User login
Break the ‘fear circuit’ in resistant panic disorder
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
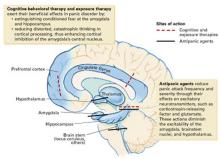
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
When initial therapy fails to control a patient’s panic attacks, a neuroanatomic model of anxiety disorders may help. This model proposes that panic sufferers have an abnormally sensitive brain “fear circuit.”1 It suggests why both medications and cognitive-behavioral therapy (CBT) are effective for treating panic disorder (PD) and can be used as a guide to more successful treatment.
This article explains the fear circuit model and describes how to determine whether initial drug treatment of panic symptoms has been adequate. It offers evidence-and experience-based dosing ranges, augmentation strategies, tips for antidepressant titration, and solutions to the most common inadequate response problems.
HOW THE FEAR CIRCUIT WORKS
Panic disorder may occur with or without agoraphobia. The diagnosis requires recurrent, unexpected panic attacks (Table 1), with at least one attack followed by 1 month or more of:
- persistent concern about having additional attacks
- worry about the implications of the attack
- or significant change in behavior related to the attack.
Panic disorder is usually accompanied by phobic avoidance and anticipatory anxiety, and it often coexists with other psychiatric disorders. Anxiety disorders may share a common genetic vulnerability. Childhood experiences, gender, and life events may increase or decrease the probability that a biologically vulnerable individual will develop an anxiety disorder or depression.1
Table 1
Panic attacks: The core symptom of panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and peak within 10 minutes: |
|
| Source: DSM-IV-TR |
Fear circuit model. PD’s pathophysiology is not completely understood, but evidence suggests that an overactive brain alarm network may increase vulnerability for PD (Box).1,2 Individual patients require different intensities of treatment to normalize their panic symptoms:
Mild to moderate PD (characterized by little or no avoidance and no comorbid disorders) often responds to either medication or CBT. A single intervention—such as using CBT to enhance the cortical inhibitory effects or using medication to reduce the amygdala’s reactivity—may suffice for symptomatic relief.
Severe or complicated PD (characterized by frequent panic attacks, significant agoraphobia, and comorbid anxiety disorders or depression) may require high medication dosages, intense CBT/exposure therapy, or both to normalize more severely disrupted communication among the fear circuit’s components.
ASSESSING TREATMENT OUTCOME
The goal of treatment is remission: a return to functioning without illness-related impairment or loss of quality of life, as if the patient had never been ill. In clinical practice, we can use validated, patient-rated assessment tools to document improvement in panic-related impairment, patient satisfaction, and quality of life—the real targets of treatment. Two useful tools are the Sheehan Disability Scale3 and the Quality of Life Enjoyment and Satisfaction Questionnaire.4
With adequate treatment, achieving remission can take several months or more; without it, remission may never occur. The following guidelines can help ensure that you provide adequate treatment.
What is adequate CBT? When patients’ symptoms fail to respond to CBT, the first step is to examine whether inadequate treatment is the culprit. At least 10 weekly CBT sessions administered by a “qualified professional” has been suggested as an adequate CBT trial for PD.5 Unfortunately, qualified CBT therapists are not always available. If CBT referral is not an option, clinicians can provide patients with at least some elements of CBT, such as education about PD, information resources, and self-exposure instruction as indicated. For more information on CBT for PD, see Related Resources.
What is adequate drug treatment? Noncompliance with medication because a patient fears adverse effects or has insufficient information can easily thwart treatment. Before treatment begins, therefore, it is important to establish your credibility. Provide the patient with information about PD, its treatment options, and what to expect so that he or she can collaborate in treatment (Table 2).
An inherited, abnormally active brain alarm mechanism—or “fear circuit”—may explain panic disorder, according to a theoretical neuroanatomic model.1 Its hub is the central nucleus of the amygdala, which coordinates fear responses via pathways communicating with the hippocampus, thalamus, hypothalamus, brainstem, and cortical processing areas.
The amygdala mediates acute emotional responses, including fear and anxiety. The hypothalamus mediates physiologic changes connected with emotions, such as release of stress hormones and some changes in heart rate. The prefrontal cortex is involved in thinking and memory and may be instrumental in predicting the consequences of rewards or punishments. In vulnerable individuals, defects in coordinating the sensory input among these brain regions may cause the central nucleus to discharge, resulting in a panic attack.
Medication and cognitive-behavioral therapy may reduce fear circuit reactivity and prevent panic attacks by acting at different components of the fear circuit. When the amygdala’s central nucleus no longer overreacts to sensory input, anticipatory anxiety and phobic avoidance usually dissipate over time.2,3 Thus, the fear circuit model integrates the clinical observation that both cognitive-behavioral therapy and medication are effective for treating panic.1
Abnormal interactions among components of this oversensitive fear circuit also may occur in social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, and depression.1 In these disorders, communication patterns among the parts of the hypothesized circuit may be disrupted in different ways. The clinical observation that anxious individuals often become depressed when under stress is consistent with this model and with the literature.
Antidepressants are preferred as first-line treatment of PD, even in nondepressed patients. Selective serotonin reuptake inhibitors (SSRIs) are recommended for PD because of their comparable efficacy and tolerability compared with older antipanic agents.6 SSRIs are also effective against other anxiety disorders likely to co-occur with PD.7
Many panic patients are exquisitely sensitive to activation by initial antidepressant dosages. Activation rarely occurs in other disorders, so its appearance suggests that your diagnosis is correct. Clinical strategies to help you manage antidepressant titration are suggested in Table 3.
