User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Magic Wand Initiative Empowers Dermatologists to Innovate
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.
Are You Unwittingly Aiding the Rise of Superfungi?
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Coming Soon: The First mRNA Vaccine for Melanoma?
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Efficacy of Topical Clascoterone for Acne Increased Over Time, Analysis Shows
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
Resistance Training Formats Compared in Patients With PsA
TOPLINE:
Exercise with weight machines or elastic resistance bands yielded similar improvements in strength and function in adults with psoriatic arthritis (PsA) after 12 weeks.
METHODOLOGY:
- Researchers recruited 41 adults aged 18-65 years with PsA who were then randomized to a functional training group (FT) or a resistance exercise group (RE) for 12 weeks of twice-weekly, 55-minute sessions under the supervision of a physical trainer.
- Functional training involved the use of elastic bands to work upper body, lower body, and trunk muscles including the biceps, triceps, back quadriceps, glutes, and hips; the RE used weight machines instead of bands.
- Participants were evaluated at baseline and after 6 and 12 weeks of training sessions; the primary outcome was functional status based on the Health Assessment Questionnaire for the Spondyloarthropathies (HAQ-S).
- Secondary outcomes included the Bath Ankylosing Spondylitis Functional Index (BASFI) to assess functional capacity, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Disease Activity Score in 28 joints (DAS28) to assess disease activity, and the Short Form 36 (SF-36) to measure quality of life.
TAKEAWAY:
- Participants in both groups showed significant improvement from baseline on the primary outcome measure, with no significant differences between the groups on the primary outcome of function or secondary measures of function and disease activity after 12 weeks.
- Significant intragroup changes occurred between times for both groups on the HAQ-S, BASFI, BASDAI, and DAS28 (P = .001, .007, .001, and .001, respectively).
- Improvement in quality of life was significant from baseline and similar between the FT and RE, with the exception of the “social aspects” domain, for which only the FT showed significant improvement.
- No intervention-related adverse events were reported in either group.
IN PRACTICE:
Despite the absence of consensus guidelines on the use and effectiveness of FT and RE, “we can conclude that both FT and RE have similar effectiveness in improving functional capacity, functional status, disease activity, general quality of life, and muscle strength in patients with psoriatic arthritis,” the researchers wrote.
SOURCE:
The study was led by Diego Roger Silva, MD, of the Universidade Federal de São Paulo, Brazil, and published online in Advances in Rheumatology.
LIMITATIONS:
The study population was recruited from outpatient clinics, and the mean age of 52 years was higher than in previous studies; the study also lacked long-term follow-up data.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Exercise with weight machines or elastic resistance bands yielded similar improvements in strength and function in adults with psoriatic arthritis (PsA) after 12 weeks.
METHODOLOGY:
- Researchers recruited 41 adults aged 18-65 years with PsA who were then randomized to a functional training group (FT) or a resistance exercise group (RE) for 12 weeks of twice-weekly, 55-minute sessions under the supervision of a physical trainer.
- Functional training involved the use of elastic bands to work upper body, lower body, and trunk muscles including the biceps, triceps, back quadriceps, glutes, and hips; the RE used weight machines instead of bands.
- Participants were evaluated at baseline and after 6 and 12 weeks of training sessions; the primary outcome was functional status based on the Health Assessment Questionnaire for the Spondyloarthropathies (HAQ-S).
- Secondary outcomes included the Bath Ankylosing Spondylitis Functional Index (BASFI) to assess functional capacity, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Disease Activity Score in 28 joints (DAS28) to assess disease activity, and the Short Form 36 (SF-36) to measure quality of life.
TAKEAWAY:
- Participants in both groups showed significant improvement from baseline on the primary outcome measure, with no significant differences between the groups on the primary outcome of function or secondary measures of function and disease activity after 12 weeks.
- Significant intragroup changes occurred between times for both groups on the HAQ-S, BASFI, BASDAI, and DAS28 (P = .001, .007, .001, and .001, respectively).
- Improvement in quality of life was significant from baseline and similar between the FT and RE, with the exception of the “social aspects” domain, for which only the FT showed significant improvement.
- No intervention-related adverse events were reported in either group.
IN PRACTICE:
Despite the absence of consensus guidelines on the use and effectiveness of FT and RE, “we can conclude that both FT and RE have similar effectiveness in improving functional capacity, functional status, disease activity, general quality of life, and muscle strength in patients with psoriatic arthritis,” the researchers wrote.
SOURCE:
The study was led by Diego Roger Silva, MD, of the Universidade Federal de São Paulo, Brazil, and published online in Advances in Rheumatology.
LIMITATIONS:
The study population was recruited from outpatient clinics, and the mean age of 52 years was higher than in previous studies; the study also lacked long-term follow-up data.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Exercise with weight machines or elastic resistance bands yielded similar improvements in strength and function in adults with psoriatic arthritis (PsA) after 12 weeks.
METHODOLOGY:
- Researchers recruited 41 adults aged 18-65 years with PsA who were then randomized to a functional training group (FT) or a resistance exercise group (RE) for 12 weeks of twice-weekly, 55-minute sessions under the supervision of a physical trainer.
- Functional training involved the use of elastic bands to work upper body, lower body, and trunk muscles including the biceps, triceps, back quadriceps, glutes, and hips; the RE used weight machines instead of bands.
- Participants were evaluated at baseline and after 6 and 12 weeks of training sessions; the primary outcome was functional status based on the Health Assessment Questionnaire for the Spondyloarthropathies (HAQ-S).
