User login
Follow the Money
I eagerly await results from SHM’s survey of hospitalist productivity and compensation every two years. I’m most curious about whether a typical hospitalist has experienced an improvement in his/her “juice to squeeze ratio” (aka compensation per unit of work).
I was pleased to see in the recently released “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement” that average hospitalist salaries increased the most for any two-year interval since we began surveying in 1997. If you haven’t seen the survey results, go to SHM’s Web site www.hospitalmedicine.org. Production remained flat, while compensation increased to an average of $188,500. (The survey showed an adjusted mean annual compensation of $193,300, and a median salary of $183,900. See complete survey for explanation regarding the adjusted mean, which refers to data for hospitalists who care for adult patients only.)
The 2008 survey has a couple of findings even more compelling than the gratifying improvement in compensation:
- 37% of HMG leaders did not know their annual expenses; and
- 35% of HMG leaders did not know their annual professional fee revenues.
Think about this for a minute. One-third of hospitalist group leaders don’t know enough about their own practice’s financial picture to know high-level details related to income and expenses. We only can presume an even larger portion of non-leader hospitalists don’t know these things about their practice.
These numbers are disconcerting, and they’re even a little worse than the numbers reported two years ago. How can this be?
Behind the Numbers
My first inclination is to look for reasons the data are misleading. Maybe some leaders chose to respond by indicating they don’t know these numbers, when in fact they do have the numbers but were just too busy to look them up and complete that part of the survey. So they might be better informed than the survey suggests, but just too busy to demonstrate it.
Or, some group leaders in large organizations, like Kaiser, may track and account for productivity and financial health in ways that differ from a typical practice. They may know a lot about their practice, but the metrics the survey asks for aren’t relevant to them.
Maybe the survey results are misleading and group leaders know a lot more about their practice financials than these numbers suggest. Well, maybe.
Unfortunately, in my consulting work up close and personal with hundreds of practices, I regularly meet group leaders who don’t see financial accountability as one of their duties. I think the survey numbers may be a reasonably accurate reflection of reality.
I typically ask group leaders things like what portion of their practice budget is funded by professional fee collections vs. payment from the hospital (or other “sponsoring organization”) and what the pro fee collection rate is. As in the survey, a large portion don’t know. They often say it’s up to someone else to keep track of those numbers and worry about the practice budget. I worry that a leader with such a hands-off approach to the practice budget can’t be very effective.
I also ask leaders things like what is their most important duty as group leader. “Making the schedule” is too often the disappointing answer. Clearly the schedule is a critical part of operating a practice, but in many practices it is reasonable, even optimal, to have a clerical person manage the schedule, or rotate responsibility for creating it among all members of the group. This frees some time the leader can spend on other activities like managing the group’s financial performance, among other things.
What Leaders Do
The ideal hospitalist practice leader’s job description will vary from place to place. It includes many things in addition to ensuring the schedule gets created. There are a handful of things that should probably be on every leader’s list. For my money, this leader should:
- Understand where the money comes from, where it goes, and what portion comes from professional fee collections vs. other sources. Also, to ensure all members of the group are updated on financial parameters regularly;
- Put in place mechanisms to ensure the hospitalists provide high-quality care to patients;
- Facilitate communication among hospitalists, hospital personnel, and medical staff to foster effective working relationships and facilitate problem-solving and conflict resolution;
- Proactively identify opportunities for the practice to enhance the service it provides to its constituents and the organization in general, and negotiating a reasonable balance between such opportunities and the practice’s resources and clinical expertise;
- Serve as a point of contact for referring primary care physicians;
- Representing the group when working and negotiating with the hospital administration; and
- Take an active role in recruitment while addressing behavior and performance issues within the practice.
Whether the leader handles these issues alone, delegates responsibility but still provides oversight, or forms a committee with other hospitalists, will vary from place to place. In every case, though, the leader should make sure these things are happening effectively.
Our field is young, and I think tends to attract people who want to avoid managing a complex practice. Perhaps it is no surprise some leaders may not be handling their job optimally. Fortunately, help is available.
Any group leader who wants to function more effectively can do several things. First, start talking to other practice leaders in your hospital. You could ask the lead doctor in another group what he/she regards as the most important components of their leadership role, and strategies that person used to become an effective leader.
Additionally, SHM has a highly regarded Leadership Academy designed to provide group leaders with the skills and resources required to successfully lead and manage a hospital medicine program now and in the future.
Each group leader should periodically step back from the day-to-day work to think about whether his/her time and energy is optimally allocated. Is the mix of clinical and administrative work reasonable? Does the leader devote time to activities (e.g., making the schedule) that could be handed off to others?
The standards used to differentiate between an effective and ineffective leader are hard to pin down and will vary a lot depending on the characteristics of a practice. Still, a comprehensive understanding of the practice’s budget and financial performance should probably be on everyone’s list. I hope the next SHM survey in late 2009 shows a lot more group leaders know things like their group’s annual expenses and revenues. We’ll see. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
I eagerly await results from SHM’s survey of hospitalist productivity and compensation every two years. I’m most curious about whether a typical hospitalist has experienced an improvement in his/her “juice to squeeze ratio” (aka compensation per unit of work).
I was pleased to see in the recently released “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement” that average hospitalist salaries increased the most for any two-year interval since we began surveying in 1997. If you haven’t seen the survey results, go to SHM’s Web site www.hospitalmedicine.org. Production remained flat, while compensation increased to an average of $188,500. (The survey showed an adjusted mean annual compensation of $193,300, and a median salary of $183,900. See complete survey for explanation regarding the adjusted mean, which refers to data for hospitalists who care for adult patients only.)
The 2008 survey has a couple of findings even more compelling than the gratifying improvement in compensation:
- 37% of HMG leaders did not know their annual expenses; and
- 35% of HMG leaders did not know their annual professional fee revenues.
Think about this for a minute. One-third of hospitalist group leaders don’t know enough about their own practice’s financial picture to know high-level details related to income and expenses. We only can presume an even larger portion of non-leader hospitalists don’t know these things about their practice.
These numbers are disconcerting, and they’re even a little worse than the numbers reported two years ago. How can this be?
Behind the Numbers
My first inclination is to look for reasons the data are misleading. Maybe some leaders chose to respond by indicating they don’t know these numbers, when in fact they do have the numbers but were just too busy to look them up and complete that part of the survey. So they might be better informed than the survey suggests, but just too busy to demonstrate it.
Or, some group leaders in large organizations, like Kaiser, may track and account for productivity and financial health in ways that differ from a typical practice. They may know a lot about their practice, but the metrics the survey asks for aren’t relevant to them.
Maybe the survey results are misleading and group leaders know a lot more about their practice financials than these numbers suggest. Well, maybe.
Unfortunately, in my consulting work up close and personal with hundreds of practices, I regularly meet group leaders who don’t see financial accountability as one of their duties. I think the survey numbers may be a reasonably accurate reflection of reality.
I typically ask group leaders things like what portion of their practice budget is funded by professional fee collections vs. payment from the hospital (or other “sponsoring organization”) and what the pro fee collection rate is. As in the survey, a large portion don’t know. They often say it’s up to someone else to keep track of those numbers and worry about the practice budget. I worry that a leader with such a hands-off approach to the practice budget can’t be very effective.
I also ask leaders things like what is their most important duty as group leader. “Making the schedule” is too often the disappointing answer. Clearly the schedule is a critical part of operating a practice, but in many practices it is reasonable, even optimal, to have a clerical person manage the schedule, or rotate responsibility for creating it among all members of the group. This frees some time the leader can spend on other activities like managing the group’s financial performance, among other things.
What Leaders Do
The ideal hospitalist practice leader’s job description will vary from place to place. It includes many things in addition to ensuring the schedule gets created. There are a handful of things that should probably be on every leader’s list. For my money, this leader should:
- Understand where the money comes from, where it goes, and what portion comes from professional fee collections vs. other sources. Also, to ensure all members of the group are updated on financial parameters regularly;
- Put in place mechanisms to ensure the hospitalists provide high-quality care to patients;
- Facilitate communication among hospitalists, hospital personnel, and medical staff to foster effective working relationships and facilitate problem-solving and conflict resolution;
- Proactively identify opportunities for the practice to enhance the service it provides to its constituents and the organization in general, and negotiating a reasonable balance between such opportunities and the practice’s resources and clinical expertise;
- Serve as a point of contact for referring primary care physicians;
- Representing the group when working and negotiating with the hospital administration; and
- Take an active role in recruitment while addressing behavior and performance issues within the practice.
Whether the leader handles these issues alone, delegates responsibility but still provides oversight, or forms a committee with other hospitalists, will vary from place to place. In every case, though, the leader should make sure these things are happening effectively.
Our field is young, and I think tends to attract people who want to avoid managing a complex practice. Perhaps it is no surprise some leaders may not be handling their job optimally. Fortunately, help is available.
Any group leader who wants to function more effectively can do several things. First, start talking to other practice leaders in your hospital. You could ask the lead doctor in another group what he/she regards as the most important components of their leadership role, and strategies that person used to become an effective leader.
Additionally, SHM has a highly regarded Leadership Academy designed to provide group leaders with the skills and resources required to successfully lead and manage a hospital medicine program now and in the future.
Each group leader should periodically step back from the day-to-day work to think about whether his/her time and energy is optimally allocated. Is the mix of clinical and administrative work reasonable? Does the leader devote time to activities (e.g., making the schedule) that could be handed off to others?
The standards used to differentiate between an effective and ineffective leader are hard to pin down and will vary a lot depending on the characteristics of a practice. Still, a comprehensive understanding of the practice’s budget and financial performance should probably be on everyone’s list. I hope the next SHM survey in late 2009 shows a lot more group leaders know things like their group’s annual expenses and revenues. We’ll see. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
I eagerly await results from SHM’s survey of hospitalist productivity and compensation every two years. I’m most curious about whether a typical hospitalist has experienced an improvement in his/her “juice to squeeze ratio” (aka compensation per unit of work).
I was pleased to see in the recently released “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement” that average hospitalist salaries increased the most for any two-year interval since we began surveying in 1997. If you haven’t seen the survey results, go to SHM’s Web site www.hospitalmedicine.org. Production remained flat, while compensation increased to an average of $188,500. (The survey showed an adjusted mean annual compensation of $193,300, and a median salary of $183,900. See complete survey for explanation regarding the adjusted mean, which refers to data for hospitalists who care for adult patients only.)
The 2008 survey has a couple of findings even more compelling than the gratifying improvement in compensation:
- 37% of HMG leaders did not know their annual expenses; and
- 35% of HMG leaders did not know their annual professional fee revenues.
Think about this for a minute. One-third of hospitalist group leaders don’t know enough about their own practice’s financial picture to know high-level details related to income and expenses. We only can presume an even larger portion of non-leader hospitalists don’t know these things about their practice.
These numbers are disconcerting, and they’re even a little worse than the numbers reported two years ago. How can this be?
Behind the Numbers
My first inclination is to look for reasons the data are misleading. Maybe some leaders chose to respond by indicating they don’t know these numbers, when in fact they do have the numbers but were just too busy to look them up and complete that part of the survey. So they might be better informed than the survey suggests, but just too busy to demonstrate it.
Or, some group leaders in large organizations, like Kaiser, may track and account for productivity and financial health in ways that differ from a typical practice. They may know a lot about their practice, but the metrics the survey asks for aren’t relevant to them.
Maybe the survey results are misleading and group leaders know a lot more about their practice financials than these numbers suggest. Well, maybe.
Unfortunately, in my consulting work up close and personal with hundreds of practices, I regularly meet group leaders who don’t see financial accountability as one of their duties. I think the survey numbers may be a reasonably accurate reflection of reality.
I typically ask group leaders things like what portion of their practice budget is funded by professional fee collections vs. payment from the hospital (or other “sponsoring organization”) and what the pro fee collection rate is. As in the survey, a large portion don’t know. They often say it’s up to someone else to keep track of those numbers and worry about the practice budget. I worry that a leader with such a hands-off approach to the practice budget can’t be very effective.
I also ask leaders things like what is their most important duty as group leader. “Making the schedule” is too often the disappointing answer. Clearly the schedule is a critical part of operating a practice, but in many practices it is reasonable, even optimal, to have a clerical person manage the schedule, or rotate responsibility for creating it among all members of the group. This frees some time the leader can spend on other activities like managing the group’s financial performance, among other things.
What Leaders Do
The ideal hospitalist practice leader’s job description will vary from place to place. It includes many things in addition to ensuring the schedule gets created. There are a handful of things that should probably be on every leader’s list. For my money, this leader should:
- Understand where the money comes from, where it goes, and what portion comes from professional fee collections vs. other sources. Also, to ensure all members of the group are updated on financial parameters regularly;
- Put in place mechanisms to ensure the hospitalists provide high-quality care to patients;
- Facilitate communication among hospitalists, hospital personnel, and medical staff to foster effective working relationships and facilitate problem-solving and conflict resolution;
- Proactively identify opportunities for the practice to enhance the service it provides to its constituents and the organization in general, and negotiating a reasonable balance between such opportunities and the practice’s resources and clinical expertise;
- Serve as a point of contact for referring primary care physicians;
- Representing the group when working and negotiating with the hospital administration; and
- Take an active role in recruitment while addressing behavior and performance issues within the practice.
Whether the leader handles these issues alone, delegates responsibility but still provides oversight, or forms a committee with other hospitalists, will vary from place to place. In every case, though, the leader should make sure these things are happening effectively.
Our field is young, and I think tends to attract people who want to avoid managing a complex practice. Perhaps it is no surprise some leaders may not be handling their job optimally. Fortunately, help is available.
Any group leader who wants to function more effectively can do several things. First, start talking to other practice leaders in your hospital. You could ask the lead doctor in another group what he/she regards as the most important components of their leadership role, and strategies that person used to become an effective leader.
Additionally, SHM has a highly regarded Leadership Academy designed to provide group leaders with the skills and resources required to successfully lead and manage a hospital medicine program now and in the future.
Each group leader should periodically step back from the day-to-day work to think about whether his/her time and energy is optimally allocated. Is the mix of clinical and administrative work reasonable? Does the leader devote time to activities (e.g., making the schedule) that could be handed off to others?
The standards used to differentiate between an effective and ineffective leader are hard to pin down and will vary a lot depending on the characteristics of a practice. Still, a comprehensive understanding of the practice’s budget and financial performance should probably be on everyone’s list. I hope the next SHM survey in late 2009 shows a lot more group leaders know things like their group’s annual expenses and revenues. We’ll see. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Promise or Insanity?
Insanity is doing the same thing over and over again and expecting different results.—Albert Einstein
A hospitalist is defined as a provider whose primary professional focus is the general medical care of hospitalized patients.1
While this allows a concise, usable characterization of a hospitalist, it’s not the whole story. If it were, medical residents, nurses, and inpatient pharmacists all would be hospitalists.
Indeed, a traditional internist with a large hospital practice could reasonably deem him or herself a hospitalist. What defines what a hospitalist does, or should be doing—and how, if at all, is that different than what a traditional internist does in the hospital?
Education Deficiencies
Early data suggested a stark difference between outcomes attributed to hospitalists and general internists who rotated between the clinic and the hospital.
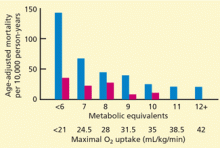
An early experience from the academic environment showed a hospitalist teaching model, when compared with a traditional teaching service, resulted in a 0.6-day length-of-stay (LOS) reduction and a cost savings of $700 per patient with no decrement in the quality of care, clinical outcomes, or satisfaction of provider, housestaff, or patient.2
Similar findings were revealed when community teaching and non-teaching hospitals transitioned to the hospitalist model.3-5 A 2002 review of 19 hospitalist studies revealed an average decreased LOS of 17% coupled with a 1% reduction in hospital costs per case.6
The year 2002 also saw, for the first time, published data that the hospitalist model could reduce in-hospital and 30-day mortality rates.7,8 Together with a 2004 paper showing reductions in minor post-operative complications with hospitalist comanagement of orthopedic patients, these studies suggested hospitalists’ care transcended mere cost savings, improving quality measures as well.9
More recently, however, Lindenauer, et al., found important but less robust differences between hospitalists and non-hospitalists.10 As compared with traditional internists, hospitalists reduced LOS 0.4 days and cost per patient by $268.
While these moderate reductions in LOS and cost versus traditional internists are statistically and clinically significant, they are less vigorous than previous findings. Despite some methodological concerns, this largest investigation—in terms of hospital sites (45), patients (76,926) and hospitalists (284)—revealed no demonstrable improvements in the quality outcomes measured.
Similarly, another recent publication found consultation, provided by medical subspecialists or hospitalists, did not improve glycemic control, rate of appropriate venous thromboembolism (VTE) prophylaxis or perioperative beta-blocker use compared with patients cared for by surgeons alone.11,12
While it is tempting to think hospitalists have re-engineered the systems of care to the point that any provider can fluently and adroitly care for patients, continued reports of less-than-optimal hospital outcomes do not support this hypothesis. More likely, the variance in the early and recent studies relates to the egress from the hospital of less capable or engaged non-hospitalist providers such that more recent findings reflect a comparator group that more closely approximates, in terms of clinical volume, hospitalists.
