User login
Physician shortage grows in latest projections
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Colonoscopy over age 75 should be ‘carefully considered’
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
FROM JAMA NETWORK OPEN
Diagnostic criteria may miss some MIS-C cases, experts say
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
How many hormones make an ideal ‘artificial pancreas?’
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Captopril questioned for diabetes patients in COVID-19 setting
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Daily Recap: Migraine affects pregnancy planning; FDA okays urothelial carcinoma therapy
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Pubovaginal sling during urethral diverticulectomy reduces stress incontinence
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Self-measured BP monitoring at home ‘more important than ever’
.
“With fewer patients visiting medical offices during the COVID-19 pandemic, SMBP monitoring is more important than ever for people at risk for hypertension and uncontrolled BP,” writing group chair Daichi Shimbo, MD, said in a statement.
“There should be investment in creating and supporting the infrastructure for expanding self-measured BP monitoring, as well as increasing coverage for patient- and provider-related costs,” Dr. Shimbo, director, The Columbia Hypertension Center, Columbia University Irving Medical Center, New York, said in an interview.
The statement, Self-Measured Blood Pressure Monitoring at Home, was published June 22 in Circulation.
It provides “contemporary information” on the use, efficacy, and cost-effectiveness of SMBP at home for the diagnosis and management of hypertension.
The writing group noted that hypertension is one of the most important risk factors for cardiovascular disease. Several American and international guidelines support the use of SMBP.
“Indications include the diagnosis of white-coat hypertension and masked hypertension and the identification of white-coat effect and masked uncontrolled hypertension. Other indications include confirming the diagnosis of resistant hypertension and detecting morning hypertension,” the group pointed out.
Use validated devices
Devices that are validated for clinical accuracy should be used for SMBP monitoring, the writing group advised. Validated devices that use the oscillometric method are preferred, and a standardized BP measurement (with appropriately sized cuffs) and monitoring protocol should be followed.
The group noted that meta-analyses of randomized trials indicate that SMBP monitoring is associated with a reduction in BP and improved BP control, and the benefits are greatest when it is used along with other interventions, such as education and counseling, that can be delivered via phone or telehealth visits by nurses and care coordinators.
There are “sufficient data” to indicate that adding SMBP monitoring to office-based monitoring is cost-effective compared with office BP monitoring alone or usual care in patients with high office BP, the writing group said.
Potential cost savings associated with SMBP monitoring include a reduction in office visit follow-ups as a result of improved BP control, avoidance of possible overtreatment in patients with white-coat hypertension, and improvement in quality of life.
They noted that randomized controlled trials assessing the impact of SMBP monitoring on cardiovascular outcomes are needed.
Barriers to widespread use
The use of SMBP monitoring is “essential” for the self-management of hypertension and has “great appeal” for expanding the benefits of cardiovascular prevention, the writing group said. They acknowledged, however, that transitioning from solely office-based BP management to a strategy that includes SMBP monitoring is not without actual and potential barriers.
The group recommends addressing these barriers by:
- Educating patients and providers about the benefits of SMBP monitoring and the optimal approaches for SMBP monitoring.
- Establishing clinical core competency criteria to ensure high-quality SMBP monitoring is supported in clinical practice.
- Incorporating cointerventions that increase the effectiveness of SMBP monitoring, including behavioral change management and counseling, communication of treatment recommendations back to patients, medication management, and prescription and adherence monitoring.
- Creating systems for SMBP readings to be transferred from devices to electronic health records.
- Improving public and private health insurance coverage of validated SMBP monitoring devices prescribed by a health care provider.
- Reimbursing providers for costs associated with training patients, transmitting BP data, interpreting and reporting BP readings, and delivering cointerventions.
Increasing the use of SMBP monitoring is a major focus area of Target: BP – a national initiative of the AHA and AMA launched in response to the high prevalence of uncontrolled BP.
Target: BP helps health care organizations and care teams improve BP control rates through the evidence-based MAP BP Program.
MAP is an acronym that stands for Measure BP accurately every time it’s measured, Act rapidly to manage uncontrolled BP, and Partner with patients to promote BP self-management.
