
| Upper gastrointestinal bleeding, gastritis, reflux | 2 (15) | 4 (27) | 14 (11) |
| Syncope | 2 (15) | 0 | 5 (4) |
| Acute renal failure | 0 | 0 | 5 (4) |
| Pancreatitis | 0 | 1 (7) | 6 (5) |
| Venous thromboembolism | 2 (15) | 1 (7) | 3 (2) |
| Chronic obstructive pulmonary disease | 0 | 0 | 4 (3) |
| Diabetic ketoacidosis | 1 (8) | 1 (7) | 3 (2) |
| Pyelonephritis | 0 | 0 | 5 (4) |
| Pneumonia | 2 (15) | 1 (7) | 5 (4) |
| Comorbidities | |||
| Diabetes | 5 (38) | 9 (60) | 41 (33) |
| Hypertension | 1 (8) | 9 (60) | 55 (44) |
| Dyslipidemia | 6 (46) | 2 (13) | 26 (21) |
| Tobacco | 5 (38) | 7 (47) | 55 (44) |
| Chronic obstructive pulmonary disease | 2 (15) | 4 (27) | 15 (12) |
| Congestive heart failure | 2 (15) | 2 (13) | 13 (10) |
| Coronary heart disease | 3 (23) | 3 (20) | 21 (17) |
Time‐Motion Study
Thirteen patients were studied. Of the 315 patient‐hours observed, 71% were categorized as downtime, 15% as provider time, and 14% as busy time. The proportion of downtime ranged from a low of 0.65 (SE 0.04) on hospital Day 2 to a high of 0.76 (SE 0.06) on hospital Day 4, but the differences in downtime proportions by day did not reach statistical significance (P = .65; Fig. 2). The lowest percentage of downtime observed in any patient on any day was 39%. The 125 hours of downtime observed consisted of 1317 separate blocks of time, 80% of which were less than 15 minutes in duration, 14% of which were 15 to 30 minutes in duration, and 6% of which exceeded 30 minutes in duration.

Thirty‐six full days of observation, defined as greater than 7 hours of observation in 1 day, were used to assess the amount of time spent with providers. Of the 60 minutes/day (IQR = 44) that patients spent with health care providers, 21 minutes/day (IQR = 34) was spent with phlebotomists, physical or occupational therapists, dieticians, or social workers, 25 minutes/day (IQR = 25) was spent with patients' nurses, and a median of only 9 minutes/day (IQR = 11) was spent with their physicians.
Questionnaire
A total of 311 questionnaires were administered to the 138 consenting participants. Irrespective of the day of testing, 79% to 97% strongly agreed or agreed with the 4 statements (Fig. 3). In response to the first statementI feel well enough to learnpatient scores increased steadily over the 6 days of hospitalization patients were surveyed (coefficient = 0.15, P = .004). On hospital day 1, the mean score was 3.85 (SE 0.08), and by day 6 the mean score had increased to 4.75 (SE 0.08) However, there was no significant change over time in patients' desire to learn, self‐perceived time available to learn, or importance placed on learning during their hospital stay.

