User login
Male alopecia agents ranked by efficacy in meta-analysis
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Hairstyling Practices to Prevent Hair Damage and Alopecia in Women of African Descent
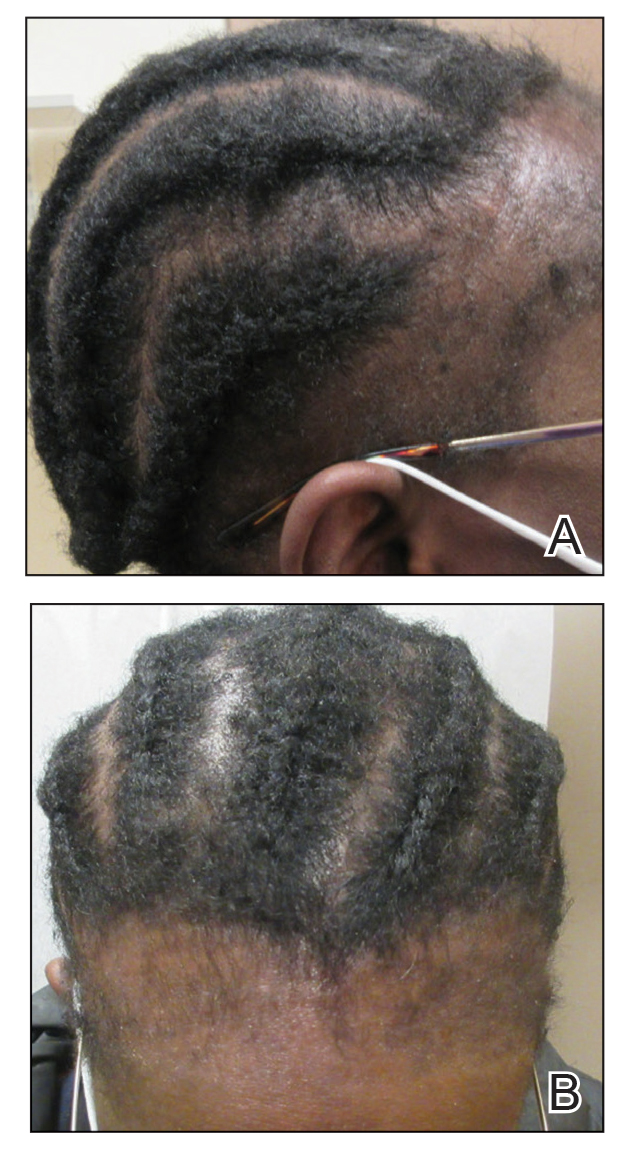
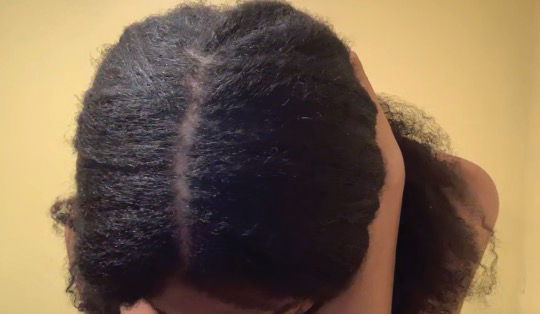
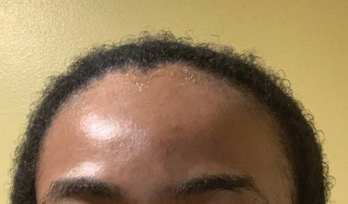
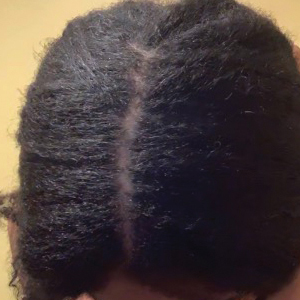
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Severe Acute Systemic Reaction After the First Injections of Ixekizumab
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
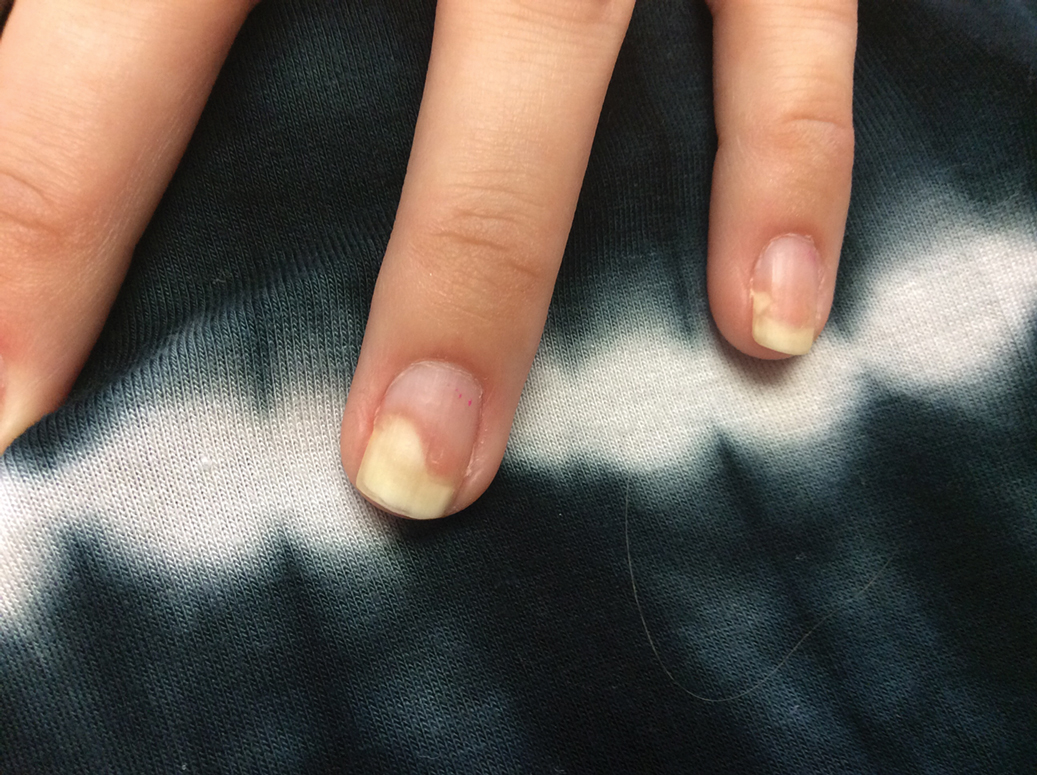
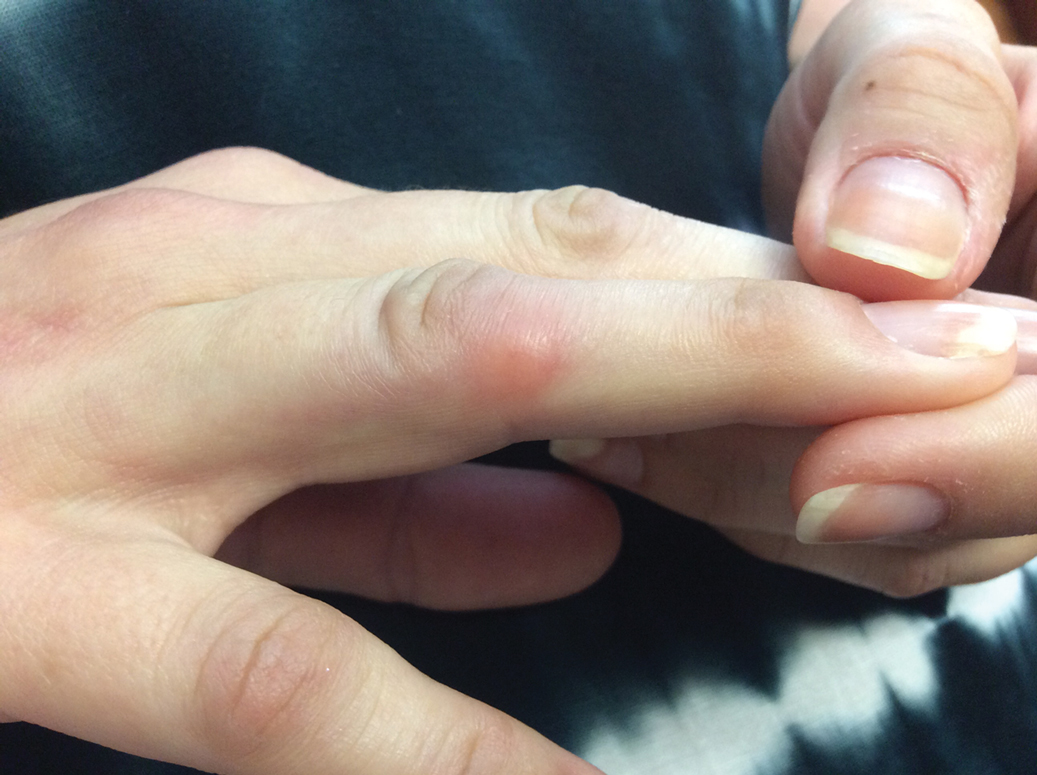
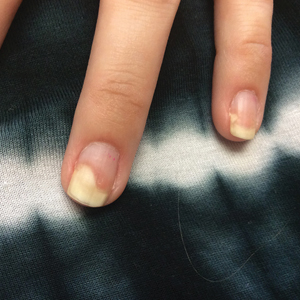
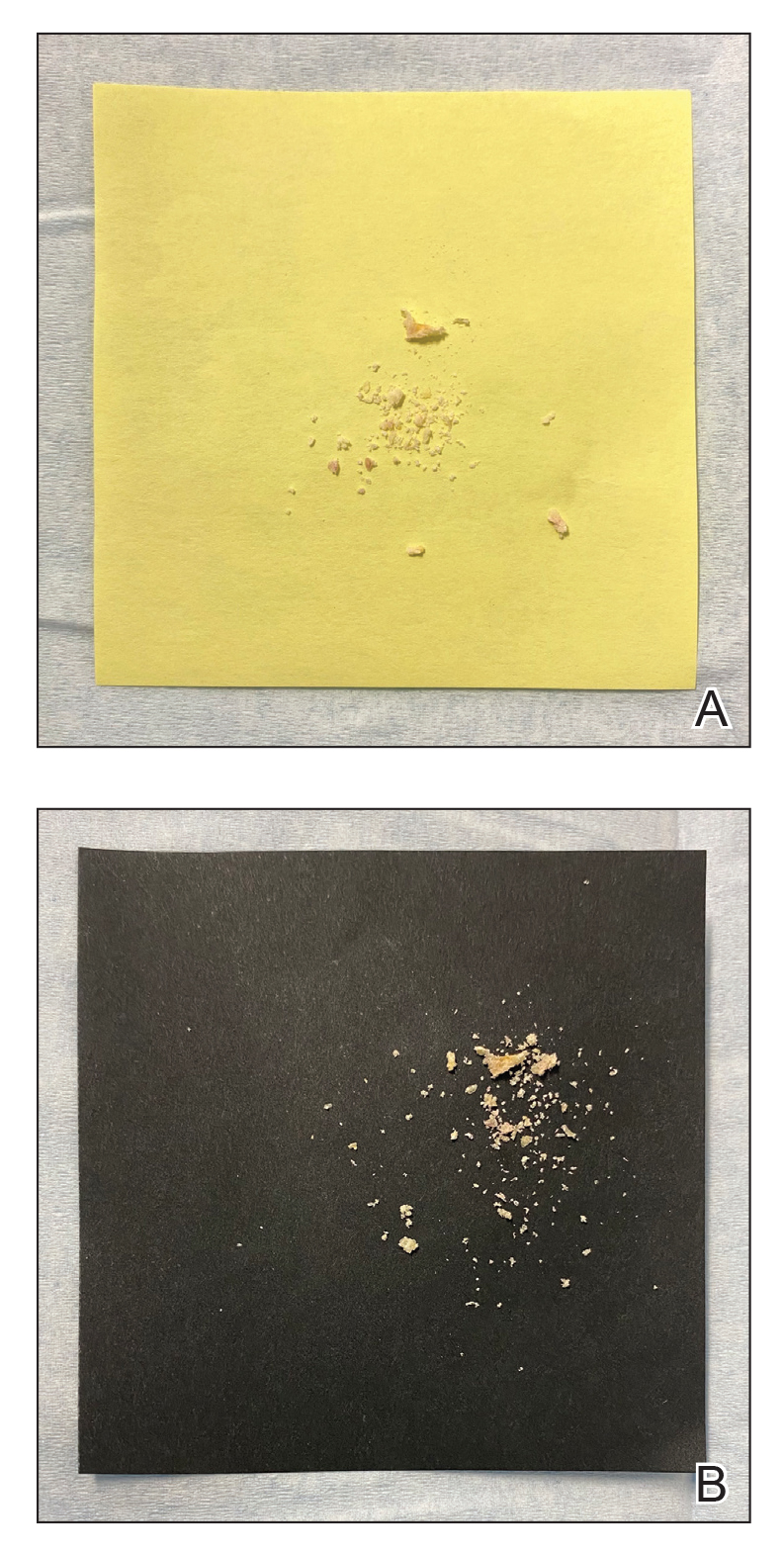
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Practice Points
- Psoriasis is an autoimmune disorder with a predominance of CD4+ and CD8+ T cells that release cytokines, such as tumor necrosis factor 11α and interleukins, which promote inflammation in the skin and joints and is associated with systemic inflammation predisposing patients to cardiovascular disease.
- Common adverse effects of most biologic medications for psoriasis include injection-site pain and rash, fever, malaise, back pain, urticaria and flushing, edema, dyspnea, and nausea.
- Ixekizumab is a humanized IL-17A antagonist intended for adults with moderate to severe psoriasis. Certain rare side effects specific to ixekizumab include inflammatory bowel disease, thrombocytopenia, severe injection-site reactions, and candidiasis.
- Acute onycholysis and acute exacerbation of arthritis/dactylitis are rare side effects of ixekizumab therapy.
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
A Contrasting Dark Background for Nail Sampling
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Alopecia tied to a threefold increased risk for dementia
Alopecia areata (AA) has been linked to a significantly increased risk for dementia, new research shows.
