User login
Before pandemic, rheumatologists saw small salary increase
COVID-19 has changed many things in the medical landscape as practices have closed, many physicians are transitioning to telemedicine, and EDs struggle to provide safe environments for their employees.
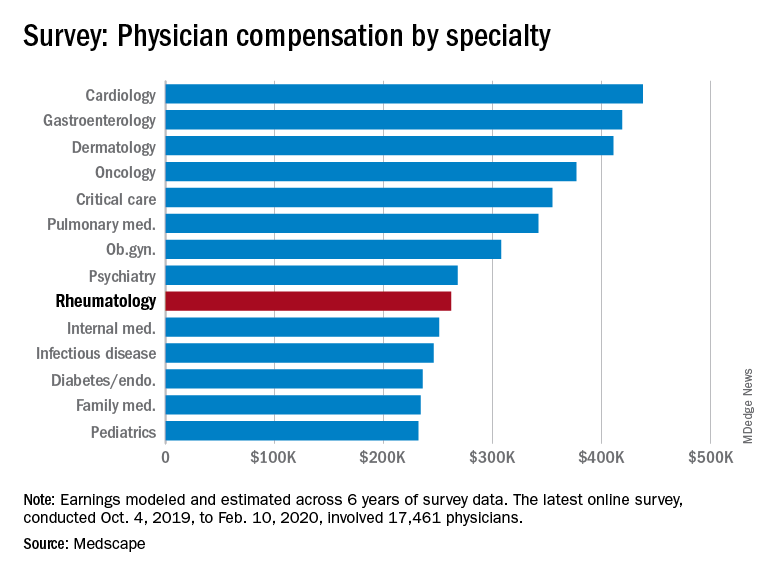
Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, illustrates what rheumatology looked like just before the coronavirus arrived.
Rheumatologists saw a small increase in average salary in 2020, rising from $259,000 in 2019 to $262,000, an increase of 1.16%. In comparison, average income for all specialists was $346,000 in this year’s survey, up by 1.5% from the $341,000 earned in 2019, Medscape reported. Male rheumatologists earned significantly more than women at $288,000 versus $240,000.
Prospects for next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
Before the pandemic, 55% of rheumatologists felt they were fairly compensated. This was about average among the 29 specialties included in the survey, which ranged from nephrology at 44% to oncology, emergency medicine, and radiology at 67%.
Rheumatologists were more likely than the average physician to report that “having so many rules and regulations” was the most challenging part of their job, according to the survey. A similar number of rheumatologists said that “gratitude/relationships with patients” was the most rewarding part of the job at 27%.
When asked if they would choose medicine again, 79% of rheumatologists said yes, slightly more than the 77% for all physicians; 81% said that they’d choose rheumatology again.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
COVID-19 has changed many things in the medical landscape as practices have closed, many physicians are transitioning to telemedicine, and EDs struggle to provide safe environments for their employees.

Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, illustrates what rheumatology looked like just before the coronavirus arrived.
Rheumatologists saw a small increase in average salary in 2020, rising from $259,000 in 2019 to $262,000, an increase of 1.16%. In comparison, average income for all specialists was $346,000 in this year’s survey, up by 1.5% from the $341,000 earned in 2019, Medscape reported. Male rheumatologists earned significantly more than women at $288,000 versus $240,000.
Prospects for next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
Before the pandemic, 55% of rheumatologists felt they were fairly compensated. This was about average among the 29 specialties included in the survey, which ranged from nephrology at 44% to oncology, emergency medicine, and radiology at 67%.
Rheumatologists were more likely than the average physician to report that “having so many rules and regulations” was the most challenging part of their job, according to the survey. A similar number of rheumatologists said that “gratitude/relationships with patients” was the most rewarding part of the job at 27%.
When asked if they would choose medicine again, 79% of rheumatologists said yes, slightly more than the 77% for all physicians; 81% said that they’d choose rheumatology again.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
COVID-19 has changed many things in the medical landscape as practices have closed, many physicians are transitioning to telemedicine, and EDs struggle to provide safe environments for their employees.

Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, illustrates what rheumatology looked like just before the coronavirus arrived.
Rheumatologists saw a small increase in average salary in 2020, rising from $259,000 in 2019 to $262,000, an increase of 1.16%. In comparison, average income for all specialists was $346,000 in this year’s survey, up by 1.5% from the $341,000 earned in 2019, Medscape reported. Male rheumatologists earned significantly more than women at $288,000 versus $240,000.
Prospects for next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
Before the pandemic, 55% of rheumatologists felt they were fairly compensated. This was about average among the 29 specialties included in the survey, which ranged from nephrology at 44% to oncology, emergency medicine, and radiology at 67%.
Rheumatologists were more likely than the average physician to report that “having so many rules and regulations” was the most challenging part of their job, according to the survey. A similar number of rheumatologists said that “gratitude/relationships with patients” was the most rewarding part of the job at 27%.