Interview
Fifteen interviews were conducted. Representative comments are presented in Table 4. Responses generally indicated that the patients had anxieties and uncertainties about their health and safety after discharge. Most participants wanted to know more about the condition for which they had been hospitalized, including information pertaining to management, prevention, etiology, and prognosis of their disease. Diabetic patients asked for information on insulin dosing, nutrition, and the effect of the disease on their bodies.
| Theme | Sample quotes |
|---|---|
| Preferred topics | |
| Self‐management | I need to know what to do when I go home, how to take care of it. Medical peopledon't give enough information to the patient and patient's family so they can help themselves. You need to encourage patients to help themselves, take some responsibility for themselves. |
| Prevention of disease recurrence or progression | It's okay to tell people that they have something and give them medicine, but also tell them what they can do to prevent it or make it less painful. I've known about bronchitis for many years, but didn't know it would affect my heart. |
| What's happening to me? | Am I going to diehow long? |
| Just fix me | I came to the hospital to get fixed, not educated. I'm results‐oriented, not cause‐oriented. |
| Preferred learning methods | |
| One‐to‐one didactics with MDs | I'd like one‐to‐one time with someone who has the time to listen. One to one with doctors who can explain what can happen, what to take, what not to take. |
| Family involvement | Get the family involved so the family understands the limitations of the person, how medications affect them. To say a person has a heart condition is a very vague statement. If they [family] understand more, it's better. |
| Groups | A group of people with similar illnessI like groups where everyone listens. I'd participate in groups at the hospital but not at home. |
| Video | Hospital TV is not meeting my needs. |
| Printed material | A doctor or nurse tell me what's going on and then also handouts on dietary and nutrition. |
| Electronic learning | I learned a lot through the encyclopedia of family health care, and through Web sites |
Patients preferred to pick their own topics for education rather than having topics chosen for them. Patients also showed in interest in prevention. One diabetic patient wanted to know how to prevent her children from becoming diabetic, and a cardiac patient wanted education on heart disease prevention. Other recurrent themes included the desire to know what was causing their illness and information about prognosis.
Almost all the patients were interested in more than one type of learning experience. The most frequently cited preference was to have a doctor or other knowledgeable health care professional answer questions specific to their individual situation. Video and group learning were each mentioned by approximately half the participants. Most patients thought that having family present during educational discussions was important.
Video was the most frequently mentioned learning tool, and patients thought it would be useful to have this modality available in the hospital as well as the home. Two patients expressed interest in computerized learning (one of whom had used health Web sites before his hospitalization). Most patients wanted handouts or reading material in addition to other methods of communicating information. Although many patients said they felt comfortable discussing their problems in group settings, some did not.
DISCUSSION
The important findings of this study are that hospitalized patients have a substantial amount of time available for health education and a considerable willingness and interest in participating in health educational activities. We found that although there was a great deal of time available on all days of hospitalization studied, patients felt increasingly well enough to participate in educational activities through their hospital stay.
We are unaware of other studies that have attempted to quantify the amount of time hospitalized patients are available for educational activities or whether they feel capable of participating in these activities. McBride10 found that 95% of hospitalized patients supported a health‐promoting hospital and that almost 80% wanted to modify at least 1 aspect of their lifestyle. Martin and colleagues11 found that patient satisfaction was improved by a patient‐centered unit incorporating dedicated nursing staff to promote patient involvement and provide personalized care and education. Barber‐Parker9 suggested that high patient acuity, short durations of hospitalization, and lack of patient availability because of testing and treatment limited the opportunities that patients had for health education during their hospitalization. These conclusions were reached on the basis of surveys of nurses' perceptions, however, rather than on direct observations or assessments of patients' perceptions.
Our findings suggest that many types of patient educational approaches may be needed to achieve maximal effectiveness and that regardless of the specific approach employed, the focus should be on the primary reason for a patient's hospitalization, what the hospitalization meant, why it happened and what the patient can do to prevent hospitalization from occurring in the future.
Transitions in care have been identified as periods in which communication lapses occur and outcomes can be adversely affected.12 A recent study by Epstein and colleagues13 found that almost 12% of patients had new or worsening symptoms of disease within the first few days after discharge from the hospital and that 22% either did not pick up their medications or understand how to take them (consistent with the observations of Kerzman and colleagues).5 The most common action taken in response to these findings was nurse‐mediated patient education. Our study indicates there is potential for further educational processes in hospitals, which may improve the safety of transitions from a hospital setting to outpatient care.
Although many disease management programs have been studied in the outpatient setting,1417 very few have been extended into hospitals. Accordingly, hospitalists are ideally suited to develop and implement disease management programs in concert with outpatient efforts.18 Our study suggests there is an underutilized opportunity for hospital‐based physicians and other health care providers to work with patients at a time when they are uniquely focused on their own health and free from many of the time constraints of their normal lives.
Although JCAHO mandates that hospitalized patients receive education and training specific to the patient's needs and as appropriate to the care, treatment and services provided,19 there is a paucity of data describing the educational processes in US hospitals. Johansson and colleagues20 conducted a survey in a Finnish hospital where patient education is also mandated. Written materials were given to about 55% of the patients. Demonstration and practice were used with about one third, whereas the Internet and videotapes were used for fewer than 10%. Although patients underwent educational activities throughout their hospitalization, and most were satisfied with the process, Johansson and colleagues found that only 59% felt that what they knew about their care was sufficient, almost a third felt they did not know enough about the side effects of their medical care, and almost half felt they did not have sufficient input into what they were being taught.
Although we found a large amount of time that might be used for patient education during a hospitalization, this time was commonly limited to 15‐minute blocks, as has been noted previously.9 This observation implies that educational activities should be designed so they can be conducted over short periods and/or stopped for short periods when interruptions occur or that the processes of care during a hospitalization should be altered to create larger blocks of continuous time available for educational activities.
A number of issues could have biased our results. Only 66% of the patients who were approached to participate agreed to do so. Because those declining may have been sicker and because sicker patients may require more diagnostic testing or more invasive treatment, we may have overestimated both the amount of downtime available and the willingness of patients to participate in educational activities. If we assume, however, that all patients who refused to participate either disagreed or strongly disagreed with the statements in the questionnaire regarding their interest in educational activities, the fraction of patients agreeing or strongly agreeing with idea that they were well enough and interested would still be 57% to 75% of the population sampled. Accordingly, this potential bias, if it occurred, would not alter our conclusions.
The time‐motion studies were only performed between 8:00 AM and 5:00 PM, such that the resulting data do not reflect any diagnostic testing, therapeutic interventions, or contact with health care providers that occurred at other times. This may have contributed to the strikingly small amount of time that patients spent with their physicians and nurses. If patient time after 5:00 PM and before 8:00 AM had been observed and included, it is likely that the absolute amount of time spent with physicians and nurses would increase, whereas the overall proportion of patient time spent with providers would decrease.
We were also only able to collect data on 13 time‐motion subjects. This limited sample size from a single institution may not be representative of all hospitalized non‐ICU patients on general medical wards. Accordingly, we make no claims that our data can be generalized to the entire population of patients admitted to non‐ICU medical services. However, the results of our surveys, which sampled a much broader patient population and supported our time‐motion findings, suggest that our time‐motion findings are likely to be representative of significant underutilized time and motivation for patient education in the hospital setting.
Also, it is important to note that although the time‐motion studies were only done with 13 patients, these studies are extremely labor intensive and are rarely done with much larger samples. In addition, the SDs on the data collected from the time‐motion studies were quite small. It is possible that if a larger sample were studied, the percentage of free time might be larger or smaller than what we observed for the 13 patients we studied. However, it would be quite unlikely that the amount of free time would be so small (eg, 10%‐15%) that it would invalidate our conclusion that considerable time is available for patient education over and above what currently occurs in most hospital settings.
A patient's self‐perception of his or her ability to learn may not reflect that patient's true cognitive readiness to do so. JCAHO requirements mandate that nurses be trained to assess patients for their ability to learn and to do so as part of the admission process. After reviewing all day 1 patient responses to our questionnaires, in no instance did a nurse assess a patient as having a barrier to learning when the patient had reported feeling well enough to learn. Accordingly, although we performed no direct tests of patients' ability to learn, this retrospective independent assessment did not suggest that patients systematically overestimated their ability to learn.
Finding that hospitalized patients are unoccupied for approximately 70% of their daytime hours and that most patients are both highly motivated to learn and have few barriers to doing so indicates that educational activities during hospitalizations have substantial potential for expansion. The current structure for educating hospitalized patients should be supplemented to take these findings into account.
Acknowledgements
We thank Dr. John Steiner and Dr. Sheena Bull for their assistance in study design as well as the development of the questionnaire and interview tools. We also thank Carolyn Nowels for her assistance with the qualitative data analysis. The assistance of Dr. Bull and Dr. Steiner was made possible through NHLBI grant U01HL079160, and funding for data collection was made possible by the University of Colorado at Denver and Health Sciences Center Department of Medicine, Division of General Internal Medicine small grants program.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
- ,.Randomized controlled trial of smoking cessation intervention after admission for coronary heart disease.BMJ.2003;327:1254–1257.
- .Treatment of tobacco use and dependence.N Engl J Med.2002;346:506–512.
- ,, et al.A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report.JAMA.2000;283:3244–3254.
- ,,, et al.National Institutes of Health state‐of‐the science conference statement: tobacco use: prevention, cessation and control.Ann Intern Med.2006;145:839–844.
- ,,.What do discharged patients know about their medications?Patient Educ Couns.2005;56:276–282.
- Joint Commission on Accreditation of Healthcare Organizations.A Comprehensive Review for the Development and Testing for National Implementation of Hospital Core Measures.2006:1–40.
- .ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines.J Am Coll Cardiol.2005;46:1–82.
- JCAHO data. Available: https://cimprod.uhc.edu/CoreMeasures/Products/DownloadSystem/WebPages/ViewReportsDownloadList.aspx.
- .Integrating patient teaching into bedside patient care: a participant‐observation study of hospital nurses.Patient Educ Couns.2002;48:107–113.
- .Health promotion in the acute hospital setting: the receptivity of adult in‐patients.Patient Educ Couns.2004;54:73–78.
- ,,,,,.Randomized trial of a patient‐centered hospital unit.Patient Educ Couns.1998;34:125–133.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. HYPERLINK “javascript:AL_get(this,%20'jour',%20'JAMA.');”JAMA.2007;297:831–841.
- ,,,,.Frequency of new or worsening symptoms in the posthospitalization period.J Hosp Med.2007;2:58–68.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288:2469–2475.
- ,,.Effectiveness of self‐management training in type 2 diabetes.Diabetes Care.2001;24:561–587.
- ,,.Quality of life assessment after patient education a randomized controlled study on asthma and chronic obstructive pulmonary disease.Am J Respir Crit Care Med.2001;95:56–63.
- ,.The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee.J Rheumatol.1997;24:1378–1383.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- The Joint Commission on Accreditation of Healthcare Organizations Comprehensive Accreditation Manual for Hospitals: The Official Handbook. January2007. p.152
- ,,,.Need for change in patient education: a Finnish survey from the patient's perspective.Patient Educ Couns.2003;51:239–245.
Copyright © 2008 Society of Hospital Medicine
Esophageal Perforation, Complication of EGD
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19

If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24
If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28
A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19


If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24

If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28

A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19


If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24

If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28

A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.
A “Super” case of longevity
A 109‐year‐old woman was admitted to the hospital for mild congestive heart failure and bradycardia. She scored 30 out of 30 on the Mini‐mental status exam. Further conversations revealed that she was a native of San Francisco and that she was 9 years old during the earthquake of 1906. She was 109 years old during hospitalization (Fig. 1). About 5 months after the hospitalization, she celebrated her 110th birthday (Fig. 2).


A centenarian is a person who has lived to the age of 100, and a supercentenarian is a person who has reached the age of 110. The number of centenarians in the United States was counted as 50,454 in the 2000 Census, increased from the 37,306 reported in 1990.1 The US Census Bureau estimates the current number of centenarians to be 79,682 and projects an increase to 580,605 by the year 2040.2 The number of supercentenarians was reported as 1400 in the 2000 Census.3
Hospital physicians can expect to see a growing number of centenarians in their practice as this segment of the population continues to increase.
A 109‐year‐old woman was admitted to the hospital for mild congestive heart failure and bradycardia. She scored 30 out of 30 on the Mini‐mental status exam. Further conversations revealed that she was a native of San Francisco and that she was 9 years old during the earthquake of 1906. She was 109 years old during hospitalization (Fig. 1). About 5 months after the hospitalization, she celebrated her 110th birthday (Fig. 2).