After controlling for an array of potential confounders, investigators found a threefold higher risk of developing any form of dementia and a fourfold higher risk of developing Alzheimer’s disease (AD) in those with AA versus the controls.
“AA shares a similar inflammatory signature with dementia and has great psychological impacts that lead to poor social engagement,” lead author Cheng-Yuan Li, MD, MSc, of the department of dermatology, Taipei (Taiwan) Veterans General Hospital.
“Poor social engagement and shared inflammatory cytokines might both be important links between AA and dementia,” said Dr. Li, who is also affiliated with the School of Medicine and the Institute of Brain Science at National Yang Ming Chiao Tung University, Taipei.
The study was published online Oct. 26, 2021, in the Journal of Clinical Psychiatry (doi: 10.4088/JCP.21m13931).
Significant psychological impact
Patients with AA often experience anxiety and depression, possibly caused by the negative emotional and psychological impact of the hair loss and partial or even complete baldness associated with the disease, the authors noted.
However, AA is also associated with an array of other atopic and autoimmune diseases, including psoriasis and systemic lupus erythematosus (SLE).
Epidemiologic research has suggested a link between dementia and autoimmune diseases such as psoriasis and SLE, with some evidence suggesting that autoimmune and inflammatory mechanisms may “play a role” in the development of AD.
Dementia in general and AD in particular, “have been shown to include an inflammatory component” that may share some of the same mediators seen in AA (eg, IL-1 beta, IL-6, and tumor necrosis factor–alpha).
Moreover, “the great negative psychosocial impact of AA might result in poor social engagement, a typical risk factor for dementia,” said Dr. Li. The investigators sought to investigate whether patients with AA actually do have a higher dementia risk than individuals without AA.
The researchers used data from the Taiwan National Health Insurance Research Database, comparing 2,534 patients with AA against 25,340 controls matched for age, sex, residence, income, dementia-related comorbidities, systemic steroid use, and annual outpatient visits. Participants were enrolled between 1998 and 2011 and followed to the end of 2013.
The mean age of the cohort was 53.9 years, and a little over half (57.6%) were female. The most common comorbidity was hypertension (32.3%), followed by dyslipidemia (27%) and diabetes (15.4%).
Dual intervention
After adjusting for potential confounders, those with AA were more likely to develop dementia, AD, and unspecified dementia, compared with controls. They also had a numerically higher risk for vascular dementia, compared with controls, but it was not statistically significant.
When participants were stratified by age, investigators found a significant association between AA and higher risk for any dementia as well as unspecified dementia in individuals of all ages and an increased risk for AD in patients with dementia age at onset of 65 years and older.
The mean age of dementia diagnosis was considerably younger in patients with AA versus controls (73.4 vs. 78.9 years, P = .002). The risk for any dementia and unspecified dementia was higher in patients of both sexes, but the risk for AD was higher only in male patients.
Sensitivity analyses that excluded the first year or first 3 years of observation yielded similar and consistent findings.
“Intervention targeting poor social engagement and inflammatory cytokines may be beneficial to AA-associated dementia,” said Dr. Li.
“Physicians should be more aware of this possible association, help reduce disease discrimination among the public, and encourage more social engagement for AA patients,” he said.
“Further studies are needed to elucidate the underlying pathophysiology between AA and dementia risk,” he added.
No cause and effect
Commenting on the study, Heather M. Snyder, PhD, vice president of medical and scientific affairs, Alzheimer’s Association, said, “We continue to learn about and better understand factors that may increase or decrease a person’s risk of dementia.”
“While we know the immune system plays a role in Alzheimer’s and other dementia, we are still investigating links between, and impact of, autoimmune diseases – like alopecia areata, rheumatoid arthritis, and others – on our overall health and our brains, [which] may eventually give us important information on risk reduction strategies as well,” said Dr. Snyder, who was not involved in the research.
She cautioned that although the study did show a correlation between AA and dementia risk, this does not equate to a demonstration of cause and effect.
At present, “the message for clinicians is that when a patient comes to your office with complaints about their memory, they should, No. 1, be taken seriously; and, No. 2, receive a thorough evaluation that takes into account the many factors that may lead to cognitive decline,” Dr. Snyder said.
The study was supported by a grant from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. Dr. Li, coauthors, and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alopecia areata (AA) has been linked to a significantly increased risk for dementia, new research shows.
After controlling for an array of potential confounders, investigators found a threefold higher risk of developing any form of dementia and a fourfold higher risk of developing Alzheimer’s disease (AD) in those with AA versus the controls.
“AA shares a similar inflammatory signature with dementia and has great psychological impacts that lead to poor social engagement,” lead author Cheng-Yuan Li, MD, MSc, of the department of dermatology, Taipei (Taiwan) Veterans General Hospital.
“Poor social engagement and shared inflammatory cytokines might both be important links between AA and dementia,” said Dr. Li, who is also affiliated with the School of Medicine and the Institute of Brain Science at National Yang Ming Chiao Tung University, Taipei.
The study was published online Oct. 26, 2021, in the Journal of Clinical Psychiatry (doi: 10.4088/JCP.21m13931).
Significant psychological impact
Patients with AA often experience anxiety and depression, possibly caused by the negative emotional and psychological impact of the hair loss and partial or even complete baldness associated with the disease, the authors noted.
However, AA is also associated with an array of other atopic and autoimmune diseases, including psoriasis and systemic lupus erythematosus (SLE).
Epidemiologic research has suggested a link between dementia and autoimmune diseases such as psoriasis and SLE, with some evidence suggesting that autoimmune and inflammatory mechanisms may “play a role” in the development of AD.
Dementia in general and AD in particular, “have been shown to include an inflammatory component” that may share some of the same mediators seen in AA (eg, IL-1 beta, IL-6, and tumor necrosis factor–alpha).
Moreover, “the great negative psychosocial impact of AA might result in poor social engagement, a typical risk factor for dementia,” said Dr. Li. The investigators sought to investigate whether patients with AA actually do have a higher dementia risk than individuals without AA.
The researchers used data from the Taiwan National Health Insurance Research Database, comparing 2,534 patients with AA against 25,340 controls matched for age, sex, residence, income, dementia-related comorbidities, systemic steroid use, and annual outpatient visits. Participants were enrolled between 1998 and 2011 and followed to the end of 2013.
The mean age of the cohort was 53.9 years, and a little over half (57.6%) were female. The most common comorbidity was hypertension (32.3%), followed by dyslipidemia (27%) and diabetes (15.4%).
Dual intervention
After adjusting for potential confounders, those with AA were more likely to develop dementia, AD, and unspecified dementia, compared with controls. They also had a numerically higher risk for vascular dementia, compared with controls, but it was not statistically significant.
When participants were stratified by age, investigators found a significant association between AA and higher risk for any dementia as well as unspecified dementia in individuals of all ages and an increased risk for AD in patients with dementia age at onset of 65 years and older.
The mean age of dementia diagnosis was considerably younger in patients with AA versus controls (73.4 vs. 78.9 years, P = .002). The risk for any dementia and unspecified dementia was higher in patients of both sexes, but the risk for AD was higher only in male patients.