Table 2
Prescription for success in treating panic disorder
| Relieve patient of perceived burden of being ill Explain the disorder’s familial/genetic origins Describe the fear circuit model Include spouse or significant other in treatment |
| Build patient-physician collaboration Explain potential medication side effects Describe the usual pattern of symptom relief (stop panic attacks → reduce anticipatory anxiety → decrease phobia) Estimate a time frame for improvement Map out next steps if first try is unsuccessful Be available, especially at first |
| Address patient’s long-term medication concerns Discuss safety, long-term efficacy Frame treatment as a pathway to independence from panic attacks Use analogy of diabetes or hypertension to explain that medication is for managing symptoms, rather than a cure Discuss tapering medication after sustained improvement (12 to 18 months) to determine continued need for medication |
In clinical settings, two naturalistic studies suggested that more-favorable outcomes are associated with antipanic medication dosages shown in Table 4 as “possibly effective”—and that most patients with poor medication response received inadequate treatment.8,9Table 4 ’s dosages come from those two studies—published before the efficacy studies of SSRIs in PD—and from later studies of SSRIs and the selective norepinephrine-serotonin reuptake inhibitor (SNRI) venlafaxine.7,8,10
The lower end of the “probably effective” range in Table 4 represents the lowest dose levels generally expected to be effective for PD. Not all agents in the table are FDA-approved for PD, nor are the dosages of approved agents necessarily “within labeling.” Some patients’ symptoms may resolve at higher or lower dosages.
Table 3
Tips to help the patient tolerate antidepressant titration
| Be pre-emptive Before starting therapy, explain that low initial dosing and flexible titration help to control unpleasant but medically safe “jitteriness” known as antidepressant-induced activation Tell the patient that activation rarely occurs in disorders other than PD (“Its appearance suggests that the diagnosis is correct and that we’re likely on the right track”) |
| Be reassuring Tell the patient, “You control the gas peddle—I’ll help you steer” (to an effective dose) |
| Be cautious Start with 25 to 50% of the usual antidepressant initial dosage for depression (Table 4); if too activating, reduce and advance more gradually Activation usually dissipates in 1 to 2 weeks; over time, larger dosage increments are often possible |
| Be attentive Use benzodiazepines or beta blockers as needed to attenuate activation |
Some patients require months to reach and maintain the “probably effective” dosage for at least 6 weeks. Short-term benzodiazepines can be used to control panic symptoms during antidepressant titration, then tapered off.11 We categorize patients who are unable to tolerate an “adequate dose” as not having had a therapeutic trial—not as treatment failures.
No controlled studies of PD have examined the success rate of switching to a second antidepressant after a first one has been ineffective.12 In clinical practice, we may try two different SSRIs and venlafaxine. When switching agents, we usually co-administer the treatments for a few weeks, titrate the second agent upward gradually, then taper and discontinue the first agent over 2 to 4 weeks. We use short-term benzodiazepines as needed.
Partial improvement. Sometimes overall symptoms improve meaningfully, but bothersome panic symptoms remain. Clinical response may improve sufficiently if you raise the medication dosage in increments while monitoring for safety and tolerability. Address medicolegal concerns by documenting in the patient’s chart:
- your rationale for prescribing dosages that exceed FDA guidelines
- that you discussed possible risks versus benefits with the patient, and the patient agrees to the treatment.
When in doubt about using dosages that exceed FDA guidelines for patients with unusually resistant panic symptoms, obtain consultation from an expert or colleague.
Table 4
Recommended drug dosages for panic disorder
| Class/agent | Possibly effective (mg/d) | Probably effective (mg/d) | High dosage (mg/d) | Initial dosage (mg/d) | Confidence level |
|---|---|---|---|---|---|
| SSRIs | |||||
| Citalopram | <20 | 20-60 | >60 | 10 | ++ |
| Escitalopram | <10 | 10-30 | >30 | 5 | ++++ |
| Fluoxetine | <40 | 40-80 | >80 | 10 | ++ |
| Fluvoxamine | <150 | 150-300 | >300 | 25 | ++++ |
| Paroxetine* | <40 | 40-60 | >60 | 5-10 | ++++ |
| Sertraline* | <150 | 150-300 | >300 | 12.5-25 | ++++ |
| SNRI | |||||
| Venlafaxine | <150 | 150-300 | >300 | 18.75-37.5 | ++ |
| Benzodiazepines | |||||
| Alprazolam* | <2 | 2-8 | >8 | 0.5-1.0 | ++++ |
| Clonazepam* | <1 | 2-4 | >4 | 0.25-0.5 | ++++ |
| Tricyclics | |||||
| Clomipramine | <100 | 100-200 | >200 | 10 | ++++ |
| Desipramine | <150 | 150-300 | >300 | 10 | ++ |
| Imipramine | <150 | 150-300 | >300 | 10 | ++++ |
| MAOIs | |||||
| Phenelzine | <45 | 45-90 | >90 | 15 | +++ |
| Tranylcypromine | <30 | 30-70 | >70 | 10 | + |
| Antiepileptics | |||||
| Gabapentin | 100-200 | 600-3,400 | ++ | ||
| Valproate (VPA) | 250-500 | 1,000-2,000 | ++ | ||
| * FDA-approved for treating panic disorder | |||||
| Confidence: | |||||
| + (uncontrolled series) | |||||
| ++ (at least 1 controlled study) | |||||
| +++ (>1 controlled study) | |||||
| ++++ (Unequivocal) | |||||
Using benzodiazepines. As noted above, adjunctive use of benzodiazepines while initiating antidepressant therapy can help extremely anxious or medication-sensitive patients.11 Many clinicians coadminister benzodiazepines with antidepressants over the longer term.7 As a primary treatment, benzodiazepines may be useful for patients who could not tolerate or did not respond to at least two or three antidepressant trials.