A centenarian is a person who has lived to the age of 100, and a supercentenarian is a person who has reached the age of 110. The number of centenarians in the United States was counted as 50,454 in the 2000 Census, increased from the 37,306 reported in 1990.1 The US Census Bureau estimates the current number of centenarians to be 79,682 and projects an increase to 580,605 by the year 2040.2 The number of supercentenarians was reported as 1400 in the 2000 Census.3
Hospital physicians can expect to see a growing number of centenarians in their practice as this segment of the population continues to increase.
A 109‐year‐old woman was admitted to the hospital for mild congestive heart failure and bradycardia. She scored 30 out of 30 on the Mini‐mental status exam. Further conversations revealed that she was a native of San Francisco and that she was 9 years old during the earthquake of 1906. She was 109 years old during hospitalization (Fig. 1). About 5 months after the hospitalization, she celebrated her 110th birthday (Fig. 2).


A centenarian is a person who has lived to the age of 100, and a supercentenarian is a person who has reached the age of 110. The number of centenarians in the United States was counted as 50,454 in the 2000 Census, increased from the 37,306 reported in 1990.1 The US Census Bureau estimates the current number of centenarians to be 79,682 and projects an increase to 580,605 by the year 2040.2 The number of supercentenarians was reported as 1400 in the 2000 Census.3
Hospital physicians can expect to see a growing number of centenarians in their practice as this segment of the population continues to increase.
Evidence in Prevention of Secondary Stroke
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
Copyright © 2008 Society of Hospital Medicine
Systems Approach to Stroke Care
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16
Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16

Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16

Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
Copyright © 2008 Society of Hospital Medicine
Challenging Patient Cases
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
Copyright © 2008 Society of Hospital Medicine
Hospitalist Role in Stroke Prevention
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11
MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11

MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11

MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
Copyright © 2008 Society of Hospital Medicine
10 Ways to Help Your Case
Even following the best practices, some patients will suffer adverse outcomes—and some of those patients will bring a lawsuit. Knowing that some of you either are defending claims against you or that you may have to defend a claim in the future, we wanted to provide you with a bit of practical advice that may ease the burden of litigation.
1) Engage: Many physicians want to put a lawsuit out of their mind and “let the lawyer handle it.” Just as a patient can’t cure a cancer by ignoring it, avoiding a lawsuit is not going to make it go away.
While much of the legal work takes place on a day-to-day basis without your participation, you need to remember that this is your lawsuit, not your lawyer’s lawsuit. If you do not engage with your lawyer and help the lawyer shape the defense, your lawyer may end up presenting the wrong theories. More importantly, spending time with your lawyer will help them understand your personality and the way you interact with your patients. If your lawyer doesn’t know you very well, it’s very difficult for the lawyer to build rapport between you and the jurors, who ultimately will determine the outcome of the lawsuit.
2) Teach: Many defense lawyers have picked up a fair amount of medical knowledge during our careers, but few of us have practiced medicine. As you certainly know, the fact that your lawyer has read surgical textbooks doesn’t make them qualified to perform surgery.
Because you have cared for thousands of patients, you know more about your area of medical expertise than we can ever hope to gain in the course of defending a lawsuit. Teach us the medicine that will enable us to understand how and why you made important decisions while caring for the plaintiff. Ultimately, our success at trial depends on our ability to convince juries that your decisions were thoughtful and reasonable, but we can’t do that without your help.
3) Select: In almost every medical malpractice case, the parties will endorse physicians to provide the jury with expert testimony about the medical issues. These experts become important witnesses because they help the jury understand the relevant standards of care and determine whether an allegedly negligent act caused the plaintiff to suffer an injury.
You probably know the well-respected practitioners in your field who would make credible and persuasive witnesses. Help us identify them and persuade them to serve as experts on your behalf.
4) Prepare: During the course of a lawsuit, one of the most critical events is your deposition. During your deposition, the opposing lawyer will attempt to “lock you in” on the key issues in the case and prevent you from changing your testimony at the time of trial. Consequently, you have to be well prepared for your deposition, both in terms of knowing the facts of the plaintiff’s care (which may have been rendered several years earlier) and in knowing the medical principles that applied to the plaintiff’s care.
You must demand your lawyer adequately prepare you for the deposition by reviewing these matters and preparing you for the deposition process. You need to understand how lawyers frame questions in the hopes of obtaining responses that will come back to haunt you. If you haven’t devoted the time and energy necessary for you to understand and feel comfortable with the process before sitting down for the deposition, you’re in trouble.
5) Attend: Your deposition is the only event before trial that you legally are required to attend. As a defendant, however, you have a right to attend any other deposition that takes place before trial, including the deposition of the plaintiffs and the opposing experts.
If you attend the plaintiff’s deposition, you will have the firsthand ability to hear that person’s story, and you then have the ability to suggest areas where your lawyer can challenge the plaintiff’s recollection. If you attend the opposing expert’s deposition, you similarly have the ability to hear that person’s criticisms, and you can suggest areas where your lawyer can challenge the factual or medical basis for the opinions.
6) Demonstrate: Contrary to television depictions, a trial can be a long and boring process, particularly when there’s nothing to capture the jury’s attention. Jurors have a hard time following a witness’s testimony when it consists solely of questions and answers.
This problem can be compounded when the testimony consists of technical medical information. To prevent boredom and inattention, we want to engage the jurors—and you can help us do it. Give us props, whether in the form of anatomic models, instruments used during the procedure, photographs, charts, or animations that will allow us to capture the jury’s imagination.
7) Communicate: Lawyers and doctors work in different environments. For example, you have the ability to order a test and receive the results within hours, but lawyers generally have weeks to respond to an opposing party’s requests for information. Doctors often receive results that are quantifiable and measurable—but ambiguity and nuance are a lawyer’s stock in trade.
You will be frustrated as you go through the litigation, and you need to have clear and open channels of communication with your lawyer.
Just as your patients depend upon you to orient them within an unfamiliar and frightening environment, your lawyer should help you understand what’s happening in your case. If you don’t have enough information to make intelligent decisions, you should ask for more.
8) Trust: While it’s vital to engage in the process and understand how the lawsuit is proceeding, you need to remember you are not a lawyer. There will be times when your lawyer will have to make judgment calls, and you need to give your lawyer the ability to make those decisions.
Please don’t misunderstand: You have a right to make informed decisions, but a lawyer will make hundreds of judgment calls in the course of a trial, such as whether to dismiss a potential juror, pursue a certain line of questioning with a witness, or introduce a particular exhibit. Some of your lawyer’s recommendations may seem counterintuitive to you, but the courtroom is our operating room.
9) Defend: Most jurors come to the courtroom with some skepticism of medical malpractice claims. One of the reasons for this skepticism is jurors generally like their own physicians and want to believe the medical system functions properly. When they hear a plaintiff’s claim that they were injured through medical negligence, they want the physicians involved in the care to explain how the injury occurred and why it wasn’t the physicians’ fault.
You need to be able to stand up, look the jurors in the eye, explain that your care was appropriate, and withstand an attorney’s attempts to impeach your credibility. If you are unwilling to stand up and fight for yourself and your care, there’s little reason to expect the jurors will fight on your behalf once they begin their deliberations.
10) Relax: This may be the most important tip of all. Lawsuits impose a tremendous amount of stress upon all of the participants, but especially upon a physician whose care is under fire.
We’ve represented physicians who have become so stressed and frustrated by the litigation process that it has overwhelmed them and harmed their ability to provide high-quality care for their ongoing patients.
Some physicians resort to alcohol or other substances to cope with stress. This is the worst possible scenario because it increases the likelihood that you will face another lawsuit in the future.
You need to recognize the stress imposed by a lawsuit, take care of yourself, take care of your practice, and seek help when appropriate. Almost every state has a peer-counseling program for physicians that offers specialized and confidential assistance for physicians. Contact your local medical association for a referral to one of these organizations. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Even following the best practices, some patients will suffer adverse outcomes—and some of those patients will bring a lawsuit. Knowing that some of you either are defending claims against you or that you may have to defend a claim in the future, we wanted to provide you with a bit of practical advice that may ease the burden of litigation.
1) Engage: Many physicians want to put a lawsuit out of their mind and “let the lawyer handle it.” Just as a patient can’t cure a cancer by ignoring it, avoiding a lawsuit is not going to make it go away.
While much of the legal work takes place on a day-to-day basis without your participation, you need to remember that this is your lawsuit, not your lawyer’s lawsuit. If you do not engage with your lawyer and help the lawyer shape the defense, your lawyer may end up presenting the wrong theories. More importantly, spending time with your lawyer will help them understand your personality and the way you interact with your patients. If your lawyer doesn’t know you very well, it’s very difficult for the lawyer to build rapport between you and the jurors, who ultimately will determine the outcome of the lawsuit.
2) Teach: Many defense lawyers have picked up a fair amount of medical knowledge during our careers, but few of us have practiced medicine. As you certainly know, the fact that your lawyer has read surgical textbooks doesn’t make them qualified to perform surgery.
Because you have cared for thousands of patients, you know more about your area of medical expertise than we can ever hope to gain in the course of defending a lawsuit. Teach us the medicine that will enable us to understand how and why you made important decisions while caring for the plaintiff. Ultimately, our success at trial depends on our ability to convince juries that your decisions were thoughtful and reasonable, but we can’t do that without your help.
3) Select: In almost every medical malpractice case, the parties will endorse physicians to provide the jury with expert testimony about the medical issues. These experts become important witnesses because they help the jury understand the relevant standards of care and determine whether an allegedly negligent act caused the plaintiff to suffer an injury.
You probably know the well-respected practitioners in your field who would make credible and persuasive witnesses. Help us identify them and persuade them to serve as experts on your behalf.
4) Prepare: During the course of a lawsuit, one of the most critical events is your deposition. During your deposition, the opposing lawyer will attempt to “lock you in” on the key issues in the case and prevent you from changing your testimony at the time of trial. Consequently, you have to be well prepared for your deposition, both in terms of knowing the facts of the plaintiff’s care (which may have been rendered several years earlier) and in knowing the medical principles that applied to the plaintiff’s care.
You must demand your lawyer adequately prepare you for the deposition by reviewing these matters and preparing you for the deposition process. You need to understand how lawyers frame questions in the hopes of obtaining responses that will come back to haunt you. If you haven’t devoted the time and energy necessary for you to understand and feel comfortable with the process before sitting down for the deposition, you’re in trouble.
5) Attend: Your deposition is the only event before trial that you legally are required to attend. As a defendant, however, you have a right to attend any other deposition that takes place before trial, including the deposition of the plaintiffs and the opposing experts.
If you attend the plaintiff’s deposition, you will have the firsthand ability to hear that person’s story, and you then have the ability to suggest areas where your lawyer can challenge the plaintiff’s recollection. If you attend the opposing expert’s deposition, you similarly have the ability to hear that person’s criticisms, and you can suggest areas where your lawyer can challenge the factual or medical basis for the opinions.