It’s time to reconsider how we document the merit of hospitalists. Continuing to benchmark hospitalists against non-hospitalists will not tell us if inpatient care is becoming safer, only how one group is doing compared with another. Nor will it necessarily lead to improvements in the quality of care.
To fulfill the promise of the hospitalist model, we need to ensure hospitalists are doing it better, not just better than an external comparator group. As such, it would be more valuable to evaluate hospitalists today versus those five years ago. If, as I suspect, there would be little difference in the clinical outcomes between a new hospitalist (or one in practice for three years) in 2003 and one in 2008 and we accept that hospitalist care has yet to achieve its pinnacle then we must adopt a new path. This will require redesigning the way we train hospitalists.
The ineffectiveness of our current training system is playing out in Dr. Lindenauer’s New England Journal of Medicine paper last year. He found hospitalist outcomes are only marginally better than their similarly trained traditional internist colleagues. To expect differences is to succumb to Albert Einstein’s definition of insanity. We simply cannot expect hospitalists to improve the quality of care with the same set of tools that didn’t allow our predecessors to do so.
Hospitalist-Focused Curricula
Several studies have evaluated the gap between internal medicine (IM) training and hospital medicine practice. A 2007 paper reported that nearly 30% of a community hospitalist practice consisted of areas of under emphasis in traditional IM training.13
These include consultative medicine (6.4% of practice) and the care of the patients with neurological (13.4%), orthopedic (6.4%), or general surgical (2.2%) issues. Additionally, nearly 50% of their practice consisted of patients older than 65, with the largest subset of patients ages 75-84.
Yet, most IM residency training programs do not adequately train housestaff to care for these types of patients and problems. Plauth, et al., documented areas of educational deficiencies by surveying several hundred IM-trained hospitalists about their preparedness to practice hospital medicine following residency training.14
The respondents reported feeling unprepared to care for the type and amount of neurology, geriatrics, palliative care and consultative and perioperative medicine they encountered.
Additionally, they were ill-equipped for the myriad quality improvement and systems and transitions-of-care issues they faced daily.
The “2005-2006 SHM Survey: State of the Hospital Medicine Movement” further highlighted the level of hospitalist non-clinical work, showing that 86% of hospitalist groups engage in quality improvement, 72% contribute practice guidelines, 54% work in utilization review, and 54% are involved in developing electronic medical records and provider order entry.15
For the hospitalist model to deliver outcomes superior to our traditional care model, we will need to create training programs that provide hospitalists with the skills current IM graduates do not possess.
Training programs must evolve to include the necessary clinical and non-clinical aspects of this new medical specialty. Hospitalists have populated the American healthcare landscape for more than a decade, yet very few training programs support innovation in the field of hospital medicine.
It is past time for IM educators, many of whom are hospitalists, to bridge this educational chasm through curricular reform. Short of this, the hospital medicine movement will achieve its pinnacle well short of its promise. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Society of Hospital Medicine. General information about SHM. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/GeneralInformation/General_Information.htm. Accessed April 25, 2008.
- Wachter RB, Katz P, Showstack J, Bindman AB, Goldman L. Reorganizing an academic medical service. JAMA. 1998;279:1560-1565.
- Diamond HS, Goldberg E, Janosky JE. The effect of full-time faculty hospitalists on the efficiency of care at a community teaching hospital. Ann Intern Med. 1998;129:197-203.
- Freese RB. The Park Nicollet experience in establishing a hospitalist system. Ann Intern Med. 1999;130:350-354.
- Craig DE, Hartka L, Likosky WH, Caplan WM, Litsky P, Smithey J. Implementation of a hospitalist system in a large health maintenance organization: The Kaiser Permanente experience. Ann Intern Med. 1999;130:355-359.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Meltzer D, Manning W, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866-874.
- Auerbach AD, Wachter RM, Katz P, et al. Implementation of a voluntary hospitalist service at a community teaching hospital: Improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-865.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty. Ann Intern Med. 2004;141:28-38.
- Lindenauer PK, Rothber MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalist, general internists, and family physicians. N Engl J Med. 2007;357:2589-600.
- Dr Andrew Auerbach, personal communication, January 7, 2008.
- Auerbach AD, Rasic MA, Sehgal N, Ide B, Stone B, Maselli J. Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery. Arch Intern Med. 2007;167:2338-2344.
- Glasheen JJ, Epstein KR, Siegal E, Kutner J, Prochazka AV. The spectrum of community-based hospitalist practice, a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM et al. Hospitalist’s perceptions of their residency training needs: Results of a national survey. Am J Med. 2001;111:247-254.
- Society of Hospital Medicine. 2005-2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://dev.hospitalmedicine.org/AM/Template.cfm?Section=Survey&Template=/CM/ContentDisplay.cfm&ContentID=14352. Accessed April 28, 2008.
Insanity is doing the same thing over and over again and expecting different results.—Albert Einstein
A hospitalist is defined as a provider whose primary professional focus is the general medical care of hospitalized patients.1
While this allows a concise, usable characterization of a hospitalist, it’s not the whole story. If it were, medical residents, nurses, and inpatient pharmacists all would be hospitalists.
Indeed, a traditional internist with a large hospital practice could reasonably deem him or herself a hospitalist. What defines what a hospitalist does, or should be doing—and how, if at all, is that different than what a traditional internist does in the hospital?
Education Deficiencies
Early data suggested a stark difference between outcomes attributed to hospitalists and general internists who rotated between the clinic and the hospital.
An early experience from the academic environment showed a hospitalist teaching model, when compared with a traditional teaching service, resulted in a 0.6-day length-of-stay (LOS) reduction and a cost savings of $700 per patient with no decrement in the quality of care, clinical outcomes, or satisfaction of provider, housestaff, or patient.2
Similar findings were revealed when community teaching and non-teaching hospitals transitioned to the hospitalist model.3-5 A 2002 review of 19 hospitalist studies revealed an average decreased LOS of 17% coupled with a 1% reduction in hospital costs per case.6
The year 2002 also saw, for the first time, published data that the hospitalist model could reduce in-hospital and 30-day mortality rates.7,8 Together with a 2004 paper showing reductions in minor post-operative complications with hospitalist comanagement of orthopedic patients, these studies suggested hospitalists’ care transcended mere cost savings, improving quality measures as well.9
More recently, however, Lindenauer, et al., found important but less robust differences between hospitalists and non-hospitalists.10 As compared with traditional internists, hospitalists reduced LOS 0.4 days and cost per patient by $268.
While these moderate reductions in LOS and cost versus traditional internists are statistically and clinically significant, they are less vigorous than previous findings. Despite some methodological concerns, this largest investigation—in terms of hospital sites (45), patients (76,926) and hospitalists (284)—revealed no demonstrable improvements in the quality outcomes measured.
Similarly, another recent publication found consultation, provided by medical subspecialists or hospitalists, did not improve glycemic control, rate of appropriate venous thromboembolism (VTE) prophylaxis or perioperative beta-blocker use compared with patients cared for by surgeons alone.11,12
While it is tempting to think hospitalists have re-engineered the systems of care to the point that any provider can fluently and adroitly care for patients, continued reports of less-than-optimal hospital outcomes do not support this hypothesis. More likely, the variance in the early and recent studies relates to the egress from the hospital of less capable or engaged non-hospitalist providers such that more recent findings reflect a comparator group that more closely approximates, in terms of clinical volume, hospitalists.
It’s time to reconsider how we document the merit of hospitalists. Continuing to benchmark hospitalists against non-hospitalists will not tell us if inpatient care is becoming safer, only how one group is doing compared with another. Nor will it necessarily lead to improvements in the quality of care.
To fulfill the promise of the hospitalist model, we need to ensure hospitalists are doing it better, not just better than an external comparator group. As such, it would be more valuable to evaluate hospitalists today versus those five years ago. If, as I suspect, there would be little difference in the clinical outcomes between a new hospitalist (or one in practice for three years) in 2003 and one in 2008 and we accept that hospitalist care has yet to achieve its pinnacle then we must adopt a new path. This will require redesigning the way we train hospitalists.
The ineffectiveness of our current training system is playing out in Dr. Lindenauer’s New England Journal of Medicine paper last year. He found hospitalist outcomes are only marginally better than their similarly trained traditional internist colleagues. To expect differences is to succumb to Albert Einstein’s definition of insanity. We simply cannot expect hospitalists to improve the quality of care with the same set of tools that didn’t allow our predecessors to do so.
Hospitalist-Focused Curricula
Several studies have evaluated the gap between internal medicine (IM) training and hospital medicine practice. A 2007 paper reported that nearly 30% of a community hospitalist practice consisted of areas of under emphasis in traditional IM training.13
These include consultative medicine (6.4% of practice) and the care of the patients with neurological (13.4%), orthopedic (6.4%), or general surgical (2.2%) issues. Additionally, nearly 50% of their practice consisted of patients older than 65, with the largest subset of patients ages 75-84.
Yet, most IM residency training programs do not adequately train housestaff to care for these types of patients and problems. Plauth, et al., documented areas of educational deficiencies by surveying several hundred IM-trained hospitalists about their preparedness to practice hospital medicine following residency training.14
The respondents reported feeling unprepared to care for the type and amount of neurology, geriatrics, palliative care and consultative and perioperative medicine they encountered.
Additionally, they were ill-equipped for the myriad quality improvement and systems and transitions-of-care issues they faced daily.
The “2005-2006 SHM Survey: State of the Hospital Medicine Movement” further highlighted the level of hospitalist non-clinical work, showing that 86% of hospitalist groups engage in quality improvement, 72% contribute practice guidelines, 54% work in utilization review, and 54% are involved in developing electronic medical records and provider order entry.15
For the hospitalist model to deliver outcomes superior to our traditional care model, we will need to create training programs that provide hospitalists with the skills current IM graduates do not possess.
Training programs must evolve to include the necessary clinical and non-clinical aspects of this new medical specialty. Hospitalists have populated the American healthcare landscape for more than a decade, yet very few training programs support innovation in the field of hospital medicine.
It is past time for IM educators, many of whom are hospitalists, to bridge this educational chasm through curricular reform. Short of this, the hospital medicine movement will achieve its pinnacle well short of its promise. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Society of Hospital Medicine. General information about SHM. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/GeneralInformation/General_Information.htm. Accessed April 25, 2008.
- Wachter RB, Katz P, Showstack J, Bindman AB, Goldman L. Reorganizing an academic medical service. JAMA. 1998;279:1560-1565.
- Diamond HS, Goldberg E, Janosky JE. The effect of full-time faculty hospitalists on the efficiency of care at a community teaching hospital. Ann Intern Med. 1998;129:197-203.
- Freese RB. The Park Nicollet experience in establishing a hospitalist system. Ann Intern Med. 1999;130:350-354.
- Craig DE, Hartka L, Likosky WH, Caplan WM, Litsky P, Smithey J. Implementation of a hospitalist system in a large health maintenance organization: The Kaiser Permanente experience. Ann Intern Med. 1999;130:355-359.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Meltzer D, Manning W, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866-874.
- Auerbach AD, Wachter RM, Katz P, et al. Implementation of a voluntary hospitalist service at a community teaching hospital: Improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-865.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty. Ann Intern Med. 2004;141:28-38.
- Lindenauer PK, Rothber MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalist, general internists, and family physicians. N Engl J Med. 2007;357:2589-600.
- Dr Andrew Auerbach, personal communication, January 7, 2008.
- Auerbach AD, Rasic MA, Sehgal N, Ide B, Stone B, Maselli J. Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery. Arch Intern Med. 2007;167:2338-2344.
- Glasheen JJ, Epstein KR, Siegal E, Kutner J, Prochazka AV. The spectrum of community-based hospitalist practice, a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM et al. Hospitalist’s perceptions of their residency training needs: Results of a national survey. Am J Med. 2001;111:247-254.
- Society of Hospital Medicine. 2005-2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://dev.hospitalmedicine.org/AM/Template.cfm?Section=Survey&Template=/CM/ContentDisplay.cfm&ContentID=14352. Accessed April 28, 2008.
Insanity is doing the same thing over and over again and expecting different results.—Albert Einstein
A hospitalist is defined as a provider whose primary professional focus is the general medical care of hospitalized patients.1
While this allows a concise, usable characterization of a hospitalist, it’s not the whole story. If it were, medical residents, nurses, and inpatient pharmacists all would be hospitalists.
Indeed, a traditional internist with a large hospital practice could reasonably deem him or herself a hospitalist. What defines what a hospitalist does, or should be doing—and how, if at all, is that different than what a traditional internist does in the hospital?
Education Deficiencies
Early data suggested a stark difference between outcomes attributed to hospitalists and general internists who rotated between the clinic and the hospital.
An early experience from the academic environment showed a hospitalist teaching model, when compared with a traditional teaching service, resulted in a 0.6-day length-of-stay (LOS) reduction and a cost savings of $700 per patient with no decrement in the quality of care, clinical outcomes, or satisfaction of provider, housestaff, or patient.2
Similar findings were revealed when community teaching and non-teaching hospitals transitioned to the hospitalist model.3-5 A 2002 review of 19 hospitalist studies revealed an average decreased LOS of 17% coupled with a 1% reduction in hospital costs per case.6
The year 2002 also saw, for the first time, published data that the hospitalist model could reduce in-hospital and 30-day mortality rates.7,8 Together with a 2004 paper showing reductions in minor post-operative complications with hospitalist comanagement of orthopedic patients, these studies suggested hospitalists’ care transcended mere cost savings, improving quality measures as well.9
More recently, however, Lindenauer, et al., found important but less robust differences between hospitalists and non-hospitalists.10 As compared with traditional internists, hospitalists reduced LOS 0.4 days and cost per patient by $268.
While these moderate reductions in LOS and cost versus traditional internists are statistically and clinically significant, they are less vigorous than previous findings. Despite some methodological concerns, this largest investigation—in terms of hospital sites (45), patients (76,926) and hospitalists (284)—revealed no demonstrable improvements in the quality outcomes measured.
Similarly, another recent publication found consultation, provided by medical subspecialists or hospitalists, did not improve glycemic control, rate of appropriate venous thromboembolism (VTE) prophylaxis or perioperative beta-blocker use compared with patients cared for by surgeons alone.11,12
While it is tempting to think hospitalists have re-engineered the systems of care to the point that any provider can fluently and adroitly care for patients, continued reports of less-than-optimal hospital outcomes do not support this hypothesis. More likely, the variance in the early and recent studies relates to the egress from the hospital of less capable or engaged non-hospitalist providers such that more recent findings reflect a comparator group that more closely approximates, in terms of clinical volume, hospitalists.
It’s time to reconsider how we document the merit of hospitalists. Continuing to benchmark hospitalists against non-hospitalists will not tell us if inpatient care is becoming safer, only how one group is doing compared with another. Nor will it necessarily lead to improvements in the quality of care.
To fulfill the promise of the hospitalist model, we need to ensure hospitalists are doing it better, not just better than an external comparator group. As such, it would be more valuable to evaluate hospitalists today versus those five years ago. If, as I suspect, there would be little difference in the clinical outcomes between a new hospitalist (or one in practice for three years) in 2003 and one in 2008 and we accept that hospitalist care has yet to achieve its pinnacle then we must adopt a new path. This will require redesigning the way we train hospitalists.
The ineffectiveness of our current training system is playing out in Dr. Lindenauer’s New England Journal of Medicine paper last year. He found hospitalist outcomes are only marginally better than their similarly trained traditional internist colleagues. To expect differences is to succumb to Albert Einstein’s definition of insanity. We simply cannot expect hospitalists to improve the quality of care with the same set of tools that didn’t allow our predecessors to do so.
Hospitalist-Focused Curricula
Several studies have evaluated the gap between internal medicine (IM) training and hospital medicine practice. A 2007 paper reported that nearly 30% of a community hospitalist practice consisted of areas of under emphasis in traditional IM training.13
These include consultative medicine (6.4% of practice) and the care of the patients with neurological (13.4%), orthopedic (6.4%), or general surgical (2.2%) issues. Additionally, nearly 50% of their practice consisted of patients older than 65, with the largest subset of patients ages 75-84.
Yet, most IM residency training programs do not adequately train housestaff to care for these types of patients and problems. Plauth, et al., documented areas of educational deficiencies by surveying several hundred IM-trained hospitalists about their preparedness to practice hospital medicine following residency training.14
The respondents reported feeling unprepared to care for the type and amount of neurology, geriatrics, palliative care and consultative and perioperative medicine they encountered.
Additionally, they were ill-equipped for the myriad quality improvement and systems and transitions-of-care issues they faced daily.
The “2005-2006 SHM Survey: State of the Hospital Medicine Movement” further highlighted the level of hospitalist non-clinical work, showing that 86% of hospitalist groups engage in quality improvement, 72% contribute practice guidelines, 54% work in utilization review, and 54% are involved in developing electronic medical records and provider order entry.15
For the hospitalist model to deliver outcomes superior to our traditional care model, we will need to create training programs that provide hospitalists with the skills current IM graduates do not possess.