Sensitivity analyses that excluded the first year or first 3 years of observation yielded similar and consistent findings.
“Intervention targeting poor social engagement and inflammatory cytokines may be beneficial to AA-associated dementia,” said Dr. Li.
“Physicians should be more aware of this possible association, help reduce disease discrimination among the public, and encourage more social engagement for AA patients,” he said.
“Further studies are needed to elucidate the underlying pathophysiology between AA and dementia risk,” he added.
No cause and effect
Commenting on the study, Heather M. Snyder, PhD, vice president of medical and scientific affairs, Alzheimer’s Association, said, “We continue to learn about and better understand factors that may increase or decrease a person’s risk of dementia.”
“While we know the immune system plays a role in Alzheimer’s and other dementia, we are still investigating links between, and impact of, autoimmune diseases – like alopecia areata, rheumatoid arthritis, and others – on our overall health and our brains, [which] may eventually give us important information on risk reduction strategies as well,” said Dr. Snyder, who was not involved in the research.
She cautioned that although the study did show a correlation between AA and dementia risk, this does not equate to a demonstration of cause and effect.
At present, “the message for clinicians is that when a patient comes to your office with complaints about their memory, they should, No. 1, be taken seriously; and, No. 2, receive a thorough evaluation that takes into account the many factors that may lead to cognitive decline,” Dr. Snyder said.
The study was supported by a grant from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. Dr. Li, coauthors, and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alopecia areata (AA) has been linked to a significantly increased risk for dementia, new research shows.
After controlling for an array of potential confounders, investigators found a threefold higher risk of developing any form of dementia and a fourfold higher risk of developing Alzheimer’s disease (AD) in those with AA versus the controls.
“AA shares a similar inflammatory signature with dementia and has great psychological impacts that lead to poor social engagement,” lead author Cheng-Yuan Li, MD, MSc, of the department of dermatology, Taipei (Taiwan) Veterans General Hospital.
“Poor social engagement and shared inflammatory cytokines might both be important links between AA and dementia,” said Dr. Li, who is also affiliated with the School of Medicine and the Institute of Brain Science at National Yang Ming Chiao Tung University, Taipei.
The study was published online Oct. 26, 2021, in the Journal of Clinical Psychiatry (doi: 10.4088/JCP.21m13931).
Significant psychological impact
Patients with AA often experience anxiety and depression, possibly caused by the negative emotional and psychological impact of the hair loss and partial or even complete baldness associated with the disease, the authors noted.
However, AA is also associated with an array of other atopic and autoimmune diseases, including psoriasis and systemic lupus erythematosus (SLE).
Epidemiologic research has suggested a link between dementia and autoimmune diseases such as psoriasis and SLE, with some evidence suggesting that autoimmune and inflammatory mechanisms may “play a role” in the development of AD.
Dementia in general and AD in particular, “have been shown to include an inflammatory component” that may share some of the same mediators seen in AA (eg, IL-1 beta, IL-6, and tumor necrosis factor–alpha).
Moreover, “the great negative psychosocial impact of AA might result in poor social engagement, a typical risk factor for dementia,” said Dr. Li. The investigators sought to investigate whether patients with AA actually do have a higher dementia risk than individuals without AA.
The researchers used data from the Taiwan National Health Insurance Research Database, comparing 2,534 patients with AA against 25,340 controls matched for age, sex, residence, income, dementia-related comorbidities, systemic steroid use, and annual outpatient visits. Participants were enrolled between 1998 and 2011 and followed to the end of 2013.
The mean age of the cohort was 53.9 years, and a little over half (57.6%) were female. The most common comorbidity was hypertension (32.3%), followed by dyslipidemia (27%) and diabetes (15.4%).
Dual intervention
After adjusting for potential confounders, those with AA were more likely to develop dementia, AD, and unspecified dementia, compared with controls. They also had a numerically higher risk for vascular dementia, compared with controls, but it was not statistically significant.
When participants were stratified by age, investigators found a significant association between AA and higher risk for any dementia as well as unspecified dementia in individuals of all ages and an increased risk for AD in patients with dementia age at onset of 65 years and older.
The mean age of dementia diagnosis was considerably younger in patients with AA versus controls (73.4 vs. 78.9 years, P = .002). The risk for any dementia and unspecified dementia was higher in patients of both sexes, but the risk for AD was higher only in male patients.
Sensitivity analyses that excluded the first year or first 3 years of observation yielded similar and consistent findings.
“Intervention targeting poor social engagement and inflammatory cytokines may be beneficial to AA-associated dementia,” said Dr. Li.
“Physicians should be more aware of this possible association, help reduce disease discrimination among the public, and encourage more social engagement for AA patients,” he said.
“Further studies are needed to elucidate the underlying pathophysiology between AA and dementia risk,” he added.
No cause and effect
Commenting on the study, Heather M. Snyder, PhD, vice president of medical and scientific affairs, Alzheimer’s Association, said, “We continue to learn about and better understand factors that may increase or decrease a person’s risk of dementia.”
“While we know the immune system plays a role in Alzheimer’s and other dementia, we are still investigating links between, and impact of, autoimmune diseases – like alopecia areata, rheumatoid arthritis, and others – on our overall health and our brains, [which] may eventually give us important information on risk reduction strategies as well,” said Dr. Snyder, who was not involved in the research.
She cautioned that although the study did show a correlation between AA and dementia risk, this does not equate to a demonstration of cause and effect.
At present, “the message for clinicians is that when a patient comes to your office with complaints about their memory, they should, No. 1, be taken seriously; and, No. 2, receive a thorough evaluation that takes into account the many factors that may lead to cognitive decline,” Dr. Snyder said.
The study was supported by a grant from Taipei Veterans General Hospital and the Ministry of Science and Technology, Taiwan. Dr. Li, coauthors, and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Management of Pediatric Nail Psoriasis
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Practice Points
- No clinical trials assessing the management of pediatric nail psoriasis currently are present in the literature. Limited information on the treatment of pediatric nail psoriasis exists, mostly in the form of small case series and case reports.
- As more agents are approved for on-label use in plaque psoriasis in pediatric patients, gradually more real-life data on their efficacy for nail psoriasis in children are expected to come to light.
Hair follicle miniaturization common in persistent chemo-induced alopecia, case series suggests
and treatment with minoxidil (sometimes with antiandrogen therapy) was associated with improved hair density, according to a recently published retrospective case series.
“An improvement in hair density was observed in most of the patients treated with topical minoxidil or LDOM [low-dose oral minoxidil], with a more favorable outcome seen with LDOM with or without antiandrogens,” reported Bevin Bhoyrul, MBBS, of Sinclair Dermatology in Melbourne and coauthors from the United Kingdom and Germany.
The findings, published in JAMA Dermatology, suggest that pCIA “may be at least partly reversible,” they wrote.
The investigators analyzed the clinicopathologic characteristics of pCIA in 100 patients presenting to the hair clinics, as well as the results of trichoscopy performed in 90 of the patients and biopsies in 18. The researchers also assessed the effectiveness of treatment in 49 of these patients who met their criteria of completing at least 6 months of therapy with minoxidil.