6) Demonstrate: Contrary to television depictions, a trial can be a long and boring process, particularly when there’s nothing to capture the jury’s attention. Jurors have a hard time following a witness’s testimony when it consists solely of questions and answers.
This problem can be compounded when the testimony consists of technical medical information. To prevent boredom and inattention, we want to engage the jurors—and you can help us do it. Give us props, whether in the form of anatomic models, instruments used during the procedure, photographs, charts, or animations that will allow us to capture the jury’s imagination.
7) Communicate: Lawyers and doctors work in different environments. For example, you have the ability to order a test and receive the results within hours, but lawyers generally have weeks to respond to an opposing party’s requests for information. Doctors often receive results that are quantifiable and measurable—but ambiguity and nuance are a lawyer’s stock in trade.
You will be frustrated as you go through the litigation, and you need to have clear and open channels of communication with your lawyer.
Just as your patients depend upon you to orient them within an unfamiliar and frightening environment, your lawyer should help you understand what’s happening in your case. If you don’t have enough information to make intelligent decisions, you should ask for more.
8) Trust: While it’s vital to engage in the process and understand how the lawsuit is proceeding, you need to remember you are not a lawyer. There will be times when your lawyer will have to make judgment calls, and you need to give your lawyer the ability to make those decisions.
Please don’t misunderstand: You have a right to make informed decisions, but a lawyer will make hundreds of judgment calls in the course of a trial, such as whether to dismiss a potential juror, pursue a certain line of questioning with a witness, or introduce a particular exhibit. Some of your lawyer’s recommendations may seem counterintuitive to you, but the courtroom is our operating room.
9) Defend: Most jurors come to the courtroom with some skepticism of medical malpractice claims. One of the reasons for this skepticism is jurors generally like their own physicians and want to believe the medical system functions properly. When they hear a plaintiff’s claim that they were injured through medical negligence, they want the physicians involved in the care to explain how the injury occurred and why it wasn’t the physicians’ fault.
You need to be able to stand up, look the jurors in the eye, explain that your care was appropriate, and withstand an attorney’s attempts to impeach your credibility. If you are unwilling to stand up and fight for yourself and your care, there’s little reason to expect the jurors will fight on your behalf once they begin their deliberations.
10) Relax: This may be the most important tip of all. Lawsuits impose a tremendous amount of stress upon all of the participants, but especially upon a physician whose care is under fire.
We’ve represented physicians who have become so stressed and frustrated by the litigation process that it has overwhelmed them and harmed their ability to provide high-quality care for their ongoing patients.
Some physicians resort to alcohol or other substances to cope with stress. This is the worst possible scenario because it increases the likelihood that you will face another lawsuit in the future.
You need to recognize the stress imposed by a lawsuit, take care of yourself, take care of your practice, and seek help when appropriate. Almost every state has a peer-counseling program for physicians that offers specialized and confidential assistance for physicians. Contact your local medical association for a referral to one of these organizations. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Even following the best practices, some patients will suffer adverse outcomes—and some of those patients will bring a lawsuit. Knowing that some of you either are defending claims against you or that you may have to defend a claim in the future, we wanted to provide you with a bit of practical advice that may ease the burden of litigation.
1) Engage: Many physicians want to put a lawsuit out of their mind and “let the lawyer handle it.” Just as a patient can’t cure a cancer by ignoring it, avoiding a lawsuit is not going to make it go away.
While much of the legal work takes place on a day-to-day basis without your participation, you need to remember that this is your lawsuit, not your lawyer’s lawsuit. If you do not engage with your lawyer and help the lawyer shape the defense, your lawyer may end up presenting the wrong theories. More importantly, spending time with your lawyer will help them understand your personality and the way you interact with your patients. If your lawyer doesn’t know you very well, it’s very difficult for the lawyer to build rapport between you and the jurors, who ultimately will determine the outcome of the lawsuit.
2) Teach: Many defense lawyers have picked up a fair amount of medical knowledge during our careers, but few of us have practiced medicine. As you certainly know, the fact that your lawyer has read surgical textbooks doesn’t make them qualified to perform surgery.
Because you have cared for thousands of patients, you know more about your area of medical expertise than we can ever hope to gain in the course of defending a lawsuit. Teach us the medicine that will enable us to understand how and why you made important decisions while caring for the plaintiff. Ultimately, our success at trial depends on our ability to convince juries that your decisions were thoughtful and reasonable, but we can’t do that without your help.
3) Select: In almost every medical malpractice case, the parties will endorse physicians to provide the jury with expert testimony about the medical issues. These experts become important witnesses because they help the jury understand the relevant standards of care and determine whether an allegedly negligent act caused the plaintiff to suffer an injury.
You probably know the well-respected practitioners in your field who would make credible and persuasive witnesses. Help us identify them and persuade them to serve as experts on your behalf.
4) Prepare: During the course of a lawsuit, one of the most critical events is your deposition. During your deposition, the opposing lawyer will attempt to “lock you in” on the key issues in the case and prevent you from changing your testimony at the time of trial. Consequently, you have to be well prepared for your deposition, both in terms of knowing the facts of the plaintiff’s care (which may have been rendered several years earlier) and in knowing the medical principles that applied to the plaintiff’s care.
You must demand your lawyer adequately prepare you for the deposition by reviewing these matters and preparing you for the deposition process. You need to understand how lawyers frame questions in the hopes of obtaining responses that will come back to haunt you. If you haven’t devoted the time and energy necessary for you to understand and feel comfortable with the process before sitting down for the deposition, you’re in trouble.
5) Attend: Your deposition is the only event before trial that you legally are required to attend. As a defendant, however, you have a right to attend any other deposition that takes place before trial, including the deposition of the plaintiffs and the opposing experts.
If you attend the plaintiff’s deposition, you will have the firsthand ability to hear that person’s story, and you then have the ability to suggest areas where your lawyer can challenge the plaintiff’s recollection. If you attend the opposing expert’s deposition, you similarly have the ability to hear that person’s criticisms, and you can suggest areas where your lawyer can challenge the factual or medical basis for the opinions.
6) Demonstrate: Contrary to television depictions, a trial can be a long and boring process, particularly when there’s nothing to capture the jury’s attention. Jurors have a hard time following a witness’s testimony when it consists solely of questions and answers.
This problem can be compounded when the testimony consists of technical medical information. To prevent boredom and inattention, we want to engage the jurors—and you can help us do it. Give us props, whether in the form of anatomic models, instruments used during the procedure, photographs, charts, or animations that will allow us to capture the jury’s imagination.
7) Communicate: Lawyers and doctors work in different environments. For example, you have the ability to order a test and receive the results within hours, but lawyers generally have weeks to respond to an opposing party’s requests for information. Doctors often receive results that are quantifiable and measurable—but ambiguity and nuance are a lawyer’s stock in trade.
You will be frustrated as you go through the litigation, and you need to have clear and open channels of communication with your lawyer.
Just as your patients depend upon you to orient them within an unfamiliar and frightening environment, your lawyer should help you understand what’s happening in your case. If you don’t have enough information to make intelligent decisions, you should ask for more.
8) Trust: While it’s vital to engage in the process and understand how the lawsuit is proceeding, you need to remember you are not a lawyer. There will be times when your lawyer will have to make judgment calls, and you need to give your lawyer the ability to make those decisions.
Please don’t misunderstand: You have a right to make informed decisions, but a lawyer will make hundreds of judgment calls in the course of a trial, such as whether to dismiss a potential juror, pursue a certain line of questioning with a witness, or introduce a particular exhibit. Some of your lawyer’s recommendations may seem counterintuitive to you, but the courtroom is our operating room.
9) Defend: Most jurors come to the courtroom with some skepticism of medical malpractice claims. One of the reasons for this skepticism is jurors generally like their own physicians and want to believe the medical system functions properly. When they hear a plaintiff’s claim that they were injured through medical negligence, they want the physicians involved in the care to explain how the injury occurred and why it wasn’t the physicians’ fault.
You need to be able to stand up, look the jurors in the eye, explain that your care was appropriate, and withstand an attorney’s attempts to impeach your credibility. If you are unwilling to stand up and fight for yourself and your care, there’s little reason to expect the jurors will fight on your behalf once they begin their deliberations.
10) Relax: This may be the most important tip of all. Lawsuits impose a tremendous amount of stress upon all of the participants, but especially upon a physician whose care is under fire.
We’ve represented physicians who have become so stressed and frustrated by the litigation process that it has overwhelmed them and harmed their ability to provide high-quality care for their ongoing patients.
Some physicians resort to alcohol or other substances to cope with stress. This is the worst possible scenario because it increases the likelihood that you will face another lawsuit in the future.
You need to recognize the stress imposed by a lawsuit, take care of yourself, take care of your practice, and seek help when appropriate. Almost every state has a peer-counseling program for physicians that offers specialized and confidential assistance for physicians. Contact your local medical association for a referral to one of these organizations. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Document Your Decisions
For all the differences highlighted in my April and May columns studying the 1995 and 1997 documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) and the American Medical Association (AMA), decision making remains consistent in both.