Training programs must evolve to include the necessary clinical and non-clinical aspects of this new medical specialty. Hospitalists have populated the American healthcare landscape for more than a decade, yet very few training programs support innovation in the field of hospital medicine.
It is past time for IM educators, many of whom are hospitalists, to bridge this educational chasm through curricular reform. Short of this, the hospital medicine movement will achieve its pinnacle well short of its promise. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Society of Hospital Medicine. General information about SHM. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/GeneralInformation/General_Information.htm. Accessed April 25, 2008.
- Wachter RB, Katz P, Showstack J, Bindman AB, Goldman L. Reorganizing an academic medical service. JAMA. 1998;279:1560-1565.
- Diamond HS, Goldberg E, Janosky JE. The effect of full-time faculty hospitalists on the efficiency of care at a community teaching hospital. Ann Intern Med. 1998;129:197-203.
- Freese RB. The Park Nicollet experience in establishing a hospitalist system. Ann Intern Med. 1999;130:350-354.
- Craig DE, Hartka L, Likosky WH, Caplan WM, Litsky P, Smithey J. Implementation of a hospitalist system in a large health maintenance organization: The Kaiser Permanente experience. Ann Intern Med. 1999;130:355-359.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494.
- Meltzer D, Manning W, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866-874.
- Auerbach AD, Wachter RM, Katz P, et al. Implementation of a voluntary hospitalist service at a community teaching hospital: Improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-865.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty. Ann Intern Med. 2004;141:28-38.
- Lindenauer PK, Rothber MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalist, general internists, and family physicians. N Engl J Med. 2007;357:2589-600.
- Dr Andrew Auerbach, personal communication, January 7, 2008.
- Auerbach AD, Rasic MA, Sehgal N, Ide B, Stone B, Maselli J. Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery. Arch Intern Med. 2007;167:2338-2344.
- Glasheen JJ, Epstein KR, Siegal E, Kutner J, Prochazka AV. The spectrum of community-based hospitalist practice, a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM et al. Hospitalist’s perceptions of their residency training needs: Results of a national survey. Am J Med. 2001;111:247-254.
- Society of Hospital Medicine. 2005-2006 SHM Survey: State of the Hospital Medicine Movement. Available at: http://dev.hospitalmedicine.org/AM/Template.cfm?Section=Survey&Template=/CM/ContentDisplay.cfm&ContentID=14352. Accessed April 28, 2008.
Medicine’s Guiding Team
Change is in the air. Some pundits point to a new healthcare system; others point to something a little less dramatic on the edges. No matter how one views it, change definitely is afoot.
The last time I recall a similar feeling was 1993. There was a general feeling then among many in healthcare that a unique convergence of events might result in healthcare reform. Because of the similarities between 1993 and 2008, many people are naturally wondering whether the atmosphere is similar enough to again result in reform this time.
What do we know about healthcare in 2008 as opposed to 1993? Well, an even greater share of the United States economy is based on healthcare. The quality and patient-safety movement has arrived. There is a greater discussion about pay for performance. The effects of consumerism are being felt by all healthcare providers. There is evidence the United States does not have the best healthcare.1 There still are some physician shortages, and predictions of greater shortages, albeit in different areas then 1993. So, if anything, the burning platform for change appears brighter in 2008 than in 1993.
As I reflect on these facts and the differences between then and now, I think of the principles of change management.2,3 Establishing the burning platform or the sense of urgency is only the first step in change. It is a vital one, but if the next steps are not completed, hard-wired change does not occur.
The second tenet of change management is that you must pull together a guiding team. There must be a powerful group guiding the change—one with leadership skills, credibility, communications ability, authority, analytical skills, and sense of urgency.
This is the main difference between 1993 and 2008 and one that convinces me we are on the road to change in healthcare. For the biggest difference is you. In 1993, there were several hundred hospitalists in the United States. Now, there are approximately 20,000 and a robust professional society to help manage and lead the group.
You are the guiding team. Why? In many mature hospital medicine programs, hospitalists account for the majority of a hospital’s admissions. Add this to the fact that more than 30% of healthcare is spent on hospital care. The result is that hospitalists through their pens control a significant amount of the healthcare market. Hospitalists have the leadership, authority, and credibility to be the guiding team. And when I see the tremendous skills of hospitalists in guiding new programs and serving as medical staff leaders, I am convinced hospitalists are the nation’s guiding team in healthcare reform.
The third step in change management principles is deciding what to do. There must be a unified vision and strategy. I am not as confident this vision is fully formed yet and hence one of the reasons we won’t get change immediately. To create a unified vision and strategy, we need additional innovation in hospital care. Granted, hospital medicine is a relatively recent innovation; we are far from done developing the right care mode for hospitalized patients.
What is the area we need to innovate in the most? Our practices. While there is much innovation occurring in hospital medicine, we need to continue aggressively pursuing new methods of care delivery. Year after year at our annual meetings, we see tremendous evidence of innovation in the numerous abstracts presented. Still, we must try to take it up to a new level. The present way of doing things isn’t sustainable. We cannot completely care for patients by merely working harder in our current care-delivery model. Working differently or fundamentally redesigning our jobs will help us. It will help us see more patients and deliver greater quality, all while maintaining high degrees of personal and professional satisfaction.
Do not leave the innovation up to others. Each of us must continue to assess how we deliver care through a team model. We must evaluate how to better integrate with midlevel providers. We must lead in transitions of care and discharge planning. We need to re-examine the basic model of physician-patient care. We must make sure residency and post-residency training prepare hospitalists for all of this. Finally, we need to innovate on how hospital administrators and hospitalists work together to improve quality and patient safety.
Don’t forget to share those innovations with SHM. SHM is your conduit to change healthcare—and if you take things to the third step of change management, SHM easily can help you through the remaining steps. TH
Dr. Cawley is president of SHM.
References
- Davis K, Schoen C, Schoenbaum SC, et al. Mirror, mirror on the wall: an international update on the comparative performance of American health care. Commonwealth Fund: May 2007.
- Kotter J. Leading change. Boston: Harvard Business Press; 1996.
- Kotter J, Rathgebar H. Our iceberg is melting: changing and succeeding under any condition. New York: St. Martin’s Press; 2005.
Change is in the air. Some pundits point to a new healthcare system; others point to something a little less dramatic on the edges. No matter how one views it, change definitely is afoot.
The last time I recall a similar feeling was 1993. There was a general feeling then among many in healthcare that a unique convergence of events might result in healthcare reform. Because of the similarities between 1993 and 2008, many people are naturally wondering whether the atmosphere is similar enough to again result in reform this time.
What do we know about healthcare in 2008 as opposed to 1993? Well, an even greater share of the United States economy is based on healthcare. The quality and patient-safety movement has arrived. There is a greater discussion about pay for performance. The effects of consumerism are being felt by all healthcare providers. There is evidence the United States does not have the best healthcare.1 There still are some physician shortages, and predictions of greater shortages, albeit in different areas then 1993. So, if anything, the burning platform for change appears brighter in 2008 than in 1993.
As I reflect on these facts and the differences between then and now, I think of the principles of change management.2,3 Establishing the burning platform or the sense of urgency is only the first step in change. It is a vital one, but if the next steps are not completed, hard-wired change does not occur.
The second tenet of change management is that you must pull together a guiding team. There must be a powerful group guiding the change—one with leadership skills, credibility, communications ability, authority, analytical skills, and sense of urgency.
This is the main difference between 1993 and 2008 and one that convinces me we are on the road to change in healthcare. For the biggest difference is you. In 1993, there were several hundred hospitalists in the United States. Now, there are approximately 20,000 and a robust professional society to help manage and lead the group.
You are the guiding team. Why? In many mature hospital medicine programs, hospitalists account for the majority of a hospital’s admissions. Add this to the fact that more than 30% of healthcare is spent on hospital care. The result is that hospitalists through their pens control a significant amount of the healthcare market. Hospitalists have the leadership, authority, and credibility to be the guiding team. And when I see the tremendous skills of hospitalists in guiding new programs and serving as medical staff leaders, I am convinced hospitalists are the nation’s guiding team in healthcare reform.
The third step in change management principles is deciding what to do. There must be a unified vision and strategy. I am not as confident this vision is fully formed yet and hence one of the reasons we won’t get change immediately. To create a unified vision and strategy, we need additional innovation in hospital care. Granted, hospital medicine is a relatively recent innovation; we are far from done developing the right care mode for hospitalized patients.
What is the area we need to innovate in the most? Our practices. While there is much innovation occurring in hospital medicine, we need to continue aggressively pursuing new methods of care delivery. Year after year at our annual meetings, we see tremendous evidence of innovation in the numerous abstracts presented. Still, we must try to take it up to a new level. The present way of doing things isn’t sustainable. We cannot completely care for patients by merely working harder in our current care-delivery model. Working differently or fundamentally redesigning our jobs will help us. It will help us see more patients and deliver greater quality, all while maintaining high degrees of personal and professional satisfaction.
Do not leave the innovation up to others. Each of us must continue to assess how we deliver care through a team model. We must evaluate how to better integrate with midlevel providers. We must lead in transitions of care and discharge planning. We need to re-examine the basic model of physician-patient care. We must make sure residency and post-residency training prepare hospitalists for all of this. Finally, we need to innovate on how hospital administrators and hospitalists work together to improve quality and patient safety.
Don’t forget to share those innovations with SHM. SHM is your conduit to change healthcare—and if you take things to the third step of change management, SHM easily can help you through the remaining steps. TH
Dr. Cawley is president of SHM.
References
- Davis K, Schoen C, Schoenbaum SC, et al. Mirror, mirror on the wall: an international update on the comparative performance of American health care. Commonwealth Fund: May 2007.
- Kotter J. Leading change. Boston: Harvard Business Press; 1996.
- Kotter J, Rathgebar H. Our iceberg is melting: changing and succeeding under any condition. New York: St. Martin’s Press; 2005.
Change is in the air. Some pundits point to a new healthcare system; others point to something a little less dramatic on the edges. No matter how one views it, change definitely is afoot.
The last time I recall a similar feeling was 1993. There was a general feeling then among many in healthcare that a unique convergence of events might result in healthcare reform. Because of the similarities between 1993 and 2008, many people are naturally wondering whether the atmosphere is similar enough to again result in reform this time.
What do we know about healthcare in 2008 as opposed to 1993? Well, an even greater share of the United States economy is based on healthcare. The quality and patient-safety movement has arrived. There is a greater discussion about pay for performance. The effects of consumerism are being felt by all healthcare providers. There is evidence the United States does not have the best healthcare.1 There still are some physician shortages, and predictions of greater shortages, albeit in different areas then 1993. So, if anything, the burning platform for change appears brighter in 2008 than in 1993.
As I reflect on these facts and the differences between then and now, I think of the principles of change management.2,3 Establishing the burning platform or the sense of urgency is only the first step in change. It is a vital one, but if the next steps are not completed, hard-wired change does not occur.
The second tenet of change management is that you must pull together a guiding team. There must be a powerful group guiding the change—one with leadership skills, credibility, communications ability, authority, analytical skills, and sense of urgency.
This is the main difference between 1993 and 2008 and one that convinces me we are on the road to change in healthcare. For the biggest difference is you. In 1993, there were several hundred hospitalists in the United States. Now, there are approximately 20,000 and a robust professional society to help manage and lead the group.
You are the guiding team. Why? In many mature hospital medicine programs, hospitalists account for the majority of a hospital’s admissions. Add this to the fact that more than 30% of healthcare is spent on hospital care. The result is that hospitalists through their pens control a significant amount of the healthcare market. Hospitalists have the leadership, authority, and credibility to be the guiding team. And when I see the tremendous skills of hospitalists in guiding new programs and serving as medical staff leaders, I am convinced hospitalists are the nation’s guiding team in healthcare reform.
The third step in change management principles is deciding what to do. There must be a unified vision and strategy. I am not as confident this vision is fully formed yet and hence one of the reasons we won’t get change immediately. To create a unified vision and strategy, we need additional innovation in hospital care. Granted, hospital medicine is a relatively recent innovation; we are far from done developing the right care mode for hospitalized patients.
What is the area we need to innovate in the most? Our practices. While there is much innovation occurring in hospital medicine, we need to continue aggressively pursuing new methods of care delivery. Year after year at our annual meetings, we see tremendous evidence of innovation in the numerous abstracts presented. Still, we must try to take it up to a new level. The present way of doing things isn’t sustainable. We cannot completely care for patients by merely working harder in our current care-delivery model. Working differently or fundamentally redesigning our jobs will help us. It will help us see more patients and deliver greater quality, all while maintaining high degrees of personal and professional satisfaction.
Do not leave the innovation up to others. Each of us must continue to assess how we deliver care through a team model. We must evaluate how to better integrate with midlevel providers. We must lead in transitions of care and discharge planning. We need to re-examine the basic model of physician-patient care. We must make sure residency and post-residency training prepare hospitalists for all of this. Finally, we need to innovate on how hospital administrators and hospitalists work together to improve quality and patient safety.
Don’t forget to share those innovations with SHM. SHM is your conduit to change healthcare—and if you take things to the third step of change management, SHM easily can help you through the remaining steps. TH
Dr. Cawley is president of SHM.
References
- Davis K, Schoen C, Schoenbaum SC, et al. Mirror, mirror on the wall: an international update on the comparative performance of American health care. Commonwealth Fund: May 2007.
- Kotter J. Leading change. Boston: Harvard Business Press; 1996.
- Kotter J, Rathgebar H. Our iceberg is melting: changing and succeeding under any condition. New York: St. Martin’s Press; 2005.
What is the best intervention to help hospitalized patients quit smoking?
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
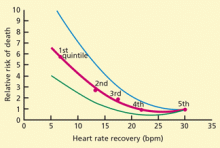
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
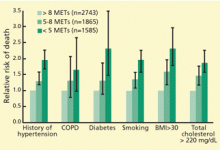
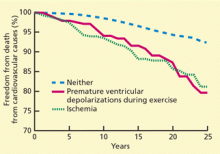
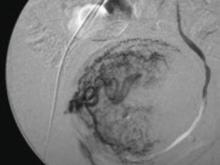
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
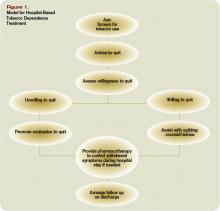
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
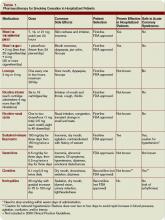
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.
The Ultrasound Advantage
The iPhone may be the latest “it” gadget, but a flurry of recent innovation has given portable ultrasound devices a healthy buzz within the biomedical community.
Beyond the gee-whiz factor, though, a growing number of studies demonstrate the everyday value of putting portable units in the hands of hospitalists.
“The big news has been the tiny portable scanner,” says Stephen Smith, a biomedical engineer at Duke University, in North Carolina, and a pioneer in ultrasound technology. Siemens recently introduced a hand-held device called the Acuson P10, which weighs 1.6 pounds, retails for $9,499 and can fit within a hospitalist’s coat pocket. Not to be outdone, GE has announced plans to introduce an ultrasound unit no bigger than an iPod.
Smith and his collaborators have taken the technology one step farther. They incorporate electrocardial leads on the unit’s transducer face to permit electrocardiograms and a microphone to let hospitalists use the ultrasound like a stethoscope.
Eric Isaacs, MD, a clinical professor of medicine at San Francisco General Hospital, says he routinely uses ultrasound for vascular access “to ensure the safety of procedures that we previously performed either blind or by anatomical landmarks.” Beyond improving the accuracy of placing central and peripheral lines, he says, “the reason we are using ultrasound more now is that the machine is so portable. The radiologists are no longer in the hospital 24 hours a day, and so by necessity we are using the tools that were previously only accessible from 9 to 5.”
Range of Uses
Among the reports recognizing ultrasound’s value, he cited a 2003 study in the British Medical Journal affirming the technology’s superiority to relying on physical landmarks in gaining central venous access, resulting in a lower technical failure rate, reduced complications, and faster access.1 Dr. Issacs says ultrasound also has helped guide procedures such as thorancentesis and paracentesis, other applications once confined to radiology. “It’s something that’s allowing me to do at the bedside what I would otherwise have to wait several hours for,” he says.
For heart patients, he says, a hospitalist can bring ultrasound to the bedside during a cardiac arrest to inspect cardiac motion and fluid, and monitor the patient’s hydration status by examining the size of the inferior vena cava. Internists likewise could examine the size of a patient’s aorta to look for signs of an aneurism, especially for a patient experiencing abdominal pain in the middle of the night. “Quite frankly, it seems like the only limit to ultrasound use is imagination,” Dr. Isaacs says.
Robert Rodriguez, MD, a clinical professor of medicine and emergency medicine at San Francisco General Hospital, says he uses ultrasound on 25% of the patients he sees on an in-patient basis. His biggest use, he says, is for placing central lines—though that could soon change.