Almost all patients in their series – 92% – were treated with taxanes and had more severe alopecia than those who weren’t exposed to taxanes (a median Sinclair scale grade of 4 vs. 2). Defined as absent or incomplete hair regrowth 6 months or more after completion of chemotherapy, pCIA has been increasingly reported in the literature, the authors note.
Of the 100 patients, all but one of whom were women, 39 had globally-reduced hair density that also involved the occipital area (diffuse alopecia), and 55 patients had thinning of the centroparietal scalp hair in a female pattern hair loss (FPHL) distribution. Patients presented between November 2011 and February 2020 and had a mean age of 54. The Sinclair scale, which grades from 1 to 5, was used to assess the severity of hair loss in these patients.
Five female patients had bitemporal recession or balding of the crown in a male pattern hair loss (MPHL) distribution, and the one male patient had extensive baldness resembling Hamilton-Norwood type VII.
The vast majority of patients who had trichoscopy performed – 88% – had trichoscopic features that were “indistinguishable from those of androgenetic alopecia,” most commonly hair shaft diameter variability, increased vellus hairs, and predominant single-hair follicular units, the authors reported.
Of the 18 patients who had biopsies, 14 had androgenetic alopecia-like features with decreased terminal hairs, increased vellus hairs, and fibrous streamers. The reduced terminal-to-vellus ratio characterizes hair follicle miniaturization, a hallmark of androgenetic alopecia, they said. (Two patients had cicatricial alopecia, and two had features of both.)
“The predominant phenotypes of pCIA show prominent vellus hairs both clinically and histologically, suggesting that terminal hair follicles undergo miniaturization,” Dr. Bhoyrul and coauthors wrote. Among the 49 patients who completed 6 months or more of treatment, the median Sinclair grade improved from 4 to 3 in 21 patients who received topical minoxidil for a median duration of 17 months; from 4 to 2.5 in 18 patients who received LDOM for a median duration of 29 months; and from 5 to 3 in 10 patients who received LDOM combined with an antiandrogen, such as spironolactone, for a median of 33 months.
Almost three-quarters of the patients in the series received adjuvant hormone therapy, which is independently associated with hair loss, the authors noted. However, there was no statistically significant difference in the pattern or severity of alopecia between patients who were treated with endocrine therapy and those who weren’t.
Asked to comment on the study and on the care of patients with pCIA, Maria K. Hordinsky, MD, professor and chair of dermatology at the University of Minnesota, Minneapolis, and an expert in hair diseases, said the case series points to the value of biopsies in patients with pCIA.
“Some patients really do have a loss of hair follicles,” she said. “But if you do a biopsy and see this miniaturization of the hair follicles, then we have tools to stimulate the hair follicles to become more normal. ... These patients can be successfully treated.”
For patients who do not want to do a biopsy, a therapeutic trial is acceptable. “But knowing helps set expectations for people,” she said. “If the follicles are really small, it will take months [of therapy].”
In addition to topical minoxidil, which she said “is always a good tool,” and LDOM, which is “becoming very popular,” Dr. Hordinsky has used low-level laser light successfully. She cautioned against the use of spironolactone and other hair-growth promoting therapies with potentially significant hormonal impacts unless there is discussion between the dermatologist, oncologist, and patient.
The authors of the case series called in their conclusion for wider use of hair-protective strategies such as scalp hypothermia. But Dr. Hordinsky said that, in the United States, there are divergent opinions among oncologists and among cancer centers on the use of scalp cooling and whether or not it might lessen response to chemotherapy.
More research is needed, she noted, on chemotherapy-induced hair loss in patients of different races and ethnicities. Of the 100 patients in the case series, 91 were European; others were Afro Caribbean, Middle Eastern, and South Asian.
Dr. Bhoyrul is supported by the Geoffrey Dowling Fellowship from the British Association of Dermatologists. One coauthor disclosed serving as a principal investigator and/or scientific board member for various pharmaceutical companies, outside of the submitted study. There were no other disclosures reported. Dr. Hordinsky, the immediate past president of the American Hair Research Society and a section editor for hair diseases in UpToDate, had no relevant disclosures.
and treatment with minoxidil (sometimes with antiandrogen therapy) was associated with improved hair density, according to a recently published retrospective case series.
“An improvement in hair density was observed in most of the patients treated with topical minoxidil or LDOM [low-dose oral minoxidil], with a more favorable outcome seen with LDOM with or without antiandrogens,” reported Bevin Bhoyrul, MBBS, of Sinclair Dermatology in Melbourne and coauthors from the United Kingdom and Germany.
The findings, published in JAMA Dermatology, suggest that pCIA “may be at least partly reversible,” they wrote.
The investigators analyzed the clinicopathologic characteristics of pCIA in 100 patients presenting to the hair clinics, as well as the results of trichoscopy performed in 90 of the patients and biopsies in 18. The researchers also assessed the effectiveness of treatment in 49 of these patients who met their criteria of completing at least 6 months of therapy with minoxidil.
Almost all patients in their series – 92% – were treated with taxanes and had more severe alopecia than those who weren’t exposed to taxanes (a median Sinclair scale grade of 4 vs. 2). Defined as absent or incomplete hair regrowth 6 months or more after completion of chemotherapy, pCIA has been increasingly reported in the literature, the authors note.
Of the 100 patients, all but one of whom were women, 39 had globally-reduced hair density that also involved the occipital area (diffuse alopecia), and 55 patients had thinning of the centroparietal scalp hair in a female pattern hair loss (FPHL) distribution. Patients presented between November 2011 and February 2020 and had a mean age of 54. The Sinclair scale, which grades from 1 to 5, was used to assess the severity of hair loss in these patients.
Five female patients had bitemporal recession or balding of the crown in a male pattern hair loss (MPHL) distribution, and the one male patient had extensive baldness resembling Hamilton-Norwood type VII.
The vast majority of patients who had trichoscopy performed – 88% – had trichoscopic features that were “indistinguishable from those of androgenetic alopecia,” most commonly hair shaft diameter variability, increased vellus hairs, and predominant single-hair follicular units, the authors reported.
Of the 18 patients who had biopsies, 14 had androgenetic alopecia-like features with decreased terminal hairs, increased vellus hairs, and fibrous streamers. The reduced terminal-to-vellus ratio characterizes hair follicle miniaturization, a hallmark of androgenetic alopecia, they said. (Two patients had cicatricial alopecia, and two had features of both.)
“The predominant phenotypes of pCIA show prominent vellus hairs both clinically and histologically, suggesting that terminal hair follicles undergo miniaturization,” Dr. Bhoyrul and coauthors wrote. Among the 49 patients who completed 6 months or more of treatment, the median Sinclair grade improved from 4 to 3 in 21 patients who received topical minoxidil for a median duration of 17 months; from 4 to 2.5 in 18 patients who received LDOM for a median duration of 29 months; and from 5 to 3 in 10 patients who received LDOM combined with an antiandrogen, such as spironolactone, for a median of 33 months.
Almost three-quarters of the patients in the series received adjuvant hormone therapy, which is independently associated with hair loss, the authors noted. However, there was no statistically significant difference in the pattern or severity of alopecia between patients who were treated with endocrine therapy and those who weren’t.