Table 5
Solving inadequate response to initial SSRI treatment of panic disorder
| Problem | Differential diagnosis | Suggested solutions |
|---|---|---|
| Persistent panic attacks | Unexpected attacks Inadequate treatment or duration Situational attacks Medical condition Other psychiatric disorder | ≥Threshold dose for 6 weeks Try second SSRI Try venlafaxine CBT/exposure therapy Address specific conditions Rule out social phobia, OCD, PTSD |
| Persistent nonpanic anxiety | Medication-related Activation (SSRI or SNRI) Akathisia from SSRI Comorbid GAD Interdose BZD rebound BZD or alcohol withdrawal Residual anxiety | Adjust dosage, add BZD or beta blocker Adjust dosage, add beta blocker or BZD Increase antidepressant dosage, add BZD Switch to longer-acting agent Assess and treat as indicated Add/increase BZD |
| Residual phobia | Agoraphobia | CBT/exposure, adjust medication |
| Other disorders | Depression Bipolar disorder Personality disorders Medical disorder | Aggressive antidepressant treatment ±BZDs Mood stabilizer and antidepressant ±BZDs Specific psychotherapy Review and modify treatment as indicated |
| Environmental event or stressor(s) | Review work, family events, patient perception of stressor | Family/spouse interview and education Environmental hygiene as indicated Brief adjustment in treatment plan(s) as needed |
| Poor adherence | Drug sexual side effects Inadequate patient or family understanding of panic disorder and its treatment | Try bupropion, sildenafil, amantadine, switch agents Patient/family education Make resource materials available |
| BZD: Benzodiazepine | ||
| CBT: Cognitive-behavioral therapy | ||
| GAD: Generalized anxiety disorder | ||
| OCD: Obsessive-compulsive disorder | ||
| PTSD: Posttraumatic stress disorder | ||
| SNRI: Serotonin-norepinephrine reuptake inhibitor | ||
| SSRI: Selective serotonin reuptake inhibitor | ||
Because benzodiazepine monotherapy does not reliably protect against depression, we advise clinicians to encourage patients to self-monitor and report any signs of emerging depression. Avoid benzodiazepines in patients with a history of alcohol or substance abuse.7
Other agents. Once the mainstay of antipanic treatment, tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) are seldom used today because of their side effects, toxicity in overdose, and—for MAOIs—tyramine-restricted diet. Their usefulness in resistant panic is probably limited to last-ditch efforts.
DISSECTING TREATMENT FAILURE
In uncomplicated PD, lack of improvement after two or more adequate medication trials is unusual. If you observe minimal or no improvement, review carefully for other causes of anxiety or factors that can complicate PD treatment (Table 5).
If no other cause for the persistent symptom(s) is apparent, the fear circuit model may help you decide how to modify or enhance medication treatment, add CBT, or both.
For example:
- If panic attacks persist, advancing the medication dosage (if tolerated and acceptably safe) may help. Consider increasing the dosage, augmenting, or switching to a different agent.
- If persistent attacks are consistently cued to feared situations, try intervening with moreaggressive exposure therapy. Consider whether other disorders such as unrecognized social anxiety disorder, obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD) may be perpetuating the fearful avoidance.
- If the patient is depressed, consider that depression-related social withdrawal may be causing the avoidance symptoms. Aggressive antidepressant pharmacotherapy is strongly suggested.
AUGMENTATION STRATEGIES
Medication for CBT failure. Only two controlled studies have examined adding an adequate dose of medication after patients failed to respond to exposure/CBT alone:
- One study of 18 hospitalized patients with agoraphobia who failed a course of behavioral psychodynamic therapy reported improvement when clomipramine, 150 mg/d, was given for 3 weeks.13
- In a study of 43 patients who failed initial CBT, greater improvement was reported in patients who received CBT plus paroxetine, 40 mg/d, compared with those who received placebo while continuing CBT.14
Augmentation in drug therapy. Only one controlled study has examined augmentation therapy after lack of response to an SSRI—in this case 8 weeks of fluoxetine after two undefined “antidepressant failures.” When pindolol, 2.5 mg tid, or placebo were added to the fluoxetine therapy, the 13 patients who received pindolol improved clinically and statistically more on several standardized ratings than the 12 who received placebo.15
An 8-week, open-label trial showed beneficial effects of olanzapine, up to 20 mg/d, in patients with well-described treatment-resistant PD.16
Other well-described treatment adjustments reported to benefit nonresponsive PD include:
- Adding fluoxetine to a TCA or adding a TCA to fluoxetine, for TCA/SSRI combination therapy17
- Switching to the selective norepinephrine reuptake inhibitor reboxetine, 2 to 8 mg/d for 6 weeks after inadequate paroxetine or fluoxetine response (average of 8 weeks, maximum dosage 40 mg/d).18 (Note: Reboxetine is not available in the United States.)