- Secondary outcomes included the Bath Ankylosing Spondylitis Functional Index (BASFI) to assess functional capacity, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Disease Activity Score in 28 joints (DAS28) to assess disease activity, and the Short Form 36 (SF-36) to measure quality of life.
TAKEAWAY:
- Participants in both groups showed significant improvement from baseline on the primary outcome measure, with no significant differences between the groups on the primary outcome of function or secondary measures of function and disease activity after 12 weeks.
- Significant intragroup changes occurred between times for both groups on the HAQ-S, BASFI, BASDAI, and DAS28 (P = .001, .007, .001, and .001, respectively).
- Improvement in quality of life was significant from baseline and similar between the FT and RE, with the exception of the “social aspects” domain, for which only the FT showed significant improvement.
- No intervention-related adverse events were reported in either group.
IN PRACTICE:
Despite the absence of consensus guidelines on the use and effectiveness of FT and RE, “we can conclude that both FT and RE have similar effectiveness in improving functional capacity, functional status, disease activity, general quality of life, and muscle strength in patients with psoriatic arthritis,” the researchers wrote.
SOURCE:
The study was led by Diego Roger Silva, MD, of the Universidade Federal de São Paulo, Brazil, and published online in Advances in Rheumatology.
LIMITATIONS:
The study population was recruited from outpatient clinics, and the mean age of 52 years was higher than in previous studies; the study also lacked long-term follow-up data.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Autoimmune Diseases and Perinatal Depression May Share Two-Way Link
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
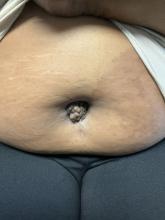
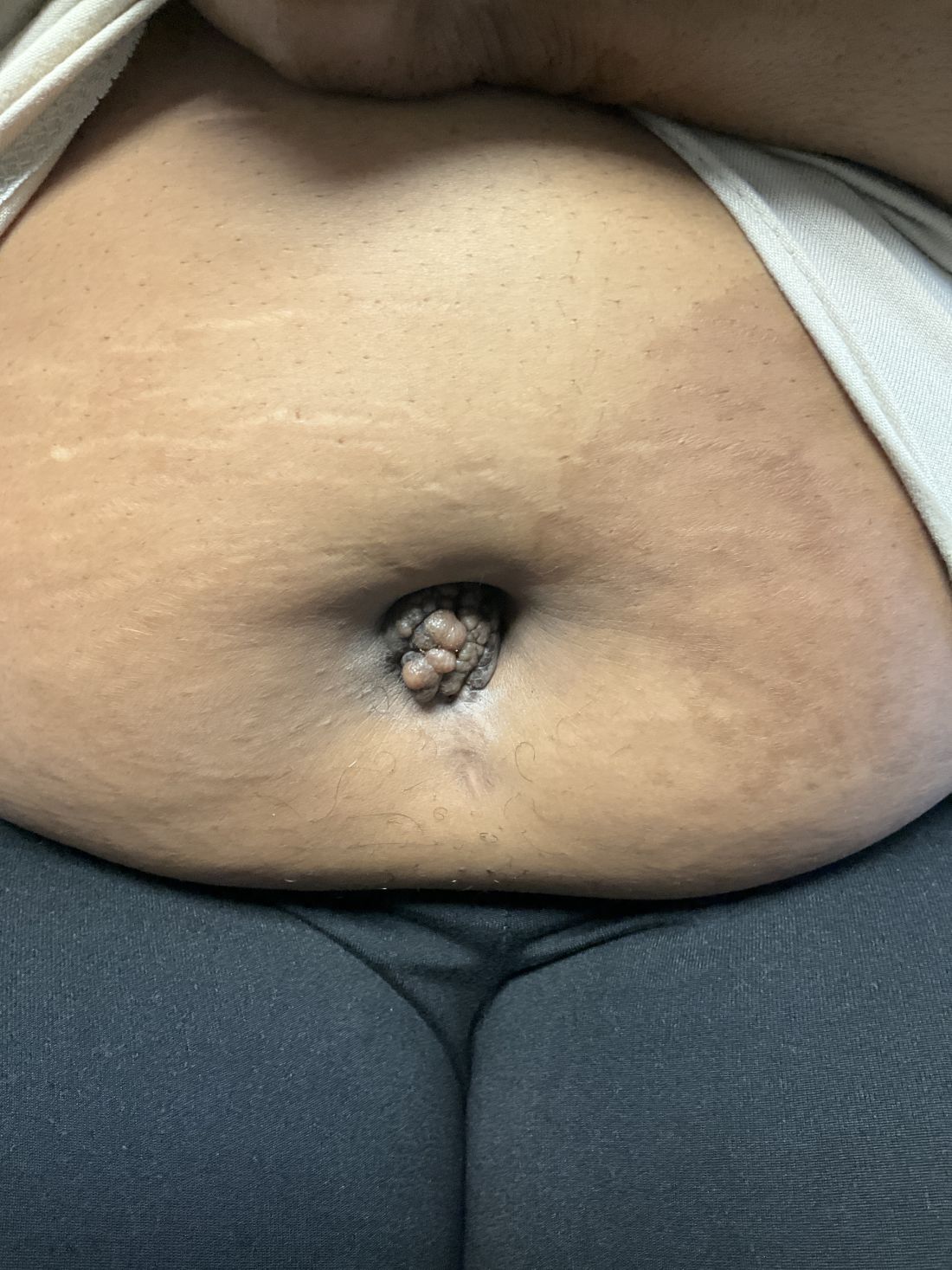
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Panel Recommends Small Bump in 2025 Medicare Physician Pay
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.