Asked to comment on the study and on the care of patients with pCIA, Maria K. Hordinsky, MD, professor and chair of dermatology at the University of Minnesota, Minneapolis, and an expert in hair diseases, said the case series points to the value of biopsies in patients with pCIA.
“Some patients really do have a loss of hair follicles,” she said. “But if you do a biopsy and see this miniaturization of the hair follicles, then we have tools to stimulate the hair follicles to become more normal. ... These patients can be successfully treated.”
For patients who do not want to do a biopsy, a therapeutic trial is acceptable. “But knowing helps set expectations for people,” she said. “If the follicles are really small, it will take months [of therapy].”
In addition to topical minoxidil, which she said “is always a good tool,” and LDOM, which is “becoming very popular,” Dr. Hordinsky has used low-level laser light successfully. She cautioned against the use of spironolactone and other hair-growth promoting therapies with potentially significant hormonal impacts unless there is discussion between the dermatologist, oncologist, and patient.
The authors of the case series called in their conclusion for wider use of hair-protective strategies such as scalp hypothermia. But Dr. Hordinsky said that, in the United States, there are divergent opinions among oncologists and among cancer centers on the use of scalp cooling and whether or not it might lessen response to chemotherapy.
More research is needed, she noted, on chemotherapy-induced hair loss in patients of different races and ethnicities. Of the 100 patients in the case series, 91 were European; others were Afro Caribbean, Middle Eastern, and South Asian.
Dr. Bhoyrul is supported by the Geoffrey Dowling Fellowship from the British Association of Dermatologists. One coauthor disclosed serving as a principal investigator and/or scientific board member for various pharmaceutical companies, outside of the submitted study. There were no other disclosures reported. Dr. Hordinsky, the immediate past president of the American Hair Research Society and a section editor for hair diseases in UpToDate, had no relevant disclosures.
and treatment with minoxidil (sometimes with antiandrogen therapy) was associated with improved hair density, according to a recently published retrospective case series.
“An improvement in hair density was observed in most of the patients treated with topical minoxidil or LDOM [low-dose oral minoxidil], with a more favorable outcome seen with LDOM with or without antiandrogens,” reported Bevin Bhoyrul, MBBS, of Sinclair Dermatology in Melbourne and coauthors from the United Kingdom and Germany.
The findings, published in JAMA Dermatology, suggest that pCIA “may be at least partly reversible,” they wrote.
The investigators analyzed the clinicopathologic characteristics of pCIA in 100 patients presenting to the hair clinics, as well as the results of trichoscopy performed in 90 of the patients and biopsies in 18. The researchers also assessed the effectiveness of treatment in 49 of these patients who met their criteria of completing at least 6 months of therapy with minoxidil.
Almost all patients in their series – 92% – were treated with taxanes and had more severe alopecia than those who weren’t exposed to taxanes (a median Sinclair scale grade of 4 vs. 2). Defined as absent or incomplete hair regrowth 6 months or more after completion of chemotherapy, pCIA has been increasingly reported in the literature, the authors note.
Of the 100 patients, all but one of whom were women, 39 had globally-reduced hair density that also involved the occipital area (diffuse alopecia), and 55 patients had thinning of the centroparietal scalp hair in a female pattern hair loss (FPHL) distribution. Patients presented between November 2011 and February 2020 and had a mean age of 54. The Sinclair scale, which grades from 1 to 5, was used to assess the severity of hair loss in these patients.
Five female patients had bitemporal recession or balding of the crown in a male pattern hair loss (MPHL) distribution, and the one male patient had extensive baldness resembling Hamilton-Norwood type VII.
The vast majority of patients who had trichoscopy performed – 88% – had trichoscopic features that were “indistinguishable from those of androgenetic alopecia,” most commonly hair shaft diameter variability, increased vellus hairs, and predominant single-hair follicular units, the authors reported.
Of the 18 patients who had biopsies, 14 had androgenetic alopecia-like features with decreased terminal hairs, increased vellus hairs, and fibrous streamers. The reduced terminal-to-vellus ratio characterizes hair follicle miniaturization, a hallmark of androgenetic alopecia, they said. (Two patients had cicatricial alopecia, and two had features of both.)
“The predominant phenotypes of pCIA show prominent vellus hairs both clinically and histologically, suggesting that terminal hair follicles undergo miniaturization,” Dr. Bhoyrul and coauthors wrote. Among the 49 patients who completed 6 months or more of treatment, the median Sinclair grade improved from 4 to 3 in 21 patients who received topical minoxidil for a median duration of 17 months; from 4 to 2.5 in 18 patients who received LDOM for a median duration of 29 months; and from 5 to 3 in 10 patients who received LDOM combined with an antiandrogen, such as spironolactone, for a median of 33 months.
Almost three-quarters of the patients in the series received adjuvant hormone therapy, which is independently associated with hair loss, the authors noted. However, there was no statistically significant difference in the pattern or severity of alopecia between patients who were treated with endocrine therapy and those who weren’t.
Asked to comment on the study and on the care of patients with pCIA, Maria K. Hordinsky, MD, professor and chair of dermatology at the University of Minnesota, Minneapolis, and an expert in hair diseases, said the case series points to the value of biopsies in patients with pCIA.
“Some patients really do have a loss of hair follicles,” she said. “But if you do a biopsy and see this miniaturization of the hair follicles, then we have tools to stimulate the hair follicles to become more normal. ... These patients can be successfully treated.”
For patients who do not want to do a biopsy, a therapeutic trial is acceptable. “But knowing helps set expectations for people,” she said. “If the follicles are really small, it will take months [of therapy].”
In addition to topical minoxidil, which she said “is always a good tool,” and LDOM, which is “becoming very popular,” Dr. Hordinsky has used low-level laser light successfully. She cautioned against the use of spironolactone and other hair-growth promoting therapies with potentially significant hormonal impacts unless there is discussion between the dermatologist, oncologist, and patient.
The authors of the case series called in their conclusion for wider use of hair-protective strategies such as scalp hypothermia. But Dr. Hordinsky said that, in the United States, there are divergent opinions among oncologists and among cancer centers on the use of scalp cooling and whether or not it might lessen response to chemotherapy.
More research is needed, she noted, on chemotherapy-induced hair loss in patients of different races and ethnicities. Of the 100 patients in the case series, 91 were European; others were Afro Caribbean, Middle Eastern, and South Asian.
Dr. Bhoyrul is supported by the Geoffrey Dowling Fellowship from the British Association of Dermatologists. One coauthor disclosed serving as a principal investigator and/or scientific board member for various pharmaceutical companies, outside of the submitted study. There were no other disclosures reported. Dr. Hordinsky, the immediate past president of the American Hair Research Society and a section editor for hair diseases in UpToDate, had no relevant disclosures.
FROM JAMA DERMATOLOGY
JAK inhibitor provides impressive hair growth for patients with alopecia areata
, according to the results of two phase 3 trials presented at the European Academy of Dermatology and Venereology (EADV) 2021 Annual Meeting.
In both trials, severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of greater than or equal to 50, was an enrollment requirement. The primary endpoint was a SALT score of less than or equal to 20, signifying 80% scalp coverage.
