User login
Purple-red papules on foot
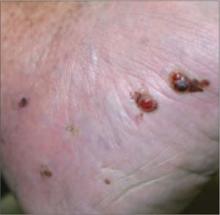
An 88-year-old Caucasian man of Italian ancestry came into our clinic with multiple, painful purple-red “growths” on his left foot that he’d had for several years (FIGURE 1).
The patient had no systemic complaints (no fever, chills, weight loss, night sweats). He had a history of hypertension, a cardiac valve replacement, and chronic back pain (secondary to a motor vehicle accident). He was taking warfarin and nadolol.
The patient had multiple, 0.1– to 0.5-cm purple-red papules and nodules on the dorsal and plantar surfaces of the left foot, with associated moderate lower extremity edema and mottled dyspigmentation.
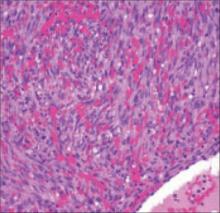
We did a punch biopsy, which showed a nodular neoplasm composed of moderately plump, spindle-shaped cells in short interweaving fascicles and numerous extravasated erythrocytes in the spaces (“vascular slits”) between the spindle-shaped cells (FIGURE 2).
FIGURE 1
Painful papules and nodules
FIGURE 2
Hematoxylin/eosin stain
What is your diagnosis?
How would you manage this condition?
Diagnosis: Kaposi’s sarcoma
Classic Kaposi’s sarcoma is a rare mesenchymal tumor most often seen in elderly men of Mediterranean or Ashkenazi Jewish origin with an annual incidence in the United States of between 0.02% and 0.06%, with a peak occurring in the 5th to 8th decade of life.1 (Two-thirds of cases develop after the age of 50.) Population-based studies in the United States have shown a male-to-female ratio of 4:1.1
First described by the Hungarian dermatologist Moritz Kaposi in 1872, Kaposi’s sarcoma assumed prominence during the emerging HIV epidemic and is now the most common tumor in patients with acquired immune deficiency syndrome (AIDS).2
Recent research has implicated the human herpes virus–8 (HHV–8) as an inductive agent (necessary though not sufficient) in all epidemiologic subsets of the disease.2
There are 4 principal clinical variants of Kaposi’s sarcoma:
- classic (or chronic),
- African endemic (includes childhood lymphadenopathic),
- transplant-associated, and
- AIDS-related.
What you’ll see
Clinically, classic Kaposi’s sarcoma often first manifests as blue-red, well-demarcated, painless macules confined to the distal lower extremities.3 These slow-growing lesions may enlarge to forms papules and plaques, or progress to nodules and tumors. Unilateral involvement is often observed at the outset of the disease, with potential centripetal spread occurring late-in-course.3
Early lesions are generally soft, spongy, and “angiomatous,” while in the advanced state, lesional skin becomes hard, solid, and brown in color.3 Edema of the surrounding tissue is common. In addition to the skin, classic Kaposi’s sarcoma also involves mucosal sites (especially the oral and gastrointestinal mucosae).
Differential includes melanocytic nevus
A differential diagnosis for classic Kaposi’s sarcoma includes stasis dermatitis (“acroangiodermatitis”), melanocytic nevus, pyogenic granuloma, hemangioma, granuloma annulare, arthropod assault, and dermatofibroma/dermatofibrosarcoma protuberans (DF/DFSP).
Melanocytic nevi, pyogenic granuloma, hemangioma, granuloma annulare, and DF/DFSP ordinarily feature single lesions, while Kaposi’s sarcoma has multiple lesions. An arthropod assault is pruritic, and stasis dermatitis typically has dilated/varicose veins.
Histology will confirm your suspicions
While epidemiological and clinical factors may suggest classic Kaposi’s sarcoma, a final diagnosis ultimately rests on confirmatory histology. The pathology of classic Kaposi’s sarcoma (like all of the variant subtypes) is based solely on stage of the lesion.
Early patch-stage lesions exhibit papillary dermal proliferation of small, angulated vessels lined by bland endothelial cells with an accompanying sparse infiltrate of lymphocytes and plasma cells.
As the disease progresses to the plaque stage, the vascular proliferation expands into the reticular dermis and subcutis. The transition to nodular Kaposi’s sarcoma develops when a population of spindle cells expressing endothelial markers occurs between the “vascular slits” (FIGURE 2).
Chemotherapy for rapidly progressive disease
There is minimal evidence-based data for the treatment of Kaposi’s sarcoma. Treatment options for limited disease include surgical excision, cryotherapy, laser ablation, topical retinoids (alitretinoin), interferon-alpha, and radiation.1
If rapidly progressive disease (>10 new lesions per month) exists, the most effective treatment remains systemic chemotherapy (vincristine, doxorubicin, vinblastine,4 bleomycin,4 or paclitaxel5). The benefits of chemotherapy can last for months—and even years.
Liquid nitrogen cryotherapy does the trick
We treated our patient with liquid nitrogen cryotherapy that was applied at regular 4- to 6-week intervals over several months. After 3 months, our patient’s lesions were nearly resolved. We followed him monthly thereafter.
Correspondence
John Patrick Welsh, MD, Associates in Dermatology, 4727 Friendship Avenue, Suite 300, Pittsburgh, PA 15224-1778; jp_welsh@hotmail.com.
1. Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500-517.
2. Pellet C, Kerob D, Dupuy A, et al. Kaposi’s sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi’s sarcoma. J Invest Dermatol. 2006;126:621-627.
3. Schwartz R. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
4. Brambilla L, Miedico A, Ferrucci S, et al. Combination of vinblastine and bleomycin as first line therapy in advanced classic Kaposi’s sarcoma. J Eur Acad Dermatol Venereol. 2006;20:1090-1094.
5. Baskan EB, Tunali S, Adim SB, et al. Treatment of advanced classic Kaposi’s sarcoma with weekly low-dose paclitaxel therapy. Int J Dermatol. 2006;45:1441-1443.
An 88-year-old Caucasian man of Italian ancestry came into our clinic with multiple, painful purple-red “growths” on his left foot that he’d had for several years (FIGURE 1).
The patient had no systemic complaints (no fever, chills, weight loss, night sweats). He had a history of hypertension, a cardiac valve replacement, and chronic back pain (secondary to a motor vehicle accident). He was taking warfarin and nadolol.
The patient had multiple, 0.1– to 0.5-cm purple-red papules and nodules on the dorsal and plantar surfaces of the left foot, with associated moderate lower extremity edema and mottled dyspigmentation.
We did a punch biopsy, which showed a nodular neoplasm composed of moderately plump, spindle-shaped cells in short interweaving fascicles and numerous extravasated erythrocytes in the spaces (“vascular slits”) between the spindle-shaped cells (FIGURE 2).
FIGURE 1
Painful papules and nodules
FIGURE 2
Hematoxylin/eosin stain
What is your diagnosis?
How would you manage this condition?
Diagnosis: Kaposi’s sarcoma
Classic Kaposi’s sarcoma is a rare mesenchymal tumor most often seen in elderly men of Mediterranean or Ashkenazi Jewish origin with an annual incidence in the United States of between 0.02% and 0.06%, with a peak occurring in the 5th to 8th decade of life.1 (Two-thirds of cases develop after the age of 50.) Population-based studies in the United States have shown a male-to-female ratio of 4:1.1
First described by the Hungarian dermatologist Moritz Kaposi in 1872, Kaposi’s sarcoma assumed prominence during the emerging HIV epidemic and is now the most common tumor in patients with acquired immune deficiency syndrome (AIDS).2
Recent research has implicated the human herpes virus–8 (HHV–8) as an inductive agent (necessary though not sufficient) in all epidemiologic subsets of the disease.2
There are 4 principal clinical variants of Kaposi’s sarcoma:
- classic (or chronic),
- African endemic (includes childhood lymphadenopathic),
- transplant-associated, and
- AIDS-related.
What you’ll see
Clinically, classic Kaposi’s sarcoma often first manifests as blue-red, well-demarcated, painless macules confined to the distal lower extremities.3 These slow-growing lesions may enlarge to forms papules and plaques, or progress to nodules and tumors. Unilateral involvement is often observed at the outset of the disease, with potential centripetal spread occurring late-in-course.3
Early lesions are generally soft, spongy, and “angiomatous,” while in the advanced state, lesional skin becomes hard, solid, and brown in color.3 Edema of the surrounding tissue is common. In addition to the skin, classic Kaposi’s sarcoma also involves mucosal sites (especially the oral and gastrointestinal mucosae).
Differential includes melanocytic nevus
A differential diagnosis for classic Kaposi’s sarcoma includes stasis dermatitis (“acroangiodermatitis”), melanocytic nevus, pyogenic granuloma, hemangioma, granuloma annulare, arthropod assault, and dermatofibroma/dermatofibrosarcoma protuberans (DF/DFSP).
Melanocytic nevi, pyogenic granuloma, hemangioma, granuloma annulare, and DF/DFSP ordinarily feature single lesions, while Kaposi’s sarcoma has multiple lesions. An arthropod assault is pruritic, and stasis dermatitis typically has dilated/varicose veins.
Histology will confirm your suspicions
While epidemiological and clinical factors may suggest classic Kaposi’s sarcoma, a final diagnosis ultimately rests on confirmatory histology. The pathology of classic Kaposi’s sarcoma (like all of the variant subtypes) is based solely on stage of the lesion.
Early patch-stage lesions exhibit papillary dermal proliferation of small, angulated vessels lined by bland endothelial cells with an accompanying sparse infiltrate of lymphocytes and plasma cells.
As the disease progresses to the plaque stage, the vascular proliferation expands into the reticular dermis and subcutis. The transition to nodular Kaposi’s sarcoma develops when a population of spindle cells expressing endothelial markers occurs between the “vascular slits” (FIGURE 2).
Chemotherapy for rapidly progressive disease
There is minimal evidence-based data for the treatment of Kaposi’s sarcoma. Treatment options for limited disease include surgical excision, cryotherapy, laser ablation, topical retinoids (alitretinoin), interferon-alpha, and radiation.1
If rapidly progressive disease (>10 new lesions per month) exists, the most effective treatment remains systemic chemotherapy (vincristine, doxorubicin, vinblastine,4 bleomycin,4 or paclitaxel5). The benefits of chemotherapy can last for months—and even years.
Liquid nitrogen cryotherapy does the trick
We treated our patient with liquid nitrogen cryotherapy that was applied at regular 4- to 6-week intervals over several months. After 3 months, our patient’s lesions were nearly resolved. We followed him monthly thereafter.
Correspondence
John Patrick Welsh, MD, Associates in Dermatology, 4727 Friendship Avenue, Suite 300, Pittsburgh, PA 15224-1778; jp_welsh@hotmail.com.
An 88-year-old Caucasian man of Italian ancestry came into our clinic with multiple, painful purple-red “growths” on his left foot that he’d had for several years (FIGURE 1).
The patient had no systemic complaints (no fever, chills, weight loss, night sweats). He had a history of hypertension, a cardiac valve replacement, and chronic back pain (secondary to a motor vehicle accident). He was taking warfarin and nadolol.
The patient had multiple, 0.1– to 0.5-cm purple-red papules and nodules on the dorsal and plantar surfaces of the left foot, with associated moderate lower extremity edema and mottled dyspigmentation.
We did a punch biopsy, which showed a nodular neoplasm composed of moderately plump, spindle-shaped cells in short interweaving fascicles and numerous extravasated erythrocytes in the spaces (“vascular slits”) between the spindle-shaped cells (FIGURE 2).
FIGURE 1
Painful papules and nodules
FIGURE 2
Hematoxylin/eosin stain
What is your diagnosis?
How would you manage this condition?
Diagnosis: Kaposi’s sarcoma
Classic Kaposi’s sarcoma is a rare mesenchymal tumor most often seen in elderly men of Mediterranean or Ashkenazi Jewish origin with an annual incidence in the United States of between 0.02% and 0.06%, with a peak occurring in the 5th to 8th decade of life.1 (Two-thirds of cases develop after the age of 50.) Population-based studies in the United States have shown a male-to-female ratio of 4:1.1
First described by the Hungarian dermatologist Moritz Kaposi in 1872, Kaposi’s sarcoma assumed prominence during the emerging HIV epidemic and is now the most common tumor in patients with acquired immune deficiency syndrome (AIDS).2
Recent research has implicated the human herpes virus–8 (HHV–8) as an inductive agent (necessary though not sufficient) in all epidemiologic subsets of the disease.2
There are 4 principal clinical variants of Kaposi’s sarcoma:
- classic (or chronic),
- African endemic (includes childhood lymphadenopathic),
- transplant-associated, and
- AIDS-related.
What you’ll see
Clinically, classic Kaposi’s sarcoma often first manifests as blue-red, well-demarcated, painless macules confined to the distal lower extremities.3 These slow-growing lesions may enlarge to forms papules and plaques, or progress to nodules and tumors. Unilateral involvement is often observed at the outset of the disease, with potential centripetal spread occurring late-in-course.3
Early lesions are generally soft, spongy, and “angiomatous,” while in the advanced state, lesional skin becomes hard, solid, and brown in color.3 Edema of the surrounding tissue is common. In addition to the skin, classic Kaposi’s sarcoma also involves mucosal sites (especially the oral and gastrointestinal mucosae).
Differential includes melanocytic nevus
A differential diagnosis for classic Kaposi’s sarcoma includes stasis dermatitis (“acroangiodermatitis”), melanocytic nevus, pyogenic granuloma, hemangioma, granuloma annulare, arthropod assault, and dermatofibroma/dermatofibrosarcoma protuberans (DF/DFSP).
Melanocytic nevi, pyogenic granuloma, hemangioma, granuloma annulare, and DF/DFSP ordinarily feature single lesions, while Kaposi’s sarcoma has multiple lesions. An arthropod assault is pruritic, and stasis dermatitis typically has dilated/varicose veins.
Histology will confirm your suspicions
While epidemiological and clinical factors may suggest classic Kaposi’s sarcoma, a final diagnosis ultimately rests on confirmatory histology. The pathology of classic Kaposi’s sarcoma (like all of the variant subtypes) is based solely on stage of the lesion.
Early patch-stage lesions exhibit papillary dermal proliferation of small, angulated vessels lined by bland endothelial cells with an accompanying sparse infiltrate of lymphocytes and plasma cells.
As the disease progresses to the plaque stage, the vascular proliferation expands into the reticular dermis and subcutis. The transition to nodular Kaposi’s sarcoma develops when a population of spindle cells expressing endothelial markers occurs between the “vascular slits” (FIGURE 2).
Chemotherapy for rapidly progressive disease
There is minimal evidence-based data for the treatment of Kaposi’s sarcoma. Treatment options for limited disease include surgical excision, cryotherapy, laser ablation, topical retinoids (alitretinoin), interferon-alpha, and radiation.1
If rapidly progressive disease (>10 new lesions per month) exists, the most effective treatment remains systemic chemotherapy (vincristine, doxorubicin, vinblastine,4 bleomycin,4 or paclitaxel5). The benefits of chemotherapy can last for months—and even years.
Liquid nitrogen cryotherapy does the trick
We treated our patient with liquid nitrogen cryotherapy that was applied at regular 4- to 6-week intervals over several months. After 3 months, our patient’s lesions were nearly resolved. We followed him monthly thereafter.
Correspondence
John Patrick Welsh, MD, Associates in Dermatology, 4727 Friendship Avenue, Suite 300, Pittsburgh, PA 15224-1778; jp_welsh@hotmail.com.
1. Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500-517.
2. Pellet C, Kerob D, Dupuy A, et al. Kaposi’s sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi’s sarcoma. J Invest Dermatol. 2006;126:621-627.
3. Schwartz R. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
4. Brambilla L, Miedico A, Ferrucci S, et al. Combination of vinblastine and bleomycin as first line therapy in advanced classic Kaposi’s sarcoma. J Eur Acad Dermatol Venereol. 2006;20:1090-1094.
5. Baskan EB, Tunali S, Adim SB, et al. Treatment of advanced classic Kaposi’s sarcoma with weekly low-dose paclitaxel therapy. Int J Dermatol. 2006;45:1441-1443.
1. Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500-517.
2. Pellet C, Kerob D, Dupuy A, et al. Kaposi’s sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi’s sarcoma. J Invest Dermatol. 2006;126:621-627.
3. Schwartz R. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
4. Brambilla L, Miedico A, Ferrucci S, et al. Combination of vinblastine and bleomycin as first line therapy in advanced classic Kaposi’s sarcoma. J Eur Acad Dermatol Venereol. 2006;20:1090-1094.
5. Baskan EB, Tunali S, Adim SB, et al. Treatment of advanced classic Kaposi’s sarcoma with weekly low-dose paclitaxel therapy. Int J Dermatol. 2006;45:1441-1443.
How to make exercise counseling more effective: Lessons from rural America
- To help overweight patients and those with a sedentary lifestyle to adopt and stick with an exercise regimen, develop a detailed and realistic plan with their help, and follow up with them periodically to see how they’re doing.
Purpose: Exercise counseling by primary care physicians has been shown to improve physical activity in patients. However, the prevalence and effectiveness of physician counseling is unknown in rural populations that are at increased risk for chronic diseases.
Methods: Using a population-based telephone survey at baseline and again at 1-year follow-up, we assessed physical activity behavior among 1141 adults (75% female, 95% white) living within 12 rural communities of Missouri, Tennessee, and Arkansas. We tested the association between physician counseling and patients meeting current physical activity recommendations using logistic regression analysis controlling for demographic variables.
Results: Participants who saw a doctor for regular care were 54% more likely to be physically active (adjusted odds ratio [aOR]=1.54; 95% confidence interval [CI], 1.04-2.28). Overweight adults (body mass index [BMI]=25-29.9 kg/m2) who had been advised by their physician to exercise more were nearly 5 times more likely to meet physical activity recommendations if their doctor helped develop an exercise plan (aOR=4.99; 95% CI, 1.69-14.73).
Overweight individuals who received additional follow-up with the exercise plan from their doctor had a 5½-fold increase in likelihood of meeting physical activity recommendations (P<.05).
In the overall sample, patients were significantly more likely to initiate (P=.01) and maintain (P=.002) physical activity when the physician prescribed and followed up on an exercise plan.
Conclusion: This longitudinal study provides evidence that exercise counseling is most effective when the physician presents the counseling as a plan or prescription and when he or she follows up with the patient on it.
Simply telling sedentary patients that they need to exercise may not help them much. If the goal is to inspire action, a more effective approach would be to help them devise a plan for exercise and then inquire periodically about how it’s going. That premise was the basis for our study.
There’s good reason to get your patients moving
Physical inactivity is an independent risk factor for the most prevalent chronic diseases, including obesity, cardiovascular disease, and type 2 diabetes. Physical activity at moderate or vigorous intensities reduces stress and depressive symptoms, controls high blood pressure and cholesterol levels, improves sleep, reduces or reverses weight gain, and prevents or controls chronic diseases.1 Based on these benefits, all physicians are encouraged to counsel sedentary patients to increase activity levels.2
National disease prevention objectives of Healthy People 2010 call for physicians to counsel at-risk patients on health behaviors such as physical activity and diet.3 Knowledge of patients’ families, environments, and communities makes primary care physicians uniquely suited to give effective advice,4 and physician counseling is known to positively influence patients’ health-related behavior.5-12
To date, findings on counseling effectiveness have been mixed. Unfortunately, previous controlled trials of primary care physicians counseling adult patients on physical activity have varied in quality and yielded mixed results.13 Therefore, in its Guide to Clinical Preventive Services, the US Preventive Services Task Force did not recommend for or against behavioral counseling in primary care settings to promote physical activity.14 The guidelines state that existing studies do not provide a clear picture of which counseling components are effective.13
A population-based study by Glasgow and colleagues suggests that follow-up support by the physician may be needed to change physical activity behavior. Generalizations were limited, though, by the cross-sectional study design and post hoc analysis.15
More research is also needed to determine which strategies help patients stay physically active, a necessary component to sustaining the health benefits of exercise.16 Unfortunately, few primary care physicians counsel overweight or inactive patients on the benefits of diet and physical activity, let alone assist them with long-term follow-through.7,8,11,16-18
Why we chose to study a rural population
Rural Americans are among the groups at highest risk for chronic diseases. On average they are older, less educated, and poorer than their urban counterparts.19,20 And rural residents walk 13% less than suburbanites.21 According to the Rural Healthy People 2010 survey, 5 of the top 10 health concerns are chronic conditions that can be prevented or ameliorated with adequate physical activity.22
Studies have shown that healthy adults believe their health care providers are a credible source of information, and that they are motivated to comply with physician advice.23 Nondisabled adults believe their physicians want them to be physically active.19 However, to our knowledge, no study has examined the effects of physician counseling on physical activity behavior for patients at increased risk for chronic diseases in rural areas.
Our objectives. The first objective of this study was to identify the prevalence of specific components of physician counseling in a tri-state sample of at-risk, rural adults using telephone survey data. A second objective was to measure the longitudinal relationship between physician counseling and physical activity.
Methods
The Saint Louis University Institutional Review Board approved this study.
Study population and design
This study reports on baseline and 1-year follow-up telephone survey data collected as part of a larger 3-year intervention study in 12 rural communities from Missouri (6), Tennessee (4), and Arkansas (2). Project WOW (Walk the Ozarks to Wellness) aims to promote walking among overweight rural adults by integrating individual, interpersonal, and community-level interventions. Methods and details of the intervention are described in detail elsewhere.24 The target communities ranged in population from 766 to 12,993 adults, and in geographic area from 1.4 to 16.1 square miles.25
At baseline in the summer of 2003, we used a modified version of the Behavioral Risk Factor Surveillance System (BRFSS) interview protocol26,27 and randomly dialed telephone numbers to recruit participants residing within a 2-mile radius of a walking trail. In all, 2470 English-speaking adults, ages 18 and older, completed the baseline survey (65.2% response rate).28 Sampling was proportionate to community size.
At follow-up in the summer of 2004, participants identified at baseline completed the same telephone survey used a year earlier. This time, 1531 participants completed the survey (62.0% response rate).28
Demographic variables included age, sex, education, race/ethnicity, and annual combined household income (TABLE 1). Overweight and obesity, based on self-reported height and weight, were defined by a body mass index (BMI) of 25 to 29.9 kg/m2 and 30 kg/m2 or greater, respectively.
TABLE 1
The population sample
| PATIENT CHARACTERISTICS | FOLLOW-UP SURVEY (N =1141) % |
|---|---|
| Female | 74.6 |
| White, non-Hispanic | 94.6 |
| Education | |
| Less than high school | 9.7* |
| High school graduate | 30.8 |
| Some college | 23.9 |
| College graduate | 35.6 |
| Age | |
| 18-24 | 6.4* |
| 25-44 | 35.4 |
| 45-64 | 39.1 |
| 65+ | 19.1 |
| Annual household combined income (n=1102) | |
| < $25,000 31.7* | |
| ‡ $25,000 | 68.3 |
| Body mass index (n=1107) | |
| Normal (<25) | 43.7 |
| Overweight (25-29.9) | 31.5 |
| Obese (‡ 30) | 24.8 |
| Physician encounters/counseling | |
| Has doctor for regular care | 87.8* |
| In usual year, has seen doctor ≤1 time | 28.2* |
| Has been advised to exercise more | 35.1 |
| Doctor helped develop plan to exercise more | 33.8 |
| Doctor followed up on plan to exercise more | 46.3 |
| * Significant P value <.001 between responders and nonresponders at 1-year follow-up. | |
Measurement of dependent and independent variables
The survey instrument incorporated questions from the BRFSS, as well as questions developed by researchers from San Diego; Sumter County, South Carolina; and St. Louis.29-34 Psychometric properties of the questions and scales are reported elsewhere.35 The survey instrument contained 106 questions, including skip patterns; the average administration time was 34 minutes.
We assessed physician counseling about exercise with 5 questions from the survey (TABLE 2). These questions were modeled on the “4 As” counseling approach (Ask, Advise, Assist, and Arrange follow-up) recommended by the National Cancer Institute36 and used in a previous, similar BRFSS-based telephone survey.15 The questions were:
- Do you have a doctor whom you see for regular health care?
- In a usual year, how often do you see your doctor?
- Have you been advised within the last year by a doctor to exercise more?
- Has your doctor helped you to develop a plan to increase exercise?
- Has your doctor followed up with you at subsequent visits to see how you increased exercise?
We administered the questions at baseline and at follow-up. For our analyses, we used patient reports of physician counseling at 1-year follow-up, which covered all counseling received in the past 12 months.
We considered respondents to have met recommendations for physical activity if they had engaged in prescribed moderate or vigorous physical activities, or had walked for exercise 150 minutes a week.