“I work with a population that has a very high percentage of injection drug abuse, in whom it’s very difficult to find even a peripheral vein,” he says. At least once a day, he uses ultrasound to locate the brachial vein for such peripheral lines, circumventing the need for a central line through the subclavian vein and the risk of a pneumothorax. “In the past, we would have to put in a central line for just about anything,” he says. “And now we can put in a peripheral line that saves them the risk.”
Another benefit, he says, is in breeding better patient interactions—for example, with gallstones. “You can say to the patient: ‘This is the gallbladder, these are the stones in the gallbladder, this is what’s causing the pain,’” he says. “I think patients appreciate being able to see that firsthand. I think they also appreciate that it’s going to lessen their likelihood of having a complication.”
At the University of Chicago Medical Center, cardiologist Kirk Spencer, MD, says ultrasound procedures still are performed mainly by sonographers and cardiologists. He hopes to change that with a slew of studies demonstrating the feasibility of putting portable ultrasound in the hands of internists.
In one study, hospitalized patients indicated for echocardiography received an echocardiogram, while all others were examined with ultrasound. “We found a significant number of cardio pathologies,” Dr. Spencer says. The findings, he says, were independent of specific medical complaints, such as endocrinology or orthopedic problems.2 “If you were sick enough to get in the hospital, there was a chance that you had a significant cardiac problem that needed to be addressed,” he says.
The study that most excites Dr. Spencer was presented at the 2007 IEEE International Ultrasonics Symposium in October.2 It looked at using ultrasound before releasing a cardiac patient. “One of the biggest problems, one of the most common diagnoses is congestive heart failure,” he says, with a six-month readmission rate of 30% to 40%. Giving ultrasound devices to internists allowed them to look at the amount of fluid around the heart of each cardiac patient.
“The patients who got readmitted all had more fluid detected by ultrasound,” Dr. Spencer says. “So we can do that and say, ‘Hey, you need to stay in the hospital two more days. But if that prevents you from coming back in six months, then that’s a good thing.’”
In patients diagnosed with congestive heart failure, he and his collaborators found, the mean fluid volume was higher for those who were later readmitted. Dr. Spencer plans to pick a reasonable cut-off value and prospectively test whether delaying the release of patients whose fluid levels exceed that value can cut readmission rates.
Most of the battery-operated units used by the medical center weigh between 6 to 10 pounds and cost between $12,000 and $20,000, he says. The devices, about twice the thickness of an iBook, can easily be carried on a shoulder strap. Echocardiogram machines, by contrast, weigh about 300 pounds, must be plugged in and retail for about $250,000.
Concerns, Obstacles
Dr. Spencer cautioned that ultrasound shouldn’t replace echocardiograms or other tools. “So no one is proposing that this would replace a full exam,” he says. “What we’re hoping is that this would detect things that have gone missing or would help ask very specific questions at the bedside.” His studies suggest the approach works well as long as the questions are simple: “Is there fluid or not? Is the heart good or bad?’ But not: ‘Is there an infection?’”
Beyond cardiology and the emergency room, Dr. Spencer says ultrasound has obvious imaging uses in the ICU. The dichotomy, he says, is that imaging intensive care patients can be especially difficult due to their edema, wounds, and lack of mobility. “That area has not blossomed as well as it could have,” he says.
Even so, the burgeoning number of applications for ultrasound “really has huge potential for good,” says Harvey Nisenbaum, MD, an associate professor of radiology at the University of Pennsylvania School of Medicine and president-elect of the American Institute of Ultrasound in Medicine (AIUM). “But the problem is that it’s an art form in the sense that it’s not automated.” No two ultrasound images will be identical, for example, because each depends upon the probe’s precise location. The key, Dr. Nisenbaum says, is proper training under agreed-upon guidelines, followed by continuing education and the maintenance of a hospitalist’s competency.
The AIUM, Nisenbaum says, is working to develop standard credentialing criteria for a range of ultrasound applications to help unify what has been a patchwork approach. Another limitation, he says, has been the lack of Food and Drug Administration (FDA) approval for ultrasound contrast agents Optison and Definity for noncardiac applications. Several deaths have been linked to the use of the intravenous agents in the sickest patients.
The institute is working with the FDA on trying to get the reagents approved for broader use, as they are in other countries. Nisenbaum cautioned the process likely will take a while. Once approved, getting a reimbursement code established for insurance purposes could take even longer.
A further obstacle, according to Dr. Spencer, is the lack of resolution surrounding medical legal issues. “Are we going to agree that this is like a physical examination?” he asks. “It’s unclear whether the medical legal community is going to accept that with ultrasound,” he says.
For cardiology applications, at least, he wonders if the push for reimbursement is such a good thing. “General internists are under incredible pressure [for billing],” he says. “They’re in a really tough spot, and so there would be enormous pressure to get reimbursed for every ultrasound.” As it is, he says, Medicare is targeting echocardiogram as an overutilized reimbursement item. “I hope the reason we’re using this is because we’re examining the patients anyway and this would allow us to find things that we might have missed,” he says. “It’s a better way of examining people, not a new technique for generating revenues. I think that would be a disaster.”
Jeffrey Wiese, MD, SHM board member and associate dean of graduate medical education at Tulane University School of Medicine’s Section of General Internal Medicine and Geriatrics in New Orleans, began putting ultrasound in the hands of his hospitalists and residents in 2007. It’s the “100% right thing to do,” he advises hospitalist groups. “It can be a meaningful way of improving safety. I hope that everyody would move that way.”
Dr. Wiese says residents began using ultrasound more and more for extra visualization during procedures.
“The reason we got into this was straightaway safety, independent of [Centers for Medicare and Medicaid Services] codes and billing—particularly regarding thoracentesis and internal lines,’’ he says. His hospitalists use SonaSite’s MicroMaxx system, “which was a key piece in the way of being able to bill. For all CMT just like endoscopy and bronchoscopy, you have to provide images of the procedure to prove you did it. With the MicroMaxx machine, it allows you to insert a USB and pull down images, take them to a print machine, print them out, and put them in a chart.”
Dr. Wiese touts the sheer amount of what hospitalists can use ultrasound for. “You can do echoes and abdominal ultrasound—not at the level of the radiology room or the cardiology lab, but you can get a quick look,” he says.
Should other hospitalist programs go in the same direction? “From a quality perspective there’s no question you go down that road,” Dr. Wiese asserts. “You do the math: How much does one pneumothorax cost? That’s especially true if [a] pneuothorax finds its way to CMS. One pneuomothorax that you prevent probably pays for your [$20,000-$30,000] machine. That’s even before you get into issues of billing for the use of it, which I think is a secondary way of funding the purchase.”
Forging Ahead
In the meantime, researchers are focusing on ever-diverse applications and smaller units.
At the Mayo Clinic in Jacksonville, Fla., director of regional anesthesia Steven Clendenen, MD, has pioneered the use of ultrasound for guiding nerve blocks.3 The imaging has “totally revolutionized” how the hospital manages pain, he says. As yet, the device still is cart-based, though he expects its size to shrink considerably. “You remember the first calculators, how big they were, and now look at them,” he says.
Beyond working toward miniaturized ultrasound units, Duke’s Smith has been developing real-time three-dimensional angiograms of blood vessels in the brain, a potential boon for stroke diagnoses.4 Another project may bring hospital-based ultrasound full circle: a device that produces a 3-D stereo-image, “like in the IMAX theater,” he says.5 Smith and his colleagues have modified a commercial scanner, “so the target comes out of the screen at you.” Among the many potential uses, expectant parents could see a 3-D stereo view of the developing fetus—something not even the iPhone can offer. TH
Bryn Nelson is a science journalist based in New York.
References
- Hind, D, Calvert, N, McWilliams, R, Davidson, A, Paisley, S, Beverley, C, Thomas, S. Ultrasonic locating devices for central venous cannulation: meta-analysis. Br Med J. 2003;327(7411):361.
- Fedson, S, Neithardt, G, Thomas, P, et al. Unsuspected clinically important findings detected with a small portable ultrasound device in patients admitted to a general medicine service. J Am Soc Echocardiogr. 2003;16(9):901-905.
- Feinglass NG, Clendenen SR, Torp KD, Wang RD, Castello R, Greengrass RA. Real-time three-dimensional ultrasound for continuous popliteal blockade: a case report and image description. Anesth Analg. 2007;105(1):272-274.
- Smith SW, Chu K, Idriss SF, Ivancevich NM, Light ED, Wolf PD. Feasibility Study: Real time 3D ultrasound imaging of the brain. Ultras Med Biol. 2004;30:1365-1371.
- Noble JR, Fronheiser MP, Smith SW. Real-time Stereo 3D Ultrasound. Ultrason Imaging. 2006;28:245-254.
The iPhone may be the latest “it” gadget, but a flurry of recent innovation has given portable ultrasound devices a healthy buzz within the biomedical community.
Beyond the gee-whiz factor, though, a growing number of studies demonstrate the everyday value of putting portable units in the hands of hospitalists.
“The big news has been the tiny portable scanner,” says Stephen Smith, a biomedical engineer at Duke University, in North Carolina, and a pioneer in ultrasound technology. Siemens recently introduced a hand-held device called the Acuson P10, which weighs 1.6 pounds, retails for $9,499 and can fit within a hospitalist’s coat pocket. Not to be outdone, GE has announced plans to introduce an ultrasound unit no bigger than an iPod.
Smith and his collaborators have taken the technology one step farther. They incorporate electrocardial leads on the unit’s transducer face to permit electrocardiograms and a microphone to let hospitalists use the ultrasound like a stethoscope.
Eric Isaacs, MD, a clinical professor of medicine at San Francisco General Hospital, says he routinely uses ultrasound for vascular access “to ensure the safety of procedures that we previously performed either blind or by anatomical landmarks.” Beyond improving the accuracy of placing central and peripheral lines, he says, “the reason we are using ultrasound more now is that the machine is so portable. The radiologists are no longer in the hospital 24 hours a day, and so by necessity we are using the tools that were previously only accessible from 9 to 5.”
Range of Uses
Among the reports recognizing ultrasound’s value, he cited a 2003 study in the British Medical Journal affirming the technology’s superiority to relying on physical landmarks in gaining central venous access, resulting in a lower technical failure rate, reduced complications, and faster access.1 Dr. Issacs says ultrasound also has helped guide procedures such as thorancentesis and paracentesis, other applications once confined to radiology. “It’s something that’s allowing me to do at the bedside what I would otherwise have to wait several hours for,” he says.
For heart patients, he says, a hospitalist can bring ultrasound to the bedside during a cardiac arrest to inspect cardiac motion and fluid, and monitor the patient’s hydration status by examining the size of the inferior vena cava. Internists likewise could examine the size of a patient’s aorta to look for signs of an aneurism, especially for a patient experiencing abdominal pain in the middle of the night. “Quite frankly, it seems like the only limit to ultrasound use is imagination,” Dr. Isaacs says.
Robert Rodriguez, MD, a clinical professor of medicine and emergency medicine at San Francisco General Hospital, says he uses ultrasound on 25% of the patients he sees on an in-patient basis. His biggest use, he says, is for placing central lines—though that could soon change.
“I work with a population that has a very high percentage of injection drug abuse, in whom it’s very difficult to find even a peripheral vein,” he says. At least once a day, he uses ultrasound to locate the brachial vein for such peripheral lines, circumventing the need for a central line through the subclavian vein and the risk of a pneumothorax. “In the past, we would have to put in a central line for just about anything,” he says. “And now we can put in a peripheral line that saves them the risk.”
Another benefit, he says, is in breeding better patient interactions—for example, with gallstones. “You can say to the patient: ‘This is the gallbladder, these are the stones in the gallbladder, this is what’s causing the pain,’” he says. “I think patients appreciate being able to see that firsthand. I think they also appreciate that it’s going to lessen their likelihood of having a complication.”
At the University of Chicago Medical Center, cardiologist Kirk Spencer, MD, says ultrasound procedures still are performed mainly by sonographers and cardiologists. He hopes to change that with a slew of studies demonstrating the feasibility of putting portable ultrasound in the hands of internists.
In one study, hospitalized patients indicated for echocardiography received an echocardiogram, while all others were examined with ultrasound. “We found a significant number of cardio pathologies,” Dr. Spencer says. The findings, he says, were independent of specific medical complaints, such as endocrinology or orthopedic problems.2 “If you were sick enough to get in the hospital, there was a chance that you had a significant cardiac problem that needed to be addressed,” he says.
The study that most excites Dr. Spencer was presented at the 2007 IEEE International Ultrasonics Symposium in October.2 It looked at using ultrasound before releasing a cardiac patient. “One of the biggest problems, one of the most common diagnoses is congestive heart failure,” he says, with a six-month readmission rate of 30% to 40%. Giving ultrasound devices to internists allowed them to look at the amount of fluid around the heart of each cardiac patient.
“The patients who got readmitted all had more fluid detected by ultrasound,” Dr. Spencer says. “So we can do that and say, ‘Hey, you need to stay in the hospital two more days. But if that prevents you from coming back in six months, then that’s a good thing.’”
In patients diagnosed with congestive heart failure, he and his collaborators found, the mean fluid volume was higher for those who were later readmitted. Dr. Spencer plans to pick a reasonable cut-off value and prospectively test whether delaying the release of patients whose fluid levels exceed that value can cut readmission rates.
Most of the battery-operated units used by the medical center weigh between 6 to 10 pounds and cost between $12,000 and $20,000, he says. The devices, about twice the thickness of an iBook, can easily be carried on a shoulder strap. Echocardiogram machines, by contrast, weigh about 300 pounds, must be plugged in and retail for about $250,000.
Concerns, Obstacles
Dr. Spencer cautioned that ultrasound shouldn’t replace echocardiograms or other tools. “So no one is proposing that this would replace a full exam,” he says. “What we’re hoping is that this would detect things that have gone missing or would help ask very specific questions at the bedside.” His studies suggest the approach works well as long as the questions are simple: “Is there fluid or not? Is the heart good or bad?’ But not: ‘Is there an infection?’”
Beyond cardiology and the emergency room, Dr. Spencer says ultrasound has obvious imaging uses in the ICU. The dichotomy, he says, is that imaging intensive care patients can be especially difficult due to their edema, wounds, and lack of mobility. “That area has not blossomed as well as it could have,” he says.
Even so, the burgeoning number of applications for ultrasound “really has huge potential for good,” says Harvey Nisenbaum, MD, an associate professor of radiology at the University of Pennsylvania School of Medicine and president-elect of the American Institute of Ultrasound in Medicine (AIUM). “But the problem is that it’s an art form in the sense that it’s not automated.” No two ultrasound images will be identical, for example, because each depends upon the probe’s precise location. The key, Dr. Nisenbaum says, is proper training under agreed-upon guidelines, followed by continuing education and the maintenance of a hospitalist’s competency.
The AIUM, Nisenbaum says, is working to develop standard credentialing criteria for a range of ultrasound applications to help unify what has been a patchwork approach. Another limitation, he says, has been the lack of Food and Drug Administration (FDA) approval for ultrasound contrast agents Optison and Definity for noncardiac applications. Several deaths have been linked to the use of the intravenous agents in the sickest patients.
The institute is working with the FDA on trying to get the reagents approved for broader use, as they are in other countries. Nisenbaum cautioned the process likely will take a while. Once approved, getting a reimbursement code established for insurance purposes could take even longer.
A further obstacle, according to Dr. Spencer, is the lack of resolution surrounding medical legal issues. “Are we going to agree that this is like a physical examination?” he asks. “It’s unclear whether the medical legal community is going to accept that with ultrasound,” he says.
For cardiology applications, at least, he wonders if the push for reimbursement is such a good thing. “General internists are under incredible pressure [for billing],” he says. “They’re in a really tough spot, and so there would be enormous pressure to get reimbursed for every ultrasound.” As it is, he says, Medicare is targeting echocardiogram as an overutilized reimbursement item. “I hope the reason we’re using this is because we’re examining the patients anyway and this would allow us to find things that we might have missed,” he says. “It’s a better way of examining people, not a new technique for generating revenues. I think that would be a disaster.”
Jeffrey Wiese, MD, SHM board member and associate dean of graduate medical education at Tulane University School of Medicine’s Section of General Internal Medicine and Geriatrics in New Orleans, began putting ultrasound in the hands of his hospitalists and residents in 2007. It’s the “100% right thing to do,” he advises hospitalist groups. “It can be a meaningful way of improving safety. I hope that everyody would move that way.”
Dr. Wiese says residents began using ultrasound more and more for extra visualization during procedures.
“The reason we got into this was straightaway safety, independent of [Centers for Medicare and Medicaid Services] codes and billing—particularly regarding thoracentesis and internal lines,’’ he says. His hospitalists use SonaSite’s MicroMaxx system, “which was a key piece in the way of being able to bill. For all CMT just like endoscopy and bronchoscopy, you have to provide images of the procedure to prove you did it. With the MicroMaxx machine, it allows you to insert a USB and pull down images, take them to a print machine, print them out, and put them in a chart.”
Dr. Wiese touts the sheer amount of what hospitalists can use ultrasound for. “You can do echoes and abdominal ultrasound—not at the level of the radiology room or the cardiology lab, but you can get a quick look,” he says.
