User login
Rounding up the usual suspects
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
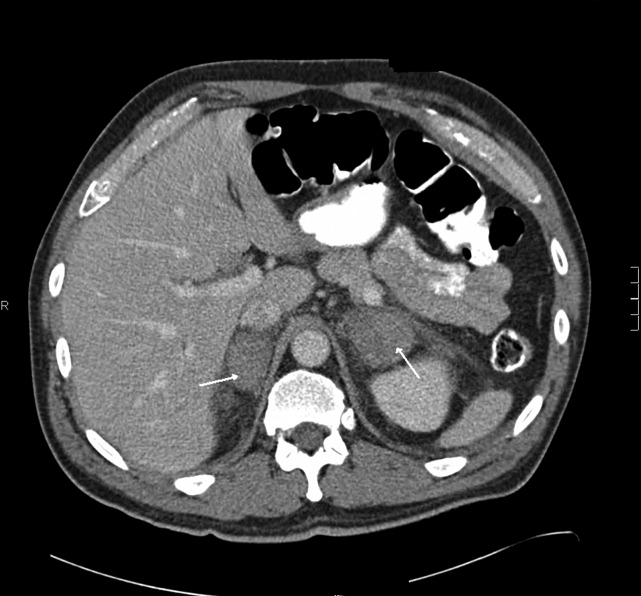
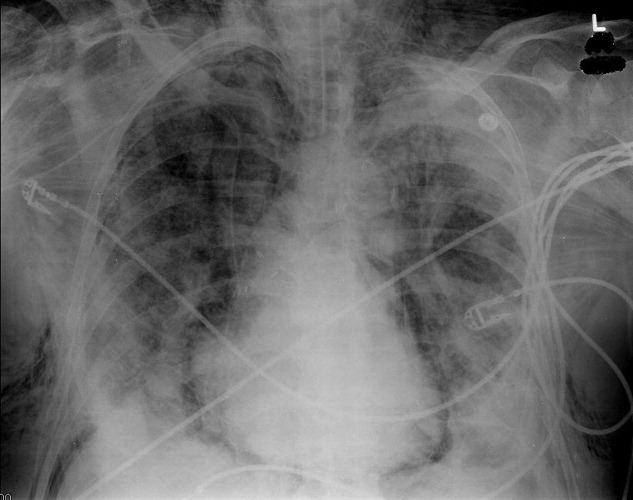
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Unscripted
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was <20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal <1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, <5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was <20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal <1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, <5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was <20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal <1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, <5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
Bilateral Adrenal Hemorrhage Complication
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.
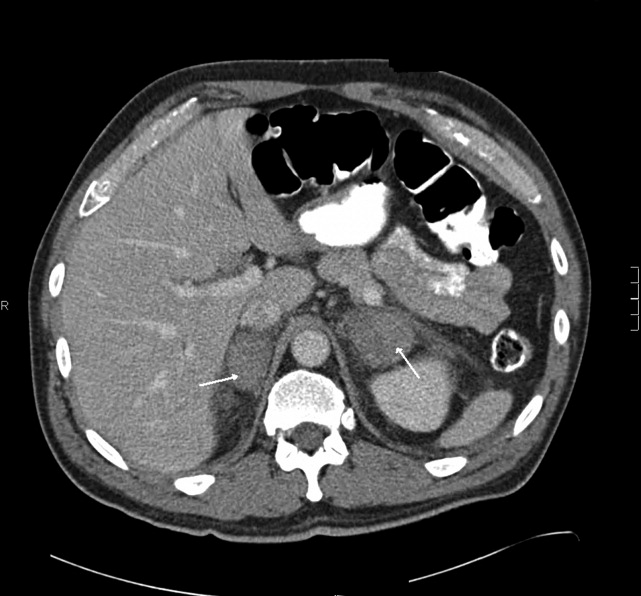
Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.

Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
A 52‐year‐old man presented to the emergency department (ED) from a skilled nursing facility with a complaint of bilateral upper‐quadrant abdominal pain of 48 hours' duration. The pain was sharp, nonradiating, constant, and was associated with nausea, vomiting, and constipation. The patient denied any fever, back pain, dysuria, melena, or hematochezia. In the rehabilitation facility the patient had been initially evaluated for this pain. He was given laxatives and stool softeners for presumed constipation but these measures had not been effective. A computed tomography (CT) scan of the abdomen had only showed stool in the colon and he was sent to the ED for further evaluation.
Apart from severe degenerative joint disease in both his knees he was in good health. He was in the skilled nursing facility (SNF) for rehabilitation for bilateral knee replacement surgery done 9 days prior to this presentation. His postoperative course was unremarkable. He had been maintained on prophylaxis for venous thromboembolism with enoxaparin since postoperative day 1 at a daily dose of 40 mg subcutaneously, and was transferred to the SNF on postoperative day 6 on the same dose. His was receiving oxycodone and Tylenol for pain. He was on no other medications.
Vital signs on presentation revealed a temperature of 97.5F, a heart rate of 100 beats per minute, a respiratory rate of 16 breaths per minute, and a blood pressure of 136/69 mmHg. He was alert and oriented and in mild distress from the abdominal pain. Examination was normal except for tenderness in the upper quadrants of the abdomen though no rigidity or rebound tenderness were noted. Routine chemistries were normal except for sodium of 134 mg/dL. His white count, hemoglobin, hematocrit, and platelet levels were noted to be at 17.5K/L, 10 g/dL, 30%, and 345K/L, respectively, and were stable with regard to his discharge laboratory values. His serum eosinophil level was normal. A complete workup for hypercoagulable state and bleeding disorders including assays for antibodies associated with heparin‐induced thrombocytopenia were negative. He was admitted for further evaluation and treatment.
The patient had another CT scan of the abdomen (Figure 1), which when compared to the one done at the SNF 2 days prior showed markedly enlarged bilateral adrenal glands suggestive of bilateral acute adrenal hemorrhage. The enoxaparin was discontinued and empiric steroid replacement therapy was begun. A random cortisol level was normal but a cosyntropin stimulation test showed an absolute increase in cortisol level of only 0.8 g/dL at both 30 and 60 minutes after administration of 250 g of cosyntropin. An investigation was undertaken to determine if the patient had any prior risk factors for bleeding. There was no evidence of infection and a comprehensive evaluation for bleeding, and coagulation disorders was normal. The bilateral adrenal hemorrhage was attributed to the use of enoxaparin in the postoperative setting. Unfortunately, the patient subsequently developed a deep venous thrombosis in his lower extremity and an inferior vena cava (IVC) filter was placed before discharge. He was doing well 6 months later, and is still continued on glucocorticoid and mineralocorticoid replacement therapy and follows up with endocrinology as an outpatient.

Discussion
Bilateral adrenal hemorrhage is usually associated with massive sepsis from Gram‐negative organisms such as Neisseria meningitides, Pseudomonas aeroginosa, Escherichia coli, and Bacteroides fragilis. Rupert Waterhouse, in 1911, was the first person to describe a patient with severe meningococcal sepsis resulting in acute adrenal hemorrhage and collapse. This was also later described independently by Carl Friderichsen in 1918, and is now referred to as the Waterhouse‐Friderichsen syndrome. Other causes include antiphospholipid antibody syndrome, heparin‐associated thrombocytopenia (HIT), and severe physical stress. Bilateral adrenal hemorrhage can also spontaneously occur in the postoperative period, especially after cardiothoracic or orthopedic surgery. This phenomenon may be related to the frequent use of prophylactic anticoagulants after these types of procedures.