Moderate physical activity was defined (according to the current CDC recommendations) as 30 cumulative minutes of moderate-intensity activity (brisk walking or jogging) at least 5 days per week.1
Vigorous physical activity was defined as 20 minutes of vigorous-intensity activity (running) at least 3 days per week.1
The sample was limited to participants who completed the baseline and follow-up surveys, and who reported at follow-up that they had no physical impairment that prevented walking (n=1141).
Statistical analysis
To evaluate how physician counseling would change a patient’s physical activity between baseline and 1-year follow-up, we used multivariate logistic regression analysis. In accordance with the questions asked in the survey, we defined 5 potential predictors of a patient’s decision to start exercising and keep exercising:
- Patient has seen a doctor for regular care.
- In a usual year, patient has seen a doctor once or less.
- Patient has been advised to exercise more.
- Doctor helped develop a plan to exercise more.
- Doctor followed up on plan to exercise more
For every patient who met physical activity recommendations at the 1-year follow-up, we performed regression analysis on each of these 5 measures, adjusting for baseline physical activity and potential confounders of age, education, and sex. This method allowed us to examine the independent effect of physician counseling on physical activity at 1-year follow-up.
The number of respondents analyzed differed according to the measure being examined. For example, we included all respondents in analyzing the effect of visit frequency and the effect of being advised to exercise more (n=1141). However, only those respondents who reported being advised to exercise more were included in the analyses of a physician helping to develop an exercise plan and physician follow-up in supporting exercise behavior (n=402).
To determine whether physician counseling was consistent across BMI categories and income (dichotomized at $25,000), we performed stratified analyses on the multilevel logistic regressions.
TABLE 2
What increased the likelihood of exercise? Regular medical care, a physician-assisted exercise plan, and physician follow-up (n=1141)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS | |
|---|---|---|
| aOR* | 95% CI | |
| 1. Do you have a doctor whom you see for regular health care? | ||
| Yes | 1.54 | 1.04-2.28 |
| No | Ref | |
| 2. In a usual year, how often do you see your doctor? | ||
| Once a year or less | 1.41 | 1.02-1.95 |
| Twice a year or more | Ref | |
| 3. Have you been advised within the last year by a doctor to exercise more? | ||
| Yes | 0.68 | 0.52-0.90 |
| No | Ref | |
| Of those advised to exercise more (n=402): 4. Has your doctor helped you to develop a plan to increase exercise? | ||
| Yes | 1.93 | 1.19-3.15 |
| No | Ref | |
| 5. Has your doctor followed up with you at subsequent visits to see how you increased exercise? | ||
| Yes | 2.84 | 1.78-4.53 |
| No | Ref | |
| aOR, adjusted odds ratio; CI, confidence interval; Ref, reference group. | ||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | ||
Results
The final cohort consisted of 1141 adults (TABLE 2). Those who did not respond to the follow-up survey were significantly more likely to be younger (P<.001), have less than a high school education (P<.001), and have an annual household combined income <$25,000 (P<.001). Those completing the follow-up survey were more likely to have a doctor for regular care (P<.001), although they saw their doctor, on average, significantly less per year than nonrespondents (P<.001).
Ninety percent of the cohort sample reported having a doctor whom they saw for regular care. Within the last year, 35% had been advised by their doctor to exercise more. Of those who had been so advised, 34% received help from their physician in developing a plan to increase exercise, and 46% were queried at subsequent visits as to how they were progressing with their exercise program.
After adjusting for age, sex, education, and baseline physical activity, we found that those who had a doctor for regular care were 54% more likely to be physically active than those who reported not having a doctor for regular care (aOR=1.54; 95% CI, 1.04-2.28). If the advising physician also developed a plan with the patient to increase exercise, there was nearly a 2-fold increase in physical activity compared with those who received only advice to exercise more (aOR=1.93; 95% CI, 1.19-3.15). If the physician followed up with the exercise plan at subsequent visits, the likelihood of physical activity increased further (aOR=2.84; 95% CI, 1.78-4.53) compared with those who did not receive follow-up from the physician.
Results of stratified analysis by BMI status are shown in TABLE 3. Individuals at normal weight were significantly more likely to be physically active if they had a physician for regular care (aOR=2.76; 95% CI, 1.49-5.13). Overweight adults (BMI 25-29.9 kg/m2) who had been advised by their physician to exercise more were significantly more likely to attain recommended levels of physical activity if their doctor helped develop an exercise plan than were those given more general advice about exercise (aOR=4.99; 95% CI, 1.69-14.73). Overweight individuals who received further counseling with follow-up inquiries were 5.64 times more likely to be physically active (95% CI, 2.10-15.17). A physician-developed exercise plan did not appreciably improve physical activity in obese adults (BMI ≥30 kg/m2); however, benefit in this group was demonstrated when physicians prescribed and followed up with the exercise plan (aOR=2.13; 95% CI, 1.10-4.12). Stratified analysis by income status provided no clear pattern (data not shown).
TABLE 3
What role does BMI play in patients achieving activity goals? (n=1107)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS* (aOR [95% CI]) | ||
|---|---|---|---|
| NORMAL (BMI<25) | OVERWEIGHT (BMI 25-29.9) | OBESE (BMI ≥30) | |
| Has seen a doctor for regular care | 2.76 (1.49-5.13) | 1.08 (0.51-2.29) | 1.34 (0.62-2.91) |
| In a usual year, has seen a doctor ≤1 time | 1.98 (1.13-3.47) | 0.95 (0.54-1.68) | 0.95 (0.47-1.92) |
| Has been advised to exercise more | 1.02 (0.56-1.85) | 0.75 (0.46-1.25) | 0.77 (0.46-1.31) |
| Doctor helped develop a plan to exercise more | 0.69 (0.22-2.18) | 4.99 (1.69-14.73) | 1.76 (0.87-3.56) |
| Doctor followed up on plan to exercise more | 2.59 (0.80-8.36) | 5.64 (2.10-15.17) | 2.13 (1.10-4.12) |
| aOR, adjusted odds ratio; BMI, body-mass index; CI, confidence interval. | |||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | |||
Discussion
Findings from our analyses support the need for more detailed and more frequent exercise counseling (including follow-up) by rural primary care physicians. In our study, physicians’ counsel was most effective when presented as a plan or prescription that was followed up with periodic inquiries. Patients’ initiation and maintenance of physical activity were significantly associated with physicians’ follow-up of exercise plans. Those who were merely “advised” to exercise more were less likely to meet physical activity recommendations. This illustrates the importance of detailed physician counseling over simple advice to exercise more.
Over 80% of normal-weight individuals, who comprised more than 40% of the sample, reported that their physician had not suggested they exercise more. There are many possible explanations for these reports. Rural populations are relatively isolated and slow to adopt changes. Thus patients may be unaware of new recommendations for physical activity and their significant benefit for disease prevention, and therefore unlikely to discuss such matters with their physician. Physicians also may perceive normal-weight individuals as healthy regardless of their actual health behaviors. On the other hand, 1 study showed that patients with disease risk factors (eg, high cholesterol, elevated BMI) were more likely to be counseled on preventive health behaviors.37
With overweight patients, who are at increased risk of developing chronic diseases, physician counseling strengthened their resolve significantly. Overweight individuals who received directives from their physician (a plan to increase exercise and subsequent follow-up) were 5½ times more likely to be physically active than those who received less counseling.
Obese patients did not receive counseling as often as overweight patients, or benefit from it as much when given, perhaps due to the presence of comorbidities. However, many studies show that regardless of BMI status, physical activity reduces all-cause mortality.38-41
Interestingly, our results showed that seeing a doctor less than once a year was associated with increases in physical activity. Patients who see their physician once a year may be going for annual wellness exams, providing more opportunity to discuss health behavior.
Overall, patients are counseled less often/thoroughly than needed. Our findings agree with those of a previous statewide study that used Missouri BRFSS data to assess the extent to which overweight or physically inactive people received advice from their physicians concerning these risk factors.42 Although most Missouri residents who were overweight or inactive reported seeing their physician within the past year, less than half said their doctors advised them to alter their risk behavior(s).42 Our findings are also consistent with a recent nationwide study by Ma and colleagues that focused on adults with obesity, diabetes, or other related conditions.43 Participants from across the United States reported receiving counseling for physical activity in <30% of visits to private physician offices and hospital outpatient departments.43
Our study was unique in that it examined a tri-state sample of the nation’s rural population for both evidence and effectiveness of physician counseling. It is one of very few studies using a longitudinal design, strengthening the associations found. Causality is limited, however, due to the multifaceted design of the intervention program from which the data were obtained. Future research should evaluate varying degrees of physician counseling and other indirect measures of its impact.
Limitations of the study
Our observational cohort design and the large, randomly selected sample resulted in fewer limitations than were seen with previous similar studies. However, our study had several limitations.
- Recall bias may be present. We assessed counseling with patient memory alone; we made no attempts to interview physicians or audit charts.
- Self-reported height and weight data tend to underestimate the prevalence of obesity.44,45 Resultant misclassification of overweight subjects as being at normal weight could have skewed the stratified analysis.
- The external validity of the physician-counseling questions we used has not been formally confirmed. Given the demographics of the analytic sample (ie, mostly female, white, low income), it would be appropriate to generalize our findings only to similar, rural populations.
Barriers to counseling, and means of removing them
Primary care physicians—rural or urban—are no doubt aware of the health risks associated with physical inactivity. However, the barriers physicians face in counseling at-risk patients overwhelm most efforts. These barriers include lack of time, inadequate provider counseling skills and training, perceived ineffectiveness and nonadherence, patient comorbidities, and lack of organizational support and reimbursement.46-48
Intervention programs and tools have been developed to help health care providers overcome time, skill, and training barriers. These programs, available even to rural providers, have proven effective.49,50 (Go to www.paceproject.org/Home.html and click on “Projects” to learn about the PACE program.) However, application of such skills and tools may be more successful if training is incorporated into medical school curricula and residency training programs rather than through CME endeavors.49 This would require medical institutions and organizations to prioritize the direct link between healthy lifestyle behaviors and disease prevention and the vital role physicians play in underscoring this link.
Finally, health care policy makers and systems must be persuaded to address the lack of organizational support and reimbursement that prevents physicians from counseling at-risk patients on unhealthy lifestyle behaviors. Responsible payers and providers should aggressively explore low-cost ways to promote physical activity and weight loss in primary care settings, to stem the tide of obesity-related chronic diseases. At the local level, physicians can team up to support policies that may enhance preventive counseling efforts2—increasing access to places for activity, encouraging physical activity programming in communities, schools, and organizations, and physical environment enhancements such as safe sidewalks, adequate lighting, and improved zoning.44,51,44,52
Acknowledgments
We thank the communities that are participating in the ongoing intervention study. For their assistance in data collection, we thank the Department of Health Management and Informatics, Behavioral Risk Research Unit at the University of Missouri, Columbia.
Funding/support
This study was funded through the National Institutes of Health grant NIDDK #5 R18 DK061706 and the Centers for Disease Control and Prevention contract U48/CCU710806 (Centers for Research and Demonstration of Health Promotion and Disease Prevention). Human subjects approval was obtained from the Saint Louis University Institutional Review Board.
Correspondence
Sarah L. Lovegreen, MPH, Prevention Research Center and Department of Community Health, Saint Louis University School of Public Health, 3545 Lafayette Ave., Salus Center, Suite 300, St. Louis, MO 63146; slove green@oasisnet.org.
1. US Department of Health and Human Services. Physical Activity and Health: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, 1996.
2. Chakravarthy MV, Joyner MJ, Booth FW. An obligation for primary care physicians to prescribe physical activity to sedentary patients to reduce the risk of chronic health conditions. Mayo Clin Proc 2002;77:165-173.
3. US. Department of Health and Human Services. Health People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Iverson D, Fielding J, Crow R, Christenson G. The promotion of physical activity in the U.S. population: the status of programs in medical, worksite, community, and school settings. Public Health Rep 1985;100:212-224.
5. Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med 2000;9:426-433.
6. The Writing Group for the Activity Counseling Trial Research Group. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA 2001;286:677-687.
7. Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professionals advising obese patients to lose weight? JAMA 1999;282:1576-1578.
8. Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225-233.
9. Sallis JF, Patrick K, Calfas KJ. Counseling patients/clients about physical activity and nutrition. Weight Control Digest 1999;9:843, 846-850.
10. Calfas KJ, Zabinski MF, Rupp J. Practical nutrition assessment in primary care settings: a review. Am J Prev Med. 2000;18:289-299.
11. Marcus BH, Goldstein MG, Jette A, et al. Training physicians to conduct physical activity counseling. Prev Med 1997;26:382-388.
12. Godin R, Shephard RJ. An evaluation of the potential role of the physician in influencing community exercise behavior. Am J Health Prom 1990;4:255-259.
13. Eden KB, Orleans CT, Mulrow CD, Pender NJ, Teutsch SM. Does counseling by clinicians improve physical activity? A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:208-215.
14. US. Preventive Services Task Force. Behavioral Counseling in Primary Care to Promote Physical Activity: Recommendations and Rationale. Rockville, MD: Agency for Healthcare Research and Quality; July 2002. Available at: http://www.ahrq.gov/clinic/3rduspstf/physactivity/physactrr.htm. Accessed April 24, 2008.
15. Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med 2001;21:189-196.
16. Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol 2000;19(1 suppl):32-41.
17. Stafford RS, Farhat JH, Misra B, Schoenfeld DA. National patterns of physician activities related to obesity management. Arch Fam Med 2000;9:631-638.
18. Potter MB, Vu JD, Croughan-Minihane M. Weight management what patients want from their primary care physician. J Fam Pract 2001;50:513-518.
19. Sobal J, Troiana RP, Frongillo EA, Jr. Rural-urban differences in obesity. Rural Sociol 1996;2:289-305.
20. Miller MK, Stokes CS, Clifford WB. A comparison of the rural-urban mortality differential for deaths from all causes, cardiovascular disease and cancer. J Rural Health 1987;3(2):23-34.
21. Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Med Sci Sports Exerc 2003;35:1529-36.
22. Gamm L, Hutchison L, Bellamy G, et al. Rural healthy people 2010: identifying rural health priorities and models for practice. J Rural Health 2002;18(1):9-14.
23. Schappert SM. National ambulatory medical care survey: 1991 Summary. Hyattsville, MD: US Department of Health and Human Services. Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 1993.
24. Brownson R, Hagood L, Lovegreen S, et al. A multilevel, ecological approach to promoting walking in rural communities. Prev Med 2005;41:837-842.
25. US. Census Bureau. Census 2000. Available at: http://www.census.gov/main/www/cen2000.html. Accessed November 2004.
26. Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med 1985;1(6):9-14.
27. Remington PL, Smith MY, Williamson DF, Anda RF, Gentry EM, Hogelin GC. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981-87. Public Health Rep 1988;103:366-375.
28. Council of American Survey Research Organizations (CASRO) Task Force on Completion Rates. On the Definitions of Response Rates. Special Report. New York, NY: Council of American Survey Organizations; 1982.
29. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss/. Accessed November 2004.
30. Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health 2003;93:1552-1558.
31. Eyler AA, Brownson RC, Donatelle RJ, King AC, Brown D, Sallis JF. Physical activity social support and middle- and older-aged minority women: results from a US survey. Soc Sci Med 1999;49:781-789.
32. King AC, Castro C, Wilcox S, Eyler AA, Sallis JF, Brownson RC. Personal and environmental factors associated with physical inactivity among different racial-ethnic groups of US middle- aged and older-aged women. Health Psychol 2000;19:354-364.
33. Ainsworth BE, Bassett DR, Jr, Strath SJ, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc 2000;32(9 suppl):S457-S464.
34. Brownson RC, Baker EA, Housemann RA, Brennan LK, Bacak SJ. Environmental and policy determinants of physical activity in the United States. Am J Public Health 2001;91:1995-2003.
35. Brownson RC, Chang JJ, Eyler AA. Measuring the environment for friendliness toward physical activity: a comparison of the reliability of three questionnaires. Am J Public Health 2004;94:473-483.
36. Manely M, Epps R, Husten C, Glynn T, Shopland D. Clinical interventions in tobacco control: a National Cancer Institute training program for physicians. JAMA 1991;266:3172-3173.
37. Kreuter MW, Scharff DP, Brennan LK, Lukwago SN. Physician recommendations for diet and physical activity: which patients get advised to change? Prev Med 1997;26:825-833.
38. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694-2703.
39. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nut 1999;69:373-380.
40. Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the Lipid Research Clinics Study. Am J Epidemiol 2002;156:832-841.
41. Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight and obese men. JAMA 1999;282:1547-1553.
42. Friedman C, Brownson RC, Peterson DE, Wilkerson JC. Physician advice to reduce chronic disease risk factors. Am J Prev Med 1994;10:367-371.
43. Ma J, Urizar GG, Alehegn T, Stafford R. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med 2004;39:815-822.
44. Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol 1982;115:223-230.
45. Kuskowska-Wolk A, Karlsson P, Stolt M, Rossner S. The predictive validity of body mass index based on self-reported weight and height. Int J Obes 1989;13:441-453.
46. Kushner RF. Barriers to providing nutritional counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546-552.
47. Rogers LQ, Bailey JE, Gutin B, et al. Teaching resi-dent physicians to provide physician counseling: a needs assessment. Acad Med 2002;77:841-844.
48. Hiss RG. Barriers to care in non-insulin-dependent diabetes mellitus. The Michigan experience. Ann Intern Med 1996;124:146-148.
49. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multi-component program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med 2002;34:153-161.
50. Sallis JF, Patrick K, Calfas KJ, et al. A multi-media behavior change program for nutrition and physical activity in primary care: PACE+ for adults. Homeostasis 1999;39:196-202.
51. Health GW, Brownson RC, Kruger J, et al. The effectiveness of urban design and land use and transport policies and practices to increase physical activity: a systematic review. J Phys Act Health 2006;3(suppl 1):555-576.
52. Sallis JF, Glanz K. The role of built environment in physical activity, eating and obesity in childhood. Future Child 2006 Spring;16(1):89-108.
- To help overweight patients and those with a sedentary lifestyle to adopt and stick with an exercise regimen, develop a detailed and realistic plan with their help, and follow up with them periodically to see how they’re doing.
Purpose: Exercise counseling by primary care physicians has been shown to improve physical activity in patients. However, the prevalence and effectiveness of physician counseling is unknown in rural populations that are at increased risk for chronic diseases.
Methods: Using a population-based telephone survey at baseline and again at 1-year follow-up, we assessed physical activity behavior among 1141 adults (75% female, 95% white) living within 12 rural communities of Missouri, Tennessee, and Arkansas. We tested the association between physician counseling and patients meeting current physical activity recommendations using logistic regression analysis controlling for demographic variables.
Results: Participants who saw a doctor for regular care were 54% more likely to be physically active (adjusted odds ratio [aOR]=1.54; 95% confidence interval [CI], 1.04-2.28). Overweight adults (body mass index [BMI]=25-29.9 kg/m2) who had been advised by their physician to exercise more were nearly 5 times more likely to meet physical activity recommendations if their doctor helped develop an exercise plan (aOR=4.99; 95% CI, 1.69-14.73).
Overweight individuals who received additional follow-up with the exercise plan from their doctor had a 5½-fold increase in likelihood of meeting physical activity recommendations (P<.05).
In the overall sample, patients were significantly more likely to initiate (P=.01) and maintain (P=.002) physical activity when the physician prescribed and followed up on an exercise plan.
Conclusion: This longitudinal study provides evidence that exercise counseling is most effective when the physician presents the counseling as a plan or prescription and when he or she follows up with the patient on it.
Simply telling sedentary patients that they need to exercise may not help them much. If the goal is to inspire action, a more effective approach would be to help them devise a plan for exercise and then inquire periodically about how it’s going. That premise was the basis for our study.
There’s good reason to get your patients moving
Physical inactivity is an independent risk factor for the most prevalent chronic diseases, including obesity, cardiovascular disease, and type 2 diabetes. Physical activity at moderate or vigorous intensities reduces stress and depressive symptoms, controls high blood pressure and cholesterol levels, improves sleep, reduces or reverses weight gain, and prevents or controls chronic diseases.1 Based on these benefits, all physicians are encouraged to counsel sedentary patients to increase activity levels.2
National disease prevention objectives of Healthy People 2010 call for physicians to counsel at-risk patients on health behaviors such as physical activity and diet.3 Knowledge of patients’ families, environments, and communities makes primary care physicians uniquely suited to give effective advice,4 and physician counseling is known to positively influence patients’ health-related behavior.5-12
To date, findings on counseling effectiveness have been mixed. Unfortunately, previous controlled trials of primary care physicians counseling adult patients on physical activity have varied in quality and yielded mixed results.13 Therefore, in its Guide to Clinical Preventive Services, the US Preventive Services Task Force did not recommend for or against behavioral counseling in primary care settings to promote physical activity.14 The guidelines state that existing studies do not provide a clear picture of which counseling components are effective.13
A population-based study by Glasgow and colleagues suggests that follow-up support by the physician may be needed to change physical activity behavior. Generalizations were limited, though, by the cross-sectional study design and post hoc analysis.15
More research is also needed to determine which strategies help patients stay physically active, a necessary component to sustaining the health benefits of exercise.16 Unfortunately, few primary care physicians counsel overweight or inactive patients on the benefits of diet and physical activity, let alone assist them with long-term follow-through.7,8,11,16-18
Why we chose to study a rural population
Rural Americans are among the groups at highest risk for chronic diseases. On average they are older, less educated, and poorer than their urban counterparts.19,20 And rural residents walk 13% less than suburbanites.21 According to the Rural Healthy People 2010 survey, 5 of the top 10 health concerns are chronic conditions that can be prevented or ameliorated with adequate physical activity.22
Studies have shown that healthy adults believe their health care providers are a credible source of information, and that they are motivated to comply with physician advice.23 Nondisabled adults believe their physicians want them to be physically active.19 However, to our knowledge, no study has examined the effects of physician counseling on physical activity behavior for patients at increased risk for chronic diseases in rural areas.
Our objectives. The first objective of this study was to identify the prevalence of specific components of physician counseling in a tri-state sample of at-risk, rural adults using telephone survey data. A second objective was to measure the longitudinal relationship between physician counseling and physical activity.
Methods
The Saint Louis University Institutional Review Board approved this study.
Study population and design
This study reports on baseline and 1-year follow-up telephone survey data collected as part of a larger 3-year intervention study in 12 rural communities from Missouri (6), Tennessee (4), and Arkansas (2). Project WOW (Walk the Ozarks to Wellness) aims to promote walking among overweight rural adults by integrating individual, interpersonal, and community-level interventions. Methods and details of the intervention are described in detail elsewhere.24 The target communities ranged in population from 766 to 12,993 adults, and in geographic area from 1.4 to 16.1 square miles.25
At baseline in the summer of 2003, we used a modified version of the Behavioral Risk Factor Surveillance System (BRFSS) interview protocol26,27 and randomly dialed telephone numbers to recruit participants residing within a 2-mile radius of a walking trail. In all, 2470 English-speaking adults, ages 18 and older, completed the baseline survey (65.2% response rate).28 Sampling was proportionate to community size.
At follow-up in the summer of 2004, participants identified at baseline completed the same telephone survey used a year earlier. This time, 1531 participants completed the survey (62.0% response rate).28
Demographic variables included age, sex, education, race/ethnicity, and annual combined household income (TABLE 1). Overweight and obesity, based on self-reported height and weight, were defined by a body mass index (BMI) of 25 to 29.9 kg/m2 and 30 kg/m2 or greater, respectively.
TABLE 1
The population sample
| PATIENT CHARACTERISTICS | FOLLOW-UP SURVEY (N =1141) % |
|---|---|
| Female | 74.6 |
| White, non-Hispanic | 94.6 |
| Education | |
| Less than high school | 9.7* |
| High school graduate | 30.8 |
| Some college | 23.9 |
| College graduate | 35.6 |
| Age | |
| 18-24 | 6.4* |
| 25-44 | 35.4 |
| 45-64 | 39.1 |
| 65+ | 19.1 |
| Annual household combined income (n=1102) | |
| < $25,000 31.7* | |
| ‡ $25,000 | 68.3 |
| Body mass index (n=1107) | |
| Normal (<25) | 43.7 |
| Overweight (25-29.9) | 31.5 |
| Obese (‡ 30) | 24.8 |
| Physician encounters/counseling | |
| Has doctor for regular care | 87.8* |
| In usual year, has seen doctor ≤1 time | 28.2* |
| Has been advised to exercise more | 35.1 |
| Doctor helped develop plan to exercise more | 33.8 |
| Doctor followed up on plan to exercise more | 46.3 |
| * Significant P value <.001 between responders and nonresponders at 1-year follow-up. | |
Measurement of dependent and independent variables
The survey instrument incorporated questions from the BRFSS, as well as questions developed by researchers from San Diego; Sumter County, South Carolina; and St. Louis.29-34 Psychometric properties of the questions and scales are reported elsewhere.35 The survey instrument contained 106 questions, including skip patterns; the average administration time was 34 minutes.
