User login
Today’s top news highlights: ACE inhibitors in COVID patients, fewer AMI admissions, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Out of the pipeline: Remdesivir
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
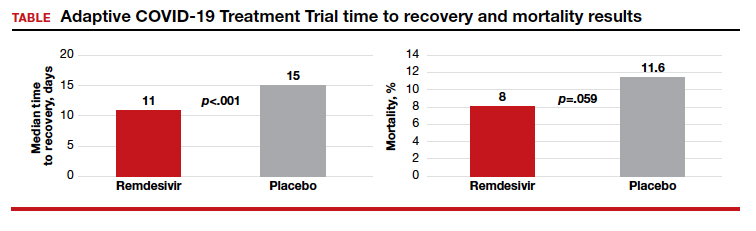
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
The ‘Three Rs’ of email effectiveness
Resist, Reorganize, and Respond
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
Resist, Reorganize, and Respond
Resist, Reorganize, and Respond
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
Internists least likely to choose their specialty again, survey shows
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Is anemia due to folate deficiency a myth?
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
FDA approves twice-daily formulation of key thalassemia drug
Chiesi Global Rare Diseases announced that the Food and Drug Administration has approved a new formulation of deferiprone (Ferriprox) for the treatment of patients with transfusional iron overload caused by thalassemia syndromes when current chelation therapy is inadequate. The new formulation of twice-a-day Ferriprox 1,000 mg oral tablets eliminates the midday dose, according to a company press release.
“A treatment option that reduces serum ferritin, cardiac iron, and liver iron with an established safety profile and now twice-a-day tablet dosing can represent a significant advantage for patients,” stated Thomas Coates, MD, in the press release. Dr. Coates is the section head of hematology at Children’s Hospital Los Angeles.
Deferiprone was originally approved by the FDA in 2011 for the treatment of transfusional iron overload caused by thalassemia syndromes. Ferriprox contains a label warning that it can cause agranulocytosis that can lead to serious infections and death. As neutropenia may precede the development of agranulocytosis, the warning advises measurement of the absolute neutrophil count before starting Ferriprox and monitoring the ANC weekly on therapy. In addition, Ferriprox should be interrupted if infection develops, and the ANC should be monitored more frequently.
As Ferriprox can cause fetal harm, women of reproductive potential should be advised to use an effective method of contraception during treatment and for at least 6 months after the last dose, according to the company release.
Chiesi Global Rare Diseases announced that the Food and Drug Administration has approved a new formulation of deferiprone (Ferriprox) for the treatment of patients with transfusional iron overload caused by thalassemia syndromes when current chelation therapy is inadequate. The new formulation of twice-a-day Ferriprox 1,000 mg oral tablets eliminates the midday dose, according to a company press release.
“A treatment option that reduces serum ferritin, cardiac iron, and liver iron with an established safety profile and now twice-a-day tablet dosing can represent a significant advantage for patients,” stated Thomas Coates, MD, in the press release. Dr. Coates is the section head of hematology at Children’s Hospital Los Angeles.
Deferiprone was originally approved by the FDA in 2011 for the treatment of transfusional iron overload caused by thalassemia syndromes. Ferriprox contains a label warning that it can cause agranulocytosis that can lead to serious infections and death. As neutropenia may precede the development of agranulocytosis, the warning advises measurement of the absolute neutrophil count before starting Ferriprox and monitoring the ANC weekly on therapy. In addition, Ferriprox should be interrupted if infection develops, and the ANC should be monitored more frequently.
As Ferriprox can cause fetal harm, women of reproductive potential should be advised to use an effective method of contraception during treatment and for at least 6 months after the last dose, according to the company release.
Chiesi Global Rare Diseases announced that the Food and Drug Administration has approved a new formulation of deferiprone (Ferriprox) for the treatment of patients with transfusional iron overload caused by thalassemia syndromes when current chelation therapy is inadequate. The new formulation of twice-a-day Ferriprox 1,000 mg oral tablets eliminates the midday dose, according to a company press release.
“A treatment option that reduces serum ferritin, cardiac iron, and liver iron with an established safety profile and now twice-a-day tablet dosing can represent a significant advantage for patients,” stated Thomas Coates, MD, in the press release. Dr. Coates is the section head of hematology at Children’s Hospital Los Angeles.
Deferiprone was originally approved by the FDA in 2011 for the treatment of transfusional iron overload caused by thalassemia syndromes. Ferriprox contains a label warning that it can cause agranulocytosis that can lead to serious infections and death. As neutropenia may precede the development of agranulocytosis, the warning advises measurement of the absolute neutrophil count before starting Ferriprox and monitoring the ANC weekly on therapy. In addition, Ferriprox should be interrupted if infection develops, and the ANC should be monitored more frequently.
