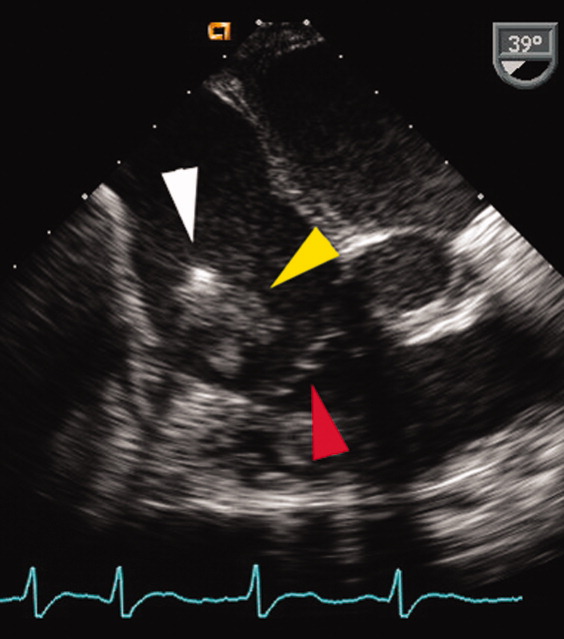
User login
REIMBURSEMENT ADVISER
If the patient can pinpoint which quadrant the pain is in, a better option is to report 789.0X (abdominal pain; the fifth [X] digit reports the site, such as left lower-quadrant or right upper-quadrant, etc.). Using this code more specifically identifies the complaint and location; I have found that fewer payers deny a US scan when this code is reported.
Problem with -52 modifier for US follicle evaluation
Can you comment on our coding strategies for these services?
Among payers that recognize -52, almost all put the claim into manual review before payment. If you are being paid a reduced amount, have you compared it with the reimbursement you might be getting by reporting 76857 instead? Note that neither code 76857 (which specifies checking for follicles) nor code 76815 (which specifies a limited exam such as you would perform for a quick cervical check on a pregnant patient) specifies the approach—in other words, the word “pelvic” does not imply strictly a transabdominal approach. These codes can therefore be used to report either an abdominal or transvaginal scan. In my opinion, either code more accurately describes the procedures that you are performing.
Dx/procedure mismatch when checking for fibroids
Also, understand that the duplex procedures are only reported when you are trying to characterize the pattern and direction of blood flow in arteries or veins. This year, CPT clarified that, although evaluation of vascular structures using both color and spectral Doppler is reportable separately, color Doppler alone, when performed for identification of anatomic structures in conjunction with a real-time US exam, cannot be reported separately.
Last, the code you are billing, 93975, represents a complete study. Examination of a single fibroid within the uterus constitutes a limited study, billed using 93976.
If the patient can pinpoint which quadrant the pain is in, a better option is to report 789.0X (abdominal pain; the fifth [X] digit reports the site, such as left lower-quadrant or right upper-quadrant, etc.). Using this code more specifically identifies the complaint and location; I have found that fewer payers deny a US scan when this code is reported.
Problem with -52 modifier for US follicle evaluation
Can you comment on our coding strategies for these services?
Among payers that recognize -52, almost all put the claim into manual review before payment. If you are being paid a reduced amount, have you compared it with the reimbursement you might be getting by reporting 76857 instead? Note that neither code 76857 (which specifies checking for follicles) nor code 76815 (which specifies a limited exam such as you would perform for a quick cervical check on a pregnant patient) specifies the approach—in other words, the word “pelvic” does not imply strictly a transabdominal approach. These codes can therefore be used to report either an abdominal or transvaginal scan. In my opinion, either code more accurately describes the procedures that you are performing.
Dx/procedure mismatch when checking for fibroids
Also, understand that the duplex procedures are only reported when you are trying to characterize the pattern and direction of blood flow in arteries or veins. This year, CPT clarified that, although evaluation of vascular structures using both color and spectral Doppler is reportable separately, color Doppler alone, when performed for identification of anatomic structures in conjunction with a real-time US exam, cannot be reported separately.
Last, the code you are billing, 93975, represents a complete study. Examination of a single fibroid within the uterus constitutes a limited study, billed using 93976.
If the patient can pinpoint which quadrant the pain is in, a better option is to report 789.0X (abdominal pain; the fifth [X] digit reports the site, such as left lower-quadrant or right upper-quadrant, etc.). Using this code more specifically identifies the complaint and location; I have found that fewer payers deny a US scan when this code is reported.
Problem with -52 modifier for US follicle evaluation
Can you comment on our coding strategies for these services?
Among payers that recognize -52, almost all put the claim into manual review before payment. If you are being paid a reduced amount, have you compared it with the reimbursement you might be getting by reporting 76857 instead? Note that neither code 76857 (which specifies checking for follicles) nor code 76815 (which specifies a limited exam such as you would perform for a quick cervical check on a pregnant patient) specifies the approach—in other words, the word “pelvic” does not imply strictly a transabdominal approach. These codes can therefore be used to report either an abdominal or transvaginal scan. In my opinion, either code more accurately describes the procedures that you are performing.
Dx/procedure mismatch when checking for fibroids
Also, understand that the duplex procedures are only reported when you are trying to characterize the pattern and direction of blood flow in arteries or veins. This year, CPT clarified that, although evaluation of vascular structures using both color and spectral Doppler is reportable separately, color Doppler alone, when performed for identification of anatomic structures in conjunction with a real-time US exam, cannot be reported separately.
Last, the code you are billing, 93975, represents a complete study. Examination of a single fibroid within the uterus constitutes a limited study, billed using 93976.
Researchers elucidate mechanism of heparin contaminant
Researchers have discovered the mechanism behind the deaths and adverse events that occurred in patients receiving contaminated heparin.
In March, a team led by Ram Sasisekharan, PhD, of Massachusetts Institute of Technology in Cambridge, identified the contaminant responsible for the numerous adverse events and 81 deaths that have occurred since November 2007 in patients receiving heparin.
Now, Dr Sasisekharan and colleagues have identified the mechanism by which the contaminant, oversulfated chondroitin sulfate (OSCS), works. This finding was published in New England Journal of Medicine April 24.
The researchers found that OSCS activated the kinin-kallikrein pathway in human plasma, which can lead to the generation of the potent vasoactive mediator bradykinin. In addition, OSCS induced generation of C3a and C5a, which are potent anaphylatoxins derived from complement proteins.
Dr Sasisekharan’s team arrived at these conclusions by testing 29 lots of heparin obtained from the FDA. Thirteen of these lots had been associated with adverse events. A laboratory lot was also included to serve as a control.
In a blinded fashion, the researchers screened the heparin for the existence of OSCS. They then tested the effects heparin contaminated with 19.3% wt/wt OSCS had on human plasma.
At 2.5 µg/mL and 25 µg/mL, contaminated heparin showed activation of kallikrein, while the same doses of uncontaminated heparin did not.
At 250 µg/mL, the contaminated heparin did not demonstrate activation of kallikrein. Dr Sasisekharan and colleagues said this suggests that, at a high concentration, heparin may inhibit or cause the depletion of factor XII.
The researchers next examined the contaminated heparin for its ability to generate C3a and C5a. At 5 µg/mL and 50 µg/mL, contaminated heparin generated C5a, whereas the same doses of uncontaminated heparin did not. At 500 µg/mL, the contaminated heparin did not generate significant amounts of C5a.
Dr Sasisekharan and colleagues also found that activation of C3a and C5a were linked and dependent upon fluid-phase activation of factor XII.
To ensure the accuracy of these results, the researchers created synthetic OSCS via chemical sulfonation of chondroitin sulfate. This synthetic OSCS behaved in the same manner as the OSCS found in the contaminated lots of heparin— demonstrating activation of kallikrein and generating C3a and C5a.
In an attempt to better understand the effects of OSCS, the team tested their results on swine. Swine were chosen because their reactions to contaminated heparin were similar to those observed in humans.
Each pig received an infusion of 5mg of one of the following substances: control heparin, contaminated heparin, chondroitin sulfate A, or synthetic OSCS. The researchers monitored the pigs’ vital signs for an hour before the animals were euthanized. The team collected blood samples at baseline and 5, 10, 20, 40, and 60 minutes.
Six pigs received contaminated heparin. Of these, 2 experienced at least a 30% drop in blood pressure within the first 30 minutes after infusion. One pig experienced hypotension for more than 15 minutes.
In the pigs that received synthetic OSCS, adverse events were more severe. This was expected, as the dose of OSCS in this group was higher than that in the group of pigs receiving contaminated heparin. All pigs given synthetic OSCS experienced a profound drop in blood pressure and an increased heart rate. One pig had difficulty breathing.
None of the pigs given control heparin or chondroitin sulfate A experienced any adverse events.
Dr Sasisekharan and colleagues said the results of this study suggest that a simple in vitro bioassay could complement the tests currently used in the screening of heparin. This bioassay would uncover the presence of OSCS and other polysulfated contaminants that might cause patients harm. ![]()
Researchers have discovered the mechanism behind the deaths and adverse events that occurred in patients receiving contaminated heparin.
In March, a team led by Ram Sasisekharan, PhD, of Massachusetts Institute of Technology in Cambridge, identified the contaminant responsible for the numerous adverse events and 81 deaths that have occurred since November 2007 in patients receiving heparin.
Now, Dr Sasisekharan and colleagues have identified the mechanism by which the contaminant, oversulfated chondroitin sulfate (OSCS), works. This finding was published in New England Journal of Medicine April 24.
The researchers found that OSCS activated the kinin-kallikrein pathway in human plasma, which can lead to the generation of the potent vasoactive mediator bradykinin. In addition, OSCS induced generation of C3a and C5a, which are potent anaphylatoxins derived from complement proteins.
Dr Sasisekharan’s team arrived at these conclusions by testing 29 lots of heparin obtained from the FDA. Thirteen of these lots had been associated with adverse events. A laboratory lot was also included to serve as a control.
In a blinded fashion, the researchers screened the heparin for the existence of OSCS. They then tested the effects heparin contaminated with 19.3% wt/wt OSCS had on human plasma.
At 2.5 µg/mL and 25 µg/mL, contaminated heparin showed activation of kallikrein, while the same doses of uncontaminated heparin did not.
At 250 µg/mL, the contaminated heparin did not demonstrate activation of kallikrein. Dr Sasisekharan and colleagues said this suggests that, at a high concentration, heparin may inhibit or cause the depletion of factor XII.
The researchers next examined the contaminated heparin for its ability to generate C3a and C5a. At 5 µg/mL and 50 µg/mL, contaminated heparin generated C5a, whereas the same doses of uncontaminated heparin did not. At 500 µg/mL, the contaminated heparin did not generate significant amounts of C5a.
Dr Sasisekharan and colleagues also found that activation of C3a and C5a were linked and dependent upon fluid-phase activation of factor XII.
To ensure the accuracy of these results, the researchers created synthetic OSCS via chemical sulfonation of chondroitin sulfate. This synthetic OSCS behaved in the same manner as the OSCS found in the contaminated lots of heparin— demonstrating activation of kallikrein and generating C3a and C5a.
In an attempt to better understand the effects of OSCS, the team tested their results on swine. Swine were chosen because their reactions to contaminated heparin were similar to those observed in humans.
Each pig received an infusion of 5mg of one of the following substances: control heparin, contaminated heparin, chondroitin sulfate A, or synthetic OSCS. The researchers monitored the pigs’ vital signs for an hour before the animals were euthanized. The team collected blood samples at baseline and 5, 10, 20, 40, and 60 minutes.
Six pigs received contaminated heparin. Of these, 2 experienced at least a 30% drop in blood pressure within the first 30 minutes after infusion. One pig experienced hypotension for more than 15 minutes.
In the pigs that received synthetic OSCS, adverse events were more severe. This was expected, as the dose of OSCS in this group was higher than that in the group of pigs receiving contaminated heparin. All pigs given synthetic OSCS experienced a profound drop in blood pressure and an increased heart rate. One pig had difficulty breathing.
None of the pigs given control heparin or chondroitin sulfate A experienced any adverse events.
Dr Sasisekharan and colleagues said the results of this study suggest that a simple in vitro bioassay could complement the tests currently used in the screening of heparin. This bioassay would uncover the presence of OSCS and other polysulfated contaminants that might cause patients harm. ![]()
Researchers have discovered the mechanism behind the deaths and adverse events that occurred in patients receiving contaminated heparin.
In March, a team led by Ram Sasisekharan, PhD, of Massachusetts Institute of Technology in Cambridge, identified the contaminant responsible for the numerous adverse events and 81 deaths that have occurred since November 2007 in patients receiving heparin.
Now, Dr Sasisekharan and colleagues have identified the mechanism by which the contaminant, oversulfated chondroitin sulfate (OSCS), works. This finding was published in New England Journal of Medicine April 24.
The researchers found that OSCS activated the kinin-kallikrein pathway in human plasma, which can lead to the generation of the potent vasoactive mediator bradykinin. In addition, OSCS induced generation of C3a and C5a, which are potent anaphylatoxins derived from complement proteins.
Dr Sasisekharan’s team arrived at these conclusions by testing 29 lots of heparin obtained from the FDA. Thirteen of these lots had been associated with adverse events. A laboratory lot was also included to serve as a control.
In a blinded fashion, the researchers screened the heparin for the existence of OSCS. They then tested the effects heparin contaminated with 19.3% wt/wt OSCS had on human plasma.
At 2.5 µg/mL and 25 µg/mL, contaminated heparin showed activation of kallikrein, while the same doses of uncontaminated heparin did not.
At 250 µg/mL, the contaminated heparin did not demonstrate activation of kallikrein. Dr Sasisekharan and colleagues said this suggests that, at a high concentration, heparin may inhibit or cause the depletion of factor XII.
The researchers next examined the contaminated heparin for its ability to generate C3a and C5a. At 5 µg/mL and 50 µg/mL, contaminated heparin generated C5a, whereas the same doses of uncontaminated heparin did not. At 500 µg/mL, the contaminated heparin did not generate significant amounts of C5a.
Dr Sasisekharan and colleagues also found that activation of C3a and C5a were linked and dependent upon fluid-phase activation of factor XII.
To ensure the accuracy of these results, the researchers created synthetic OSCS via chemical sulfonation of chondroitin sulfate. This synthetic OSCS behaved in the same manner as the OSCS found in the contaminated lots of heparin— demonstrating activation of kallikrein and generating C3a and C5a.
In an attempt to better understand the effects of OSCS, the team tested their results on swine. Swine were chosen because their reactions to contaminated heparin were similar to those observed in humans.
Each pig received an infusion of 5mg of one of the following substances: control heparin, contaminated heparin, chondroitin sulfate A, or synthetic OSCS. The researchers monitored the pigs’ vital signs for an hour before the animals were euthanized. The team collected blood samples at baseline and 5, 10, 20, 40, and 60 minutes.
Six pigs received contaminated heparin. Of these, 2 experienced at least a 30% drop in blood pressure within the first 30 minutes after infusion. One pig experienced hypotension for more than 15 minutes.
In the pigs that received synthetic OSCS, adverse events were more severe. This was expected, as the dose of OSCS in this group was higher than that in the group of pigs receiving contaminated heparin. All pigs given synthetic OSCS experienced a profound drop in blood pressure and an increased heart rate. One pig had difficulty breathing.
None of the pigs given control heparin or chondroitin sulfate A experienced any adverse events.
Dr Sasisekharan and colleagues said the results of this study suggest that a simple in vitro bioassay could complement the tests currently used in the screening of heparin. This bioassay would uncover the presence of OSCS and other polysulfated contaminants that might cause patients harm. ![]()
ASHP–SHM Statement on Hospitalist–Pharmacist Collaboration
POSITION
The American Society of Health‐System Pharmacists (ASHP) and the Society of Hospital Medicine (SHM) believe that the rapidly emerging hospitalist model of inpatient care offers new and significant opportunities to optimize patient care through collaboration among hospitalists, hospital pharmacists (hereinafter, pharmacists), and other health care providers. The emerging model of care allows for deeper professional relationships among health care providers and promotes a shared interest in and responsibility for direct patient care, indirect patient care, and service activities. ASHP and SHM encourage hospitalists, pharmacists, and health care executives to seek out ways to foster collaboration between hospitalists and pharmacists.
The purpose of this consensus statement is to promote an understanding of the ways hospitalists and pharmacists can jointly optimize the care provided to patients in hospitals, examine opportunities for improving hospitalistpharmacist alliances that enhance patient care, suggest future directions for collaboration, and identify aspects of such collaboration that warrant further research.
BACKGROUND
Increases in health care spending and the expanding influence of managed care in the late 1980s and early 1990s resulted in calls for more efficient health care. The movement toward greater efficiency resulted in more emphasis on ambulatory care, fewer hospital admissions, shortened hospital stays, and an overall increase in the acuity of illness of hospitalized patients. The emphasis on ambulatory care increased the number and complexity of physician office visits, and the changing characteristics of office‐ and hospital‐based care placed significant demands on primary care physicians and contributed to the rise of hospital medicine.
In 1996, the term hospitalist was introduced into the health care lexicon.1 A hospitalist was defined as an inpatient physician who manages the care of hospitalized patients and facilitates the transfer of their care back to the primary care physician. The Society of Hospital Medicine has since defined a hospitalist as a physician whose primary professional focus is the general medical care of hospitalized patients and whose activities may include patient care, teaching, research, and leadership related to hospital medicine.2
The past decade has seen rapid growth of the number of hospitalists and the use of hospitalists by US hospitals.3 In 2005, 70% of hospitals with more than 200 beds used hospitalist services, and there were more than 16,000 hospitalists in practice.4 An estimated 20,000 hospitalists were practicing at more than 2600 US hospitals in 2007.5
Initially, many physicians expressed concern about the potential for hospitalists to interfere in the relationship between the patient and the primary care physician, as well as about the potential negative impact on continuity of care.6 However, subsequent studies demonstrated increasing acceptance of hospitalists by primary care physicians, with as many as 89% considering the hospitalist model to be superior to the historical model of hospital care provided by primary care physicians or by specialists working on rotations.7, 8 Numerous studies demonstrate the value of hospitalists in improving quality of care, decreasing hospital costs and length of stay, and reducing hospital readmissions.921
As early as 1921, hospital pharmacists in the American Pharmaceutical Association (now the American Pharmacists Association) had formed a committee to address their distinct concerns. During the 1930s, hospital pharmacists began to organize state organizations and to adhere to a set of minimum standards of practice. In 1942, the American Society of Hospital Pharmacists (now the American Society of Health‐System Pharmacists) was formed to establish minimum standards of pharmaceutical services in hospitals, provide interchange among pharmacists, promote new pharmaceutical techniques, and aid the medical profession in extending the economic and rational use of medications.22 As of 2005, there were approximately 50,000 pharmacists practicing in US hospitals.23
The modern mission of hospital pharmacy departments is to ensure optimal outcomes from the use of medicines.24 Although the focus of hospital pharmacy has traditionally been on the safe dispensing of medications, direct patient care by pharmacists (clinical pharmacy) has always been a component of hospital pharmacy practice. Following the rise of pharmaceutical care in the 1980s,25 these pharmacist services have expanded greatly. It has been estimated that 35%‐40% of hospital pharmacists are devoted to providing clinical services.23 A systematic review in 2006 documented improved outcomes when clinical pharmacists interacted with the health care team on patient rounds, interviewed patients, reconciled medications, and provided discharge counseling and follow‐up.26 These findings support those of other studies in which specific clinical pharmacy services were associated with improved therapeutic and economic outcomes.2731
OPPORTUNITIES FOR COLLABORATION BETWEEN PHARMACISTS AND HOSPITALISTS
Pharmacists and hospitalists have shared interests that provide strong incentives for collaboration. All health care professionals share, first, a commitment to and responsibility for providing safe and effective patient care. Physicians, pharmacists, and other health care providers have long collaborated in providing direct patient care. The emerging hospitalist model of care offers more opportunities for collaboration because pharmacists and hospitalists also share interest in and responsibility for indirect patient care and service activitiesdeveloping the institutional policies, processes, and infrastructure that support patient care.
Direct patient care activities typically performed by hospitalists include obtaining patient histories, conducting physical examinations, making diagnoses, developing treatment plans, monitoring patients' responses to therapy, performing follow‐up hospital visits, participating in family meetings, and providing discharge instructions.32 Specific clinical pharmacy services that have been associated with improved health care outcomes include providing drug information, managing medication protocols and adverse drug reactions, participating in medical rounds, gathering admission medication histories, interviewing patients, reconciling patient medications, and providing discharge counseling and follow‐up.2631
Pharmacists should be involved in the care of hospitalized patients and can collaborate with hospitalists in numerous ways, including:
-
Providing consultative services that foster appropriate, evidence‐based medication selection (eg, during rounds),
-
Providing drug information to physicians, nurses, and other clinicians,
-
Managing medication protocols under collaborative practice agreements,
-
Assisting in the development of treatment protocols,
-
Monitoring therapeutic responses (including laboratory test results),
-
Continuously assessing for and managing adverse drug reactions,
-
Gathering medication histories,
-
Reconciling medications as patients move across the continuum of hospital care, and
-
Providing patient and caretaker education, including discharge counseling and follow‐up.
Both hospitalists and pharmacists have a responsibility to ensure continuity as patients move across settings of care.
In addition to their direct patient care activities, hospitalists add value through their efforts in hospital service activities, student and resident education, and research. Typical service activities include participating in quality‐improvement and safety initiatives, developing institutional guidelines and protocols for the treatment of specific diseases, serving on hospital committees (eg, the pharmacy and therapeutics [P&T] committee), and working with others to introduce new technologies to the hospital setting.33, 34
Pharmacists also participate in hospital service activities, education, and research. For example, pharmacists serve on the P&T committee and are directly involved in managing the formulary system that guides an institution's medication use. As medication experts, pharmacists contribute to the development and implementation of patient care guidelines and other medication‐use policies. Pharmacist expertise is also integral to many quality‐improvement efforts (eg, surgical infection prophylaxis) and to technology initiatives (eg, bedside medication scanning and computerized prescriber‐order‐entry systems). Pharmacist provision of in‐service education on medications and medication use is invaluable for all health care providers.
These overlapping responsibilities provide hospitalists and pharmacists with opportunities to collaborate on activities that can have a profound effect on care in the hospital. Hospitalists and pharmacists can work together to ensure that care is evidence based, cost‐effective, and adherent to national guidelines; establish an institutional culture of safety; develop and implement quality‐improvement initiatives; meet accreditation standards; and, in many cases, foster the institution's education and research initiatives. Health professional education and research offers the opportunity to improve patient care provided not just by a single hospital but by other facilities as well.