We assessed physician counseling about exercise with 5 questions from the survey (TABLE 2). These questions were modeled on the “4 As” counseling approach (Ask, Advise, Assist, and Arrange follow-up) recommended by the National Cancer Institute36 and used in a previous, similar BRFSS-based telephone survey.15 The questions were:
- Do you have a doctor whom you see for regular health care?
- In a usual year, how often do you see your doctor?
- Have you been advised within the last year by a doctor to exercise more?
- Has your doctor helped you to develop a plan to increase exercise?
- Has your doctor followed up with you at subsequent visits to see how you increased exercise?
We administered the questions at baseline and at follow-up. For our analyses, we used patient reports of physician counseling at 1-year follow-up, which covered all counseling received in the past 12 months.
We considered respondents to have met recommendations for physical activity if they had engaged in prescribed moderate or vigorous physical activities, or had walked for exercise 150 minutes a week.
Moderate physical activity was defined (according to the current CDC recommendations) as 30 cumulative minutes of moderate-intensity activity (brisk walking or jogging) at least 5 days per week.1
Vigorous physical activity was defined as 20 minutes of vigorous-intensity activity (running) at least 3 days per week.1
The sample was limited to participants who completed the baseline and follow-up surveys, and who reported at follow-up that they had no physical impairment that prevented walking (n=1141).
Statistical analysis
To evaluate how physician counseling would change a patient’s physical activity between baseline and 1-year follow-up, we used multivariate logistic regression analysis. In accordance with the questions asked in the survey, we defined 5 potential predictors of a patient’s decision to start exercising and keep exercising:
- Patient has seen a doctor for regular care.
- In a usual year, patient has seen a doctor once or less.
- Patient has been advised to exercise more.
- Doctor helped develop a plan to exercise more.
- Doctor followed up on plan to exercise more
For every patient who met physical activity recommendations at the 1-year follow-up, we performed regression analysis on each of these 5 measures, adjusting for baseline physical activity and potential confounders of age, education, and sex. This method allowed us to examine the independent effect of physician counseling on physical activity at 1-year follow-up.
The number of respondents analyzed differed according to the measure being examined. For example, we included all respondents in analyzing the effect of visit frequency and the effect of being advised to exercise more (n=1141). However, only those respondents who reported being advised to exercise more were included in the analyses of a physician helping to develop an exercise plan and physician follow-up in supporting exercise behavior (n=402).
To determine whether physician counseling was consistent across BMI categories and income (dichotomized at $25,000), we performed stratified analyses on the multilevel logistic regressions.
TABLE 2
What increased the likelihood of exercise? Regular medical care, a physician-assisted exercise plan, and physician follow-up (n=1141)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS | |
|---|---|---|
| aOR* | 95% CI | |
| 1. Do you have a doctor whom you see for regular health care? | ||
| Yes | 1.54 | 1.04-2.28 |
| No | Ref | |
| 2. In a usual year, how often do you see your doctor? | ||
| Once a year or less | 1.41 | 1.02-1.95 |
| Twice a year or more | Ref | |
| 3. Have you been advised within the last year by a doctor to exercise more? | ||
| Yes | 0.68 | 0.52-0.90 |
| No | Ref | |
| Of those advised to exercise more (n=402): 4. Has your doctor helped you to develop a plan to increase exercise? | ||
| Yes | 1.93 | 1.19-3.15 |
| No | Ref | |
| 5. Has your doctor followed up with you at subsequent visits to see how you increased exercise? | ||
| Yes | 2.84 | 1.78-4.53 |
| No | Ref | |
| aOR, adjusted odds ratio; CI, confidence interval; Ref, reference group. | ||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | ||
Results
The final cohort consisted of 1141 adults (TABLE 2). Those who did not respond to the follow-up survey were significantly more likely to be younger (P<.001), have less than a high school education (P<.001), and have an annual household combined income <$25,000 (P<.001). Those completing the follow-up survey were more likely to have a doctor for regular care (P<.001), although they saw their doctor, on average, significantly less per year than nonrespondents (P<.001).
Ninety percent of the cohort sample reported having a doctor whom they saw for regular care. Within the last year, 35% had been advised by their doctor to exercise more. Of those who had been so advised, 34% received help from their physician in developing a plan to increase exercise, and 46% were queried at subsequent visits as to how they were progressing with their exercise program.
After adjusting for age, sex, education, and baseline physical activity, we found that those who had a doctor for regular care were 54% more likely to be physically active than those who reported not having a doctor for regular care (aOR=1.54; 95% CI, 1.04-2.28). If the advising physician also developed a plan with the patient to increase exercise, there was nearly a 2-fold increase in physical activity compared with those who received only advice to exercise more (aOR=1.93; 95% CI, 1.19-3.15). If the physician followed up with the exercise plan at subsequent visits, the likelihood of physical activity increased further (aOR=2.84; 95% CI, 1.78-4.53) compared with those who did not receive follow-up from the physician.
Results of stratified analysis by BMI status are shown in TABLE 3. Individuals at normal weight were significantly more likely to be physically active if they had a physician for regular care (aOR=2.76; 95% CI, 1.49-5.13). Overweight adults (BMI 25-29.9 kg/m2) who had been advised by their physician to exercise more were significantly more likely to attain recommended levels of physical activity if their doctor helped develop an exercise plan than were those given more general advice about exercise (aOR=4.99; 95% CI, 1.69-14.73). Overweight individuals who received further counseling with follow-up inquiries were 5.64 times more likely to be physically active (95% CI, 2.10-15.17). A physician-developed exercise plan did not appreciably improve physical activity in obese adults (BMI ≥30 kg/m2); however, benefit in this group was demonstrated when physicians prescribed and followed up with the exercise plan (aOR=2.13; 95% CI, 1.10-4.12). Stratified analysis by income status provided no clear pattern (data not shown).
TABLE 3
What role does BMI play in patients achieving activity goals? (n=1107)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS* (aOR [95% CI]) | ||
|---|---|---|---|
| NORMAL (BMI<25) | OVERWEIGHT (BMI 25-29.9) | OBESE (BMI ≥30) | |
| Has seen a doctor for regular care | 2.76 (1.49-5.13) | 1.08 (0.51-2.29) | 1.34 (0.62-2.91) |
| In a usual year, has seen a doctor ≤1 time | 1.98 (1.13-3.47) | 0.95 (0.54-1.68) | 0.95 (0.47-1.92) |
| Has been advised to exercise more | 1.02 (0.56-1.85) | 0.75 (0.46-1.25) | 0.77 (0.46-1.31) |
| Doctor helped develop a plan to exercise more | 0.69 (0.22-2.18) | 4.99 (1.69-14.73) | 1.76 (0.87-3.56) |
| Doctor followed up on plan to exercise more | 2.59 (0.80-8.36) | 5.64 (2.10-15.17) | 2.13 (1.10-4.12) |
| aOR, adjusted odds ratio; BMI, body-mass index; CI, confidence interval. | |||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | |||
Discussion
Findings from our analyses support the need for more detailed and more frequent exercise counseling (including follow-up) by rural primary care physicians. In our study, physicians’ counsel was most effective when presented as a plan or prescription that was followed up with periodic inquiries. Patients’ initiation and maintenance of physical activity were significantly associated with physicians’ follow-up of exercise plans. Those who were merely “advised” to exercise more were less likely to meet physical activity recommendations. This illustrates the importance of detailed physician counseling over simple advice to exercise more.
Over 80% of normal-weight individuals, who comprised more than 40% of the sample, reported that their physician had not suggested they exercise more. There are many possible explanations for these reports. Rural populations are relatively isolated and slow to adopt changes. Thus patients may be unaware of new recommendations for physical activity and their significant benefit for disease prevention, and therefore unlikely to discuss such matters with their physician. Physicians also may perceive normal-weight individuals as healthy regardless of their actual health behaviors. On the other hand, 1 study showed that patients with disease risk factors (eg, high cholesterol, elevated BMI) were more likely to be counseled on preventive health behaviors.37
With overweight patients, who are at increased risk of developing chronic diseases, physician counseling strengthened their resolve significantly. Overweight individuals who received directives from their physician (a plan to increase exercise and subsequent follow-up) were 5½ times more likely to be physically active than those who received less counseling.
Obese patients did not receive counseling as often as overweight patients, or benefit from it as much when given, perhaps due to the presence of comorbidities. However, many studies show that regardless of BMI status, physical activity reduces all-cause mortality.38-41
Interestingly, our results showed that seeing a doctor less than once a year was associated with increases in physical activity. Patients who see their physician once a year may be going for annual wellness exams, providing more opportunity to discuss health behavior.
Overall, patients are counseled less often/thoroughly than needed. Our findings agree with those of a previous statewide study that used Missouri BRFSS data to assess the extent to which overweight or physically inactive people received advice from their physicians concerning these risk factors.42 Although most Missouri residents who were overweight or inactive reported seeing their physician within the past year, less than half said their doctors advised them to alter their risk behavior(s).42 Our findings are also consistent with a recent nationwide study by Ma and colleagues that focused on adults with obesity, diabetes, or other related conditions.43 Participants from across the United States reported receiving counseling for physical activity in <30% of visits to private physician offices and hospital outpatient departments.43
Our study was unique in that it examined a tri-state sample of the nation’s rural population for both evidence and effectiveness of physician counseling. It is one of very few studies using a longitudinal design, strengthening the associations found. Causality is limited, however, due to the multifaceted design of the intervention program from which the data were obtained. Future research should evaluate varying degrees of physician counseling and other indirect measures of its impact.
Limitations of the study
Our observational cohort design and the large, randomly selected sample resulted in fewer limitations than were seen with previous similar studies. However, our study had several limitations.
- Recall bias may be present. We assessed counseling with patient memory alone; we made no attempts to interview physicians or audit charts.
- Self-reported height and weight data tend to underestimate the prevalence of obesity.44,45 Resultant misclassification of overweight subjects as being at normal weight could have skewed the stratified analysis.
- The external validity of the physician-counseling questions we used has not been formally confirmed. Given the demographics of the analytic sample (ie, mostly female, white, low income), it would be appropriate to generalize our findings only to similar, rural populations.
Barriers to counseling, and means of removing them
Primary care physicians—rural or urban—are no doubt aware of the health risks associated with physical inactivity. However, the barriers physicians face in counseling at-risk patients overwhelm most efforts. These barriers include lack of time, inadequate provider counseling skills and training, perceived ineffectiveness and nonadherence, patient comorbidities, and lack of organizational support and reimbursement.46-48
Intervention programs and tools have been developed to help health care providers overcome time, skill, and training barriers. These programs, available even to rural providers, have proven effective.49,50 (Go to www.paceproject.org/Home.html and click on “Projects” to learn about the PACE program.) However, application of such skills and tools may be more successful if training is incorporated into medical school curricula and residency training programs rather than through CME endeavors.49 This would require medical institutions and organizations to prioritize the direct link between healthy lifestyle behaviors and disease prevention and the vital role physicians play in underscoring this link.
Finally, health care policy makers and systems must be persuaded to address the lack of organizational support and reimbursement that prevents physicians from counseling at-risk patients on unhealthy lifestyle behaviors. Responsible payers and providers should aggressively explore low-cost ways to promote physical activity and weight loss in primary care settings, to stem the tide of obesity-related chronic diseases. At the local level, physicians can team up to support policies that may enhance preventive counseling efforts2—increasing access to places for activity, encouraging physical activity programming in communities, schools, and organizations, and physical environment enhancements such as safe sidewalks, adequate lighting, and improved zoning.44,51,44,52
Acknowledgments
We thank the communities that are participating in the ongoing intervention study. For their assistance in data collection, we thank the Department of Health Management and Informatics, Behavioral Risk Research Unit at the University of Missouri, Columbia.
Funding/support
This study was funded through the National Institutes of Health grant NIDDK #5 R18 DK061706 and the Centers for Disease Control and Prevention contract U48/CCU710806 (Centers for Research and Demonstration of Health Promotion and Disease Prevention). Human subjects approval was obtained from the Saint Louis University Institutional Review Board.
Correspondence
Sarah L. Lovegreen, MPH, Prevention Research Center and Department of Community Health, Saint Louis University School of Public Health, 3545 Lafayette Ave., Salus Center, Suite 300, St. Louis, MO 63146; slove green@oasisnet.org.
- To help overweight patients and those with a sedentary lifestyle to adopt and stick with an exercise regimen, develop a detailed and realistic plan with their help, and follow up with them periodically to see how they’re doing.
Purpose: Exercise counseling by primary care physicians has been shown to improve physical activity in patients. However, the prevalence and effectiveness of physician counseling is unknown in rural populations that are at increased risk for chronic diseases.
Methods: Using a population-based telephone survey at baseline and again at 1-year follow-up, we assessed physical activity behavior among 1141 adults (75% female, 95% white) living within 12 rural communities of Missouri, Tennessee, and Arkansas. We tested the association between physician counseling and patients meeting current physical activity recommendations using logistic regression analysis controlling for demographic variables.
Results: Participants who saw a doctor for regular care were 54% more likely to be physically active (adjusted odds ratio [aOR]=1.54; 95% confidence interval [CI], 1.04-2.28). Overweight adults (body mass index [BMI]=25-29.9 kg/m2) who had been advised by their physician to exercise more were nearly 5 times more likely to meet physical activity recommendations if their doctor helped develop an exercise plan (aOR=4.99; 95% CI, 1.69-14.73).
Overweight individuals who received additional follow-up with the exercise plan from their doctor had a 5½-fold increase in likelihood of meeting physical activity recommendations (P<.05).
In the overall sample, patients were significantly more likely to initiate (P=.01) and maintain (P=.002) physical activity when the physician prescribed and followed up on an exercise plan.
Conclusion: This longitudinal study provides evidence that exercise counseling is most effective when the physician presents the counseling as a plan or prescription and when he or she follows up with the patient on it.
Simply telling sedentary patients that they need to exercise may not help them much. If the goal is to inspire action, a more effective approach would be to help them devise a plan for exercise and then inquire periodically about how it’s going. That premise was the basis for our study.
There’s good reason to get your patients moving
Physical inactivity is an independent risk factor for the most prevalent chronic diseases, including obesity, cardiovascular disease, and type 2 diabetes. Physical activity at moderate or vigorous intensities reduces stress and depressive symptoms, controls high blood pressure and cholesterol levels, improves sleep, reduces or reverses weight gain, and prevents or controls chronic diseases.1 Based on these benefits, all physicians are encouraged to counsel sedentary patients to increase activity levels.2
National disease prevention objectives of Healthy People 2010 call for physicians to counsel at-risk patients on health behaviors such as physical activity and diet.3 Knowledge of patients’ families, environments, and communities makes primary care physicians uniquely suited to give effective advice,4 and physician counseling is known to positively influence patients’ health-related behavior.5-12
To date, findings on counseling effectiveness have been mixed. Unfortunately, previous controlled trials of primary care physicians counseling adult patients on physical activity have varied in quality and yielded mixed results.13 Therefore, in its Guide to Clinical Preventive Services, the US Preventive Services Task Force did not recommend for or against behavioral counseling in primary care settings to promote physical activity.14 The guidelines state that existing studies do not provide a clear picture of which counseling components are effective.13
A population-based study by Glasgow and colleagues suggests that follow-up support by the physician may be needed to change physical activity behavior. Generalizations were limited, though, by the cross-sectional study design and post hoc analysis.15
More research is also needed to determine which strategies help patients stay physically active, a necessary component to sustaining the health benefits of exercise.16 Unfortunately, few primary care physicians counsel overweight or inactive patients on the benefits of diet and physical activity, let alone assist them with long-term follow-through.7,8,11,16-18
Why we chose to study a rural population
Rural Americans are among the groups at highest risk for chronic diseases. On average they are older, less educated, and poorer than their urban counterparts.19,20 And rural residents walk 13% less than suburbanites.21 According to the Rural Healthy People 2010 survey, 5 of the top 10 health concerns are chronic conditions that can be prevented or ameliorated with adequate physical activity.22
Studies have shown that healthy adults believe their health care providers are a credible source of information, and that they are motivated to comply with physician advice.23 Nondisabled adults believe their physicians want them to be physically active.19 However, to our knowledge, no study has examined the effects of physician counseling on physical activity behavior for patients at increased risk for chronic diseases in rural areas.
Our objectives. The first objective of this study was to identify the prevalence of specific components of physician counseling in a tri-state sample of at-risk, rural adults using telephone survey data. A second objective was to measure the longitudinal relationship between physician counseling and physical activity.
Methods
The Saint Louis University Institutional Review Board approved this study.
Study population and design
This study reports on baseline and 1-year follow-up telephone survey data collected as part of a larger 3-year intervention study in 12 rural communities from Missouri (6), Tennessee (4), and Arkansas (2). Project WOW (Walk the Ozarks to Wellness) aims to promote walking among overweight rural adults by integrating individual, interpersonal, and community-level interventions. Methods and details of the intervention are described in detail elsewhere.24 The target communities ranged in population from 766 to 12,993 adults, and in geographic area from 1.4 to 16.1 square miles.25
At baseline in the summer of 2003, we used a modified version of the Behavioral Risk Factor Surveillance System (BRFSS) interview protocol26,27 and randomly dialed telephone numbers to recruit participants residing within a 2-mile radius of a walking trail. In all, 2470 English-speaking adults, ages 18 and older, completed the baseline survey (65.2% response rate).28 Sampling was proportionate to community size.
At follow-up in the summer of 2004, participants identified at baseline completed the same telephone survey used a year earlier. This time, 1531 participants completed the survey (62.0% response rate).28
Demographic variables included age, sex, education, race/ethnicity, and annual combined household income (TABLE 1). Overweight and obesity, based on self-reported height and weight, were defined by a body mass index (BMI) of 25 to 29.9 kg/m2 and 30 kg/m2 or greater, respectively.
TABLE 1
The population sample
| PATIENT CHARACTERISTICS | FOLLOW-UP SURVEY (N =1141) % |
|---|---|
| Female | 74.6 |
| White, non-Hispanic | 94.6 |
| Education | |
| Less than high school | 9.7* |
| High school graduate | 30.8 |
| Some college | 23.9 |
| College graduate | 35.6 |
| Age | |
| 18-24 | 6.4* |
| 25-44 | 35.4 |
| 45-64 | 39.1 |
| 65+ | 19.1 |
| Annual household combined income (n=1102) | |
| < $25,000 31.7* | |
| ‡ $25,000 | 68.3 |
| Body mass index (n=1107) | |
| Normal (<25) | 43.7 |
| Overweight (25-29.9) | 31.5 |
| Obese (‡ 30) | 24.8 |
| Physician encounters/counseling | |
| Has doctor for regular care | 87.8* |
| In usual year, has seen doctor ≤1 time | 28.2* |
| Has been advised to exercise more | 35.1 |
| Doctor helped develop plan to exercise more | 33.8 |
| Doctor followed up on plan to exercise more | 46.3 |
| * Significant P value <.001 between responders and nonresponders at 1-year follow-up. | |
Measurement of dependent and independent variables
The survey instrument incorporated questions from the BRFSS, as well as questions developed by researchers from San Diego; Sumter County, South Carolina; and St. Louis.29-34 Psychometric properties of the questions and scales are reported elsewhere.35 The survey instrument contained 106 questions, including skip patterns; the average administration time was 34 minutes.
We assessed physician counseling about exercise with 5 questions from the survey (TABLE 2). These questions were modeled on the “4 As” counseling approach (Ask, Advise, Assist, and Arrange follow-up) recommended by the National Cancer Institute36 and used in a previous, similar BRFSS-based telephone survey.15 The questions were:
- Do you have a doctor whom you see for regular health care?
- In a usual year, how often do you see your doctor?
- Have you been advised within the last year by a doctor to exercise more?
- Has your doctor helped you to develop a plan to increase exercise?
- Has your doctor followed up with you at subsequent visits to see how you increased exercise?
We administered the questions at baseline and at follow-up. For our analyses, we used patient reports of physician counseling at 1-year follow-up, which covered all counseling received in the past 12 months.
We considered respondents to have met recommendations for physical activity if they had engaged in prescribed moderate or vigorous physical activities, or had walked for exercise 150 minutes a week.
Moderate physical activity was defined (according to the current CDC recommendations) as 30 cumulative minutes of moderate-intensity activity (brisk walking or jogging) at least 5 days per week.1
Vigorous physical activity was defined as 20 minutes of vigorous-intensity activity (running) at least 3 days per week.1
The sample was limited to participants who completed the baseline and follow-up surveys, and who reported at follow-up that they had no physical impairment that prevented walking (n=1141).
Statistical analysis
To evaluate how physician counseling would change a patient’s physical activity between baseline and 1-year follow-up, we used multivariate logistic regression analysis. In accordance with the questions asked in the survey, we defined 5 potential predictors of a patient’s decision to start exercising and keep exercising:
- Patient has seen a doctor for regular care.
- In a usual year, patient has seen a doctor once or less.
- Patient has been advised to exercise more.
- Doctor helped develop a plan to exercise more.
- Doctor followed up on plan to exercise more
For every patient who met physical activity recommendations at the 1-year follow-up, we performed regression analysis on each of these 5 measures, adjusting for baseline physical activity and potential confounders of age, education, and sex. This method allowed us to examine the independent effect of physician counseling on physical activity at 1-year follow-up.
The number of respondents analyzed differed according to the measure being examined. For example, we included all respondents in analyzing the effect of visit frequency and the effect of being advised to exercise more (n=1141). However, only those respondents who reported being advised to exercise more were included in the analyses of a physician helping to develop an exercise plan and physician follow-up in supporting exercise behavior (n=402).
To determine whether physician counseling was consistent across BMI categories and income (dichotomized at $25,000), we performed stratified analyses on the multilevel logistic regressions.
TABLE 2
What increased the likelihood of exercise? Regular medical care, a physician-assisted exercise plan, and physician follow-up (n=1141)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS | |
|---|---|---|
| aOR* | 95% CI | |
| 1. Do you have a doctor whom you see for regular health care? | ||
| Yes | 1.54 | 1.04-2.28 |
| No | Ref | |
| 2. In a usual year, how often do you see your doctor? | ||
| Once a year or less | 1.41 | 1.02-1.95 |
| Twice a year or more | Ref | |
| 3. Have you been advised within the last year by a doctor to exercise more? | ||
| Yes | 0.68 | 0.52-0.90 |
| No | Ref | |
| Of those advised to exercise more (n=402): 4. Has your doctor helped you to develop a plan to increase exercise? | ||
| Yes | 1.93 | 1.19-3.15 |
| No | Ref | |
| 5. Has your doctor followed up with you at subsequent visits to see how you increased exercise? | ||
| Yes | 2.84 | 1.78-4.53 |
| No | Ref | |
| aOR, adjusted odds ratio; CI, confidence interval; Ref, reference group. | ||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | ||
Results
The final cohort consisted of 1141 adults (TABLE 2). Those who did not respond to the follow-up survey were significantly more likely to be younger (P<.001), have less than a high school education (P<.001), and have an annual household combined income <$25,000 (P<.001). Those completing the follow-up survey were more likely to have a doctor for regular care (P<.001), although they saw their doctor, on average, significantly less per year than nonrespondents (P<.001).
Ninety percent of the cohort sample reported having a doctor whom they saw for regular care. Within the last year, 35% had been advised by their doctor to exercise more. Of those who had been so advised, 34% received help from their physician in developing a plan to increase exercise, and 46% were queried at subsequent visits as to how they were progressing with their exercise program.
After adjusting for age, sex, education, and baseline physical activity, we found that those who had a doctor for regular care were 54% more likely to be physically active than those who reported not having a doctor for regular care (aOR=1.54; 95% CI, 1.04-2.28). If the advising physician also developed a plan with the patient to increase exercise, there was nearly a 2-fold increase in physical activity compared with those who received only advice to exercise more (aOR=1.93; 95% CI, 1.19-3.15). If the physician followed up with the exercise plan at subsequent visits, the likelihood of physical activity increased further (aOR=2.84; 95% CI, 1.78-4.53) compared with those who did not receive follow-up from the physician.