As Ferriprox can cause fetal harm, women of reproductive potential should be advised to use an effective method of contraception during treatment and for at least 6 months after the last dose, according to the company release.
Progressive multiple sclerosis linked to faster retinal layer thinning
Key clinical point: Inner nuclear layer (INL) and outer nuclear layer (ONL) measures may be novel biomarkers of neurodegeneration in patients with progressive multiple sclerosis (PMS).
Major finding: Independent of age, PMS vs. relapsing-remitting MS (RRMS) was associated with a faster thinning of peri-papillary retinal nerve fiber layer (β = −0.34%/year; P less than .001), ganglion cell+inner plexiform layer (β = −0.27%/year; P less than .001), INL (β = −0.10%/year; P = .01), and ONL (β = −0.13%/year; P = .01).
Study details: In all, 178 RRMS, 186 PMS, and 66 control participants were followed for a median of 3.7 years; retinal imaging was performed with spectral-domain optical coherence tomography.
Disclosures: This study was funded by the NIH/NINDS, National MS Society, Race to Erase MS, Walters Foundation, and ACTRIMS. The authors declared no conflict of interest.
Citation: Sotirchos ES et al. Ann Neurol. 2020 Apr 13. doi: 10.1002/ana.25738.
Key clinical point: Inner nuclear layer (INL) and outer nuclear layer (ONL) measures may be novel biomarkers of neurodegeneration in patients with progressive multiple sclerosis (PMS).
Major finding: Independent of age, PMS vs. relapsing-remitting MS (RRMS) was associated with a faster thinning of peri-papillary retinal nerve fiber layer (β = −0.34%/year; P less than .001), ganglion cell+inner plexiform layer (β = −0.27%/year; P less than .001), INL (β = −0.10%/year; P = .01), and ONL (β = −0.13%/year; P = .01).
Study details: In all, 178 RRMS, 186 PMS, and 66 control participants were followed for a median of 3.7 years; retinal imaging was performed with spectral-domain optical coherence tomography.
Disclosures: This study was funded by the NIH/NINDS, National MS Society, Race to Erase MS, Walters Foundation, and ACTRIMS. The authors declared no conflict of interest.
Citation: Sotirchos ES et al. Ann Neurol. 2020 Apr 13. doi: 10.1002/ana.25738.
Key clinical point: Inner nuclear layer (INL) and outer nuclear layer (ONL) measures may be novel biomarkers of neurodegeneration in patients with progressive multiple sclerosis (PMS).
Major finding: Independent of age, PMS vs. relapsing-remitting MS (RRMS) was associated with a faster thinning of peri-papillary retinal nerve fiber layer (β = −0.34%/year; P less than .001), ganglion cell+inner plexiform layer (β = −0.27%/year; P less than .001), INL (β = −0.10%/year; P = .01), and ONL (β = −0.13%/year; P = .01).
Study details: In all, 178 RRMS, 186 PMS, and 66 control participants were followed for a median of 3.7 years; retinal imaging was performed with spectral-domain optical coherence tomography.
Disclosures: This study was funded by the NIH/NINDS, National MS Society, Race to Erase MS, Walters Foundation, and ACTRIMS. The authors declared no conflict of interest.
Citation: Sotirchos ES et al. Ann Neurol. 2020 Apr 13. doi: 10.1002/ana.25738.
Newly diagnosed MS patients more likely to have impaired cognitive function
Key clinical point: Patients with newly diagnosed multiple sclerosis (MS) or clinically isolated syndrome (CIS) are more likely to have subtly impaired cognitive function irrespective of race/ethnicity.
Major finding: Mean oral Symbol Digit Modalities Test (SDMT) scores were lower in patients with MS/CIS vs. control participants (52.2 vs. 58.3; P less than .0001). Independent predictors of lower oral SDMT scores included being black (β = −5.97) or Hispanic (β = −3.06), having MS (β = −6.04), lower educational attainment (β = −5.02), and having a household income ≤$65,000 (β = −2.28). No significant interaction was found between race/ethnicity and having MS on SDMT scores (P = .41).
Study details: 1,174 adult patients (mean age, 40.7 years) from the MS Sunshine Study were included in this analysis (MS/CIS cases, n = 554; matched control participants, n = 620).
Disclosures: This study was supported in part by the National Institute of Neurologic Disorders and Stroke. The presenting author received personal compensation or funding from Genzyme, Biogen, Serrono, MedDay, and Novartis, and grant support from the NIH, the National MS Society, California Community Foundation, and the Charitable Guthy-Jackson Foundation.
Citation: Amezcua L et al. Neurology. 2020 Mar 9. doi: 10.1212/WNL.0000000000009210.
Key clinical point: Patients with newly diagnosed multiple sclerosis (MS) or clinically isolated syndrome (CIS) are more likely to have subtly impaired cognitive function irrespective of race/ethnicity.