Physician documentation addresses the complexity of the patient’s condition in terms of the number of diagnoses and/or treatment options, the amount and/or complexity of data ordered/reviewed, and the risk of complications/morbidity/mortality. The “diagnoses” and “data” categories follow a point system (see Table 1, below) determined by local Medicare contractors, whereas the “risk” category utilizes a universal table to define medical and/or procedural risks for the patient. The final result of complexity is classified as straightforward, low, moderate, or high.
A complete and accurate description of the patient’s condition should be conveyed through the plan of care. While acuity and severity may be inferred by a physician’s colleagues from particular pieces of information included in the record (e.g., critical lab values), the importance of this information may be lost on auditors and medical record reviewers. This article will assist in explaining the categories of medical decision making, as well as provide documentation tips to best represent patient complexity.
Diagnoses, Care Options
The plan of care outlines problems the physician personally manages and those that affect their management options, even if another physician directly oversees the problem. For example, the hospitalist may primarily manage a patient’s diabetes while the nephrologist manages renal insufficiency. Since the renal insufficiency may affect the hospitalist’s plan for diabetic management, the hospitalist receives credit for the documented renal insufficiency diagnosis and hospitalist-related care plan.
Physicians should address all problems in the documentation for each encounter regardless of any changes to the treatment plan. Credit is provided for each problem that has an associated plan, even if the plan states “continue same treatment.” Additional credit is provided when the treatment to be “continued” is referenced somewhere in the progress note (e.g., in the history).
The amount of credit varies depending upon the problem type. An established problem, defined as having a care plan established by the physician or someone from the same group practice during the current hospitalization, is considered less complex than an undiagnosed new problem for which a prognosis cannot be determined. Severity of the problem affects the weight of complexity. A stable, improving problem is not as complex as a progressing problem.
When documenting diagnoses/treatment options:
- Identify all problems managed or addressed during each encounter;
- Identify problems as stable or progressing, when appropriate;
- Indicate differential diagnoses when the problem remains undefined; and
- Indicate the management/treatment option(s) for each problem.
When documentation indicates a continuation of current management options (e.g., “continue meds”), be sure the management options to be continued are noted somewhere in the progress note for that encounter (e.g., medication list).
Data Ordered/Reviewed
“Data” order/review comes in many forms: pathology/laboratory testing, radiology, and medicine-based diagnostics. Although an intuitive part of medical practice, the data section of the progress note is often underdocumented by physicians. Pertinent orders or results may be noted in the visit record, but most of the background interactions and communications involving testing are undetected when reviewing the progress note.
When documenting amount and/or complexity of data:
- Specify tests ordered and rationale in the physician’s progress note or make an entry that refers to another auditor-accessible location for ordered tests and studies;
- Test review may be documented by including a brief entry in the progress note (e.g., “decreased Hgb” or “CXR shows NAD”), or by dating and initialing the report;
- Physicians receive credit for reviewing old records or obtaining history from someone other than the patient, when necessary, as long as a summary of the review or discussion is documented in the medical record; and
- Indicate when images, tracings, or specimens are “personally reviewed” by the physician.
Discussion of unexpected or contradictory test results with the performing physician should be summarized in the medical record.
Risks of Complication
Risk is viewed in light of the patient’s presenting problem, diagnostic procedures ordered, and management options selected.
Risk is graded as minimal, low, moderate, and high with corresponding items that help to differentiate each level (see Table 2, right). The single highest item in any given risk category determines the risk level.
Chronic conditions and invasive procedures expose the patient to more risk than acute, uncomplicated illnesses or non-invasive procedures, respectively. As in the diagnoses/treatment options category, a stable or improving problem poses less risk than a progressing problem. Medication risk varies with the type and degree of potential adverse effects associated with each medication.
When documenting risk:
- Indicate status of all problems in the plan of care; identify them as stable, worsening, exacerbating (mild or severe), etc.;
- Document all diagnostic procedures being considered;
- Identify surgical risk factors involving co-morbid conditions, when appropriate; and
- Associate the labs ordered to monitor for toxicity with the corresponding. medication (e.g., “Continue coumadin, monitor PT/INR”). A patient maintains the same level of risk for a given medication whether the dosage is increased, decreased, or continued without change.
Determine Complexity
To determine the final complexity of medical decision making, two of three categories must be met. For example, if a physician satisfies the requirements for “multiple” diagnoses/treatment options, “minimal” data, and “high” risk, the physician achieves moderate complexity decision-making.
Remember that decision-making is just one of three components of evaluation and management services, along with history and exam.
Determining the final visit level (e.g., 9922x) depends upon each of these three key components for initial hospital care and consultations, and two key components for subsequent hospital care. However, medical decision making always should drive visit level selection as it is the best representation of medical necessity for the service involved.
Contributory Factors
In addition to the three categories of medical decision making, a payer (e.g., TrailblazerHealth) may consider contributory factors when determining patient complexity and selecting visit levels.
For example, the nature of the presenting problem may play a role when reviewing claims for subsequent hospital care codes (99231-99233). Found in the code descriptors of the CPT manual, problems are identified as:
- 99231: Stable, recovering or improving;
- 99232: Responding inadequately to therapy or developed a minor complication; and
- 99233: Unstable or has developed a significant complication or a significant new problem.
Although this is not a general requirement, it represents a locally established standard for reviewing claims for medical necessity. It should not be used exclusively to determine the visit level.
Be sure to query your payer’s policy via written communication or Web site posting (e.g., www.trailblazerhealth.com/Publications/Job%20Aid/medical%20necessity.pdf) for guidance on how payers review documentation. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
For all the differences highlighted in my April and May columns studying the 1995 and 1997 documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) and the American Medical Association (AMA), decision making remains consistent in both.
Physician documentation addresses the complexity of the patient’s condition in terms of the number of diagnoses and/or treatment options, the amount and/or complexity of data ordered/reviewed, and the risk of complications/morbidity/mortality. The “diagnoses” and “data” categories follow a point system (see Table 1, below) determined by local Medicare contractors, whereas the “risk” category utilizes a universal table to define medical and/or procedural risks for the patient. The final result of complexity is classified as straightforward, low, moderate, or high.
A complete and accurate description of the patient’s condition should be conveyed through the plan of care. While acuity and severity may be inferred by a physician’s colleagues from particular pieces of information included in the record (e.g., critical lab values), the importance of this information may be lost on auditors and medical record reviewers. This article will assist in explaining the categories of medical decision making, as well as provide documentation tips to best represent patient complexity.
Diagnoses, Care Options
The plan of care outlines problems the physician personally manages and those that affect their management options, even if another physician directly oversees the problem. For example, the hospitalist may primarily manage a patient’s diabetes while the nephrologist manages renal insufficiency. Since the renal insufficiency may affect the hospitalist’s plan for diabetic management, the hospitalist receives credit for the documented renal insufficiency diagnosis and hospitalist-related care plan.
Physicians should address all problems in the documentation for each encounter regardless of any changes to the treatment plan. Credit is provided for each problem that has an associated plan, even if the plan states “continue same treatment.” Additional credit is provided when the treatment to be “continued” is referenced somewhere in the progress note (e.g., in the history).
The amount of credit varies depending upon the problem type. An established problem, defined as having a care plan established by the physician or someone from the same group practice during the current hospitalization, is considered less complex than an undiagnosed new problem for which a prognosis cannot be determined. Severity of the problem affects the weight of complexity. A stable, improving problem is not as complex as a progressing problem.
When documenting diagnoses/treatment options:
- Identify all problems managed or addressed during each encounter;
- Identify problems as stable or progressing, when appropriate;
- Indicate differential diagnoses when the problem remains undefined; and
- Indicate the management/treatment option(s) for each problem.
When documentation indicates a continuation of current management options (e.g., “continue meds”), be sure the management options to be continued are noted somewhere in the progress note for that encounter (e.g., medication list).
Data Ordered/Reviewed
“Data” order/review comes in many forms: pathology/laboratory testing, radiology, and medicine-based diagnostics. Although an intuitive part of medical practice, the data section of the progress note is often underdocumented by physicians. Pertinent orders or results may be noted in the visit record, but most of the background interactions and communications involving testing are undetected when reviewing the progress note.
When documenting amount and/or complexity of data:
- Specify tests ordered and rationale in the physician’s progress note or make an entry that refers to another auditor-accessible location for ordered tests and studies;
- Test review may be documented by including a brief entry in the progress note (e.g., “decreased Hgb” or “CXR shows NAD”), or by dating and initialing the report;
- Physicians receive credit for reviewing old records or obtaining history from someone other than the patient, when necessary, as long as a summary of the review or discussion is documented in the medical record; and
- Indicate when images, tracings, or specimens are “personally reviewed” by the physician.
Discussion of unexpected or contradictory test results with the performing physician should be summarized in the medical record.
Risks of Complication
Risk is viewed in light of the patient’s presenting problem, diagnostic procedures ordered, and management options selected.
Risk is graded as minimal, low, moderate, and high with corresponding items that help to differentiate each level (see Table 2, right). The single highest item in any given risk category determines the risk level.