OPPORTUNITIES TO IMPROVE COLLABORATION
ASHP and SHM believe that there are opportunities for improving collaboration between hospitalists and pharmacists. Barriers to collaboration include real and perceived professional boundaries, poor integration of technology systems, inadequate pharmacist and hospitalist staffing, time constraints, inadequate funding and resources, lack of third‐party compensation for clinical pharmacy services, and the competing obligations weighing on both professions.
Real and perceived professional boundaries can be addressed by clear communication and by enhanced interdisciplinary educational opportunities for all members of the health care team.3538 ASHP and SHM believe that while hospitalists should serve as the primary leaders of hospital care teams, all health care professionals should be willing to assume a leadership role in treating patients and, when appropriate, accept leadership by other team members. Like all members of the care team, pharmacists require timely access to hospitalists for consultation, as well as access to patient information. The vital flow of information and communication among health care providers should be conducive to collaborating and improving patient outcomes. ASHP and SHM believe that properly applied, well‐integrated technologies (eg, electronic medical records and personal digital assistants with clinical decision support systems, including drug information) can enhance communication among all members of the health care team.
Hospitalists and pharmacists can work together to overcome limitations created by inadequate funding and staffing by providing evidence to health care executives of the value of clinical pharmacist positions and pharmacisthospitalist collaboration. This evidence should examine the impact of these positions and such collaboration on therapeutic, safety, humanistic, and economic outcomes. Collaboration among all members of the health care team would also be encouraged by reforming the current fee‐for‐service reimbursement practices to base payment for care delivery on overall treatment goals (eg, a payment rate based on diagnosis).
CONCLUSIONS
An interdisciplinary approach to health care that includes physicians, pharmacists, nurses, and other health care professionals will improve the quality of patient care. Hospitalists and pharmacists need to collaborate with each other and with other health care professionals to optimize outcomes in hospitalized patients. ASHP and SHM believe that hospitalistpharmacist alliances should be encouraged and that the systems and technologies that enable collaboration and the incentives for such collaboration should be enhanced.
Acknowledgements
The following individuals and organizations are acknowledged for reviewing draft versions of this statement: Nicole M. Allcock, PharmD, BCPS; American Academy of Physician Assistants (AAPA); American Nurses Association (ANA); American Society of Consultant Pharmacists (ASCP); Philip Anderson, PharmD, FASHP; Linda C. Annecchini, MS, FASHP; John A. Armitstead, MS, FASHP; Carol Bickford, PhD. (ANA); Michael L. Brandt, BS, PharmD; John Bridges, PharmD; Tim R. Brown, PharmD; Gail M. Burniske, PharmD, BCPS; Margaret Chrymko, PharmD, FASHP; Steve Crane (AAPA); Karren Crowson, MBA; Lourdes M. Cuellar, MS, FASHP; Michele Danish, PharmD; Neil Davis; Jean Douglas, PharmD; Jillian James Foster, PharmD; Georgia W. Fox, PharmD; Nicole Gara (AAPA); Kathleen M. Gura, PharmD, BCNSP, FASHP; Stuart T. Haines, PharmD, FCCP, FASHP; Tom Hall, PharmD; John Hertig; Philip E. Johnson, MS, FASHP; Thomas J. Johnson, PharmD, BCPS; Michael Kelly, PharmD; Patricia Kienle, MPA, FASHP; Kathrin C. Kucharski, PharmD, BCPS; Sharon Kulesz (AAPA); Timothy R. Lanese, MBA, FASHP, FACHE; Bob McNellis, MPH, PA (AAPA); Joe Miller, MD (SHM); Rima Mohammad, PharmD, BCPS; Lynette R. Moser, PharmD; Joe E. Ness, MHA; Scott Oxenhandler, MD; Charles D. Ponte, PharmD, BC‐ADM, BCPS, CDE, FAPhA, FASHP, FCCP; James A. Ponto, MS, BCNP, FASHP; Michael D. Sanborn, MS; Phil Saucedo, MBA; Kenneth H. Schell, PharmD, FASHP, FCSHP; Edward C. Seidl, PharmD; Michele F. Shepherd, PharmD, MS, BCPS, FASHP; Jonalan Smith, PharmD (ASCP); Kelly M. Smith, PharmD; Miriam A. Mobley Smith, PharmD; Edward Stemley, MS, PharmD; Joe Strain, PharmD; James A. Trovato, PharmD, MBA, BCOP; Jennifer Tryon, PharmD, MS; Laura Wachter, BS, PharmD; William E. Wade, PharmD, FASHP, FCCP; Paul C. Walker, PharmD; Larry Wellikson, MD (SHM); Karl G. Williams, JD, MS; and John L. Woon, PharmD, FASHP.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed May 29,2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- AHA Hospital Statistics.Chicago:American Hospital Association;2005.
- Hospital medicine specialty shows 20 percent growth. SHM analysis of 2005 American Hospital Association survey data. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases130:368–372.
- ,,, et al.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160:2902–2908.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,, et al.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,, et al.Implementation of a hospitalist system in a large health maintenance organization: the Kaiser Permanente experience.Ann Intern Med.1999;130:355–359.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,, et al.Comparison of hospitalists and primary care internists in the care of patients with pneumonia.J Gen Intern Med.1999;14(suppl):S118.
- ,,, et al.Comparing hospitalists' and community‐based primary care physicians' care of patients with pneumonia.J Gen Intern Med.2001;16(suppl):S215.
- ,,, et al.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105:478–484.
- ,,, et al.Outcomes of hospitalization in pediatric patients insured by HMOs: comparison of care by hospitalists and traditional academic providers.Pediatr Res.2000;47:204A. Abstract.
- ,,, et al.Impact of a managed care hospitalist system in academic pediatrics.Pediatr Res.2000;47:228A. Abstract.
- ,,, et al.Cost savings for patients with acute conditions cared for by pediatric hospitalists in a tertiary care center.Pediatr Res.2001;49:125A. Abstract.
- .Overview of the history of pharmacy in the United States. In:Brown TR, ed.Handbook of Institutional Pharmacy Practice.Bethesda, MD:American Society of Health‐System Pharmacists;2006:19–32.
- ,,.ASHP national survey of pharmacy practice in hospital settings: dispensing and administration—2005.Am J Health‐Syst Pharm.2006;63:327–345.
- .Perspectives on Hilton Head.Am J Hosp Pharm.1986;43:1439–1443.
- American Society of Hospital Pharmacists.ASHP statement on pharmaceutical care.Am J Hosp Pharm.1993;50:1720–1723.
- ,,, et al.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,.Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing.Pharmacotherapy.2001;21:129–141.
- ,,.Clinical pharmacy services, hospital pharmacy staffing, and medication errors in United States hospitals.Pharmacotherapy.2002;22:134–147.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Evidence of the economic benefit of clinical pharmacy services: 1996‐2000.Pharmacotherapy.2003;23:113–132.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,, et al.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- Committee on the Health Professions Education Summit.Health professions education: a bridge to quality.Washington, DC:National Academy Press;2003.
- ,,, et al.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001:31:228–237.
- ,,.Multiprofessional learning: the attitudes of medical, nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,.Team working: palliative care as a model of interdisciplinary practice.Med J Aust.2003;179:S32–S34.
POSITION
The American Society of Health‐System Pharmacists (ASHP) and the Society of Hospital Medicine (SHM) believe that the rapidly emerging hospitalist model of inpatient care offers new and significant opportunities to optimize patient care through collaboration among hospitalists, hospital pharmacists (hereinafter, pharmacists), and other health care providers. The emerging model of care allows for deeper professional relationships among health care providers and promotes a shared interest in and responsibility for direct patient care, indirect patient care, and service activities. ASHP and SHM encourage hospitalists, pharmacists, and health care executives to seek out ways to foster collaboration between hospitalists and pharmacists.
The purpose of this consensus statement is to promote an understanding of the ways hospitalists and pharmacists can jointly optimize the care provided to patients in hospitals, examine opportunities for improving hospitalistpharmacist alliances that enhance patient care, suggest future directions for collaboration, and identify aspects of such collaboration that warrant further research.
BACKGROUND
Increases in health care spending and the expanding influence of managed care in the late 1980s and early 1990s resulted in calls for more efficient health care. The movement toward greater efficiency resulted in more emphasis on ambulatory care, fewer hospital admissions, shortened hospital stays, and an overall increase in the acuity of illness of hospitalized patients. The emphasis on ambulatory care increased the number and complexity of physician office visits, and the changing characteristics of office‐ and hospital‐based care placed significant demands on primary care physicians and contributed to the rise of hospital medicine.
In 1996, the term hospitalist was introduced into the health care lexicon.1 A hospitalist was defined as an inpatient physician who manages the care of hospitalized patients and facilitates the transfer of their care back to the primary care physician. The Society of Hospital Medicine has since defined a hospitalist as a physician whose primary professional focus is the general medical care of hospitalized patients and whose activities may include patient care, teaching, research, and leadership related to hospital medicine.2
The past decade has seen rapid growth of the number of hospitalists and the use of hospitalists by US hospitals.3 In 2005, 70% of hospitals with more than 200 beds used hospitalist services, and there were more than 16,000 hospitalists in practice.4 An estimated 20,000 hospitalists were practicing at more than 2600 US hospitals in 2007.5
Initially, many physicians expressed concern about the potential for hospitalists to interfere in the relationship between the patient and the primary care physician, as well as about the potential negative impact on continuity of care.6 However, subsequent studies demonstrated increasing acceptance of hospitalists by primary care physicians, with as many as 89% considering the hospitalist model to be superior to the historical model of hospital care provided by primary care physicians or by specialists working on rotations.7, 8 Numerous studies demonstrate the value of hospitalists in improving quality of care, decreasing hospital costs and length of stay, and reducing hospital readmissions.921
As early as 1921, hospital pharmacists in the American Pharmaceutical Association (now the American Pharmacists Association) had formed a committee to address their distinct concerns. During the 1930s, hospital pharmacists began to organize state organizations and to adhere to a set of minimum standards of practice. In 1942, the American Society of Hospital Pharmacists (now the American Society of Health‐System Pharmacists) was formed to establish minimum standards of pharmaceutical services in hospitals, provide interchange among pharmacists, promote new pharmaceutical techniques, and aid the medical profession in extending the economic and rational use of medications.22 As of 2005, there were approximately 50,000 pharmacists practicing in US hospitals.23
The modern mission of hospital pharmacy departments is to ensure optimal outcomes from the use of medicines.24 Although the focus of hospital pharmacy has traditionally been on the safe dispensing of medications, direct patient care by pharmacists (clinical pharmacy) has always been a component of hospital pharmacy practice. Following the rise of pharmaceutical care in the 1980s,25 these pharmacist services have expanded greatly. It has been estimated that 35%‐40% of hospital pharmacists are devoted to providing clinical services.23 A systematic review in 2006 documented improved outcomes when clinical pharmacists interacted with the health care team on patient rounds, interviewed patients, reconciled medications, and provided discharge counseling and follow‐up.26 These findings support those of other studies in which specific clinical pharmacy services were associated with improved therapeutic and economic outcomes.2731
OPPORTUNITIES FOR COLLABORATION BETWEEN PHARMACISTS AND HOSPITALISTS
Pharmacists and hospitalists have shared interests that provide strong incentives for collaboration. All health care professionals share, first, a commitment to and responsibility for providing safe and effective patient care. Physicians, pharmacists, and other health care providers have long collaborated in providing direct patient care. The emerging hospitalist model of care offers more opportunities for collaboration because pharmacists and hospitalists also share interest in and responsibility for indirect patient care and service activitiesdeveloping the institutional policies, processes, and infrastructure that support patient care.
Direct patient care activities typically performed by hospitalists include obtaining patient histories, conducting physical examinations, making diagnoses, developing treatment plans, monitoring patients' responses to therapy, performing follow‐up hospital visits, participating in family meetings, and providing discharge instructions.32 Specific clinical pharmacy services that have been associated with improved health care outcomes include providing drug information, managing medication protocols and adverse drug reactions, participating in medical rounds, gathering admission medication histories, interviewing patients, reconciling patient medications, and providing discharge counseling and follow‐up.2631
Pharmacists should be involved in the care of hospitalized patients and can collaborate with hospitalists in numerous ways, including:
-
Providing consultative services that foster appropriate, evidence‐based medication selection (eg, during rounds),
-
Providing drug information to physicians, nurses, and other clinicians,
-
Managing medication protocols under collaborative practice agreements,
-
Assisting in the development of treatment protocols,
-
Monitoring therapeutic responses (including laboratory test results),
-
Continuously assessing for and managing adverse drug reactions,
-
Gathering medication histories,
-
Reconciling medications as patients move across the continuum of hospital care, and
-
Providing patient and caretaker education, including discharge counseling and follow‐up.
Both hospitalists and pharmacists have a responsibility to ensure continuity as patients move across settings of care.
In addition to their direct patient care activities, hospitalists add value through their efforts in hospital service activities, student and resident education, and research. Typical service activities include participating in quality‐improvement and safety initiatives, developing institutional guidelines and protocols for the treatment of specific diseases, serving on hospital committees (eg, the pharmacy and therapeutics [P&T] committee), and working with others to introduce new technologies to the hospital setting.33, 34
Pharmacists also participate in hospital service activities, education, and research. For example, pharmacists serve on the P&T committee and are directly involved in managing the formulary system that guides an institution's medication use. As medication experts, pharmacists contribute to the development and implementation of patient care guidelines and other medication‐use policies. Pharmacist expertise is also integral to many quality‐improvement efforts (eg, surgical infection prophylaxis) and to technology initiatives (eg, bedside medication scanning and computerized prescriber‐order‐entry systems). Pharmacist provision of in‐service education on medications and medication use is invaluable for all health care providers.
These overlapping responsibilities provide hospitalists and pharmacists with opportunities to collaborate on activities that can have a profound effect on care in the hospital. Hospitalists and pharmacists can work together to ensure that care is evidence based, cost‐effective, and adherent to national guidelines; establish an institutional culture of safety; develop and implement quality‐improvement initiatives; meet accreditation standards; and, in many cases, foster the institution's education and research initiatives. Health professional education and research offers the opportunity to improve patient care provided not just by a single hospital but by other facilities as well.
OPPORTUNITIES TO IMPROVE COLLABORATION
ASHP and SHM believe that there are opportunities for improving collaboration between hospitalists and pharmacists. Barriers to collaboration include real and perceived professional boundaries, poor integration of technology systems, inadequate pharmacist and hospitalist staffing, time constraints, inadequate funding and resources, lack of third‐party compensation for clinical pharmacy services, and the competing obligations weighing on both professions.
Real and perceived professional boundaries can be addressed by clear communication and by enhanced interdisciplinary educational opportunities for all members of the health care team.3538 ASHP and SHM believe that while hospitalists should serve as the primary leaders of hospital care teams, all health care professionals should be willing to assume a leadership role in treating patients and, when appropriate, accept leadership by other team members. Like all members of the care team, pharmacists require timely access to hospitalists for consultation, as well as access to patient information. The vital flow of information and communication among health care providers should be conducive to collaborating and improving patient outcomes. ASHP and SHM believe that properly applied, well‐integrated technologies (eg, electronic medical records and personal digital assistants with clinical decision support systems, including drug information) can enhance communication among all members of the health care team.
Hospitalists and pharmacists can work together to overcome limitations created by inadequate funding and staffing by providing evidence to health care executives of the value of clinical pharmacist positions and pharmacisthospitalist collaboration. This evidence should examine the impact of these positions and such collaboration on therapeutic, safety, humanistic, and economic outcomes. Collaboration among all members of the health care team would also be encouraged by reforming the current fee‐for‐service reimbursement practices to base payment for care delivery on overall treatment goals (eg, a payment rate based on diagnosis).
CONCLUSIONS
An interdisciplinary approach to health care that includes physicians, pharmacists, nurses, and other health care professionals will improve the quality of patient care. Hospitalists and pharmacists need to collaborate with each other and with other health care professionals to optimize outcomes in hospitalized patients. ASHP and SHM believe that hospitalistpharmacist alliances should be encouraged and that the systems and technologies that enable collaboration and the incentives for such collaboration should be enhanced.
Acknowledgements
The following individuals and organizations are acknowledged for reviewing draft versions of this statement: Nicole M. Allcock, PharmD, BCPS; American Academy of Physician Assistants (AAPA); American Nurses Association (ANA); American Society of Consultant Pharmacists (ASCP); Philip Anderson, PharmD, FASHP; Linda C. Annecchini, MS, FASHP; John A. Armitstead, MS, FASHP; Carol Bickford, PhD. (ANA); Michael L. Brandt, BS, PharmD; John Bridges, PharmD; Tim R. Brown, PharmD; Gail M. Burniske, PharmD, BCPS; Margaret Chrymko, PharmD, FASHP; Steve Crane (AAPA); Karren Crowson, MBA; Lourdes M. Cuellar, MS, FASHP; Michele Danish, PharmD; Neil Davis; Jean Douglas, PharmD; Jillian James Foster, PharmD; Georgia W. Fox, PharmD; Nicole Gara (AAPA); Kathleen M. Gura, PharmD, BCNSP, FASHP; Stuart T. Haines, PharmD, FCCP, FASHP; Tom Hall, PharmD; John Hertig; Philip E. Johnson, MS, FASHP; Thomas J. Johnson, PharmD, BCPS; Michael Kelly, PharmD; Patricia Kienle, MPA, FASHP; Kathrin C. Kucharski, PharmD, BCPS; Sharon Kulesz (AAPA); Timothy R. Lanese, MBA, FASHP, FACHE; Bob McNellis, MPH, PA (AAPA); Joe Miller, MD (SHM); Rima Mohammad, PharmD, BCPS; Lynette R. Moser, PharmD; Joe E. Ness, MHA; Scott Oxenhandler, MD; Charles D. Ponte, PharmD, BC‐ADM, BCPS, CDE, FAPhA, FASHP, FCCP; James A. Ponto, MS, BCNP, FASHP; Michael D. Sanborn, MS; Phil Saucedo, MBA; Kenneth H. Schell, PharmD, FASHP, FCSHP; Edward C. Seidl, PharmD; Michele F. Shepherd, PharmD, MS, BCPS, FASHP; Jonalan Smith, PharmD (ASCP); Kelly M. Smith, PharmD; Miriam A. Mobley Smith, PharmD; Edward Stemley, MS, PharmD; Joe Strain, PharmD; James A. Trovato, PharmD, MBA, BCOP; Jennifer Tryon, PharmD, MS; Laura Wachter, BS, PharmD; William E. Wade, PharmD, FASHP, FCCP; Paul C. Walker, PharmD; Larry Wellikson, MD (SHM); Karl G. Williams, JD, MS; and John L. Woon, PharmD, FASHP.
POSITION
The American Society of Health‐System Pharmacists (ASHP) and the Society of Hospital Medicine (SHM) believe that the rapidly emerging hospitalist model of inpatient care offers new and significant opportunities to optimize patient care through collaboration among hospitalists, hospital pharmacists (hereinafter, pharmacists), and other health care providers. The emerging model of care allows for deeper professional relationships among health care providers and promotes a shared interest in and responsibility for direct patient care, indirect patient care, and service activities. ASHP and SHM encourage hospitalists, pharmacists, and health care executives to seek out ways to foster collaboration between hospitalists and pharmacists.
The purpose of this consensus statement is to promote an understanding of the ways hospitalists and pharmacists can jointly optimize the care provided to patients in hospitals, examine opportunities for improving hospitalistpharmacist alliances that enhance patient care, suggest future directions for collaboration, and identify aspects of such collaboration that warrant further research.
BACKGROUND
Increases in health care spending and the expanding influence of managed care in the late 1980s and early 1990s resulted in calls for more efficient health care. The movement toward greater efficiency resulted in more emphasis on ambulatory care, fewer hospital admissions, shortened hospital stays, and an overall increase in the acuity of illness of hospitalized patients. The emphasis on ambulatory care increased the number and complexity of physician office visits, and the changing characteristics of office‐ and hospital‐based care placed significant demands on primary care physicians and contributed to the rise of hospital medicine.
In 1996, the term hospitalist was introduced into the health care lexicon.1 A hospitalist was defined as an inpatient physician who manages the care of hospitalized patients and facilitates the transfer of their care back to the primary care physician. The Society of Hospital Medicine has since defined a hospitalist as a physician whose primary professional focus is the general medical care of hospitalized patients and whose activities may include patient care, teaching, research, and leadership related to hospital medicine.2
The past decade has seen rapid growth of the number of hospitalists and the use of hospitalists by US hospitals.3 In 2005, 70% of hospitals with more than 200 beds used hospitalist services, and there were more than 16,000 hospitalists in practice.4 An estimated 20,000 hospitalists were practicing at more than 2600 US hospitals in 2007.5
Initially, many physicians expressed concern about the potential for hospitalists to interfere in the relationship between the patient and the primary care physician, as well as about the potential negative impact on continuity of care.6 However, subsequent studies demonstrated increasing acceptance of hospitalists by primary care physicians, with as many as 89% considering the hospitalist model to be superior to the historical model of hospital care provided by primary care physicians or by specialists working on rotations.7, 8 Numerous studies demonstrate the value of hospitalists in improving quality of care, decreasing hospital costs and length of stay, and reducing hospital readmissions.921
As early as 1921, hospital pharmacists in the American Pharmaceutical Association (now the American Pharmacists Association) had formed a committee to address their distinct concerns. During the 1930s, hospital pharmacists began to organize state organizations and to adhere to a set of minimum standards of practice. In 1942, the American Society of Hospital Pharmacists (now the American Society of Health‐System Pharmacists) was formed to establish minimum standards of pharmaceutical services in hospitals, provide interchange among pharmacists, promote new pharmaceutical techniques, and aid the medical profession in extending the economic and rational use of medications.22 As of 2005, there were approximately 50,000 pharmacists practicing in US hospitals.23
The modern mission of hospital pharmacy departments is to ensure optimal outcomes from the use of medicines.24 Although the focus of hospital pharmacy has traditionally been on the safe dispensing of medications, direct patient care by pharmacists (clinical pharmacy) has always been a component of hospital pharmacy practice. Following the rise of pharmaceutical care in the 1980s,25 these pharmacist services have expanded greatly. It has been estimated that 35%‐40% of hospital pharmacists are devoted to providing clinical services.23 A systematic review in 2006 documented improved outcomes when clinical pharmacists interacted with the health care team on patient rounds, interviewed patients, reconciled medications, and provided discharge counseling and follow‐up.26 These findings support those of other studies in which specific clinical pharmacy services were associated with improved therapeutic and economic outcomes.2731
OPPORTUNITIES FOR COLLABORATION BETWEEN PHARMACISTS AND HOSPITALISTS
Pharmacists and hospitalists have shared interests that provide strong incentives for collaboration. All health care professionals share, first, a commitment to and responsibility for providing safe and effective patient care. Physicians, pharmacists, and other health care providers have long collaborated in providing direct patient care. The emerging hospitalist model of care offers more opportunities for collaboration because pharmacists and hospitalists also share interest in and responsibility for indirect patient care and service activitiesdeveloping the institutional policies, processes, and infrastructure that support patient care.