When asked if they would choose medicine again, 79% of rheumatologists said yes, slightly more than the 77% for all physicians; 81% said that they’d choose rheumatology again.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
Pulmonology, critical care earnings on the upswing before pandemic
As the COVID spring progresses, the days before the pandemic may seem like a dream: Practices were open, waiting rooms were full of unmasked people, and PPE was plentiful.
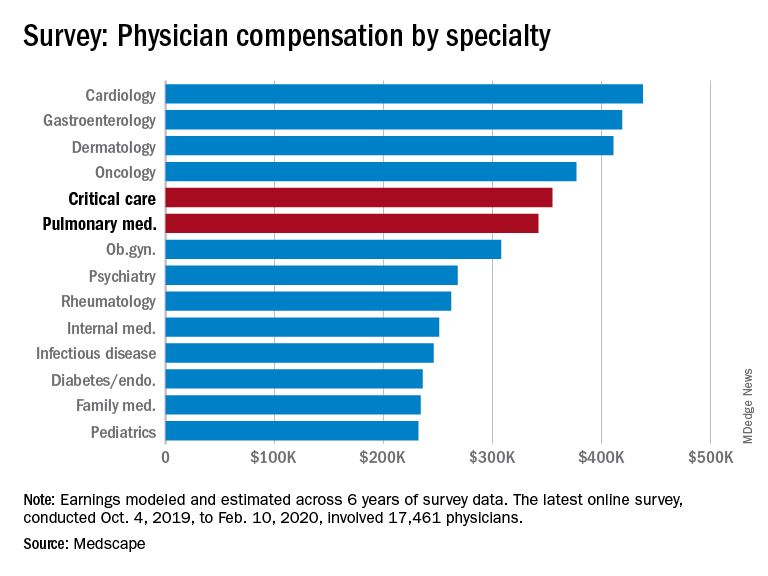
Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, shows what pulmonology and critical care looked like just before the coronavirus arrived.
Back then, earnings were up. Average compensation reported by pulmonologists was up from $331,000 in 2019 to $342,000 this year, a 3.3% increase. For intensivists, earnings rose from $349,000 to $355,000, or 1.7%. Average income for all specialists was $346,000 in this year’s survey – 1.5% higher than the $341,000 earned in 2019, Medscape reported.
Prospects for the next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
One problem area for intensivists, even before the pandemic, was paperwork and administration. Of the 26 specialties for which data are available, critical care was highest for amount of time spent on paperwork, at 19.1 hours per week. Those in pulmonary medicine spent 15.6 hours per week, which also happened to be the average for all specialists, the survey data show.
Both specialties also ranked high in denied/resubmitted claims: Intensivists were fourth among the 27 types of specialists with reliable data, with 20% of claims denied, and pulmonologists were tied for eighth at 18%, Medscape said.
Only 50% of pulmonologists surveyed said that they were being fairly compensated, putting them 26th among the 29 specialties on that list. Those in critical care medicine were 13th, with a 59% positive response, Medscape reported.
In the end, though, it looks like you can’t keep a good pulmonologist or intensivist down. When asked if they would choose medicine again, 83% of pulmonologists said yes, just one percentage point behind a three-way tie for first. Intensivists were just a little further down the list at 81%, according to the survey.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
As the COVID spring progresses, the days before the pandemic may seem like a dream: Practices were open, waiting rooms were full of unmasked people, and PPE was plentiful.

Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, shows what pulmonology and critical care looked like just before the coronavirus arrived.
Back then, earnings were up. Average compensation reported by pulmonologists was up from $331,000 in 2019 to $342,000 this year, a 3.3% increase. For intensivists, earnings rose from $349,000 to $355,000, or 1.7%. Average income for all specialists was $346,000 in this year’s survey – 1.5% higher than the $341,000 earned in 2019, Medscape reported.
Prospects for the next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
One problem area for intensivists, even before the pandemic, was paperwork and administration. Of the 26 specialties for which data are available, critical care was highest for amount of time spent on paperwork, at 19.1 hours per week. Those in pulmonary medicine spent 15.6 hours per week, which also happened to be the average for all specialists, the survey data show.
Both specialties also ranked high in denied/resubmitted claims: Intensivists were fourth among the 27 types of specialists with reliable data, with 20% of claims denied, and pulmonologists were tied for eighth at 18%, Medscape said.
Only 50% of pulmonologists surveyed said that they were being fairly compensated, putting them 26th among the 29 specialties on that list. Those in critical care medicine were 13th, with a 59% positive response, Medscape reported.
In the end, though, it looks like you can’t keep a good pulmonologist or intensivist down. When asked if they would choose medicine again, 83% of pulmonologists said yes, just one percentage point behind a three-way tie for first. Intensivists were just a little further down the list at 81%, according to the survey.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
As the COVID spring progresses, the days before the pandemic may seem like a dream: Practices were open, waiting rooms were full of unmasked people, and PPE was plentiful.

Medscape’s latest physician survey, conducted from Oct. 4, 2019, to Feb. 10, 2020, shows what pulmonology and critical care looked like just before the coronavirus arrived.