Major finding: Mean oral Symbol Digit Modalities Test (SDMT) scores were lower in patients with MS/CIS vs. control participants (52.2 vs. 58.3; P less than .0001). Independent predictors of lower oral SDMT scores included being black (β = −5.97) or Hispanic (β = −3.06), having MS (β = −6.04), lower educational attainment (β = −5.02), and having a household income ≤$65,000 (β = −2.28). No significant interaction was found between race/ethnicity and having MS on SDMT scores (P = .41).
Study details: 1,174 adult patients (mean age, 40.7 years) from the MS Sunshine Study were included in this analysis (MS/CIS cases, n = 554; matched control participants, n = 620).
Disclosures: This study was supported in part by the National Institute of Neurologic Disorders and Stroke. The presenting author received personal compensation or funding from Genzyme, Biogen, Serrono, MedDay, and Novartis, and grant support from the NIH, the National MS Society, California Community Foundation, and the Charitable Guthy-Jackson Foundation.
Citation: Amezcua L et al. Neurology. 2020 Mar 9. doi: 10.1212/WNL.0000000000009210.
Key clinical point: Patients with newly diagnosed multiple sclerosis (MS) or clinically isolated syndrome (CIS) are more likely to have subtly impaired cognitive function irrespective of race/ethnicity.
Major finding: Mean oral Symbol Digit Modalities Test (SDMT) scores were lower in patients with MS/CIS vs. control participants (52.2 vs. 58.3; P less than .0001). Independent predictors of lower oral SDMT scores included being black (β = −5.97) or Hispanic (β = −3.06), having MS (β = −6.04), lower educational attainment (β = −5.02), and having a household income ≤$65,000 (β = −2.28). No significant interaction was found between race/ethnicity and having MS on SDMT scores (P = .41).
Study details: 1,174 adult patients (mean age, 40.7 years) from the MS Sunshine Study were included in this analysis (MS/CIS cases, n = 554; matched control participants, n = 620).
Disclosures: This study was supported in part by the National Institute of Neurologic Disorders and Stroke. The presenting author received personal compensation or funding from Genzyme, Biogen, Serrono, MedDay, and Novartis, and grant support from the NIH, the National MS Society, California Community Foundation, and the Charitable Guthy-Jackson Foundation.
Citation: Amezcua L et al. Neurology. 2020 Mar 9. doi: 10.1212/WNL.0000000000009210.
T1-hypointense count corrected by T2/FLAIR lesion volume indicates clinical severity in MS
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Key clinical point: The number of T1-hypointense areas (T1-count), corrected for T2-fluid-attenuated inversion recovery (FLAIR)-hyperintense lesion volume, reflects the disease severity and activity in patients with multiple sclerosis (MS).
Major finding: T1-count (Spearman’s correlation coefficient [rho] = 0.51, P less than .001), gray-matter atrophy (rho = 0.40; P less than .01), and white-matter atrophy (rho = 0.49; P less than .001) significantly correlated with the expanded disability status scale. T1-count divided by FLAIR lesion volume correlated with the MS severity score (rho = 0.60; P less than .001).
Study details: This study included 42 patients with MS who were treated in a single university hospital in Japan; each patient underwent brain volumetry and was followed-up for more than 3 years until 2017.
Disclosures: Ichiro Nakashima was funded by JSPS KAKENHI. The authors declared no conflict of interest.
Citation: Akaishi T et al. PLoS One. 2020 Apr 3. doi: 10.1371/journal.pone.0231225.
Exclusive breastfeeding lowers the risk of postpartum MS relapse
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.
Key clinical point: Most women diagnosed with multiple sclerosis (MS) can have children without incurring an increased risk of relapses and should be encouraged to breastfeed exclusively as this lowers the risk of postpartum relapses.
Major finding: The annualized relapse rates (ARRs) declined from 0.37 before pregnancy to 0.14 during pregnancy (P less than .0001), with no rebound disease activity in the postpartum period. ARR was found to be 0.27 at 3 months postpartum and 0.37 at 4-6 months, matching prepregnancy rates. Exclusive breastfeeding for at least 2 months after delivery reduced the risk of relapse in the first 6 months postpartum (adjusted hazard ratio, 0.37; P = .0093).
Study details: This study evaluated the electronic health records of 466 pregnancies among 375 women with MS and their infants at the Kaiser Permanente Southern and Northern California between 2008 and 2016.
Disclosures: This study was supported by the National Multiple Sclerosis Society. Annette Langer-Gould has received grant support and awards from the NIH, the Patient-Centered Outcomes Research Institute, and the National MS Society; and currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from the ICER. The remaining authors declared no conflict of interest.
Citation: Langer-Gould A et al. Neurology. 2020 Apr 13. doi: 10.1212/WNL.0000000000009374.