Direct patient care activities typically performed by hospitalists include obtaining patient histories, conducting physical examinations, making diagnoses, developing treatment plans, monitoring patients' responses to therapy, performing follow‐up hospital visits, participating in family meetings, and providing discharge instructions.32 Specific clinical pharmacy services that have been associated with improved health care outcomes include providing drug information, managing medication protocols and adverse drug reactions, participating in medical rounds, gathering admission medication histories, interviewing patients, reconciling patient medications, and providing discharge counseling and follow‐up.2631
Pharmacists should be involved in the care of hospitalized patients and can collaborate with hospitalists in numerous ways, including:
-
Providing consultative services that foster appropriate, evidence‐based medication selection (eg, during rounds),
-
Providing drug information to physicians, nurses, and other clinicians,
-
Managing medication protocols under collaborative practice agreements,
-
Assisting in the development of treatment protocols,
-
Monitoring therapeutic responses (including laboratory test results),
-
Continuously assessing for and managing adverse drug reactions,
-
Gathering medication histories,
-
Reconciling medications as patients move across the continuum of hospital care, and
-
Providing patient and caretaker education, including discharge counseling and follow‐up.
Both hospitalists and pharmacists have a responsibility to ensure continuity as patients move across settings of care.
In addition to their direct patient care activities, hospitalists add value through their efforts in hospital service activities, student and resident education, and research. Typical service activities include participating in quality‐improvement and safety initiatives, developing institutional guidelines and protocols for the treatment of specific diseases, serving on hospital committees (eg, the pharmacy and therapeutics [P&T] committee), and working with others to introduce new technologies to the hospital setting.33, 34
Pharmacists also participate in hospital service activities, education, and research. For example, pharmacists serve on the P&T committee and are directly involved in managing the formulary system that guides an institution's medication use. As medication experts, pharmacists contribute to the development and implementation of patient care guidelines and other medication‐use policies. Pharmacist expertise is also integral to many quality‐improvement efforts (eg, surgical infection prophylaxis) and to technology initiatives (eg, bedside medication scanning and computerized prescriber‐order‐entry systems). Pharmacist provision of in‐service education on medications and medication use is invaluable for all health care providers.
These overlapping responsibilities provide hospitalists and pharmacists with opportunities to collaborate on activities that can have a profound effect on care in the hospital. Hospitalists and pharmacists can work together to ensure that care is evidence based, cost‐effective, and adherent to national guidelines; establish an institutional culture of safety; develop and implement quality‐improvement initiatives; meet accreditation standards; and, in many cases, foster the institution's education and research initiatives. Health professional education and research offers the opportunity to improve patient care provided not just by a single hospital but by other facilities as well.
OPPORTUNITIES TO IMPROVE COLLABORATION
ASHP and SHM believe that there are opportunities for improving collaboration between hospitalists and pharmacists. Barriers to collaboration include real and perceived professional boundaries, poor integration of technology systems, inadequate pharmacist and hospitalist staffing, time constraints, inadequate funding and resources, lack of third‐party compensation for clinical pharmacy services, and the competing obligations weighing on both professions.
Real and perceived professional boundaries can be addressed by clear communication and by enhanced interdisciplinary educational opportunities for all members of the health care team.3538 ASHP and SHM believe that while hospitalists should serve as the primary leaders of hospital care teams, all health care professionals should be willing to assume a leadership role in treating patients and, when appropriate, accept leadership by other team members. Like all members of the care team, pharmacists require timely access to hospitalists for consultation, as well as access to patient information. The vital flow of information and communication among health care providers should be conducive to collaborating and improving patient outcomes. ASHP and SHM believe that properly applied, well‐integrated technologies (eg, electronic medical records and personal digital assistants with clinical decision support systems, including drug information) can enhance communication among all members of the health care team.
Hospitalists and pharmacists can work together to overcome limitations created by inadequate funding and staffing by providing evidence to health care executives of the value of clinical pharmacist positions and pharmacisthospitalist collaboration. This evidence should examine the impact of these positions and such collaboration on therapeutic, safety, humanistic, and economic outcomes. Collaboration among all members of the health care team would also be encouraged by reforming the current fee‐for‐service reimbursement practices to base payment for care delivery on overall treatment goals (eg, a payment rate based on diagnosis).
CONCLUSIONS
An interdisciplinary approach to health care that includes physicians, pharmacists, nurses, and other health care professionals will improve the quality of patient care. Hospitalists and pharmacists need to collaborate with each other and with other health care professionals to optimize outcomes in hospitalized patients. ASHP and SHM believe that hospitalistpharmacist alliances should be encouraged and that the systems and technologies that enable collaboration and the incentives for such collaboration should be enhanced.
Acknowledgements
The following individuals and organizations are acknowledged for reviewing draft versions of this statement: Nicole M. Allcock, PharmD, BCPS; American Academy of Physician Assistants (AAPA); American Nurses Association (ANA); American Society of Consultant Pharmacists (ASCP); Philip Anderson, PharmD, FASHP; Linda C. Annecchini, MS, FASHP; John A. Armitstead, MS, FASHP; Carol Bickford, PhD. (ANA); Michael L. Brandt, BS, PharmD; John Bridges, PharmD; Tim R. Brown, PharmD; Gail M. Burniske, PharmD, BCPS; Margaret Chrymko, PharmD, FASHP; Steve Crane (AAPA); Karren Crowson, MBA; Lourdes M. Cuellar, MS, FASHP; Michele Danish, PharmD; Neil Davis; Jean Douglas, PharmD; Jillian James Foster, PharmD; Georgia W. Fox, PharmD; Nicole Gara (AAPA); Kathleen M. Gura, PharmD, BCNSP, FASHP; Stuart T. Haines, PharmD, FCCP, FASHP; Tom Hall, PharmD; John Hertig; Philip E. Johnson, MS, FASHP; Thomas J. Johnson, PharmD, BCPS; Michael Kelly, PharmD; Patricia Kienle, MPA, FASHP; Kathrin C. Kucharski, PharmD, BCPS; Sharon Kulesz (AAPA); Timothy R. Lanese, MBA, FASHP, FACHE; Bob McNellis, MPH, PA (AAPA); Joe Miller, MD (SHM); Rima Mohammad, PharmD, BCPS; Lynette R. Moser, PharmD; Joe E. Ness, MHA; Scott Oxenhandler, MD; Charles D. Ponte, PharmD, BC‐ADM, BCPS, CDE, FAPhA, FASHP, FCCP; James A. Ponto, MS, BCNP, FASHP; Michael D. Sanborn, MS; Phil Saucedo, MBA; Kenneth H. Schell, PharmD, FASHP, FCSHP; Edward C. Seidl, PharmD; Michele F. Shepherd, PharmD, MS, BCPS, FASHP; Jonalan Smith, PharmD (ASCP); Kelly M. Smith, PharmD; Miriam A. Mobley Smith, PharmD; Edward Stemley, MS, PharmD; Joe Strain, PharmD; James A. Trovato, PharmD, MBA, BCOP; Jennifer Tryon, PharmD, MS; Laura Wachter, BS, PharmD; William E. Wade, PharmD, FASHP, FCCP; Paul C. Walker, PharmD; Larry Wellikson, MD (SHM); Karl G. Williams, JD, MS; and John L. Woon, PharmD, FASHP.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed May 29,2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- AHA Hospital Statistics.Chicago:American Hospital Association;2005.
- Hospital medicine specialty shows 20 percent growth. SHM analysis of 2005 American Hospital Association survey data. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases130:368–372.
- ,,, et al.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160:2902–2908.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,, et al.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,, et al.Implementation of a hospitalist system in a large health maintenance organization: the Kaiser Permanente experience.Ann Intern Med.1999;130:355–359.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,, et al.Comparison of hospitalists and primary care internists in the care of patients with pneumonia.J Gen Intern Med.1999;14(suppl):S118.
- ,,, et al.Comparing hospitalists' and community‐based primary care physicians' care of patients with pneumonia.J Gen Intern Med.2001;16(suppl):S215.
- ,,, et al.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105:478–484.
- ,,, et al.Outcomes of hospitalization in pediatric patients insured by HMOs: comparison of care by hospitalists and traditional academic providers.Pediatr Res.2000;47:204A. Abstract.
- ,,, et al.Impact of a managed care hospitalist system in academic pediatrics.Pediatr Res.2000;47:228A. Abstract.
- ,,, et al.Cost savings for patients with acute conditions cared for by pediatric hospitalists in a tertiary care center.Pediatr Res.2001;49:125A. Abstract.
- .Overview of the history of pharmacy in the United States. In:Brown TR, ed.Handbook of Institutional Pharmacy Practice.Bethesda, MD:American Society of Health‐System Pharmacists;2006:19–32.
- ,,.ASHP national survey of pharmacy practice in hospital settings: dispensing and administration—2005.Am J Health‐Syst Pharm.2006;63:327–345.
- .Perspectives on Hilton Head.Am J Hosp Pharm.1986;43:1439–1443.
- American Society of Hospital Pharmacists.ASHP statement on pharmaceutical care.Am J Hosp Pharm.1993;50:1720–1723.
- ,,, et al.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,.Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing.Pharmacotherapy.2001;21:129–141.
- ,,.Clinical pharmacy services, hospital pharmacy staffing, and medication errors in United States hospitals.Pharmacotherapy.2002;22:134–147.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Evidence of the economic benefit of clinical pharmacy services: 1996‐2000.Pharmacotherapy.2003;23:113–132.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,, et al.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- Committee on the Health Professions Education Summit.Health professions education: a bridge to quality.Washington, DC:National Academy Press;2003.
- ,,, et al.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001:31:228–237.
- ,,.Multiprofessional learning: the attitudes of medical, nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,.Team working: palliative care as a model of interdisciplinary practice.Med J Aust.2003;179:S32–S34.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Definition of a hospitalist. Available at: www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed May 29,2007.
- ,,, et al.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- AHA Hospital Statistics.Chicago:American Hospital Association;2005.
- Hospital medicine specialty shows 20 percent growth. SHM analysis of 2005 American Hospital Association survey data. Available at: www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases130:368–372.
- ,,, et al.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109:648–653.
- ,,, et al.Friend or foe? How primary care physicians perceive hospitalists.Arch Intern Med.2000;160:2902–2908.
- ,,, et al.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,, et al.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,, et al.Implementation of a hospitalist system in a large health maintenance organization: the Kaiser Permanente experience.Ann Intern Med.1999;130:355–359.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,, et al.Comparison of hospitalists and primary care internists in the care of patients with pneumonia.J Gen Intern Med.1999;14(suppl):S118.
- ,,, et al.Comparing hospitalists' and community‐based primary care physicians' care of patients with pneumonia.J Gen Intern Med.2001;16(suppl):S215.
- ,,, et al.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,, et al.The impact of an inpatient physician program on quality, utilization, and satisfaction.Am J Manag Care.2000;6:549–555.
- ,.Evaluation of a pediatric hospitalist service: impact on length of stay and hospital charges.Pediatrics.2000;105:478–484.
- ,,, et al.Outcomes of hospitalization in pediatric patients insured by HMOs: comparison of care by hospitalists and traditional academic providers.Pediatr Res.2000;47:204A. Abstract.
- ,,, et al.Impact of a managed care hospitalist system in academic pediatrics.Pediatr Res.2000;47:228A. Abstract.
- ,,, et al.Cost savings for patients with acute conditions cared for by pediatric hospitalists in a tertiary care center.Pediatr Res.2001;49:125A. Abstract.
- .Overview of the history of pharmacy in the United States. In:Brown TR, ed.Handbook of Institutional Pharmacy Practice.Bethesda, MD:American Society of Health‐System Pharmacists;2006:19–32.
- ,,.ASHP national survey of pharmacy practice in hospital settings: dispensing and administration—2005.Am J Health‐Syst Pharm.2006;63:327–345.
- .Perspectives on Hilton Head.Am J Hosp Pharm.1986;43:1439–1443.
- American Society of Hospital Pharmacists.ASHP statement on pharmaceutical care.Am J Hosp Pharm.1993;50:1720–1723.
- ,,, et al.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,.Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing.Pharmacotherapy.2001;21:129–141.
- ,,.Clinical pharmacy services, hospital pharmacy staffing, and medication errors in United States hospitals.Pharmacotherapy.2002;22:134–147.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Evidence of the economic benefit of clinical pharmacy services: 1996‐2000.Pharmacotherapy.2003;23:113–132.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,, et al.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111:247–254.
- Committee on the Health Professions Education Summit.Health professions education: a bridge to quality.Washington, DC:National Academy Press;2003.
- ,,, et al.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001:31:228–237.
- ,,.Multiprofessional learning: the attitudes of medical, nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,.Team working: palliative care as a model of interdisciplinary practice.Med J Aust.2003;179:S32–S34.
Handoffs
It had been a turbulent year. Death and disease in the family had taken a toll on my personal life. Though I was a newlywed, life was anything but bliss. That month I was the resident in the cardiac intensive care unit (CICU); a challenging rotation, where sleep was a luxury and the long nights on call added to the strain on my relationship with my wife. It was on one of those nights that I met Mr. and Mrs. Dubinski.
Mr. Dubinski was a pleasant man who looked younger than his 75 years. He had been brought to the hospital because his implantable cardioverter defibrillator (ICD) had fired twice that night. He was in good spirits and chatting amiably with his son. I asked him how he was doing. His pleasant expression changed to a worried one. I have been rather upset for the last few days, worried about my wife, he said.
It turned out that over the last few days Mrs. Dubinski had not been feeling well. This had troubled Mr. Dubinski, and he was often preoccupied with concerns about her. The couple had been married 55 years and had never spent a day apart. They had waited to seek medical advice. Her pain was intermittent, and they thought it would pass; they had some appointments coming up, and they thought they could wait it out. That night, Mrs. Dubinski had a particularly severe episode of pain that bothered her greatly and worried Mr. Dubinski even more. He said that he felt as though he was beginning to pass out, and as he began to faint, he felt a funny feeling in his chest. He had never had a shock from the ICD before, and he didn't know what happened. He sat down to compose himself and felt the same funny feeling in his chest again and also felt lightheaded. He described it, saying, I felt like I was going to explode from the inside. Concerned about his unusual symptoms and her worsening pain, Mr. and Mrs. Dubinski decided to come to the hospital.
Mr. Dubinski's electrocardiogram revealed many premature ventricular complexes (PVCs), and I suspected that one of these had triggered a malignant arrhythmia, which resulted in the device firing. He would need monitoring, and his ICD would be interrogated in the morning to ensure that it was functioning properly. I reassured Mr. Dubinski that the device seemed to have done what it was meant to do. It had almost certainly saved his life. He was relieved to hear this but wanted me to reassure his wife that even though he was going to the CICU, he was all right and it was nothing serious.
As I was wheeling Mr. Dubinski up, I walked past the nurse taking care of his wife. She pulled me aside for a moment and said, Looks like you'll be taking her, too; her troponin just came back at 5.96.
Mrs. Dubinski was a thin, older woman who looked uncomfortable. For about a week, she had been experiencing intermittent pain in her chest and abdomen and just felt that something was not right. Tonight her chest pain did not get better spontaneously, and she had a particularly long episode of pain that radiated to her left arm. She said she felt like she was going to explode from the inside. It was uncanny how she used the same words and expressions that her husband did. I suppose after 55 years of marriage, it should not have been surprising to me, but it was. When they had gotten to the emergency room Mrs. Dubinski had told the doctor about her own complaints. He ordered an electrocardiogram, which showed subtle changes consistent with myocardial ischemia. Her lab data confirmed that she was having a heart attack.
Mrs. Dubinski asked me what was going on. I gently explained to her that she was having a small heart attack. The stuttering episodes of chest pain in the past week probably meant that it had been coming on for a few days now. We could see some evidence of heart damage in her blood tests and the subtle changes in her electrocardiogram. I expected her to ask me more questions about the heart attack or what we were going to next. Instead, she said, Please don't tell my husband. It will only worry him more. I reassured her that I understood her concerns and told her that she was also going to be admitted to the CICU. She was fine with this, more worried about her husband than herself. Once in the CICU I kept my word to Mrs. Dubinski and told Mr. Dubinski a partial truththat his wife was being admitted for observation because we were worried about her.
I was genuinely touched by the deep bond between Mr. and Mrs. Dubinski. It amazed me to see that a man's heart could be stimulated by his wife's suffering in such a way that would have taken his life if not for his ICD. One could say that Mr. Dubinski was anxious about his wife's health, which led to an increased sympathetic drive and higher catecholamine levels. But as a young man at the beginning of a relationship with my wife, I thought there was much more here. Tonight, perhaps, because he cared so deeply, a PVC occurred right during the vulnerable period of the cardiac cycle in a person with a vulnerable heart, and a potentially lethal ventricular arrhythmia had ensued. And tonight my heart was also vulnerable, and I was moved. I thought of all the storms they must have weathered in their 55 years together and the love they had forged. It gave me hope for my own fledgling marriage and made me hope that one day my wife and I would be able to look back on many years of life together like Mr. and Mrs. Dubinski could, with 2 hearts beating as 1.
I had the privilege to know this couple for only 1 call night. By the time I was back on the CICU, Mrs. Dubinski had been transferred to another facility for angioplasty, and Mr. Dubinski had been discharged. Yet that was enough time for me to take part in the care of 2 amazing people and to witness the majesty of their love.
Note: Dubinski is a fictitious name.
It had been a turbulent year. Death and disease in the family had taken a toll on my personal life. Though I was a newlywed, life was anything but bliss. That month I was the resident in the cardiac intensive care unit (CICU); a challenging rotation, where sleep was a luxury and the long nights on call added to the strain on my relationship with my wife. It was on one of those nights that I met Mr. and Mrs. Dubinski.
Mr. Dubinski was a pleasant man who looked younger than his 75 years. He had been brought to the hospital because his implantable cardioverter defibrillator (ICD) had fired twice that night. He was in good spirits and chatting amiably with his son. I asked him how he was doing. His pleasant expression changed to a worried one. I have been rather upset for the last few days, worried about my wife, he said.
It turned out that over the last few days Mrs. Dubinski had not been feeling well. This had troubled Mr. Dubinski, and he was often preoccupied with concerns about her. The couple had been married 55 years and had never spent a day apart. They had waited to seek medical advice. Her pain was intermittent, and they thought it would pass; they had some appointments coming up, and they thought they could wait it out. That night, Mrs. Dubinski had a particularly severe episode of pain that bothered her greatly and worried Mr. Dubinski even more. He said that he felt as though he was beginning to pass out, and as he began to faint, he felt a funny feeling in his chest. He had never had a shock from the ICD before, and he didn't know what happened. He sat down to compose himself and felt the same funny feeling in his chest again and also felt lightheaded. He described it, saying, I felt like I was going to explode from the inside. Concerned about his unusual symptoms and her worsening pain, Mr. and Mrs. Dubinski decided to come to the hospital.
Mr. Dubinski's electrocardiogram revealed many premature ventricular complexes (PVCs), and I suspected that one of these had triggered a malignant arrhythmia, which resulted in the device firing. He would need monitoring, and his ICD would be interrogated in the morning to ensure that it was functioning properly. I reassured Mr. Dubinski that the device seemed to have done what it was meant to do. It had almost certainly saved his life. He was relieved to hear this but wanted me to reassure his wife that even though he was going to the CICU, he was all right and it was nothing serious.
As I was wheeling Mr. Dubinski up, I walked past the nurse taking care of his wife. She pulled me aside for a moment and said, Looks like you'll be taking her, too; her troponin just came back at 5.96.
Mrs. Dubinski was a thin, older woman who looked uncomfortable. For about a week, she had been experiencing intermittent pain in her chest and abdomen and just felt that something was not right. Tonight her chest pain did not get better spontaneously, and she had a particularly long episode of pain that radiated to her left arm. She said she felt like she was going to explode from the inside. It was uncanny how she used the same words and expressions that her husband did. I suppose after 55 years of marriage, it should not have been surprising to me, but it was. When they had gotten to the emergency room Mrs. Dubinski had told the doctor about her own complaints. He ordered an electrocardiogram, which showed subtle changes consistent with myocardial ischemia. Her lab data confirmed that she was having a heart attack.
Mrs. Dubinski asked me what was going on. I gently explained to her that she was having a small heart attack. The stuttering episodes of chest pain in the past week probably meant that it had been coming on for a few days now. We could see some evidence of heart damage in her blood tests and the subtle changes in her electrocardiogram. I expected her to ask me more questions about the heart attack or what we were going to next. Instead, she said, Please don't tell my husband. It will only worry him more. I reassured her that I understood her concerns and told her that she was also going to be admitted to the CICU. She was fine with this, more worried about her husband than herself. Once in the CICU I kept my word to Mrs. Dubinski and told Mr. Dubinski a partial truththat his wife was being admitted for observation because we were worried about her.
I was genuinely touched by the deep bond between Mr. and Mrs. Dubinski. It amazed me to see that a man's heart could be stimulated by his wife's suffering in such a way that would have taken his life if not for his ICD. One could say that Mr. Dubinski was anxious about his wife's health, which led to an increased sympathetic drive and higher catecholamine levels. But as a young man at the beginning of a relationship with my wife, I thought there was much more here. Tonight, perhaps, because he cared so deeply, a PVC occurred right during the vulnerable period of the cardiac cycle in a person with a vulnerable heart, and a potentially lethal ventricular arrhythmia had ensued. And tonight my heart was also vulnerable, and I was moved. I thought of all the storms they must have weathered in their 55 years together and the love they had forged. It gave me hope for my own fledgling marriage and made me hope that one day my wife and I would be able to look back on many years of life together like Mr. and Mrs. Dubinski could, with 2 hearts beating as 1.
I had the privilege to know this couple for only 1 call night. By the time I was back on the CICU, Mrs. Dubinski had been transferred to another facility for angioplasty, and Mr. Dubinski had been discharged. Yet that was enough time for me to take part in the care of 2 amazing people and to witness the majesty of their love.
Note: Dubinski is a fictitious name.
It had been a turbulent year. Death and disease in the family had taken a toll on my personal life. Though I was a newlywed, life was anything but bliss. That month I was the resident in the cardiac intensive care unit (CICU); a challenging rotation, where sleep was a luxury and the long nights on call added to the strain on my relationship with my wife. It was on one of those nights that I met Mr. and Mrs. Dubinski.