Back then, earnings were up. Average compensation reported by pulmonologists was up from $331,000 in 2019 to $342,000 this year, a 3.3% increase. For intensivists, earnings rose from $349,000 to $355,000, or 1.7%. Average income for all specialists was $346,000 in this year’s survey – 1.5% higher than the $341,000 earned in 2019, Medscape reported.
Prospects for the next year, however, are grim. “We found out that we have a 10% salary decrease effective May 2 to Dec. 25. Our bonus will be based on clinical productivity, and since our numbers are down, that is likely to go away,” a pediatric emergency physician told Medscape.
One problem area for intensivists, even before the pandemic, was paperwork and administration. Of the 26 specialties for which data are available, critical care was highest for amount of time spent on paperwork, at 19.1 hours per week. Those in pulmonary medicine spent 15.6 hours per week, which also happened to be the average for all specialists, the survey data show.
Both specialties also ranked high in denied/resubmitted claims: Intensivists were fourth among the 27 types of specialists with reliable data, with 20% of claims denied, and pulmonologists were tied for eighth at 18%, Medscape said.
Only 50% of pulmonologists surveyed said that they were being fairly compensated, putting them 26th among the 29 specialties on that list. Those in critical care medicine were 13th, with a 59% positive response, Medscape reported.
In the end, though, it looks like you can’t keep a good pulmonologist or intensivist down. When asked if they would choose medicine again, 83% of pulmonologists said yes, just one percentage point behind a three-way tie for first. Intensivists were just a little further down the list at 81%, according to the survey.
The respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
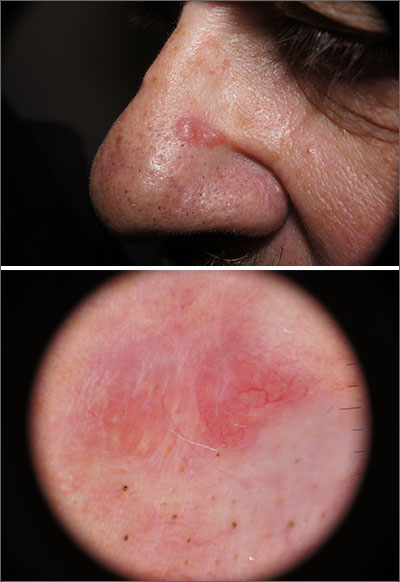
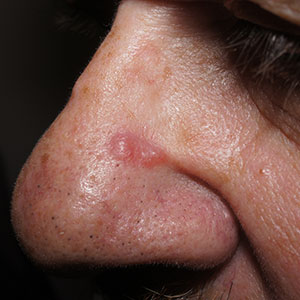
Pink nose lesion
The punch biopsy revealed dense lymphoid hyperplasia consistent with pseudolymphoma. The punch biopsy sample underwent notable immunohistochemical analysis to exclude lymphoma and was remarkable for a polyclonal set of B and T lymphocytes in a benign ratio, without epidermotropism.
Pseudolymphoma is an inflammatory response often induced by an arthropod bite, or occasionally, medication. Both T cell predominant and B cell predominant pseudolymphoma occur. Most present as a 5- to 30-mm pink papule on the head. They are rare below the waist and more common in young adults.
Basal cell carcinoma (BCC) is such a common diagnosis for a novel pink neoplasm on the face of an older adult that it is easy to forget the many other rare tumors and benign lesions (such as this one) present this way. An expanded differential diagnosis included cutaneous lymphoma, amelanotic melanoma, and granulomatous rosacea.
In this case, dermoscopy of the lesion (shown above) also was of limited help because BCC features of arborized vessels and shiny white lines were evident. There was a lack of more specific dermoscopic features such as spoke wheel–like structures, leaflike structures, or blue clods. This lack of more specific features might be the subtle clue to think beyond BCC when approaching the diagnosis. The history of rapid change was somewhat atypical for a BCC, which tends to grow more slowly. Patient history about the timing of facial lesions can be poor, but this patient’s history fit the ultimate diagnosis well.
This patient was treated with a superpotent topical steroid, clobetasol ointment 0.05% bid for 3 weeks. As a general rule, use of such a strong steroid is avoided on the face because of the risk of steroid atrophy, but in the case of pseudolymphoma or lymphoma, an exception is allowed. An alternative would be intralesional triamcinolone at a 5 to 10 mg/mL concentration. At follow-up 6 weeks later, the lesion was completely resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Miguel D, Peckruhn M, Elsner P. Treatment of cutaneous pseudolymphoma: a systematic review. Acta Derm Venereol. 2018;98:310-317.
The punch biopsy revealed dense lymphoid hyperplasia consistent with pseudolymphoma. The punch biopsy sample underwent notable immunohistochemical analysis to exclude lymphoma and was remarkable for a polyclonal set of B and T lymphocytes in a benign ratio, without epidermotropism.