Results of stratified analysis by BMI status are shown in TABLE 3. Individuals at normal weight were significantly more likely to be physically active if they had a physician for regular care (aOR=2.76; 95% CI, 1.49-5.13). Overweight adults (BMI 25-29.9 kg/m2) who had been advised by their physician to exercise more were significantly more likely to attain recommended levels of physical activity if their doctor helped develop an exercise plan than were those given more general advice about exercise (aOR=4.99; 95% CI, 1.69-14.73). Overweight individuals who received further counseling with follow-up inquiries were 5.64 times more likely to be physically active (95% CI, 2.10-15.17). A physician-developed exercise plan did not appreciably improve physical activity in obese adults (BMI ≥30 kg/m2); however, benefit in this group was demonstrated when physicians prescribed and followed up with the exercise plan (aOR=2.13; 95% CI, 1.10-4.12). Stratified analysis by income status provided no clear pattern (data not shown).
TABLE 3
What role does BMI play in patients achieving activity goals? (n=1107)
| PHYSICIAN ENCOUNTERS/COUNSELING | PATIENTS MEETING PHYSICAL ACTIVITY RECOMMENDATIONS* (aOR [95% CI]) | ||
|---|---|---|---|
| NORMAL (BMI<25) | OVERWEIGHT (BMI 25-29.9) | OBESE (BMI ≥30) | |
| Has seen a doctor for regular care | 2.76 (1.49-5.13) | 1.08 (0.51-2.29) | 1.34 (0.62-2.91) |
| In a usual year, has seen a doctor ≤1 time | 1.98 (1.13-3.47) | 0.95 (0.54-1.68) | 0.95 (0.47-1.92) |
| Has been advised to exercise more | 1.02 (0.56-1.85) | 0.75 (0.46-1.25) | 0.77 (0.46-1.31) |
| Doctor helped develop a plan to exercise more | 0.69 (0.22-2.18) | 4.99 (1.69-14.73) | 1.76 (0.87-3.56) |
| Doctor followed up on plan to exercise more | 2.59 (0.80-8.36) | 5.64 (2.10-15.17) | 2.13 (1.10-4.12) |
| aOR, adjusted odds ratio; BMI, body-mass index; CI, confidence interval. | |||
| *Adjusted for sex, age, educational attainment, and baseline physical activity. | |||
Discussion
Findings from our analyses support the need for more detailed and more frequent exercise counseling (including follow-up) by rural primary care physicians. In our study, physicians’ counsel was most effective when presented as a plan or prescription that was followed up with periodic inquiries. Patients’ initiation and maintenance of physical activity were significantly associated with physicians’ follow-up of exercise plans. Those who were merely “advised” to exercise more were less likely to meet physical activity recommendations. This illustrates the importance of detailed physician counseling over simple advice to exercise more.
Over 80% of normal-weight individuals, who comprised more than 40% of the sample, reported that their physician had not suggested they exercise more. There are many possible explanations for these reports. Rural populations are relatively isolated and slow to adopt changes. Thus patients may be unaware of new recommendations for physical activity and their significant benefit for disease prevention, and therefore unlikely to discuss such matters with their physician. Physicians also may perceive normal-weight individuals as healthy regardless of their actual health behaviors. On the other hand, 1 study showed that patients with disease risk factors (eg, high cholesterol, elevated BMI) were more likely to be counseled on preventive health behaviors.37
With overweight patients, who are at increased risk of developing chronic diseases, physician counseling strengthened their resolve significantly. Overweight individuals who received directives from their physician (a plan to increase exercise and subsequent follow-up) were 5½ times more likely to be physically active than those who received less counseling.
Obese patients did not receive counseling as often as overweight patients, or benefit from it as much when given, perhaps due to the presence of comorbidities. However, many studies show that regardless of BMI status, physical activity reduces all-cause mortality.38-41
Interestingly, our results showed that seeing a doctor less than once a year was associated with increases in physical activity. Patients who see their physician once a year may be going for annual wellness exams, providing more opportunity to discuss health behavior.
Overall, patients are counseled less often/thoroughly than needed. Our findings agree with those of a previous statewide study that used Missouri BRFSS data to assess the extent to which overweight or physically inactive people received advice from their physicians concerning these risk factors.42 Although most Missouri residents who were overweight or inactive reported seeing their physician within the past year, less than half said their doctors advised them to alter their risk behavior(s).42 Our findings are also consistent with a recent nationwide study by Ma and colleagues that focused on adults with obesity, diabetes, or other related conditions.43 Participants from across the United States reported receiving counseling for physical activity in <30% of visits to private physician offices and hospital outpatient departments.43
Our study was unique in that it examined a tri-state sample of the nation’s rural population for both evidence and effectiveness of physician counseling. It is one of very few studies using a longitudinal design, strengthening the associations found. Causality is limited, however, due to the multifaceted design of the intervention program from which the data were obtained. Future research should evaluate varying degrees of physician counseling and other indirect measures of its impact.
Limitations of the study
Our observational cohort design and the large, randomly selected sample resulted in fewer limitations than were seen with previous similar studies. However, our study had several limitations.
- Recall bias may be present. We assessed counseling with patient memory alone; we made no attempts to interview physicians or audit charts.
- Self-reported height and weight data tend to underestimate the prevalence of obesity.44,45 Resultant misclassification of overweight subjects as being at normal weight could have skewed the stratified analysis.
- The external validity of the physician-counseling questions we used has not been formally confirmed. Given the demographics of the analytic sample (ie, mostly female, white, low income), it would be appropriate to generalize our findings only to similar, rural populations.
Barriers to counseling, and means of removing them
Primary care physicians—rural or urban—are no doubt aware of the health risks associated with physical inactivity. However, the barriers physicians face in counseling at-risk patients overwhelm most efforts. These barriers include lack of time, inadequate provider counseling skills and training, perceived ineffectiveness and nonadherence, patient comorbidities, and lack of organizational support and reimbursement.46-48
Intervention programs and tools have been developed to help health care providers overcome time, skill, and training barriers. These programs, available even to rural providers, have proven effective.49,50 (Go to www.paceproject.org/Home.html and click on “Projects” to learn about the PACE program.) However, application of such skills and tools may be more successful if training is incorporated into medical school curricula and residency training programs rather than through CME endeavors.49 This would require medical institutions and organizations to prioritize the direct link between healthy lifestyle behaviors and disease prevention and the vital role physicians play in underscoring this link.
Finally, health care policy makers and systems must be persuaded to address the lack of organizational support and reimbursement that prevents physicians from counseling at-risk patients on unhealthy lifestyle behaviors. Responsible payers and providers should aggressively explore low-cost ways to promote physical activity and weight loss in primary care settings, to stem the tide of obesity-related chronic diseases. At the local level, physicians can team up to support policies that may enhance preventive counseling efforts2—increasing access to places for activity, encouraging physical activity programming in communities, schools, and organizations, and physical environment enhancements such as safe sidewalks, adequate lighting, and improved zoning.44,51,44,52
Acknowledgments
We thank the communities that are participating in the ongoing intervention study. For their assistance in data collection, we thank the Department of Health Management and Informatics, Behavioral Risk Research Unit at the University of Missouri, Columbia.
Funding/support
This study was funded through the National Institutes of Health grant NIDDK #5 R18 DK061706 and the Centers for Disease Control and Prevention contract U48/CCU710806 (Centers for Research and Demonstration of Health Promotion and Disease Prevention). Human subjects approval was obtained from the Saint Louis University Institutional Review Board.
Correspondence
Sarah L. Lovegreen, MPH, Prevention Research Center and Department of Community Health, Saint Louis University School of Public Health, 3545 Lafayette Ave., Salus Center, Suite 300, St. Louis, MO 63146; slove green@oasisnet.org.
1. US Department of Health and Human Services. Physical Activity and Health: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, 1996.
2. Chakravarthy MV, Joyner MJ, Booth FW. An obligation for primary care physicians to prescribe physical activity to sedentary patients to reduce the risk of chronic health conditions. Mayo Clin Proc 2002;77:165-173.
3. US. Department of Health and Human Services. Health People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Iverson D, Fielding J, Crow R, Christenson G. The promotion of physical activity in the U.S. population: the status of programs in medical, worksite, community, and school settings. Public Health Rep 1985;100:212-224.
5. Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med 2000;9:426-433.
6. The Writing Group for the Activity Counseling Trial Research Group. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA 2001;286:677-687.
7. Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professionals advising obese patients to lose weight? JAMA 1999;282:1576-1578.
8. Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225-233.
9. Sallis JF, Patrick K, Calfas KJ. Counseling patients/clients about physical activity and nutrition. Weight Control Digest 1999;9:843, 846-850.
10. Calfas KJ, Zabinski MF, Rupp J. Practical nutrition assessment in primary care settings: a review. Am J Prev Med. 2000;18:289-299.
11. Marcus BH, Goldstein MG, Jette A, et al. Training physicians to conduct physical activity counseling. Prev Med 1997;26:382-388.
12. Godin R, Shephard RJ. An evaluation of the potential role of the physician in influencing community exercise behavior. Am J Health Prom 1990;4:255-259.
13. Eden KB, Orleans CT, Mulrow CD, Pender NJ, Teutsch SM. Does counseling by clinicians improve physical activity? A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:208-215.
14. US. Preventive Services Task Force. Behavioral Counseling in Primary Care to Promote Physical Activity: Recommendations and Rationale. Rockville, MD: Agency for Healthcare Research and Quality; July 2002. Available at: http://www.ahrq.gov/clinic/3rduspstf/physactivity/physactrr.htm. Accessed April 24, 2008.
15. Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med 2001;21:189-196.
16. Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol 2000;19(1 suppl):32-41.
17. Stafford RS, Farhat JH, Misra B, Schoenfeld DA. National patterns of physician activities related to obesity management. Arch Fam Med 2000;9:631-638.
18. Potter MB, Vu JD, Croughan-Minihane M. Weight management what patients want from their primary care physician. J Fam Pract 2001;50:513-518.
19. Sobal J, Troiana RP, Frongillo EA, Jr. Rural-urban differences in obesity. Rural Sociol 1996;2:289-305.
20. Miller MK, Stokes CS, Clifford WB. A comparison of the rural-urban mortality differential for deaths from all causes, cardiovascular disease and cancer. J Rural Health 1987;3(2):23-34.
21. Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Med Sci Sports Exerc 2003;35:1529-36.
22. Gamm L, Hutchison L, Bellamy G, et al. Rural healthy people 2010: identifying rural health priorities and models for practice. J Rural Health 2002;18(1):9-14.
23. Schappert SM. National ambulatory medical care survey: 1991 Summary. Hyattsville, MD: US Department of Health and Human Services. Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 1993.
24. Brownson R, Hagood L, Lovegreen S, et al. A multilevel, ecological approach to promoting walking in rural communities. Prev Med 2005;41:837-842.
25. US. Census Bureau. Census 2000. Available at: http://www.census.gov/main/www/cen2000.html. Accessed November 2004.
26. Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med 1985;1(6):9-14.
27. Remington PL, Smith MY, Williamson DF, Anda RF, Gentry EM, Hogelin GC. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981-87. Public Health Rep 1988;103:366-375.
28. Council of American Survey Research Organizations (CASRO) Task Force on Completion Rates. On the Definitions of Response Rates. Special Report. New York, NY: Council of American Survey Organizations; 1982.
29. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss/. Accessed November 2004.
30. Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health 2003;93:1552-1558.
31. Eyler AA, Brownson RC, Donatelle RJ, King AC, Brown D, Sallis JF. Physical activity social support and middle- and older-aged minority women: results from a US survey. Soc Sci Med 1999;49:781-789.
32. King AC, Castro C, Wilcox S, Eyler AA, Sallis JF, Brownson RC. Personal and environmental factors associated with physical inactivity among different racial-ethnic groups of US middle- aged and older-aged women. Health Psychol 2000;19:354-364.
33. Ainsworth BE, Bassett DR, Jr, Strath SJ, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc 2000;32(9 suppl):S457-S464.
34. Brownson RC, Baker EA, Housemann RA, Brennan LK, Bacak SJ. Environmental and policy determinants of physical activity in the United States. Am J Public Health 2001;91:1995-2003.
35. Brownson RC, Chang JJ, Eyler AA. Measuring the environment for friendliness toward physical activity: a comparison of the reliability of three questionnaires. Am J Public Health 2004;94:473-483.
36. Manely M, Epps R, Husten C, Glynn T, Shopland D. Clinical interventions in tobacco control: a National Cancer Institute training program for physicians. JAMA 1991;266:3172-3173.
37. Kreuter MW, Scharff DP, Brennan LK, Lukwago SN. Physician recommendations for diet and physical activity: which patients get advised to change? Prev Med 1997;26:825-833.
38. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694-2703.
39. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nut 1999;69:373-380.
40. Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the Lipid Research Clinics Study. Am J Epidemiol 2002;156:832-841.
41. Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight and obese men. JAMA 1999;282:1547-1553.
42. Friedman C, Brownson RC, Peterson DE, Wilkerson JC. Physician advice to reduce chronic disease risk factors. Am J Prev Med 1994;10:367-371.
43. Ma J, Urizar GG, Alehegn T, Stafford R. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med 2004;39:815-822.
44. Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol 1982;115:223-230.
45. Kuskowska-Wolk A, Karlsson P, Stolt M, Rossner S. The predictive validity of body mass index based on self-reported weight and height. Int J Obes 1989;13:441-453.
46. Kushner RF. Barriers to providing nutritional counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546-552.
47. Rogers LQ, Bailey JE, Gutin B, et al. Teaching resi-dent physicians to provide physician counseling: a needs assessment. Acad Med 2002;77:841-844.
48. Hiss RG. Barriers to care in non-insulin-dependent diabetes mellitus. The Michigan experience. Ann Intern Med 1996;124:146-148.
49. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multi-component program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med 2002;34:153-161.
50. Sallis JF, Patrick K, Calfas KJ, et al. A multi-media behavior change program for nutrition and physical activity in primary care: PACE+ for adults. Homeostasis 1999;39:196-202.
51. Health GW, Brownson RC, Kruger J, et al. The effectiveness of urban design and land use and transport policies and practices to increase physical activity: a systematic review. J Phys Act Health 2006;3(suppl 1):555-576.
52. Sallis JF, Glanz K. The role of built environment in physical activity, eating and obesity in childhood. Future Child 2006 Spring;16(1):89-108.
1. US Department of Health and Human Services. Physical Activity and Health: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, 1996.
2. Chakravarthy MV, Joyner MJ, Booth FW. An obligation for primary care physicians to prescribe physical activity to sedentary patients to reduce the risk of chronic health conditions. Mayo Clin Proc 2002;77:165-173.
3. US. Department of Health and Human Services. Health People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: U.S. Government Printing Office, November 2000.
4. Iverson D, Fielding J, Crow R, Christenson G. The promotion of physical activity in the U.S. population: the status of programs in medical, worksite, community, and school settings. Public Health Rep 1985;100:212-224.
5. Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med 2000;9:426-433.
6. The Writing Group for the Activity Counseling Trial Research Group. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA 2001;286:677-687.
7. Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professionals advising obese patients to lose weight? JAMA 1999;282:1576-1578.
8. Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225-233.
9. Sallis JF, Patrick K, Calfas KJ. Counseling patients/clients about physical activity and nutrition. Weight Control Digest 1999;9:843, 846-850.
10. Calfas KJ, Zabinski MF, Rupp J. Practical nutrition assessment in primary care settings: a review. Am J Prev Med. 2000;18:289-299.
11. Marcus BH, Goldstein MG, Jette A, et al. Training physicians to conduct physical activity counseling. Prev Med 1997;26:382-388.
12. Godin R, Shephard RJ. An evaluation of the potential role of the physician in influencing community exercise behavior. Am J Health Prom 1990;4:255-259.
13. Eden KB, Orleans CT, Mulrow CD, Pender NJ, Teutsch SM. Does counseling by clinicians improve physical activity? A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:208-215.
14. US. Preventive Services Task Force. Behavioral Counseling in Primary Care to Promote Physical Activity: Recommendations and Rationale. Rockville, MD: Agency for Healthcare Research and Quality; July 2002. Available at: http://www.ahrq.gov/clinic/3rduspstf/physactivity/physactrr.htm. Accessed April 24, 2008.
15. Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med 2001;21:189-196.
16. Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol 2000;19(1 suppl):32-41.
17. Stafford RS, Farhat JH, Misra B, Schoenfeld DA. National patterns of physician activities related to obesity management. Arch Fam Med 2000;9:631-638.
18. Potter MB, Vu JD, Croughan-Minihane M. Weight management what patients want from their primary care physician. J Fam Pract 2001;50:513-518.
19. Sobal J, Troiana RP, Frongillo EA, Jr. Rural-urban differences in obesity. Rural Sociol 1996;2:289-305.
20. Miller MK, Stokes CS, Clifford WB. A comparison of the rural-urban mortality differential for deaths from all causes, cardiovascular disease and cancer. J Rural Health 1987;3(2):23-34.
21. Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Med Sci Sports Exerc 2003;35:1529-36.
22. Gamm L, Hutchison L, Bellamy G, et al. Rural healthy people 2010: identifying rural health priorities and models for practice. J Rural Health 2002;18(1):9-14.
23. Schappert SM. National ambulatory medical care survey: 1991 Summary. Hyattsville, MD: US Department of Health and Human Services. Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics; 1993.
24. Brownson R, Hagood L, Lovegreen S, et al. A multilevel, ecological approach to promoting walking in rural communities. Prev Med 2005;41:837-842.
25. US. Census Bureau. Census 2000. Available at: http://www.census.gov/main/www/cen2000.html. Accessed November 2004.
26. Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med 1985;1(6):9-14.
27. Remington PL, Smith MY, Williamson DF, Anda RF, Gentry EM, Hogelin GC. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981-87. Public Health Rep 1988;103:366-375.
28. Council of American Survey Research Organizations (CASRO) Task Force on Completion Rates. On the Definitions of Response Rates. Special Report. New York, NY: Council of American Survey Organizations; 1982.
29. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss/. Accessed November 2004.
30. Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health 2003;93:1552-1558.
31. Eyler AA, Brownson RC, Donatelle RJ, King AC, Brown D, Sallis JF. Physical activity social support and middle- and older-aged minority women: results from a US survey. Soc Sci Med 1999;49:781-789.
32. King AC, Castro C, Wilcox S, Eyler AA, Sallis JF, Brownson RC. Personal and environmental factors associated with physical inactivity among different racial-ethnic groups of US middle- aged and older-aged women. Health Psychol 2000;19:354-364.
33. Ainsworth BE, Bassett DR, Jr, Strath SJ, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc 2000;32(9 suppl):S457-S464.
34. Brownson RC, Baker EA, Housemann RA, Brennan LK, Bacak SJ. Environmental and policy determinants of physical activity in the United States. Am J Public Health 2001;91:1995-2003.
35. Brownson RC, Chang JJ, Eyler AA. Measuring the environment for friendliness toward physical activity: a comparison of the reliability of three questionnaires. Am J Public Health 2004;94:473-483.
36. Manely M, Epps R, Husten C, Glynn T, Shopland D. Clinical interventions in tobacco control: a National Cancer Institute training program for physicians. JAMA 1991;266:3172-3173.
37. Kreuter MW, Scharff DP, Brennan LK, Lukwago SN. Physician recommendations for diet and physical activity: which patients get advised to change? Prev Med 1997;26:825-833.
38. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694-2703.
39. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nut 1999;69:373-380.
40. Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the Lipid Research Clinics Study. Am J Epidemiol 2002;156:832-841.
41. Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight and obese men. JAMA 1999;282:1547-1553.
42. Friedman C, Brownson RC, Peterson DE, Wilkerson JC. Physician advice to reduce chronic disease risk factors. Am J Prev Med 1994;10:367-371.
43. Ma J, Urizar GG, Alehegn T, Stafford R. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med 2004;39:815-822.
44. Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol 1982;115:223-230.
45. Kuskowska-Wolk A, Karlsson P, Stolt M, Rossner S. The predictive validity of body mass index based on self-reported weight and height. Int J Obes 1989;13:441-453.
46. Kushner RF. Barriers to providing nutritional counseling by physicians: a survey of primary care practitioners. Prev Med 1995;24:546-552.
47. Rogers LQ, Bailey JE, Gutin B, et al. Teaching resi-dent physicians to provide physician counseling: a needs assessment. Acad Med 2002;77:841-844.
48. Hiss RG. Barriers to care in non-insulin-dependent diabetes mellitus. The Michigan experience. Ann Intern Med 1996;124:146-148.
49. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multi-component program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med 2002;34:153-161.
50. Sallis JF, Patrick K, Calfas KJ, et al. A multi-media behavior change program for nutrition and physical activity in primary care: PACE+ for adults. Homeostasis 1999;39:196-202.
51. Health GW, Brownson RC, Kruger J, et al. The effectiveness of urban design and land use and transport policies and practices to increase physical activity: a systematic review. J Phys Act Health 2006;3(suppl 1):555-576.
52. Sallis JF, Glanz K. The role of built environment in physical activity, eating and obesity in childhood. Future Child 2006 Spring;16(1):89-108.
Malpractice minute
Could a patient’s violent act
have been prevented?
THE PATIENT. A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type.
CASE FACTS. The patient killed his developmentally disabled niece, age 26.
THE VICTIM’S FAMILY’S CLAIM. The death would not have occurred if the patient had been civilly committed or heavily medicated.
THE BEHAVIORAL HEALTH AUTHORITY’S DEFENSE. The violent act was unforeseeable, and the patient was compliant with treatment. The victim’s mother should not have left the disabled woman alone with the patient.
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Could a patient’s violent act
have been prevented?
THE PATIENT. A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type.
CASE FACTS. The patient killed his developmentally disabled niece, age 26.
THE VICTIM’S FAMILY’S CLAIM. The death would not have occurred if the patient had been civilly committed or heavily medicated.
THE BEHAVIORAL HEALTH AUTHORITY’S DEFENSE. The violent act was unforeseeable, and the patient was compliant with treatment. The victim’s mother should not have left the disabled woman alone with the patient.
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
Could a patient’s violent act
have been prevented?
THE PATIENT. A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type.
CASE FACTS. The patient killed his developmentally disabled niece, age 26.
THE VICTIM’S FAMILY’S CLAIM. The death would not have occurred if the patient had been civilly committed or heavily medicated.
THE BEHAVIORAL HEALTH AUTHORITY’S DEFENSE. The violent act was unforeseeable, and the patient was compliant with treatment. The victim’s mother should not have left the disabled woman alone with the patient.
Submit your verdict and find out how the court ruled. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Violence risk: Is clinical judgment enough?
Dear Dr. Mossman:
Multiple studies support the reliability and validity of actuarial measures—such as the Historical, Clinical, and Risk Management (HCR-20) risk assessment scheme—to assess violence risk, whereas physicians’ clinical judgment is highly variable. Should clinicians use actuarial measures to assess a patient’s risk of violence? Could it be considered negligent not to use actuarial measures?—Submitted by “Dr. S”
In the 30 years since the Tarasoff decision—which held that psychiatrists have a duty to protect individuals who are being threatened with bodily harm by a patient1—assessing patients’ risk of future violence has become an accepted part of mental health practice.2 Dr. S has asked 2 sophisticated questions about risk assessment. The short answer is that although so-called “actuarial” techniques for assessing risk are valuable, psychiatrists who do not use them are not practicing negligently. To explain why, this article discusses:
- the difference between “clinical” and “actuarial” judgment
- the HCR-20’s strengths and weaknesses
- actuarial measures and negligence.
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Clinical vs actuarial judgment
In the 1970s and 1980s, mental health professionals believed they could not accurately predict violence.3 We now know this is not correct. Since the 1990s, when researchers adopted better methods for gauging the accuracy of risk assessments,4-6 research has shown that mental health clinicians can assess dangerousness with clearly-better-than-chance accuracy, whether the assessment covers just the next few days, several months, or years.4
Over the same period, psychologists recognized that when it comes to making predictions, clinical judgment—making predictions by putting together information in one’s head—often is inferior to using simple formulae derived from empirically demonstrated relationships between data and outcome.7 This approach—“actuarial” judgment—is how insurance companies use data to calculate risk.
By the late 1990s, psychologists had developed actuarial risk assessment instruments (ARAIs)8 that could accurately rank the likelihood of various forms of violence. Table 1 lists some well-known ARAIs and the populations for which they were designed. In clinical practice, psychiatrists usually focus on risk posed by psychiatric patients. The HCR-209 was designed to help evaluate this type of risk.
Table 1
Examples of actuarial risk assessment instruments (ARAIs)
| ARAI | Risk assessed |
|---|---|
| HCR-209 | Violence in psychiatric populations, such as formerly hospitalized patients |
| Classification Of Violence Risk (COVR) | Violence by civil psychiatric patients following discharge into the community |
| Violence Risk Assessment Guide (VRAG) | Violent recidivism by formerly incarcerated offenders |
| Static-99 | Recidivism by sex offenders |
HCR-20’s pros and cons
The HCR-20 has 20 items:
- 10 concerning the patient’s history
- 5 related to clinical factors
- 5 that deal with risk management (Table 2).