Mr. Dubinski was a pleasant man who looked younger than his 75 years. He had been brought to the hospital because his implantable cardioverter defibrillator (ICD) had fired twice that night. He was in good spirits and chatting amiably with his son. I asked him how he was doing. His pleasant expression changed to a worried one. I have been rather upset for the last few days, worried about my wife, he said.
It turned out that over the last few days Mrs. Dubinski had not been feeling well. This had troubled Mr. Dubinski, and he was often preoccupied with concerns about her. The couple had been married 55 years and had never spent a day apart. They had waited to seek medical advice. Her pain was intermittent, and they thought it would pass; they had some appointments coming up, and they thought they could wait it out. That night, Mrs. Dubinski had a particularly severe episode of pain that bothered her greatly and worried Mr. Dubinski even more. He said that he felt as though he was beginning to pass out, and as he began to faint, he felt a funny feeling in his chest. He had never had a shock from the ICD before, and he didn't know what happened. He sat down to compose himself and felt the same funny feeling in his chest again and also felt lightheaded. He described it, saying, I felt like I was going to explode from the inside. Concerned about his unusual symptoms and her worsening pain, Mr. and Mrs. Dubinski decided to come to the hospital.
Mr. Dubinski's electrocardiogram revealed many premature ventricular complexes (PVCs), and I suspected that one of these had triggered a malignant arrhythmia, which resulted in the device firing. He would need monitoring, and his ICD would be interrogated in the morning to ensure that it was functioning properly. I reassured Mr. Dubinski that the device seemed to have done what it was meant to do. It had almost certainly saved his life. He was relieved to hear this but wanted me to reassure his wife that even though he was going to the CICU, he was all right and it was nothing serious.
As I was wheeling Mr. Dubinski up, I walked past the nurse taking care of his wife. She pulled me aside for a moment and said, Looks like you'll be taking her, too; her troponin just came back at 5.96.
Mrs. Dubinski was a thin, older woman who looked uncomfortable. For about a week, she had been experiencing intermittent pain in her chest and abdomen and just felt that something was not right. Tonight her chest pain did not get better spontaneously, and she had a particularly long episode of pain that radiated to her left arm. She said she felt like she was going to explode from the inside. It was uncanny how she used the same words and expressions that her husband did. I suppose after 55 years of marriage, it should not have been surprising to me, but it was. When they had gotten to the emergency room Mrs. Dubinski had told the doctor about her own complaints. He ordered an electrocardiogram, which showed subtle changes consistent with myocardial ischemia. Her lab data confirmed that she was having a heart attack.
Mrs. Dubinski asked me what was going on. I gently explained to her that she was having a small heart attack. The stuttering episodes of chest pain in the past week probably meant that it had been coming on for a few days now. We could see some evidence of heart damage in her blood tests and the subtle changes in her electrocardiogram. I expected her to ask me more questions about the heart attack or what we were going to next. Instead, she said, Please don't tell my husband. It will only worry him more. I reassured her that I understood her concerns and told her that she was also going to be admitted to the CICU. She was fine with this, more worried about her husband than herself. Once in the CICU I kept my word to Mrs. Dubinski and told Mr. Dubinski a partial truththat his wife was being admitted for observation because we were worried about her.
I was genuinely touched by the deep bond between Mr. and Mrs. Dubinski. It amazed me to see that a man's heart could be stimulated by his wife's suffering in such a way that would have taken his life if not for his ICD. One could say that Mr. Dubinski was anxious about his wife's health, which led to an increased sympathetic drive and higher catecholamine levels. But as a young man at the beginning of a relationship with my wife, I thought there was much more here. Tonight, perhaps, because he cared so deeply, a PVC occurred right during the vulnerable period of the cardiac cycle in a person with a vulnerable heart, and a potentially lethal ventricular arrhythmia had ensued. And tonight my heart was also vulnerable, and I was moved. I thought of all the storms they must have weathered in their 55 years together and the love they had forged. It gave me hope for my own fledgling marriage and made me hope that one day my wife and I would be able to look back on many years of life together like Mr. and Mrs. Dubinski could, with 2 hearts beating as 1.
I had the privilege to know this couple for only 1 call night. By the time I was back on the CICU, Mrs. Dubinski had been transferred to another facility for angioplasty, and Mr. Dubinski had been discharged. Yet that was enough time for me to take part in the care of 2 amazing people and to witness the majesty of their love.
Note: Dubinski is a fictitious name.
Dexmedetomidine for Sedation in Children
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.
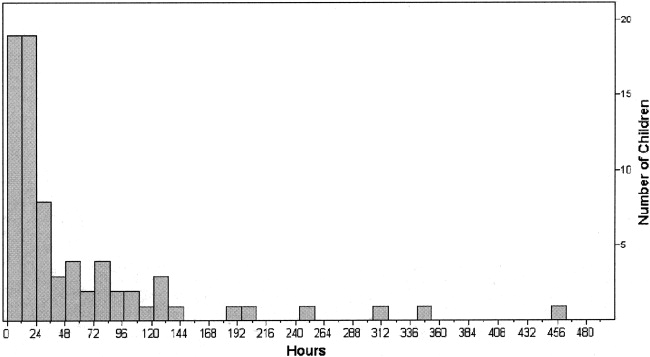
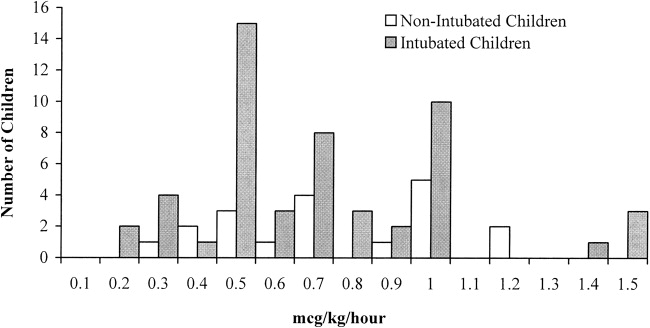
| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.


| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.


| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
Copyright © 2008 Society of Hospital Medicine
Obstructive Jaundice
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |
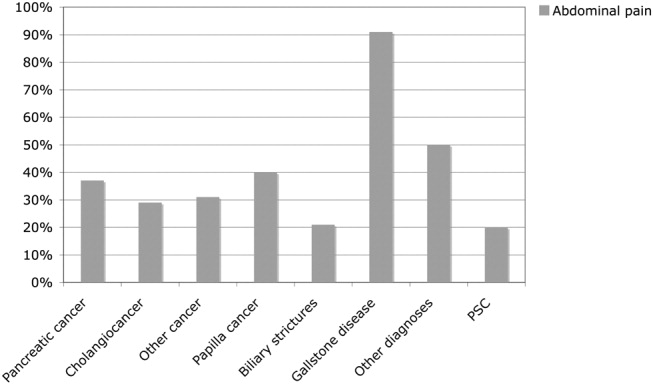
Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |
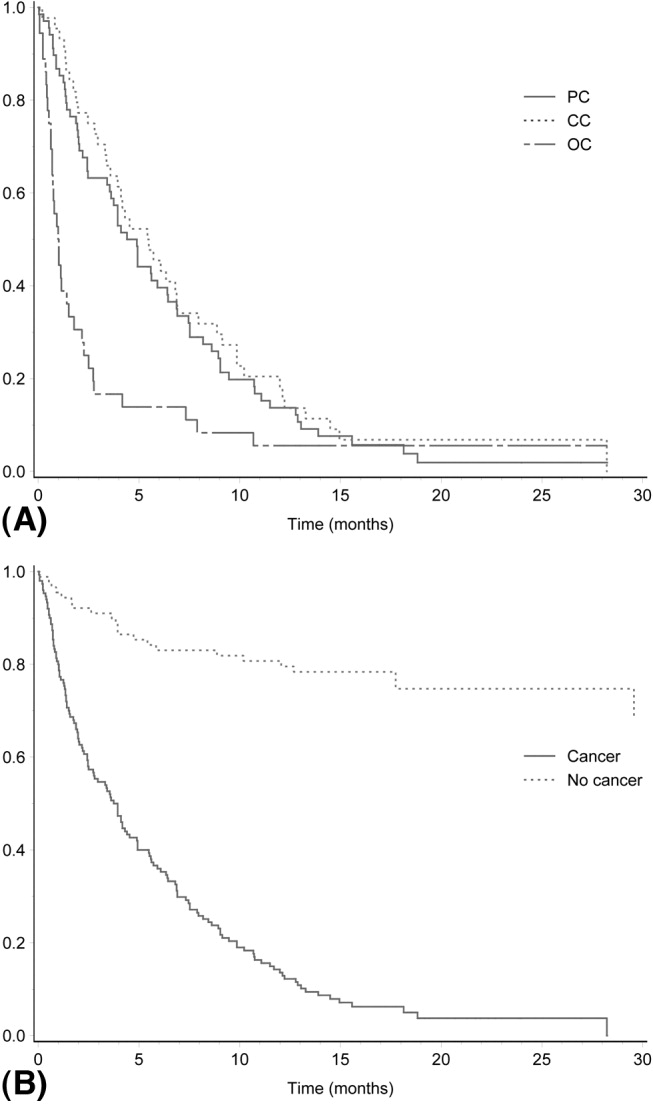
DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |

Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |

DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |

Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |

DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
Copyright © 2008 Society of Hospital Medicine
Hospital Mortality of Schizophrenics
Previous studies have found that the total mortality of schizophrenic patients was higher than that of the general population.14 The all‐cause mortality for schizophrenic patients was 2 to 4 times that of the general population.57 Further, not only was the mortality of schizophrenic patients from suicide and traumatic causes higher than that of the general population, but mortality from natural causes was higher as well.8, 9 Of the specific causes of death of schizophrenic patients, suicide was the most important.10 In one study, suicidal mortality was approximately 20 times that of the general population.1 For natural causes, the most common cause of death was diseases of the circulatory system, followed by diseases of the respiratory system, the digestive system, and malignant neoplasm.2, 11 Overall, schizophrenic patients had a life expectancy about 20% shorter than that of the general population.1
However, few studies have investigated mortality among schizophrenic patients who were admitted to acute care hospitals. Studies involving large samples of inpatients with schizophrenia in general (nonpsychiatric) hospitals have also been scarce. The specific causes of death of hospitalized schizophrenic patients have not been investigated thoroughly. In addition, little is known about risk factors associated with the mortality of schizophrenic patients during acute care hospitalization. In this study, we analyzed hospital mortality of schizophrenic patients admitted to an acute care community teaching hospital and compared mortality between schizophrenic and nonschizophrenic patients. Our mortality data included patients presenting with suicidal attempts who died during their admission. We also determined significant factors associated with this increased mortality and the specific causes of death of these patients.
METHODS
Study Patients
We studied all patients admitted to Okinawa Chubu Hospital from January 1, 1987, to December 31, 2004. Okinawa Chubu is a community teaching hospital that provides primary to tertiary care to a population of approximately 400,000 in Okinawa, Japan. Nearly all patients admitted during the study period were Japanese (more than 99.5%).
We identified all schizophrenic patients through the computerized inpatient registry. This registry includes the hospital discharge summary electronic database, which is updated and reviewed by certified hospital record technicians using the ICD‐9 coding scheme (International Classification of Diseases, 9th Revision, and Clinical Modification). The diagnosis of schizophrenia was documented by staff psychiatrists, who provided basic psychiatric care for these patients in inpatient or outpatient settings. The study was approved by the Institutional Review Board of Okinawa Chubu Hospital.
Data Collection
We obtained basic demographic data on all patients admitted during the study period using the computerized inpatient registry. Data were extracted on demographics, discharge outcome (survival or nonsurvival), route of admission, direct transfer from psychiatric hospitals, admitting department, intensive care unit admission, liaison psychiatrist consultation, length of hospital stay (days), and diagnostic classifications based on the ICD‐9. Routes of admission included the emergency department (ED) and outpatient clinic. From the computerized record, we also determined causes of death of schizophrenic as well as nonschizophrenic patients. We performed a manual chart review to determine specific causes of death of schizophrenic patients because the administrative data included the ICD‐9 coding scheme for admitting diagnosis but did not include information on specific causes of death.
Statistical Analysis
We obtained the standardized mortality ratio of schizophrenic patients compared with general patient population during each admission year and calculated the 95% confidence interval (95% CI) using sex‐stratified 5‐year bands and the exact Poisson distribution method. We also analyzed risk factors associated with hospital mortality. In unadjusted logistic regression analyses, we calculated the odds ratios (ORs) and 95% CIs of the demographic and clinical variables for hospital mortality among all schizophrenic patients. We then performed multivariable‐adjusted logistic regression analysis to identify significant risk factors associated with increased hospital mortality, adjusted for demographic and clinical variables. A 2‐sided P value < .05 was considered statistically significant. All statistical analyses were performed using Stata Software Version 8.2 (StataCorp LP, College Station, TX).
RESULTS
We identified a total of 189,049 general nonschizophrenic patients admitted to Okinawa Chubu Hospital during the 18‐year period (Table 1). Of these, 7528 patients died during hospitalization, with an overall hospital mortality rate of 4.0% (95% CI, 3.9%4.1%). There were 55 deaths among 1108 schizophrenic patients admitted to Okinawa Chubu Hospital during the same period. Schizophrenic patients had an overall hospital mortality rate of 5.0% (95% CI, 3.8%6.4%). The hospital mortality rate was 4.4% (95% CI, 2.9%6.5%) for schizophrenic women and 5.5% (95% CI, 3.8%7.7%) for schizophrenic men.
| Clinical characteristic | Schizophrenics n = 1108 | Non‐schizophrenics n = 189049 | p‐value** |
|---|---|---|---|
| |||
| Age, mean (SD) | 48.7 (14.2) | 40.0 (28.8) | <0.001 |
| Women, n (%) | 543 (49.0) | 101218 (53.5) | 0.0026 |
| Transferred from psychiatric hospital, n (%) | 254 (22.9) | N/A | |
| Admission through ED, n (%) | 853 (77.0) | 111109 (58.8) | <0.001 |
| Admitted department, n (%) | |||
| Internal medicine | 643 (58.0) | 65657 (34.7) | |
| General surgery | 222 (20.0) | 43036 (22.8) | |
| Other departments | 243 (21.9) | 80356 (42.5) | |
| Intensive care unit admission, n (%) | 157 (14.2) | 9900 (5.2) | <0.001 |
| Liaison psychiatrist consultation, n (%) | 239 (21.6) | N/A | |
| Length of hospital stay (days), mean (SD) | 25.2 (44.1) | 18.0 (42.1) | <0.001 |
| Admission diagnosis, n (%) | |||
| Infectious diseases without organ involvement | 13 (1.2) | 8009 (4.2) | |
| Malignant neoplasms | 92 (8.3) | 18481 (9.8) | |
| Endocrine, nutritional and metabolic diseases | 63 (5.7) | 3389 (1.8) | |
| Hematologic diseases | 11 (1.0) | 1364 (0.7) | |
| Mental diseases except schizophrenia | 32 (2.9) | 673 (0.4) | |
| Diseases of the nervous system | 28 (2.5) | 8433 (4.5) | |
| Diseases of the circulatory system | 108 (9.8) | 23163 (12.3) | |
| Diseases of the respiratory system | 133 (12.0) | 31212 (16.5) | |
| Diseases of the digestive system | 120 (10.8) | 15570 (8.2) | |
| Diseases of the genitourinary system | 44 (4.0) | 13816 (7.3) | |
| Complications of pregnancy | 46 (4.2) | 17132 (9.1) | |
| Diseases of the skin | 23 (2.1) | 2582 (1.4) | |
| Diseases of the musculoskeletal system | 20 (1.8) | 4221 (2.2) | |
| Congenital anomalies | 1 (0.1) | 3496 (1.8) | |
| Complications in the perinatal period | 1 (0.1) | 4178 (2.2) | |
| Ill‐defined conditions | 40 (3.6) | 4677 (2.5) | |
| Injury and poisoning | 190 (17.1) | 16083 (8.5) | |
| Social reasons | 5 (0.5) | 66 (0.0) | |
| Indeterminate | 138 (12.5) | 12504 (6.6) | |
Comparing the schizophrenic patients and general patients admitted to Okinawa Chubu Hospital during the same period showed no significant differences in acute hospital mortality, with an overall standardized mortality ratio of 1.294 (95% CI, 0.9751.684) based on sex‐stratified 5‐year bands using the exact Poisson distribution method. However, there were no deaths among schizophrenics in 2004, which we considered an outlier. Thus, when excluding data on mortality in 2004 from the analysis, we obtained a significant standardized mortality ratio of 1.421 (95% CI, 1.0901.884) for the schizophrenic patients.
Table 1 also shows the clinical characteristics of schizophrenic and nonschizophrenic patients admitted to the hospital. Admissions of schizophrenic patients showed that 643 patients (58%) were admitted to the department of internal medicine, 222 patients (20%) to the department of surgery, and 243 patients (22%) to other departments. The most common admission diagnoses of schizophrenics were: 190 patients (17%) diagnosed with injury and poisoning, 133 (12%) with diseases of the respiratory system, and 120 (11%) with diseases of the digestive system. Patients with an admitting diagnosis of injury and poisoning included those who had attempted suicide, although the exact number of patients with suicidal tendencies was unclear in this registry data. Comparison of the clinical characteristics of schizophrenic and nonschizophrenic patients indicated that schizophrenics were more likely to be older, have a longer hospital stay, be male, be admitted through the emergency department, and be admitted to the intensive care unit.
Table 2 presents the specific causes of death of hospitalized schizophrenic and nonschizophrenic patients based on ICD‐9 coding. Forty‐five schizophrenic patients (81.8%; 95% CI, 69.1%90.9%) died from natural causes (all deaths excluding injury and poisoning). The most frequent of all causes of death (>2 total cases) were suicide (n = 8; 14.5%), malignant lymphoma or leukemia (6; 10.9%), stroke (5; 9.0%), and sepsis (4; 7.3%). Suicide was the cause of 14.5% (95% CI, 6.5%26.7%) of all deaths of schizophrenic patients. The initial hospital presentations of patients with schizophrenia whose suicide attempts were successful included burns (3), brain injury (1), drug overdose (1), organophosphate pesticide ingestion (1), hanging (1), and drowning (1). Although 2 of the patients had attempted suicide while hospitalized at a psychiatric hospital, we did not identify any patients who succeeded in killing themselves while hospitalized at the acute care general hospital. There were 2 deaths of schizophrenic patients with neuroleptic malignant syndrome in the study period. Nonschizophrenic patients who died from injury and poisoning (n = 407; 5.4%) included patients who had committed suicide, although the exact number of nonschizophrenic patients were successful suicides was unclear in these registry data.
| Schizophrenic patients | Non‐schizophrenic patients | ||||
|---|---|---|---|---|---|
| Rank | Cause | No. (%) | Rank | Cause | No. (%) |
| |||||
| 1 | Suicide | 8 (14.5) | 1 | Malignant neoplasms | 2646 (35.1) |
| 2 | Malignant lymphoma or leukemia | 6 (10.9) | 2 | Diseases of the circulatory system | 1769 (23.5) |
| 3 | Stroke | 5 (9.0) | 3 | Diseases of the respiratory system | 787 (10.5) |
| 4 | Sepsis | 4 (7.3) | 4 | Diseases of the digestive system | 427 (5.7) |
| 5 | Lung cancer | 2 (3.6) | 5 | Injury and poisoning | 407 (5.4) |
| 6 | Acute myocardial infarction | 2 (3.6) | 6 | Sepsis | 262 (3.5) |
| 7 | Pneumonia | 2 (3.6) | 7 | Diseases of the genitourinary system | 150 (2.0) |
| 8 | Uterine cancer | 2 (3.6) | 8 | Diseases of the nervous system | 119 (1.6) |
| 9 | Neuroleptic malignant syndrome | 2 (3.6) | 9 | Endocrine, nutritional, and metabolic diseases | 86 (1.1) |
| 10 | Other causes | 22 (40.0) | 10 | Others | 875 (11.6) |
| Total | 55 (100) | Total | 7528 (100) | ||
Table 3 shows the logistic regression analyses for variables associated with acute hospital mortality of schizophrenic patients. In unadjusted analysis, the significant variables associated with the increased mortality included malignant neoplasm, diseases of the circulatory system, and older age. There was no significant difference in mortality between female and male schizophrenic patients. Unadjusted analysis showed that the mortality of schizophrenic patients directly transferred from a psychiatric hospital was not increased.
| Clinical characteristic | Unadjusted analysis odds ratio (95% CI) | Multivariable analysis odds ratio (95% CI) |
|---|---|---|
| ||
| Malignant neoplasms | 5.83 (3.14 10.83) | 12.93 (5.67 29.51) |
| Admission through ED | 1.29 (0.62 2.68) | 3.30 (1.33 8.20) |
| Diseases of the circulatory system | 2.17 (1.06 4.43) | 2.63 (1.20 5.77) |
| Intensive care unit admission | 1.37 (0.68 2.78) | 1.43 (0.65 3.11) |
| Male gender | 1.26 (0.73 2.17) | 1.39 (0.77 2.50) |
| Older age | 1.02 (1.00 1.04) | 1.01 (0.99 1.03) |
| Longer hospital stay | 1.00 (0.99 1.01) | 1.00 (0.99 1.01) |
| Liaison psychiatrist consultation | 0.43 (0.18 1.02) | 0.45 (0.18 1.11) |
| Transferred from psychiatric hospital | 0.48 (0.21 1.07) | 0.37 (0.16 0.87) |
In multivariable‐adjusted analysis (Table 3), the significant variables associated with increased mortality included malignant neoplasm (OR 12.93; 95% CI, 5.6729.51), diseases of the circulatory system (OR 2.63; 95% CI, 1.205.77), and admission through an emergency department (OR 3.30; 95% CI, 1.338.20). Further, a significant variable associated with decreased mortality was direct transfer from a psychiatric hospital (OR 0.37; 95% CI, 0.160.87). Consultation with a liaison psychiatrist in our hospital was not associated with decreased mortality.
DISCUSSION
The results of our study suggest that schizophrenic and nonschizophrenic patients were admitted with similar levels of medical pathology to an acute care hospital and that they responded comparably to inpatient medical care. The crude hospital mortality rate of schizophrenic patients admitted to our acute care hospital in Japan was 5.0%, whereas the crude mortality rate of nonschizophrenic patients during the same period was 4.0%. There was a nearly significant trend toward an increase in the overall standardized mortality ratio of schizophrenic patients compared with nonschizophrenic patients. Significant risk factors for the increased mortality of the schizophrenic patients were malignant neoplasm, cardiovascular disease, admission through an emergency department, and not transferred directly from a psychiatric hospital.