Pseudolymphoma is an inflammatory response often induced by an arthropod bite, or occasionally, medication. Both T cell predominant and B cell predominant pseudolymphoma occur. Most present as a 5- to 30-mm pink papule on the head. They are rare below the waist and more common in young adults.
Basal cell carcinoma (BCC) is such a common diagnosis for a novel pink neoplasm on the face of an older adult that it is easy to forget the many other rare tumors and benign lesions (such as this one) present this way. An expanded differential diagnosis included cutaneous lymphoma, amelanotic melanoma, and granulomatous rosacea.
In this case, dermoscopy of the lesion (shown above) also was of limited help because BCC features of arborized vessels and shiny white lines were evident. There was a lack of more specific dermoscopic features such as spoke wheel–like structures, leaflike structures, or blue clods. This lack of more specific features might be the subtle clue to think beyond BCC when approaching the diagnosis. The history of rapid change was somewhat atypical for a BCC, which tends to grow more slowly. Patient history about the timing of facial lesions can be poor, but this patient’s history fit the ultimate diagnosis well.
This patient was treated with a superpotent topical steroid, clobetasol ointment 0.05% bid for 3 weeks. As a general rule, use of such a strong steroid is avoided on the face because of the risk of steroid atrophy, but in the case of pseudolymphoma or lymphoma, an exception is allowed. An alternative would be intralesional triamcinolone at a 5 to 10 mg/mL concentration. At follow-up 6 weeks later, the lesion was completely resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The punch biopsy revealed dense lymphoid hyperplasia consistent with pseudolymphoma. The punch biopsy sample underwent notable immunohistochemical analysis to exclude lymphoma and was remarkable for a polyclonal set of B and T lymphocytes in a benign ratio, without epidermotropism.
Pseudolymphoma is an inflammatory response often induced by an arthropod bite, or occasionally, medication. Both T cell predominant and B cell predominant pseudolymphoma occur. Most present as a 5- to 30-mm pink papule on the head. They are rare below the waist and more common in young adults.
Basal cell carcinoma (BCC) is such a common diagnosis for a novel pink neoplasm on the face of an older adult that it is easy to forget the many other rare tumors and benign lesions (such as this one) present this way. An expanded differential diagnosis included cutaneous lymphoma, amelanotic melanoma, and granulomatous rosacea.
In this case, dermoscopy of the lesion (shown above) also was of limited help because BCC features of arborized vessels and shiny white lines were evident. There was a lack of more specific dermoscopic features such as spoke wheel–like structures, leaflike structures, or blue clods. This lack of more specific features might be the subtle clue to think beyond BCC when approaching the diagnosis. The history of rapid change was somewhat atypical for a BCC, which tends to grow more slowly. Patient history about the timing of facial lesions can be poor, but this patient’s history fit the ultimate diagnosis well.
This patient was treated with a superpotent topical steroid, clobetasol ointment 0.05% bid for 3 weeks. As a general rule, use of such a strong steroid is avoided on the face because of the risk of steroid atrophy, but in the case of pseudolymphoma or lymphoma, an exception is allowed. An alternative would be intralesional triamcinolone at a 5 to 10 mg/mL concentration. At follow-up 6 weeks later, the lesion was completely resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Miguel D, Peckruhn M, Elsner P. Treatment of cutaneous pseudolymphoma: a systematic review. Acta Derm Venereol. 2018;98:310-317.
Miguel D, Peckruhn M, Elsner P. Treatment of cutaneous pseudolymphoma: a systematic review. Acta Derm Venereol. 2018;98:310-317.
FDA expands approval of atezolizumab in NSCLC
The Food and Drug Administration has expanded the approved indication for atezolizumab (Tecentriq) in patients with non–small cell lung cancer (NSCLC).
Atezolizumab is now approved as first-line monotherapy for adults with metastatic NSCLC whose tumors are EGFR and ALK wild-type but have high PD-L1 expression (PD-L1 stained ≥50% of tumor cells or PD-L1 stained tumor-infiltrating immune cells covering ≥10% of the tumor area).
The FDA also approved the VENTANA PD-L1 (SP142) Assay as a companion diagnostic to identify patients with NSCLC who are eligible for treatment with atezolizumab.
The drug was evaluated in the IMpower110 trial (NCT02409342), which enrolled patients with stage IV, PD-L1–positive (tumor cells [TC] ≥1% or immune cells [IC] ≥1%) NSCLC who had received no prior chemotherapy for metastatic disease.
The patients were randomized to receive atezolizumab at 1,200 mg every 3 weeks (n = 286) or platinum-based chemotherapy (n = 263), which consisted of carboplatin or cisplatin with either pemetrexed or gemcitabine, until disease progression or unacceptable toxicity.
Overall survival was superior in the atezolizumab arm, but only among patients with high PD-L1 expression (TC ≥50% or IC ≥10%). The median overall survival was 20.2 months among PD-L1–high patients in the atezolizumab arm and 13.1 months among PD-L1–high patients in the chemotherapy arm (hazard ratio, 0.59; P = .0106). There was no significant difference in overall survival between the treatment arms for patients in the other two PD-L1 subgroups – TC ≥5% or IC ≥5% and TC ≥1% or IC ≥1%.
