User login
Expert shares his approach to treating warts in children
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
FROM PEDIATRIC DERMATOLOGY 2020
Geographical hot spots for early-onset colon cancer
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Use of nonopioid pain meds is on the rise
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.
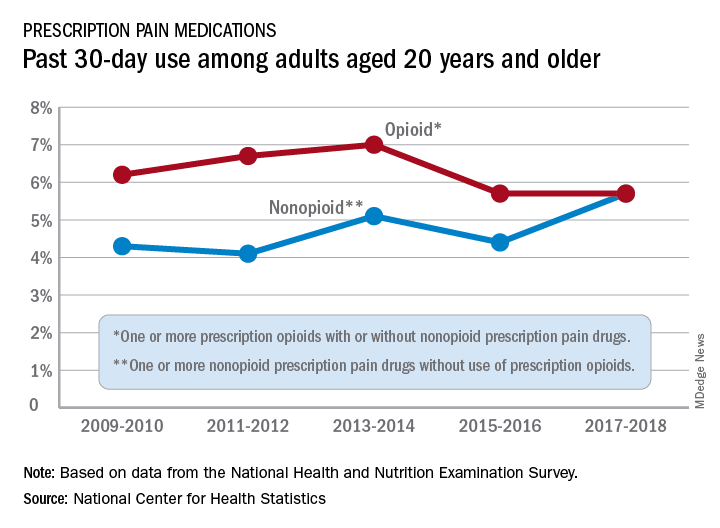
At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
HIV does not appear to worsen COVID-19 outcomes
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
FROM AIDS 2020
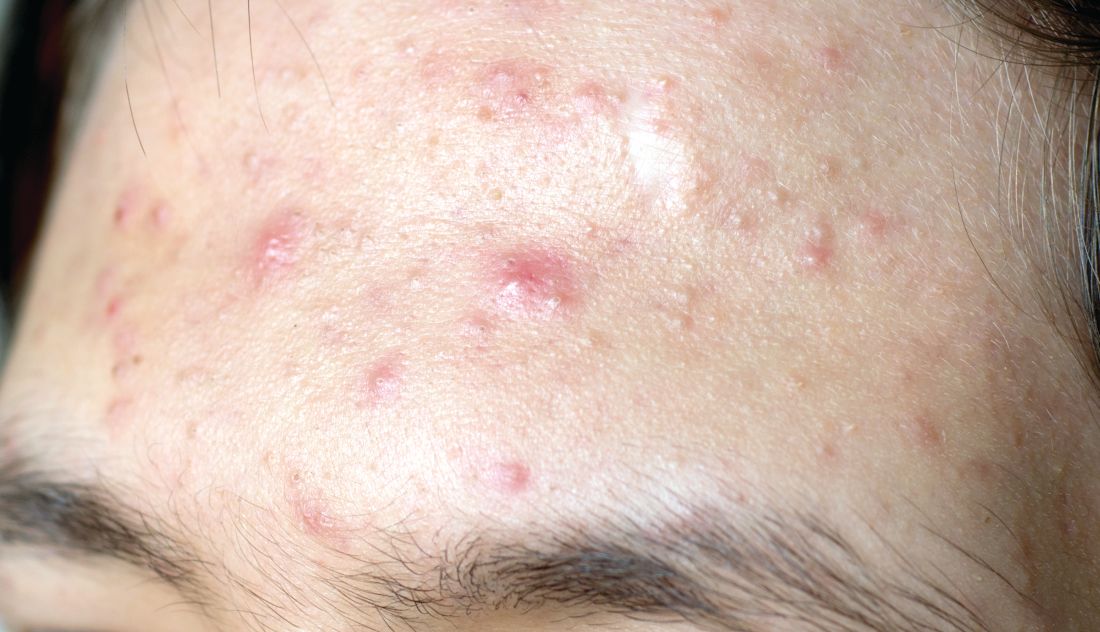
Treat acne aggressively upfront, expert advises
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
FROM PEDIATRIC DERMATOLOGY 2020
High percentage of stimulant use found in opioid ED cases
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
FROM CPDD 2020
Antihypertensives linked to reduced risk of colorectal cancer
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Daily Recap: Lifestyle vs. genes in breast cancer showdown; Big pharma sues over insulin affordability law
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Higher stroke rates seen among patients with COVID-19 compared with influenza
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
, according to a retrospective cohort study conducted at New York–Presbyterian Hospital and Weill Cornell Medicine, New York. “These findings suggest that clinicians should be vigilant for symptoms and signs of acute ischemic stroke in patients with COVID-19 so that time-sensitive interventions, such as thrombolysis and thrombectomy, can be instituted if possible to reduce the burden of long-term disability,” wrote Alexander E. Merkler and colleagues. Their report is in JAMA Neurology.