- Using open-label gabapentin, 600 to 2,400 mg/d, after two SSRI treatment failures.19
- Adding the dopamine receptor agonist pramipexole, 1.0 to 1.5 mg/d, to various antipanic medications.20
Augmenting an SSRI with pindolol or supplementing unsuccessful behavioral treatment with “probably effective” dosages of paroxetine or clomipramine could be recommended with some confidence, although more definitive studies are needed. As outlined above, some strategies17-20 might be considered if a patient fails to respond to two or more adequate medication trials. Anecdotal reports are difficult to assess but may be clinically useful when other treatment options have been exhausted.
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic New York: Guilford Press, 1988.
- Craske MG, DeCola JP, Sachs AD, Pontillo DC. Panic control treatment of agoraphobia. J Anxiety Disord 2003;17:321-33.
- National Institute for Mental Health: Panic Disorder http://www.nimh.nih.gov/publicat/fearandtrauma.cfm
- Anxiety Disorders Association of America http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Olanzapine • Zyprexa
- Phenelzine • Nardil
- Pindolol • Visken
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Reboxetine • Vestra
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
Dr. Lydiard receives research support from GlaxoSmithKline, Eli Lilly and Co., Organon, Sanofi-Synthelabo, Cephalon, UCB Pharma, and Merck & Co. and he is a speaker for or consultant to Pfizer Inc., Eli Lilly and Co., Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, and Forest Pharmaceuticals.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
1. Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revisited. Am J Psychiatry 2000;157:493-505.
2. Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry 1998;44:1264-76.
3. Sheehan DV. The anxiety disease. New York: Charles Scribner and Sons, 1983;151.-
4. Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull 1998;34:183-9.
5. Otto MW. Psychosocial approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
6. Gorman JM, Shear MK, McIntyre JS, Zarin DA. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. Am J Psychiatry 1998;155(May supplement).
7. Lydiard RB, Otto MW, Milrod B. Panic disorder treatment. In: Gabbard, GO (ed). Treatment of psychiatric disorders (3rd ed). Washington, DC: American Psychiatric Press, Inc, 2001;1447-82.
8. Simon NM, Safrens SA, Otto MW, et al. Outcome with pharmacotherapy in a naturalistic study of panic disorder. J Affect Disord 2002;69:201-8.
9. Yonkers KA, Ellison J, Shera D, et al. Description of antipanic therapy in a prospective longitudinal study. J Clin Psychopharmacol 1996;16:223-32.
10. Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: results from a double-blind, placebo-controlled study. Psychopharmacol Bull 1996;32:667-70.
11. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry 2001;58:681-6.
12. Simon NM. Pharmacological approach to treatment-resistant anxiety disorders (presentation). Chantilly, VA: Anxiety Disorders Association of America conference on novel approaches to treatment of refractory anxiety disorders, June 15-16, 2003.
13. Hoffart A, Due-Madsen J, Lande B, et al. Clomipramine in the treatment of agoraphobic inpatients resistant to behavioral therapy. J Clin Psychiatry 1993;54:481-7.
14. Kampman M, Keijsers GP, Hoogduin CA, Hendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry 2002;63:772-7.
15. Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2000;20:556-9.
16. Hollifield M, Thompson P, Uhlenluth E. Potential efficacy and safety of olanzapine in refractory panic disorder (presentation). San Francisco: American Psychiatric Association annual meeting, 2003.
17. Tiffon L, Coplan J, Papp L, Gorman J. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry 1994;55:66-9.
18. Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in treatment-refractory patients with panic disorder: an open-label study. Hum Psychopharmacol 2002;17:329-33.
19. Chiu S. Gabapentin treatment response in SSRI-refractory panic disorder (presentation) San Francisco: American Psychiatric Association annual meeting, 2003.
20. Marazziti D, Presta S, Pfanner C, et al. Pramipexole augmentation in panic with agoraphobia. Am J Psychiatry 2001;158:498-9.
Germ warfare: Arm young patients to fight obsessive-compulsive disorder
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
Adam, age 10, is extremely distressed at school. Because of obsessional contamination fears, he avoids contact with other children and refuses to eat in the cafeteria. He washes his hands 20 times per day and changes his clothes at least three times daily.
His primary obsessions involve contact with bodily fluids—such as saliva or feces—and excessive concerns that this contamination would cause him serious illness.
Adam’s parents say their son’s worries about dirt and germs began when he entered kindergarten. They sought treatment for him 2 years ago, and he has been receiving outpatient psychotherapy since then. They have brought him to an anxiety disorders specialty clinic for evaluation because his obsessive-compulsive symptoms are worsening,
When treating patients such as Adam, our approach is to use cognitive-behavioral therapy (CBT) and adjunctive drug therapies to relieve their symptoms and help them reclaim their lives. Diagnosis of pediatric OCD is often delayed, and few children receive state-of-the-art treatment.1 The good news, however, is that skillful CBT combined, as needed, with medication is highly effective.
Although family dysfunction does not cause OCD, families affect and are affected by OCD. Control struggles over the child’s rituals are common, as are differences of opinion about how to cope with OCD symptoms. It is important to address these issues early in treatment, as helping the family combat the disorder—rather than each other—is crucial to effective treatment.
Parents need to know that neither they nor the child are to blame. OCD is a neurobehavioral illness, and treatment is most effective when the patient, therapist, and family are aligned to combat it. Families are often entangled in the child’s OCD symptoms, and disentangling them by eliminating their role in ritualizing (such as giving excessive reassurance) is important to address in therapy.