Serious adverse events occurred in 28% of patients receiving atezolizumab. The most frequent of these were pneumonia (2.8%), chronic obstructive pulmonary disease (2.1%), and pneumonitis (2.1%). Fatal adverse events in the atezolizumab arm included unexplained death, aspiration, chronic obstructive pulmonary disease, pulmonary embolism, acute myocardial infarction, cardiac arrest, mechanical ileus, sepsis, cerebral infraction, and device occlusion (one patient each).
For more details on atezolizumab, see the full prescribing information.
The FDA has granted the approval of atezolizumab to Genentech and the approval of the VENTANA PD-L1 (SP142) Assay to Ventana Medical Systems.
The Food and Drug Administration has expanded the approved indication for atezolizumab (Tecentriq) in patients with non–small cell lung cancer (NSCLC).
Atezolizumab is now approved as first-line monotherapy for adults with metastatic NSCLC whose tumors are EGFR and ALK wild-type but have high PD-L1 expression (PD-L1 stained ≥50% of tumor cells or PD-L1 stained tumor-infiltrating immune cells covering ≥10% of the tumor area).
The FDA also approved the VENTANA PD-L1 (SP142) Assay as a companion diagnostic to identify patients with NSCLC who are eligible for treatment with atezolizumab.
The drug was evaluated in the IMpower110 trial (NCT02409342), which enrolled patients with stage IV, PD-L1–positive (tumor cells [TC] ≥1% or immune cells [IC] ≥1%) NSCLC who had received no prior chemotherapy for metastatic disease.
The patients were randomized to receive atezolizumab at 1,200 mg every 3 weeks (n = 286) or platinum-based chemotherapy (n = 263), which consisted of carboplatin or cisplatin with either pemetrexed or gemcitabine, until disease progression or unacceptable toxicity.
Overall survival was superior in the atezolizumab arm, but only among patients with high PD-L1 expression (TC ≥50% or IC ≥10%). The median overall survival was 20.2 months among PD-L1–high patients in the atezolizumab arm and 13.1 months among PD-L1–high patients in the chemotherapy arm (hazard ratio, 0.59; P = .0106). There was no significant difference in overall survival between the treatment arms for patients in the other two PD-L1 subgroups – TC ≥5% or IC ≥5% and TC ≥1% or IC ≥1%.
Serious adverse events occurred in 28% of patients receiving atezolizumab. The most frequent of these were pneumonia (2.8%), chronic obstructive pulmonary disease (2.1%), and pneumonitis (2.1%). Fatal adverse events in the atezolizumab arm included unexplained death, aspiration, chronic obstructive pulmonary disease, pulmonary embolism, acute myocardial infarction, cardiac arrest, mechanical ileus, sepsis, cerebral infraction, and device occlusion (one patient each).
For more details on atezolizumab, see the full prescribing information.
The FDA has granted the approval of atezolizumab to Genentech and the approval of the VENTANA PD-L1 (SP142) Assay to Ventana Medical Systems.
The Food and Drug Administration has expanded the approved indication for atezolizumab (Tecentriq) in patients with non–small cell lung cancer (NSCLC).
Atezolizumab is now approved as first-line monotherapy for adults with metastatic NSCLC whose tumors are EGFR and ALK wild-type but have high PD-L1 expression (PD-L1 stained ≥50% of tumor cells or PD-L1 stained tumor-infiltrating immune cells covering ≥10% of the tumor area).
The FDA also approved the VENTANA PD-L1 (SP142) Assay as a companion diagnostic to identify patients with NSCLC who are eligible for treatment with atezolizumab.
The drug was evaluated in the IMpower110 trial (NCT02409342), which enrolled patients with stage IV, PD-L1–positive (tumor cells [TC] ≥1% or immune cells [IC] ≥1%) NSCLC who had received no prior chemotherapy for metastatic disease.
The patients were randomized to receive atezolizumab at 1,200 mg every 3 weeks (n = 286) or platinum-based chemotherapy (n = 263), which consisted of carboplatin or cisplatin with either pemetrexed or gemcitabine, until disease progression or unacceptable toxicity.
Overall survival was superior in the atezolizumab arm, but only among patients with high PD-L1 expression (TC ≥50% or IC ≥10%). The median overall survival was 20.2 months among PD-L1–high patients in the atezolizumab arm and 13.1 months among PD-L1–high patients in the chemotherapy arm (hazard ratio, 0.59; P = .0106). There was no significant difference in overall survival between the treatment arms for patients in the other two PD-L1 subgroups – TC ≥5% or IC ≥5% and TC ≥1% or IC ≥1%.
Serious adverse events occurred in 28% of patients receiving atezolizumab. The most frequent of these were pneumonia (2.8%), chronic obstructive pulmonary disease (2.1%), and pneumonitis (2.1%). Fatal adverse events in the atezolizumab arm included unexplained death, aspiration, chronic obstructive pulmonary disease, pulmonary embolism, acute myocardial infarction, cardiac arrest, mechanical ileus, sepsis, cerebral infraction, and device occlusion (one patient each).