Should other hospitalist programs go in the same direction? “From a quality perspective there’s no question you go down that road,” Dr. Wiese asserts. “You do the math: How much does one pneumothorax cost? That’s especially true if [a] pneuothorax finds its way to CMS. One pneuomothorax that you prevent probably pays for your [$20,000-$30,000] machine. That’s even before you get into issues of billing for the use of it, which I think is a secondary way of funding the purchase.”
Forging Ahead
In the meantime, researchers are focusing on ever-diverse applications and smaller units.
At the Mayo Clinic in Jacksonville, Fla., director of regional anesthesia Steven Clendenen, MD, has pioneered the use of ultrasound for guiding nerve blocks.3 The imaging has “totally revolutionized” how the hospital manages pain, he says. As yet, the device still is cart-based, though he expects its size to shrink considerably. “You remember the first calculators, how big they were, and now look at them,” he says.
Beyond working toward miniaturized ultrasound units, Duke’s Smith has been developing real-time three-dimensional angiograms of blood vessels in the brain, a potential boon for stroke diagnoses.4 Another project may bring hospital-based ultrasound full circle: a device that produces a 3-D stereo-image, “like in the IMAX theater,” he says.5 Smith and his colleagues have modified a commercial scanner, “so the target comes out of the screen at you.” Among the many potential uses, expectant parents could see a 3-D stereo view of the developing fetus—something not even the iPhone can offer. TH
Bryn Nelson is a science journalist based in New York.
References
- Hind, D, Calvert, N, McWilliams, R, Davidson, A, Paisley, S, Beverley, C, Thomas, S. Ultrasonic locating devices for central venous cannulation: meta-analysis. Br Med J. 2003;327(7411):361.
- Fedson, S, Neithardt, G, Thomas, P, et al. Unsuspected clinically important findings detected with a small portable ultrasound device in patients admitted to a general medicine service. J Am Soc Echocardiogr. 2003;16(9):901-905.
- Feinglass NG, Clendenen SR, Torp KD, Wang RD, Castello R, Greengrass RA. Real-time three-dimensional ultrasound for continuous popliteal blockade: a case report and image description. Anesth Analg. 2007;105(1):272-274.
- Smith SW, Chu K, Idriss SF, Ivancevich NM, Light ED, Wolf PD. Feasibility Study: Real time 3D ultrasound imaging of the brain. Ultras Med Biol. 2004;30:1365-1371.
- Noble JR, Fronheiser MP, Smith SW. Real-time Stereo 3D Ultrasound. Ultrason Imaging. 2006;28:245-254.
The iPhone may be the latest “it” gadget, but a flurry of recent innovation has given portable ultrasound devices a healthy buzz within the biomedical community.
Beyond the gee-whiz factor, though, a growing number of studies demonstrate the everyday value of putting portable units in the hands of hospitalists.
“The big news has been the tiny portable scanner,” says Stephen Smith, a biomedical engineer at Duke University, in North Carolina, and a pioneer in ultrasound technology. Siemens recently introduced a hand-held device called the Acuson P10, which weighs 1.6 pounds, retails for $9,499 and can fit within a hospitalist’s coat pocket. Not to be outdone, GE has announced plans to introduce an ultrasound unit no bigger than an iPod.
Smith and his collaborators have taken the technology one step farther. They incorporate electrocardial leads on the unit’s transducer face to permit electrocardiograms and a microphone to let hospitalists use the ultrasound like a stethoscope.
Eric Isaacs, MD, a clinical professor of medicine at San Francisco General Hospital, says he routinely uses ultrasound for vascular access “to ensure the safety of procedures that we previously performed either blind or by anatomical landmarks.” Beyond improving the accuracy of placing central and peripheral lines, he says, “the reason we are using ultrasound more now is that the machine is so portable. The radiologists are no longer in the hospital 24 hours a day, and so by necessity we are using the tools that were previously only accessible from 9 to 5.”
Range of Uses
Among the reports recognizing ultrasound’s value, he cited a 2003 study in the British Medical Journal affirming the technology’s superiority to relying on physical landmarks in gaining central venous access, resulting in a lower technical failure rate, reduced complications, and faster access.1 Dr. Issacs says ultrasound also has helped guide procedures such as thorancentesis and paracentesis, other applications once confined to radiology. “It’s something that’s allowing me to do at the bedside what I would otherwise have to wait several hours for,” he says.
For heart patients, he says, a hospitalist can bring ultrasound to the bedside during a cardiac arrest to inspect cardiac motion and fluid, and monitor the patient’s hydration status by examining the size of the inferior vena cava. Internists likewise could examine the size of a patient’s aorta to look for signs of an aneurism, especially for a patient experiencing abdominal pain in the middle of the night. “Quite frankly, it seems like the only limit to ultrasound use is imagination,” Dr. Isaacs says.
Robert Rodriguez, MD, a clinical professor of medicine and emergency medicine at San Francisco General Hospital, says he uses ultrasound on 25% of the patients he sees on an in-patient basis. His biggest use, he says, is for placing central lines—though that could soon change.
“I work with a population that has a very high percentage of injection drug abuse, in whom it’s very difficult to find even a peripheral vein,” he says. At least once a day, he uses ultrasound to locate the brachial vein for such peripheral lines, circumventing the need for a central line through the subclavian vein and the risk of a pneumothorax. “In the past, we would have to put in a central line for just about anything,” he says. “And now we can put in a peripheral line that saves them the risk.”
Another benefit, he says, is in breeding better patient interactions—for example, with gallstones. “You can say to the patient: ‘This is the gallbladder, these are the stones in the gallbladder, this is what’s causing the pain,’” he says. “I think patients appreciate being able to see that firsthand. I think they also appreciate that it’s going to lessen their likelihood of having a complication.”
At the University of Chicago Medical Center, cardiologist Kirk Spencer, MD, says ultrasound procedures still are performed mainly by sonographers and cardiologists. He hopes to change that with a slew of studies demonstrating the feasibility of putting portable ultrasound in the hands of internists.
In one study, hospitalized patients indicated for echocardiography received an echocardiogram, while all others were examined with ultrasound. “We found a significant number of cardio pathologies,” Dr. Spencer says. The findings, he says, were independent of specific medical complaints, such as endocrinology or orthopedic problems.2 “If you were sick enough to get in the hospital, there was a chance that you had a significant cardiac problem that needed to be addressed,” he says.
The study that most excites Dr. Spencer was presented at the 2007 IEEE International Ultrasonics Symposium in October.2 It looked at using ultrasound before releasing a cardiac patient. “One of the biggest problems, one of the most common diagnoses is congestive heart failure,” he says, with a six-month readmission rate of 30% to 40%. Giving ultrasound devices to internists allowed them to look at the amount of fluid around the heart of each cardiac patient.
“The patients who got readmitted all had more fluid detected by ultrasound,” Dr. Spencer says. “So we can do that and say, ‘Hey, you need to stay in the hospital two more days. But if that prevents you from coming back in six months, then that’s a good thing.’”
In patients diagnosed with congestive heart failure, he and his collaborators found, the mean fluid volume was higher for those who were later readmitted. Dr. Spencer plans to pick a reasonable cut-off value and prospectively test whether delaying the release of patients whose fluid levels exceed that value can cut readmission rates.
Most of the battery-operated units used by the medical center weigh between 6 to 10 pounds and cost between $12,000 and $20,000, he says. The devices, about twice the thickness of an iBook, can easily be carried on a shoulder strap. Echocardiogram machines, by contrast, weigh about 300 pounds, must be plugged in and retail for about $250,000.
Concerns, Obstacles
Dr. Spencer cautioned that ultrasound shouldn’t replace echocardiograms or other tools. “So no one is proposing that this would replace a full exam,” he says. “What we’re hoping is that this would detect things that have gone missing or would help ask very specific questions at the bedside.” His studies suggest the approach works well as long as the questions are simple: “Is there fluid or not? Is the heart good or bad?’ But not: ‘Is there an infection?’”
Beyond cardiology and the emergency room, Dr. Spencer says ultrasound has obvious imaging uses in the ICU. The dichotomy, he says, is that imaging intensive care patients can be especially difficult due to their edema, wounds, and lack of mobility. “That area has not blossomed as well as it could have,” he says.
Even so, the burgeoning number of applications for ultrasound “really has huge potential for good,” says Harvey Nisenbaum, MD, an associate professor of radiology at the University of Pennsylvania School of Medicine and president-elect of the American Institute of Ultrasound in Medicine (AIUM). “But the problem is that it’s an art form in the sense that it’s not automated.” No two ultrasound images will be identical, for example, because each depends upon the probe’s precise location. The key, Dr. Nisenbaum says, is proper training under agreed-upon guidelines, followed by continuing education and the maintenance of a hospitalist’s competency.
The AIUM, Nisenbaum says, is working to develop standard credentialing criteria for a range of ultrasound applications to help unify what has been a patchwork approach. Another limitation, he says, has been the lack of Food and Drug Administration (FDA) approval for ultrasound contrast agents Optison and Definity for noncardiac applications. Several deaths have been linked to the use of the intravenous agents in the sickest patients.
The institute is working with the FDA on trying to get the reagents approved for broader use, as they are in other countries. Nisenbaum cautioned the process likely will take a while. Once approved, getting a reimbursement code established for insurance purposes could take even longer.
A further obstacle, according to Dr. Spencer, is the lack of resolution surrounding medical legal issues. “Are we going to agree that this is like a physical examination?” he asks. “It’s unclear whether the medical legal community is going to accept that with ultrasound,” he says.
For cardiology applications, at least, he wonders if the push for reimbursement is such a good thing. “General internists are under incredible pressure [for billing],” he says. “They’re in a really tough spot, and so there would be enormous pressure to get reimbursed for every ultrasound.” As it is, he says, Medicare is targeting echocardiogram as an overutilized reimbursement item. “I hope the reason we’re using this is because we’re examining the patients anyway and this would allow us to find things that we might have missed,” he says. “It’s a better way of examining people, not a new technique for generating revenues. I think that would be a disaster.”
Jeffrey Wiese, MD, SHM board member and associate dean of graduate medical education at Tulane University School of Medicine’s Section of General Internal Medicine and Geriatrics in New Orleans, began putting ultrasound in the hands of his hospitalists and residents in 2007. It’s the “100% right thing to do,” he advises hospitalist groups. “It can be a meaningful way of improving safety. I hope that everyody would move that way.”
Dr. Wiese says residents began using ultrasound more and more for extra visualization during procedures.
“The reason we got into this was straightaway safety, independent of [Centers for Medicare and Medicaid Services] codes and billing—particularly regarding thoracentesis and internal lines,’’ he says. His hospitalists use SonaSite’s MicroMaxx system, “which was a key piece in the way of being able to bill. For all CMT just like endoscopy and bronchoscopy, you have to provide images of the procedure to prove you did it. With the MicroMaxx machine, it allows you to insert a USB and pull down images, take them to a print machine, print them out, and put them in a chart.”
Dr. Wiese touts the sheer amount of what hospitalists can use ultrasound for. “You can do echoes and abdominal ultrasound—not at the level of the radiology room or the cardiology lab, but you can get a quick look,” he says.
Should other hospitalist programs go in the same direction? “From a quality perspective there’s no question you go down that road,” Dr. Wiese asserts. “You do the math: How much does one pneumothorax cost? That’s especially true if [a] pneuothorax finds its way to CMS. One pneuomothorax that you prevent probably pays for your [$20,000-$30,000] machine. That’s even before you get into issues of billing for the use of it, which I think is a secondary way of funding the purchase.”
Forging Ahead
In the meantime, researchers are focusing on ever-diverse applications and smaller units.
At the Mayo Clinic in Jacksonville, Fla., director of regional anesthesia Steven Clendenen, MD, has pioneered the use of ultrasound for guiding nerve blocks.3 The imaging has “totally revolutionized” how the hospital manages pain, he says. As yet, the device still is cart-based, though he expects its size to shrink considerably. “You remember the first calculators, how big they were, and now look at them,” he says.
Beyond working toward miniaturized ultrasound units, Duke’s Smith has been developing real-time three-dimensional angiograms of blood vessels in the brain, a potential boon for stroke diagnoses.4 Another project may bring hospital-based ultrasound full circle: a device that produces a 3-D stereo-image, “like in the IMAX theater,” he says.5 Smith and his colleagues have modified a commercial scanner, “so the target comes out of the screen at you.” Among the many potential uses, expectant parents could see a 3-D stereo view of the developing fetus—something not even the iPhone can offer. TH
Bryn Nelson is a science journalist based in New York.
References
- Hind, D, Calvert, N, McWilliams, R, Davidson, A, Paisley, S, Beverley, C, Thomas, S. Ultrasonic locating devices for central venous cannulation: meta-analysis. Br Med J. 2003;327(7411):361.
- Fedson, S, Neithardt, G, Thomas, P, et al. Unsuspected clinically important findings detected with a small portable ultrasound device in patients admitted to a general medicine service. J Am Soc Echocardiogr. 2003;16(9):901-905.
- Feinglass NG, Clendenen SR, Torp KD, Wang RD, Castello R, Greengrass RA. Real-time three-dimensional ultrasound for continuous popliteal blockade: a case report and image description. Anesth Analg. 2007;105(1):272-274.
- Smith SW, Chu K, Idriss SF, Ivancevich NM, Light ED, Wolf PD. Feasibility Study: Real time 3D ultrasound imaging of the brain. Ultras Med Biol. 2004;30:1365-1371.
- Noble JR, Fronheiser MP, Smith SW. Real-time Stereo 3D Ultrasound. Ultrason Imaging. 2006;28:245-254.
Know the Score
With hospitals facing increasing pressure to improve safety based on measurements, hospitalists need to build a key role in improving quality by developing safety scorecards, say leading hospital medicine experts.
A framework for designing scorecards was recently suggested by researchers at Johns Hopkins University in Baltimore in an article published by the Journal of the American Medical Association.1 The commentary suggests a framework to help healthcare organizations develop safety scorecards, evaluate their validity, and understand measures appropriate to present as rates.
Their framework is intended to build scorecards that monitor progress in improving patient safety over time or relative to a benchmark. The authors urged organizations to think of safety on a continuum and look for improvements, rather than regard practices as either safe or unsafe. They also stated that their term “safety scorecard” acknowledges an overlap between quality and safety.
To build their framework, the researchers adapted elements of the “Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice,” to address three key questions:
- Is the measure important?
- Is the measure valid? and
- Can the measure be used to improve safety in healthcare organizations?
The resulting worksheet to evaluate a scorecard guides hospitals through questions aimed at determining whether their institution meets the three criteria.
Initial Reaction
“This worksheet would be very important to follow, a good step forward in efforts to improve quality because its questions make clear where a group might be falling down in developing a scorecard,” says hospitalist Brian Bossard, MD, director of Inpatient Physician Associates at BryanLGH Medical Center in Lincoln, Neb. Dr. Bossard, who is also the medical staff quality designee, says he believes large national hospitalist groups should be involved in planning scorecards as part of a multidisciplinary team.
Other experts also tout hospitalists’ importance in the vanguard of creating these vital instruments.
“Hospitalists should be on or chairing safety committees, and there should be investments in training them in these areas,” says Eric Kupersmith, MD, division head of hospital medicine and assistant professor of medicine for the Cooper Health System, University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School in Newark. “Because we are frontline physicians experiencing what is happening with patients and orchestrating as well as delivering care, we should provide feedback.”
Dr. Kupersmith, who is on his facility’s patient safety committee and has participated in a root-cause analysis of hand-offs as well as worked on medical reconciliation and pneumonia core measure performance improvement, says SHM “should help take the lead in bringing together specialists, administrators, and nurses with hospitalists who should have a major impact in designing a scorecard as a society.”
As hospitalists mature as clinicians, they become system- and process-oriented, says Dr. Kupersmith. As a result, “We should be part of re-engineering efforts because of our experience bringing people together,” he says. “We’re in a good position to analyze the process.”
Use with Care
There is also value in hospitalists’ anecdotal experiences, Dr. Kupersmith says, but “there needs to be a filter between anecdotes and a facility’s leadership to ensure that the information provided is broadly important. Decisions shouldn’t be made on anecdotes, but creative ideas can come from them.”
Randy Ferrance, DC, MD, and chief of the medical staff at Riverside Tappahannock Hospital in Tappahan-nock, Va., regards anecdotal information in much the same light. “It should be seen as guidelines, not rules,” he cautions. “There is still an art to medicine even though it is clearly science. Sometimes the best available evidence may be anecdotal. It’s not hard data, but it can be valuable.”
Dr. Ferrance at one time chaired his hospital’s quality improvement committee; the panel now reports to him. He believes hospitalists are fortunate that “we became a specialty after evidence-based medicine really came to the forefront. We are fortunate to have the backing of much hard data.”
Still, he acknowledges the difficulty of establishing proof that an action affects patient outcome. “It’s hard, but what we can do is look at what might help result in things like decreased morbidity, length of stays and complications, and a faster return to a patient’s normal functions. Then we might see influence on patient outcomes.”