The first case report of bilateral adrenal hemorrhage secondary to use of anticoagulants was described in 1947, and the first case report of successful resuscitation after corticosteroid administration in a patient with bilateral adrenal hemorrhage secondary to anticoagulant use was described by Thorn in 1956.1 A review of the literature demonstrates multiple case reports of adrenal hemorrhage reported in the postoperative period, particularly after joint arthroplasty, and especially after knee replacement surgeries. Most of the recent cases have been associated with use of prophylactic low‐dose heparin or low‐molecular‐weight heparin at the time of adrenal hemorrhage. In a study of 157 case reports of individuals with bilateral hemorrhage (including 22 autopsies), 48 cases were associated with administration of anticoagulants, although the dose and effect were not specified.2 Amador et al.1 showed that out of 4325 autopsies performed from 1949 to 1962 in their institution, 30 cases were found of bilateral hemorrhage, of which 10 were receiving heparin at presumably prophylactic doses; 5 of these patients were also receiving dicumarol.
Mayo Clinic investigators performed a retrospective review of all cases of adrenal hemorrhage over a period of 25 years at their hospital, and found 141 cases of adrenal hemorrhage, of which 78 were bilateral and 63 were unilateral,3 and in 67 patients the condition was diagnosed at autopsy. In this study 14 patients had adrenal hemorrhage in the postoperative period in the absence of lupus anticoagulant or HIT; there was no specific mention in this study of the use of postoperative anticoagulants. Finally, a multicenter case control study was undertaken by Kovacs et al.4 to assess putative risk factors for development of bilateral massive adrenal hemorrhage. In the multivariate analysis, thrombocytopenia, exposure to heparin, and sepsis were found to be strongly associated with risk of hemorrhage. Of 23 patients with bilateral, massive adrenal hemorrhage, 16 had been exposed to heparin, and at least 6 were on exclusively subcutaneous heparin. The authors concluded that heparin exposure was a much bigger risk factor than other coagulopathies, and those exposed to heparin of any route or type for 4 to 6 days and those exposed for more than 6 days were about 17 and 34 times, respectively, more likely to develop bilateral hemorrhage than those who had less than 4 days or no exposure.
The clinical presentation of adrenal insufficiency due to bilateral adrenal hemorrhage is often nonspecific. Symptoms may include abdominal pain, back pain, fever, nausea, vomiting, weakness, obtundation, confusion, and hypotensionall of which are also common postoperative symptoms and can be missed or ignored.5 Rao et al.6 profiled the clinical presentation of 64 cases of bilateral hemorrhage and found the following: abdominal, flank, back, or chest pain (86%); anorexia, nausea, or vomiting (47%); psychiatric symptoms (42%); fever (66%); hypotension recognized before shock episode (19%); and abdominal rigidity or rebound (22%). Adrenal insufficiency becomes clinically evident once 90% of the gland is destroyed. About 50% of patients do not manifest typical laboratory abnormalities, so a high degree of suspicion is necessary to diagnose the condition.3 Also, the laboratory diagnosis of adrenal insufficiency using random cortisol levels is unreliable, as reference ranges in patients experiencing stress (as in the postoperative period) have not been well studied or established. In patients with bilateral hemorrhage postoperatively on prophylactic anticoagulants, the coagulation profile is usually within normal limits and there is typically no evidence of spontaneous bleeding elsewhere. In later stages, the typical laboratory findings of abnormal adrenal function such as hypokalemia, hyponatremia, declining cortisol levels, and an inappropriate response to adrenocorticotropic hormone stimulation test may be seen. A significant drop in hemoglobin secondary to hemorrhage may also be encountered in some patients secondary to the bleed.
CT is the most reliable and extensively used imaging modality for making the diagnosis, although magnetic resonance imaging (MRI) or ultrasound may also be utilized. Early in the course of adrenal hemorrhage, CT findings may be negative, and repeated imaging is appropriate when clinical suspicion is high. The presence of bilateral adrenal enlargement with increased signal attenuation suggests bilateral adrenal hemorrhage. MRI can both characterize adrenal hematomas, and estimate their age.7, 8
Postoperative adrenal hemorrhage and insufficiency is easily treatable and has excellent outcomes; survivors will need lifelong corticosteroid replacement (and usually mineralocorticoid replacement as well). In the Mayo Clinic study, survival was 100% with treatment vs. 17% without treatment. In comparison, sepsis‐induced or stress‐induced adrenal insufficiency has poor outcomes despite adequate treatment (9% survival with treatment vs. 6% survival without treatment).3 Death can occur within hours to days of symptoms if untreated. Treatment includes timely initiation of adrenal hormone replacement and reversal of coagulopathies.
Postoperative venous thromboembolism (VTE) prophylaxis with anticoagulants is the appropriate care in many cases, but, along with the postoperative state itself, also appears to be a risk factor for this unusual condition. Postoperative bilateral adrenal hemorrhage is rare and potentially fatal. Early identification and prompt initiation of steroid replacement therapy and reversal of coagulopathies can prove to be lifesaving. Making this diagnosis can be very challenging, as the clinical presentation and laboratory findings of adrenal hemorrhage are vague and nonspecific and mimic many nonlife threatening postoperative complications. Radiological diagnosis by CT may initially be normal and thus further confound the diagnosis. Hence, providers should remain vigilant for associated complications even with low‐dose prophylactic heparin or low‐molecular‐weight heparin in postoperative patients, and prompt, presumptive treatment with corticosteroids should be started while awaiting confirmation by imaging and laboratory testing.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
- .Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.Ann Intern Med.1965;63(4):559–571.
- ,,,,,.Adrenal hemorrhage in the adult.Medicine.1978;57(3):211–221.
- ,,.Adrenal hemorrhage: a 25‐year experience at the Mayo Clinic.Mayo Clin Proc.2001;76(2):161–168.
- ,,.Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case‐control method.Medicine.2001;80(1):45–53.
- .Bilateral massive adrenal hemorrhage.Med Clin North Am.1995;79(1):107–129.
- ,,.Bilateral massive adrenal hemorrhage: early recognition and treatment.Ann Intern Med.1989;110(3):227–235.
- ,,, et al.Imaging of nontraumatic hemorrhage of the adrenal gland.Radiographics.1999;19(4):949–963.
- ,,,,.Spontaneous unilateral adrenal hemorrhage: computerized tomography and magnetic resonance imaging findings in 8 cases.J Urol.1995;154(5):1647–1651.
Pneumomediastinum and Pneumopericardium
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.