Although the overall standardized mortality ratio between schizophrenic and nonschizophrenic patients was not statistically significant in this study population, our reanalysis excluding the probable outlier data for 2004 did show a significant increase in the mortality of schizophrenic patients. Previous community‐based cohort studies, including a Japanese study, have consistently shown that the mortality of patients with schizophrenia was higher than that of the general population.12, 13 A meta‐analysis also suggested that schizophrenic patients had a significantly higher mortality from suicide and traumatic death as well as natural causes.14 In a recent study in Sweden, natural cause of death was found to be the main cause of excess deaths.15 Natural causes were also likely to be important in our study, because deaths from natural causes were 82% of all deaths of schizophrenic patients in settings of acute care hospitalization in Okinawa, Japan.
Suicide was the most important cause of death among our reported schizophrenic patients (14.5% of all deaths). In another survey, suicide was also the most frequent cause of death (36%).16 We may need improved and vigilant suicide prevention programs and better control of the psychiatric symptoms of these patients. In addition, there may be subgroups of schizophrenic patients at higher risk for suicide who should be targeted for suicide prevention. For instance, previous studies have suggested that the need for psychosedative medication at discharge from a psychiatric hospital and multiple previous hospitalizations increased the risk of suicide.17, 18 Suicide risk may also be increased in the first year after discharge from a psychiatric hospital.10, 17, 18
Our study showed that malignant neoplasm and cardiovascular disease were significantly associated with increased hospital mortality of schizophrenic patients, although malignant lymphoma/leukemia was the most frequent specific cause of death from malignant neoplasm. One previous study showed that fatal smoking‐related diseases were more prominent in schizophrenic patients than in the general population.19, 20 Attention to designing health educational programs specifically for schizophrenic patients, including healthy diet, smoking cessation, and physical exercise may be necessary. Smoking cessation may be important because a high percentage of schizophrenic patients in Japan are smokers.13
In our study survival of patients coming from psychiatric hospitals was greater than that of those coming from the community. In contrast, a study in Italy suggested that longer psychiatric hospitalization and chronic custodial care at psychiatric hospitals were risk factors for death of schizophrenic patients.11 The Japanese psychiatric management system is usually based on a model in which half the schizophrenic patients are managed in psychiatric hospitals and half are managed in community outpatient psychiatric clinics. Schizophrenic patients who are followed as outpatients may not receive adequate preventive care for common medical illnesses in Japan. Psychiatric hospitalization may provide an opportunity for preventive medicine for these patients.
The suicide rate of 14.5% among all causes of death suggests the need to focus on suicide prevention in schizophrenic patients. Prevention efforts should focus on suicidal tendencies, smoking, and other cardiovascular risk factors. Because most schizophrenic patients are followed regularly by practicing psychiatrists on a long‐term basis, we encourage practicing psychiatrists to use preventive health programs as a regular part of their treatment plans.21 However, we need to recognize that the psychiatric conditions could limit their ability to communicate symptoms of comorbid conditions. In addition, schizophrenic patients sometimes even refuse to undergo treatment for any illness they may have. These barriers that make it difficult to provide preventive care may be challenging issues for health care providers of psychiatric services.
Early recognition of comorbid medical conditions and the subsequent referral to acute care hospitals in a timely manner may be necessary for the improvement of care to reduce the mortality of schizophrenic patients. One review article suggested that schizophrenic patients suffered from more comorbid medical illnesses, which were largely undiagnosed and untreated and which may cause or exacerbate psychiatric symptoms.22 However, there may be multiple barriers to optimal primary medical care for these patients. In patients with schizophrenia, atypical presentation may be common; schizophrenic patients may be less symptomatic for localized symptoms and signs.23 There may be system‐based and politically based disparities between psychiatric hospitals and general hospitals in the treatment of schizophrenic patients depending on the country and region.22 Both political advocacy and development of primary care programs may be instituted to efficiently meet the health needs of these patients.
We did not find any significant differences in hospital mortality between patients with liaison psychiatrist consultation and those without. Liaison psychiatrists at our hospital received consultations from inpatient medical care teams and provided advice to 20% of admitted schizophrenic patients. Although their advice appears to be useful for controlling psychiatric symptoms, their consultations may not be significantly important for lowering hospital mortality.
Our study conducted in Japan may have implications for physicians working as general internists, especially hospitalists, in other countries. This may be the first study to comprehensively assess acute hospital care among schizophrenic patients. We determined common causes of and several risk factors associated with acute care mortality. These findings may help to identify schizophrenic patients at risk of dying when caring for these patients in acute care hospitals. In addition, the importance of preventive programs focusing on suicide would also be applicable to other countries.
We interpreted our results according to whether they are clinically significant. First, we performed the study at a single institution in Okinawa, Japan. Thus, our findings would require external confirmation for their generalizability to other acute care hospital settings. Second, different systems of health care in different countries may influence not only overall mortality but also hospital mortality. Comparative studies of hospital mortality at acute care general hospitals in different countries would be helpful. Third, we analyzed inpatient hospital mortality rather than long‐term mortality, including follow‐up, after hospital discharge. Further studies are needed to determine whether there may be excess mortality after patients are discharged from acute care hospitals. Fourth, our study used administrative data. Possible misclassification in coding disease and clinical characteristics may limit the utility of administrative data for interpreting the results.
In summary, this study may be the first report on the mortality of schizophrenic patients in acute care hospitalization. There was a nearly significant trend towards an increase in the standardized mortality of schizophrenic patients compared with that of general patients. Malignant neoplasm and cardiovascular diseases were significant factors associated with increased mortality. Suicide was the most frequent cause of death in this patient population.
Acknowledgements
We thank Mr. Masahito Taira and Ms. Noriko Irei for their excellent support of our data analysis and Mrs. Tomoko Yonaha for her excellent secretarial support.
- ,.Mortality in a cohort of patients with schizophrenia: a record linkage study.Can J Psychiatry.1991;36:239–245.
- ,,, et al.Excess mortality among long‐stay psychiatric patients in northern Finland.Soc Psychiatry Psychiatr Epidemiol.2003;38:297–304.
- ,,, et al.Mortality among patients with schizophrenia and reduced psychiatric hospital care.Psychol Med.2005;35:725–732.
- ,,, et al.Mortality in psychiatric patients 5 to 21 years after hospital admission in Italy.Psychol Med.2002;32:227–237.
- ,,, et al.Mental disorders and cause‐specific mortality.Br J Psychiatry.2001;179:498–502.
- ,,, et al.Mortality in Western Australian psychiatric patients.Soc Psychiatry Psychiatr Epidemiol.2000;35:341–347.
- ,.Mortality among discharged psychiatric patients in Finland.Acta Psychiatr Scand.1999;99:102–109.
- ,,.Mortality in long‐stay patients from psychiatric hospitals in Italy—results from the Qualyop Project.Soc Psychiatry Psychiatr Epidemiol.2003;38:385–389.
- ,,.Total mortality in people admitted to a psychiatric hospital.Br J Psychiatry.1997;170:186–190.
- ,,, et al.Where schizophrenic patients commit suicide: a review of suicide among inpatients and former inpatients.Int J Psychiatry Med.2005;35:171–190.
- ,,, et al.Mortality in psychiatric hospital patients: a cohort analysis of prognostic factors.Int J Epidemiol.1997;26:1227–1235
- ,.Excess mortality of mental disorder.Br J Psychiatry.1998;173:11–53.
- ,,, et al.Mortality in psychiatric patients, with a specific focus on cancer mortality associated with schizophrenia.Int J Epidemiol.1995;24:366–372.
- .Excess mortality of schizophrenia. A meta‐analysis.Br J Psychiatry.1997;171:502–508.
- ,,, et al.Mortality and causes of death in schizophrenia in Stockholm county, Sweden.Schizophr Res.2000;45:21–28.
- ,,, et al.Mortality among patients in psychiatric hospitals in Germany.Acta Psychiatr Scand.1995;91:174–179.
- ,,, et al.Mortality in chronic schizophrenia during decreasing number of psychiatric beds in Finland.Schizophrenia Research2002;54:265–275.
- ,,.Cause‐specific mortality in psychiatric patients after deinstitutionalisation.Br J Psychiatry.2001;179:438–443.
- ,,.Causes of the excess mortality of schizophrenia.Br J Psychiatry.2000;177:212–217.
- ,,, et al.Mortality after discharge from long‐term psychiatric care in Scotland, 1977–94: a retrospective cohort study.BMC Public Health.2003;3:30.
- ,,.Prevalence of physical illness among psychiatric inpatients who die of natural causes.Psychiatr Serv.1998;49:788–793.
- ,,.Mortality and medical comorbidity among psychiatric patients: a review.Psychiatr Serv.1996;47:1356–1363.
- .The “silent” acute abdomen of schizophrenia.J Med Soc N J.1981;78:679–680.
Previous studies have found that the total mortality of schizophrenic patients was higher than that of the general population.14 The all‐cause mortality for schizophrenic patients was 2 to 4 times that of the general population.57 Further, not only was the mortality of schizophrenic patients from suicide and traumatic causes higher than that of the general population, but mortality from natural causes was higher as well.8, 9 Of the specific causes of death of schizophrenic patients, suicide was the most important.10 In one study, suicidal mortality was approximately 20 times that of the general population.1 For natural causes, the most common cause of death was diseases of the circulatory system, followed by diseases of the respiratory system, the digestive system, and malignant neoplasm.2, 11 Overall, schizophrenic patients had a life expectancy about 20% shorter than that of the general population.1
However, few studies have investigated mortality among schizophrenic patients who were admitted to acute care hospitals. Studies involving large samples of inpatients with schizophrenia in general (nonpsychiatric) hospitals have also been scarce. The specific causes of death of hospitalized schizophrenic patients have not been investigated thoroughly. In addition, little is known about risk factors associated with the mortality of schizophrenic patients during acute care hospitalization. In this study, we analyzed hospital mortality of schizophrenic patients admitted to an acute care community teaching hospital and compared mortality between schizophrenic and nonschizophrenic patients. Our mortality data included patients presenting with suicidal attempts who died during their admission. We also determined significant factors associated with this increased mortality and the specific causes of death of these patients.
METHODS
Study Patients
We studied all patients admitted to Okinawa Chubu Hospital from January 1, 1987, to December 31, 2004. Okinawa Chubu is a community teaching hospital that provides primary to tertiary care to a population of approximately 400,000 in Okinawa, Japan. Nearly all patients admitted during the study period were Japanese (more than 99.5%).
We identified all schizophrenic patients through the computerized inpatient registry. This registry includes the hospital discharge summary electronic database, which is updated and reviewed by certified hospital record technicians using the ICD‐9 coding scheme (International Classification of Diseases, 9th Revision, and Clinical Modification). The diagnosis of schizophrenia was documented by staff psychiatrists, who provided basic psychiatric care for these patients in inpatient or outpatient settings. The study was approved by the Institutional Review Board of Okinawa Chubu Hospital.
Data Collection
We obtained basic demographic data on all patients admitted during the study period using the computerized inpatient registry. Data were extracted on demographics, discharge outcome (survival or nonsurvival), route of admission, direct transfer from psychiatric hospitals, admitting department, intensive care unit admission, liaison psychiatrist consultation, length of hospital stay (days), and diagnostic classifications based on the ICD‐9. Routes of admission included the emergency department (ED) and outpatient clinic. From the computerized record, we also determined causes of death of schizophrenic as well as nonschizophrenic patients. We performed a manual chart review to determine specific causes of death of schizophrenic patients because the administrative data included the ICD‐9 coding scheme for admitting diagnosis but did not include information on specific causes of death.
Statistical Analysis
We obtained the standardized mortality ratio of schizophrenic patients compared with general patient population during each admission year and calculated the 95% confidence interval (95% CI) using sex‐stratified 5‐year bands and the exact Poisson distribution method. We also analyzed risk factors associated with hospital mortality. In unadjusted logistic regression analyses, we calculated the odds ratios (ORs) and 95% CIs of the demographic and clinical variables for hospital mortality among all schizophrenic patients. We then performed multivariable‐adjusted logistic regression analysis to identify significant risk factors associated with increased hospital mortality, adjusted for demographic and clinical variables. A 2‐sided P value < .05 was considered statistically significant. All statistical analyses were performed using Stata Software Version 8.2 (StataCorp LP, College Station, TX).
RESULTS
We identified a total of 189,049 general nonschizophrenic patients admitted to Okinawa Chubu Hospital during the 18‐year period (Table 1). Of these, 7528 patients died during hospitalization, with an overall hospital mortality rate of 4.0% (95% CI, 3.9%4.1%). There were 55 deaths among 1108 schizophrenic patients admitted to Okinawa Chubu Hospital during the same period. Schizophrenic patients had an overall hospital mortality rate of 5.0% (95% CI, 3.8%6.4%). The hospital mortality rate was 4.4% (95% CI, 2.9%6.5%) for schizophrenic women and 5.5% (95% CI, 3.8%7.7%) for schizophrenic men.
| Clinical characteristic | Schizophrenics n = 1108 | Non‐schizophrenics n = 189049 | p‐value** |
|---|---|---|---|
| |||
| Age, mean (SD) | 48.7 (14.2) | 40.0 (28.8) | <0.001 |
| Women, n (%) | 543 (49.0) | 101218 (53.5) | 0.0026 |
| Transferred from psychiatric hospital, n (%) | 254 (22.9) | N/A | |
| Admission through ED, n (%) | 853 (77.0) | 111109 (58.8) | <0.001 |
| Admitted department, n (%) | |||
| Internal medicine | 643 (58.0) | 65657 (34.7) | |
| General surgery | 222 (20.0) | 43036 (22.8) | |
| Other departments | 243 (21.9) | 80356 (42.5) | |
| Intensive care unit admission, n (%) | 157 (14.2) | 9900 (5.2) | <0.001 |
| Liaison psychiatrist consultation, n (%) | 239 (21.6) | N/A | |
| Length of hospital stay (days), mean (SD) | 25.2 (44.1) | 18.0 (42.1) | <0.001 |
| Admission diagnosis, n (%) | |||
| Infectious diseases without organ involvement | 13 (1.2) | 8009 (4.2) | |
| Malignant neoplasms | 92 (8.3) | 18481 (9.8) | |
| Endocrine, nutritional and metabolic diseases | 63 (5.7) | 3389 (1.8) | |
| Hematologic diseases | 11 (1.0) | 1364 (0.7) | |
| Mental diseases except schizophrenia | 32 (2.9) | 673 (0.4) | |
| Diseases of the nervous system | 28 (2.5) | 8433 (4.5) | |
| Diseases of the circulatory system | 108 (9.8) | 23163 (12.3) | |
| Diseases of the respiratory system | 133 (12.0) | 31212 (16.5) | |
| Diseases of the digestive system | 120 (10.8) | 15570 (8.2) | |
| Diseases of the genitourinary system | 44 (4.0) | 13816 (7.3) | |
| Complications of pregnancy | 46 (4.2) | 17132 (9.1) | |
| Diseases of the skin | 23 (2.1) | 2582 (1.4) | |
| Diseases of the musculoskeletal system | 20 (1.8) | 4221 (2.2) | |
| Congenital anomalies | 1 (0.1) | 3496 (1.8) | |
| Complications in the perinatal period | 1 (0.1) | 4178 (2.2) | |
| Ill‐defined conditions | 40 (3.6) | 4677 (2.5) | |
| Injury and poisoning | 190 (17.1) | 16083 (8.5) | |
| Social reasons | 5 (0.5) | 66 (0.0) | |
| Indeterminate | 138 (12.5) | 12504 (6.6) | |
Comparing the schizophrenic patients and general patients admitted to Okinawa Chubu Hospital during the same period showed no significant differences in acute hospital mortality, with an overall standardized mortality ratio of 1.294 (95% CI, 0.9751.684) based on sex‐stratified 5‐year bands using the exact Poisson distribution method. However, there were no deaths among schizophrenics in 2004, which we considered an outlier. Thus, when excluding data on mortality in 2004 from the analysis, we obtained a significant standardized mortality ratio of 1.421 (95% CI, 1.0901.884) for the schizophrenic patients.
Table 1 also shows the clinical characteristics of schizophrenic and nonschizophrenic patients admitted to the hospital. Admissions of schizophrenic patients showed that 643 patients (58%) were admitted to the department of internal medicine, 222 patients (20%) to the department of surgery, and 243 patients (22%) to other departments. The most common admission diagnoses of schizophrenics were: 190 patients (17%) diagnosed with injury and poisoning, 133 (12%) with diseases of the respiratory system, and 120 (11%) with diseases of the digestive system. Patients with an admitting diagnosis of injury and poisoning included those who had attempted suicide, although the exact number of patients with suicidal tendencies was unclear in this registry data. Comparison of the clinical characteristics of schizophrenic and nonschizophrenic patients indicated that schizophrenics were more likely to be older, have a longer hospital stay, be male, be admitted through the emergency department, and be admitted to the intensive care unit.
Table 2 presents the specific causes of death of hospitalized schizophrenic and nonschizophrenic patients based on ICD‐9 coding. Forty‐five schizophrenic patients (81.8%; 95% CI, 69.1%90.9%) died from natural causes (all deaths excluding injury and poisoning). The most frequent of all causes of death (>2 total cases) were suicide (n = 8; 14.5%), malignant lymphoma or leukemia (6; 10.9%), stroke (5; 9.0%), and sepsis (4; 7.3%). Suicide was the cause of 14.5% (95% CI, 6.5%26.7%) of all deaths of schizophrenic patients. The initial hospital presentations of patients with schizophrenia whose suicide attempts were successful included burns (3), brain injury (1), drug overdose (1), organophosphate pesticide ingestion (1), hanging (1), and drowning (1). Although 2 of the patients had attempted suicide while hospitalized at a psychiatric hospital, we did not identify any patients who succeeded in killing themselves while hospitalized at the acute care general hospital. There were 2 deaths of schizophrenic patients with neuroleptic malignant syndrome in the study period. Nonschizophrenic patients who died from injury and poisoning (n = 407; 5.4%) included patients who had committed suicide, although the exact number of nonschizophrenic patients were successful suicides was unclear in these registry data.
| Schizophrenic patients | Non‐schizophrenic patients | ||||
|---|---|---|---|---|---|
| Rank | Cause | No. (%) | Rank | Cause | No. (%) |
| |||||
| 1 | Suicide | 8 (14.5) | 1 | Malignant neoplasms | 2646 (35.1) |
| 2 | Malignant lymphoma or leukemia | 6 (10.9) | 2 | Diseases of the circulatory system | 1769 (23.5) |
| 3 | Stroke | 5 (9.0) | 3 | Diseases of the respiratory system | 787 (10.5) |
| 4 | Sepsis | 4 (7.3) | 4 | Diseases of the digestive system | 427 (5.7) |
| 5 | Lung cancer | 2 (3.6) | 5 | Injury and poisoning | 407 (5.4) |
| 6 | Acute myocardial infarction | 2 (3.6) | 6 | Sepsis | 262 (3.5) |
| 7 | Pneumonia | 2 (3.6) | 7 | Diseases of the genitourinary system | 150 (2.0) |
| 8 | Uterine cancer | 2 (3.6) | 8 | Diseases of the nervous system | 119 (1.6) |
| 9 | Neuroleptic malignant syndrome | 2 (3.6) | 9 | Endocrine, nutritional, and metabolic diseases | 86 (1.1) |
| 10 | Other causes | 22 (40.0) | 10 | Others | 875 (11.6) |
| Total | 55 (100) | Total | 7528 (100) | ||
Table 3 shows the logistic regression analyses for variables associated with acute hospital mortality of schizophrenic patients. In unadjusted analysis, the significant variables associated with the increased mortality included malignant neoplasm, diseases of the circulatory system, and older age. There was no significant difference in mortality between female and male schizophrenic patients. Unadjusted analysis showed that the mortality of schizophrenic patients directly transferred from a psychiatric hospital was not increased.
| Clinical characteristic | Unadjusted analysis odds ratio (95% CI) | Multivariable analysis odds ratio (95% CI) |
|---|---|---|
| ||
| Malignant neoplasms | 5.83 (3.14 10.83) | 12.93 (5.67 29.51) |
| Admission through ED | 1.29 (0.62 2.68) | 3.30 (1.33 8.20) |
| Diseases of the circulatory system | 2.17 (1.06 4.43) | 2.63 (1.20 5.77) |
| Intensive care unit admission | 1.37 (0.68 2.78) | 1.43 (0.65 3.11) |
| Male gender | 1.26 (0.73 2.17) | 1.39 (0.77 2.50) |
| Older age | 1.02 (1.00 1.04) | 1.01 (0.99 1.03) |
| Longer hospital stay | 1.00 (0.99 1.01) | 1.00 (0.99 1.01) |
| Liaison psychiatrist consultation | 0.43 (0.18 1.02) | 0.45 (0.18 1.11) |
| Transferred from psychiatric hospital | 0.48 (0.21 1.07) | 0.37 (0.16 0.87) |
In multivariable‐adjusted analysis (Table 3), the significant variables associated with increased mortality included malignant neoplasm (OR 12.93; 95% CI, 5.6729.51), diseases of the circulatory system (OR 2.63; 95% CI, 1.205.77), and admission through an emergency department (OR 3.30; 95% CI, 1.338.20). Further, a significant variable associated with decreased mortality was direct transfer from a psychiatric hospital (OR 0.37; 95% CI, 0.160.87). Consultation with a liaison psychiatrist in our hospital was not associated with decreased mortality.
DISCUSSION
The results of our study suggest that schizophrenic and nonschizophrenic patients were admitted with similar levels of medical pathology to an acute care hospital and that they responded comparably to inpatient medical care. The crude hospital mortality rate of schizophrenic patients admitted to our acute care hospital in Japan was 5.0%, whereas the crude mortality rate of nonschizophrenic patients during the same period was 4.0%. There was a nearly significant trend toward an increase in the overall standardized mortality ratio of schizophrenic patients compared with nonschizophrenic patients. Significant risk factors for the increased mortality of the schizophrenic patients were malignant neoplasm, cardiovascular disease, admission through an emergency department, and not transferred directly from a psychiatric hospital.
Although the overall standardized mortality ratio between schizophrenic and nonschizophrenic patients was not statistically significant in this study population, our reanalysis excluding the probable outlier data for 2004 did show a significant increase in the mortality of schizophrenic patients. Previous community‐based cohort studies, including a Japanese study, have consistently shown that the mortality of patients with schizophrenia was higher than that of the general population.12, 13 A meta‐analysis also suggested that schizophrenic patients had a significantly higher mortality from suicide and traumatic death as well as natural causes.14 In a recent study in Sweden, natural cause of death was found to be the main cause of excess deaths.15 Natural causes were also likely to be important in our study, because deaths from natural causes were 82% of all deaths of schizophrenic patients in settings of acute care hospitalization in Okinawa, Japan.