Chronic conditions and invasive procedures expose the patient to more risk than acute, uncomplicated illnesses or non-invasive procedures, respectively. As in the diagnoses/treatment options category, a stable or improving problem poses less risk than a progressing problem. Medication risk varies with the type and degree of potential adverse effects associated with each medication.
When documenting risk:
- Indicate status of all problems in the plan of care; identify them as stable, worsening, exacerbating (mild or severe), etc.;
- Document all diagnostic procedures being considered;
- Identify surgical risk factors involving co-morbid conditions, when appropriate; and
- Associate the labs ordered to monitor for toxicity with the corresponding. medication (e.g., “Continue coumadin, monitor PT/INR”). A patient maintains the same level of risk for a given medication whether the dosage is increased, decreased, or continued without change.
Determine Complexity
To determine the final complexity of medical decision making, two of three categories must be met. For example, if a physician satisfies the requirements for “multiple” diagnoses/treatment options, “minimal” data, and “high” risk, the physician achieves moderate complexity decision-making.
Remember that decision-making is just one of three components of evaluation and management services, along with history and exam.
Determining the final visit level (e.g., 9922x) depends upon each of these three key components for initial hospital care and consultations, and two key components for subsequent hospital care. However, medical decision making always should drive visit level selection as it is the best representation of medical necessity for the service involved.
Contributory Factors
In addition to the three categories of medical decision making, a payer (e.g., TrailblazerHealth) may consider contributory factors when determining patient complexity and selecting visit levels.
For example, the nature of the presenting problem may play a role when reviewing claims for subsequent hospital care codes (99231-99233). Found in the code descriptors of the CPT manual, problems are identified as:
- 99231: Stable, recovering or improving;
- 99232: Responding inadequately to therapy or developed a minor complication; and
- 99233: Unstable or has developed a significant complication or a significant new problem.
Although this is not a general requirement, it represents a locally established standard for reviewing claims for medical necessity. It should not be used exclusively to determine the visit level.
Be sure to query your payer’s policy via written communication or Web site posting (e.g., www.trailblazerhealth.com/Publications/Job%20Aid/medical%20necessity.pdf) for guidance on how payers review documentation. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
For all the differences highlighted in my April and May columns studying the 1995 and 1997 documentation guidelines set forth by the Centers for Medicare and Medicaid Services (CMS) and the American Medical Association (AMA), decision making remains consistent in both.
Physician documentation addresses the complexity of the patient’s condition in terms of the number of diagnoses and/or treatment options, the amount and/or complexity of data ordered/reviewed, and the risk of complications/morbidity/mortality. The “diagnoses” and “data” categories follow a point system (see Table 1, below) determined by local Medicare contractors, whereas the “risk” category utilizes a universal table to define medical and/or procedural risks for the patient. The final result of complexity is classified as straightforward, low, moderate, or high.
A complete and accurate description of the patient’s condition should be conveyed through the plan of care. While acuity and severity may be inferred by a physician’s colleagues from particular pieces of information included in the record (e.g., critical lab values), the importance of this information may be lost on auditors and medical record reviewers. This article will assist in explaining the categories of medical decision making, as well as provide documentation tips to best represent patient complexity.
Diagnoses, Care Options
The plan of care outlines problems the physician personally manages and those that affect their management options, even if another physician directly oversees the problem. For example, the hospitalist may primarily manage a patient’s diabetes while the nephrologist manages renal insufficiency. Since the renal insufficiency may affect the hospitalist’s plan for diabetic management, the hospitalist receives credit for the documented renal insufficiency diagnosis and hospitalist-related care plan.
Physicians should address all problems in the documentation for each encounter regardless of any changes to the treatment plan. Credit is provided for each problem that has an associated plan, even if the plan states “continue same treatment.” Additional credit is provided when the treatment to be “continued” is referenced somewhere in the progress note (e.g., in the history).
The amount of credit varies depending upon the problem type. An established problem, defined as having a care plan established by the physician or someone from the same group practice during the current hospitalization, is considered less complex than an undiagnosed new problem for which a prognosis cannot be determined. Severity of the problem affects the weight of complexity. A stable, improving problem is not as complex as a progressing problem.
When documenting diagnoses/treatment options:
- Identify all problems managed or addressed during each encounter;
- Identify problems as stable or progressing, when appropriate;
- Indicate differential diagnoses when the problem remains undefined; and
- Indicate the management/treatment option(s) for each problem.
When documentation indicates a continuation of current management options (e.g., “continue meds”), be sure the management options to be continued are noted somewhere in the progress note for that encounter (e.g., medication list).
Data Ordered/Reviewed
“Data” order/review comes in many forms: pathology/laboratory testing, radiology, and medicine-based diagnostics. Although an intuitive part of medical practice, the data section of the progress note is often underdocumented by physicians. Pertinent orders or results may be noted in the visit record, but most of the background interactions and communications involving testing are undetected when reviewing the progress note.
When documenting amount and/or complexity of data:
- Specify tests ordered and rationale in the physician’s progress note or make an entry that refers to another auditor-accessible location for ordered tests and studies;
- Test review may be documented by including a brief entry in the progress note (e.g., “decreased Hgb” or “CXR shows NAD”), or by dating and initialing the report;
- Physicians receive credit for reviewing old records or obtaining history from someone other than the patient, when necessary, as long as a summary of the review or discussion is documented in the medical record; and
- Indicate when images, tracings, or specimens are “personally reviewed” by the physician.
Discussion of unexpected or contradictory test results with the performing physician should be summarized in the medical record.
Risks of Complication
Risk is viewed in light of the patient’s presenting problem, diagnostic procedures ordered, and management options selected.
Risk is graded as minimal, low, moderate, and high with corresponding items that help to differentiate each level (see Table 2, right). The single highest item in any given risk category determines the risk level.
Chronic conditions and invasive procedures expose the patient to more risk than acute, uncomplicated illnesses or non-invasive procedures, respectively. As in the diagnoses/treatment options category, a stable or improving problem poses less risk than a progressing problem. Medication risk varies with the type and degree of potential adverse effects associated with each medication.
When documenting risk:
- Indicate status of all problems in the plan of care; identify them as stable, worsening, exacerbating (mild or severe), etc.;
- Document all diagnostic procedures being considered;
- Identify surgical risk factors involving co-morbid conditions, when appropriate; and
- Associate the labs ordered to monitor for toxicity with the corresponding. medication (e.g., “Continue coumadin, monitor PT/INR”). A patient maintains the same level of risk for a given medication whether the dosage is increased, decreased, or continued without change.
Determine Complexity
To determine the final complexity of medical decision making, two of three categories must be met. For example, if a physician satisfies the requirements for “multiple” diagnoses/treatment options, “minimal” data, and “high” risk, the physician achieves moderate complexity decision-making.
Remember that decision-making is just one of three components of evaluation and management services, along with history and exam.
Determining the final visit level (e.g., 9922x) depends upon each of these three key components for initial hospital care and consultations, and two key components for subsequent hospital care. However, medical decision making always should drive visit level selection as it is the best representation of medical necessity for the service involved.
Contributory Factors
In addition to the three categories of medical decision making, a payer (e.g., TrailblazerHealth) may consider contributory factors when determining patient complexity and selecting visit levels.
For example, the nature of the presenting problem may play a role when reviewing claims for subsequent hospital care codes (99231-99233). Found in the code descriptors of the CPT manual, problems are identified as:
- 99231: Stable, recovering or improving;
- 99232: Responding inadequately to therapy or developed a minor complication; and
- 99233: Unstable or has developed a significant complication or a significant new problem.
Although this is not a general requirement, it represents a locally established standard for reviewing claims for medical necessity. It should not be used exclusively to determine the visit level.
Be sure to query your payer’s policy via written communication or Web site posting (e.g., www.trailblazerhealth.com/Publications/Job%20Aid/medical%20necessity.pdf) for guidance on how payers review documentation. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
Are Patients Satisfied?
Have you seen what your discharged patients are saying about your hospital?
Now that patient satisfaction data is public, you can rest assured others are looking at how your facility stacks up against neighboring hospitals on doctor communication, pain management, and more.
As of late March, patient satisfaction information is available on the Centers for Medicare and Medicaid Services (CMS) Hospital Compare consumer Web site (www.hospitalcompare.hhs.gov). This allows for a new level of transparency about the quality of care hospitals provide.
“This is an opportunity,” says Mark V. Williams, MD, director of the hospital medicine program at Northwestern University’s Feinberg School of Medicine in Chicago.
“Hospitalists ought to look up the information on their hospitals and, if they’re not doing well, go to their administrators and say they want to help bring those standings up.”
Satisfaction Defined
What is patient satisfaction? The Hospital Compare site terms this information “Survey of Patients’ Hospital Experiences” and offers a straight percentage of patient satisfaction for 10 areas, including these summary measures:
- How well nurses and doctors in the hospital communicated with the patient;
- How responsive hospital staff were to the patient’s needs;
- How well hospital staff helped the patient manage pain;
- How well the staff communicated with the patient about medicines; and
- Whether pertinent information was provided when the patient was discharged.
Additional items address the cleanliness and quietness of the patient’s room, as well as the patient’s overall rating of the hospital and whether the patient would recommend the hospital to others.
About the Survey
The CMS patient satisfaction percentages are compiled from hospital responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). This is the first national, standardized, publicly reported survey of patients’ perspectives of hospital care.