This research had no commercial funding. Dr. Shimbo has disclosed no relevant conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article originally appeared on Medscape.com.
.
“With fewer patients visiting medical offices during the COVID-19 pandemic, SMBP monitoring is more important than ever for people at risk for hypertension and uncontrolled BP,” writing group chair Daichi Shimbo, MD, said in a statement.
“There should be investment in creating and supporting the infrastructure for expanding self-measured BP monitoring, as well as increasing coverage for patient- and provider-related costs,” Dr. Shimbo, director, The Columbia Hypertension Center, Columbia University Irving Medical Center, New York, said in an interview.
The statement, Self-Measured Blood Pressure Monitoring at Home, was published June 22 in Circulation.
It provides “contemporary information” on the use, efficacy, and cost-effectiveness of SMBP at home for the diagnosis and management of hypertension.
The writing group noted that hypertension is one of the most important risk factors for cardiovascular disease. Several American and international guidelines support the use of SMBP.
“Indications include the diagnosis of white-coat hypertension and masked hypertension and the identification of white-coat effect and masked uncontrolled hypertension. Other indications include confirming the diagnosis of resistant hypertension and detecting morning hypertension,” the group pointed out.
Use validated devices
Devices that are validated for clinical accuracy should be used for SMBP monitoring, the writing group advised. Validated devices that use the oscillometric method are preferred, and a standardized BP measurement (with appropriately sized cuffs) and monitoring protocol should be followed.
The group noted that meta-analyses of randomized trials indicate that SMBP monitoring is associated with a reduction in BP and improved BP control, and the benefits are greatest when it is used along with other interventions, such as education and counseling, that can be delivered via phone or telehealth visits by nurses and care coordinators.
There are “sufficient data” to indicate that adding SMBP monitoring to office-based monitoring is cost-effective compared with office BP monitoring alone or usual care in patients with high office BP, the writing group said.
Potential cost savings associated with SMBP monitoring include a reduction in office visit follow-ups as a result of improved BP control, avoidance of possible overtreatment in patients with white-coat hypertension, and improvement in quality of life.
They noted that randomized controlled trials assessing the impact of SMBP monitoring on cardiovascular outcomes are needed.
Barriers to widespread use
The use of SMBP monitoring is “essential” for the self-management of hypertension and has “great appeal” for expanding the benefits of cardiovascular prevention, the writing group said. They acknowledged, however, that transitioning from solely office-based BP management to a strategy that includes SMBP monitoring is not without actual and potential barriers.
The group recommends addressing these barriers by:
- Educating patients and providers about the benefits of SMBP monitoring and the optimal approaches for SMBP monitoring.
- Establishing clinical core competency criteria to ensure high-quality SMBP monitoring is supported in clinical practice.
- Incorporating cointerventions that increase the effectiveness of SMBP monitoring, including behavioral change management and counseling, communication of treatment recommendations back to patients, medication management, and prescription and adherence monitoring.
- Creating systems for SMBP readings to be transferred from devices to electronic health records.
- Improving public and private health insurance coverage of validated SMBP monitoring devices prescribed by a health care provider.
- Reimbursing providers for costs associated with training patients, transmitting BP data, interpreting and reporting BP readings, and delivering cointerventions.
Increasing the use of SMBP monitoring is a major focus area of Target: BP – a national initiative of the AHA and AMA launched in response to the high prevalence of uncontrolled BP.
Target: BP helps health care organizations and care teams improve BP control rates through the evidence-based MAP BP Program.
MAP is an acronym that stands for Measure BP accurately every time it’s measured, Act rapidly to manage uncontrolled BP, and Partner with patients to promote BP self-management.
This research had no commercial funding. Dr. Shimbo has disclosed no relevant conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article originally appeared on Medscape.com.
.
“With fewer patients visiting medical offices during the COVID-19 pandemic, SMBP monitoring is more important than ever for people at risk for hypertension and uncontrolled BP,” writing group chair Daichi Shimbo, MD, said in a statement.