To use the HCR-20 as an exercise of true actuarial judgment, you would base your opinion of a patient’s risk of violence solely on the HCR-20 score, without regard for other patient factors. However, the HCR-20’s developers think this approach “may be unreasonable, unethical, and illegal.”9 One reason is that the HCR-20 omits obvious signs of potential violence, such as a clearly stated threat with unambiguous intent to act.
For example, if a patient is doing well in the hospital (and has a low score on HCR-20 clinical items), a psychiatrist might assume the patient will cause few problems after discharge. But if the risk management items generate a high score, the psychiatrist should realize that these factors raise the patient’s violence risk and may require additional intervention—perhaps a different type of community placement or special effort to help the patient follow up with out-patient treatment.
Table 2
Items from the Historical, Clinical, and Risk Management (HCR-20)
| Historical items | Clinical items | Risk management items |
|---|---|---|
| H1 Previous violence | C1 Lack of insight | R1 Plans lack feasibility |
| H2 Young age at first incident | C2 Negative attitudes | R2 Exposure to destabilizers |
| H3 Relationship instability | C3 Active symptoms of major mental illness | R3 Lack of personal support |
| H4 Employment problems | C4 Impulsivity | R4 Noncompliance with remediation attempts |
| H5 Substance use problems | C5 Unresponsive to treatment | R5 Stress |
| H6 Major mental illness | ||
| H7 Psychopathy | ||
| H8 Early maladjustment | ||
| H9 Personality disorder | ||
| H10 Prior supervision failure | ||
| Score each item 0, 1, or 2, depending on how closely the patient matches the described characteristic. For example, when scoring item C3 (active symptoms of major mental illness), a patient gets 0 for “no active symptoms,” 1 for “possible/less serious active symptoms,” or 2 for “definite/serious active symptoms.” An individual can receive a total HCR-20 score of 0 to 40. The higher the score, the higher likelihood of violence in the coming months. | ||
| Source: Reprinted with permission from Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia, Canada: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997 | ||
Is not using ARAIs negligent?
Some writers believe that using ARAIs should12 or may soon13 become the standard of care. Why, then, do psychiatrists seldom use ARAIs in their clinical work? Partly it is because clinicians rarely receive adequate training in assessing violence risk or the science supporting it. After a 5-hour training module featuring the HCR-20, psychiatry residents could better identify factors that affect violence risk, organize their reasoning, and come up with risk management strategies.2
Psychiatrists may have other reasons for not using ARAIs that make clinical sense. Although ARAIs can rank individuals’ violence risk, the probabilities of violence associated with each rank aren’t substantial enough to justify differences in management.14 Scientifically, it’s interesting to know that we can separate patients into groups with “low” (9%) and “high” (49%) risks of violence.15 But would you want to manage these patients differently? Most psychiatrists probably would not feel comfortable ignoring a 9% risk of violence.
To avoid negligence, psychiatrists need only “exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession.”17 Psychiatrists seldom use ARAIs,12 so failing to use them cannot constitute malpractice. As Simon points out, a practicing psychiatrist’s role is to treat patients, not predict violence. He concludes, “at this time, the standard of care does not require the average or reasonable psychiatrist to use actuarial assessment instruments in the evaluation and treatment of potentially violent patients.”16
1. Tarasoff vs Regents of the University of California, 551 P. 2d 334 (Cal. 1976).
2. McNiel DE, Chamberlain JR, Weaver CM, et al. Impact of clinical training on violence risk assessment. Am J Psychiatry 2008;165:195-200.
3. Monahan J. The clinical prediction of violent behavior. Washington, DC: National Institute of Mental Health; 1981.
4. Mossman D. Assessing predictions of violence: being accurate about accuracy. J Consult Clin Psychol 1994;62:783-92.
5. Rice ME, Harris GT. Violent recidivism: assessing predictive validity. J Consult Clin Psychol 1995;63:737-48.
6. Gardner W, Lidz CW, Mulvey EP, Shaw EC. Clinical versus actuarial predictions of violence in patients with mental illness. J Consult Clin Psychol 1996;64:602-9.
7. Dawes RM, Faust D, Meehl PE. Clinical versus actuarial judgment. Science 1989;243:1668-74.
8. Hart SD, Michie C, Cooke DJ. Precision of actuarial risk assessment instruments: evaluating the ‘margins of error’ of group v. individual predictions of violence. Brit J Psychiatry 2007;190:60-5.
9. Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997.
10. Quinsey VL, Harris GT, Rice ME, Cormier CA. Violent offenders: appraising and managing risk. 2nd ed. Washington, DC: American Psychological Association; 2006.
11. Hanson RK, Morton-Bourgon KE. The accuracy of recidivism risk assessments for sexual offenders: a meta-analysis. Ottawa, Canada: Public Safety Canada; 2007. Available at: http://www.publicsafety.gc.ca/res/cor/rep/_fl/crp2007-01-en.pdf. Accessed April 21, 2008.
12. Swanson JW. Preventing the unpredicted: managing violence risk in mental health care. Psychiatr Serv 2008;59:191-3.
13. Lamberg L. New tools aid violence risk assessment. JAMA 2007;298(5):499-501.
14. Mossman D. Commentary: assessing the risk of violence—are “accurate” predictions useful? J Am Acad Psychiatry Law 2000;28:272-81.
15. Monahan J, Steadman HJ, Robbins PC, et al. An actuarial model of violence risk assessment for persons with mental disorders. Psychiatr Serv 2005;56:810-15.
16. Simon RI. The myth of “imminent” violence in psychiatry and the law. Univ Cincinnati L Rev 2006;75:631-43.
17. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
Dear Dr. Mossman:
Multiple studies support the reliability and validity of actuarial measures—such as the Historical, Clinical, and Risk Management (HCR-20) risk assessment scheme—to assess violence risk, whereas physicians’ clinical judgment is highly variable. Should clinicians use actuarial measures to assess a patient’s risk of violence? Could it be considered negligent not to use actuarial measures?—Submitted by “Dr. S”
In the 30 years since the Tarasoff decision—which held that psychiatrists have a duty to protect individuals who are being threatened with bodily harm by a patient1—assessing patients’ risk of future violence has become an accepted part of mental health practice.2 Dr. S has asked 2 sophisticated questions about risk assessment. The short answer is that although so-called “actuarial” techniques for assessing risk are valuable, psychiatrists who do not use them are not practicing negligently. To explain why, this article discusses:
- the difference between “clinical” and “actuarial” judgment
- the HCR-20’s strengths and weaknesses
- actuarial measures and negligence.
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Clinical vs actuarial judgment
In the 1970s and 1980s, mental health professionals believed they could not accurately predict violence.3 We now know this is not correct. Since the 1990s, when researchers adopted better methods for gauging the accuracy of risk assessments,4-6 research has shown that mental health clinicians can assess dangerousness with clearly-better-than-chance accuracy, whether the assessment covers just the next few days, several months, or years.4
Over the same period, psychologists recognized that when it comes to making predictions, clinical judgment—making predictions by putting together information in one’s head—often is inferior to using simple formulae derived from empirically demonstrated relationships between data and outcome.7 This approach—“actuarial” judgment—is how insurance companies use data to calculate risk.
By the late 1990s, psychologists had developed actuarial risk assessment instruments (ARAIs)8 that could accurately rank the likelihood of various forms of violence. Table 1 lists some well-known ARAIs and the populations for which they were designed. In clinical practice, psychiatrists usually focus on risk posed by psychiatric patients. The HCR-209 was designed to help evaluate this type of risk.
Table 1
Examples of actuarial risk assessment instruments (ARAIs)
| ARAI | Risk assessed |
|---|---|
| HCR-209 | Violence in psychiatric populations, such as formerly hospitalized patients |
| Classification Of Violence Risk (COVR) | Violence by civil psychiatric patients following discharge into the community |
| Violence Risk Assessment Guide (VRAG) | Violent recidivism by formerly incarcerated offenders |
| Static-99 | Recidivism by sex offenders |
HCR-20’s pros and cons
The HCR-20 has 20 items:
- 10 concerning the patient’s history
- 5 related to clinical factors
- 5 that deal with risk management (Table 2).
To use the HCR-20 as an exercise of true actuarial judgment, you would base your opinion of a patient’s risk of violence solely on the HCR-20 score, without regard for other patient factors. However, the HCR-20’s developers think this approach “may be unreasonable, unethical, and illegal.”9 One reason is that the HCR-20 omits obvious signs of potential violence, such as a clearly stated threat with unambiguous intent to act.
For example, if a patient is doing well in the hospital (and has a low score on HCR-20 clinical items), a psychiatrist might assume the patient will cause few problems after discharge. But if the risk management items generate a high score, the psychiatrist should realize that these factors raise the patient’s violence risk and may require additional intervention—perhaps a different type of community placement or special effort to help the patient follow up with out-patient treatment.
Table 2
Items from the Historical, Clinical, and Risk Management (HCR-20)
| Historical items | Clinical items | Risk management items |
|---|---|---|
| H1 Previous violence | C1 Lack of insight | R1 Plans lack feasibility |
| H2 Young age at first incident | C2 Negative attitudes | R2 Exposure to destabilizers |
| H3 Relationship instability | C3 Active symptoms of major mental illness | R3 Lack of personal support |
| H4 Employment problems | C4 Impulsivity | R4 Noncompliance with remediation attempts |
| H5 Substance use problems | C5 Unresponsive to treatment | R5 Stress |
| H6 Major mental illness | ||
| H7 Psychopathy | ||
| H8 Early maladjustment | ||
| H9 Personality disorder | ||
| H10 Prior supervision failure | ||
| Score each item 0, 1, or 2, depending on how closely the patient matches the described characteristic. For example, when scoring item C3 (active symptoms of major mental illness), a patient gets 0 for “no active symptoms,” 1 for “possible/less serious active symptoms,” or 2 for “definite/serious active symptoms.” An individual can receive a total HCR-20 score of 0 to 40. The higher the score, the higher likelihood of violence in the coming months. | ||
| Source: Reprinted with permission from Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia, Canada: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997 | ||
Is not using ARAIs negligent?
Some writers believe that using ARAIs should12 or may soon13 become the standard of care. Why, then, do psychiatrists seldom use ARAIs in their clinical work? Partly it is because clinicians rarely receive adequate training in assessing violence risk or the science supporting it. After a 5-hour training module featuring the HCR-20, psychiatry residents could better identify factors that affect violence risk, organize their reasoning, and come up with risk management strategies.2
Psychiatrists may have other reasons for not using ARAIs that make clinical sense. Although ARAIs can rank individuals’ violence risk, the probabilities of violence associated with each rank aren’t substantial enough to justify differences in management.14 Scientifically, it’s interesting to know that we can separate patients into groups with “low” (9%) and “high” (49%) risks of violence.15 But would you want to manage these patients differently? Most psychiatrists probably would not feel comfortable ignoring a 9% risk of violence.
To avoid negligence, psychiatrists need only “exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession.”17 Psychiatrists seldom use ARAIs,12 so failing to use them cannot constitute malpractice. As Simon points out, a practicing psychiatrist’s role is to treat patients, not predict violence. He concludes, “at this time, the standard of care does not require the average or reasonable psychiatrist to use actuarial assessment instruments in the evaluation and treatment of potentially violent patients.”16
Dear Dr. Mossman:
Multiple studies support the reliability and validity of actuarial measures—such as the Historical, Clinical, and Risk Management (HCR-20) risk assessment scheme—to assess violence risk, whereas physicians’ clinical judgment is highly variable. Should clinicians use actuarial measures to assess a patient’s risk of violence? Could it be considered negligent not to use actuarial measures?—Submitted by “Dr. S”
In the 30 years since the Tarasoff decision—which held that psychiatrists have a duty to protect individuals who are being threatened with bodily harm by a patient1—assessing patients’ risk of future violence has become an accepted part of mental health practice.2 Dr. S has asked 2 sophisticated questions about risk assessment. The short answer is that although so-called “actuarial” techniques for assessing risk are valuable, psychiatrists who do not use them are not practicing negligently. To explain why, this article discusses:
- the difference between “clinical” and “actuarial” judgment
- the HCR-20’s strengths and weaknesses
- actuarial measures and negligence.
- Submit your malpractice-related questions to Dr. Mossman at douglas.mossman@dowdenhealth.com.
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Clinical vs actuarial judgment
In the 1970s and 1980s, mental health professionals believed they could not accurately predict violence.3 We now know this is not correct. Since the 1990s, when researchers adopted better methods for gauging the accuracy of risk assessments,4-6 research has shown that mental health clinicians can assess dangerousness with clearly-better-than-chance accuracy, whether the assessment covers just the next few days, several months, or years.4
Over the same period, psychologists recognized that when it comes to making predictions, clinical judgment—making predictions by putting together information in one’s head—often is inferior to using simple formulae derived from empirically demonstrated relationships between data and outcome.7 This approach—“actuarial” judgment—is how insurance companies use data to calculate risk.
By the late 1990s, psychologists had developed actuarial risk assessment instruments (ARAIs)8 that could accurately rank the likelihood of various forms of violence. Table 1 lists some well-known ARAIs and the populations for which they were designed. In clinical practice, psychiatrists usually focus on risk posed by psychiatric patients. The HCR-209 was designed to help evaluate this type of risk.
Table 1
Examples of actuarial risk assessment instruments (ARAIs)
| ARAI | Risk assessed |
|---|---|
| HCR-209 | Violence in psychiatric populations, such as formerly hospitalized patients |
| Classification Of Violence Risk (COVR) | Violence by civil psychiatric patients following discharge into the community |
| Violence Risk Assessment Guide (VRAG) | Violent recidivism by formerly incarcerated offenders |
| Static-99 | Recidivism by sex offenders |
HCR-20’s pros and cons
The HCR-20 has 20 items:
- 10 concerning the patient’s history
- 5 related to clinical factors
- 5 that deal with risk management (Table 2).
To use the HCR-20 as an exercise of true actuarial judgment, you would base your opinion of a patient’s risk of violence solely on the HCR-20 score, without regard for other patient factors. However, the HCR-20’s developers think this approach “may be unreasonable, unethical, and illegal.”9 One reason is that the HCR-20 omits obvious signs of potential violence, such as a clearly stated threat with unambiguous intent to act.
For example, if a patient is doing well in the hospital (and has a low score on HCR-20 clinical items), a psychiatrist might assume the patient will cause few problems after discharge. But if the risk management items generate a high score, the psychiatrist should realize that these factors raise the patient’s violence risk and may require additional intervention—perhaps a different type of community placement or special effort to help the patient follow up with out-patient treatment.
Table 2
Items from the Historical, Clinical, and Risk Management (HCR-20)
| Historical items | Clinical items | Risk management items |
|---|---|---|
| H1 Previous violence | C1 Lack of insight | R1 Plans lack feasibility |
| H2 Young age at first incident | C2 Negative attitudes | R2 Exposure to destabilizers |
| H3 Relationship instability | C3 Active symptoms of major mental illness | R3 Lack of personal support |
| H4 Employment problems | C4 Impulsivity | R4 Noncompliance with remediation attempts |
| H5 Substance use problems | C5 Unresponsive to treatment | R5 Stress |
| H6 Major mental illness | ||
| H7 Psychopathy | ||
| H8 Early maladjustment | ||
| H9 Personality disorder | ||
| H10 Prior supervision failure | ||
| Score each item 0, 1, or 2, depending on how closely the patient matches the described characteristic. For example, when scoring item C3 (active symptoms of major mental illness), a patient gets 0 for “no active symptoms,” 1 for “possible/less serious active symptoms,” or 2 for “definite/serious active symptoms.” An individual can receive a total HCR-20 score of 0 to 40. The higher the score, the higher likelihood of violence in the coming months. | ||
| Source: Reprinted with permission from Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia, Canada: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997 | ||
Is not using ARAIs negligent?
Some writers believe that using ARAIs should12 or may soon13 become the standard of care. Why, then, do psychiatrists seldom use ARAIs in their clinical work? Partly it is because clinicians rarely receive adequate training in assessing violence risk or the science supporting it. After a 5-hour training module featuring the HCR-20, psychiatry residents could better identify factors that affect violence risk, organize their reasoning, and come up with risk management strategies.2
Psychiatrists may have other reasons for not using ARAIs that make clinical sense. Although ARAIs can rank individuals’ violence risk, the probabilities of violence associated with each rank aren’t substantial enough to justify differences in management.14 Scientifically, it’s interesting to know that we can separate patients into groups with “low” (9%) and “high” (49%) risks of violence.15 But would you want to manage these patients differently? Most psychiatrists probably would not feel comfortable ignoring a 9% risk of violence.
To avoid negligence, psychiatrists need only “exercise the skill, knowledge, and care normally possessed and exercised by other members of their profession.”17 Psychiatrists seldom use ARAIs,12 so failing to use them cannot constitute malpractice. As Simon points out, a practicing psychiatrist’s role is to treat patients, not predict violence. He concludes, “at this time, the standard of care does not require the average or reasonable psychiatrist to use actuarial assessment instruments in the evaluation and treatment of potentially violent patients.”16
1. Tarasoff vs Regents of the University of California, 551 P. 2d 334 (Cal. 1976).
2. McNiel DE, Chamberlain JR, Weaver CM, et al. Impact of clinical training on violence risk assessment. Am J Psychiatry 2008;165:195-200.
3. Monahan J. The clinical prediction of violent behavior. Washington, DC: National Institute of Mental Health; 1981.
4. Mossman D. Assessing predictions of violence: being accurate about accuracy. J Consult Clin Psychol 1994;62:783-92.
5. Rice ME, Harris GT. Violent recidivism: assessing predictive validity. J Consult Clin Psychol 1995;63:737-48.
6. Gardner W, Lidz CW, Mulvey EP, Shaw EC. Clinical versus actuarial predictions of violence in patients with mental illness. J Consult Clin Psychol 1996;64:602-9.
7. Dawes RM, Faust D, Meehl PE. Clinical versus actuarial judgment. Science 1989;243:1668-74.
8. Hart SD, Michie C, Cooke DJ. Precision of actuarial risk assessment instruments: evaluating the ‘margins of error’ of group v. individual predictions of violence. Brit J Psychiatry 2007;190:60-5.
9. Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997.
10. Quinsey VL, Harris GT, Rice ME, Cormier CA. Violent offenders: appraising and managing risk. 2nd ed. Washington, DC: American Psychological Association; 2006.
11. Hanson RK, Morton-Bourgon KE. The accuracy of recidivism risk assessments for sexual offenders: a meta-analysis. Ottawa, Canada: Public Safety Canada; 2007. Available at: http://www.publicsafety.gc.ca/res/cor/rep/_fl/crp2007-01-en.pdf. Accessed April 21, 2008.
12. Swanson JW. Preventing the unpredicted: managing violence risk in mental health care. Psychiatr Serv 2008;59:191-3.
13. Lamberg L. New tools aid violence risk assessment. JAMA 2007;298(5):499-501.
14. Mossman D. Commentary: assessing the risk of violence—are “accurate” predictions useful? J Am Acad Psychiatry Law 2000;28:272-81.
15. Monahan J, Steadman HJ, Robbins PC, et al. An actuarial model of violence risk assessment for persons with mental disorders. Psychiatr Serv 2005;56:810-15.
16. Simon RI. The myth of “imminent” violence in psychiatry and the law. Univ Cincinnati L Rev 2006;75:631-43.
17. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
1. Tarasoff vs Regents of the University of California, 551 P. 2d 334 (Cal. 1976).
2. McNiel DE, Chamberlain JR, Weaver CM, et al. Impact of clinical training on violence risk assessment. Am J Psychiatry 2008;165:195-200.
3. Monahan J. The clinical prediction of violent behavior. Washington, DC: National Institute of Mental Health; 1981.
4. Mossman D. Assessing predictions of violence: being accurate about accuracy. J Consult Clin Psychol 1994;62:783-92.
5. Rice ME, Harris GT. Violent recidivism: assessing predictive validity. J Consult Clin Psychol 1995;63:737-48.
6. Gardner W, Lidz CW, Mulvey EP, Shaw EC. Clinical versus actuarial predictions of violence in patients with mental illness. J Consult Clin Psychol 1996;64:602-9.
7. Dawes RM, Faust D, Meehl PE. Clinical versus actuarial judgment. Science 1989;243:1668-74.
8. Hart SD, Michie C, Cooke DJ. Precision of actuarial risk assessment instruments: evaluating the ‘margins of error’ of group v. individual predictions of violence. Brit J Psychiatry 2007;190:60-5.
9. Webster CD, Douglas KS, Eaves D, Hart SD. HCR-20: assessing risk for violence, version 2. Burnaby, British Columbia: Simon Fraser University, Mental Health, Law, and Policy Institute; 1997.
10. Quinsey VL, Harris GT, Rice ME, Cormier CA. Violent offenders: appraising and managing risk. 2nd ed. Washington, DC: American Psychological Association; 2006.
11. Hanson RK, Morton-Bourgon KE. The accuracy of recidivism risk assessments for sexual offenders: a meta-analysis. Ottawa, Canada: Public Safety Canada; 2007. Available at: http://www.publicsafety.gc.ca/res/cor/rep/_fl/crp2007-01-en.pdf. Accessed April 21, 2008.
12. Swanson JW. Preventing the unpredicted: managing violence risk in mental health care. Psychiatr Serv 2008;59:191-3.
13. Lamberg L. New tools aid violence risk assessment. JAMA 2007;298(5):499-501.
14. Mossman D. Commentary: assessing the risk of violence—are “accurate” predictions useful? J Am Acad Psychiatry Law 2000;28:272-81.
15. Monahan J, Steadman HJ, Robbins PC, et al. An actuarial model of violence risk assessment for persons with mental disorders. Psychiatr Serv 2005;56:810-15.
16. Simon RI. The myth of “imminent” violence in psychiatry and the law. Univ Cincinnati L Rev 2006;75:631-43.
17. Dobbs DB. The law of torts. St. Paul, MN: West Group; 2000:269.
Double jeopardy: How to treat kids with comorbid anxiety and ADHD
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Aaron, age 10, has been diagnosed with an anxiety disorder and attention-deficit/hyperactivity disorder (ADHD) but is not being treated with medication because his parents do not believe in psychopharmacology. They bring him to a specialized child anxiety clinic and ask for “urgent CBT” because his behavior at school is out of control.
Aaron rearranges the therapist’s office furniture during much of the assessment interview. He also acknowledges many anxiety symptoms. The therapist doubts that cognitive-behavioral therapy (CBT) would help without other interventions.
Children with anxiety disorders and ADHD—a common comorbid presentation—tend to be more impaired than those with either condition alone.1 Effective treatment usually requires 4 components (Table 1), including medication plus behavioral or cognitive-behavioral therapy. This article discusses clinical issues related to each component and describes how to successfully combine them into a treatment plan.
Table 1
Comorbid ADHD and anxiety: 4 treatment components
| Successful treatment usually involves combining 4 components: | |
| |
| Make individual adjustments as needed, depending on the child’s symptom profile, social context, and developmental level | |
| ADHD: attention-deficit/hyperactivity disorder | |
Medication options
Stimulants, atomoxetine, and selective serotonin reuptake inhibitors (SSRIs) have been advocated for children with anxiety and ADHD. Given the high risk of behavioral disinhibition with SSRIs in children,2 stimulants or atomoxetine are suggested as first-line medications.3,4
Stimulants target ADHD symptoms primarily, but anxiety decreases in some children (24% in a recent trial) as ADHD symptoms are controlled.4 Because it is a selective norepinephrine reuptake inhibitor (SNRI), atomoxetine may target both ADHD and anxiety symptoms. When initiating these medications, “start low and go slow.” Recommended dosing is no different for children with ADHD and anxiety than for those with ADHD alone (Table 2).5
Stimulant response rates for children with ADHD and anxiety vary among studies. Some report lower response rates than for children with ADHD alone and possibly more treatment-emergent side effects.6 The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD (MTA) found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes (Box 1).7,8 Adding intensive psychosocial intervention to stimulant treatment appeared to yield greater improvements in anxious children with ADHD, compared with stimulants alone.8
Cognitive impairments related to inattention do not consistently improve with stimulant treatment.9 This is clinically important because children with ADHD and comorbid anxiety disorders can be very cognitively impaired.10
Add an SSRI? Monotherapy is simpler and usually more acceptable to families, but a placebo-controlled study examined adding an SSRI (fluvoxamine) to methylphenidate treatment.4 Children with anxiety and ADHD who received adjunctive fluvoxamine did no better than those who received methylphenidate plus placebo.
Atomoxetine. A large, randomized, controlled trial of atomoxetine in this population found good tolerability and statistically significant reductions in ADHD and anxiety symptoms compared with placebo. Effect size was greater for ADHD symptoms than for anxiety symptoms,11 however, which supports smaller trials that show more consistent evidence of atomoxetine reducing ADHD symptoms than anxiety symptoms.