Suicide was the most important cause of death among our reported schizophrenic patients (14.5% of all deaths). In another survey, suicide was also the most frequent cause of death (36%).16 We may need improved and vigilant suicide prevention programs and better control of the psychiatric symptoms of these patients. In addition, there may be subgroups of schizophrenic patients at higher risk for suicide who should be targeted for suicide prevention. For instance, previous studies have suggested that the need for psychosedative medication at discharge from a psychiatric hospital and multiple previous hospitalizations increased the risk of suicide.17, 18 Suicide risk may also be increased in the first year after discharge from a psychiatric hospital.10, 17, 18
Our study showed that malignant neoplasm and cardiovascular disease were significantly associated with increased hospital mortality of schizophrenic patients, although malignant lymphoma/leukemia was the most frequent specific cause of death from malignant neoplasm. One previous study showed that fatal smoking‐related diseases were more prominent in schizophrenic patients than in the general population.19, 20 Attention to designing health educational programs specifically for schizophrenic patients, including healthy diet, smoking cessation, and physical exercise may be necessary. Smoking cessation may be important because a high percentage of schizophrenic patients in Japan are smokers.13
In our study survival of patients coming from psychiatric hospitals was greater than that of those coming from the community. In contrast, a study in Italy suggested that longer psychiatric hospitalization and chronic custodial care at psychiatric hospitals were risk factors for death of schizophrenic patients.11 The Japanese psychiatric management system is usually based on a model in which half the schizophrenic patients are managed in psychiatric hospitals and half are managed in community outpatient psychiatric clinics. Schizophrenic patients who are followed as outpatients may not receive adequate preventive care for common medical illnesses in Japan. Psychiatric hospitalization may provide an opportunity for preventive medicine for these patients.
The suicide rate of 14.5% among all causes of death suggests the need to focus on suicide prevention in schizophrenic patients. Prevention efforts should focus on suicidal tendencies, smoking, and other cardiovascular risk factors. Because most schizophrenic patients are followed regularly by practicing psychiatrists on a long‐term basis, we encourage practicing psychiatrists to use preventive health programs as a regular part of their treatment plans.21 However, we need to recognize that the psychiatric conditions could limit their ability to communicate symptoms of comorbid conditions. In addition, schizophrenic patients sometimes even refuse to undergo treatment for any illness they may have. These barriers that make it difficult to provide preventive care may be challenging issues for health care providers of psychiatric services.
Early recognition of comorbid medical conditions and the subsequent referral to acute care hospitals in a timely manner may be necessary for the improvement of care to reduce the mortality of schizophrenic patients. One review article suggested that schizophrenic patients suffered from more comorbid medical illnesses, which were largely undiagnosed and untreated and which may cause or exacerbate psychiatric symptoms.22 However, there may be multiple barriers to optimal primary medical care for these patients. In patients with schizophrenia, atypical presentation may be common; schizophrenic patients may be less symptomatic for localized symptoms and signs.23 There may be system‐based and politically based disparities between psychiatric hospitals and general hospitals in the treatment of schizophrenic patients depending on the country and region.22 Both political advocacy and development of primary care programs may be instituted to efficiently meet the health needs of these patients.
We did not find any significant differences in hospital mortality between patients with liaison psychiatrist consultation and those without. Liaison psychiatrists at our hospital received consultations from inpatient medical care teams and provided advice to 20% of admitted schizophrenic patients. Although their advice appears to be useful for controlling psychiatric symptoms, their consultations may not be significantly important for lowering hospital mortality.
Our study conducted in Japan may have implications for physicians working as general internists, especially hospitalists, in other countries. This may be the first study to comprehensively assess acute hospital care among schizophrenic patients. We determined common causes of and several risk factors associated with acute care mortality. These findings may help to identify schizophrenic patients at risk of dying when caring for these patients in acute care hospitals. In addition, the importance of preventive programs focusing on suicide would also be applicable to other countries.
We interpreted our results according to whether they are clinically significant. First, we performed the study at a single institution in Okinawa, Japan. Thus, our findings would require external confirmation for their generalizability to other acute care hospital settings. Second, different systems of health care in different countries may influence not only overall mortality but also hospital mortality. Comparative studies of hospital mortality at acute care general hospitals in different countries would be helpful. Third, we analyzed inpatient hospital mortality rather than long‐term mortality, including follow‐up, after hospital discharge. Further studies are needed to determine whether there may be excess mortality after patients are discharged from acute care hospitals. Fourth, our study used administrative data. Possible misclassification in coding disease and clinical characteristics may limit the utility of administrative data for interpreting the results.
In summary, this study may be the first report on the mortality of schizophrenic patients in acute care hospitalization. There was a nearly significant trend towards an increase in the standardized mortality of schizophrenic patients compared with that of general patients. Malignant neoplasm and cardiovascular diseases were significant factors associated with increased mortality. Suicide was the most frequent cause of death in this patient population.
Acknowledgements
We thank Mr. Masahito Taira and Ms. Noriko Irei for their excellent support of our data analysis and Mrs. Tomoko Yonaha for her excellent secretarial support.
Previous studies have found that the total mortality of schizophrenic patients was higher than that of the general population.14 The all‐cause mortality for schizophrenic patients was 2 to 4 times that of the general population.57 Further, not only was the mortality of schizophrenic patients from suicide and traumatic causes higher than that of the general population, but mortality from natural causes was higher as well.8, 9 Of the specific causes of death of schizophrenic patients, suicide was the most important.10 In one study, suicidal mortality was approximately 20 times that of the general population.1 For natural causes, the most common cause of death was diseases of the circulatory system, followed by diseases of the respiratory system, the digestive system, and malignant neoplasm.2, 11 Overall, schizophrenic patients had a life expectancy about 20% shorter than that of the general population.1
However, few studies have investigated mortality among schizophrenic patients who were admitted to acute care hospitals. Studies involving large samples of inpatients with schizophrenia in general (nonpsychiatric) hospitals have also been scarce. The specific causes of death of hospitalized schizophrenic patients have not been investigated thoroughly. In addition, little is known about risk factors associated with the mortality of schizophrenic patients during acute care hospitalization. In this study, we analyzed hospital mortality of schizophrenic patients admitted to an acute care community teaching hospital and compared mortality between schizophrenic and nonschizophrenic patients. Our mortality data included patients presenting with suicidal attempts who died during their admission. We also determined significant factors associated with this increased mortality and the specific causes of death of these patients.
METHODS
Study Patients
We studied all patients admitted to Okinawa Chubu Hospital from January 1, 1987, to December 31, 2004. Okinawa Chubu is a community teaching hospital that provides primary to tertiary care to a population of approximately 400,000 in Okinawa, Japan. Nearly all patients admitted during the study period were Japanese (more than 99.5%).
We identified all schizophrenic patients through the computerized inpatient registry. This registry includes the hospital discharge summary electronic database, which is updated and reviewed by certified hospital record technicians using the ICD‐9 coding scheme (International Classification of Diseases, 9th Revision, and Clinical Modification). The diagnosis of schizophrenia was documented by staff psychiatrists, who provided basic psychiatric care for these patients in inpatient or outpatient settings. The study was approved by the Institutional Review Board of Okinawa Chubu Hospital.
Data Collection
We obtained basic demographic data on all patients admitted during the study period using the computerized inpatient registry. Data were extracted on demographics, discharge outcome (survival or nonsurvival), route of admission, direct transfer from psychiatric hospitals, admitting department, intensive care unit admission, liaison psychiatrist consultation, length of hospital stay (days), and diagnostic classifications based on the ICD‐9. Routes of admission included the emergency department (ED) and outpatient clinic. From the computerized record, we also determined causes of death of schizophrenic as well as nonschizophrenic patients. We performed a manual chart review to determine specific causes of death of schizophrenic patients because the administrative data included the ICD‐9 coding scheme for admitting diagnosis but did not include information on specific causes of death.
Statistical Analysis
We obtained the standardized mortality ratio of schizophrenic patients compared with general patient population during each admission year and calculated the 95% confidence interval (95% CI) using sex‐stratified 5‐year bands and the exact Poisson distribution method. We also analyzed risk factors associated with hospital mortality. In unadjusted logistic regression analyses, we calculated the odds ratios (ORs) and 95% CIs of the demographic and clinical variables for hospital mortality among all schizophrenic patients. We then performed multivariable‐adjusted logistic regression analysis to identify significant risk factors associated with increased hospital mortality, adjusted for demographic and clinical variables. A 2‐sided P value < .05 was considered statistically significant. All statistical analyses were performed using Stata Software Version 8.2 (StataCorp LP, College Station, TX).
RESULTS
We identified a total of 189,049 general nonschizophrenic patients admitted to Okinawa Chubu Hospital during the 18‐year period (Table 1). Of these, 7528 patients died during hospitalization, with an overall hospital mortality rate of 4.0% (95% CI, 3.9%4.1%). There were 55 deaths among 1108 schizophrenic patients admitted to Okinawa Chubu Hospital during the same period. Schizophrenic patients had an overall hospital mortality rate of 5.0% (95% CI, 3.8%6.4%). The hospital mortality rate was 4.4% (95% CI, 2.9%6.5%) for schizophrenic women and 5.5% (95% CI, 3.8%7.7%) for schizophrenic men.
| Clinical characteristic | Schizophrenics n = 1108 | Non‐schizophrenics n = 189049 | p‐value** |
|---|---|---|---|
| |||
| Age, mean (SD) | 48.7 (14.2) | 40.0 (28.8) | <0.001 |
| Women, n (%) | 543 (49.0) | 101218 (53.5) | 0.0026 |
| Transferred from psychiatric hospital, n (%) | 254 (22.9) | N/A | |
| Admission through ED, n (%) | 853 (77.0) | 111109 (58.8) | <0.001 |
| Admitted department, n (%) | |||
| Internal medicine | 643 (58.0) | 65657 (34.7) | |
| General surgery | 222 (20.0) | 43036 (22.8) | |
| Other departments | 243 (21.9) | 80356 (42.5) | |
| Intensive care unit admission, n (%) | 157 (14.2) | 9900 (5.2) | <0.001 |
| Liaison psychiatrist consultation, n (%) | 239 (21.6) | N/A | |
| Length of hospital stay (days), mean (SD) | 25.2 (44.1) | 18.0 (42.1) | <0.001 |
| Admission diagnosis, n (%) | |||
| Infectious diseases without organ involvement | 13 (1.2) | 8009 (4.2) | |
| Malignant neoplasms | 92 (8.3) | 18481 (9.8) | |
| Endocrine, nutritional and metabolic diseases | 63 (5.7) | 3389 (1.8) | |
| Hematologic diseases | 11 (1.0) | 1364 (0.7) | |
| Mental diseases except schizophrenia | 32 (2.9) | 673 (0.4) | |
| Diseases of the nervous system | 28 (2.5) | 8433 (4.5) | |
| Diseases of the circulatory system | 108 (9.8) | 23163 (12.3) | |
| Diseases of the respiratory system | 133 (12.0) | 31212 (16.5) | |
| Diseases of the digestive system | 120 (10.8) | 15570 (8.2) | |
| Diseases of the genitourinary system | 44 (4.0) | 13816 (7.3) | |
| Complications of pregnancy | 46 (4.2) | 17132 (9.1) | |
| Diseases of the skin | 23 (2.1) | 2582 (1.4) | |
| Diseases of the musculoskeletal system | 20 (1.8) | 4221 (2.2) | |
| Congenital anomalies | 1 (0.1) | 3496 (1.8) | |
| Complications in the perinatal period | 1 (0.1) | 4178 (2.2) | |
| Ill‐defined conditions | 40 (3.6) | 4677 (2.5) | |
| Injury and poisoning | 190 (17.1) | 16083 (8.5) | |
| Social reasons | 5 (0.5) | 66 (0.0) | |
| Indeterminate | 138 (12.5) | 12504 (6.6) | |
Comparing the schizophrenic patients and general patients admitted to Okinawa Chubu Hospital during the same period showed no significant differences in acute hospital mortality, with an overall standardized mortality ratio of 1.294 (95% CI, 0.9751.684) based on sex‐stratified 5‐year bands using the exact Poisson distribution method. However, there were no deaths among schizophrenics in 2004, which we considered an outlier. Thus, when excluding data on mortality in 2004 from the analysis, we obtained a significant standardized mortality ratio of 1.421 (95% CI, 1.0901.884) for the schizophrenic patients.
Table 1 also shows the clinical characteristics of schizophrenic and nonschizophrenic patients admitted to the hospital. Admissions of schizophrenic patients showed that 643 patients (58%) were admitted to the department of internal medicine, 222 patients (20%) to the department of surgery, and 243 patients (22%) to other departments. The most common admission diagnoses of schizophrenics were: 190 patients (17%) diagnosed with injury and poisoning, 133 (12%) with diseases of the respiratory system, and 120 (11%) with diseases of the digestive system. Patients with an admitting diagnosis of injury and poisoning included those who had attempted suicide, although the exact number of patients with suicidal tendencies was unclear in this registry data. Comparison of the clinical characteristics of schizophrenic and nonschizophrenic patients indicated that schizophrenics were more likely to be older, have a longer hospital stay, be male, be admitted through the emergency department, and be admitted to the intensive care unit.
Table 2 presents the specific causes of death of hospitalized schizophrenic and nonschizophrenic patients based on ICD‐9 coding. Forty‐five schizophrenic patients (81.8%; 95% CI, 69.1%90.9%) died from natural causes (all deaths excluding injury and poisoning). The most frequent of all causes of death (>2 total cases) were suicide (n = 8; 14.5%), malignant lymphoma or leukemia (6; 10.9%), stroke (5; 9.0%), and sepsis (4; 7.3%). Suicide was the cause of 14.5% (95% CI, 6.5%26.7%) of all deaths of schizophrenic patients. The initial hospital presentations of patients with schizophrenia whose suicide attempts were successful included burns (3), brain injury (1), drug overdose (1), organophosphate pesticide ingestion (1), hanging (1), and drowning (1). Although 2 of the patients had attempted suicide while hospitalized at a psychiatric hospital, we did not identify any patients who succeeded in killing themselves while hospitalized at the acute care general hospital. There were 2 deaths of schizophrenic patients with neuroleptic malignant syndrome in the study period. Nonschizophrenic patients who died from injury and poisoning (n = 407; 5.4%) included patients who had committed suicide, although the exact number of nonschizophrenic patients were successful suicides was unclear in these registry data.
| Schizophrenic patients | Non‐schizophrenic patients | ||||
|---|---|---|---|---|---|
| Rank | Cause | No. (%) | Rank | Cause | No. (%) |
| |||||
| 1 | Suicide | 8 (14.5) | 1 | Malignant neoplasms | 2646 (35.1) |
| 2 | Malignant lymphoma or leukemia | 6 (10.9) | 2 | Diseases of the circulatory system | 1769 (23.5) |
| 3 | Stroke | 5 (9.0) | 3 | Diseases of the respiratory system | 787 (10.5) |
| 4 | Sepsis | 4 (7.3) | 4 | Diseases of the digestive system | 427 (5.7) |
| 5 | Lung cancer | 2 (3.6) | 5 | Injury and poisoning | 407 (5.4) |
| 6 | Acute myocardial infarction | 2 (3.6) | 6 | Sepsis | 262 (3.5) |
| 7 | Pneumonia | 2 (3.6) | 7 | Diseases of the genitourinary system | 150 (2.0) |
| 8 | Uterine cancer | 2 (3.6) | 8 | Diseases of the nervous system | 119 (1.6) |
| 9 | Neuroleptic malignant syndrome | 2 (3.6) | 9 | Endocrine, nutritional, and metabolic diseases | 86 (1.1) |
| 10 | Other causes | 22 (40.0) | 10 | Others | 875 (11.6) |
| Total | 55 (100) | Total | 7528 (100) | ||
Table 3 shows the logistic regression analyses for variables associated with acute hospital mortality of schizophrenic patients. In unadjusted analysis, the significant variables associated with the increased mortality included malignant neoplasm, diseases of the circulatory system, and older age. There was no significant difference in mortality between female and male schizophrenic patients. Unadjusted analysis showed that the mortality of schizophrenic patients directly transferred from a psychiatric hospital was not increased.
| Clinical characteristic | Unadjusted analysis odds ratio (95% CI) | Multivariable analysis odds ratio (95% CI) |
|---|---|---|
| ||
| Malignant neoplasms | 5.83 (3.14 10.83) | 12.93 (5.67 29.51) |
| Admission through ED | 1.29 (0.62 2.68) | 3.30 (1.33 8.20) |
| Diseases of the circulatory system | 2.17 (1.06 4.43) | 2.63 (1.20 5.77) |
| Intensive care unit admission | 1.37 (0.68 2.78) | 1.43 (0.65 3.11) |
| Male gender | 1.26 (0.73 2.17) | 1.39 (0.77 2.50) |
| Older age | 1.02 (1.00 1.04) | 1.01 (0.99 1.03) |
| Longer hospital stay | 1.00 (0.99 1.01) | 1.00 (0.99 1.01) |
| Liaison psychiatrist consultation | 0.43 (0.18 1.02) | 0.45 (0.18 1.11) |
| Transferred from psychiatric hospital | 0.48 (0.21 1.07) | 0.37 (0.16 0.87) |
In multivariable‐adjusted analysis (Table 3), the significant variables associated with increased mortality included malignant neoplasm (OR 12.93; 95% CI, 5.6729.51), diseases of the circulatory system (OR 2.63; 95% CI, 1.205.77), and admission through an emergency department (OR 3.30; 95% CI, 1.338.20). Further, a significant variable associated with decreased mortality was direct transfer from a psychiatric hospital (OR 0.37; 95% CI, 0.160.87). Consultation with a liaison psychiatrist in our hospital was not associated with decreased mortality.
DISCUSSION
The results of our study suggest that schizophrenic and nonschizophrenic patients were admitted with similar levels of medical pathology to an acute care hospital and that they responded comparably to inpatient medical care. The crude hospital mortality rate of schizophrenic patients admitted to our acute care hospital in Japan was 5.0%, whereas the crude mortality rate of nonschizophrenic patients during the same period was 4.0%. There was a nearly significant trend toward an increase in the overall standardized mortality ratio of schizophrenic patients compared with nonschizophrenic patients. Significant risk factors for the increased mortality of the schizophrenic patients were malignant neoplasm, cardiovascular disease, admission through an emergency department, and not transferred directly from a psychiatric hospital.
Although the overall standardized mortality ratio between schizophrenic and nonschizophrenic patients was not statistically significant in this study population, our reanalysis excluding the probable outlier data for 2004 did show a significant increase in the mortality of schizophrenic patients. Previous community‐based cohort studies, including a Japanese study, have consistently shown that the mortality of patients with schizophrenia was higher than that of the general population.12, 13 A meta‐analysis also suggested that schizophrenic patients had a significantly higher mortality from suicide and traumatic death as well as natural causes.14 In a recent study in Sweden, natural cause of death was found to be the main cause of excess deaths.15 Natural causes were also likely to be important in our study, because deaths from natural causes were 82% of all deaths of schizophrenic patients in settings of acute care hospitalization in Okinawa, Japan.
Suicide was the most important cause of death among our reported schizophrenic patients (14.5% of all deaths). In another survey, suicide was also the most frequent cause of death (36%).16 We may need improved and vigilant suicide prevention programs and better control of the psychiatric symptoms of these patients. In addition, there may be subgroups of schizophrenic patients at higher risk for suicide who should be targeted for suicide prevention. For instance, previous studies have suggested that the need for psychosedative medication at discharge from a psychiatric hospital and multiple previous hospitalizations increased the risk of suicide.17, 18 Suicide risk may also be increased in the first year after discharge from a psychiatric hospital.10, 17, 18
Our study showed that malignant neoplasm and cardiovascular disease were significantly associated with increased hospital mortality of schizophrenic patients, although malignant lymphoma/leukemia was the most frequent specific cause of death from malignant neoplasm. One previous study showed that fatal smoking‐related diseases were more prominent in schizophrenic patients than in the general population.19, 20 Attention to designing health educational programs specifically for schizophrenic patients, including healthy diet, smoking cessation, and physical exercise may be necessary. Smoking cessation may be important because a high percentage of schizophrenic patients in Japan are smokers.13
In our study survival of patients coming from psychiatric hospitals was greater than that of those coming from the community. In contrast, a study in Italy suggested that longer psychiatric hospitalization and chronic custodial care at psychiatric hospitals were risk factors for death of schizophrenic patients.11 The Japanese psychiatric management system is usually based on a model in which half the schizophrenic patients are managed in psychiatric hospitals and half are managed in community outpatient psychiatric clinics. Schizophrenic patients who are followed as outpatients may not receive adequate preventive care for common medical illnesses in Japan. Psychiatric hospitalization may provide an opportunity for preventive medicine for these patients.
The suicide rate of 14.5% among all causes of death suggests the need to focus on suicide prevention in schizophrenic patients. Prevention efforts should focus on suicidal tendencies, smoking, and other cardiovascular risk factors. Because most schizophrenic patients are followed regularly by practicing psychiatrists on a long‐term basis, we encourage practicing psychiatrists to use preventive health programs as a regular part of their treatment plans.21 However, we need to recognize that the psychiatric conditions could limit their ability to communicate symptoms of comorbid conditions. In addition, schizophrenic patients sometimes even refuse to undergo treatment for any illness they may have. These barriers that make it difficult to provide preventive care may be challenging issues for health care providers of psychiatric services.
Early recognition of comorbid medical conditions and the subsequent referral to acute care hospitals in a timely manner may be necessary for the improvement of care to reduce the mortality of schizophrenic patients. One review article suggested that schizophrenic patients suffered from more comorbid medical illnesses, which were largely undiagnosed and untreated and which may cause or exacerbate psychiatric symptoms.22 However, there may be multiple barriers to optimal primary medical care for these patients. In patients with schizophrenia, atypical presentation may be common; schizophrenic patients may be less symptomatic for localized symptoms and signs.23 There may be system‐based and politically based disparities between psychiatric hospitals and general hospitals in the treatment of schizophrenic patients depending on the country and region.22 Both political advocacy and development of primary care programs may be instituted to efficiently meet the health needs of these patients.
We did not find any significant differences in hospital mortality between patients with liaison psychiatrist consultation and those without. Liaison psychiatrists at our hospital received consultations from inpatient medical care teams and provided advice to 20% of admitted schizophrenic patients. Although their advice appears to be useful for controlling psychiatric symptoms, their consultations may not be significantly important for lowering hospital mortality.