“There should be investment in creating and supporting the infrastructure for expanding self-measured BP monitoring, as well as increasing coverage for patient- and provider-related costs,” Dr. Shimbo, director, The Columbia Hypertension Center, Columbia University Irving Medical Center, New York, said in an interview.
The statement, Self-Measured Blood Pressure Monitoring at Home, was published June 22 in Circulation.
It provides “contemporary information” on the use, efficacy, and cost-effectiveness of SMBP at home for the diagnosis and management of hypertension.
The writing group noted that hypertension is one of the most important risk factors for cardiovascular disease. Several American and international guidelines support the use of SMBP.
“Indications include the diagnosis of white-coat hypertension and masked hypertension and the identification of white-coat effect and masked uncontrolled hypertension. Other indications include confirming the diagnosis of resistant hypertension and detecting morning hypertension,” the group pointed out.
Use validated devices
Devices that are validated for clinical accuracy should be used for SMBP monitoring, the writing group advised. Validated devices that use the oscillometric method are preferred, and a standardized BP measurement (with appropriately sized cuffs) and monitoring protocol should be followed.
The group noted that meta-analyses of randomized trials indicate that SMBP monitoring is associated with a reduction in BP and improved BP control, and the benefits are greatest when it is used along with other interventions, such as education and counseling, that can be delivered via phone or telehealth visits by nurses and care coordinators.
There are “sufficient data” to indicate that adding SMBP monitoring to office-based monitoring is cost-effective compared with office BP monitoring alone or usual care in patients with high office BP, the writing group said.
Potential cost savings associated with SMBP monitoring include a reduction in office visit follow-ups as a result of improved BP control, avoidance of possible overtreatment in patients with white-coat hypertension, and improvement in quality of life.
They noted that randomized controlled trials assessing the impact of SMBP monitoring on cardiovascular outcomes are needed.
Barriers to widespread use
The use of SMBP monitoring is “essential” for the self-management of hypertension and has “great appeal” for expanding the benefits of cardiovascular prevention, the writing group said. They acknowledged, however, that transitioning from solely office-based BP management to a strategy that includes SMBP monitoring is not without actual and potential barriers.
The group recommends addressing these barriers by:
- Educating patients and providers about the benefits of SMBP monitoring and the optimal approaches for SMBP monitoring.
- Establishing clinical core competency criteria to ensure high-quality SMBP monitoring is supported in clinical practice.
- Incorporating cointerventions that increase the effectiveness of SMBP monitoring, including behavioral change management and counseling, communication of treatment recommendations back to patients, medication management, and prescription and adherence monitoring.
- Creating systems for SMBP readings to be transferred from devices to electronic health records.
- Improving public and private health insurance coverage of validated SMBP monitoring devices prescribed by a health care provider.
- Reimbursing providers for costs associated with training patients, transmitting BP data, interpreting and reporting BP readings, and delivering cointerventions.
Increasing the use of SMBP monitoring is a major focus area of Target: BP – a national initiative of the AHA and AMA launched in response to the high prevalence of uncontrolled BP.
Target: BP helps health care organizations and care teams improve BP control rates through the evidence-based MAP BP Program.
MAP is an acronym that stands for Measure BP accurately every time it’s measured, Act rapidly to manage uncontrolled BP, and Partner with patients to promote BP self-management.
This research had no commercial funding. Dr. Shimbo has disclosed no relevant conflicts of interest. A complete list of disclosures for the writing group is available with the original article.
A version of this article originally appeared on Medscape.com.
Lawmakers question mental health disclosure rules
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
State medical licensing queries criticized
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
FROM A HOUSE ENERGY AND COMMERCE’S HEALTH SUBCOMMITTEE HEARING
The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C)
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14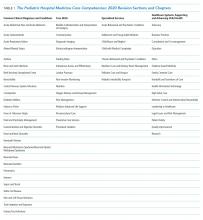
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.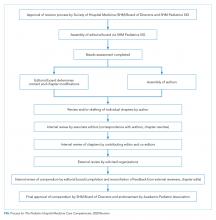
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
© 2020 Society of Hospital Medicine