Scaling family involvement is part of the “art” of CBT, and it will remain so until empiric studies determine the family’s role in the treatment plan.2
‘Contaminated’ mother.
Adam becomes distressed when he comes in contact with objects that have been touched by others (such as doorknobs). He is especially anxious when these items are associated with public bathrooms or sick people.
Adam’s mother is a family physician who has daily patient contact. In the last 6 months, Adam has insisted that his mother change her work clothes before she enters his room, touches him, prepares his food, or handles his possessions.
As in Adam’s case, the family often gets caught up in a child or adolescent’s obsessive rituals (Box 1).2 After a detailed discussion with Adam and his parents and because his symptoms were severe, we recommended combined treatment with sertraline and CBT. Adam was willing to consider CBT and medication because he recognized that he was having increasing difficulty doing the things he wanted to do in school and at home.
SNAPSHOT OF PEDIATRIC OCD
Approximately 1 in 200 children and adolescents suffer from clinically significant OCD.3 They experience intrusive thoughts, urges, or images to which they respond with dysphoria-reducing behaviors or rituals.
Common obsessions include:
- fear of dirt or germs
- fear of harm to oneself or someone else
- or a persistent need to complete something “just so.”
Corresponding compulsions include hand washing, checking, and repeating or arranging.
OCD appears more common in boys than in girls. Onset occurs in two modes: first at age 9 for boys and age 12 for girls, followed by a second mode in late adolescence or early adulthood.
Two practice guidelines address OCD in youth: an independent expert consensus guideline4 and the American Academy of Child and Adolescent Psychiatry’s practice parameters for OCD.5
For uncomplicated OCD, these guidelines recommend CBT as first-line treatment. If symptoms do not respond after six to eight sessions, a selective serotonin reuptake inhibitor (SSRI) is added to CBT.
For complicated OCD, medication is considered an appropriate initial treatment. Complicated OCD includes patients who:
- display severe symptoms—such as with scores >30 on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS)
- or have comorbidity such as depression or panic disorder that is likely to complicate treatment.
KEYS TO SUCCESSFUL TREATMENT
OCD is remarkably resistant to insight-oriented psychotherapy and other nondirective therapies. The benefits of CBT, however, are well-established, with reported response rates of >80% in pilot studies.6,7 Although confirming studies have yet to be conducted, successful CBT for pediatric OCD appears to include four elements (Table 1).
Exposure and response prevention (EX/RP) is central to psychosocial treatment of OCD.7,8 In specialized centers, exposure can be applied intensively (three to five times per week for 3 to 4 weeks).9 In most practices, however, exposure is more gradual (weekly for 12 to 20 weeks). With repeated exposure, the child’s anxiety decreases until he or she no longer fears contact with the targeted stimuli.8,10
Not ‘misbehavior.’ Children—and less commonly adolescents—with this disorder may not view their obsessions as senseless or their compulsions as excessive. Even when insight is clearly present, young OCD patients often hide their symptoms because of embarrassment or fear of being punished for their behavior.
Response predictors. A key to CBT in children or adolescents is that they come to see obsessions and compulsions as symptoms of an illness. The symptoms, therefore, require a skillfully applied “antidote,” as taught by the clinician and implemented by the child, family, and others on the child’s behalf. Besides overt rituals, three response predictors include the patient’s:
- desire to eliminate symptoms
- ability to monitor and report symptoms
- willingness to cooperate with treatment.
Table 1
Pediatric OCD: 4 keys to successful cognitive therapy
| Treat OCD as a neurobehavioral disorder, not a misbehavior |
| Help the child develop a “tool kit” to manage dysphoria and faulty thinking |
| Expose the patient to anxiety-producing stimuli until he or she becomes desensitized and can refrain from the usual compulsive responses (exposure and response prevention) |
| Educate family members and school personnel |
CBT may be difficult with patients younger than age 6 and will invariably involve training the parents to serve as “coaches;” a CBT protocol for patients ages 4 to 6 is under investigation (H. Leonard, personal communication). CBT also can be adapted for patients with intellectual deficits.11
A ‘tool kit.’ Successful exposure therapy for OCD relies on equipping children and adolescents with the knowledge and skills to battle the illness. They often have tried unsuccessfully to resist OCD’s compulsions and must be convinced that EX/RP techniques will work. Using a “tool kit” concept reminds young patients that they have the implements they need to combat OCD (Table 2).
A ‘germ ladder’ and ‘fear thermometer.’
Adam’s tools include a stimulus hierarchy called a “germ ladder,” which the therapist and Adam create collaboratively. It ranks stimuli from low (his own doorknob) to very high (public toilets, sinks, and door handles).
As part of his treatment, Adam begins to touch objects in his room and house while voluntarily refraining from ritualizing. He uses another tool—a fear thermometer—to record his distress level on a scale of 1 to 10 during and after these exposures.
Adam discovers that when he comes into contact with less-threatening items his fear ratings typically return to baseline within 20 to 30 minutes. This insight helps him modify his assessment of the risk they pose.