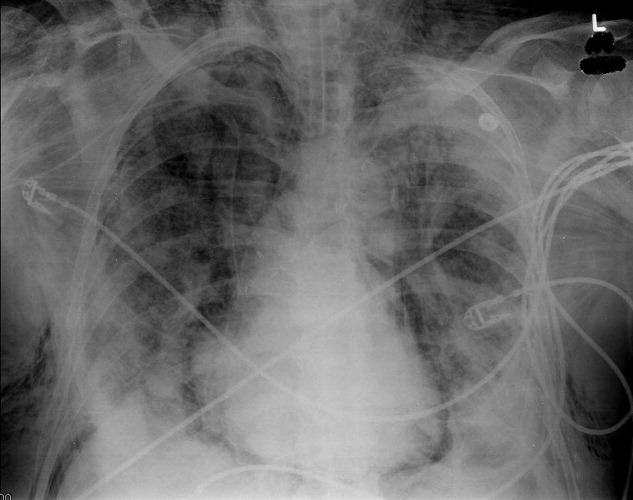
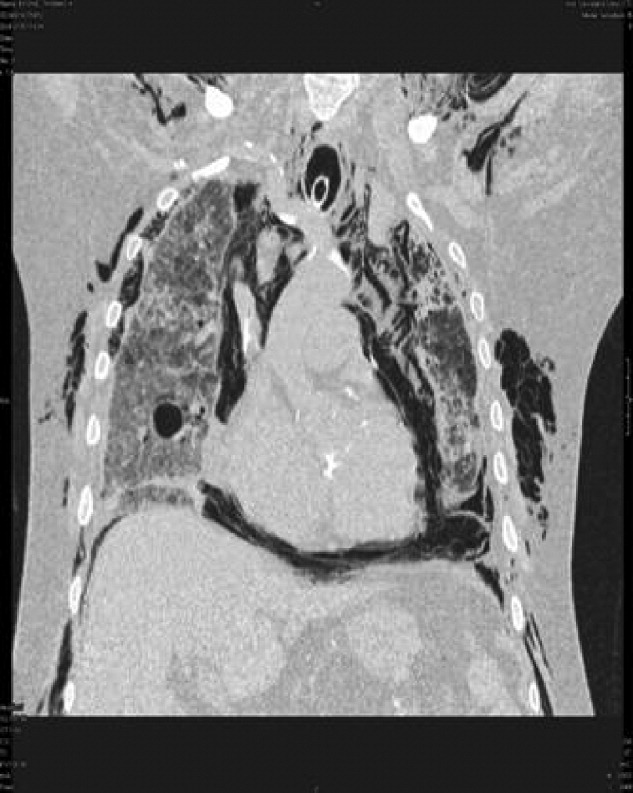
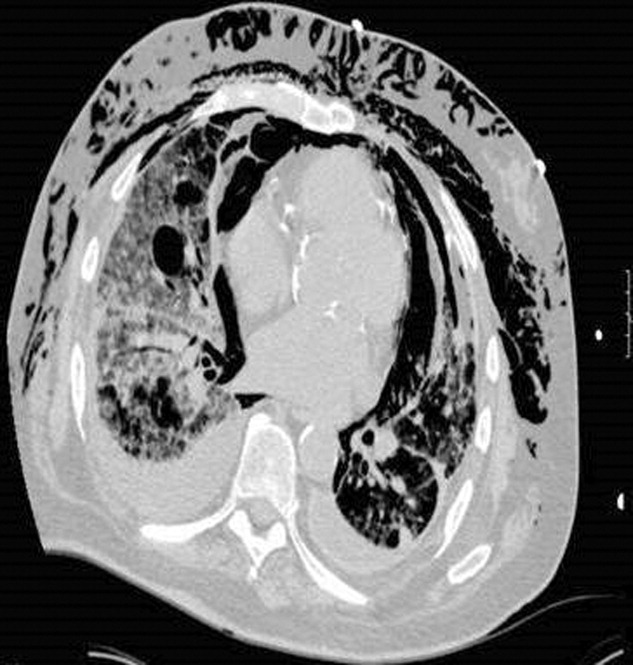
In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.



In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
A 73‐year‐old male presented with acute congestive heart failure and non‐ST elevation myocardial infarction. His initial chest x‐ray and computed tomography (CT) demonstrated pulmonary vascular congestion and alveolar infiltrates, and he promptly underwent cardiac catheterization with placement of a coronary stent. Subsequently, his respiratory status deteriorated, and repeat films and chest CT demonstrated extensive pneumomediastinum and pneumopericardium (Figures 13). The patient was intubated, and bronchoscopy and upper gastrointestinal (GI) endoscopy were performed, but demonstrated no evidence of perforation that could cause such an air leak. There was no evidence of tamponade, clinically or on echocardiogram. His condition worsened abruptly, and he expired following a cardiac arrest. Postmortem, the team considered that the extensive air leak could have been caused by catheterization, stent placement, central line placement, or mediastinitis or pericarditis causing microscopic fistulae. The patient's tracheal aspirate and biopsy grew Candida albicans but no evidence of invasive candidiasis was found on autopsy. No definitive etiology was found.



In contrast to pneumomediastinum, pneumopericardium is a rare condition and its pathophysiology is not well understood. Most cases have been reported in newborns receiving mechanical ventilation. In adults, the condition occurs due to chest trauma, or can be iatrogenic secondary to laparoscopy, bronchoscopy, or endotracheal intubation. There have been case reports of pneumopericardium after cardiac catheterization and central line placement.1, 2 Other causes include lung transplant, esophageal perforation, severe asthma, positive pressure ventilation, and pericarditis (eg, histoplasmosis and tuberculosis).3, 4 Clinical findings include distant heart sounds, shifting precordial tympany, and a succussion splash with metallic tinkling (known as mill wheel murmur) in hydropneumopericardium.5 Chest CT can distinguish pneumopericardium from pneumomediastinum: with the former, the air changes position when the patient adopts a supine position.6 Cardiac tamponade can occur in up to 37% of cases, and pericardiocentesis or pericardial tube drainage in these cases can be lifesaving.7
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.
- ,,,,.[Cardiac tamponade and central venous catheterization].Ann Fr Anesth Reanim.1992;11:201–204. [French]
- ,,,.Pneumopericardium as a complication of balloon atrial septostomy.Pediatr Cardiol.1987;8:135–137.
- ,,,,.Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review.Heart.2002;88:e5.
- ,.Tension pneumopericardium: a case report and a review of the literature.Am Surg.2006;72:330–331.
- Symptoms and signs of syndromes associated with mill wheel murmurs.NC Med J.1988;49:569–572.
- ,.Pneumomediastinum: old signs and new signs.AJR Am J Roentgenol.1996;166:1041–1048.
- ,,,,.Cardiac tamponade without pericardial effusion after blunt chest trauma.Am Heart J.1996;131:198–200.
Development of an electronic medical record smart set form to increase standardization, consistency, and compliance with ACC/AHA perioperative guidelines
Development of a perioperative electronic medical record research and quality improvement database
Perioperative beta-blockers in noncardiac surgery: Evolution of the evidence
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
KEY POINTS
- Beta-blockers reduce perioperative ischemia, but the benefit may be only in high-risk patients undergoing high-risk surgery. Currently, the best evidence supports their use in two groups: patients undergoing vascular surgery who have known ischemic heart disease or multiple risk factors for it, and patients who are already on beta-blockers.
- The Perioperative Ischemic Evaluation (POISE) findings suggest that beta-blockers should be used in the immediate preoperative period only with great caution, after ensuring that the patient is clinically stable and without evidence of infection, hypovolemia, anemia, or other conditions that could make heart-rate titration misleading or use of the drug dangerous.