Similar to antidepressants with the SNRI chemical structure, atomoxetine’s effectiveness for a given child takes several weeks to determine. This can be a problem in children who are highly distressed or impaired and require rapid symptomatic improvement.
Recommendation. Consider a stimulant or atomoxetine initially for children with anxiety disorders and ADHD, and seek concurrent behavioral or cognitive-behavioral therapy. Caution families that:
- >1 medication trial might be needed, as response may not be as consistent as in children with ADHD alone
- medication-related improvements in ADHD symptoms will not necessarily be associated with reduced anxiety symptoms or improved academic ability
- improvements with atomoxetine might not be evident for several weeks.
Table 2
Medication dosing for children with ADHD*
| Medication | Recommended starting dosage | Recommended maximum dosage | 5 most common side effects in descending prevalence |
|---|---|---|---|
| Stimulants | |||
| Methylphenidate hydrochloride (Ritalin) | 5 mg tid | Total 60 mg/d | Insomnia, nervousness, decreased appetite, dizziness, nausea |
| Methylphenidate hydrochloride (Concerta) | 18 mg every morning | 54 mg every morning | Headache, abdominal pain, decreased appetite, vomiting, insomnia |
| Dextroamphetamine sulfate (Dexedrine) | 5 mg every morning | Total 40 mg/d | Palpitations, restlessness, dizziness, dry mouth, decreased appetite |
| Mixed amphetamine salts (Adderall) | 10 mg every morning | 30 mg every morning | Decreased appetite, insomnia, abdominal pain, emotional lability, vomiting |
| Nonstimulant | |||
| Atomoxetine (Strattera) | 0.5 mg/kg/d | 1.2 mg/kg/d | Decreased appetite, dizziness, stomach upset, fatigue, irritability |
| ADHD: attention-deficit/hyperactivity disorder | |||
| * Recommended dosing is no different for children with ADHD and anxiety than for children with only ADHD | |||
| Source: Reference 5 | |||
Psychological intervention
CBT has been shown effective for child-hood anxiety disorders in randomized controlled trials,12 but even those that included children with comorbid ADHD required that an anxiety disorder be the primary, most impairing diagnosis.13 Thus, little is known about CBT’s effectiveness for children with anxiety plus ADHD. Given the evidence for cognitive deficits in comorbid anxiety and ADHD10 and the challenge of working with highly distractible children, one would expect CBT to be more difficult in this population.
The potential for distraction to adversely affect learning of coping strategies is higher in group than in individual therapy, and children with anxiety and ADHD can be disruptive to other children in CBT groups. Consider individual CBT, and seek a therapist who has experience with this population. Having the child on medication for ADHD symptoms usually helps reduce these symptoms’ impact on sessions.
For children younger than about age 8 or too cognitively impaired to benefit from CBT, behavioral intervention alone may be helpful. The largely behavioral psychosocial intervention in the MTA study of ADHD children age 7 to 9 (Box 2)8,14 helped many of those with comorbid anxiety.
Although programs as intense as that used in the MTA study rarely are provided in community practice, consider behavior modification. For example:
- To reduce anxiety, have the child follow regular, predictable routines, and reward the child for gradually facing previously avoided situations.
- To reduce distractibility in class, have the child sit near the teacher, break work into small chunks, and reward completion of each chunk.
Even small improvements in the child’s home or school behavior may reduce negative interactions with others and the attendant effects on self-esteem.
CASE CONTINUED: Weighing the options
The therapist seeing Aaron’s family listens to their concerns about medication and reassures them that their son will not be denied psychotherapy. She tells them, however, that psychotherapy will not address his urgent school problems and is unlikely to work in the absence of medication, given Aaron’s behavior in the office. The therapist provides accurate information about the risks and benefits of medication and CBT, and the parents agree to think about all treatment options.
By the next office visit, the school has threatened to suspend Aaron. He and his parents agree to combined treatment with a stimulant medication and CBT and to having the therapist provide a behavioral consultation at the school.
shows best outcomes for ADHD with anxiety
The National Institute of Mental Health’s Multimodal Treatment Study of Children with ADHD—the largest study to date—found that comorbid anxiety did not adversely affect behavioral response to stimulants but did moderate outcomes.
In the parallel group design study, 579 ADHD children age 7 to 9 were enrolled at 6 treatment sites, thoroughly assessed, then randomly assigned to 4 groups: medication treatment alone, intensive psychosocial treatment alone, a combination of both treatments, or usual community care. The first 3 interventions were designed to reflect best practices for each approach, and these children were closely monitored and studied for 14 months. All 4 groups were reassessed periodically for 24 months, evaluating multiple outcomes.
For the total sample, combined and medication treatment were more effective than psychosocial treatment and community care. For ADHD children with comorbid anxiety disorders:
- combined treatment was more effective than either medication treatment alone or psychosocial treatment alone
- both monotherapies were superior to community care.
ADHD: attention-deficit/hyperactivity disorder
Family psychoeducation
With families of children with behavioral challenges, adopt a patient, educational approach rather than acquiescing to their wishes or arguing with them. Either can result in treatment failure. Discuss potential benefits and risks of all treatment options and the impact of comorbidity on treatment.
Parents’ rigid insistence on a particular course of action—such as refusing psychopharmacology—may be caused by anxiety or misinformation. Elicit the source of any anxiety, and provide realistic information and reassurance if possible.
Anxiety in family members may be constitutional—as anxiety is highly heritable15—or relate to aspects of treatment. Families may feel overwhelmed by:
- their child having 2 disorders rather than 1
- your suggestion to start medical and nonmedical intervention together
- hearing about the possibility of multiple medication trials.
Negotiating medication. Discuss with the family the difficulties of a child learning CBT strategies when ADHD is not well-controlled and the cognitive difficulties in many of these children that may necessitate individualized CBT. If the family remains reluctant to consider combining medication with CBT, try contracting for a limited number of CBT sessions (perhaps 3 or 4) before re-evaluating the need for medication.
The child’s perceptions (and potential anxieties) about his or her difficulties also must be understood, validated, and addressed. Children are more likely to engage in a treatment if they participate in the decision to adopt it.
Anxiety can heighten vigilance in the child or the parents to treatment-emergent side effects, which you may exacerbate by providing exhaustive lists of potential ad-verse events. Limit discussion to serious side effects—with emphasis on their rarity—and those that are common.
ADHD traits in families can affect treatment success. Because of their own distractibility and organizational difficulties, parents with ADHD traits may have difficulty ensuring the child’s medication adherence and treatment participation.16
Behavior modification can require a high degree of consistency in parents’ behavior toward the child. This may be difficult to achieve in families where:
- 1 or both parents are inattentive because of ADHD
- a high degree of conflict exists between parents.
To help these families, provide reminder calls about appointments and schedule sessions at a consistent time. To improve consistency of medication use:
- combine medication administration with an essential daily activity
- check adherence with pill counts or other means.
If the child participates in CBT, provide separate notebooks for in-session and homework exercises—anticipating some loss of homework notebooks.
Individualizing care
Individualized care is important to return each child to his or her best possible level of functioning. The child’s symptom profile, environment, and developmental level can affect treatment.
For example, in a child whose ADHD-related impairment is substantial but whose anxiety-related impairment is mild, pharmacotherapy for ADHD and some pa-rental guidance may be adequate to manage remaining anxiety symptoms.17 As mentioned, some children show decreased anxiety as their ADHD is better controlled.4 Conversely, if ADHD-related impairment is mild but the child is highly anxious, consider CBT alone—preferably on an individual basis—provided the child can manage the cognitive aspects of therapy.
School personnel can monitor change in relation to various interventions, as many of these children’s symptoms manifest in the classroom. Behavioral interventions are more likely to succeed if they are administered consistently across home and school environments8 and teachers participate in behavior modification.
To elicit cooperation from school personnel, listen to their concerns and observations and help them understand the child’s difficulties and the rationale for various treatments. This approach often reduces negative feedback toward the child, a benefit that may further improve outcomes.
Attention to peer relationships and social stressors is often needed. Because of their multiple difficulties, these children may lack social skills and be shunned by their peers.1 You may need to help them develop social skills and reconnect with their peers after symptoms are well-controlled.
Poverty or lack of social support can affect treatment. Children with ADHD and anxiety usually need multiple interventions, and it is difficult for families to at-tend to these consistently when struggling with social stressors.
The 14-month intensive behavioral intervention used in the National Institute of Mental Health’s Multimodal Treatment Study (MTA) of 579 children age 7 to 9 with ADHD included:
- weekly parent training initially, decreasing to monthly by the end
- biweekly teacher consultations in behavior management
- 8-week full-day therapeutic summer program for children, focusing on behavioral and cognitive behavioral intervention
- 12-week half-time behaviorally trained paraprofessional aide in the classroom to generalize gains from summer program
- parent coaching on collaborating with teacher long-term so therapeutic consultation could be faded.
ADHD: attention-deficit/hyperactivity disorder
Adolescent adjustments. ADHD and anxiety often are diagnosed in the early school years, so anticipate developmental effects on treatment as the child enters adolescence. Adolescents value autonomy and may need to be more involved in treatment decisions than younger children.
Ask about and address family disagreements about treatment options, which may reduce adherence. You may need to talk about peer pressure to “not take drugs” by clearly differentiating the reasons some people take street drugs and the reasons for taking prescribed medication. Also discuss in a frank, nonjudgmental manner the risks of experimenting with street drugs (especially with prescribed medication) or of “sharing” one’s medications with friends.
Increased cognitive sophistication in adolescence may increase the potential benefit of CBT, so explore this option with the teen, especially if it was not attempted in the past.
Related resources
- American Academy of Child and Adolescent Psychiatry. “ADHD—a guide for families,” under the Resources for Families tab. www.aacap.org.
- Watkins C. Stimulant medication and ADHD. www.ncpamd.com/Stimulants.htm.
- Manassis K. Keys to parenting your anxious child. 2nd ed. Hauppauge, NY: Barron’s Educational Series, Inc.; 2008.
Drug brand names
- Atomoxetine • Strattera
- Dextroamphetamine • Dexedrine
- Fluvoxamine • Luvox
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
Disclosures
Dr. Manassis reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
1. Bowen R, Chavira DA, Bailey K, et al. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res 2008;157:201-9.
2. Walkup JT, Labellarte MJ, Riddle MA, et al. Searching for moderators and mediators of pharmacological treatment in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2003;42:13-21.
3. Wiesegger G, Kienbacher C, Pellegrini E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) and comorbid disorders. Neuropsychiatr 2007;21:187-206.
4. Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2005;44:418-27.
5. Compendium of pharmaceuticals and specialties. Ottawa, Canada: Canadian Pharmacists Association; 2008.
6. Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J Child Neurol 2007;22:538-42.
7. Wells KC, Pelham WE, Kotkin RA, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. J Abnorm Child Psychol 2000;28:483-505.
8. March JS, Swanson JM, Arnold EL, et al. Anxiety as a predictor and outcome variable in the Multimodal Treatment Study of Children with ADHD (MTA). J Abnorm Child Psychol 2000;28:527-41.
9. Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without anxiety. J Am Acad Child Adolesc Psychiatry 1995;34:886-96.
10. Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behav Brain Funct 2007;3-4.
11. Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007;46:1119-27.
12. Compton SN, March JS, Brent D, et al. Cognitive behavioural psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930-59.
13. Manassis K, Mendlowitz SL, Scapillato D, et al. Group and individual cognitive-behavioral therapy for childhood anxiety disorders: a randomized trial. J Am Acad Child Adolesc Psychiatry 2002;41:1423-30.
14. Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997;54:865-70.
15. Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science 1990;240:167-71.
16. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann 2008;37:19-26.
17. Manassis K, Monga S. therapeutic approach to children and adolescents with anxiety disorders and associated comorbid conditions. J Am Acad Child Adolesc Psychiatry 2001;40:115-7.
Study shows drug is safe and effective in cardiac patients
The direct thrombin inhibitor bivalirudin (Angiomax) reduces major bleeding and increases short-term survival in certain cardiac patients, according to a study published in the New England Journal of Medicine.
Bivalirudin was associated with a lower rate of major bleeding and death from cardiac and other causes at 30 days from treatment initiation. This was in comparison to treatment with heparin plus glycoprotein IIb/IIIa inhibitors.
Both therapies were tested in patients with ST-segment elevation myocardial infarction who were undergoing primary percutaneous coronary intervention (PCI).
Gregg Stone, MD, of Columbia University Medical Center, and colleagues randomized 3602 patients to receive either bivalirudin (n=1800) or heparin plus glycoprotein IIb/IIIa inhibitors (n=1802).
The 2 primary endpoints of the study were major bleeding and combined clinical events. Combined events included death, reinfarction, target-vessel revascularization for ischemia, and stroke.
Bivalirudin reduced the overall rate of combined events, as compared to heparin plus glycoprotein IIb/IIIa inhibitors (9.2% vs 12.1%, respectively). However, when evaluated separately, there were no significant differences in the incidence of these events between the 2 treatment groups.
The rate of reinfarction was the same in both groups (1.8%). The rate of stroke was insignificantly higher in the bivalirudin group (0.7% vs 0.6%). The same was true of target-vessel revascularization; the rate was 2.6% in bivalirudin patients and 1.9% in the other patient group.
Patients on bivalirudin experienced a lower rate of major bleeding than patients on heparin plus glycoprotein IIb/IIIa inhibitors (4.9% vs 8.3%, respectively). When large hematomas were excluded, the rates of major bleeding were reduced to 4.7% and 7.8%, respectively.
Bivalirudin also reduced the incidence of hemorrhagic complications, the development of thrombocytopenia, and the need for blood transfusions.
The rate of cardiac-related death at 30 days was significantly lower for bivalirudin patients than for patients who received heparin plus glycoprotein IIb/IIIa inhibitors (1.8% vs 2.9%, respectively). The rate of death from any cause was also lower in bivalirudin patients (2.1% vs 3.1%).
Bivalirudin increased the rate of acute stent thrombosis within 24 hours of treatment initiation (1.3% vs 0.3%). However, between 24 hours and 30 days, the incidence of acute stent thrombosis was lower in the bivalirudin patients than in those on heparin plus glycoprotein IIb/IIIa inhibitors (1.2% vs. 1.7%, respectively).
This study, known as HORIZONS-AMI, was sponsored, in part, by The Medicines Company, makers of bivalirudin (Angiomax). ![]()
The direct thrombin inhibitor bivalirudin (Angiomax) reduces major bleeding and increases short-term survival in certain cardiac patients, according to a study published in the New England Journal of Medicine.
Bivalirudin was associated with a lower rate of major bleeding and death from cardiac and other causes at 30 days from treatment initiation. This was in comparison to treatment with heparin plus glycoprotein IIb/IIIa inhibitors.
Both therapies were tested in patients with ST-segment elevation myocardial infarction who were undergoing primary percutaneous coronary intervention (PCI).
Gregg Stone, MD, of Columbia University Medical Center, and colleagues randomized 3602 patients to receive either bivalirudin (n=1800) or heparin plus glycoprotein IIb/IIIa inhibitors (n=1802).
The 2 primary endpoints of the study were major bleeding and combined clinical events. Combined events included death, reinfarction, target-vessel revascularization for ischemia, and stroke.
Bivalirudin reduced the overall rate of combined events, as compared to heparin plus glycoprotein IIb/IIIa inhibitors (9.2% vs 12.1%, respectively). However, when evaluated separately, there were no significant differences in the incidence of these events between the 2 treatment groups.
The rate of reinfarction was the same in both groups (1.8%). The rate of stroke was insignificantly higher in the bivalirudin group (0.7% vs 0.6%). The same was true of target-vessel revascularization; the rate was 2.6% in bivalirudin patients and 1.9% in the other patient group.
Patients on bivalirudin experienced a lower rate of major bleeding than patients on heparin plus glycoprotein IIb/IIIa inhibitors (4.9% vs 8.3%, respectively). When large hematomas were excluded, the rates of major bleeding were reduced to 4.7% and 7.8%, respectively.
Bivalirudin also reduced the incidence of hemorrhagic complications, the development of thrombocytopenia, and the need for blood transfusions.
The rate of cardiac-related death at 30 days was significantly lower for bivalirudin patients than for patients who received heparin plus glycoprotein IIb/IIIa inhibitors (1.8% vs 2.9%, respectively). The rate of death from any cause was also lower in bivalirudin patients (2.1% vs 3.1%).
Bivalirudin increased the rate of acute stent thrombosis within 24 hours of treatment initiation (1.3% vs 0.3%). However, between 24 hours and 30 days, the incidence of acute stent thrombosis was lower in the bivalirudin patients than in those on heparin plus glycoprotein IIb/IIIa inhibitors (1.2% vs. 1.7%, respectively).
This study, known as HORIZONS-AMI, was sponsored, in part, by The Medicines Company, makers of bivalirudin (Angiomax). ![]()
The direct thrombin inhibitor bivalirudin (Angiomax) reduces major bleeding and increases short-term survival in certain cardiac patients, according to a study published in the New England Journal of Medicine.
Bivalirudin was associated with a lower rate of major bleeding and death from cardiac and other causes at 30 days from treatment initiation. This was in comparison to treatment with heparin plus glycoprotein IIb/IIIa inhibitors.
Both therapies were tested in patients with ST-segment elevation myocardial infarction who were undergoing primary percutaneous coronary intervention (PCI).
Gregg Stone, MD, of Columbia University Medical Center, and colleagues randomized 3602 patients to receive either bivalirudin (n=1800) or heparin plus glycoprotein IIb/IIIa inhibitors (n=1802).
The 2 primary endpoints of the study were major bleeding and combined clinical events. Combined events included death, reinfarction, target-vessel revascularization for ischemia, and stroke.
Bivalirudin reduced the overall rate of combined events, as compared to heparin plus glycoprotein IIb/IIIa inhibitors (9.2% vs 12.1%, respectively). However, when evaluated separately, there were no significant differences in the incidence of these events between the 2 treatment groups.
The rate of reinfarction was the same in both groups (1.8%). The rate of stroke was insignificantly higher in the bivalirudin group (0.7% vs 0.6%). The same was true of target-vessel revascularization; the rate was 2.6% in bivalirudin patients and 1.9% in the other patient group.
Patients on bivalirudin experienced a lower rate of major bleeding than patients on heparin plus glycoprotein IIb/IIIa inhibitors (4.9% vs 8.3%, respectively). When large hematomas were excluded, the rates of major bleeding were reduced to 4.7% and 7.8%, respectively.
Bivalirudin also reduced the incidence of hemorrhagic complications, the development of thrombocytopenia, and the need for blood transfusions.
The rate of cardiac-related death at 30 days was significantly lower for bivalirudin patients than for patients who received heparin plus glycoprotein IIb/IIIa inhibitors (1.8% vs 2.9%, respectively). The rate of death from any cause was also lower in bivalirudin patients (2.1% vs 3.1%).
Bivalirudin increased the rate of acute stent thrombosis within 24 hours of treatment initiation (1.3% vs 0.3%). However, between 24 hours and 30 days, the incidence of acute stent thrombosis was lower in the bivalirudin patients than in those on heparin plus glycoprotein IIb/IIIa inhibitors (1.2% vs. 1.7%, respectively).
This study, known as HORIZONS-AMI, was sponsored, in part, by The Medicines Company, makers of bivalirudin (Angiomax). ![]()
Midlife Menopause Management
Supplement Editor:
Holly L. Thacker, MD, FACP
Contents
Assessing benefits and risks of hormone therapy in 2008: New evidence, especially with regard to the heart
Howard N. Hodis, MD
Update on nonhormonal approaches to menopausal management
Marjorie R. Jenkins, MD, and Andrea L. Sikon, MD, FACP
Case studies and clinical considerations in menopausal management: Putting the latest data into practice
Margaret McKenzie, MD, FACOG; Andrea L. Sikon, MD, FACP; Holly L. Thacker, MD, FACP; Margery Gass, MD; Howard N. Hodis, MD; and Marjorie R. Jenkins, MD
Supplement Editor:
Holly L. Thacker, MD, FACP
Contents
Assessing benefits and risks of hormone therapy in 2008: New evidence, especially with regard to the heart
Howard N. Hodis, MD
Update on nonhormonal approaches to menopausal management
Marjorie R. Jenkins, MD, and Andrea L. Sikon, MD, FACP
Case studies and clinical considerations in menopausal management: Putting the latest data into practice
Margaret McKenzie, MD, FACOG; Andrea L. Sikon, MD, FACP; Holly L. Thacker, MD, FACP; Margery Gass, MD; Howard N. Hodis, MD; and Marjorie R. Jenkins, MD
Supplement Editor:
Holly L. Thacker, MD, FACP
Contents
Assessing benefits and risks of hormone therapy in 2008: New evidence, especially with regard to the heart
Howard N. Hodis, MD
Update on nonhormonal approaches to menopausal management
Marjorie R. Jenkins, MD, and Andrea L. Sikon, MD, FACP
Case studies and clinical considerations in menopausal management: Putting the latest data into practice
Margaret McKenzie, MD, FACOG; Andrea L. Sikon, MD, FACP; Holly L. Thacker, MD, FACP; Margery Gass, MD; Howard N. Hodis, MD; and Marjorie R. Jenkins, MD
Intensivists and ICUs
Intensivists and ICUs
Question: What is your opinion on a closed ICU that already has a hospitalist program but now has a new intensivist program? Certainly some patients should be cared for by critical care physicians. However I feel we play an important role throughout the hospital, including the ICU. As physicians who specialize in hospital care, I do not want to lose opportunities to care for patients in the ICU. Do you feel medicine may move toward that, especially in the larger hospitals? Or have you found a happy medium?
Eric Marsh, MD,
Carolinas Healthcare System,
Charlotte, N.C.
Dr. Hospitalist responds: In many small and rural hospitals throughout the country, generalists remain the frontline providers for ICU patients.
There is a shortage of critical care physicians in this country. Many small and rural hospitals have a difficult time recruiting sufficient numbers of critical care physicians to their medical staffs. This is not the case in most academic tertiary care medical centers, where pulmonary/critical care providers routinely care for the ICU patients.
In fact, during the past two decades, many hospitals, particularly tertiary care medical centers, have “closed” their ICUs to generalists and now use specialty-trained physicians, such as pulmonary/critical care physicians, to care for ICU patients. The reason is quality. Evidence suggests intensivists provide higher-quality, more evidence-based care to ICU patients than generalists.
Quality organizations, such as the National Quality Forum and The Leapfrog Group have actively promoted the role of intensivists in the ICU and labeled them as a marker of quality care. Hospitals are under increasing scrutiny to increase the quality of care they give patients. Reimbursement is increasingly tied to performance and quality.
I expect to see more and more hospitals “close” their ICU to generalists. To be fair, the data comparing intensivists and generalists came out before the widespread role of hospitalists in our nation’s hospitals. It would be interesting to compare the care provided by hospitalists versus intensivists in the ICU. It may be we find hospitalists fare comparably to intensivists. Until that data exist, I agree with the quality organizations. Hospitals, and more importantly patients, should rely preferentially on physicians with additional critical care training to provide care for their ICU care.
If your hospitalists are interested in continuing to provide care for patients in the ICU, I suggest you speak with the leader of the intensivist group to see how your hospitalists can work with—not in lieu of—the intensivists in the care of ICU patients.
In Pursuit of More Pay
Question: I am writing to get your advice on how to go about negotiating base pay increases. I come from a four-physician hospitalist program at the New York Hospital of Queens that has tripled its annual number of discharges since 2005 without a commensurate increase in base pay and no bonus or incentive pay. If this keeps up, we’ll continue to have high turnover always manned by junior attendings. Also, what is reasonable pay for the director of a hospitalist program?
Anne Park, DO,
hospitalist faculty,
New York Hospital of Queens
Dr. Hospitalist responds: I am writing to you from the lovely Manchester Grand Hyatt in San Diego, where I am attending the SHM Annual Meeting. I am here with nearly 1,600 of my closest friends in hospital medicine.
Meeting attendees heard SHM Senior Vice President Joe Miller reveal the results of the latest “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” I refer to this survey not only because I believe it is the latest, most accurate, and comprehensive data on hospitalist productivity and compensation, but also because I think it is the objective data you need before you start discussions about compensation for you and your hospitalist staff.
Before discussing compensation, I suggest you make sure your supervisor is well-informed of your hospitalists’ roles and responsibilities. Discussions will be more productive if your supervisor understands the value of your hospitalists. Be careful because how you go about informing your supervisor will influence the response you get.