Table 2
OCD ‘tool box’ can help patients build new behaviors
| Tool | Function |
|---|---|
| Training in exposure response prevention (EX/RP) therapy | Helps patients learn to confront rather than avoid feared stimuli |
| ‘Fear thermometer’ | Enables patients to express the intensity of their distress on a scale of 1 (lowest) to 10 (highest) |
| Positive self-statements | Teaches patients to use statements such as “I can do this” and “I’m the boss of OCD now” to build confidence that they can control their response to feared stimuli |
During office visits, he confronts similar items around the clinic, with the therapist providing encouragement and instruction for additional exposure homework. Eventually Adam works on the clinic’s public bathroom, which he perceived to be relatively clean but less so than his own bathroom. After fear in response to this bathroom is reduced, the therapist and Adam graduate to more-public facilities, such as the bathrooms at Adam’s pool and the local train station.
Exposure therapy. EX/RP is most successful when the child—rather than the therapist—chooses exposure targets from a hierarchy of fears,2 particularly when the list includes behaviors the child is resisting. In a collaborative spirit, the child takes the lead in placing items on the hierarchy and deciding when to confront them.
The therapist and child revise the hierarchy periodically, which demonstrates progress and allows them to add items as the child overcomes fears that cause less distress.
Reducing need for reassurance.
Adam has a habit of repeatedly asking his mother whether contact with particular objects in public is risky. By the third treatment session, he and the therapist agree that he will try to refrain from asking such questions.
His mother, in turn, is asked to reiterate the rationale for response prevention whenever Adam slips. She will offer encouragement and support without answering “OCD’s questions.”
ADJUNCTIVE DRUG THERAPY
While Adam is working with the behavioral therapist to reduce his anxieties and need for reassurance, he is also receiving gradually increasing dosages of sertraline. As discussed, he is considered a candidate for CBT plus medication because of his symptoms are severe. Drug treatment can benefit most pediatric OCD patients.
SSRIs. Two SSRIs are approved for pediatric OCD—fluvoxamine for ages 8 to 18 and sertraline for ages 6 to 18. Most SSRIs are likely effective for OCD in youth (Table 3),12-14 although reports have suggested a link between paroxetine and suicidality in pediatric patients. Other options may be more suitable choices unless further evidence supports the use of paroxetine as a first- or second-line agent for pediatric OCD.
Clomipramine—a nonselective tricyclic—was the first medication studied in treating OCD in children and adolescents. It is now usually considered only after two or three failed SSRI trials because of its potential for cardiac toxicity.15-17
Table 3
Suggested dosages (mg/d) for drug therapy of pediatric OCD
| Drug | Usual starting dosage | Approximate mean dosage* | Typical range | Usual maximum dosage |
|---|---|---|---|---|
| Citalopram | 20 | 40 | 20 to 60 | 80 |
| Clomipramine | 50 | 150 | 200 | 300 |
| Escitalopram | 5 | 10 | 10 to 20 | 30 |
| Fluoxetine | 20 | 40 | 40 to 60 | 80 |
| Fluvoxamine | 50 | 175 | 150 to 250 | 300 |
| Sertraline | 50 | 125 | 150 | 225 |
| *Mean dosage derived from registration trials, expert recommendation, and the authors’ clinical experience | ||||
Dosing. Fixed-dose studies suggest that dosing schedules for OCD are similar to those used for depression. For example, sertraline, 50 mg/d, or fluoxetine, 20 mg/d, are as effective as higher dosages.18
The common misconception that OCD requires higher dosages likely results from:
- increasing the dosage too early in the time-response window for a drug effect to emerge
- giving medication without concomitant exposure therapy.19
Delayed response. Although many patients respond early to an SSRI, others do not respond until 8 or even 12 weeks of treatment at therapeutic dosages. It often takes 3 to 4 weeks for evidence of benefit to emerge, so wait at least 3 weeks between dosage increases. Maintain therapeutic dosages at least 6 to 8 weeks before changing agents or beginning augmentation therapy.
Two-barrel approach.
In treating Adam, we began with sertraline, using a flexible titration schedule keyed to whether he experienced OCD symptom remission.
The starting dosage of 50 mg was titrated to 150 mg over 8 weeks while he was receiving behavioral therapy. We made adjustments with a time-response window of 2 to 3 weeks, allowing us to observe a response to each dosage escalation.
Adam’s OCD symptoms responded well to CBT plus sertraline. The maximum drug effect helped him confront the most difficult EX/RP tasks at the top of his stimulus hierarchy, which he attacked near the end of treatment.
Lessons learned. Multicenter trials have taught important lessons about drug therapy for OCD:
- OCD patients experience little or no placebo effect, unlike patients with depression.
- Clinical effects may appear as early as 2 to 3 weeks after medications are started and plateau at 10 to 12 weeks.
- Partial response is the rule; SSRIs reduce OCD symptoms by about 30%—which correlates with “moderately” to “markedly” improved ratings on patient satisfaction measures.
- Side effects and magnitude of improvement are comparable in pediatric and adult medication trials.
In some children, OCD or tic symptoms arise from or are exacerbated by group A beta hemolytic streptococcal infection (GABHS), which has been labeled pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS).21 Obsessive-compulsive symptoms are not uncommon in pediatric patients with Sydenham’s chorea, a neurologic variant of rheumatic fever. OCD is far more common in patients with rheumatic fever when chorea is present.
Acute-onset or dramatic exacerbation of OCD or tic symptoms should prompt investigation of GABHS infection. Immunomodulatory treatments—including antibiotics, plasmapheresis, or IV immunoglobulin G—may benefit some patients.22
MANAGING RESISTANT OCD
Adequate SSRI monotherapy trials fail to relieve OCD symptoms in one-third of patients. Some patients benefit from combination therapy—such as an SSRI plus risperidone—especially when comorbid schizotypal personality disorder or a tic-spectrum disorder is present.