- When feasible, beta-blockers should be started a month before surgery, titrated to a heart rate of 60 beats per minute, and continued for approximately a month. If the drug is then to be discontinued, it should be tapered slowly.
In the Literature
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
Hematocrit and Perioperative Mortality
Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007 Jun 13;297(22):2481-2488.
Several studies have outlined the risk of preoperative anemia prior to noncardiac surgery in elderly patients. These studies have not linked anemia to risk of death unless cardiac disease is present.
Anemia management remains a challenge for many hospitals and is the most important predictor of the need for blood transfusion. Transfusion increases morbidity and mortality in the perioperative setting. At the same time, little is known about the risks of polycythemia in this setting.
This retrospective cohort study used the Veterans’ Affairs National Surgical Quality Improvement Program database of 310,311 veterans 65 or older from 132 VA hospitals. It explores the relationship between abnormal levels of hematocrit and adverse events among elderly surgical patients.
The data suggest an incremental relationship between positive and negative deviation of hematocrit levels with 30-day postoperative mortality in patients 65 and older. Specifically, the study found a 1.6% increase (95% confidence interval, 1.1%-2.2%) in 30-day mortality for every percentage point of increase or decrease in hematocrit from the normal range.
Because this is an observational study of anemia and adverse events, no causal relationship can be established from this data. Hospitalists involved in perioperative care should be careful about drawing conclusions from this study alone and should not necessarily plan interventions to treat abnormal levels of hematocrit without carefully considering the risks and benefits of intervention.
Prognostic Utility of Pre-operative BNP
Feringa HH, Schouten O, Dunkelgrun M, et al. Plasma N-terminal pro-B-type natriuretic peptide as long term prognostic marker after major vascular surgery. Heart. 2007 Feb;93(2):226-231.
Traditional stratification of patients at high risk for cardiac complications and undergoing noncardiac surgery has included clinical risk index scoring and pre-operative stress testing. It is unclear if cardiac biomarkers can be used in conjunction with these measures to improve the identification of patients at risk.
Feringa and colleagues addressed this question by looking prospectively at 335 patients undergoing major vascular surgery over a two-year period. The mean age of patients was 62.2 years; 46% of patients underwent abdominal aortic aneurysm repair, and the remaining 54% received lower-extremity revascularization.
Patients had cardiac risk scores calculated based on the Revised Cardiac Risk Index (RCRI), and all patients had dobutamine stress echocardiogram (DSE) to assess for stress-induced ischemia. N-terminal pro-B-type natriuretic peptide (BNP) was measured at a mean of 12 days before surgery. Patients were followed for all-cause mortality and post-op death for a mean follow-up time of 14 months.
The authors found that NT-pro BNP performed better than the RCRI and DSE for predicting six-month mortality and cardiac events. An NT-pro BNP cut-off level of 319 ng/l was identified as optimal for predicting six-month mortality and cardiac events with 69% sensitivity and 70% specificity for mortality. Patients with levels 319 mg/l had a lower survival during the follow up period (p<0.0001).
Based on this prospective study, it appears that a preoperative elevated NT-Pro BNP is associated with long-term mortality and morbidity and could be used as an additional risk-stratification tool along with clinical risk scoring and stress testing.
Utility of Combination Medications in COPD
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007 Feb 19;146:545-555
The appropriateness of multiple long-acting inhaled medications in treating chronic obstructive pulmonary disease (COPD) is poorly studied. This study evaluated whether combining tiotropium with fluticasone-salmeterol or with salmeterol alone improves clinical outcomes in adult patients with moderate to severe COPD, as compared with tiotropium plus placebo.
This randomized, double-blind, placebo-controlled trial was set in academic and community medical centers in Canada. Researchers monitored 449 patients in the three parallel treatment groups for COPD exacerbations for 52 weeks. Analysis was done on an intention-to-treat basis. The rate of COPD exacerbations within the follow-up period (the primary outcome) was not significantly different among the three treatment groups. However, secondary outcomes, such as rates for hospitalization for COPD exacerbations, all-cause hospitalizations, health-related quality of life and lung function were significantly improved in the group receiving tiotropium and fluticasone-salmeterol.
A notable limitation was that more subjects stopped taking the study medications in the tiotropium-placebo and the tiotropium-salmeterol group. Many crossed over to treatments with inhaled corticosteroids or beta-agonists.
The results are in contrast to current guidelines, which recommend adding inhaled steroids to reduce exacerbations in moderate to severe COPD. Whether these results are due to differing statistical analysis among studies remains unclear. The authors postulate that reduction in secondary outcomes may be related to fluticasone reducing the severity of exacerbations rather than the actual number.
COPD exacerbations are among the most common diagnoses encountered by hospitalists. Most patients are treated with multiple inhaled medications to optimize their pulmonary status. Polypharmacy and the added financial burdens on the patient (particularly the elderly) are important considerations when deciding discharge medications, and the evidence of efficacy for combination inhaled medications had not been assessed as a clinical outcome prior to this study.
Benefits of Rapid Response Teams
Winters BD, Pham JC, Hunt EA et al. Rapid response systems: a systematic review. Crit Care Med. 2007 May;35(5):1238-1243.
Although the Institute for Healthcare Improvement has endorsed rapid response teams, and many hospitalist groups are involved with such systems, quality research is lacking.
Following up on the 2006 “First Consensus Conference on Medical Emergency Teams,” this meta-analysis sought to evaluate current literature to identify the effect of rapid response systems (RRS) on rates of hospital mortality and cardiac arrest.
The authors included randomized trials and observational studies in their analysis. Only eight studies met their inclusion criteria (six observational studies, one multicenter randomized trial, and one single-center randomized trial).
The pooled results did not demonstrate a statistically significant benefit of rapid-response systems in rates of hospital mortality. When rates of in-hospital cardiac arrest were analyzed, there was a weak finding in support of RRS, with the relative risk of 0.70 (confidence interval 0.56-0.92) in favor of RRSs. But the confidence interval was wide, and there was substantial heterogeneity among the included studies.
The authors conclude that “it seems premature to declare RRS as the standard of care,” and that data are lacking to justify any particular implementation scheme or composition of RRS or to support the cost-effectiveness of RRS.
Finally, they recognized the need for larger, better-designed randomized trials. However, in an accompanying editorial, Michael DeVita, MD—a pioneer in the development of RRS—rejects the use of techniques of evidence-based medicine such as multicenter trials and meta-analysis in assessing the utility of RRS. Dr. DeVita essentially says that changing the systems and culture of care within the hospital to accommodate patients with unmet critical needs must be effective in improving outcomes.
This meta-analysis is hindered by the suboptimal quality and homogeneity of studies available for assessment. Hospitalists should be aware of the limitations of the data and literature, as well as the empirical arguments raised by Dr. DeVita, when considering involvement in or designing RRS. TH
CLASSIC LIT
Perioperative Statins
Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006 Nov 6;333(7579):1149.
Recent literature and randomized trials have claimed statins decrease morbidity and mortality from cardiovascular events in patients with or at high risk of coronary artery disease. This meta-analysis sought to determine the strength of evidence leading to the recommendations that perioperative statins be used to reduce perioperative cardiovascular events.