For more details on atezolizumab, see the full prescribing information.
The FDA has granted the approval of atezolizumab to Genentech and the approval of the VENTANA PD-L1 (SP142) Assay to Ventana Medical Systems.
Today’s top news highlights: Risks & benefits of universal masking, prostate cancer rising
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively. As reported in Science, previous randomized clinical studies performed on other viruses have shown no added protection, though small sample sizes and noncompliance are limiting factors. Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus. On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects found that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” READ MORE
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Biomarkers for inflammation and thrombosis may predict deaths from COVID-19 among critically ill patients, researchers said. Their prospective cohort study of 1,150 patients hospitalized in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality. “Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York, said in a press release. The study was published in The Lancet. READ MORE
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force controversially advised in 2012 against prostate-specific antigen screening in men of all ages. “These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors of the study, published in the Journal of the National Cancer Institute, commented. “What is a surprise is that it’s every year,” said Ahmad Shabsigh, MD, a urologic oncologist at the Ohio State University Comprehensive Cancer Center. “To see it so clearly in this study is sad." READ MORE
Testicular sperm may improve IVF outcomes
Use of testicular sperm in nonazoospermic couples who had prior in vitro fertilization failure using ejaculated sperm appears to improve embryo development and rates of clinical pregnancy and live birth, a retrospective observational study has found. The findings offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” said