“The mean SALT score at entry was 85,” reported Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn. He explained that the SALT scale extends from 0 (no hair loss) to 100 (complete hair loss). About 45% of patients in the phase 3 trials had alopecia universalis.
In both trials, called BRAVE-AA1 and BRAVE-AA2, a response was seen with baricitinib after about 4 weeks. Response increased steadily through the entire 36 weeks of treatment. At the end of 36 weeks, when response curves still had an upward trajectory, the proportion of those treated with the 4-mg dose of baricitinib who had achieved a SALT score of less than or equal to 20 had reached 35.2% in BRAVE-AA1 and 32.5% in BRAVE-AA2.
The nearly identical BRAVE-AA1 and BRAVE-AA2 trials enrolled 654 and 546 patients, respectively. The patients were randomly assigned in a 3:2:2 ratio to receive baricitinib 4 mg, baricitinib 2 mg, or placebo. All treatments were taken once daily. Regrowth of eyebrow and eyelash hair were secondary outcomes.
There was a clear dose effect; hair growth increased more quickly with the 4-mg dose of baricitinib than with the 2-mg dose. The difference between the active therapy and placebo was significant by 16 weeks with the 4-mg dose. By 24 weeks, the advantage of the 2-mg dose over placebo also reached significance. The response rate with the 4-mg dose was nearly twice as great.
At the end of the 36-week trials, the proportion of patients treated with baricitinib 2 mg who achieved the primary endpoint was 21.7% and 17.3% in the BRAVE-AA1 and BRAVE-AA2 trials, respectively. Among patients taking placebo, the primary endpoint was met by 5.3% and 2.6%, respectively, at the end of the two trials.
The differences in responses with the 4-mg and the 2-mg doses were significantly higher compared with placebo (P ≤ .001 for both doses vs. placebo).
Using a scoring system for eyebrow and eyelash hair loss, the proportion of patients who achieved a score of 0 (full coverage) or 1 (minimal gaps) was again superior in both trials for patients taking the higher dose of baricitinib. This level of response was reached by about 31% to 35% of those taking the 4-mg dose in BRAVE-AA1 and BRAVE-AA2 (P ≤ .001 vs. placebo). With the lower dose, the rates were 19.1% and 13.5%, respectively. This endpoint was reached in only about 3% of patients who took placebo.
Rates of adverse events were modestly higher in the two active treatment groups in comparison with the group taking placebo. The most commonly occurring adverse events with baricitinib included upper respiratory tract infections, nasopharyngitis, urinary tract infections, and headache, according to Dr. King.
“Most of the adverse events were mild to moderate,” he said. He also reported that none of these adverse events occurred in more than 10% of patients, and there were no cases of other opportunistic infections, thromboembolic events, or gastrointestinal perforations. The discontinuation rates because of adverse events with active therapy were less than 3% in both trials.
JAK inhibitors are currently employed in the treatment of a variety of inflammatory diseases. Baricitinib is currently approved for the treatment of rheumatoid arthritis. Because specificity differs markedly for their inhibition of JAK kinases (JAK1, JAK2, JAK3, and Tyk2), these drugs do not appear to be interchangeable with regard to clinical effect.
Several case reports of hair regrowth with baricitinib led to a phase 2 trial, which was recently published in the Journal of the American Academy of Dermatology. In this trial, the therapy also yielded substantial benefit for patients with alopecia areata. The benefit of baricitinib is attributed to inhibition of JAK1 and JAK2 signaling, which has been implicated in cytokine-mediated immune dysfunction leading to damage of hair follicles.
Alopecia areata is a common disorder that can have a large adverse impact on quality of life, Dr. King noted. There is no approved therapy for this condition, so there is a large unmet need. Although longer follow-up is needed to gauge sustained efficacy and safety, he considers these results promising for a therapy with clinically meaningful benefit.
This point was reiterated by Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Service, University Hospital Miguel Servet, Zaragoza, Spain, who was moderator of the session in which Dr. King presented these data. She expressed excitement about the promise of baricitinib, particularly with regard to the substantial proportion of patients who achieved meaningful degrees of hair regrowth.
“All of us will be happy to have options for alopecia areata,” said Dr. Calzada, who predicted that the higher dose of baricitinib will be selected for clinical development, given its greater efficacy with little increase in safety concerns.
Eli Lilly provided funding for the BRAVE-AA1 and -AA2 trials. Dr. King has financial relationships with Arena, Aclaris, Bristol-Myers Squibb, Concert, Pfizer, Regeneron, Sanofi Genzyme, and Eli Lilly. Dr. Calzada has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to the results of two phase 3 trials presented at the European Academy of Dermatology and Venereology (EADV) 2021 Annual Meeting.
In both trials, severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of greater than or equal to 50, was an enrollment requirement. The primary endpoint was a SALT score of less than or equal to 20, signifying 80% scalp coverage.
“The mean SALT score at entry was 85,” reported Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn. He explained that the SALT scale extends from 0 (no hair loss) to 100 (complete hair loss). About 45% of patients in the phase 3 trials had alopecia universalis.
In both trials, called BRAVE-AA1 and BRAVE-AA2, a response was seen with baricitinib after about 4 weeks. Response increased steadily through the entire 36 weeks of treatment. At the end of 36 weeks, when response curves still had an upward trajectory, the proportion of those treated with the 4-mg dose of baricitinib who had achieved a SALT score of less than or equal to 20 had reached 35.2% in BRAVE-AA1 and 32.5% in BRAVE-AA2.
The nearly identical BRAVE-AA1 and BRAVE-AA2 trials enrolled 654 and 546 patients, respectively. The patients were randomly assigned in a 3:2:2 ratio to receive baricitinib 4 mg, baricitinib 2 mg, or placebo. All treatments were taken once daily. Regrowth of eyebrow and eyelash hair were secondary outcomes.
There was a clear dose effect; hair growth increased more quickly with the 4-mg dose of baricitinib than with the 2-mg dose. The difference between the active therapy and placebo was significant by 16 weeks with the 4-mg dose. By 24 weeks, the advantage of the 2-mg dose over placebo also reached significance. The response rate with the 4-mg dose was nearly twice as great.
At the end of the 36-week trials, the proportion of patients treated with baricitinib 2 mg who achieved the primary endpoint was 21.7% and 17.3% in the BRAVE-AA1 and BRAVE-AA2 trials, respectively. Among patients taking placebo, the primary endpoint was met by 5.3% and 2.6%, respectively, at the end of the two trials.
The differences in responses with the 4-mg and the 2-mg doses were significantly higher compared with placebo (P ≤ .001 for both doses vs. placebo).
Using a scoring system for eyebrow and eyelash hair loss, the proportion of patients who achieved a score of 0 (full coverage) or 1 (minimal gaps) was again superior in both trials for patients taking the higher dose of baricitinib. This level of response was reached by about 31% to 35% of those taking the 4-mg dose in BRAVE-AA1 and BRAVE-AA2 (P ≤ .001 vs. placebo). With the lower dose, the rates were 19.1% and 13.5%, respectively. This endpoint was reached in only about 3% of patients who took placebo.