The literature search and exclusion criteria identified 18 studies. Two were randomized controlled trials (n=177), 15 were cohort studies (n=799,632), and one was a case-control study (n=480). Of these, 12 studies enrolled patients undergoing noncardiac vascular surgery, four enrolled patients undergoing coronary bypass surgery, and two enrolled patients undergoing various surgical procedures. The 16 nonrandomized studies were rated good. The two randomized trials were rated five and two out of five using the Jadad quality scores.
The results showed that in the randomized trials the summary odds ratio (OR) for death or acute coronary syndrome during the perioperative period with statin use was 0.26 (95% confidence interval 0.07 to 0.99), but this was based on only 13 events in 177 patients and cannot be considered conclusive. In the cohort studies, the OR was 0.70 (95% confidence interval 0.57 to 0.87). Although the pooled cohort data provided a statistically significant result, these cannot be considered conclusive because the statins were not randomly allocated and the results from retrospective studies were more impressive (OR 0.65, 95% confidence interval 0.50 to 0.84) than those in the prospective cohorts (OR 0.91, 95% confidence interval 0.65 to 1.27) and dose, duration, and safety of statin use were not reported.
Limitations of this meta-analysis include that none of the studies reported patient compliance or doses of statins or cholesterol levels before and after surgery, and few reported the duration of therapy before surgery or the which statin was used. Thus, the authors were unable to demonstrate a dose-response association. They were also unable to ascertain if the benefits seen with statins in the observational studies were exaggerated owing to inclusion of patients in the nonstatin group who had their statins stopped prior to surgery, because acute statin withdrawal may be associated with cardiac events.
The authors concluded that although their meta-analysis—which included data from more than 800,000 patients—suggests considerable benefits from perioperative statin use, the evidence from the randomized trials is not definitive. They advocate only that statins be started preoperatively in eligible patients (e.g., patients with coronary artery disease, multiple cardiac risk factors, elevated LDL) who would warrant statin therapy for medical reasons independent of the proposed operation.
Electronic Alerts to Prevent Hospital-acquired VTE
Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005 Mar 10;352(10):969-977
Surveys conducted in North America and Europe have shown that prophylaxis against deep venous thrombosis (DVT) has been consistently underused in hospitalized patients despite consensus guidelines. Studies involving continuing medical education and computerized electronic alerts have shown that physician use of prophylaxis improves when such processes are in place, but have not demonstrated that they can reduce the rate of DVT.
A computer program was developed to identify consecutive hospitalized patients at increased risk for DVT. The program used eight common risk factors to determine each patient’s risk profile for DVT and each risk factor was assigned a score. A cumulative score of four or higher was used to determine patients at high risk for DVT. The computer alert program was screened daily to identify patients whose score increased to four or higher after admission into the hospital. If the cumulative risk score was at least four, the computer program reviewed the current electronic orders and active medications for the use of DVT prophylaxis.
In the study, 2,506 consecutive adult patients were identified as high risk for DVT. Further,1,255 were randomized to the intervention group—in which the responsible physician received one electronic alert about the risk of DVT—and 1,251 patients were randomized to the control group in which no alert was issued. The 120 physicians involved took care of patients in the intervention and control groups. Physicians responsible for the control group were not aware that patients were being followed for clinical events. When physicians received alerts, they had to acknowledge them and could either withhold prophylaxis or order it on the same computer screen.
Patients were followed for 90 days after the index hospitalization. The primary end point was clinically apparent DVT or pulmonary embolism (PE). Safety end points included mortality at 30 days, and the rate of hemorrhagic events at 90 days.
The results showed that prophylactic measures were ordered for 421 of the 1,255 patients in the intervention group (33.5%) and 182 of the 1,251 patients in the control group (14.5%, p <0.001). There were higher rates of both mechanical (10% versus 1.5%, p<0.001) and pharmacological (23.6% versus 13.0%, p<0.001) prophylaxis in the intervention group. The primary end point of DVT or PE at 90 days occurred in 61 patients in the intervention group (4.9%) as compared with 103 patients in the control group (8.2%).
The computer alert reduced the risk of events at 90 days by 41% (HR 0.59; 95% CI 0.43 to 0.81; P=0.001). Of the patients who received prophylaxis 5.1% had DVT or PE compared with 7.0% of those who did not. In the intervention group, DVT or PE occurred in 20 of 421 (4.8%) patients who received prophylaxis compared with 41 of 834 (4.9%) who did not receive any. In the control group, the same numbers were 11 of 182 (6.0%) and 91 of 1,069 (8.5%).
Some of this benefit might be attributed to the additional preventive measures such as physiotherapy and early ambulation in patients assigned to the intervention group. Diagnostic bias also could have played into the results. Not all patients were screened for VTE, and it is likely that symptomatic patients without prophylaxis were screened more frequently than symptomatic patients with prophylaxis. Because physicians took care of both the control and intervention group, alerts received by physicians in the control group could have influenced their decision in the control group as well.
The authors concluded that instituting computer alerts markedly reduced the rates of DVT or PE in hospitalized patients.
In the Literature
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH
Treat Atrial Flutter
Da Costa A, Thévenin J, Roche F, et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676-1681.
Radiofrequency ablation (RFA) has high success rates in atrial flutter, and American College of Cardiology/American Hospital Association guidelines classify a first episode of well-tolerated atrial flutter as a class IIa indication for RFA treatment. The LADIP trial compared RFA with the current practice of electroosmotic flow (EOF) cardioversion plus amiodarone after a first episode of symptomatic atrial flutter.
One hundred and four consecutive patients with a documented first episode of atrial flutter were enrolled over a period of 39 months. Excluded from the study were patients under the age of 70, those who had had previous antiarrythmic treatment for atrial flutter, those who had an amiodarone contraindication, patients with New York Heart Association class IV heart failure, and those who had a history of heart block. All 52 patients in group I received RFA by a standard method. Fifty-one of the 52 patients in group II underwent intracardiac stimulation, followed, if necessary, by external or internal cardioversion. All patients in group II received amiodarone as well as vitamin K antagonists.
The patients were followed up in the outpatient department at one, three, six, 12, and 18 months after randomization and at the end of the study. At each visit, arrhythmic or cardiovascular events were recorded, and a 12-lead ECG was obtained. Patients were fitted with a Holter monitor for seven days if they had recurring palpitations or symptoms. The primary outcome studied was recurrence of symptomatic atrial flutter and occurrence of atrial fibrillation.
After a mean follow-up of 13+/-6 months, atrial flutter recurred in two of the 52 (3.8%) patients in group I and 15 of 51 (29.5%) patients in group II (P<0.0001). In group I, one patient required a second, successful ablation. All the patients who recurred in group II were successfully treated using RFA. The occurrence of significant symptomatic atrial fibrillation was 8% in both groups at the end of the first year. By the end of the study, two patients in group I and one patient in group II were in chronic atrial fibrillation. When all the episodes of atrial fibrillation were counted (including those patients whose episodes lasted <10 minutes but were documented with an event monitor), the groups did not differ significantly.
