User login
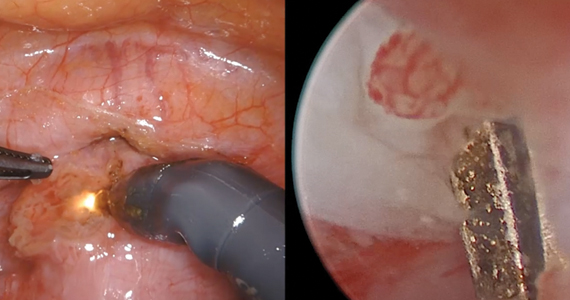
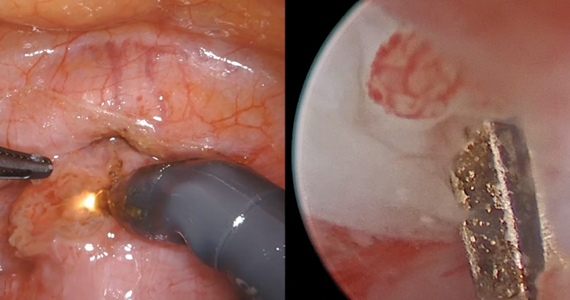
Isthmocele repair: Simultaneous hysteroscopy and robotic-assisted laparoscopy

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues


An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
Lipophilic statins linked to lower mortality in ovarian cancer
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
FROM AACR 2020
Tendyne device shows promise for mitral annular calcification
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
REPORTING FROM EUROPCR 2020
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
FROM JAMA NETWORK OPEN
Study supports changing classification of renal cell carcinoma
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
FROM CANCER
Despite guidelines, children receive opioids and steroids for pneumonia and sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
FROM PEDIATRICS
Big pharma sues to block Minnesota insulin affordability law
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
Primary care practices may lose about $68k per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Bariatric embolotherapy helps shed pounds in obese patients
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
One-year mortality after dialysis initiation nearly double prior estimates
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.