Drug switching or augmentation trials often produce only partial response and cause unnecessary suffering. A more effective strategy for many patients is to augment drug treatment with CBT until symptoms normalize.
On the other hand, augmentation is appropriate when nonresponse or partial response to SSRI monotherapy leaves a patient clinically symptomatic and functionally impaired. Clonazepam, clomipramine, and the amino acid L-tryptophan have been used successfully. Lithium and buspirone also have been tried but seem not to be effective in controlled studies in adults and anecdotal experience in youth.
When augmenting an SSRI, adding clomipramine, 25 to 50 mg/d, is a reasonable choice. However, fluoxetine or paroxetine can inhibit clomipramine metabolism by cytochrome P-450 (CYP) 2D6, with potential for cardiac arrhythmias or seizures. Sertraline or fluvoxamine are less likely to elevate clomipramine levels.20 Fluvoxamine may be the most compatible SSRI with clomipramine because it inhibits CYP 1A2—the enzyme that demethylates clomipramine to its inactive desmethyl metabolite—thereby preserving more of the active parent compound.
Clinical evidence suggests that augmentation’s success may depend in part on a patient’s comorbidities. For example, clonazepam may be particularly helpful for children with comorbid panic symptoms.
MAINTENANCE THERAPY
We typically provide 14 weekly CBT sessions, followed by monthly contacts for a few months to ensure than a patient’s gains are maintained. Standard procedure with drug therapy is to continue maintenance treatment for up to 1 year, although some have suggested continuing maintenance treatment indefinitely.
Related resources
- Chansky TE. Freeing your child from obsessive compulsive disorder: A powerful, practical program for parents of children and adolescents. New York: Crown Publishing Group, 2001.
- March JS, Mulle K. OCD in children and adolescents: A cognitive behavioral treatment manual. New York: Guilford Press, 1998.
- Moritz EK, Jablonsky J. Blink, blink, clop, clop: Why do we do things we can’t stop? An OCD storybook. Newton, MA: Professional Books, Inc, 1998.
- Wagner AP. Up and down the worry hill: A children’s book about obsessive compulsive disorder and its treatment. Rochester, NY: Lighthouse Press, Inc. 2000.
Drug brand names
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertaline • Zoloft
Disclosure
Dr. March receives research support from Pfizer Inc., Eli Lilly and Co., and Wyeth Pharmaceuticals and is a speaker for and/or consultant to Solvay Pharmaceuticals, Pfizer Inc., GlaxoSmithKline, Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Franklin and Dr. Foa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was supported in part by National Institute of Mental Health grants 1 K24 MHO1557 and 1 R10 MH55121 to Dr. March and by contributions from the Robert and Sarah Gorrell family and the Lupin Family Foundation.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
1. Kendall PC, Southam-Gerow MA. Issues in the transportability of treatment: the case of anxiety disorders in youths. J Consult Clin Psychol 1995;63(5):702-8.
2. March J, Mulle K. OCD in children and adolescents: A cognitive-behavioral treatment manual. New York: Guilford Press, 1998.
3. Flament MF, Whitaker A, Rapoport JL, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27(6):764-71.
4. March J, Frances A, Kahn D, Carpenter D. Expert consensus guidelines: treatment of obsessive-compulsive disorder. J Clin Psychiatry 1997;58(suppl 4):1-72.
5. King R, Leonard H, March J. Practice parameters for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 1998;37(10, suppl):27-45.
6. March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: a review and recommendations for treatment. J Am Acad Child Adolesc Psychiatry 1995;34(1):7-18.
7. Franklin ME, Kozak MJ, Cashman LA, et al. Cognitive-behavioral treatment of pediatric obsessive-compulsive disorder: an open clinical trial. J Am Acad Child Adolesc Psychiatry 1998;37(4):412-19.
8. March JS, Mulle K, Herbel B. Behavioral psychotherapy for children and adolescents with obsessive-compulsive disorder: an open trial of a new protocol-driven treatment package. J Am Acad Child Adolesc Psychiatry 1994;33(3):333-41.
9. Franklin ME, Tolin DF, March JS, Foa EB. Treatment of pediatric obsessive-compulsive disorder: A case example of intensive cognitive-behavioral therapy involving exposure and ritual prevention. Cognit Behav Pract 2001;8(4):297-304.
10. March J, Mulle K. Manualized cognitive-behavioral psychotherapy for obsessive-compulsive disorder in childhood: a preliminary single case study. J Anxiety Disord 1995;9(2):175-84.
11. Franklin ME, Rynn M, March JS, Foa EB. Obsessive-compulsive disorder. In: Hersen M (ed). Clinical behavior therapy: adults and children. New York: John Wiley & Sons, 2002;276-303.
12. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 1998;280(20):1752-6.
13. Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry 2001;40(2):222-9.
14. Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry 2001;40(7):773-9.
15. Leonard H, March J, Rickler K, Allen A. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
16. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder—a multicenter trial. J Am Acad Child Adolesc Psychiatry 1992;31(1):45-9.
17. Wilens TE, Biederman J, March JS, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):573-7.
18. Greist JH, Jefferson JW, Kobak KA, et al. A 1-year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 1995;10(2):57-65.