No procedure-related complications occurred in group I. In the amiodarone group, however, two patients developed hypothyroidism, one developed hyperthyroidism, and two patients had symptomatic sick sinus syndrome. There were a total of 14 deaths during the course of the study (six patients in group I and eight patients in group II); none were related to the study protocol.
This study is the largest to date showing the superiority of RFA to cardioversion plus amiodarone after the first episode of symptomatic atrial flutter. The long-term risk of subsequent atrial fibrillation was found to be similar to that of the amiodarone-treatment group. Because the mean age of patients in this study was 78, however, these findings cannot necessarily be extrapolated to younger patient populations. Further, oral amiodarone was used initially in this study. It can be argued that IV amiodarone is far more efficacious than oral forms in the acute setting. Because RFA is an invasive procedure, it is user-dependent and may be unfeasible in different care settings. Also, RFA might not be as appropriate for many symptomatic patients with atrial flutter and hemodynamic instability. Nevertheless, this study presents hospital-based physicians with an additional consideration in the acute care setting for patients with a first episode of atrial flutter.
A Transitional Care Intervention Trial
Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822-1828.
A growing body of evidence suggests that the quality of health management decreases when patients are transitioned across sites of care—particularly when they are not adequately prepared to self-manage their chronic disease, when they receive conflicting advice from various providers, or when they do not have access to their healthcare providers. Higher rates of medication errors and lack of appropriate follow up compromise patient safety during this vulnerable period. This is a particular problem for hospitalists, who introduce an additional discontinuity into the flow of patient care. Because patients and their caregivers are the only common thread moving across various sites of care, this study targeted them for an intervention designed to improve the quality of transitional care.
The study was done in collaboration with a not-for-profit capitated system in Colorado. To be eligible for the study, patients had to be over age 65 and admitted to one of the participating hospitals. Patients had to be community dwelling with no documented dementia and had to have one of eleven diagnoses selected to reflect a higher likelihood of long-term subacute care or anticoagulation, including stroke, congestive heart failure, COPD, diabetes, hip fracture, coronary artery disease, and pulmonary embolism. The intervention group comprised 379 patients, while the control group was made up of 371 patients.
The intervention model was built on four pillars derived from prior qualitative studies about care transitions:
- Assistance with medication self-management;
- A healthcare record owned and maintained by the patient;
- Timely physician follow-up; and
- A list of red flags indicative of clinical deterioration.
Intervention-group patients had access to a personal health record that included an active problem list, medications, allergies, and a list of red flags; in addition, these patients received a series of visits and telephone calls with a “transition coach,” an advanced care nurse who encouraged self-care by patients and their caregivers, facilitated communication between providers and patients, and assisted in medication review and reconciliation.
The primary outcome measure was the rate of nonelective rehospitalization at 30, 90, and 180 days after discharge from the index hospitalization. Ninety-five percent of the intervention patients and 94.9% of the control subjects were included in the analysis. Intervention patients had lower adjusted hospital readmission rates than controls at 30 (8.3% versus 11.9%) and 90 days (16.7% versus 22.5%), P=0.048 and 0.04 respectively. The result did not achieve significance at 180 days after discharge (P=0.28). Rehospitalization for the same diagnosis as the index diagnosis within 90 and 180 days of admission was 5.3% in the intervention group versus 9.8% in the control group (P=0.04) and 8.6% in the intervention group versus 13.9% (P=0.045) in the control group, respectively, but did not meet statistical significance within 30 days of readmission.
The concepts of a transition coach and a patient-maintained record are enticing, considering the amount of time hospitalists may invest in patient education and discharge planning processes. This study is different from prior studies in that it used transition coaches instead of healthcare professionals to assume the primary role in managing the post-hospitalization course, and it provided the caregiver and patient with tools that could be applied to future care transitions. The costs of intervention in this study were found to be about $74,310 for the transition coach and other related costs, compared with a semi-annual cost savings of $147,797.
The main drawbacks of the study were that the 180-day all-cause readmission rates did not achieve statistical significance, and even though the adjusted P values for all-cause 30- and 90-day readmission rates were reported to be significant, their 95% confidence interval for the odds ratio barely meets appropriate analytical criteria (OR 0.59 [0.35-1.00] and 0.64 [0.42-0.99]). Also disappointing was the fact that there was no difference in readmission rates at 30 days for the index diagnosis. Therefore, healthcare systems would likely hesitate to implement these interventions without more definitive data showing reductions in adverse outcomes and mortality rates.
Pleural Empyema in CAP Cases
Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006 Oct;119(10):877-883.
Pleural effusions complicate up to 44% of cases of community-acquired pneumonia (CAP). Of these cases, 10% develop complicated parapneumonic effusions. In the past, pleural empyema has been associated with poor outcomes and high mortality rate. Unfortunately, most of these studies were performed before the advent of newer antimicrobial agents and more modern diagnostic and therapeutic techniques.
This prospective, population-based study included all patients older than 17 who had been admitted with a diagnosis of CAP. Most of these patients were diagnosed and managed according to a “Pneumonia Critical Pathway.” Adherence to any aspect of the pathway by the admitting physician was completely voluntary.
Of 3,675 patients enrolled in the study, 47 (1.3%) were diagnosed with empyema by the attending physician—a number which correlates with previous studies. Of these, only 24 (0.7%) were ultimately classified as “definite empyema” by one or more of the following criteria:
- Presence of microorganisms on Gram stain or culture of the pleural fluid;
- Pleural fluid with a pH <7.2 plus radiographic evidence suggesting empyema; and
- Frank pus in the pleural space at time of thoracoscopy.
The remaining 23 (0.6%) patients were classified as suspected empyema.
The study then compared the patients without empyema with patients with definite empyema. Patients with definite empyema were younger, more likely to have received antibiotics before admission, and more likely to have been admitted to the ICU. Further, these patients had a higher incidence of illicit drug use and frequently presented with a history of systemic symptoms, including fevers, chills, and pleuritic chest pain. Laboratory studies—aside from elevated WBC—were not useful in distinguishing between the two groups. Also, there were no significant features on chest radiographs to separate the two groups, although in patients with complex fluid collections, 19 of 22 patients (86%) with definite empyema had computed tomography (CT) scans suggesting the diagnosis.
Streptococcus milleri was the most common pathogen, isolated in 50% of patients with definite empyema. Patients with definite empyema were more likely to have invasive diagnostic procedures and had longer hospital stays (23.5 +/- 17 days) compared with their CAP counterparts (12.4 +/- 20.2 days, P=0.007).
Clinical and laboratory features remain nonspecific and should be used with caution when differentiating between empyema and complicated pleural effusions. Diagnostic pleural effusion aspiration is essential if infection is suspected. This study also points out the greater need of ICU support in definite empyema cases that suggest a greater severity of illness.
Interestingly, definite empyema had an in-hospital mortality rate of 4.2%, compared with 10% for CAP (P<0.05). Possible reasons for this result included the fact that 50% of the empyema cases were suspected at admission and thereby received earlier antibiotic treatment and more aggressive management than CAP cases.
Rapid Response Systems: A Call for Research
Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006 Sep;34(9):2463-2478.