Under CMS’ Reporting Hospital Quality Data Annual Payment Update program, hospitals subject to Inpatient Prospective Payment System (IPPS) payment provisions must collect and submit HCAHPS data to receive their full IPPS annual payment update. Other hospitals can voluntarily participate, but there is no incentive payment.
Hospitals administer the survey to a random sample of their adult Medicare patients (across medical conditions) anywhere from 48 hours to six weeks after discharge. They are allowed to conduct the survey by mail, telephone, mail with telephone follow-up, or active interactive voice recognition, and they either can integrate the HCAHPS questions with their own patient satisfaction survey or use HCAHPS by itself. Hospitals must survey patients throughout each month of the year.
CMS began reporting HCAHPS data in March on responses of patients discharged between October 2006 and June 2007. Results will be published quarterly and will comprise the most recent four quarters of data.
To the Rescue
How will this new aspect of transparency affect hospitalists?
“Hospitals are now going to be publicly exposed, as it were, and there will be increasing pressure on how to optimize these measures,” says Dr. Williams.
For this, they are likely to turn to their hospitalists. “Especially since hospitals spend so much money on supporting their hospital medicine programs, they’re going to want to see some return on that money in the form of improvement in these numbers.”
Although the data were added to Hospital Compare for the education of current and future patients, “I don’t think consumers look at this data at all,” Dr. Williams notes. “However, I think hospitals look at it, and they’ll use it to advertise [when they have impressive ratings on measures]. On these questions, hospitals are going to begin competing with each other.”
Hospitalists should be able to help their hospitals improve on specific ratings, just as they help with current quality and outcome measures.
“A lot of hospital medicine programs have already used patient satisfaction as a metric, with their own surveys,” Dr. Williams points out.
One patient satisfaction measure in particular can be addressed by hospitalists. “For HCAHPS, discharge is the component [with the lowest scores],” says Dr. Williams. “Obviously hospitalists can have a big impact on improving those numbers.”
Your own path to improving patient satisfaction is clear: Start by checking your hospital’s numbers on Hospital Compare—and remember those numbers can change quarterly. Consider how to boost satisfaction rates for some of those measures and get the buy-in you need to make changes that will bring the percentages up and keep them up. TH
Jane Jerrard is a medical writer based in Chicago.
Have you seen what your discharged patients are saying about your hospital?
Now that patient satisfaction data is public, you can rest assured others are looking at how your facility stacks up against neighboring hospitals on doctor communication, pain management, and more.
As of late March, patient satisfaction information is available on the Centers for Medicare and Medicaid Services (CMS) Hospital Compare consumer Web site (www.hospitalcompare.hhs.gov). This allows for a new level of transparency about the quality of care hospitals provide.
“This is an opportunity,” says Mark V. Williams, MD, director of the hospital medicine program at Northwestern University’s Feinberg School of Medicine in Chicago.
“Hospitalists ought to look up the information on their hospitals and, if they’re not doing well, go to their administrators and say they want to help bring those standings up.”
Satisfaction Defined
What is patient satisfaction? The Hospital Compare site terms this information “Survey of Patients’ Hospital Experiences” and offers a straight percentage of patient satisfaction for 10 areas, including these summary measures:
- How well nurses and doctors in the hospital communicated with the patient;
- How responsive hospital staff were to the patient’s needs;
- How well hospital staff helped the patient manage pain;
- How well the staff communicated with the patient about medicines; and
- Whether pertinent information was provided when the patient was discharged.
Additional items address the cleanliness and quietness of the patient’s room, as well as the patient’s overall rating of the hospital and whether the patient would recommend the hospital to others.
About the Survey
The CMS patient satisfaction percentages are compiled from hospital responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). This is the first national, standardized, publicly reported survey of patients’ perspectives of hospital care.
Under CMS’ Reporting Hospital Quality Data Annual Payment Update program, hospitals subject to Inpatient Prospective Payment System (IPPS) payment provisions must collect and submit HCAHPS data to receive their full IPPS annual payment update. Other hospitals can voluntarily participate, but there is no incentive payment.
Hospitals administer the survey to a random sample of their adult Medicare patients (across medical conditions) anywhere from 48 hours to six weeks after discharge. They are allowed to conduct the survey by mail, telephone, mail with telephone follow-up, or active interactive voice recognition, and they either can integrate the HCAHPS questions with their own patient satisfaction survey or use HCAHPS by itself. Hospitals must survey patients throughout each month of the year.
CMS began reporting HCAHPS data in March on responses of patients discharged between October 2006 and June 2007. Results will be published quarterly and will comprise the most recent four quarters of data.
To the Rescue
How will this new aspect of transparency affect hospitalists?
“Hospitals are now going to be publicly exposed, as it were, and there will be increasing pressure on how to optimize these measures,” says Dr. Williams.
For this, they are likely to turn to their hospitalists. “Especially since hospitals spend so much money on supporting their hospital medicine programs, they’re going to want to see some return on that money in the form of improvement in these numbers.”
Although the data were added to Hospital Compare for the education of current and future patients, “I don’t think consumers look at this data at all,” Dr. Williams notes. “However, I think hospitals look at it, and they’ll use it to advertise [when they have impressive ratings on measures]. On these questions, hospitals are going to begin competing with each other.”
Hospitalists should be able to help their hospitals improve on specific ratings, just as they help with current quality and outcome measures.
“A lot of hospital medicine programs have already used patient satisfaction as a metric, with their own surveys,” Dr. Williams points out.
One patient satisfaction measure in particular can be addressed by hospitalists. “For HCAHPS, discharge is the component [with the lowest scores],” says Dr. Williams. “Obviously hospitalists can have a big impact on improving those numbers.”
Your own path to improving patient satisfaction is clear: Start by checking your hospital’s numbers on Hospital Compare—and remember those numbers can change quarterly. Consider how to boost satisfaction rates for some of those measures and get the buy-in you need to make changes that will bring the percentages up and keep them up. TH
Jane Jerrard is a medical writer based in Chicago.
Have you seen what your discharged patients are saying about your hospital?
Now that patient satisfaction data is public, you can rest assured others are looking at how your facility stacks up against neighboring hospitals on doctor communication, pain management, and more.
As of late March, patient satisfaction information is available on the Centers for Medicare and Medicaid Services (CMS) Hospital Compare consumer Web site (www.hospitalcompare.hhs.gov). This allows for a new level of transparency about the quality of care hospitals provide.
“This is an opportunity,” says Mark V. Williams, MD, director of the hospital medicine program at Northwestern University’s Feinberg School of Medicine in Chicago.
“Hospitalists ought to look up the information on their hospitals and, if they’re not doing well, go to their administrators and say they want to help bring those standings up.”
Satisfaction Defined
What is patient satisfaction? The Hospital Compare site terms this information “Survey of Patients’ Hospital Experiences” and offers a straight percentage of patient satisfaction for 10 areas, including these summary measures:
- How well nurses and doctors in the hospital communicated with the patient;
- How responsive hospital staff were to the patient’s needs;
- How well hospital staff helped the patient manage pain;
- How well the staff communicated with the patient about medicines; and
- Whether pertinent information was provided when the patient was discharged.
Additional items address the cleanliness and quietness of the patient’s room, as well as the patient’s overall rating of the hospital and whether the patient would recommend the hospital to others.
About the Survey
The CMS patient satisfaction percentages are compiled from hospital responses to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). This is the first national, standardized, publicly reported survey of patients’ perspectives of hospital care.
Under CMS’ Reporting Hospital Quality Data Annual Payment Update program, hospitals subject to Inpatient Prospective Payment System (IPPS) payment provisions must collect and submit HCAHPS data to receive their full IPPS annual payment update. Other hospitals can voluntarily participate, but there is no incentive payment.
Hospitals administer the survey to a random sample of their adult Medicare patients (across medical conditions) anywhere from 48 hours to six weeks after discharge. They are allowed to conduct the survey by mail, telephone, mail with telephone follow-up, or active interactive voice recognition, and they either can integrate the HCAHPS questions with their own patient satisfaction survey or use HCAHPS by itself. Hospitals must survey patients throughout each month of the year.
CMS began reporting HCAHPS data in March on responses of patients discharged between October 2006 and June 2007. Results will be published quarterly and will comprise the most recent four quarters of data.
To the Rescue
How will this new aspect of transparency affect hospitalists?
“Hospitals are now going to be publicly exposed, as it were, and there will be increasing pressure on how to optimize these measures,” says Dr. Williams.
For this, they are likely to turn to their hospitalists. “Especially since hospitals spend so much money on supporting their hospital medicine programs, they’re going to want to see some return on that money in the form of improvement in these numbers.”
Although the data were added to Hospital Compare for the education of current and future patients, “I don’t think consumers look at this data at all,” Dr. Williams notes. “However, I think hospitals look at it, and they’ll use it to advertise [when they have impressive ratings on measures]. On these questions, hospitals are going to begin competing with each other.”
Hospitalists should be able to help their hospitals improve on specific ratings, just as they help with current quality and outcome measures.
“A lot of hospital medicine programs have already used patient satisfaction as a metric, with their own surveys,” Dr. Williams points out.
One patient satisfaction measure in particular can be addressed by hospitalists. “For HCAHPS, discharge is the component [with the lowest scores],” says Dr. Williams. “Obviously hospitalists can have a big impact on improving those numbers.”
Your own path to improving patient satisfaction is clear: Start by checking your hospital’s numbers on Hospital Compare—and remember those numbers can change quarterly. Consider how to boost satisfaction rates for some of those measures and get the buy-in you need to make changes that will bring the percentages up and keep them up. TH
Jane Jerrard is a medical writer based in Chicago.