Rates of adverse events were modestly higher in the two active treatment groups in comparison with the group taking placebo. The most commonly occurring adverse events with baricitinib included upper respiratory tract infections, nasopharyngitis, urinary tract infections, and headache, according to Dr. King.
“Most of the adverse events were mild to moderate,” he said. He also reported that none of these adverse events occurred in more than 10% of patients, and there were no cases of other opportunistic infections, thromboembolic events, or gastrointestinal perforations. The discontinuation rates because of adverse events with active therapy were less than 3% in both trials.
JAK inhibitors are currently employed in the treatment of a variety of inflammatory diseases. Baricitinib is currently approved for the treatment of rheumatoid arthritis. Because specificity differs markedly for their inhibition of JAK kinases (JAK1, JAK2, JAK3, and Tyk2), these drugs do not appear to be interchangeable with regard to clinical effect.
Several case reports of hair regrowth with baricitinib led to a phase 2 trial, which was recently published in the Journal of the American Academy of Dermatology. In this trial, the therapy also yielded substantial benefit for patients with alopecia areata. The benefit of baricitinib is attributed to inhibition of JAK1 and JAK2 signaling, which has been implicated in cytokine-mediated immune dysfunction leading to damage of hair follicles.
Alopecia areata is a common disorder that can have a large adverse impact on quality of life, Dr. King noted. There is no approved therapy for this condition, so there is a large unmet need. Although longer follow-up is needed to gauge sustained efficacy and safety, he considers these results promising for a therapy with clinically meaningful benefit.
This point was reiterated by Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Service, University Hospital Miguel Servet, Zaragoza, Spain, who was moderator of the session in which Dr. King presented these data. She expressed excitement about the promise of baricitinib, particularly with regard to the substantial proportion of patients who achieved meaningful degrees of hair regrowth.
“All of us will be happy to have options for alopecia areata,” said Dr. Calzada, who predicted that the higher dose of baricitinib will be selected for clinical development, given its greater efficacy with little increase in safety concerns.
Eli Lilly provided funding for the BRAVE-AA1 and -AA2 trials. Dr. King has financial relationships with Arena, Aclaris, Bristol-Myers Squibb, Concert, Pfizer, Regeneron, Sanofi Genzyme, and Eli Lilly. Dr. Calzada has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to the results of two phase 3 trials presented at the European Academy of Dermatology and Venereology (EADV) 2021 Annual Meeting.
In both trials, severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of greater than or equal to 50, was an enrollment requirement. The primary endpoint was a SALT score of less than or equal to 20, signifying 80% scalp coverage.
“The mean SALT score at entry was 85,” reported Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn. He explained that the SALT scale extends from 0 (no hair loss) to 100 (complete hair loss). About 45% of patients in the phase 3 trials had alopecia universalis.
In both trials, called BRAVE-AA1 and BRAVE-AA2, a response was seen with baricitinib after about 4 weeks. Response increased steadily through the entire 36 weeks of treatment. At the end of 36 weeks, when response curves still had an upward trajectory, the proportion of those treated with the 4-mg dose of baricitinib who had achieved a SALT score of less than or equal to 20 had reached 35.2% in BRAVE-AA1 and 32.5% in BRAVE-AA2.
The nearly identical BRAVE-AA1 and BRAVE-AA2 trials enrolled 654 and 546 patients, respectively. The patients were randomly assigned in a 3:2:2 ratio to receive baricitinib 4 mg, baricitinib 2 mg, or placebo. All treatments were taken once daily. Regrowth of eyebrow and eyelash hair were secondary outcomes.
There was a clear dose effect; hair growth increased more quickly with the 4-mg dose of baricitinib than with the 2-mg dose. The difference between the active therapy and placebo was significant by 16 weeks with the 4-mg dose. By 24 weeks, the advantage of the 2-mg dose over placebo also reached significance. The response rate with the 4-mg dose was nearly twice as great.
At the end of the 36-week trials, the proportion of patients treated with baricitinib 2 mg who achieved the primary endpoint was 21.7% and 17.3% in the BRAVE-AA1 and BRAVE-AA2 trials, respectively. Among patients taking placebo, the primary endpoint was met by 5.3% and 2.6%, respectively, at the end of the two trials.
The differences in responses with the 4-mg and the 2-mg doses were significantly higher compared with placebo (P ≤ .001 for both doses vs. placebo).
Using a scoring system for eyebrow and eyelash hair loss, the proportion of patients who achieved a score of 0 (full coverage) or 1 (minimal gaps) was again superior in both trials for patients taking the higher dose of baricitinib. This level of response was reached by about 31% to 35% of those taking the 4-mg dose in BRAVE-AA1 and BRAVE-AA2 (P ≤ .001 vs. placebo). With the lower dose, the rates were 19.1% and 13.5%, respectively. This endpoint was reached in only about 3% of patients who took placebo.
Rates of adverse events were modestly higher in the two active treatment groups in comparison with the group taking placebo. The most commonly occurring adverse events with baricitinib included upper respiratory tract infections, nasopharyngitis, urinary tract infections, and headache, according to Dr. King.
“Most of the adverse events were mild to moderate,” he said. He also reported that none of these adverse events occurred in more than 10% of patients, and there were no cases of other opportunistic infections, thromboembolic events, or gastrointestinal perforations. The discontinuation rates because of adverse events with active therapy were less than 3% in both trials.
JAK inhibitors are currently employed in the treatment of a variety of inflammatory diseases. Baricitinib is currently approved for the treatment of rheumatoid arthritis. Because specificity differs markedly for their inhibition of JAK kinases (JAK1, JAK2, JAK3, and Tyk2), these drugs do not appear to be interchangeable with regard to clinical effect.
Several case reports of hair regrowth with baricitinib led to a phase 2 trial, which was recently published in the Journal of the American Academy of Dermatology. In this trial, the therapy also yielded substantial benefit for patients with alopecia areata. The benefit of baricitinib is attributed to inhibition of JAK1 and JAK2 signaling, which has been implicated in cytokine-mediated immune dysfunction leading to damage of hair follicles.
Alopecia areata is a common disorder that can have a large adverse impact on quality of life, Dr. King noted. There is no approved therapy for this condition, so there is a large unmet need. Although longer follow-up is needed to gauge sustained efficacy and safety, he considers these results promising for a therapy with clinically meaningful benefit.
This point was reiterated by Yolanda Gilaberte Calzada, MD, PhD, head of the Dermatology Service, University Hospital Miguel Servet, Zaragoza, Spain, who was moderator of the session in which Dr. King presented these data. She expressed excitement about the promise of baricitinib, particularly with regard to the substantial proportion of patients who achieved meaningful degrees of hair regrowth.
“All of us will be happy to have options for alopecia areata,” said Dr. Calzada, who predicted that the higher dose of baricitinib will be selected for clinical development, given its greater efficacy with little increase in safety concerns.
Eli Lilly provided funding for the BRAVE-AA1 and -AA2 trials. Dr. King has financial relationships with Arena, Aclaris, Bristol-Myers Squibb, Concert, Pfizer, Regeneron, Sanofi Genzyme, and Eli Lilly. Dr. Calzada has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New finasteride lawsuit brings renewed attention to psychiatric, ED adverse event reports
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.