While several recent publications have “raised the possibility” of this link, none have had an appropriate control group, noted Dr. Merkler of the department of neurology, Weill Cornell Medicine. “Further elucidation of thrombotic mechanisms in patients with COVID-19 may yield better strategies to prevent disabling thrombotic complications like ischemic stroke,” he added.
An increased risk of stroke
The study included 1,916 adults with confirmed COVID-19 (median age 64 years) who were either hospitalized or visited an emergency department between March 4 and May 2, 2020. These cases were compared with a historical cohort of 1,486 patients (median age 62 years) who were hospitalized with laboratory-confirmed influenza A or B between January 1, 2016, and May 31, 2018.
Among the patients with COVID-19, a diagnosis of cerebrovascular disease during hospitalization, a brain computed tomography (CT), or brain magnetic resonance imaging (MRI) was an indication of possible ischemic stroke. These records were then independently reviewed by two board-certified attending neurologists (with a third resolving any disagreement) to adjudicate a final stroke diagnosis. In the influenza cohort, the Cornell Acute Stroke Academic Registry (CAESAR) was used to ascertain ischemic strokes.
The study identified 31 patients with stroke among the COVID-19 cohort (1.6%; 95% confidence interval, 1.1%-2.3%) and 3 in the influenza cohort (0.2%; 95% CI, 0.0%-0.6%). After adjustment for age, sex, and race, stroke risk was almost 8 times higher in the COVID-19 cohort (OR, 7.6; 95% CI, 2.3-25.2).
This association “persisted across multiple sensitivity analyses, with the magnitude of relative associations ranging from 4.0 to 9,” wrote the authors. “This included a sensitivity analysis that adjusted for the number of vascular risk factors and ICU admissions (OR, 4.6; 95% CI, 1.4-15.7).”
The median age of patients with COVID-19 and stroke was 69 years, and the median duration of COVID-19 symptom onset to stroke diagnosis was 16 days. Stroke symptoms were the presenting complaint in only 26% of the patients, while the remainder developing stroke while hospitalized, and more than a third (35%) of all strokes occurred in patients who were mechanically ventilated with severe COVID-19. Inpatient mortality was considerably higher among patients with COVID-19 with stroke versus without (32% vs. 14%; P = .003).
In patients with COVID-19 “most ischemic strokes occurred in older age groups, those with traditional stroke risk factors, and people of color,” wrote the authors. “We also noted that initial plasma D-dimer levels were nearly 3-fold higher in those who received a diagnosis of ischemic stroke than in those who did not” (1.930 mcg/mL vs. 0.682 mcg/mL).
The authors suggested several possible explanations for the elevated risk of stroke in COVID-19. Acute viral illnesses are known to trigger inflammation, and COVID-19 in particular is associated with “a vigorous inflammatory response accompanied by coagulopathy, with elevated D-dimer levels and the frequent presence of antiphospholipid antibodies,” they wrote. The infection is also associated with more severe respiratory syndrome compared with influenza, as well as a heightened risk for complications such as atrial arrhythmias, myocardial infarction, heart failure, myocarditis, and venous thromboses, all of which likely contribute to the risk of ischemic stroke.”
COVID or conventional risk factors?
Asked to comment on the study, Benedict Michael, MBChB (Hons), MRCP (Neurol), PhD, from the United Kingdom’s Coronerve Studies Group, a collaborative initiative to study the neurological features of COVID-19, said in an interview that “this study suggests many cases of stroke are occurring in older patients with multiple existing conventional and well recognized risks for stroke, and may simply represent decompensation during sepsis.”
Dr. Michael, a senior clinician scientist fellow at the University of Liverpool and an honorary consultant neurologist at the Walton Centre, was the senior author on a recently published UK-wide surveillance study on the neurological and neuropsychiatric complications of COVID-19 (Lancet Psychiatry. 2020 Jun 25. doi: 10.1016/S2215-0366[20]30287-X).