Beyond Core Measures
Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in New York, works extensively on quality improvements. He urges hospitalists and institutions to go beyond required measures.
“A lot of what we’re doing now is imposed by government, insurers or the Joint Commission,” says Dr. Rohr. “In day-to-day work, we should look for areas to go beyond what is required.”
He believes hospitalists should look at specific issues underlying the Johns Hopkins framework’s three core questions.
For example, within the first core question “Is the measure important?” he suggests hospitalists consider what their facility’s priorities are. “There are thousands of things that could address safety,” he says. “Hospitalists should look at how a measure fits in to their organization’s priorities. At an institution known for cardiac care, look at safety measures in cardiology.”
He also suggests looking at a facility’s potential problem areas to help determine if a measure is important. “I worked at a facility that had a rule that Coumadin had to be ordered one day at a time,” says Dr. Rohr. “Since patient use of the medication was closely monitored, there were very few patients with serious bleeding.”
The point, he says, is that hospitalists should “make sure the safety process they’re interested in truly addresses a true problem and is not already in place. Try to add something of value.”
Hospitalists are in a good position to do this, he says, because of their day-to-day perspective on patients. “Hospitalists should start by looking at what their organization has addressed and what’s causing patient problems day to day and then set priorities,” he advises.
Institutional Support
—Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center, Cortland, N.Y.
Of critical importance also, Dr. Rohr says, is to see what their organization can support before deciding what safety measure to explore.
“Is it feasible to collect data to use this measure?” he asks. “That’s partly dependent on where your facility is in using electronic medical records. Does the benefit of researching and implementing outweigh the cost? You may have to spend some staff time to decide what is worthwhile.”
The last task may be easier for hospitalists working at more academic hospitals, he says, which is also an important part of trying to answer the Johns Hopkins framework’s second question: “Is the measure valid?”
It’s often hard to answer that one, Dr. Rohr acknowledges. “Research has at times shown that a process may show statistically significant improvement, but it does not show up clinically,” he says. “Aspirin and beta-blockers for heart patients, for example, has a statistically significant difference—but it is small.”
Most hospitalists try to see patients and do this kind of work on the side, he concedes: “They should do some research, but value what they see when treating patients. You have a good sense of what has helped patients.”
Julia Wright, MD, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health and medical director for hospital medicine at the University of Wisconsin Hospital in Madison, agrees.
“The expertise of our specialty is that we deliver care that is not just clinical, asking, ‘Did I meet the guidelines?’’’ she says. “We’re with patients. We should help determine how quality and safety models are addressing how care is delivered.”
She also believes hospitalists should work closely with hospital administrators on these issues. “Hospitalists have an intrinsic sense of value in delivering care,” she notes. “We are unique in that we can combine consideration of hospital goals with knowledge of care at patient levels. This provides great value to the institutions.”
Culture of Safety
While he agrees with the importance of involving hospitalists deeply in safety efforts, Dr. Kupersmith believes institutions should strive to create a culture that focuses on safety and looks at all its processes in that light.
“You shouldn’t just track hard outcomes,” he suggests. “Track the outcomes of your processes. This gives an overall sense of safety awareness in all personnel. If you focus on the process and culture, you might find a significant change in outcomes.” This also helps address the difficulty of finding data on outcomes, he says.
He agrees with the researchers’ view that safety is on a continuum, and he thinks acknowledging that can help establish an institutional culture around safety. “There is always going to be patient danger,” he says. “You want to get to a point where it is minimized because of an awareness of actions. That focus on safety will lead to less danger.”
As a result, he believes quality improvement strategies must address culture. “You need to provide education for all on safety and provide oversight and monitoring with expectations that can be tracked,” he says. “You need to create this mandate and speak in the quality language from the top. Then you start to have people bring in information that affects outcomes.” TH
Karla Feuer is a journalist based in New York.
Reference
- Pronovost PJ, Berenholtz SM, Needham DM. A framework for health care organizations to develop and evaluate a safety scorecard. JAMA. 2007;298(17):2063-2065.
With hospitals facing increasing pressure to improve safety based on measurements, hospitalists need to build a key role in improving quality by developing safety scorecards, say leading hospital medicine experts.
A framework for designing scorecards was recently suggested by researchers at Johns Hopkins University in Baltimore in an article published by the Journal of the American Medical Association.1 The commentary suggests a framework to help healthcare organizations develop safety scorecards, evaluate their validity, and understand measures appropriate to present as rates.
Their framework is intended to build scorecards that monitor progress in improving patient safety over time or relative to a benchmark. The authors urged organizations to think of safety on a continuum and look for improvements, rather than regard practices as either safe or unsafe. They also stated that their term “safety scorecard” acknowledges an overlap between quality and safety.
To build their framework, the researchers adapted elements of the “Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice,” to address three key questions:
- Is the measure important?
- Is the measure valid? and
- Can the measure be used to improve safety in healthcare organizations?
The resulting worksheet to evaluate a scorecard guides hospitals through questions aimed at determining whether their institution meets the three criteria.
Initial Reaction
“This worksheet would be very important to follow, a good step forward in efforts to improve quality because its questions make clear where a group might be falling down in developing a scorecard,” says hospitalist Brian Bossard, MD, director of Inpatient Physician Associates at BryanLGH Medical Center in Lincoln, Neb. Dr. Bossard, who is also the medical staff quality designee, says he believes large national hospitalist groups should be involved in planning scorecards as part of a multidisciplinary team.
Other experts also tout hospitalists’ importance in the vanguard of creating these vital instruments.
“Hospitalists should be on or chairing safety committees, and there should be investments in training them in these areas,” says Eric Kupersmith, MD, division head of hospital medicine and assistant professor of medicine for the Cooper Health System, University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School in Newark. “Because we are frontline physicians experiencing what is happening with patients and orchestrating as well as delivering care, we should provide feedback.”
Dr. Kupersmith, who is on his facility’s patient safety committee and has participated in a root-cause analysis of hand-offs as well as worked on medical reconciliation and pneumonia core measure performance improvement, says SHM “should help take the lead in bringing together specialists, administrators, and nurses with hospitalists who should have a major impact in designing a scorecard as a society.”
As hospitalists mature as clinicians, they become system- and process-oriented, says Dr. Kupersmith. As a result, “We should be part of re-engineering efforts because of our experience bringing people together,” he says. “We’re in a good position to analyze the process.”
Use with Care
There is also value in hospitalists’ anecdotal experiences, Dr. Kupersmith says, but “there needs to be a filter between anecdotes and a facility’s leadership to ensure that the information provided is broadly important. Decisions shouldn’t be made on anecdotes, but creative ideas can come from them.”
Randy Ferrance, DC, MD, and chief of the medical staff at Riverside Tappahannock Hospital in Tappahan-nock, Va., regards anecdotal information in much the same light. “It should be seen as guidelines, not rules,” he cautions. “There is still an art to medicine even though it is clearly science. Sometimes the best available evidence may be anecdotal. It’s not hard data, but it can be valuable.”
Dr. Ferrance at one time chaired his hospital’s quality improvement committee; the panel now reports to him. He believes hospitalists are fortunate that “we became a specialty after evidence-based medicine really came to the forefront. We are fortunate to have the backing of much hard data.”
Still, he acknowledges the difficulty of establishing proof that an action affects patient outcome. “It’s hard, but what we can do is look at what might help result in things like decreased morbidity, length of stays and complications, and a faster return to a patient’s normal functions. Then we might see influence on patient outcomes.”
Beyond Core Measures
Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in New York, works extensively on quality improvements. He urges hospitalists and institutions to go beyond required measures.
“A lot of what we’re doing now is imposed by government, insurers or the Joint Commission,” says Dr. Rohr. “In day-to-day work, we should look for areas to go beyond what is required.”
He believes hospitalists should look at specific issues underlying the Johns Hopkins framework’s three core questions.
For example, within the first core question “Is the measure important?” he suggests hospitalists consider what their facility’s priorities are. “There are thousands of things that could address safety,” he says. “Hospitalists should look at how a measure fits in to their organization’s priorities. At an institution known for cardiac care, look at safety measures in cardiology.”
He also suggests looking at a facility’s potential problem areas to help determine if a measure is important. “I worked at a facility that had a rule that Coumadin had to be ordered one day at a time,” says Dr. Rohr. “Since patient use of the medication was closely monitored, there were very few patients with serious bleeding.”
The point, he says, is that hospitalists should “make sure the safety process they’re interested in truly addresses a true problem and is not already in place. Try to add something of value.”
Hospitalists are in a good position to do this, he says, because of their day-to-day perspective on patients. “Hospitalists should start by looking at what their organization has addressed and what’s causing patient problems day to day and then set priorities,” he advises.
Institutional Support
—Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center, Cortland, N.Y.
Of critical importance also, Dr. Rohr says, is to see what their organization can support before deciding what safety measure to explore.
“Is it feasible to collect data to use this measure?” he asks. “That’s partly dependent on where your facility is in using electronic medical records. Does the benefit of researching and implementing outweigh the cost? You may have to spend some staff time to decide what is worthwhile.”
The last task may be easier for hospitalists working at more academic hospitals, he says, which is also an important part of trying to answer the Johns Hopkins framework’s second question: “Is the measure valid?”
It’s often hard to answer that one, Dr. Rohr acknowledges. “Research has at times shown that a process may show statistically significant improvement, but it does not show up clinically,” he says. “Aspirin and beta-blockers for heart patients, for example, has a statistically significant difference—but it is small.”
Most hospitalists try to see patients and do this kind of work on the side, he concedes: “They should do some research, but value what they see when treating patients. You have a good sense of what has helped patients.”
Julia Wright, MD, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health and medical director for hospital medicine at the University of Wisconsin Hospital in Madison, agrees.
“The expertise of our specialty is that we deliver care that is not just clinical, asking, ‘Did I meet the guidelines?’’’ she says. “We’re with patients. We should help determine how quality and safety models are addressing how care is delivered.”
She also believes hospitalists should work closely with hospital administrators on these issues. “Hospitalists have an intrinsic sense of value in delivering care,” she notes. “We are unique in that we can combine consideration of hospital goals with knowledge of care at patient levels. This provides great value to the institutions.”
Culture of Safety
While he agrees with the importance of involving hospitalists deeply in safety efforts, Dr. Kupersmith believes institutions should strive to create a culture that focuses on safety and looks at all its processes in that light.
“You shouldn’t just track hard outcomes,” he suggests. “Track the outcomes of your processes. This gives an overall sense of safety awareness in all personnel. If you focus on the process and culture, you might find a significant change in outcomes.” This also helps address the difficulty of finding data on outcomes, he says.
He agrees with the researchers’ view that safety is on a continuum, and he thinks acknowledging that can help establish an institutional culture around safety. “There is always going to be patient danger,” he says. “You want to get to a point where it is minimized because of an awareness of actions. That focus on safety will lead to less danger.”
As a result, he believes quality improvement strategies must address culture. “You need to provide education for all on safety and provide oversight and monitoring with expectations that can be tracked,” he says. “You need to create this mandate and speak in the quality language from the top. Then you start to have people bring in information that affects outcomes.” TH
Karla Feuer is a journalist based in New York.
Reference
- Pronovost PJ, Berenholtz SM, Needham DM. A framework for health care organizations to develop and evaluate a safety scorecard. JAMA. 2007;298(17):2063-2065.
With hospitals facing increasing pressure to improve safety based on measurements, hospitalists need to build a key role in improving quality by developing safety scorecards, say leading hospital medicine experts.
A framework for designing scorecards was recently suggested by researchers at Johns Hopkins University in Baltimore in an article published by the Journal of the American Medical Association.1 The commentary suggests a framework to help healthcare organizations develop safety scorecards, evaluate their validity, and understand measures appropriate to present as rates.
Their framework is intended to build scorecards that monitor progress in improving patient safety over time or relative to a benchmark. The authors urged organizations to think of safety on a continuum and look for improvements, rather than regard practices as either safe or unsafe. They also stated that their term “safety scorecard” acknowledges an overlap between quality and safety.
To build their framework, the researchers adapted elements of the “Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice,” to address three key questions:
- Is the measure important?
- Is the measure valid? and
- Can the measure be used to improve safety in healthcare organizations?
The resulting worksheet to evaluate a scorecard guides hospitals through questions aimed at determining whether their institution meets the three criteria.
Initial Reaction
“This worksheet would be very important to follow, a good step forward in efforts to improve quality because its questions make clear where a group might be falling down in developing a scorecard,” says hospitalist Brian Bossard, MD, director of Inpatient Physician Associates at BryanLGH Medical Center in Lincoln, Neb. Dr. Bossard, who is also the medical staff quality designee, says he believes large national hospitalist groups should be involved in planning scorecards as part of a multidisciplinary team.
Other experts also tout hospitalists’ importance in the vanguard of creating these vital instruments.
“Hospitalists should be on or chairing safety committees, and there should be investments in training them in these areas,” says Eric Kupersmith, MD, division head of hospital medicine and assistant professor of medicine for the Cooper Health System, University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School in Newark. “Because we are frontline physicians experiencing what is happening with patients and orchestrating as well as delivering care, we should provide feedback.”
Dr. Kupersmith, who is on his facility’s patient safety committee and has participated in a root-cause analysis of hand-offs as well as worked on medical reconciliation and pneumonia core measure performance improvement, says SHM “should help take the lead in bringing together specialists, administrators, and nurses with hospitalists who should have a major impact in designing a scorecard as a society.”
As hospitalists mature as clinicians, they become system- and process-oriented, says Dr. Kupersmith. As a result, “We should be part of re-engineering efforts because of our experience bringing people together,” he says. “We’re in a good position to analyze the process.”
Use with Care
There is also value in hospitalists’ anecdotal experiences, Dr. Kupersmith says, but “there needs to be a filter between anecdotes and a facility’s leadership to ensure that the information provided is broadly important. Decisions shouldn’t be made on anecdotes, but creative ideas can come from them.”
Randy Ferrance, DC, MD, and chief of the medical staff at Riverside Tappahannock Hospital in Tappahan-nock, Va., regards anecdotal information in much the same light. “It should be seen as guidelines, not rules,” he cautions. “There is still an art to medicine even though it is clearly science. Sometimes the best available evidence may be anecdotal. It’s not hard data, but it can be valuable.”
Dr. Ferrance at one time chaired his hospital’s quality improvement committee; the panel now reports to him. He believes hospitalists are fortunate that “we became a specialty after evidence-based medicine really came to the forefront. We are fortunate to have the backing of much hard data.”
Still, he acknowledges the difficulty of establishing proof that an action affects patient outcome. “It’s hard, but what we can do is look at what might help result in things like decreased morbidity, length of stays and complications, and a faster return to a patient’s normal functions. Then we might see influence on patient outcomes.”
Beyond Core Measures
Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in New York, works extensively on quality improvements. He urges hospitalists and institutions to go beyond required measures.
“A lot of what we’re doing now is imposed by government, insurers or the Joint Commission,” says Dr. Rohr. “In day-to-day work, we should look for areas to go beyond what is required.”
He believes hospitalists should look at specific issues underlying the Johns Hopkins framework’s three core questions.
For example, within the first core question “Is the measure important?” he suggests hospitalists consider what their facility’s priorities are. “There are thousands of things that could address safety,” he says. “Hospitalists should look at how a measure fits in to their organization’s priorities. At an institution known for cardiac care, look at safety measures in cardiology.”
He also suggests looking at a facility’s potential problem areas to help determine if a measure is important. “I worked at a facility that had a rule that Coumadin had to be ordered one day at a time,” says Dr. Rohr. “Since patient use of the medication was closely monitored, there were very few patients with serious bleeding.”
The point, he says, is that hospitalists should “make sure the safety process they’re interested in truly addresses a true problem and is not already in place. Try to add something of value.”
Hospitalists are in a good position to do this, he says, because of their day-to-day perspective on patients. “Hospitalists should start by looking at what their organization has addressed and what’s causing patient problems day to day and then set priorities,” he advises.
Institutional Support
—Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center, Cortland, N.Y.
Of critical importance also, Dr. Rohr says, is to see what their organization can support before deciding what safety measure to explore.
“Is it feasible to collect data to use this measure?” he asks. “That’s partly dependent on where your facility is in using electronic medical records. Does the benefit of researching and implementing outweigh the cost? You may have to spend some staff time to decide what is worthwhile.”
The last task may be easier for hospitalists working at more academic hospitals, he says, which is also an important part of trying to answer the Johns Hopkins framework’s second question: “Is the measure valid?”
It’s often hard to answer that one, Dr. Rohr acknowledges. “Research has at times shown that a process may show statistically significant improvement, but it does not show up clinically,” he says. “Aspirin and beta-blockers for heart patients, for example, has a statistically significant difference—but it is small.”
Most hospitalists try to see patients and do this kind of work on the side, he concedes: “They should do some research, but value what they see when treating patients. You have a good sense of what has helped patients.”
Julia Wright, MD, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health and medical director for hospital medicine at the University of Wisconsin Hospital in Madison, agrees.
“The expertise of our specialty is that we deliver care that is not just clinical, asking, ‘Did I meet the guidelines?’’’ she says. “We’re with patients. We should help determine how quality and safety models are addressing how care is delivered.”