19. Marks IM. Drug versus behavioral treatment of obsessive-compulsive disorder. Biolog Psychiatry 1990;28(12):1072-3.
20. Leonard HL, March J, Rickler KC, Allen AJ. Pharmacology of the selective serotonin reuptake inhibitors in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(6):725-36.
21. Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 2001;4(2):191-8.
22. Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999;354 (9185):1153-8.
The truth about anxiety disorders
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.
Too often, anxiety disorders go unrecognized or undertreated. Worse, many physicians still view an anxiety disorder as a character flaw, mirroring how society sees anxiety. The assumption is, “We all have to deal with frightening stuff. Some can take it, some can’t.”
Yet anxiety disorders are as distinct from everyday anxiety as major depressive disorder is from everyday unhappiness. Further, it is becoming increasingly clear that the consequences of anxiety disorders are serious—and that appropriate treatment can help.
Dr. Bruce Lydiard’s article provides an excellent framework for diagnosis and treatment of panic disorder. Dr. John March’s article on obsessive-compulsive disorder in children and adolescents does the same for another anxiety-related condition.
We as a profession have increased the public’s awareness of major depression; now we need to increase the public’s understanding of anxiety disorders. We need to impress upon the public and upon primary care physicians that anxiety disorders, like depressive disorders, are serious yet treatable illnesses, not character flaws. By getting this message out, we will decrease the stigma of mental illness and remove barriers to the long-await-ed effective treatments that are now becoming available.
COPD: How to manage comorbid depression and anxiety
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
Educational interventions improve outcomes for children with asthma
Asthma education interventions for children may result in modest improvement in a wide range of clinical outcomes. Interventions should target children with more severe asthma and teach them to use objective measures of lung function, such as peak flow for self-monitoring instead of symptombased self-monitoring.
Asthma education interventions for children may result in modest improvement in a wide range of clinical outcomes. Interventions should target children with more severe asthma and teach them to use objective measures of lung function, such as peak flow for self-monitoring instead of symptombased self-monitoring.
Asthma education interventions for children may result in modest improvement in a wide range of clinical outcomes. Interventions should target children with more severe asthma and teach them to use objective measures of lung function, such as peak flow for self-monitoring instead of symptombased self-monitoring.
Estrogen plus progestin may increase incidence of dementia
Estrogen plus progestin does not decrease— and may actually increase—the incidence of dementia, mild cognitive impairment, and cognitive dysfunction in elderly postmenopausal women. The effect of unopposed estrogen on these outcomes is still unknown. With these new findings and the recently reported results of the Women’s Health Initiative, for most women the benefits of estrogen plus progestin do not outweigh the risks.
Estrogen plus progestin does not decrease— and may actually increase—the incidence of dementia, mild cognitive impairment, and cognitive dysfunction in elderly postmenopausal women. The effect of unopposed estrogen on these outcomes is still unknown. With these new findings and the recently reported results of the Women’s Health Initiative, for most women the benefits of estrogen plus progestin do not outweigh the risks.
Estrogen plus progestin does not decrease— and may actually increase—the incidence of dementia, mild cognitive impairment, and cognitive dysfunction in elderly postmenopausal women. The effect of unopposed estrogen on these outcomes is still unknown. With these new findings and the recently reported results of the Women’s Health Initiative, for most women the benefits of estrogen plus progestin do not outweigh the risks.
Cervical spondylotic myelopathy: Make the difficult diagnosis, then refer for surgery
Hair Care Practices in African American Women
Prevention of Cross-Contamination When Using Microdermabrasion Equipment
Navigating the Management of Hepatitis C
Supplement Editor:
William Carey, MD
Contents
The epidemiology and natural history of hepatitis C virus infection
Nizar N. Zein, MD
Tests and screening strategies for the diagnosis of hepatitis C
William Carey, MD
Management of newly diagnosed hepatitis C virus infection
Mark W. Russo, MD, MPH; Steven L. Zacks, MD, MPH; and Michael W. Fried, MD
Treatment options for nonresponders and relapsers to initial therapy for hepatitis C
Marten Duncan, DO, and Zobair Younossi, MD, MPH
Special management challenges in hepatitis C
Jose Martagon, MD, and Steven M. Gordon, MD
Supplement Editor:
William Carey, MD
Contents
The epidemiology and natural history of hepatitis C virus infection
Nizar N. Zein, MD
Tests and screening strategies for the diagnosis of hepatitis C
William Carey, MD
Management of newly diagnosed hepatitis C virus infection
Mark W. Russo, MD, MPH; Steven L. Zacks, MD, MPH; and Michael W. Fried, MD
Treatment options for nonresponders and relapsers to initial therapy for hepatitis C
Marten Duncan, DO, and Zobair Younossi, MD, MPH
Special management challenges in hepatitis C
Jose Martagon, MD, and Steven M. Gordon, MD
Supplement Editor:
William Carey, MD
Contents
The epidemiology and natural history of hepatitis C virus infection
Nizar N. Zein, MD
Tests and screening strategies for the diagnosis of hepatitis C
William Carey, MD
Management of newly diagnosed hepatitis C virus infection
Mark W. Russo, MD, MPH; Steven L. Zacks, MD, MPH; and Michael W. Fried, MD
Treatment options for nonresponders and relapsers to initial therapy for hepatitis C
Marten Duncan, DO, and Zobair Younossi, MD, MPH
Special management challenges in hepatitis C
Jose Martagon, MD, and Steven M. Gordon, MD