The Institute for Healthcare Improvement has endorsed the concept of Rapid Response Teams (RRTs), and the 2005-2006 SHM survey indicated that 35% of responding hospitalist groups were involved with such systems. The field of in-house medical emergency teams suffers from a lack of quality research, however. Most of the existing data come from single-institution studies, and analysis is limited by a lack of standard definitions or processes. This consensus document addresses these issues and offers a “state of the literature” in RRTs, or—as the authors redefine them—rapid response systems, and attempts to frame the research agenda going forward.
The authors define an in-hospital medical emergency as a “mismatch between patient needs and resources available” and then proceed to outline the various types of responses that have been described, including medical emergency teams (METs), RRTs, and critical care outreach teams (CCO). According to the authors, a MET generally brings ICU capabilities, including procedures and medications, to the bedside, whereas an RRT is a “ramp-up” response, sometimes led by a nurse, that can rapidly assess and triage patients to a higher level of care. To be part of a complete RRS, any of these response options needs to have an adequate detection/triggering arm (“afferent”), a response arm (“efferent”), and administrative and QI components.
After establishing their suggestions for standardized nomenclature and the necessary components of a rapid response system (RRS), the authors review the literature and make several recommendations regarding areas for future research. In particular, they note that there is no data to demonstrate that one set of triggering criteria is superior to another to identify patients who will benefit from an RRS intervention; nor is there adequate literature on the relative effectiveness of the different types of responses. Finally, the authors make a formal recommendation that hospitals implement both afferent and efferent systems, although, interestingly, they do so based on evidence from single-center, historical-control trials and in spite of the lack of benefit seen in the only published multicenter randomized controlled trial (MERIT).
The authors also describe RRS as potentially inexpensive, but offer no data to support this claim. In fact, the prospect of dedicated 24-hour response personnel is probably more daunting for most institutions than the authors acknowledge. In any case, this is excellent reading for hospitalists, who will continue to be key players in the evolution of these systems, and the report is also accompanied by an outstanding bibliography.
Symptomatic Severe Carotid Stenosis: Endarterectomy Versus Stenting
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-1671.
Two large, randomized, clinical trials have established endarterectomy as the standard treatment for severe symptomatic carotid artery stenosis. The new method of carotid stenting avoids the need for general anesthesia and may cost less than surgery, but it is unclear if stenting is as effective as or safer than endarterectomy.
The authors conducted a publicly funded, randomized controlled trial in 20 academic and 10 nonacademic centers in France to compare stenting with endarterectomy in patients with symptomatic carotid stenosis. Patients were eligible if they were 18 years of age or older, had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke within 120 days of enrollment, and had a stenosis of 60% to 99% in the symptomatic carotid artery.
Patients were excluded if one of the following was present: a modified Rankin score of three or more (disabling stroke); nonatherosclerotic carotid disease; severe tandem lesions (stenosis of proximal common carotid artery or intracranial artery that was more severe than the cervical lesion); previous revascularization of the symptomatic stenosis; a history of bleeding disorder; uncontrolled hypertension or diabetes; unstable angina; contraindication to heparin, ticlopidine, or clopidogrel; life expectancy of less than two years; or percutaneous or surgical intervention within 30 days before or after the study procedure. The primary endpoint was the incidence of any stroke or death within 30 days after treatment.
The trial (EVA-3S) was stopped early, after the inclusion of 527 patients, for reasons of both safety and futility. The 30-day risk of any stroke or death was significantly higher after stenting (9.6%) than after endarterectomy (3.9%), resulting in a relative risk of 2.5 (95% CI, 1.2 to 5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5 to 4.2) and 3.4% after stenting (95% CI, 1.7 to 6.7); the relative risk was 2.2 (95% CI, 0.7 to 7.2). At six months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=0.02). Cranial nerve injury was more common after endarterectomy than after stenting.
The practice of interventional physicians has expanded in the last few years to include placement of stents—not only in coronary arteries but also in carotid arteries and other vessels. As hospitalists, we must be aware of the latest research in this changing field to provide the best evidence-based advice to our patients.
Currently, the only use of carotid stenting that has been approved by the Food and Drug Administration (FDA) is in symptomatic patients with carotid artery stenosis of 70% or more who are at high surgical risk. This FDA approval is based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, which included symptomatic patients with carotid artery stenosis exceeding 50% and asymptomatic patients, with stenosis exceeding 80%, who were at high surgical risk mainly due to severe coronary artery disease. The SAPPHIRE study showed that stenting was safer than endarterectomy mainly due to lower risk of myocardial infarction within 30 days after carotid stenting as compared with surgery. There was no significant difference in the rates of stroke or death between stenting and endarterectomy.
Why does the EVA-3S trial reported in NEJM show opposing results? The patients in the trial were different than the ones included in the SAPPHIRE study, and the periprocedural protocol was less strict. The patients in the EVA-3S trial were not at high surgical risk. Further, all patients in the EVA-3S trial had symptomatic carotid artery stenosis, whereas the majority of patients in the SAPPHIRE study were asymptomatic. Use of aspirin and clopidogrel or ticlopidine three days before carotid-artery stenting was only recommended in the EVA-3S trial but was required in the SAPPHIRE trial.
The ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), funded by the National Institutes of Health, is enrolling patients with an average surgical risk similar to those in the EVA-3S study. The CREST study, which is expected to enroll 2,500 patients, may be able to provide a more definitive answer regarding the best treatment for symptomatic patients with high-grade carotid stenosis with an average surgical risk.
In the meantime, what should we recommend to our patients? For symptomatic patients with carotid artery stenosis of 70% or more, endarterectomy is superior to medical therapy alone. For asymptomatic patients with carotid artery stenosis exceeding 60%, endarterectomy is also superior to medical therapy alone, assuming a risk of perioperative stroke or death of less than 3%. Currently, the only accepted indication for stenting is in symptomatic patients with carotid artery stenosis exceeding 70% and a high surgical risk.
D-Dimer Testing to Risk Stratify VTE Patients
Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780-1789.
D-dimer levels have been used to assist in diagnosing initial episodes of venous thromboembolism (VTE). Although not specific, D-dimer testing is very sensitive for VTE, giving it a high negative predictive value. Further, duplex ultrasound often remains abnormal after VTE, making the distinction between recurrent disease and old disease problematic when symptoms recur.
A recent study by Rathbun and colleagues investigated the use of D-dimer measurement in excluding recurrent VTE, finding that of former VTE patients presenting with symptoms, only 0.75% with a negative D-dimer level had recurrent VTE on ultrasound, compared to 6.0% with a positive test who had recurrent VTE. This study, conducted by Palareti and colleagues, tries to go a step further and assess whether D-dimer testing can be used to risk stratify VTE patients who are asymptomatic following treatment for an initial episode of VTE, as well as whether or not it can be used to determine the need to continue anticoagulation.
The PROLONG study was a multicenter prospective study of patients between 18 and 85 who had had their first episode of unprovoked, symptomatic VTE (including pulmonary embolism). Patients were enrolled in this study after completing treatment with vitamin K antagonists (VKA) for at least three months with a target INR (international normalized ratio) in the range of 2-3. Exclusion criteria included severe liver insufficiency, renal insufficiency with serum creatinine >2, or clear indications/contraindications for anticoagulation.