She also believes hospitalists should work closely with hospital administrators on these issues. “Hospitalists have an intrinsic sense of value in delivering care,” she notes. “We are unique in that we can combine consideration of hospital goals with knowledge of care at patient levels. This provides great value to the institutions.”
Culture of Safety
While he agrees with the importance of involving hospitalists deeply in safety efforts, Dr. Kupersmith believes institutions should strive to create a culture that focuses on safety and looks at all its processes in that light.
“You shouldn’t just track hard outcomes,” he suggests. “Track the outcomes of your processes. This gives an overall sense of safety awareness in all personnel. If you focus on the process and culture, you might find a significant change in outcomes.” This also helps address the difficulty of finding data on outcomes, he says.
He agrees with the researchers’ view that safety is on a continuum, and he thinks acknowledging that can help establish an institutional culture around safety. “There is always going to be patient danger,” he says. “You want to get to a point where it is minimized because of an awareness of actions. That focus on safety will lead to less danger.”
As a result, he believes quality improvement strategies must address culture. “You need to provide education for all on safety and provide oversight and monitoring with expectations that can be tracked,” he says. “You need to create this mandate and speak in the quality language from the top. Then you start to have people bring in information that affects outcomes.” TH
Karla Feuer is a journalist based in New York.
Reference
- Pronovost PJ, Berenholtz SM, Needham DM. A framework for health care organizations to develop and evaluate a safety scorecard. JAMA. 2007;298(17):2063-2065.
The exercise treadmill test: Estimating cardiovascular prognosis
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
KEY POINTS
- Of the prognostic factors, exercise duration is the one most strongly associated with risk of coronary events and death, independent of age, sex, or known presence and severity of coronary artery disease.
- A decrease in blood pressure with exercise can reflect severe coronary artery disease or left ventricular systolic dysfunction.
- A heart rate that does not increase adequately during exercise or does not recover rapidly after exercise is associated with an increased risk of death.
- Exercise training may help to improve the prognosis of patients with an abnormal hemodynamic response to exercise caused by poor general health.
Update on infectious disease prevention: Human papillomavirus, hepatitis A
How we prevent human papillomavirus (HPV) infection, and how we prevent hepatitis A following exposure to an index case have changed, based on the results of several key clinical trials published during the past year. The results of these studies should influence the measures we take in our daily practice to prevent these diseases. Here is a brief overview of these “impact” studies.
QUADRIVALENT HPV VACCINE PREVENTS CERVICAL LESIONS
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
Cervical cancer is the second most common type of cancer in women and is the leading cause of cancer-related deaths in developing countries. More than 500,000 new cases of cervical cancer are reported worldwide each year, and about 250,000 women die of it.1
Nearly all cases of cervical cancer are caused by HPVs, and the oncogenic types HPV-16 and HPV-18 together account for about 70%. These two types also cause vulvo-vaginal cancer, which accounts for about 6% of all gynecologic malignancies.2 Two other HPV types, HPV-6 and HPV-11, cause genital warts and, less often, cervical intraepithelial neoplasia and cervical invasive cancers.
Two HPV vaccines have been developed. One, sold as Cervarix, is directed against HPV-16 and HPV-18; it is not yet available in the United States. The other, sold as Gardasil, is directed against four HPV types: 6, 11, 16, and 18, and it is currently available (reviewed by Widdice and Kahn3).
The study. The Females United to Unilaterally Reduce Endo/Ectocervical Cancer (FUTURE) II study4 assessed the ability of the quadrivalent vaccine to prevent high-grade cervical lesions. Between June 2002 and September 2003, more than 12,000 women ages 15 to 26 were enrolled at 90 sites in 13 countries. Eligible women were not pregnant, had no abnormal Papanicolaou (Pap) smear, had had four or fewer lifetime sexual partners, and agreed to use effective contraception throughout the course of the study.
In a randomized, double-blind fashion, patients received vaccine or a placebo injection at day 1 and again 2 and 6 months later. They returned for follow-up 1, 6, 24, 36, and 48 months after the third injection, with Pap smears and colposcopy of cervical lesions.
The primary composite end point was the development of grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or invasive cervical carcinoma, with detection of HPV-16 or HPV-18 or both in one or more of the adjacent sections of the same lesion.
In all, 6,087 patients received vaccine and 6,080 received placebo; the two groups were well matched. About 23% had serologic evidence of exposure to either HPV-16 or HPV-18 at enrollment.
Findings. In the analysis of the data, the patients were divided into three overlapping subgroups. The first comprised women who had no serologic evidence of HPV-16 or HPV-18 infection at enrollment, who received all three injections, who remained DNA-negative at month 7, and who had no protocol violations. In this “per-protocol susceptible population,” at an average of 3 years of follow-up, lesions associated with HPV-16 or HPV-18 had developed in 42 of 5,260 women who received placebo, compared with only 1 of 5,305 who received the vaccine. The vaccine efficacy was calculated at 98% (95% confidence interval [CI] 86–100).
The second subgroup were women who had no evidence of HPV-16 or HPV-18 infection at baseline, but whose compliance with the protocol was considered imperfect. In this “unrestricted susceptible population,” the vaccine efficacy was 95% (95% CI 85–99).
The third group included all comers, regardless of whether they were already infected at baseline. In this “intention-to-treat population,” the vaccine efficacy was 44% (95% CI 26–58).
The authors concluded that in young women not previously infected with HPV-16 or HPV-18, vaccine recipients had a significantly lower occurrence of high-grade cervical intraepithelial neoplasia related to these two oncogenic HPV types.
QUADRIVALENT HPV VACCINE PREVENTS ANOGENITAL DISEASE
Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. J Engl J Med 2007; 356:1928–1943.
The study. This double-blind, placebo-controlled study5 tested the usefulness of the quadrivalent HPV vaccine to prevent anogenital disease. It included 5,400 women ages 16 to 24 and was conducted over 14 months in 2002 and 2003 at 62 sites in 16 countries. Women received vaccine or placebo at day 1 and again 2 and 6 months later, and then underwent anogenital and gynecologic examinations at intervals for up to 4 years.
The co-primary composite end points were the incidence of genital warts, vulvar or vaginal intraepithelial neoplasia or cancer, cervical intraepithelial neoplasia, cervical adenocarcinoma in situ, or cervical cancer associated with HPV types 6, 11, 16, or 18.
Findings. In all, 2,700 women were assigned to receive vaccine and 2,700 to receive placebo, and they were followed for an average of 3 years. Twenty percent had pre-existing serologic evidence of infection with one of these four HPV types. In the per-protocol population who were seronegative at day 1 and were compliant, the vaccine efficacy was 100%. In the intention-to-treat group, vaccine reduced the rate of vulvar or vaginal perianal lesions regardless of HPV type by 34%, and reduced the rate of cervical lesions regardless of type by 20%.
HPV VACCINE LIKELY COST-EFFECTIVE IN GIRLS, BUT NOT BOYS
Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
The study. In a review, Newall et al6 looked at four studies that examined the cost-effectiveness of the HPV vaccine. These studies were not perfect and had methodologic limitations because of uncertainty about vaccine efficacy, duration of protection, and the contribution of herd immunity. The studies nevertheless suggested that immunization of young girls but not young boys may be cost-effective, though they suggested the need for further research.
Findings. Three of the studies showed an incremental cost-effectiveness ratio of $14,000 to $24,000 per quality-adjusted year of life gained, which is well within the range for many preventive strategies that we employ in this country.
One of the studies examined the cost-effectiveness of immunizing males, and in that study it was found not to be cost-effective.
TAKE-HOME POINTS ON HPV VACCINATION
Quadrivalent vaccine does indeed reduce the incidence of HPV-associated cervical intra-epithelial neoplasia, vulvar and vaginal intra-epithelial neoplasia, and anogenital diseases in young women, and it is likely cost-effective.
The vaccine works only against HPV types 6, 11, 16, and 18, and 30% of cervical cancers are due to types other than HPV-16 and HPV-18. Also, vaccination is much more effective in patients not yet exposed to HPV, so it would be best to vaccinate them before they become sexually active.
The Advisory Committee on Immunization Practices voted to recommend that girls ages 11 to 12 in this country should receive vaccine.
Regrettably, many third-party payers do not yet pay for the vaccine, and the cost (around $375) must be paid out of pocket. Also, this issue remains politically charged and controversial. Some states have mandated vaccination and another 15 are presently considering legislation mandating vaccination. Such legislation has been defeated in four states.
My own practice is to offer the vaccine to 11- and 12-year old girls, and to older girls and young women (not to boys), especially if the health insurance plan covers it or if the patient or the patient’s family can afford it.
HEPATITIS A VACCINE IS AS GOOD AS IMMUNE GLOBULIN AFTER EXPOSURE
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
Before 1995, when the first hepatitis A vaccine was introduced, about 30,000 cases of hepatitis A were reported each year in the United States. This was thought to be the tip of the iceberg: since this infection is often subclinical, estimates of up to 300,000 cases per year were given.
At first, immunization against hepatitis A in this country was confined to children over age 2 in states in which hepatitis A occurred more often than the norm. In 2005, after it had become clear that the vaccine was highly effective, the Advisory Committee on Immunization Practices revised its recommendations to include immunization of children between the ages of 12 and 23 months,7 so that they would complete this two-stage vaccination procedure by the time they reached the age of 2 years. With that strategy, the annual occurrence of hepatitis A in the United States fell dramatically, to about 4,000 cases per year in 2005, the lowest number of cases reported in the last 40 years. At present, most hepatitis A infections in this country are not from casual idiosyncratic transmission but rather are food-borne.
Still, hepatitis A remains a major problem in many parts of the world. Moreover, the availability of immune globulin, the traditional recommended agent for postexposure pro-phylaxis, has been limited because only one company manufactures it and the price has steadily escalated.
The study. Investigators at the University of Michigan and in Kazakhstan compared conventional doses of immune globulin vs hepatitis A vaccine as postexposure prophylaxis, given within 14 days of exposure to index cases of hepatitis A.8 Excluded were persons under the age of 2 years or over the age of 40, those with a history of hepatitis A or vaccination, those with liver disease, and those with other contraindications. The primary end point was the development of symptomatic, laboratory-confirmed hepatitis A, defined as a positive test for immunoglobulin M antibodies to hepatitis A; transaminase levels greater than two times the upper limit of normal; and symptoms consistent with hepatitis A in the absence of another identifiable disease that occurred within 15 to 56 days of exposure to the index case.
Findings. Of 4,524 contacts randomized, only 1,414 (31%) were susceptible to hepatitis A, suggesting that the prevalence of hepatitis A in Kazakhstan was high at that time. Of these, 1,090 completed the immunization and follow-up protocol and were eligible for the final analysis. Of these, 568 received vaccine and 522 received globulin. The average age was 12 years, the average time to vaccination after exposure was 10 days; 16% of the exposures occurred in the day-care setting, and 84% of the exposures occurred from household contacts.
Symptomatic hepatitis A occurred in 4.4% of vaccine recipients vs 3.3% of immunoglobulin recipients. The authors concluded that hepatitis A vaccine met the test of noninferiority, that both strategies were highly protective, but that immunoglobulin was modestly better. Thus, in June 2007, the Advisory Committee on Immunization Practices recommended hepatitis A vaccine as the preferred regimen for postexposure prophylaxis.9
This approach has several advantages:
- Hepatitis A vaccine confers immunity and long-term protection, which globulin does not
- The supply of vaccine is abundant
- Vaccine is relatively cheap
- Vaccine is easy to give.
This study, however, does not apply to people younger than 2 years or older than 40, those who are immunocompromised, or those who have chronic liver disease. In these groups, the recommendation is still to use immunoglobulin in postexposure prophylaxis.
- CancerMondial. International Agency for Research on Cancer. www-dep.iarc.fr/. Accessed May 12, 2008.
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Widdice LE, Kahn JA. Using the new HPV vaccines in clinical practice. Cleve Clin J Med 2006; 73:929–935.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
- Advisory Committee on Immunization Practices (ACIP)Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
- Advisory Committee on Immunization Practices, US Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
How we prevent human papillomavirus (HPV) infection, and how we prevent hepatitis A following exposure to an index case have changed, based on the results of several key clinical trials published during the past year. The results of these studies should influence the measures we take in our daily practice to prevent these diseases. Here is a brief overview of these “impact” studies.
QUADRIVALENT HPV VACCINE PREVENTS CERVICAL LESIONS
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
Cervical cancer is the second most common type of cancer in women and is the leading cause of cancer-related deaths in developing countries. More than 500,000 new cases of cervical cancer are reported worldwide each year, and about 250,000 women die of it.1
Nearly all cases of cervical cancer are caused by HPVs, and the oncogenic types HPV-16 and HPV-18 together account for about 70%. These two types also cause vulvo-vaginal cancer, which accounts for about 6% of all gynecologic malignancies.2 Two other HPV types, HPV-6 and HPV-11, cause genital warts and, less often, cervical intraepithelial neoplasia and cervical invasive cancers.
Two HPV vaccines have been developed. One, sold as Cervarix, is directed against HPV-16 and HPV-18; it is not yet available in the United States. The other, sold as Gardasil, is directed against four HPV types: 6, 11, 16, and 18, and it is currently available (reviewed by Widdice and Kahn3).
The study. The Females United to Unilaterally Reduce Endo/Ectocervical Cancer (FUTURE) II study4 assessed the ability of the quadrivalent vaccine to prevent high-grade cervical lesions. Between June 2002 and September 2003, more than 12,000 women ages 15 to 26 were enrolled at 90 sites in 13 countries. Eligible women were not pregnant, had no abnormal Papanicolaou (Pap) smear, had had four or fewer lifetime sexual partners, and agreed to use effective contraception throughout the course of the study.
In a randomized, double-blind fashion, patients received vaccine or a placebo injection at day 1 and again 2 and 6 months later. They returned for follow-up 1, 6, 24, 36, and 48 months after the third injection, with Pap smears and colposcopy of cervical lesions.
The primary composite end point was the development of grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or invasive cervical carcinoma, with detection of HPV-16 or HPV-18 or both in one or more of the adjacent sections of the same lesion.
In all, 6,087 patients received vaccine and 6,080 received placebo; the two groups were well matched. About 23% had serologic evidence of exposure to either HPV-16 or HPV-18 at enrollment.
Findings. In the analysis of the data, the patients were divided into three overlapping subgroups. The first comprised women who had no serologic evidence of HPV-16 or HPV-18 infection at enrollment, who received all three injections, who remained DNA-negative at month 7, and who had no protocol violations. In this “per-protocol susceptible population,” at an average of 3 years of follow-up, lesions associated with HPV-16 or HPV-18 had developed in 42 of 5,260 women who received placebo, compared with only 1 of 5,305 who received the vaccine. The vaccine efficacy was calculated at 98% (95% confidence interval [CI] 86–100).
The second subgroup were women who had no evidence of HPV-16 or HPV-18 infection at baseline, but whose compliance with the protocol was considered imperfect. In this “unrestricted susceptible population,” the vaccine efficacy was 95% (95% CI 85–99).
The third group included all comers, regardless of whether they were already infected at baseline. In this “intention-to-treat population,” the vaccine efficacy was 44% (95% CI 26–58).
The authors concluded that in young women not previously infected with HPV-16 or HPV-18, vaccine recipients had a significantly lower occurrence of high-grade cervical intraepithelial neoplasia related to these two oncogenic HPV types.
QUADRIVALENT HPV VACCINE PREVENTS ANOGENITAL DISEASE
Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. J Engl J Med 2007; 356:1928–1943.
The study. This double-blind, placebo-controlled study5 tested the usefulness of the quadrivalent HPV vaccine to prevent anogenital disease. It included 5,400 women ages 16 to 24 and was conducted over 14 months in 2002 and 2003 at 62 sites in 16 countries. Women received vaccine or placebo at day 1 and again 2 and 6 months later, and then underwent anogenital and gynecologic examinations at intervals for up to 4 years.
The co-primary composite end points were the incidence of genital warts, vulvar or vaginal intraepithelial neoplasia or cancer, cervical intraepithelial neoplasia, cervical adenocarcinoma in situ, or cervical cancer associated with HPV types 6, 11, 16, or 18.
Findings. In all, 2,700 women were assigned to receive vaccine and 2,700 to receive placebo, and they were followed for an average of 3 years. Twenty percent had pre-existing serologic evidence of infection with one of these four HPV types. In the per-protocol population who were seronegative at day 1 and were compliant, the vaccine efficacy was 100%. In the intention-to-treat group, vaccine reduced the rate of vulvar or vaginal perianal lesions regardless of HPV type by 34%, and reduced the rate of cervical lesions regardless of type by 20%.
HPV VACCINE LIKELY COST-EFFECTIVE IN GIRLS, BUT NOT BOYS
Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
The study. In a review, Newall et al6 looked at four studies that examined the cost-effectiveness of the HPV vaccine. These studies were not perfect and had methodologic limitations because of uncertainty about vaccine efficacy, duration of protection, and the contribution of herd immunity. The studies nevertheless suggested that immunization of young girls but not young boys may be cost-effective, though they suggested the need for further research.