He said among patients in the New York study, “those with COVID and a stroke appeared to have many conventional risk factors for stroke (and often at higher percentages than COVID patients without a stroke), e.g. hypertension, overweight, diabetes, hyperlipidemia, existing vascular disease affecting the coronary arteries and atrial fibrillation. To establish evidence-based treatment pathways, clearly further studies are needed to determine the biological mechanisms underlying the seemingly higher rate of stroke with COVID-19 than influenza; but this must especially focus on those younger patients without conventional risk factors for stroke (which are largely not included in this study).”
SOURCE: Merkler AE et al. JAMA Neurol. doi: 10.1001/jamaneurol.2020.2730.
FROM JAMA NEUROLOGY
Anticoagulation in cirrhosis: Best practices
Background: Alterations to the coagulation cascade put cirrhotic patients at higher risk for bleeding and thrombotic complications.
Study design: Expert review.
Setting: Literature review.
Synopsis: The authors provide 12 best practice recommendations, including use blood products sparingly in the absence of active bleeding out of concern for raising portal pressures; low-risk paracentesis, thoracentesis, and upper endoscopy do not require routine correction of thrombocytopenia or coagulopathy; for active bleeding or high-risk procedures, correct hematocrit to above 25%, platelets to more than 50,000, and fibrinogen to above 120 mg/dL; the risk of thrombosis, including venous thromboembolism and portal vein thrombosis, is high in these patients despite elevated INR values.
As such, pharmacologic VTE prophylaxis is often underutilized in patients admitted with cirrhosis; for patients requiring therapeutic anticoagulation, direct oral anticoagulants are safe in stable patients with mild cirrhosis, but should be avoided in Child-Pugh B and C patients.
Bottom line: Cirrhotic patients do not require routine correction of coagulopathy prior to low-risk procedures.
Citation: O’Leary JG et al. AGA Clinical Practice Update: Coagulation in cirrhosis. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: Alterations to the coagulation cascade put cirrhotic patients at higher risk for bleeding and thrombotic complications.
Study design: Expert review.
Setting: Literature review.
Synopsis: The authors provide 12 best practice recommendations, including use blood products sparingly in the absence of active bleeding out of concern for raising portal pressures; low-risk paracentesis, thoracentesis, and upper endoscopy do not require routine correction of thrombocytopenia or coagulopathy; for active bleeding or high-risk procedures, correct hematocrit to above 25%, platelets to more than 50,000, and fibrinogen to above 120 mg/dL; the risk of thrombosis, including venous thromboembolism and portal vein thrombosis, is high in these patients despite elevated INR values.
As such, pharmacologic VTE prophylaxis is often underutilized in patients admitted with cirrhosis; for patients requiring therapeutic anticoagulation, direct oral anticoagulants are safe in stable patients with mild cirrhosis, but should be avoided in Child-Pugh B and C patients.
Bottom line: Cirrhotic patients do not require routine correction of coagulopathy prior to low-risk procedures.
Citation: O’Leary JG et al. AGA Clinical Practice Update: Coagulation in cirrhosis. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: Alterations to the coagulation cascade put cirrhotic patients at higher risk for bleeding and thrombotic complications.
Study design: Expert review.
Setting: Literature review.
Synopsis: The authors provide 12 best practice recommendations, including use blood products sparingly in the absence of active bleeding out of concern for raising portal pressures; low-risk paracentesis, thoracentesis, and upper endoscopy do not require routine correction of thrombocytopenia or coagulopathy; for active bleeding or high-risk procedures, correct hematocrit to above 25%, platelets to more than 50,000, and fibrinogen to above 120 mg/dL; the risk of thrombosis, including venous thromboembolism and portal vein thrombosis, is high in these patients despite elevated INR values.
As such, pharmacologic VTE prophylaxis is often underutilized in patients admitted with cirrhosis; for patients requiring therapeutic anticoagulation, direct oral anticoagulants are safe in stable patients with mild cirrhosis, but should be avoided in Child-Pugh B and C patients.
Bottom line: Cirrhotic patients do not require routine correction of coagulopathy prior to low-risk procedures.
Citation: O’Leary JG et al. AGA Clinical Practice Update: Coagulation in cirrhosis. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.