Six hundred twenty-four patients treated for VTE were enrolled in the study. All underwent compressive ultrasound in both legs to establish a baseline at the start of the study and were then instructed to stop anticoagulation. Follow-up occurred in one month, with another ultrasound to assess recurrence of VTE. Five patients were found to have VTE and were excluded. The remaining 619 patients were tested for D-dimer levels and were given thrombophilia tests. A further 11 patients were excluded due to antiphospholipid antibodies or antithrombin deficiency. Patients with factor V Leidin and G20210A mutation on the prothrombin gene were allowed to participate in the study.
Three hundred and eighty-five patients had normal D-dimer levels and were not placed on anticoagulation. The 223 patients with abnormal D-dimer levels were randomized to receive VKA (103 patients) or no treatment (120 patients). All patients were followed for minimum of 18 months. Of the 120 patients with abnormal D-dimer levels who were randomized to no treatment, 18 patients (15.0%) had recurrent VTE. Of the 103 patients with abnormal D-dimer levels who resumed anticoagulation, one had a major bleeding episode and two had recurrent VTE, for a composite result of 2.9%—a statistically significant difference (P<0.005). The group with normal D-dimer levels after initial treatment had 24 episodes of recurrent VTE (6.2%).
The study suggested that the patients with abnormal D-dimer levels who stopped anticoagulation had a statistically significant higher rate of recurrent VTE than those who continued anticoagulation. There was also a statistically significant difference in the recurrent VTE rate in the two groups who did not resume anticoagulation. Interestingly, while the absolute difference between the normal D-dimer group and the abnormal D-dimer group who resumed anticoagulation was evident (6.2% versus 2.9%), this did not reach statistical significance.
This study is promising; however, there are some caveats to take into account when trying to apply these results to current clinical practice. First, the trial was not blinded and only evaluated patients with the first unprovoked episode of VTE. It is unknown if these results will apply to secondary VTE. Older people in this study had a higher incidence of elevated D-dimer at enrollment. The authors utilized a qualitative assay for D-dimer to obtain uniform results across the multiple testing centers. Applying these results to centers that use quantitative measurements of D-dimer then becomes more difficult due to the variability inherent in the interpretation of these quantitative results. Because this study excluded patients with either severe liver disease or renal insufficiency (Cr >2.0), it remains unknown if the results are applicable to these populations.
Because D-dimer levels were only measured once at the time of the patients’ enrollment in the study, it is unknown if patients with normal levels of D-dimer might progress to abnormal D-dimer levels and, therefore, to a potentially higher risk of VTE. This question could be answered with serial testing of D-dimer levels. The study was not powered enough to detect relative risk of bleeding from anticoagulation alone. Thus, these results were taken as a composite with the VTE events.
This study argues that anticoagulation in VTE patients with abnormal D-dimer levels measured after a month of stopping a standard three-month course of anticoagulation should be continued. What is not clear is whether we should continue treating people with normal D-dimer levels. Although not statistically significant, the absolute rate of VTE of 6.2% in these patients was higher than the 2.9% rate in patients with high D-dimer levels who continued anticoagulation.
Early Administration of ACE Inhibitors in MI Patients
Borghi C, Bacchelli S, Degli Esposti D, et al. Effects of early angiotensin-converting enzyme inhibition in patients with non-ST-elevation acute anterior myocardial infarction. Am Heart J. 2006 Sep;152(3):470-477.
Angiotensin-converting enzyme inhibitors (ACEIs) have demonstrated efficacy in improving long-term survival, particularly in patients with ST-elevation MI (STEMI) with left ventricular dysfunction (LVD) and/or congestive heart failure (CHF). There is less information available from clinical trial data, however, regarding the early use of ACEIs with non-ST-elevation MI (NSTEMI) patients, who are believed to be at an overall lower risk of in-hospital morbidity and mortality than STEMI patients.
Researchers focused on the question of ACEI efficacy in NSTEMI in a post hoc analysis of the patients enrolled in the Survival of Myocardial Infarction Long-term Evaluation (SMILE) study. The original study enrolled 1,556 patients with anterior acute MI (AMI) who were admitted to 154 coronary care units in Italy. Participants were patients who presented with chest pain within 24 hours, who demonstrated electrocardiographic signs of anterior wall AMI, and who were not eligible for thrombolytic therapy or reperfusion. These patients did receive beta blockers, nitrates, analgesic agents, inotropic drugs, diuretic agents, and anticoagulation agents as deemed appropriate.
Exclusion criteria included cardiogenic shock, systolic blood pressure below 100 mm Hg, serum creatinine above 2.5 mg per deciliter, a history of CHF, prior treatment with ACEI, and contraindication to the use of ACEI. Patients were randomized to either placebo or the short-acting ACEI zofenopril, with a starting dose of 7.5 mg every 12 hours. The dose was progressively doubled until the final target dose of 30 mg twice a day was reached. Upon completion of a six-week double-blind period, the study medications were stopped, but the patients continued taking their other medications for approximately 48 additional weeks, at which time vital status was blindly obtained by questionnaire or from registry offices. The primary endpoints were the occurrence of death or CHF during the treatment period.
In this post hoc analysis, only the 526 patients with anterior MI were studied. The baseline characteristics of the placebo and zofenopril group were closely matched but were predominantly male. The primary endpoint of this analysis was the combined occurrence of death or severe CHF during the six weeks of treatment with zofenopril or placebo, both given in addition to conventional treatment. Secondary endpoints were the six-week occurrence of severe CHF, nonfatal MI or angina, and cumulative one-year mortality.
The findings of this analysis indicate a relative risk reduction (RRR) of 65% (95% CI 20%80%, 2P=0.003) of a major cardiovascular event using zofenopril in the first 6 weeks of treatment. Cumulative incidence of combined death and CHF was significantly (P=0.017) greater in the placebo group than in the group of patients given zofenopril. In addition, occurrence of severe CHF was lower in the zofenopril group (RRR 84%, 95% CI 33%97%), as was one-year mortality (RRR 43%, 95% CI 14%-57%, 2P=0.36). During the six weeks, there was a slightly lower usage of beta blockers in the zofenopril group, as well as lower usage of calcium channel blockers and diuretics in this same group at one year. Systolic blood pressure (SBP) and heart rate did not differ between the two groups.
The authors of this analysis concluded that early treatment for six weeks with zofenopril was effective in reducing death and severe CHF in non-thrombolysed anterior wall NSTEMI patients. The results were independent of SBP reduction, suggesting that zofenopril may have cardioprotective effects, preventing infarct expansion, left ventricular remodeling, and neurohormonal activation, which is involved in coronary vasoconstriction and endothelial dysfunction. Further, the relative risk reduction in composite endpoints of mortality and severe CHF exceeded that observed in the overall population in the SMILE trial (which included STEMI), drawing attention to a particular advantage of the early use of ACEI in NSTEMI patients.
Despite relevant findings, these results were derived from a post hoc analysis of the SMILE study, only including about one third of the original population. It is also a retrospective analysis, albeit recognizing the sparse availability of research in this area, thought to be related to the exclusion of such patients from most clinical trials. This analysis strongly highlights the beneficial effects of early administration ACE inhibition and should prompt prospective evaluation of these agents as first-line therapy in anterior wall NSTEMI. TH