Our study conducted in Japan may have implications for physicians working as general internists, especially hospitalists, in other countries. This may be the first study to comprehensively assess acute hospital care among schizophrenic patients. We determined common causes of and several risk factors associated with acute care mortality. These findings may help to identify schizophrenic patients at risk of dying when caring for these patients in acute care hospitals. In addition, the importance of preventive programs focusing on suicide would also be applicable to other countries.
We interpreted our results according to whether they are clinically significant. First, we performed the study at a single institution in Okinawa, Japan. Thus, our findings would require external confirmation for their generalizability to other acute care hospital settings. Second, different systems of health care in different countries may influence not only overall mortality but also hospital mortality. Comparative studies of hospital mortality at acute care general hospitals in different countries would be helpful. Third, we analyzed inpatient hospital mortality rather than long‐term mortality, including follow‐up, after hospital discharge. Further studies are needed to determine whether there may be excess mortality after patients are discharged from acute care hospitals. Fourth, our study used administrative data. Possible misclassification in coding disease and clinical characteristics may limit the utility of administrative data for interpreting the results.
In summary, this study may be the first report on the mortality of schizophrenic patients in acute care hospitalization. There was a nearly significant trend towards an increase in the standardized mortality of schizophrenic patients compared with that of general patients. Malignant neoplasm and cardiovascular diseases were significant factors associated with increased mortality. Suicide was the most frequent cause of death in this patient population.
Acknowledgements
We thank Mr. Masahito Taira and Ms. Noriko Irei for their excellent support of our data analysis and Mrs. Tomoko Yonaha for her excellent secretarial support.
- ,.Mortality in a cohort of patients with schizophrenia: a record linkage study.Can J Psychiatry.1991;36:239–245.
- ,,, et al.Excess mortality among long‐stay psychiatric patients in northern Finland.Soc Psychiatry Psychiatr Epidemiol.2003;38:297–304.
- ,,, et al.Mortality among patients with schizophrenia and reduced psychiatric hospital care.Psychol Med.2005;35:725–732.
- ,,, et al.Mortality in psychiatric patients 5 to 21 years after hospital admission in Italy.Psychol Med.2002;32:227–237.
- ,,, et al.Mental disorders and cause‐specific mortality.Br J Psychiatry.2001;179:498–502.
- ,,, et al.Mortality in Western Australian psychiatric patients.Soc Psychiatry Psychiatr Epidemiol.2000;35:341–347.
- ,.Mortality among discharged psychiatric patients in Finland.Acta Psychiatr Scand.1999;99:102–109.
- ,,.Mortality in long‐stay patients from psychiatric hospitals in Italy—results from the Qualyop Project.Soc Psychiatry Psychiatr Epidemiol.2003;38:385–389.
- ,,.Total mortality in people admitted to a psychiatric hospital.Br J Psychiatry.1997;170:186–190.
- ,,, et al.Where schizophrenic patients commit suicide: a review of suicide among inpatients and former inpatients.Int J Psychiatry Med.2005;35:171–190.
- ,,, et al.Mortality in psychiatric hospital patients: a cohort analysis of prognostic factors.Int J Epidemiol.1997;26:1227–1235
- ,.Excess mortality of mental disorder.Br J Psychiatry.1998;173:11–53.
- ,,, et al.Mortality in psychiatric patients, with a specific focus on cancer mortality associated with schizophrenia.Int J Epidemiol.1995;24:366–372.
- .Excess mortality of schizophrenia. A meta‐analysis.Br J Psychiatry.1997;171:502–508.
- ,,, et al.Mortality and causes of death in schizophrenia in Stockholm county, Sweden.Schizophr Res.2000;45:21–28.
- ,,, et al.Mortality among patients in psychiatric hospitals in Germany.Acta Psychiatr Scand.1995;91:174–179.
- ,,, et al.Mortality in chronic schizophrenia during decreasing number of psychiatric beds in Finland.Schizophrenia Research2002;54:265–275.
- ,,.Cause‐specific mortality in psychiatric patients after deinstitutionalisation.Br J Psychiatry.2001;179:438–443.
- ,,.Causes of the excess mortality of schizophrenia.Br J Psychiatry.2000;177:212–217.
- ,,, et al.Mortality after discharge from long‐term psychiatric care in Scotland, 1977–94: a retrospective cohort study.BMC Public Health.2003;3:30.
- ,,.Prevalence of physical illness among psychiatric inpatients who die of natural causes.Psychiatr Serv.1998;49:788–793.
- ,,.Mortality and medical comorbidity among psychiatric patients: a review.Psychiatr Serv.1996;47:1356–1363.
- .The “silent” acute abdomen of schizophrenia.J Med Soc N J.1981;78:679–680.
- ,.Mortality in a cohort of patients with schizophrenia: a record linkage study.Can J Psychiatry.1991;36:239–245.
- ,,, et al.Excess mortality among long‐stay psychiatric patients in northern Finland.Soc Psychiatry Psychiatr Epidemiol.2003;38:297–304.
- ,,, et al.Mortality among patients with schizophrenia and reduced psychiatric hospital care.Psychol Med.2005;35:725–732.
- ,,, et al.Mortality in psychiatric patients 5 to 21 years after hospital admission in Italy.Psychol Med.2002;32:227–237.
- ,,, et al.Mental disorders and cause‐specific mortality.Br J Psychiatry.2001;179:498–502.
- ,,, et al.Mortality in Western Australian psychiatric patients.Soc Psychiatry Psychiatr Epidemiol.2000;35:341–347.
- ,.Mortality among discharged psychiatric patients in Finland.Acta Psychiatr Scand.1999;99:102–109.
- ,,.Mortality in long‐stay patients from psychiatric hospitals in Italy—results from the Qualyop Project.Soc Psychiatry Psychiatr Epidemiol.2003;38:385–389.
- ,,.Total mortality in people admitted to a psychiatric hospital.Br J Psychiatry.1997;170:186–190.
- ,,, et al.Where schizophrenic patients commit suicide: a review of suicide among inpatients and former inpatients.Int J Psychiatry Med.2005;35:171–190.
- ,,, et al.Mortality in psychiatric hospital patients: a cohort analysis of prognostic factors.Int J Epidemiol.1997;26:1227–1235
- ,.Excess mortality of mental disorder.Br J Psychiatry.1998;173:11–53.
- ,,, et al.Mortality in psychiatric patients, with a specific focus on cancer mortality associated with schizophrenia.Int J Epidemiol.1995;24:366–372.
- .Excess mortality of schizophrenia. A meta‐analysis.Br J Psychiatry.1997;171:502–508.
- ,,, et al.Mortality and causes of death in schizophrenia in Stockholm county, Sweden.Schizophr Res.2000;45:21–28.
- ,,, et al.Mortality among patients in psychiatric hospitals in Germany.Acta Psychiatr Scand.1995;91:174–179.
- ,,, et al.Mortality in chronic schizophrenia during decreasing number of psychiatric beds in Finland.Schizophrenia Research2002;54:265–275.
- ,,.Cause‐specific mortality in psychiatric patients after deinstitutionalisation.Br J Psychiatry.2001;179:438–443.
- ,,.Causes of the excess mortality of schizophrenia.Br J Psychiatry.2000;177:212–217.
- ,,, et al.Mortality after discharge from long‐term psychiatric care in Scotland, 1977–94: a retrospective cohort study.BMC Public Health.2003;3:30.
- ,,.Prevalence of physical illness among psychiatric inpatients who die of natural causes.Psychiatr Serv.1998;49:788–793.
- ,,.Mortality and medical comorbidity among psychiatric patients: a review.Psychiatr Serv.1996;47:1356–1363.
- .The “silent” acute abdomen of schizophrenia.J Med Soc N J.1981;78:679–680.
Copyright © 2008 Society of Hospital Medicine
Solifenacin‐Induced Small Bowel Pseudo‐Obstruction
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.
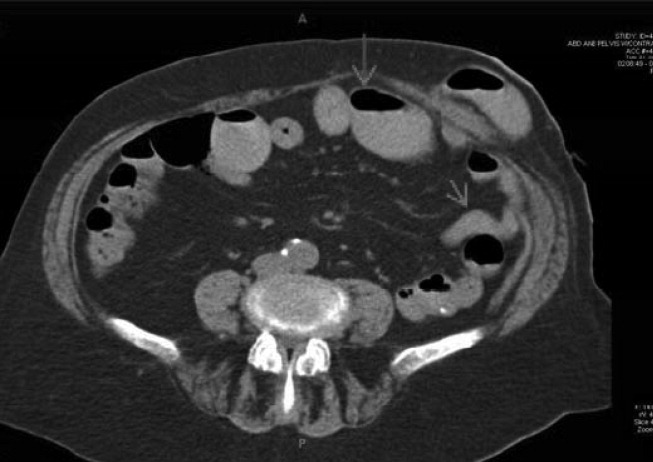
With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.

With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
Solfenacin succinate, an antimuscarinic agent, is approved for the treatment of overactive bladder and described as well tolerated in the elderly.1 We present the case of solifenacin‐induced small bowel pseudo‐obstruction in an 89‐year‐old woman.
FINDINGS
An 89‐year‐old woman with untreated stage 0 chronic lymphocytic leukemia and a history of stage III colorectal cancer treated with hemicolectomy and adjuvant capecitabine in 2003 was admitted to Johns Hopkins Hospital in 2006. She reported feeling dehydrated, nauseated, and constipated, with decreased output from her colostomy. She also noted no urine output for 4 days and felt that she had to urinate, but I can't. This coincided with a decrease in fluid intake. She denied fevers, chills, abdominal pain, or loss of appetite. While waiting to be seen in the emergency department, the patient was finally able to urinate.
She had no evidence of colon cancer recurrence, with a normal postoperative positron‐emission tomography (PET) scan in 2003, colonoscopy in 2005, and screening computerized tomography (CT) scan in 2005. She also had a history of well‐controlled hypertension and hypothyroidism, hyperlipidemia, chemotherapy‐induced neuropathy, and anxiety.
Her home medication regimen included solifenacin 5 mg once daily (started 10 days prior to her admission) for bladder overactivity, buspirone 5 mg 3 times a day, metoprolol 25 mg twice a day, pantoprazole 40 mg once daily, levothyroxine 100 g once daily, lisinopril/hydrochlorathiazide 20 mg/25 mg twice daily, gabapentin 300 mg twice a day, and fenofibrate 145 mg nightly.
The patient appeared nontoxic. Her exam was remarkable only for hypoactive bowel sounds and mild diffuse abdominal tenderness without distension or peritoneal signs. A Foley catheter was placed, and her postvoid residual was only 50 cc of urine. Her admission serum blood urea nitrogen and creatinine were 90 and 3.4 mg/dL, respectively, as compared with 18 and 0.8 mg/dL 2 months prior to presentation. A CT scan of the abdomen (Figure 1) revealed multiple dilated loops of small bowel with a transition point at the left lower quadrant ostomy site, consistent with a small bowel obstruction. A PET scan revealed no evidence of malignancy. A renal ultrasound showed no evidence of obstruction.

With cessation of solifenacin and lisinopril/hydrochlorothiazide and hydration with normal saline, her constipation resolved, as did her acute renal failure and perception of urinary retention. She began to tolerate a regular diet after 4 days of hospitalization, and her colostomy output normalized. At follow‐up 8 months after admission, her creatinine was 0.8 mg/dL, and a screening abdominal CT showed complete resolution of the small bowel obstruction.
DISCUSSION
We believe that this patient developed small bowel pseudo‐obstruction as well the feeling of urinary retention because of treatment with solifenacin, an antimuscarinic agent approved for the treatment of bladder overactivity. Her acute renal failure was a result of prerenal azotemia. This particular patient was at increased risk for developing antimuscarinic‐induced bowel obstruction because of her previous surgery and exposure to chemotherapy.
In the 4 randomized trials cited in the prescribing information for solifenacin,2 only 189 patients of the 1811 who received the active drug were greater than 75 years old. Healthy elderly patients ranging from 64 to 78 years of age (mean 68.0 years) who received 2 weeks of treatment with solifenacin 5 and 10 mg had a mean AUC024 that was approximately 20% higher than that of younger subjects.3 In the 4 12‐week double‐blind clinical trials in which 1158 patients were treated with solifenacin 10 mg, there were 3 serious intestinal adverse events: 1 patient had a fecal impaction, 1 patient had a colonic obstruction, and 1 patient had an intestinal obstruction.2 Patients receiving solifenacin 5 and 10 mg were more likely to experience constipation than those receiving placebo (5.4%, 13.4%, and 2.9%, respectively).2 Given the dearth of clinical data on patients greater than 75 years old, the effects of age on the pharmacokinetics, the higher likelihood of bowel pathology in the elderly, the increased risk of solifenacin‐induced side effects in the elderly as reported in the pooled analysis of patients at least 65 years old,4 and the small clinical benefit of solifenacin,46 physicians should seriously consider whether the benefits of solifenacin outweigh both the known and the possible risks. 0
| Patients in safety analysis (n) | Constipation, n (%) | Micturition/24 hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | Placebo | 5 mg | 10 mg | Baseline | Mean decrease from baseline | |||
| Placebo | 5 mg | 10 mg | ||||||||
| ||||||||||
| Chapple et al.6* | 267 | 279 | 268 | 5 (1.9) | 20 (7.2) | 21 (7.8) | 12.0812.32 | 1.2 | 2.19 | 2.61 |
| Cardozo et al.4* | 301 | 299 | 307 | 6 (2.0) | 11 (3.7) | 28 (9.1) | 12.0512.31 | 1.59 | 2.37 | 2.81 |
| Wagg3 | 422 | 192 | 431 | 18 (4.3) | 18 (9.4) | 78 (18.1) | 11.611.7 | 1.1 | 2.0 | 2.5 |
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.
- .Solifenacin provides effective antimuscarinic therapy for the complete management of overactive bladder.Expert Opin Pharmacother.2006;7:2421–2434.
- Yamanouchi Pharma America, Inc.United States prescribing information for solifenacin succinate (Vesicare®), November2004.
- ,,,,.Effect of age on the pharmacokinetics of solifenacin in men and women.Int J Clin Pharmacol Ther.2005;43:227–238.
- ,,.Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis.Am J Geriatr Pharmacother.2006;4(1):14–24.
- ,,, et al.Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder.J Urol.2004;172(5 Pt 1):1919–1924.
- ,,, et al.Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder.BJU Int.2004;93:303–310.
Hepatitis C–Associated Penile Necrosis
Leukocytoclastic vasculitis (LCCV) and deep venous thrombosis (DVT) are uncommon manifestations of hepatitis C and when seen, are usually associated with cryoglobulinemia. The presence of hepatitis Cassociated antiphospholipid antibodies (APLAs) such as anticardiolipin antibodies may increase the risk of deep venous thrombosis. Hepatitis Cassociated APLAs and LCCV leading to penile necrosis has not previously been reported, to our knowledge.
CASE
A previously healthy 57‐year‐old white man with hepatitis C presented with a 2‐ to 3‐day history of testicular pain and spreading, tender erythema on his left inner thigh. He reported 2 days of testicular and penile swelling and blackening of his penis 1 day prior to admission. He denied feeling ill, fevers, chills, nausea, vomiting, dysuria, hematuria, abdominal, back or penile pain or trauma, unusual sexual practices, or new medications.
His medical history was significant for IV drug use, hepatitis C infection, and hypertension in the remote past. He was in a 2‐year monogamous relationship with his female partner and denied any history of sexually transmitted diseases; however, he did report erectile dysfunction over the last few months. He worked as a bartender and reportedly drank 1 glass of wine per night. He denied tobacco or current IV drug use and did occasionally smoke marijuana. His medications included atenolol, hydrochlorothiazide, fish oil, cottonseed oil, and a multivitamin. He denied use of any herbal supplements or erectile dysfunction medications.
On physical exam he did not appear toxic. Vital signs were temperature of 37.3C, blood pressure of 155/80, pulse of 100, and O2 saturation of 97% on room air. His HEENT, cardiovascular, lung, and abdominal exams were unremarkable. He had a 5‐cm indurated, dark, erythematous lesion on his left thigh, surrounded by diffuse tracking erythema, and an erythematous and indurated suprapubic region. His uncircumcised penis was swollen and black, with a sharp demarcation near the base of the shaft (Fig. 1). A CT scan with oral and IV contrast demonstrated thickening and edema of the scrotum, suprapubic soft tissue, and penis, with asymmetric enlargement of the left corpora. Mild cirrhosis with associated small gastric varices was also noted. No thrombosis, atherosclerosis, or gas or fluid collection was noted. The bladder, prostate, and seminal vesicles were normal.
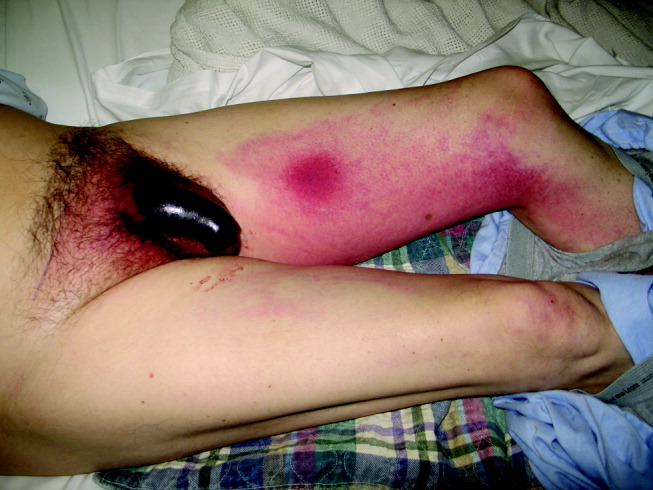
A punch biopsy of the leg lesion revealed LCCV with dense fibrin deposition throughout the vessels. Abnormal laboratory data included a mildly elevated WBC count, decreased hemoglobin, thrombocytopenia, mild hyponatremia, low albumin, mildly increased glucose, mild transaminitis, and increased bilirubin. He also had an increased aPTT, elevated ESR, positive hepatitis C PCR and antibodies, positive rheumatoid factor, and high titers of anticardiolipin IgM and anti‐B2GPI IgM.
On hospital day 2, a urological surgery was performed to remove the necrotic penile tissue, including the foreskin, down to the spared tunica albuginea. Pathology studies of the tissue specimens revealed highly vascular subcutaneous tissue with hemorrhage and focal denudation, consistent with necrosis. Following surgery, the patient's platelet count and INR returned to normal levels. No steroids or cytotoxic agents were given. On hospital day 6, the patient developed bilateral leg pain and swelling. Lower extremity doppler ultrasound examination revealed occlusive DVT of the right gastrocnemius, popliteal, and greater saphenous veins, as well thromboses in the left gastrocnemius, soleal, posterior tibial, and greater saphenous veins; thus, enoxaparin therapy was initiated. On hospital day 8, the patient returned to the operating room for a penile tunneling procedure, in which the penis was surgically inserted into the scrotum as an alternative to skin grafting. He recovered well from the surgeries and was discharged on hospital day 11 on oral anticoagulation with warfarin. At follow‐up 1 month after discharge, the patient was doing well and planned for surgery to free his penis from the scrotal sac in 2 months' time.
DISCUSSION
This case illustrates uncommon extrahepatic manifestations of hepatitis C, including leukocytoclastic vasculitis and deep venous thromboses. Our patient, with abnormal LFTs and positive hepatitis C titers, presented with tissue necrosis of the penis and an unidentifiable erythematous lesion on the leg and subsequently developed multiple deep venous thromboses during his hospital course. Initial diagnostic considerations of the penile and skin lesions included fixed drug reaction, trauma, ischemia, infection, arachnid bite, and vasculitis. The patient denied exposure to NSAIDS, antibiotics, anticonvulsants, or anticoagulants, which are commonly reported causes of fixed drug reactions. He denied trauma or spider or bug bites, was nontoxic appearing, afebrile, and had a near‐normal white blood cell count. While awaiting laboratory and biopsy results, we did not initiate pharmacological therapy because of the unknown etiology of the patient's pathology. The patient's workup revealed that his symptoms were most likely secondary to cryoglobulin‐negative hepatitis C infection with leukocytoclastic vasculitis and antiphospholipid antibodies, leading to necrosis of the penile prepucean entity that, to our knowledge, has not been reported.
Leukocytoclastic vasculitis is a complication of many diseases including Henoch‐Schnlein purpura, Wegener's granulomatosis, sepsis, ANCA‐associated vasculitis, SLE, and hepatitis C.14 Leukocytoclastic vasculitis often presents with palpable purpura but may also present with frank necrosis.4 Penile leukocytoclastic vasculitis has been reported in the literature previously5; however, most of these cases involve Wegener's granulomatosis and Henoch‐Schnlein purpura. One case series demonstrated that approximately 1% of patients with hepatitis C develop vasculitis during the course of their illness.19 There has been 1 reported case of penile leukocytoclastic vasculitis, which occurred in a patient with hepatitis C who was found to also have cryoglobulinemia.6 Our patient tested negative for cryoglobulins twice during his hospital stay and also had normal complement levels, which strongly weighs against cryoglobulinemia. One study reported that up to 75% of patients with hepatitis C who develop leukocytoclastic vasculitis will test positive for cryoglobulins6; thus, our patient's presentation with cryoglobulin‐negative leukocytoclastic vasculitis is rare.
Our patient also had a positive titer of anticardiolipin antibodies, which are a subset of APLAs. Antiphospholipid antibodies can be found in autoimmune disease, acute and chronic viral infections, and malignancy.7, 8 Furthermore, APLAs can manifest with arterial and venous thrombosis, and up to 33% of patients with hepatitis C test positive for APLAs.7 The etiology and thrombogenicity of these autoantibodies in the setting of chronic viral hepatitis is still largely unknown, but it has been hypothesized that APLAs may be an autoimmune manifestation of hepatitis C.
Our patient also tested positive for anti‐beta2‐glycoprotien‐1 antibodies, the presence of which may be associated with the occurrence of thrombotic events.9 The presence of these antibodies strengthens the likelihood that this patient's APLAs were pathogenic and likely associated with his skin necrosis as well as his numerous venous thromboses. Previously documented thromboses in patients with hepatitis C and APLAs include avascular bone necrosis, venous thromboembolism, MI, stroke, and cutaneous necrosis.10 There was 1 reported case of a patient with HIV and anticardiolipin antibodies with cutaneous necrosis and testicular thrombosis,10 however, to our knowledge there have been no reported cases of penile necrosis in association with APLAs in a patient with hepatitis C. In this case, treatment with steroids or cytotoxic agents was not warranted because of insufficient evidence to support this practice. However, lifelong anticoagulation with moderate‐intensity warfarin to prevent future thrombosis is indicated.11 These antibodies and their treatment are poorly understood, and further studies are needed to gain insight into both their development and their role in the pathogenesis of disease in patients with viral hepatitis.