Findings. Three of the studies showed an incremental cost-effectiveness ratio of $14,000 to $24,000 per quality-adjusted year of life gained, which is well within the range for many preventive strategies that we employ in this country.
One of the studies examined the cost-effectiveness of immunizing males, and in that study it was found not to be cost-effective.
TAKE-HOME POINTS ON HPV VACCINATION
Quadrivalent vaccine does indeed reduce the incidence of HPV-associated cervical intra-epithelial neoplasia, vulvar and vaginal intra-epithelial neoplasia, and anogenital diseases in young women, and it is likely cost-effective.
The vaccine works only against HPV types 6, 11, 16, and 18, and 30% of cervical cancers are due to types other than HPV-16 and HPV-18. Also, vaccination is much more effective in patients not yet exposed to HPV, so it would be best to vaccinate them before they become sexually active.
The Advisory Committee on Immunization Practices voted to recommend that girls ages 11 to 12 in this country should receive vaccine.
Regrettably, many third-party payers do not yet pay for the vaccine, and the cost (around $375) must be paid out of pocket. Also, this issue remains politically charged and controversial. Some states have mandated vaccination and another 15 are presently considering legislation mandating vaccination. Such legislation has been defeated in four states.
My own practice is to offer the vaccine to 11- and 12-year old girls, and to older girls and young women (not to boys), especially if the health insurance plan covers it or if the patient or the patient’s family can afford it.
HEPATITIS A VACCINE IS AS GOOD AS IMMUNE GLOBULIN AFTER EXPOSURE
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
Before 1995, when the first hepatitis A vaccine was introduced, about 30,000 cases of hepatitis A were reported each year in the United States. This was thought to be the tip of the iceberg: since this infection is often subclinical, estimates of up to 300,000 cases per year were given.
At first, immunization against hepatitis A in this country was confined to children over age 2 in states in which hepatitis A occurred more often than the norm. In 2005, after it had become clear that the vaccine was highly effective, the Advisory Committee on Immunization Practices revised its recommendations to include immunization of children between the ages of 12 and 23 months,7 so that they would complete this two-stage vaccination procedure by the time they reached the age of 2 years. With that strategy, the annual occurrence of hepatitis A in the United States fell dramatically, to about 4,000 cases per year in 2005, the lowest number of cases reported in the last 40 years. At present, most hepatitis A infections in this country are not from casual idiosyncratic transmission but rather are food-borne.
Still, hepatitis A remains a major problem in many parts of the world. Moreover, the availability of immune globulin, the traditional recommended agent for postexposure pro-phylaxis, has been limited because only one company manufactures it and the price has steadily escalated.
The study. Investigators at the University of Michigan and in Kazakhstan compared conventional doses of immune globulin vs hepatitis A vaccine as postexposure prophylaxis, given within 14 days of exposure to index cases of hepatitis A.8 Excluded were persons under the age of 2 years or over the age of 40, those with a history of hepatitis A or vaccination, those with liver disease, and those with other contraindications. The primary end point was the development of symptomatic, laboratory-confirmed hepatitis A, defined as a positive test for immunoglobulin M antibodies to hepatitis A; transaminase levels greater than two times the upper limit of normal; and symptoms consistent with hepatitis A in the absence of another identifiable disease that occurred within 15 to 56 days of exposure to the index case.
Findings. Of 4,524 contacts randomized, only 1,414 (31%) were susceptible to hepatitis A, suggesting that the prevalence of hepatitis A in Kazakhstan was high at that time. Of these, 1,090 completed the immunization and follow-up protocol and were eligible for the final analysis. Of these, 568 received vaccine and 522 received globulin. The average age was 12 years, the average time to vaccination after exposure was 10 days; 16% of the exposures occurred in the day-care setting, and 84% of the exposures occurred from household contacts.
Symptomatic hepatitis A occurred in 4.4% of vaccine recipients vs 3.3% of immunoglobulin recipients. The authors concluded that hepatitis A vaccine met the test of noninferiority, that both strategies were highly protective, but that immunoglobulin was modestly better. Thus, in June 2007, the Advisory Committee on Immunization Practices recommended hepatitis A vaccine as the preferred regimen for postexposure prophylaxis.9
This approach has several advantages:
- Hepatitis A vaccine confers immunity and long-term protection, which globulin does not
- The supply of vaccine is abundant
- Vaccine is relatively cheap
- Vaccine is easy to give.
This study, however, does not apply to people younger than 2 years or older than 40, those who are immunocompromised, or those who have chronic liver disease. In these groups, the recommendation is still to use immunoglobulin in postexposure prophylaxis.
How we prevent human papillomavirus (HPV) infection, and how we prevent hepatitis A following exposure to an index case have changed, based on the results of several key clinical trials published during the past year. The results of these studies should influence the measures we take in our daily practice to prevent these diseases. Here is a brief overview of these “impact” studies.
QUADRIVALENT HPV VACCINE PREVENTS CERVICAL LESIONS
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
Cervical cancer is the second most common type of cancer in women and is the leading cause of cancer-related deaths in developing countries. More than 500,000 new cases of cervical cancer are reported worldwide each year, and about 250,000 women die of it.1
Nearly all cases of cervical cancer are caused by HPVs, and the oncogenic types HPV-16 and HPV-18 together account for about 70%. These two types also cause vulvo-vaginal cancer, which accounts for about 6% of all gynecologic malignancies.2 Two other HPV types, HPV-6 and HPV-11, cause genital warts and, less often, cervical intraepithelial neoplasia and cervical invasive cancers.
Two HPV vaccines have been developed. One, sold as Cervarix, is directed against HPV-16 and HPV-18; it is not yet available in the United States. The other, sold as Gardasil, is directed against four HPV types: 6, 11, 16, and 18, and it is currently available (reviewed by Widdice and Kahn3).
The study. The Females United to Unilaterally Reduce Endo/Ectocervical Cancer (FUTURE) II study4 assessed the ability of the quadrivalent vaccine to prevent high-grade cervical lesions. Between June 2002 and September 2003, more than 12,000 women ages 15 to 26 were enrolled at 90 sites in 13 countries. Eligible women were not pregnant, had no abnormal Papanicolaou (Pap) smear, had had four or fewer lifetime sexual partners, and agreed to use effective contraception throughout the course of the study.
In a randomized, double-blind fashion, patients received vaccine or a placebo injection at day 1 and again 2 and 6 months later. They returned for follow-up 1, 6, 24, 36, and 48 months after the third injection, with Pap smears and colposcopy of cervical lesions.
The primary composite end point was the development of grade 2 or 3 cervical intraepithelial neoplasia, adenocarcinoma in situ, or invasive cervical carcinoma, with detection of HPV-16 or HPV-18 or both in one or more of the adjacent sections of the same lesion.
In all, 6,087 patients received vaccine and 6,080 received placebo; the two groups were well matched. About 23% had serologic evidence of exposure to either HPV-16 or HPV-18 at enrollment.
Findings. In the analysis of the data, the patients were divided into three overlapping subgroups. The first comprised women who had no serologic evidence of HPV-16 or HPV-18 infection at enrollment, who received all three injections, who remained DNA-negative at month 7, and who had no protocol violations. In this “per-protocol susceptible population,” at an average of 3 years of follow-up, lesions associated with HPV-16 or HPV-18 had developed in 42 of 5,260 women who received placebo, compared with only 1 of 5,305 who received the vaccine. The vaccine efficacy was calculated at 98% (95% confidence interval [CI] 86–100).
The second subgroup were women who had no evidence of HPV-16 or HPV-18 infection at baseline, but whose compliance with the protocol was considered imperfect. In this “unrestricted susceptible population,” the vaccine efficacy was 95% (95% CI 85–99).
The third group included all comers, regardless of whether they were already infected at baseline. In this “intention-to-treat population,” the vaccine efficacy was 44% (95% CI 26–58).
The authors concluded that in young women not previously infected with HPV-16 or HPV-18, vaccine recipients had a significantly lower occurrence of high-grade cervical intraepithelial neoplasia related to these two oncogenic HPV types.
QUADRIVALENT HPV VACCINE PREVENTS ANOGENITAL DISEASE
Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. J Engl J Med 2007; 356:1928–1943.
The study. This double-blind, placebo-controlled study5 tested the usefulness of the quadrivalent HPV vaccine to prevent anogenital disease. It included 5,400 women ages 16 to 24 and was conducted over 14 months in 2002 and 2003 at 62 sites in 16 countries. Women received vaccine or placebo at day 1 and again 2 and 6 months later, and then underwent anogenital and gynecologic examinations at intervals for up to 4 years.
The co-primary composite end points were the incidence of genital warts, vulvar or vaginal intraepithelial neoplasia or cancer, cervical intraepithelial neoplasia, cervical adenocarcinoma in situ, or cervical cancer associated with HPV types 6, 11, 16, or 18.
Findings. In all, 2,700 women were assigned to receive vaccine and 2,700 to receive placebo, and they were followed for an average of 3 years. Twenty percent had pre-existing serologic evidence of infection with one of these four HPV types. In the per-protocol population who were seronegative at day 1 and were compliant, the vaccine efficacy was 100%. In the intention-to-treat group, vaccine reduced the rate of vulvar or vaginal perianal lesions regardless of HPV type by 34%, and reduced the rate of cervical lesions regardless of type by 20%.
HPV VACCINE LIKELY COST-EFFECTIVE IN GIRLS, BUT NOT BOYS
Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
The study. In a review, Newall et al6 looked at four studies that examined the cost-effectiveness of the HPV vaccine. These studies were not perfect and had methodologic limitations because of uncertainty about vaccine efficacy, duration of protection, and the contribution of herd immunity. The studies nevertheless suggested that immunization of young girls but not young boys may be cost-effective, though they suggested the need for further research.
Findings. Three of the studies showed an incremental cost-effectiveness ratio of $14,000 to $24,000 per quality-adjusted year of life gained, which is well within the range for many preventive strategies that we employ in this country.
One of the studies examined the cost-effectiveness of immunizing males, and in that study it was found not to be cost-effective.
TAKE-HOME POINTS ON HPV VACCINATION
Quadrivalent vaccine does indeed reduce the incidence of HPV-associated cervical intra-epithelial neoplasia, vulvar and vaginal intra-epithelial neoplasia, and anogenital diseases in young women, and it is likely cost-effective.
The vaccine works only against HPV types 6, 11, 16, and 18, and 30% of cervical cancers are due to types other than HPV-16 and HPV-18. Also, vaccination is much more effective in patients not yet exposed to HPV, so it would be best to vaccinate them before they become sexually active.
The Advisory Committee on Immunization Practices voted to recommend that girls ages 11 to 12 in this country should receive vaccine.
Regrettably, many third-party payers do not yet pay for the vaccine, and the cost (around $375) must be paid out of pocket. Also, this issue remains politically charged and controversial. Some states have mandated vaccination and another 15 are presently considering legislation mandating vaccination. Such legislation has been defeated in four states.
My own practice is to offer the vaccine to 11- and 12-year old girls, and to older girls and young women (not to boys), especially if the health insurance plan covers it or if the patient or the patient’s family can afford it.
HEPATITIS A VACCINE IS AS GOOD AS IMMUNE GLOBULIN AFTER EXPOSURE
Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
Before 1995, when the first hepatitis A vaccine was introduced, about 30,000 cases of hepatitis A were reported each year in the United States. This was thought to be the tip of the iceberg: since this infection is often subclinical, estimates of up to 300,000 cases per year were given.
At first, immunization against hepatitis A in this country was confined to children over age 2 in states in which hepatitis A occurred more often than the norm. In 2005, after it had become clear that the vaccine was highly effective, the Advisory Committee on Immunization Practices revised its recommendations to include immunization of children between the ages of 12 and 23 months,7 so that they would complete this two-stage vaccination procedure by the time they reached the age of 2 years. With that strategy, the annual occurrence of hepatitis A in the United States fell dramatically, to about 4,000 cases per year in 2005, the lowest number of cases reported in the last 40 years. At present, most hepatitis A infections in this country are not from casual idiosyncratic transmission but rather are food-borne.
Still, hepatitis A remains a major problem in many parts of the world. Moreover, the availability of immune globulin, the traditional recommended agent for postexposure pro-phylaxis, has been limited because only one company manufactures it and the price has steadily escalated.
The study. Investigators at the University of Michigan and in Kazakhstan compared conventional doses of immune globulin vs hepatitis A vaccine as postexposure prophylaxis, given within 14 days of exposure to index cases of hepatitis A.8 Excluded were persons under the age of 2 years or over the age of 40, those with a history of hepatitis A or vaccination, those with liver disease, and those with other contraindications. The primary end point was the development of symptomatic, laboratory-confirmed hepatitis A, defined as a positive test for immunoglobulin M antibodies to hepatitis A; transaminase levels greater than two times the upper limit of normal; and symptoms consistent with hepatitis A in the absence of another identifiable disease that occurred within 15 to 56 days of exposure to the index case.
Findings. Of 4,524 contacts randomized, only 1,414 (31%) were susceptible to hepatitis A, suggesting that the prevalence of hepatitis A in Kazakhstan was high at that time. Of these, 1,090 completed the immunization and follow-up protocol and were eligible for the final analysis. Of these, 568 received vaccine and 522 received globulin. The average age was 12 years, the average time to vaccination after exposure was 10 days; 16% of the exposures occurred in the day-care setting, and 84% of the exposures occurred from household contacts.
Symptomatic hepatitis A occurred in 4.4% of vaccine recipients vs 3.3% of immunoglobulin recipients. The authors concluded that hepatitis A vaccine met the test of noninferiority, that both strategies were highly protective, but that immunoglobulin was modestly better. Thus, in June 2007, the Advisory Committee on Immunization Practices recommended hepatitis A vaccine as the preferred regimen for postexposure prophylaxis.9
This approach has several advantages:
- Hepatitis A vaccine confers immunity and long-term protection, which globulin does not
- The supply of vaccine is abundant
- Vaccine is relatively cheap
- Vaccine is easy to give.
This study, however, does not apply to people younger than 2 years or older than 40, those who are immunocompromised, or those who have chronic liver disease. In these groups, the recommendation is still to use immunoglobulin in postexposure prophylaxis.
- CancerMondial. International Agency for Research on Cancer. www-dep.iarc.fr/. Accessed May 12, 2008.
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Widdice LE, Kahn JA. Using the new HPV vaccines in clinical practice. Cleve Clin J Med 2006; 73:929–935.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
- Advisory Committee on Immunization Practices (ACIP)Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
- Advisory Committee on Immunization Practices, US Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- CancerMondial. International Agency for Research on Cancer. www-dep.iarc.fr/. Accessed May 12, 2008.
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Widdice LE, Kahn JA. Using the new HPV vaccines in clinical practice. Cleve Clin J Med 2006; 73:929–935.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Newall AT, Beutels P, Wood JG, Edmunds WJ, MacIntyre CR. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Infect Dis 2007; 7:289–296.
- Advisory Committee on Immunization Practices (ACIP)Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007; 357:1685–1694.
- Advisory Committee on Immunization Practices, US Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
Instant Poll Results
JULY 2007
Have you been drilled recently to prepare for massive obstetric hemorrhage?
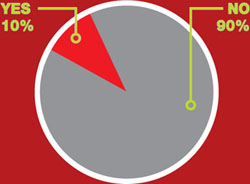
The Joint Commission recommends that labor and delivery services practice responding to common obstetric emergencies by using simulation training. Has your obstetric service had a simulation drill for massive obstetric hemorrhage during the past year?
SEPTEMBER 2007
Can you prognosticate the future of the specialty?
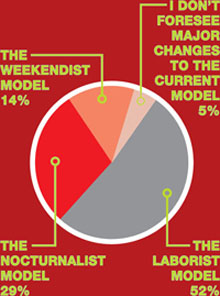
Gazing into the future, which of the following “-ist” models do you think ObGyn practices are most likely to heavily rely on to boost the career satisfaction of practicing obstetrician-gynecologists?
JULY 2007
Have you been drilled recently to prepare for massive obstetric hemorrhage?

The Joint Commission recommends that labor and delivery services practice responding to common obstetric emergencies by using simulation training. Has your obstetric service had a simulation drill for massive obstetric hemorrhage during the past year?
SEPTEMBER 2007
Can you prognosticate the future of the specialty?

Gazing into the future, which of the following “-ist” models do you think ObGyn practices are most likely to heavily rely on to boost the career satisfaction of practicing obstetrician-gynecologists?
JULY 2007
Have you been drilled recently to prepare for massive obstetric hemorrhage?

The Joint Commission recommends that labor and delivery services practice responding to common obstetric emergencies by using simulation training. Has your obstetric service had a simulation drill for massive obstetric hemorrhage during the past year?
SEPTEMBER 2007
Can you prognosticate the future of the specialty?

Gazing into the future, which of the following “-ist” models do you think ObGyn practices are most likely to heavily rely on to boost the career satisfaction of practicing obstetrician-gynecologists?
When necessity calls for treating uterine fibroids
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.