It’s important for your supervisor to know the hospitalist marketplace is highly competitive. There are more jobs than hospitalists. Many hospitals are developing hospitalist programs, and existing programs continue to expand. There are many signs of this competition for hospitalists. For example, I counted 130 vendors exhibiting at this year’s SHM Annual Meeting. About two-thirds are vendors recruiting hospitalists. This suggests hospitalists are in higher demand then ever. I didn’t need to come to San Diego to figure this out. One glance at the numerous ads in the pages preceding this section of The Hospitalist is sufficient evidence. Another sign of the times is the rise in hospitalist compensation since the results of the last survey two years ago.
Try to present this information to your supervisor in a nonconfrontational manner. Discussions will start poorly if this information is viewed as a threat the hospitalists will depart the program if you do not get your way. You alluded to “high turnover” in your group. Let your supervisor know you are presenting this information because you are concerned the cost of replacing hospitalists may exceed the cost of retaining experienced staff. Before discussions, I also suggest you think about why hospitalists have left your program. Your comments about increased discharges suggest a mismatch between compensation and job description. It is possible the discussion should be about changing the job description rather than adjusting compensation.
Once discussions begin, your supervisor will consider several factors when entertaining your request for additional compensation. Aside from individual performance, your supervisor will consider how your groups’ compensation compares to other hospitalists with similar job descriptions in the same geographic region. This is where the survey data are helpful. Most hospitalist compensation contains some element of incentive compensation. Do your best to compare apples to apples by looking at the entire compensation package, including benefits.
Your supervisor also will ask you to compare your groups’ job description to others in the area. Your hospitalists have tripled the number of discharges they did in 2005. Did other groups in the area see the same rise in productivity? What happened to their staffing and compensation? Expect these types of questions from your supervisor.
After your preparation, you may find that your hospitalists are being paid more, less, or about the same as what others are being paid for a comparable level of productivity. The latest SHM survey will assist you in preparing for your discussion. But please remember the survey just reports data. It does not indicate how much SHM thinks you should be working or how much money you should be making. It is merely a snapshot of what the market bears for hospitalists in our country. TH
Intensivists and ICUs
Question: What is your opinion on a closed ICU that already has a hospitalist program but now has a new intensivist program? Certainly some patients should be cared for by critical care physicians. However I feel we play an important role throughout the hospital, including the ICU. As physicians who specialize in hospital care, I do not want to lose opportunities to care for patients in the ICU. Do you feel medicine may move toward that, especially in the larger hospitals? Or have you found a happy medium?
Eric Marsh, MD,
Carolinas Healthcare System,
Charlotte, N.C.
Dr. Hospitalist responds: In many small and rural hospitals throughout the country, generalists remain the frontline providers for ICU patients.
There is a shortage of critical care physicians in this country. Many small and rural hospitals have a difficult time recruiting sufficient numbers of critical care physicians to their medical staffs. This is not the case in most academic tertiary care medical centers, where pulmonary/critical care providers routinely care for the ICU patients.
In fact, during the past two decades, many hospitals, particularly tertiary care medical centers, have “closed” their ICUs to generalists and now use specialty-trained physicians, such as pulmonary/critical care physicians, to care for ICU patients. The reason is quality. Evidence suggests intensivists provide higher-quality, more evidence-based care to ICU patients than generalists.
Quality organizations, such as the National Quality Forum and The Leapfrog Group have actively promoted the role of intensivists in the ICU and labeled them as a marker of quality care. Hospitals are under increasing scrutiny to increase the quality of care they give patients. Reimbursement is increasingly tied to performance and quality.
I expect to see more and more hospitals “close” their ICU to generalists. To be fair, the data comparing intensivists and generalists came out before the widespread role of hospitalists in our nation’s hospitals. It would be interesting to compare the care provided by hospitalists versus intensivists in the ICU. It may be we find hospitalists fare comparably to intensivists. Until that data exist, I agree with the quality organizations. Hospitals, and more importantly patients, should rely preferentially on physicians with additional critical care training to provide care for their ICU care.
If your hospitalists are interested in continuing to provide care for patients in the ICU, I suggest you speak with the leader of the intensivist group to see how your hospitalists can work with—not in lieu of—the intensivists in the care of ICU patients.
In Pursuit of More Pay
Question: I am writing to get your advice on how to go about negotiating base pay increases. I come from a four-physician hospitalist program at the New York Hospital of Queens that has tripled its annual number of discharges since 2005 without a commensurate increase in base pay and no bonus or incentive pay. If this keeps up, we’ll continue to have high turnover always manned by junior attendings. Also, what is reasonable pay for the director of a hospitalist program?
Anne Park, DO,
hospitalist faculty,
New York Hospital of Queens
Dr. Hospitalist responds: I am writing to you from the lovely Manchester Grand Hyatt in San Diego, where I am attending the SHM Annual Meeting. I am here with nearly 1,600 of my closest friends in hospital medicine.
Meeting attendees heard SHM Senior Vice President Joe Miller reveal the results of the latest “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” I refer to this survey not only because I believe it is the latest, most accurate, and comprehensive data on hospitalist productivity and compensation, but also because I think it is the objective data you need before you start discussions about compensation for you and your hospitalist staff.
Before discussing compensation, I suggest you make sure your supervisor is well-informed of your hospitalists’ roles and responsibilities. Discussions will be more productive if your supervisor understands the value of your hospitalists. Be careful because how you go about informing your supervisor will influence the response you get.
It’s important for your supervisor to know the hospitalist marketplace is highly competitive. There are more jobs than hospitalists. Many hospitals are developing hospitalist programs, and existing programs continue to expand. There are many signs of this competition for hospitalists. For example, I counted 130 vendors exhibiting at this year’s SHM Annual Meeting. About two-thirds are vendors recruiting hospitalists. This suggests hospitalists are in higher demand then ever. I didn’t need to come to San Diego to figure this out. One glance at the numerous ads in the pages preceding this section of The Hospitalist is sufficient evidence. Another sign of the times is the rise in hospitalist compensation since the results of the last survey two years ago.
Try to present this information to your supervisor in a nonconfrontational manner. Discussions will start poorly if this information is viewed as a threat the hospitalists will depart the program if you do not get your way. You alluded to “high turnover” in your group. Let your supervisor know you are presenting this information because you are concerned the cost of replacing hospitalists may exceed the cost of retaining experienced staff. Before discussions, I also suggest you think about why hospitalists have left your program. Your comments about increased discharges suggest a mismatch between compensation and job description. It is possible the discussion should be about changing the job description rather than adjusting compensation.
Once discussions begin, your supervisor will consider several factors when entertaining your request for additional compensation. Aside from individual performance, your supervisor will consider how your groups’ compensation compares to other hospitalists with similar job descriptions in the same geographic region. This is where the survey data are helpful. Most hospitalist compensation contains some element of incentive compensation. Do your best to compare apples to apples by looking at the entire compensation package, including benefits.
Your supervisor also will ask you to compare your groups’ job description to others in the area. Your hospitalists have tripled the number of discharges they did in 2005. Did other groups in the area see the same rise in productivity? What happened to their staffing and compensation? Expect these types of questions from your supervisor.
After your preparation, you may find that your hospitalists are being paid more, less, or about the same as what others are being paid for a comparable level of productivity. The latest SHM survey will assist you in preparing for your discussion. But please remember the survey just reports data. It does not indicate how much SHM thinks you should be working or how much money you should be making. It is merely a snapshot of what the market bears for hospitalists in our country. TH
Intensivists and ICUs
Question: What is your opinion on a closed ICU that already has a hospitalist program but now has a new intensivist program? Certainly some patients should be cared for by critical care physicians. However I feel we play an important role throughout the hospital, including the ICU. As physicians who specialize in hospital care, I do not want to lose opportunities to care for patients in the ICU. Do you feel medicine may move toward that, especially in the larger hospitals? Or have you found a happy medium?
Eric Marsh, MD,
Carolinas Healthcare System,
Charlotte, N.C.
Dr. Hospitalist responds: In many small and rural hospitals throughout the country, generalists remain the frontline providers for ICU patients.
There is a shortage of critical care physicians in this country. Many small and rural hospitals have a difficult time recruiting sufficient numbers of critical care physicians to their medical staffs. This is not the case in most academic tertiary care medical centers, where pulmonary/critical care providers routinely care for the ICU patients.
In fact, during the past two decades, many hospitals, particularly tertiary care medical centers, have “closed” their ICUs to generalists and now use specialty-trained physicians, such as pulmonary/critical care physicians, to care for ICU patients. The reason is quality. Evidence suggests intensivists provide higher-quality, more evidence-based care to ICU patients than generalists.
Quality organizations, such as the National Quality Forum and The Leapfrog Group have actively promoted the role of intensivists in the ICU and labeled them as a marker of quality care. Hospitals are under increasing scrutiny to increase the quality of care they give patients. Reimbursement is increasingly tied to performance and quality.
I expect to see more and more hospitals “close” their ICU to generalists. To be fair, the data comparing intensivists and generalists came out before the widespread role of hospitalists in our nation’s hospitals. It would be interesting to compare the care provided by hospitalists versus intensivists in the ICU. It may be we find hospitalists fare comparably to intensivists. Until that data exist, I agree with the quality organizations. Hospitals, and more importantly patients, should rely preferentially on physicians with additional critical care training to provide care for their ICU care.
If your hospitalists are interested in continuing to provide care for patients in the ICU, I suggest you speak with the leader of the intensivist group to see how your hospitalists can work with—not in lieu of—the intensivists in the care of ICU patients.
In Pursuit of More Pay
Question: I am writing to get your advice on how to go about negotiating base pay increases. I come from a four-physician hospitalist program at the New York Hospital of Queens that has tripled its annual number of discharges since 2005 without a commensurate increase in base pay and no bonus or incentive pay. If this keeps up, we’ll continue to have high turnover always manned by junior attendings. Also, what is reasonable pay for the director of a hospitalist program?
Anne Park, DO,
hospitalist faculty,
New York Hospital of Queens
Dr. Hospitalist responds: I am writing to you from the lovely Manchester Grand Hyatt in San Diego, where I am attending the SHM Annual Meeting. I am here with nearly 1,600 of my closest friends in hospital medicine.
Meeting attendees heard SHM Senior Vice President Joe Miller reveal the results of the latest “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” I refer to this survey not only because I believe it is the latest, most accurate, and comprehensive data on hospitalist productivity and compensation, but also because I think it is the objective data you need before you start discussions about compensation for you and your hospitalist staff.
Before discussing compensation, I suggest you make sure your supervisor is well-informed of your hospitalists’ roles and responsibilities. Discussions will be more productive if your supervisor understands the value of your hospitalists. Be careful because how you go about informing your supervisor will influence the response you get.
It’s important for your supervisor to know the hospitalist marketplace is highly competitive. There are more jobs than hospitalists. Many hospitals are developing hospitalist programs, and existing programs continue to expand. There are many signs of this competition for hospitalists. For example, I counted 130 vendors exhibiting at this year’s SHM Annual Meeting. About two-thirds are vendors recruiting hospitalists. This suggests hospitalists are in higher demand then ever. I didn’t need to come to San Diego to figure this out. One glance at the numerous ads in the pages preceding this section of The Hospitalist is sufficient evidence. Another sign of the times is the rise in hospitalist compensation since the results of the last survey two years ago.
Try to present this information to your supervisor in a nonconfrontational manner. Discussions will start poorly if this information is viewed as a threat the hospitalists will depart the program if you do not get your way. You alluded to “high turnover” in your group. Let your supervisor know you are presenting this information because you are concerned the cost of replacing hospitalists may exceed the cost of retaining experienced staff. Before discussions, I also suggest you think about why hospitalists have left your program. Your comments about increased discharges suggest a mismatch between compensation and job description. It is possible the discussion should be about changing the job description rather than adjusting compensation.
Once discussions begin, your supervisor will consider several factors when entertaining your request for additional compensation. Aside from individual performance, your supervisor will consider how your groups’ compensation compares to other hospitalists with similar job descriptions in the same geographic region. This is where the survey data are helpful. Most hospitalist compensation contains some element of incentive compensation. Do your best to compare apples to apples by looking at the entire compensation package, including benefits.
Your supervisor also will ask you to compare your groups’ job description to others in the area. Your hospitalists have tripled the number of discharges they did in 2005. Did other groups in the area see the same rise in productivity? What happened to their staffing and compensation? Expect these types of questions from your supervisor.
After your preparation, you may find that your hospitalists are being paid more, less, or about the same as what others are being paid for a comparable level of productivity. The latest SHM survey will assist you in preparing for your discussion. But please remember the survey just reports data. It does not indicate how much SHM thinks you should be working or how much money you should be making. It is merely a snapshot of what the market bears for hospitalists in our country. TH
Value Your Practice
The issue of practice valuation is a sensitive one. Some hospitalist practices might have significant monetary value, which could make it reasonable to ask new doctors to buy in or enable selling the practice for a profit. Still, it’s risky to assume this is the case for your practice.
Let’s examine the issue using a pair of situations I encountered not long ago. I have changed some details of the practices to more clearly illustrate a point and conceal which practices I’m describing. Both situations would have gone smoother if it was clear what the hospitalist practice was worth. But how do you assess that value?
Case No. 1
During a couple of days in 2006, I consulted with a high-performing private practice hospitalist group on the East Coast. The group was led by one of the most energetic and thoughtful leaders I’ve encountered.
Like many other private practice groups, they divided physician members of the practice into partners and non-partners (sometimes referred to as shareholder and non-shareholders in the corporation). A hospitalist who had been a full-time member of the practice for a specified period of time (two years in this case) was eligible to become partner.
This entailed a “buy-in” requiring the doctor to pay money to the practice (usually the doctor would pay using a loan from the practice, which was repaid through deductions from his/her paycheck).
For this practice, the principal benefits of partner status were having a vote in group decisions (non-partners couldn’t vote) and receiving a portion of the distribution of all corporate profits each year. These profits came from two sources:
- Money remaining after all salaries and overhead were paid; and
- Buy-in money received by the practice.
Because the partners had this “upside potential” they agreed they would cover any staff shortages by working extra shifts instead of the non-partners.
Setting things up with a buy-in to achieve partner/shareholder status seemed to make a lot of sense. After all, it is the way nearly all private-practice medical groups in other specialties are structured.
Problems soon arose when they realized there wasn’t significant “profit” available unless there happened to be two or three doctors buying into the practice in a given year.
So, the partners became disenchanted because they shouldered the burden of covering any extra shifts but didn’t get a significant profit distribution in most years. Non-partners who became eligible for partnership were choosing not to buy in because it seemed like more responsibility without more income. The group’s system began breaking down.
Keep in mind they had a terrific practice. The docs liked each other and were pleased with the group leader, were highly regarded by hospital executives and other doctors, and had a growing patient volume.
Yet, the partners were unhappy they weren’t seeing extra compensation as a reward for buying into the practice with the money, time, and effort they invested.
Despite being a desirable practice in nearly every respect, new doctors were choosing to forgo partnership status. These things were creating significant morale issues that threatened the ongoing success of the group.
So why did these problems arise?
Case No. 2
Later in 2006, I worked with a different private-practice hospitalist group out West. Their practice had been started by, and was still owned by, a “parent” medical group. As the hospitalist practice grew, everyone (hospitalists and non-hospitalist doctors in the group) agreed it made sense to have the hospitalist practice separate into its own distinct corporation. Like the practice in the first case, all parties had high regard for one another.
The problem was the non-hospitalists who invested the time and energy to start the hospitalist practice wanted the departing hospitalists to compensate the larger group.
The hospitalists could understand why the other doctors proposed a buyout but wondered what the hospitalists would get in return for paying it. The answer seemed to be not much. They weren’t confident they could recoup their investment by having future hospitalists buy in to the practice (proposing this had scared off more than one recruit), or by selling the practice to another party.
Assess Your Value
The problems faced by both these practices are a result of uncertainty about what their practices are worth.
In the first case, doctors who had the opportunity to buy into the practice were choosing not to because they believed they weren’t going to get anything in return (and had the added burden of putting themselves on the schedule more often to cover open shifts).
Likewise, in the second case the hospitalists agreed it seemed reasonable to pay the other doctors in the parent group to go out on their own. But the hospitalists worried they would never be able to recoup that money by selling shares of the practice to new partner hospitalists or selling the whole group to another entity.
It’s tricky to value any medical practice. A common approach is to put a price on tangible assets owned by the practice (e.g., buildings and equipment like computers and lab apparatus, and the accounts receivable), and the patient base (or good will) the practice has developed.
It isn’t too difficult to come up with a value for tangible assets, and most hospitalist practices have little or nothing in this category (the only hard assets I can think of that I own are my pager, stethoscope, and a couple of lab coats I never wear). Patient lists and good will are particularly difficult to place a value on. Even for a primary care practice with thousands of patient charts, there is no guarantee patients will agree to transfer their care to a purchasing doctor.
For most any kind of medical practice, including a hospitalist group, good will mainly is a function of the referral relationships doctors have developed that ensure a steady flow of patients. Since a steady flow of patients is not a problem for most hospitalist practices (too many patients is more common than too few) the value of that referral stream may not be much.
Another asset many hospitalist practices own is their contract(s) with sponsoring organizations (usually hospitals, but sometimes health plans). They provide for supplemental payments over and above professional fees the practice collects.
This is often a hospitalist practice’s most valuable asset, and it may be worth investing money to acquire. It’s the primary reason large hospitalist staffing companies are willing to pay to acquire local hospitalist practices.
Usually these contracts cannot automatically be assigned to another party without the hospital’s consent. Most hospitals’ loyalty lies with the hospitalists who provide their coverage, not with the company that may hold the contract. For example, with the hospitalists in the second case, their hospital would have been willing to immediately sign a new contract with their spin-off group to maintain their existing hospitalist coverage. The parent group’s hospital contract wasn’t worth acquiring.
All this suggests hospitalist practices may not have much monetary value. That is, an outside party probably wouldn’t pay much to buy your practice. I think this is true for the two practices I describe above. For practices like these, it is probably best to avoid having a buy-in to achieve partner status, and not diverting some practice revenue that would otherwise be used to pay salaries into a “profit” pool from which distributions are made to partners/owners periodically.
Practices Worth A Lot
I’m aware my comments might seem insulting to a group of hospitalists who have worked long and hard for several years to build what they think is a great practice. Surely it’s worth something.
Maybe your practice really does have significant value over and above the salaries the doctors earn. Perhaps you have developed proprietary operational processes that are particularly valuable and would be difficult for others to replicate without knowing your “trade secrets.” These could include things such as particularly effective ways to document, code, and collect professional fees; methods to enhance hospitalist efficiency and/or quality; unusually effective recruitment strategies; or even the ability to negotiate highly favorable contracts with payers.
Even if your practice does have remarkably effective proprietary components, you still would have to convince a buyer these valuable assets would persist after the change in ownership and the departure of key individuals. For example, you might have the best practice in the country because you’ve been able to recruit the best doctors. If I buy your practice and those excellent doctors leave, I’ve lost the unique asset that was key to the practice’s value.
Clearly there is room for a lot of debate about hospitalist practice valuation. (Search the Internet for “medical practice valuation” for a number of good articles about this.) There are many practice management companies that rely on the notion that their ideas and operations provide greater value than other practices. One such company, IPC, had a successful initial public offering of stock that found a marketplace willing to pay for its perceived value. But keep in mind that this company has many practices in many states, and much of the value may lie in the fact that the value of the whole is greater than the sum of its parts. So, unless your practice is huge and has sites in many states, I don’t think you can assume IPC’s public offering means your practice might have a similar value.
Think critically about your practice. Challenge yourself to think about what you would pay for your practice and what you would get in return. If you have a hard time coming up with clear reasons your practice has significant intangible value, you should probably avoid structuring a buy-in for new doctors or a buy-out for departing doctors. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
The issue of practice valuation is a sensitive one. Some hospitalist practices might have significant monetary value, which could make it reasonable to ask new doctors to buy in or enable selling the practice for a profit. Still, it’s risky to assume this is the case for your practice.
Let’s examine the issue using a pair of situations I encountered not long ago. I have changed some details of the practices to more clearly illustrate a point and conceal which practices I’m describing. Both situations would have gone smoother if it was clear what the hospitalist practice was worth. But how do you assess that value?
Case No. 1
During a couple of days in 2006, I consulted with a high-performing private practice hospitalist group on the East Coast. The group was led by one of the most energetic and thoughtful leaders I’ve encountered.
Like many other private practice groups, they divided physician members of the practice into partners and non-partners (sometimes referred to as shareholder and non-shareholders in the corporation). A hospitalist who had been a full-time member of the practice for a specified period of time (two years in this case) was eligible to become partner.
This entailed a “buy-in” requiring the doctor to pay money to the practice (usually the doctor would pay using a loan from the practice, which was repaid through deductions from his/her paycheck).
For this practice, the principal benefits of partner status were having a vote in group decisions (non-partners couldn’t vote) and receiving a portion of the distribution of all corporate profits each year. These profits came from two sources:
- Money remaining after all salaries and overhead were paid; and
- Buy-in money received by the practice.
Because the partners had this “upside potential” they agreed they would cover any staff shortages by working extra shifts instead of the non-partners.
Setting things up with a buy-in to achieve partner/shareholder status seemed to make a lot of sense. After all, it is the way nearly all private-practice medical groups in other specialties are structured.
Problems soon arose when they realized there wasn’t significant “profit” available unless there happened to be two or three doctors buying into the practice in a given year.
So, the partners became disenchanted because they shouldered the burden of covering any extra shifts but didn’t get a significant profit distribution in most years. Non-partners who became eligible for partnership were choosing not to buy in because it seemed like more responsibility without more income. The group’s system began breaking down.
Keep in mind they had a terrific practice. The docs liked each other and were pleased with the group leader, were highly regarded by hospital executives and other doctors, and had a growing patient volume.
Yet, the partners were unhappy they weren’t seeing extra compensation as a reward for buying into the practice with the money, time, and effort they invested.
Despite being a desirable practice in nearly every respect, new doctors were choosing to forgo partnership status. These things were creating significant morale issues that threatened the ongoing success of the group.
So why did these problems arise?
Case No. 2
Later in 2006, I worked with a different private-practice hospitalist group out West. Their practice had been started by, and was still owned by, a “parent” medical group. As the hospitalist practice grew, everyone (hospitalists and non-hospitalist doctors in the group) agreed it made sense to have the hospitalist practice separate into its own distinct corporation. Like the practice in the first case, all parties had high regard for one another.
The problem was the non-hospitalists who invested the time and energy to start the hospitalist practice wanted the departing hospitalists to compensate the larger group.
The hospitalists could understand why the other doctors proposed a buyout but wondered what the hospitalists would get in return for paying it. The answer seemed to be not much. They weren’t confident they could recoup their investment by having future hospitalists buy in to the practice (proposing this had scared off more than one recruit), or by selling the practice to another party.
Assess Your Value
The problems faced by both these practices are a result of uncertainty about what their practices are worth.
In the first case, doctors who had the opportunity to buy into the practice were choosing not to because they believed they weren’t going to get anything in return (and had the added burden of putting themselves on the schedule more often to cover open shifts).
Likewise, in the second case the hospitalists agreed it seemed reasonable to pay the other doctors in the parent group to go out on their own. But the hospitalists worried they would never be able to recoup that money by selling shares of the practice to new partner hospitalists or selling the whole group to another entity.
It’s tricky to value any medical practice. A common approach is to put a price on tangible assets owned by the practice (e.g., buildings and equipment like computers and lab apparatus, and the accounts receivable), and the patient base (or good will) the practice has developed.
It isn’t too difficult to come up with a value for tangible assets, and most hospitalist practices have little or nothing in this category (the only hard assets I can think of that I own are my pager, stethoscope, and a couple of lab coats I never wear). Patient lists and good will are particularly difficult to place a value on. Even for a primary care practice with thousands of patient charts, there is no guarantee patients will agree to transfer their care to a purchasing doctor.
For most any kind of medical practice, including a hospitalist group, good will mainly is a function of the referral relationships doctors have developed that ensure a steady flow of patients. Since a steady flow of patients is not a problem for most hospitalist practices (too many patients is more common than too few) the value of that referral stream may not be much.
Another asset many hospitalist practices own is their contract(s) with sponsoring organizations (usually hospitals, but sometimes health plans). They provide for supplemental payments over and above professional fees the practice collects.
This is often a hospitalist practice’s most valuable asset, and it may be worth investing money to acquire. It’s the primary reason large hospitalist staffing companies are willing to pay to acquire local hospitalist practices.
Usually these contracts cannot automatically be assigned to another party without the hospital’s consent. Most hospitals’ loyalty lies with the hospitalists who provide their coverage, not with the company that may hold the contract. For example, with the hospitalists in the second case, their hospital would have been willing to immediately sign a new contract with their spin-off group to maintain their existing hospitalist coverage. The parent group’s hospital contract wasn’t worth acquiring.