In summary, this patient experienced devastating complications of chronic hepatitis C infection, leading to necrosis of the penile prepuce and multiple venous thromboses. This case demonstrates that extrahepatic symptoms of hepatitis C infection, including skin manifestations secondary to leukocytoclastic vasculitis with or without cryglobulinemia, may occur. Furthermore, this case illustrates the increased risk of thrombosis and cutaneous necrosis in patients with chronic hepatitis C infection and associated antiphospholipid antibodies.
Acknowledgements
The authors acknowledge Alan Hunter, MD, Thomas DeLoughery, MD, Brittany Wilson, MD, and Kevin White, MD.
- ,.Dermatologic manifestations of hepatitis C infection.Int J Dermatol.1997;36:251–254.
- ,.Extrahepatic manifestations in patients with chronic hepatitis C virus infection.Curr Opin Rheumatol.2005;17:447–455.
- ,.The prevalence of dermatologic manifestations related to chronic hepatitis C virus infection in a study from a single center in Turkey.Acta Dermatovenerol Alp Panonica Adriat.2005;14:93–98.
- ,,,.Management of leukocytoclastic vasculitis.J Dermatolog Treat.2005;16:193–206.
- ,,, et al.Hepatitis C, cryoglbulinemia, and cutaneous vasculitis associated with unusual and serious manifestations.Am J Gastroenterol.2001;96:2489–2493.
- ,,, et al.Extrahepatic manifestations of chronic hepatitis C.Arthritis Rheumatism.1999;42:2204–2212.
- ,,.Prevalence of antiphospholipid antibodies in patients with chronic lever disease related to alcohol or hepatitis C virus: correlation with liver injury.J Lab Clin Med.1998;31:243–250.
- ,.Antiphospholipid antibodies and antiphospholipid syndrome.Curr Opin Rheumatol.1995;7:389–94.
- ,.Anticardiolipin antibodies in chronic viral hepatitis. Do they have clinical consequences?Eur J Gastroenterol Hepatol.2003;15:717–719.
- ,,, et al.Clinical features related to antiphospholipid syndrome in patients with chronic viral infections (hepatits C virus/HIV infection): description of 82 cases.CID.2004;38:1009–1016.
- ,,.Management of antiphospholipid antibody syndrome: A systematic review.JAMA.2006;295:1050–1057.
Leukocytoclastic vasculitis (LCCV) and deep venous thrombosis (DVT) are uncommon manifestations of hepatitis C and when seen, are usually associated with cryoglobulinemia. The presence of hepatitis Cassociated antiphospholipid antibodies (APLAs) such as anticardiolipin antibodies may increase the risk of deep venous thrombosis. Hepatitis Cassociated APLAs and LCCV leading to penile necrosis has not previously been reported, to our knowledge.
CASE
A previously healthy 57‐year‐old white man with hepatitis C presented with a 2‐ to 3‐day history of testicular pain and spreading, tender erythema on his left inner thigh. He reported 2 days of testicular and penile swelling and blackening of his penis 1 day prior to admission. He denied feeling ill, fevers, chills, nausea, vomiting, dysuria, hematuria, abdominal, back or penile pain or trauma, unusual sexual practices, or new medications.
His medical history was significant for IV drug use, hepatitis C infection, and hypertension in the remote past. He was in a 2‐year monogamous relationship with his female partner and denied any history of sexually transmitted diseases; however, he did report erectile dysfunction over the last few months. He worked as a bartender and reportedly drank 1 glass of wine per night. He denied tobacco or current IV drug use and did occasionally smoke marijuana. His medications included atenolol, hydrochlorothiazide, fish oil, cottonseed oil, and a multivitamin. He denied use of any herbal supplements or erectile dysfunction medications.
On physical exam he did not appear toxic. Vital signs were temperature of 37.3C, blood pressure of 155/80, pulse of 100, and O2 saturation of 97% on room air. His HEENT, cardiovascular, lung, and abdominal exams were unremarkable. He had a 5‐cm indurated, dark, erythematous lesion on his left thigh, surrounded by diffuse tracking erythema, and an erythematous and indurated suprapubic region. His uncircumcised penis was swollen and black, with a sharp demarcation near the base of the shaft (Fig. 1). A CT scan with oral and IV contrast demonstrated thickening and edema of the scrotum, suprapubic soft tissue, and penis, with asymmetric enlargement of the left corpora. Mild cirrhosis with associated small gastric varices was also noted. No thrombosis, atherosclerosis, or gas or fluid collection was noted. The bladder, prostate, and seminal vesicles were normal.

A punch biopsy of the leg lesion revealed LCCV with dense fibrin deposition throughout the vessels. Abnormal laboratory data included a mildly elevated WBC count, decreased hemoglobin, thrombocytopenia, mild hyponatremia, low albumin, mildly increased glucose, mild transaminitis, and increased bilirubin. He also had an increased aPTT, elevated ESR, positive hepatitis C PCR and antibodies, positive rheumatoid factor, and high titers of anticardiolipin IgM and anti‐B2GPI IgM.
On hospital day 2, a urological surgery was performed to remove the necrotic penile tissue, including the foreskin, down to the spared tunica albuginea. Pathology studies of the tissue specimens revealed highly vascular subcutaneous tissue with hemorrhage and focal denudation, consistent with necrosis. Following surgery, the patient's platelet count and INR returned to normal levels. No steroids or cytotoxic agents were given. On hospital day 6, the patient developed bilateral leg pain and swelling. Lower extremity doppler ultrasound examination revealed occlusive DVT of the right gastrocnemius, popliteal, and greater saphenous veins, as well thromboses in the left gastrocnemius, soleal, posterior tibial, and greater saphenous veins; thus, enoxaparin therapy was initiated. On hospital day 8, the patient returned to the operating room for a penile tunneling procedure, in which the penis was surgically inserted into the scrotum as an alternative to skin grafting. He recovered well from the surgeries and was discharged on hospital day 11 on oral anticoagulation with warfarin. At follow‐up 1 month after discharge, the patient was doing well and planned for surgery to free his penis from the scrotal sac in 2 months' time.
DISCUSSION
This case illustrates uncommon extrahepatic manifestations of hepatitis C, including leukocytoclastic vasculitis and deep venous thromboses. Our patient, with abnormal LFTs and positive hepatitis C titers, presented with tissue necrosis of the penis and an unidentifiable erythematous lesion on the leg and subsequently developed multiple deep venous thromboses during his hospital course. Initial diagnostic considerations of the penile and skin lesions included fixed drug reaction, trauma, ischemia, infection, arachnid bite, and vasculitis. The patient denied exposure to NSAIDS, antibiotics, anticonvulsants, or anticoagulants, which are commonly reported causes of fixed drug reactions. He denied trauma or spider or bug bites, was nontoxic appearing, afebrile, and had a near‐normal white blood cell count. While awaiting laboratory and biopsy results, we did not initiate pharmacological therapy because of the unknown etiology of the patient's pathology. The patient's workup revealed that his symptoms were most likely secondary to cryoglobulin‐negative hepatitis C infection with leukocytoclastic vasculitis and antiphospholipid antibodies, leading to necrosis of the penile prepucean entity that, to our knowledge, has not been reported.
Leukocytoclastic vasculitis is a complication of many diseases including Henoch‐Schnlein purpura, Wegener's granulomatosis, sepsis, ANCA‐associated vasculitis, SLE, and hepatitis C.14 Leukocytoclastic vasculitis often presents with palpable purpura but may also present with frank necrosis.4 Penile leukocytoclastic vasculitis has been reported in the literature previously5; however, most of these cases involve Wegener's granulomatosis and Henoch‐Schnlein purpura. One case series demonstrated that approximately 1% of patients with hepatitis C develop vasculitis during the course of their illness.19 There has been 1 reported case of penile leukocytoclastic vasculitis, which occurred in a patient with hepatitis C who was found to also have cryoglobulinemia.6 Our patient tested negative for cryoglobulins twice during his hospital stay and also had normal complement levels, which strongly weighs against cryoglobulinemia. One study reported that up to 75% of patients with hepatitis C who develop leukocytoclastic vasculitis will test positive for cryoglobulins6; thus, our patient's presentation with cryoglobulin‐negative leukocytoclastic vasculitis is rare.
Our patient also had a positive titer of anticardiolipin antibodies, which are a subset of APLAs. Antiphospholipid antibodies can be found in autoimmune disease, acute and chronic viral infections, and malignancy.7, 8 Furthermore, APLAs can manifest with arterial and venous thrombosis, and up to 33% of patients with hepatitis C test positive for APLAs.7 The etiology and thrombogenicity of these autoantibodies in the setting of chronic viral hepatitis is still largely unknown, but it has been hypothesized that APLAs may be an autoimmune manifestation of hepatitis C.
Our patient also tested positive for anti‐beta2‐glycoprotien‐1 antibodies, the presence of which may be associated with the occurrence of thrombotic events.9 The presence of these antibodies strengthens the likelihood that this patient's APLAs were pathogenic and likely associated with his skin necrosis as well as his numerous venous thromboses. Previously documented thromboses in patients with hepatitis C and APLAs include avascular bone necrosis, venous thromboembolism, MI, stroke, and cutaneous necrosis.10 There was 1 reported case of a patient with HIV and anticardiolipin antibodies with cutaneous necrosis and testicular thrombosis,10 however, to our knowledge there have been no reported cases of penile necrosis in association with APLAs in a patient with hepatitis C. In this case, treatment with steroids or cytotoxic agents was not warranted because of insufficient evidence to support this practice. However, lifelong anticoagulation with moderate‐intensity warfarin to prevent future thrombosis is indicated.11 These antibodies and their treatment are poorly understood, and further studies are needed to gain insight into both their development and their role in the pathogenesis of disease in patients with viral hepatitis.
In summary, this patient experienced devastating complications of chronic hepatitis C infection, leading to necrosis of the penile prepuce and multiple venous thromboses. This case demonstrates that extrahepatic symptoms of hepatitis C infection, including skin manifestations secondary to leukocytoclastic vasculitis with or without cryglobulinemia, may occur. Furthermore, this case illustrates the increased risk of thrombosis and cutaneous necrosis in patients with chronic hepatitis C infection and associated antiphospholipid antibodies.
Acknowledgements
The authors acknowledge Alan Hunter, MD, Thomas DeLoughery, MD, Brittany Wilson, MD, and Kevin White, MD.
Leukocytoclastic vasculitis (LCCV) and deep venous thrombosis (DVT) are uncommon manifestations of hepatitis C and when seen, are usually associated with cryoglobulinemia. The presence of hepatitis Cassociated antiphospholipid antibodies (APLAs) such as anticardiolipin antibodies may increase the risk of deep venous thrombosis. Hepatitis Cassociated APLAs and LCCV leading to penile necrosis has not previously been reported, to our knowledge.
CASE
A previously healthy 57‐year‐old white man with hepatitis C presented with a 2‐ to 3‐day history of testicular pain and spreading, tender erythema on his left inner thigh. He reported 2 days of testicular and penile swelling and blackening of his penis 1 day prior to admission. He denied feeling ill, fevers, chills, nausea, vomiting, dysuria, hematuria, abdominal, back or penile pain or trauma, unusual sexual practices, or new medications.
His medical history was significant for IV drug use, hepatitis C infection, and hypertension in the remote past. He was in a 2‐year monogamous relationship with his female partner and denied any history of sexually transmitted diseases; however, he did report erectile dysfunction over the last few months. He worked as a bartender and reportedly drank 1 glass of wine per night. He denied tobacco or current IV drug use and did occasionally smoke marijuana. His medications included atenolol, hydrochlorothiazide, fish oil, cottonseed oil, and a multivitamin. He denied use of any herbal supplements or erectile dysfunction medications.
On physical exam he did not appear toxic. Vital signs were temperature of 37.3C, blood pressure of 155/80, pulse of 100, and O2 saturation of 97% on room air. His HEENT, cardiovascular, lung, and abdominal exams were unremarkable. He had a 5‐cm indurated, dark, erythematous lesion on his left thigh, surrounded by diffuse tracking erythema, and an erythematous and indurated suprapubic region. His uncircumcised penis was swollen and black, with a sharp demarcation near the base of the shaft (Fig. 1). A CT scan with oral and IV contrast demonstrated thickening and edema of the scrotum, suprapubic soft tissue, and penis, with asymmetric enlargement of the left corpora. Mild cirrhosis with associated small gastric varices was also noted. No thrombosis, atherosclerosis, or gas or fluid collection was noted. The bladder, prostate, and seminal vesicles were normal.

A punch biopsy of the leg lesion revealed LCCV with dense fibrin deposition throughout the vessels. Abnormal laboratory data included a mildly elevated WBC count, decreased hemoglobin, thrombocytopenia, mild hyponatremia, low albumin, mildly increased glucose, mild transaminitis, and increased bilirubin. He also had an increased aPTT, elevated ESR, positive hepatitis C PCR and antibodies, positive rheumatoid factor, and high titers of anticardiolipin IgM and anti‐B2GPI IgM.
On hospital day 2, a urological surgery was performed to remove the necrotic penile tissue, including the foreskin, down to the spared tunica albuginea. Pathology studies of the tissue specimens revealed highly vascular subcutaneous tissue with hemorrhage and focal denudation, consistent with necrosis. Following surgery, the patient's platelet count and INR returned to normal levels. No steroids or cytotoxic agents were given. On hospital day 6, the patient developed bilateral leg pain and swelling. Lower extremity doppler ultrasound examination revealed occlusive DVT of the right gastrocnemius, popliteal, and greater saphenous veins, as well thromboses in the left gastrocnemius, soleal, posterior tibial, and greater saphenous veins; thus, enoxaparin therapy was initiated. On hospital day 8, the patient returned to the operating room for a penile tunneling procedure, in which the penis was surgically inserted into the scrotum as an alternative to skin grafting. He recovered well from the surgeries and was discharged on hospital day 11 on oral anticoagulation with warfarin. At follow‐up 1 month after discharge, the patient was doing well and planned for surgery to free his penis from the scrotal sac in 2 months' time.
DISCUSSION
This case illustrates uncommon extrahepatic manifestations of hepatitis C, including leukocytoclastic vasculitis and deep venous thromboses. Our patient, with abnormal LFTs and positive hepatitis C titers, presented with tissue necrosis of the penis and an unidentifiable erythematous lesion on the leg and subsequently developed multiple deep venous thromboses during his hospital course. Initial diagnostic considerations of the penile and skin lesions included fixed drug reaction, trauma, ischemia, infection, arachnid bite, and vasculitis. The patient denied exposure to NSAIDS, antibiotics, anticonvulsants, or anticoagulants, which are commonly reported causes of fixed drug reactions. He denied trauma or spider or bug bites, was nontoxic appearing, afebrile, and had a near‐normal white blood cell count. While awaiting laboratory and biopsy results, we did not initiate pharmacological therapy because of the unknown etiology of the patient's pathology. The patient's workup revealed that his symptoms were most likely secondary to cryoglobulin‐negative hepatitis C infection with leukocytoclastic vasculitis and antiphospholipid antibodies, leading to necrosis of the penile prepucean entity that, to our knowledge, has not been reported.
Leukocytoclastic vasculitis is a complication of many diseases including Henoch‐Schnlein purpura, Wegener's granulomatosis, sepsis, ANCA‐associated vasculitis, SLE, and hepatitis C.14 Leukocytoclastic vasculitis often presents with palpable purpura but may also present with frank necrosis.4 Penile leukocytoclastic vasculitis has been reported in the literature previously5; however, most of these cases involve Wegener's granulomatosis and Henoch‐Schnlein purpura. One case series demonstrated that approximately 1% of patients with hepatitis C develop vasculitis during the course of their illness.19 There has been 1 reported case of penile leukocytoclastic vasculitis, which occurred in a patient with hepatitis C who was found to also have cryoglobulinemia.6 Our patient tested negative for cryoglobulins twice during his hospital stay and also had normal complement levels, which strongly weighs against cryoglobulinemia. One study reported that up to 75% of patients with hepatitis C who develop leukocytoclastic vasculitis will test positive for cryoglobulins6; thus, our patient's presentation with cryoglobulin‐negative leukocytoclastic vasculitis is rare.
Our patient also had a positive titer of anticardiolipin antibodies, which are a subset of APLAs. Antiphospholipid antibodies can be found in autoimmune disease, acute and chronic viral infections, and malignancy.7, 8 Furthermore, APLAs can manifest with arterial and venous thrombosis, and up to 33% of patients with hepatitis C test positive for APLAs.7 The etiology and thrombogenicity of these autoantibodies in the setting of chronic viral hepatitis is still largely unknown, but it has been hypothesized that APLAs may be an autoimmune manifestation of hepatitis C.
Our patient also tested positive for anti‐beta2‐glycoprotien‐1 antibodies, the presence of which may be associated with the occurrence of thrombotic events.9 The presence of these antibodies strengthens the likelihood that this patient's APLAs were pathogenic and likely associated with his skin necrosis as well as his numerous venous thromboses. Previously documented thromboses in patients with hepatitis C and APLAs include avascular bone necrosis, venous thromboembolism, MI, stroke, and cutaneous necrosis.10 There was 1 reported case of a patient with HIV and anticardiolipin antibodies with cutaneous necrosis and testicular thrombosis,10 however, to our knowledge there have been no reported cases of penile necrosis in association with APLAs in a patient with hepatitis C. In this case, treatment with steroids or cytotoxic agents was not warranted because of insufficient evidence to support this practice. However, lifelong anticoagulation with moderate‐intensity warfarin to prevent future thrombosis is indicated.11 These antibodies and their treatment are poorly understood, and further studies are needed to gain insight into both their development and their role in the pathogenesis of disease in patients with viral hepatitis.
In summary, this patient experienced devastating complications of chronic hepatitis C infection, leading to necrosis of the penile prepuce and multiple venous thromboses. This case demonstrates that extrahepatic symptoms of hepatitis C infection, including skin manifestations secondary to leukocytoclastic vasculitis with or without cryglobulinemia, may occur. Furthermore, this case illustrates the increased risk of thrombosis and cutaneous necrosis in patients with chronic hepatitis C infection and associated antiphospholipid antibodies.
Acknowledgements
The authors acknowledge Alan Hunter, MD, Thomas DeLoughery, MD, Brittany Wilson, MD, and Kevin White, MD.
- ,.Dermatologic manifestations of hepatitis C infection.Int J Dermatol.1997;36:251–254.
- ,.Extrahepatic manifestations in patients with chronic hepatitis C virus infection.Curr Opin Rheumatol.2005;17:447–455.
- ,.The prevalence of dermatologic manifestations related to chronic hepatitis C virus infection in a study from a single center in Turkey.Acta Dermatovenerol Alp Panonica Adriat.2005;14:93–98.
- ,,,.Management of leukocytoclastic vasculitis.J Dermatolog Treat.2005;16:193–206.
- ,,, et al.Hepatitis C, cryoglbulinemia, and cutaneous vasculitis associated with unusual and serious manifestations.Am J Gastroenterol.2001;96:2489–2493.
- ,,, et al.Extrahepatic manifestations of chronic hepatitis C.Arthritis Rheumatism.1999;42:2204–2212.
- ,,.Prevalence of antiphospholipid antibodies in patients with chronic lever disease related to alcohol or hepatitis C virus: correlation with liver injury.J Lab Clin Med.1998;31:243–250.
- ,.Antiphospholipid antibodies and antiphospholipid syndrome.Curr Opin Rheumatol.1995;7:389–94.
- ,.Anticardiolipin antibodies in chronic viral hepatitis. Do they have clinical consequences?Eur J Gastroenterol Hepatol.2003;15:717–719.
- ,,, et al.Clinical features related to antiphospholipid syndrome in patients with chronic viral infections (hepatits C virus/HIV infection): description of 82 cases.CID.2004;38:1009–1016.
- ,,.Management of antiphospholipid antibody syndrome: A systematic review.JAMA.2006;295:1050–1057.
- ,.Dermatologic manifestations of hepatitis C infection.Int J Dermatol.1997;36:251–254.
- ,.Extrahepatic manifestations in patients with chronic hepatitis C virus infection.Curr Opin Rheumatol.2005;17:447–455.
- ,.The prevalence of dermatologic manifestations related to chronic hepatitis C virus infection in a study from a single center in Turkey.Acta Dermatovenerol Alp Panonica Adriat.2005;14:93–98.
- ,,,.Management of leukocytoclastic vasculitis.J Dermatolog Treat.2005;16:193–206.
- ,,, et al.Hepatitis C, cryoglbulinemia, and cutaneous vasculitis associated with unusual and serious manifestations.Am J Gastroenterol.2001;96:2489–2493.
- ,,, et al.Extrahepatic manifestations of chronic hepatitis C.Arthritis Rheumatism.1999;42:2204–2212.
- ,,.Prevalence of antiphospholipid antibodies in patients with chronic lever disease related to alcohol or hepatitis C virus: correlation with liver injury.J Lab Clin Med.1998;31:243–250.
- ,.Antiphospholipid antibodies and antiphospholipid syndrome.Curr Opin Rheumatol.1995;7:389–94.
- ,.Anticardiolipin antibodies in chronic viral hepatitis. Do they have clinical consequences?Eur J Gastroenterol Hepatol.2003;15:717–719.
- ,,, et al.Clinical features related to antiphospholipid syndrome in patients with chronic viral infections (hepatits C virus/HIV infection): description of 82 cases.CID.2004;38:1009–1016.
- ,,.Management of antiphospholipid antibody syndrome: A systematic review.JAMA.2006;295:1050–1057.
Unusual Cardiac Rhythm Device Infection
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.
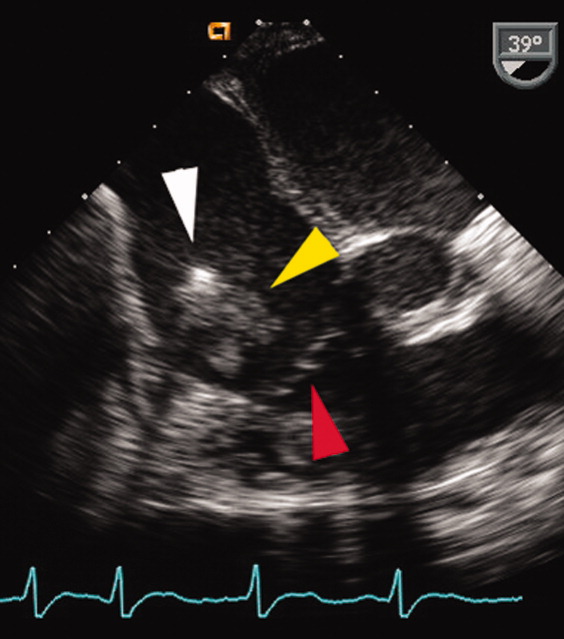
DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.


DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.


DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.