All this suggests hospitalist practices may not have much monetary value. That is, an outside party probably wouldn’t pay much to buy your practice. I think this is true for the two practices I describe above. For practices like these, it is probably best to avoid having a buy-in to achieve partner status, and not diverting some practice revenue that would otherwise be used to pay salaries into a “profit” pool from which distributions are made to partners/owners periodically.
Practices Worth A Lot
I’m aware my comments might seem insulting to a group of hospitalists who have worked long and hard for several years to build what they think is a great practice. Surely it’s worth something.
Maybe your practice really does have significant value over and above the salaries the doctors earn. Perhaps you have developed proprietary operational processes that are particularly valuable and would be difficult for others to replicate without knowing your “trade secrets.” These could include things such as particularly effective ways to document, code, and collect professional fees; methods to enhance hospitalist efficiency and/or quality; unusually effective recruitment strategies; or even the ability to negotiate highly favorable contracts with payers.
Even if your practice does have remarkably effective proprietary components, you still would have to convince a buyer these valuable assets would persist after the change in ownership and the departure of key individuals. For example, you might have the best practice in the country because you’ve been able to recruit the best doctors. If I buy your practice and those excellent doctors leave, I’ve lost the unique asset that was key to the practice’s value.
Clearly there is room for a lot of debate about hospitalist practice valuation. (Search the Internet for “medical practice valuation” for a number of good articles about this.) There are many practice management companies that rely on the notion that their ideas and operations provide greater value than other practices. One such company, IPC, had a successful initial public offering of stock that found a marketplace willing to pay for its perceived value. But keep in mind that this company has many practices in many states, and much of the value may lie in the fact that the value of the whole is greater than the sum of its parts. So, unless your practice is huge and has sites in many states, I don’t think you can assume IPC’s public offering means your practice might have a similar value.
Think critically about your practice. Challenge yourself to think about what you would pay for your practice and what you would get in return. If you have a hard time coming up with clear reasons your practice has significant intangible value, you should probably avoid structuring a buy-in for new doctors or a buy-out for departing doctors. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
The issue of practice valuation is a sensitive one. Some hospitalist practices might have significant monetary value, which could make it reasonable to ask new doctors to buy in or enable selling the practice for a profit. Still, it’s risky to assume this is the case for your practice.
Let’s examine the issue using a pair of situations I encountered not long ago. I have changed some details of the practices to more clearly illustrate a point and conceal which practices I’m describing. Both situations would have gone smoother if it was clear what the hospitalist practice was worth. But how do you assess that value?
Case No. 1
During a couple of days in 2006, I consulted with a high-performing private practice hospitalist group on the East Coast. The group was led by one of the most energetic and thoughtful leaders I’ve encountered.
Like many other private practice groups, they divided physician members of the practice into partners and non-partners (sometimes referred to as shareholder and non-shareholders in the corporation). A hospitalist who had been a full-time member of the practice for a specified period of time (two years in this case) was eligible to become partner.
This entailed a “buy-in” requiring the doctor to pay money to the practice (usually the doctor would pay using a loan from the practice, which was repaid through deductions from his/her paycheck).
For this practice, the principal benefits of partner status were having a vote in group decisions (non-partners couldn’t vote) and receiving a portion of the distribution of all corporate profits each year. These profits came from two sources:
- Money remaining after all salaries and overhead were paid; and
- Buy-in money received by the practice.
Because the partners had this “upside potential” they agreed they would cover any staff shortages by working extra shifts instead of the non-partners.
Setting things up with a buy-in to achieve partner/shareholder status seemed to make a lot of sense. After all, it is the way nearly all private-practice medical groups in other specialties are structured.
Problems soon arose when they realized there wasn’t significant “profit” available unless there happened to be two or three doctors buying into the practice in a given year.
So, the partners became disenchanted because they shouldered the burden of covering any extra shifts but didn’t get a significant profit distribution in most years. Non-partners who became eligible for partnership were choosing not to buy in because it seemed like more responsibility without more income. The group’s system began breaking down.
Keep in mind they had a terrific practice. The docs liked each other and were pleased with the group leader, were highly regarded by hospital executives and other doctors, and had a growing patient volume.
Yet, the partners were unhappy they weren’t seeing extra compensation as a reward for buying into the practice with the money, time, and effort they invested.
Despite being a desirable practice in nearly every respect, new doctors were choosing to forgo partnership status. These things were creating significant morale issues that threatened the ongoing success of the group.
So why did these problems arise?
Case No. 2
Later in 2006, I worked with a different private-practice hospitalist group out West. Their practice had been started by, and was still owned by, a “parent” medical group. As the hospitalist practice grew, everyone (hospitalists and non-hospitalist doctors in the group) agreed it made sense to have the hospitalist practice separate into its own distinct corporation. Like the practice in the first case, all parties had high regard for one another.
The problem was the non-hospitalists who invested the time and energy to start the hospitalist practice wanted the departing hospitalists to compensate the larger group.
The hospitalists could understand why the other doctors proposed a buyout but wondered what the hospitalists would get in return for paying it. The answer seemed to be not much. They weren’t confident they could recoup their investment by having future hospitalists buy in to the practice (proposing this had scared off more than one recruit), or by selling the practice to another party.
Assess Your Value
The problems faced by both these practices are a result of uncertainty about what their practices are worth.
In the first case, doctors who had the opportunity to buy into the practice were choosing not to because they believed they weren’t going to get anything in return (and had the added burden of putting themselves on the schedule more often to cover open shifts).
Likewise, in the second case the hospitalists agreed it seemed reasonable to pay the other doctors in the parent group to go out on their own. But the hospitalists worried they would never be able to recoup that money by selling shares of the practice to new partner hospitalists or selling the whole group to another entity.
It’s tricky to value any medical practice. A common approach is to put a price on tangible assets owned by the practice (e.g., buildings and equipment like computers and lab apparatus, and the accounts receivable), and the patient base (or good will) the practice has developed.
It isn’t too difficult to come up with a value for tangible assets, and most hospitalist practices have little or nothing in this category (the only hard assets I can think of that I own are my pager, stethoscope, and a couple of lab coats I never wear). Patient lists and good will are particularly difficult to place a value on. Even for a primary care practice with thousands of patient charts, there is no guarantee patients will agree to transfer their care to a purchasing doctor.
For most any kind of medical practice, including a hospitalist group, good will mainly is a function of the referral relationships doctors have developed that ensure a steady flow of patients. Since a steady flow of patients is not a problem for most hospitalist practices (too many patients is more common than too few) the value of that referral stream may not be much.
Another asset many hospitalist practices own is their contract(s) with sponsoring organizations (usually hospitals, but sometimes health plans). They provide for supplemental payments over and above professional fees the practice collects.
This is often a hospitalist practice’s most valuable asset, and it may be worth investing money to acquire. It’s the primary reason large hospitalist staffing companies are willing to pay to acquire local hospitalist practices.
Usually these contracts cannot automatically be assigned to another party without the hospital’s consent. Most hospitals’ loyalty lies with the hospitalists who provide their coverage, not with the company that may hold the contract. For example, with the hospitalists in the second case, their hospital would have been willing to immediately sign a new contract with their spin-off group to maintain their existing hospitalist coverage. The parent group’s hospital contract wasn’t worth acquiring.
All this suggests hospitalist practices may not have much monetary value. That is, an outside party probably wouldn’t pay much to buy your practice. I think this is true for the two practices I describe above. For practices like these, it is probably best to avoid having a buy-in to achieve partner status, and not diverting some practice revenue that would otherwise be used to pay salaries into a “profit” pool from which distributions are made to partners/owners periodically.
Practices Worth A Lot
I’m aware my comments might seem insulting to a group of hospitalists who have worked long and hard for several years to build what they think is a great practice. Surely it’s worth something.
Maybe your practice really does have significant value over and above the salaries the doctors earn. Perhaps you have developed proprietary operational processes that are particularly valuable and would be difficult for others to replicate without knowing your “trade secrets.” These could include things such as particularly effective ways to document, code, and collect professional fees; methods to enhance hospitalist efficiency and/or quality; unusually effective recruitment strategies; or even the ability to negotiate highly favorable contracts with payers.
Even if your practice does have remarkably effective proprietary components, you still would have to convince a buyer these valuable assets would persist after the change in ownership and the departure of key individuals. For example, you might have the best practice in the country because you’ve been able to recruit the best doctors. If I buy your practice and those excellent doctors leave, I’ve lost the unique asset that was key to the practice’s value.
Clearly there is room for a lot of debate about hospitalist practice valuation. (Search the Internet for “medical practice valuation” for a number of good articles about this.) There are many practice management companies that rely on the notion that their ideas and operations provide greater value than other practices. One such company, IPC, had a successful initial public offering of stock that found a marketplace willing to pay for its perceived value. But keep in mind that this company has many practices in many states, and much of the value may lie in the fact that the value of the whole is greater than the sum of its parts. So, unless your practice is huge and has sites in many states, I don’t think you can assume IPC’s public offering means your practice might have a similar value.
Think critically about your practice. Challenge yourself to think about what you would pay for your practice and what you would get in return. If you have a hard time coming up with clear reasons your practice has significant intangible value, you should probably avoid structuring a buy-in for new doctors or a buy-out for departing doctors. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Safety, Salary, and Saints
I just returned from the SHM annual meeting, and I am brimming with new knowledge. While we’ve tried to capture the essence of the meeting in this edition of The Hospitalist, it’s hard to describe in print the excitement, energy, and edification I encountered during those three days in San Diego.
Nearly 1,600 hospitalists descended on the Grand Hyatt on the San Diego Harbor for the six pre-courses April 3 and the two-day meeting April 4-5, marking the biggest and widest-reaching hospitalist meeting to date. Here’s just a smattering of what I learned in southern California.
I learned that Don Berwick, MD, is a healthcare visionary. In his plenary address the founder of the Institute for Healthcare Improvement and national leader of the patient safety movement gave his insights on the quality of the healthcare system and offered a challenge for hospitalists. He started by deftly outlining how Americans pay $3,000 more per capita for healthcare than other industrialized countries only to receive less access, worse care, and higher mortality rates. Clearly, more money does not equate to better care.
After noting that every system is perfectly designed to achieve the results it gets, Dr. Berwick challenged hospitalists to debunk the romantic view of the “individual as the cause of excellence” in favor of creating multidisciplinary teams and systems of care whose results do not depend on the heroism of the individual.
I learned that hospitalists are getting paid more today for the same amount of work they provided in 2005. At the same time that the average salary is up 13% to $193,300 (compared with 2005) the average number of annual encounters per hospitalist is down 4% from 2,558 in 2005 to 2,447 in 2007.
I’ve seen several interpretations of these data. The most cynical take, generally from non-hospitalists, is that this is further proof that hospitalists are overpaid compared with our non-hospitalist generalist colleagues in internal and family medicine. While these changes obviously represent a free-market response to a shortage of hospitalists, I firmly believe these higher salaries are a more accurate valuation of the work hospitalists do—and the more appropriate interpretation is that we’ve been underpaid in previous years. Now it’s time for our healthcare system to more appropriately reward our outpatient colleagues as well.
I also learned that academic hospitalists are struggling with similar issues across the country. Drs. Adrienne Bennett, Brian Lucas, and Bob Wachter, led an enthralling but sobering session surveying the challenges facing academic hospitalist groups. In many cases the vision of developing a sustainable academic model around the core tenets of research, scholarly activity, and education is being undermined by the service mandate of non-teaching clinical work.
These tensions lead to profound challenges come promotion time, a topic that Drs. Scott Flanders, David Meltzer, and Sankey Williams covered in an afternoon session.
As a director of an academic program who recently went through the promotion process, I view these two issues as critical to the health of all segments of hospital medicine, not just academics. Community hospitalist groups will encounter even larger workforce deficiencies if future hospitalists (i.e., current residents) shy away from the field because they see academic hospitalists devalued as unpromotable resident extenders and academic second-class citizens.
Speaking of workforce shortages, I learned that several highly respect leaders in hospital medicine believe this to be one of the most significant factors threatening the field. In a plenary panel discussion, Drs. Ron Greeno, John Nelson, Mike Guthrie, and John Laverty, commented that overcoming the current and future hospitalist shortage requires rethinking the current model. Dr. Greeno highlighted the need to build more efficient care models whereby hospitalists could see more patients in the same time by reducing the high levels of busy work and administrative minutiae.
Other ideas centered on the development of midlevel provider hospitalists and limiting our scope of practice. To the latter point, there was a lively debate about just how much of the traditionally “non-medical” piece of the pie hospitalists should bite off. Eric Siegal, MD, tackled this issue in a later session challenging hospitalist groups to rethink the value of further expanding the co-management model to more surgical patients while we struggle to care for the patients for whom we currently care.
I learned that Drs. Nelson and Win Whitcomb, co-founders of SHM, showed tremendous vision in their founding of this society. I had the chance to have lunch with John, and I asked him if he ever imagined that the tiny group he brought together in San Diego 11 years ago would ever grow to this—20,000 hospitalists, 6,000 SHM members, an annual meeting with 1,600 people, and a hospitalist (Russell Holman, MD, past president of SHM) seated at the table of the most influential healthcare policy meetings in Washington, D.C.
Rather than being awestruck by the development of this field and SHM, he simply noted this is exactly what he and Win foresaw more than a decade ago; this is the reason they founded SHM. That’s the kind of vision that explains why the field of hospital medicine is the fastest-growing medical specialty.
I learned that the future of hospital medicine is being defined today. Nearly 200 posters were presented at the Research, Innovations and Clinical Vignettes (RIV) Competition. When we look back 10 years from now, we will see a mature field and wonder how we got there so quickly.
That future is being constructed today by folks like Ken Epstein, MD, who presented fascinating data on the effects of fragmentation of hospitalist care, and Param Dedhia, MD, who showed that a formalized discharge toolkit could reduce emergency visits and hospital readmissions in elderly patients.
Finally, while I was away in the city named after a saint, I learned that my wife, too, is a saint. Unable to travel with me, she was landlocked in Denver with our 6-month-old son. I, like many attendees, acknowledge the families who sacrificed so their loved ones could attend the meeting. While I was socializing, learning, networking, and teaching a session, my wife was home soothing tears, changing diapers, cleaning chinfuls of cereal, and answering 3 a.m. wakeup calls. On behalf of all attendees I say thanks to all the saints who enabled us to be away charting the course of hospital medicine at Hospital Medicine 2008. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
I just returned from the SHM annual meeting, and I am brimming with new knowledge. While we’ve tried to capture the essence of the meeting in this edition of The Hospitalist, it’s hard to describe in print the excitement, energy, and edification I encountered during those three days in San Diego.
Nearly 1,600 hospitalists descended on the Grand Hyatt on the San Diego Harbor for the six pre-courses April 3 and the two-day meeting April 4-5, marking the biggest and widest-reaching hospitalist meeting to date. Here’s just a smattering of what I learned in southern California.
I learned that Don Berwick, MD, is a healthcare visionary. In his plenary address the founder of the Institute for Healthcare Improvement and national leader of the patient safety movement gave his insights on the quality of the healthcare system and offered a challenge for hospitalists. He started by deftly outlining how Americans pay $3,000 more per capita for healthcare than other industrialized countries only to receive less access, worse care, and higher mortality rates. Clearly, more money does not equate to better care.
After noting that every system is perfectly designed to achieve the results it gets, Dr. Berwick challenged hospitalists to debunk the romantic view of the “individual as the cause of excellence” in favor of creating multidisciplinary teams and systems of care whose results do not depend on the heroism of the individual.
I learned that hospitalists are getting paid more today for the same amount of work they provided in 2005. At the same time that the average salary is up 13% to $193,300 (compared with 2005) the average number of annual encounters per hospitalist is down 4% from 2,558 in 2005 to 2,447 in 2007.
I’ve seen several interpretations of these data. The most cynical take, generally from non-hospitalists, is that this is further proof that hospitalists are overpaid compared with our non-hospitalist generalist colleagues in internal and family medicine. While these changes obviously represent a free-market response to a shortage of hospitalists, I firmly believe these higher salaries are a more accurate valuation of the work hospitalists do—and the more appropriate interpretation is that we’ve been underpaid in previous years. Now it’s time for our healthcare system to more appropriately reward our outpatient colleagues as well.
I also learned that academic hospitalists are struggling with similar issues across the country. Drs. Adrienne Bennett, Brian Lucas, and Bob Wachter, led an enthralling but sobering session surveying the challenges facing academic hospitalist groups. In many cases the vision of developing a sustainable academic model around the core tenets of research, scholarly activity, and education is being undermined by the service mandate of non-teaching clinical work.
These tensions lead to profound challenges come promotion time, a topic that Drs. Scott Flanders, David Meltzer, and Sankey Williams covered in an afternoon session.
As a director of an academic program who recently went through the promotion process, I view these two issues as critical to the health of all segments of hospital medicine, not just academics. Community hospitalist groups will encounter even larger workforce deficiencies if future hospitalists (i.e., current residents) shy away from the field because they see academic hospitalists devalued as unpromotable resident extenders and academic second-class citizens.
Speaking of workforce shortages, I learned that several highly respect leaders in hospital medicine believe this to be one of the most significant factors threatening the field. In a plenary panel discussion, Drs. Ron Greeno, John Nelson, Mike Guthrie, and John Laverty, commented that overcoming the current and future hospitalist shortage requires rethinking the current model. Dr. Greeno highlighted the need to build more efficient care models whereby hospitalists could see more patients in the same time by reducing the high levels of busy work and administrative minutiae.
Other ideas centered on the development of midlevel provider hospitalists and limiting our scope of practice. To the latter point, there was a lively debate about just how much of the traditionally “non-medical” piece of the pie hospitalists should bite off. Eric Siegal, MD, tackled this issue in a later session challenging hospitalist groups to rethink the value of further expanding the co-management model to more surgical patients while we struggle to care for the patients for whom we currently care.
I learned that Drs. Nelson and Win Whitcomb, co-founders of SHM, showed tremendous vision in their founding of this society. I had the chance to have lunch with John, and I asked him if he ever imagined that the tiny group he brought together in San Diego 11 years ago would ever grow to this—20,000 hospitalists, 6,000 SHM members, an annual meeting with 1,600 people, and a hospitalist (Russell Holman, MD, past president of SHM) seated at the table of the most influential healthcare policy meetings in Washington, D.C.
Rather than being awestruck by the development of this field and SHM, he simply noted this is exactly what he and Win foresaw more than a decade ago; this is the reason they founded SHM. That’s the kind of vision that explains why the field of hospital medicine is the fastest-growing medical specialty.
I learned that the future of hospital medicine is being defined today. Nearly 200 posters were presented at the Research, Innovations and Clinical Vignettes (RIV) Competition. When we look back 10 years from now, we will see a mature field and wonder how we got there so quickly.
That future is being constructed today by folks like Ken Epstein, MD, who presented fascinating data on the effects of fragmentation of hospitalist care, and Param Dedhia, MD, who showed that a formalized discharge toolkit could reduce emergency visits and hospital readmissions in elderly patients.
Finally, while I was away in the city named after a saint, I learned that my wife, too, is a saint. Unable to travel with me, she was landlocked in Denver with our 6-month-old son. I, like many attendees, acknowledge the families who sacrificed so their loved ones could attend the meeting. While I was socializing, learning, networking, and teaching a session, my wife was home soothing tears, changing diapers, cleaning chinfuls of cereal, and answering 3 a.m. wakeup calls. On behalf of all attendees I say thanks to all the saints who enabled us to be away charting the course of hospital medicine at Hospital Medicine 2008. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
I just returned from the SHM annual meeting, and I am brimming with new knowledge. While we’ve tried to capture the essence of the meeting in this edition of The Hospitalist, it’s hard to describe in print the excitement, energy, and edification I encountered during those three days in San Diego.
Nearly 1,600 hospitalists descended on the Grand Hyatt on the San Diego Harbor for the six pre-courses April 3 and the two-day meeting April 4-5, marking the biggest and widest-reaching hospitalist meeting to date. Here’s just a smattering of what I learned in southern California.
I learned that Don Berwick, MD, is a healthcare visionary. In his plenary address the founder of the Institute for Healthcare Improvement and national leader of the patient safety movement gave his insights on the quality of the healthcare system and offered a challenge for hospitalists. He started by deftly outlining how Americans pay $3,000 more per capita for healthcare than other industrialized countries only to receive less access, worse care, and higher mortality rates. Clearly, more money does not equate to better care.
After noting that every system is perfectly designed to achieve the results it gets, Dr. Berwick challenged hospitalists to debunk the romantic view of the “individual as the cause of excellence” in favor of creating multidisciplinary teams and systems of care whose results do not depend on the heroism of the individual.
I learned that hospitalists are getting paid more today for the same amount of work they provided in 2005. At the same time that the average salary is up 13% to $193,300 (compared with 2005) the average number of annual encounters per hospitalist is down 4% from 2,558 in 2005 to 2,447 in 2007.
I’ve seen several interpretations of these data. The most cynical take, generally from non-hospitalists, is that this is further proof that hospitalists are overpaid compared with our non-hospitalist generalist colleagues in internal and family medicine. While these changes obviously represent a free-market response to a shortage of hospitalists, I firmly believe these higher salaries are a more accurate valuation of the work hospitalists do—and the more appropriate interpretation is that we’ve been underpaid in previous years. Now it’s time for our healthcare system to more appropriately reward our outpatient colleagues as well.
I also learned that academic hospitalists are struggling with similar issues across the country. Drs. Adrienne Bennett, Brian Lucas, and Bob Wachter, led an enthralling but sobering session surveying the challenges facing academic hospitalist groups. In many cases the vision of developing a sustainable academic model around the core tenets of research, scholarly activity, and education is being undermined by the service mandate of non-teaching clinical work.
These tensions lead to profound challenges come promotion time, a topic that Drs. Scott Flanders, David Meltzer, and Sankey Williams covered in an afternoon session.
As a director of an academic program who recently went through the promotion process, I view these two issues as critical to the health of all segments of hospital medicine, not just academics. Community hospitalist groups will encounter even larger workforce deficiencies if future hospitalists (i.e., current residents) shy away from the field because they see academic hospitalists devalued as unpromotable resident extenders and academic second-class citizens.
Speaking of workforce shortages, I learned that several highly respect leaders in hospital medicine believe this to be one of the most significant factors threatening the field. In a plenary panel discussion, Drs. Ron Greeno, John Nelson, Mike Guthrie, and John Laverty, commented that overcoming the current and future hospitalist shortage requires rethinking the current model. Dr. Greeno highlighted the need to build more efficient care models whereby hospitalists could see more patients in the same time by reducing the high levels of busy work and administrative minutiae.
Other ideas centered on the development of midlevel provider hospitalists and limiting our scope of practice. To the latter point, there was a lively debate about just how much of the traditionally “non-medical” piece of the pie hospitalists should bite off. Eric Siegal, MD, tackled this issue in a later session challenging hospitalist groups to rethink the value of further expanding the co-management model to more surgical patients while we struggle to care for the patients for whom we currently care.
I learned that Drs. Nelson and Win Whitcomb, co-founders of SHM, showed tremendous vision in their founding of this society. I had the chance to have lunch with John, and I asked him if he ever imagined that the tiny group he brought together in San Diego 11 years ago would ever grow to this—20,000 hospitalists, 6,000 SHM members, an annual meeting with 1,600 people, and a hospitalist (Russell Holman, MD, past president of SHM) seated at the table of the most influential healthcare policy meetings in Washington, D.C.
Rather than being awestruck by the development of this field and SHM, he simply noted this is exactly what he and Win foresaw more than a decade ago; this is the reason they founded SHM. That’s the kind of vision that explains why the field of hospital medicine is the fastest-growing medical specialty.
I learned that the future of hospital medicine is being defined today. Nearly 200 posters were presented at the Research, Innovations and Clinical Vignettes (RIV) Competition. When we look back 10 years from now, we will see a mature field and wonder how we got there so quickly.
That future is being constructed today by folks like Ken Epstein, MD, who presented fascinating data on the effects of fragmentation of hospitalist care, and Param Dedhia, MD, who showed that a formalized discharge toolkit could reduce emergency visits and hospital readmissions in elderly patients.
Finally, while I was away in the city named after a saint, I learned that my wife, too, is a saint. Unable to travel with me, she was landlocked in Denver with our 6-month-old son. I, like many attendees, acknowledge the families who sacrificed so their loved ones could attend the meeting. While I was socializing, learning, networking, and teaching a session, my wife was home soothing tears, changing diapers, cleaning chinfuls of cereal, and answering 3 a.m. wakeup calls. On behalf of all attendees I say thanks to all the saints who enabled us to be away charting the course of hospital medicine at Hospital Medicine 2008. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.