M. Blake Evans, DO, a clinical fellow in reproductive endocrinology and infertility. The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, was released ahead of a scheduled presentation at the annual American College of Obstetricians and Gynecologists meeting. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news coverage is available on MDedge.com.
The SHM 2019 Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!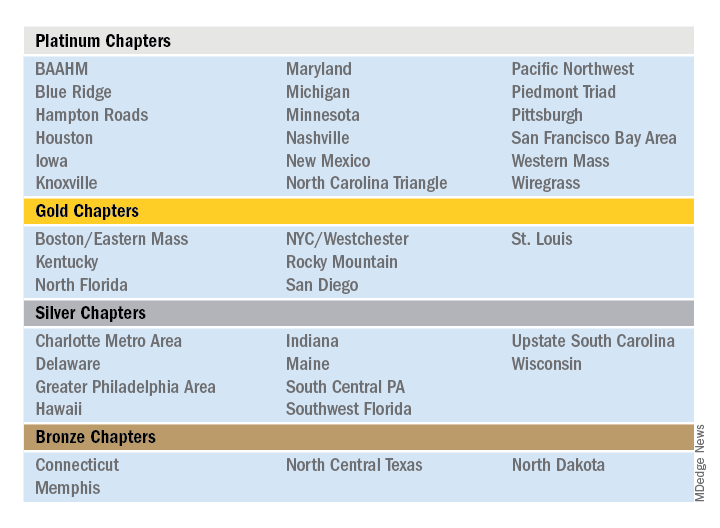
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
New selective S1P modulator FDA-approved for relapsing forms of multiple sclerosis1
Paid content by 
Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
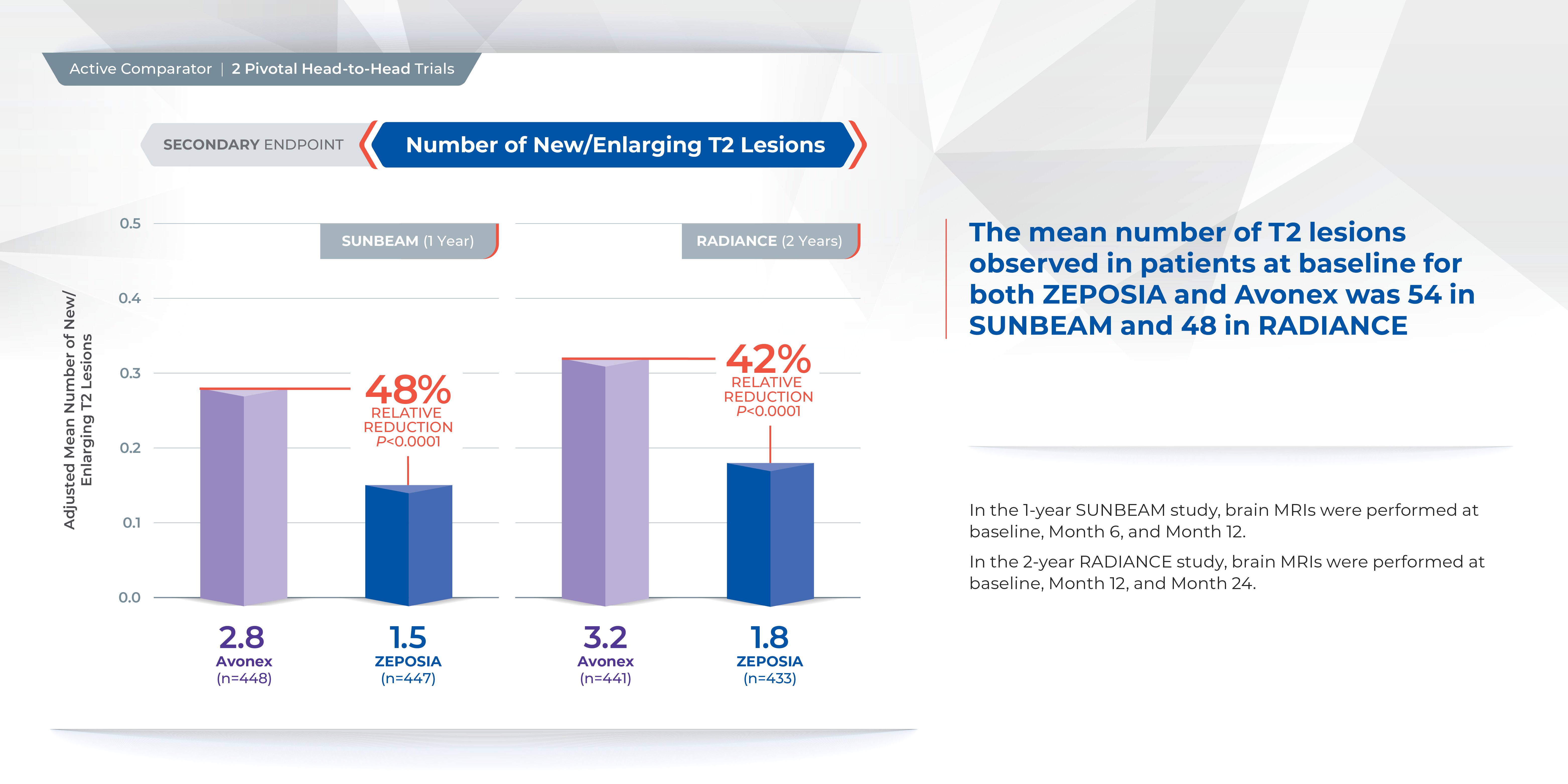
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1
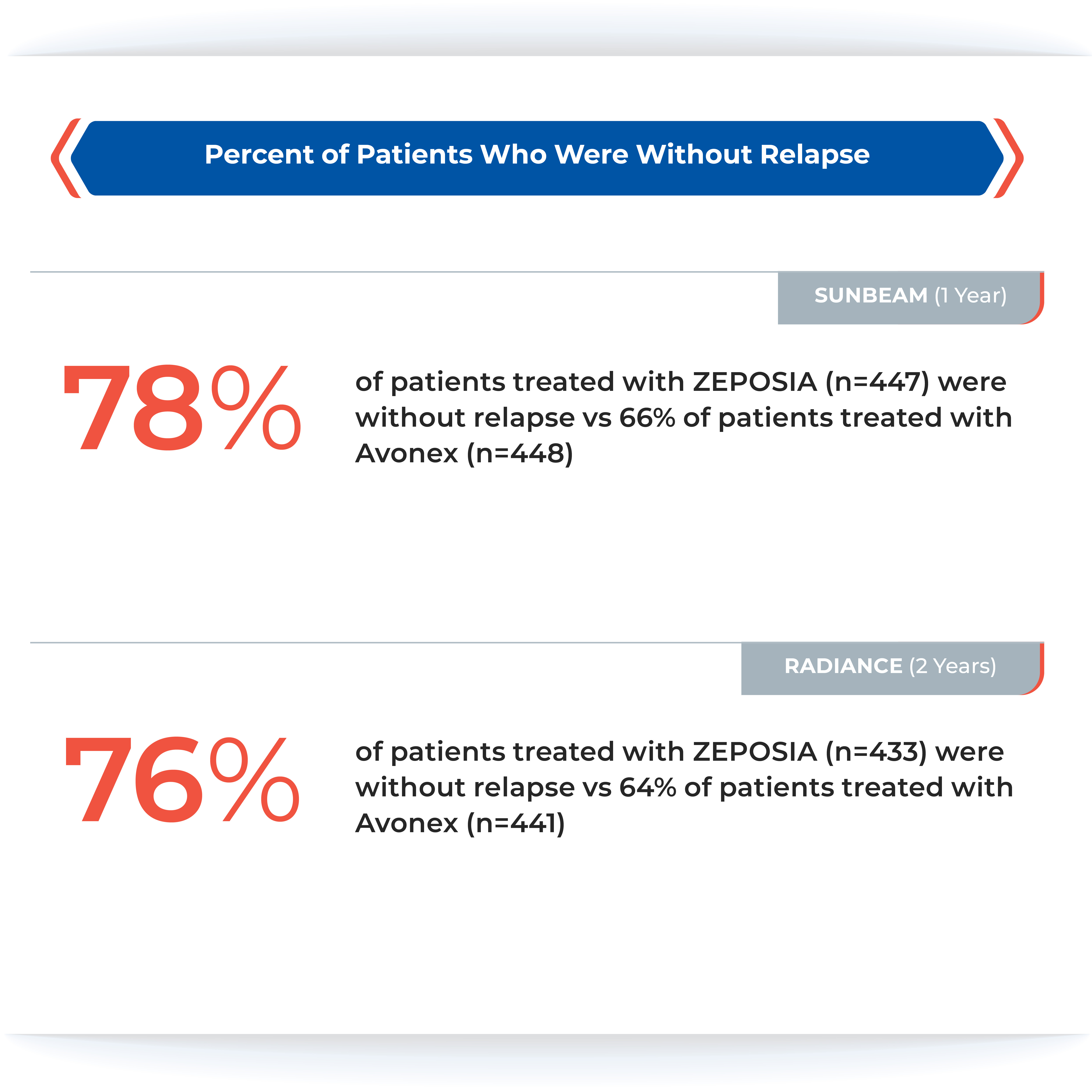
Proven superiority in reducing brain lesions
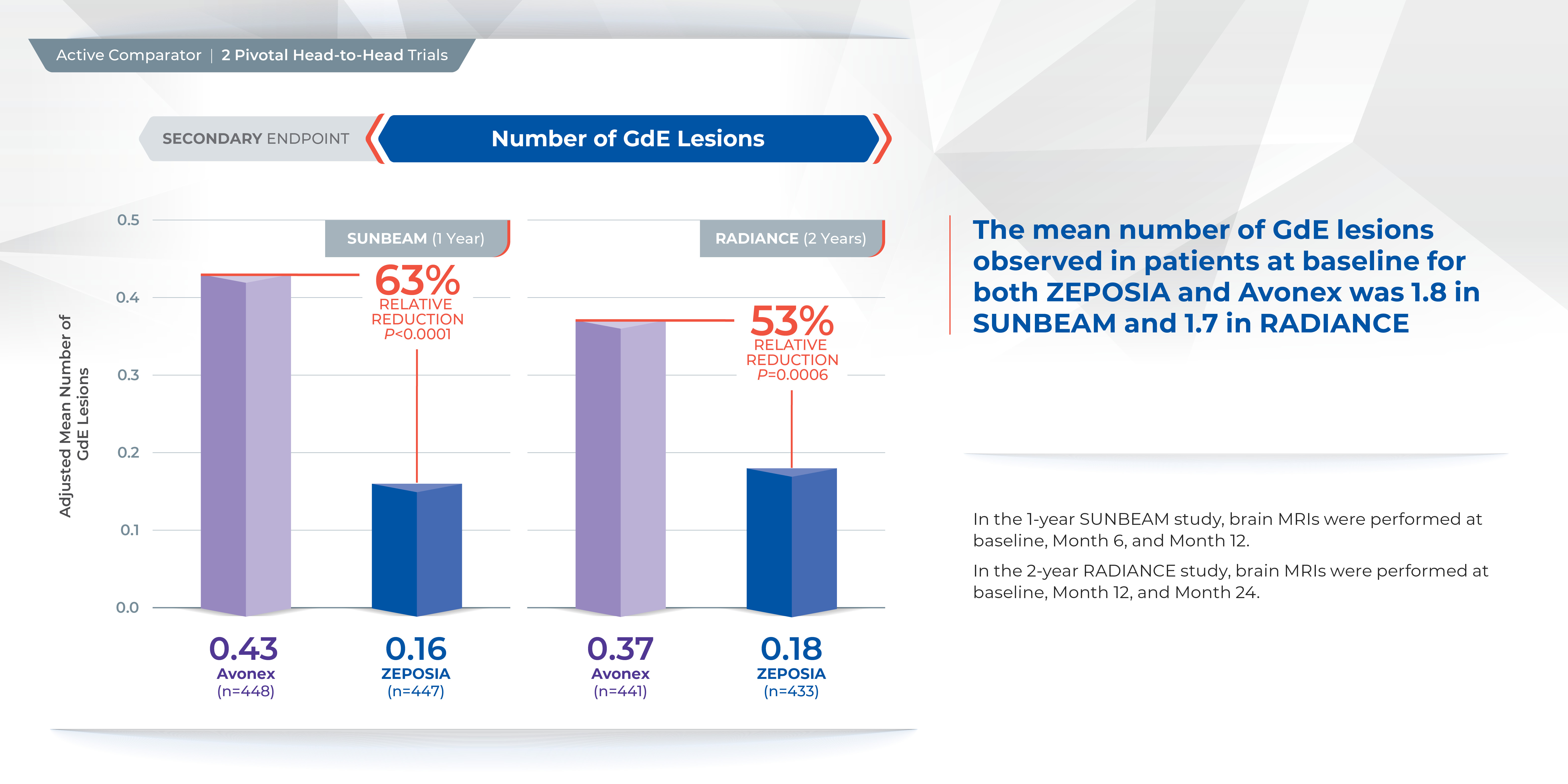
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1
In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1
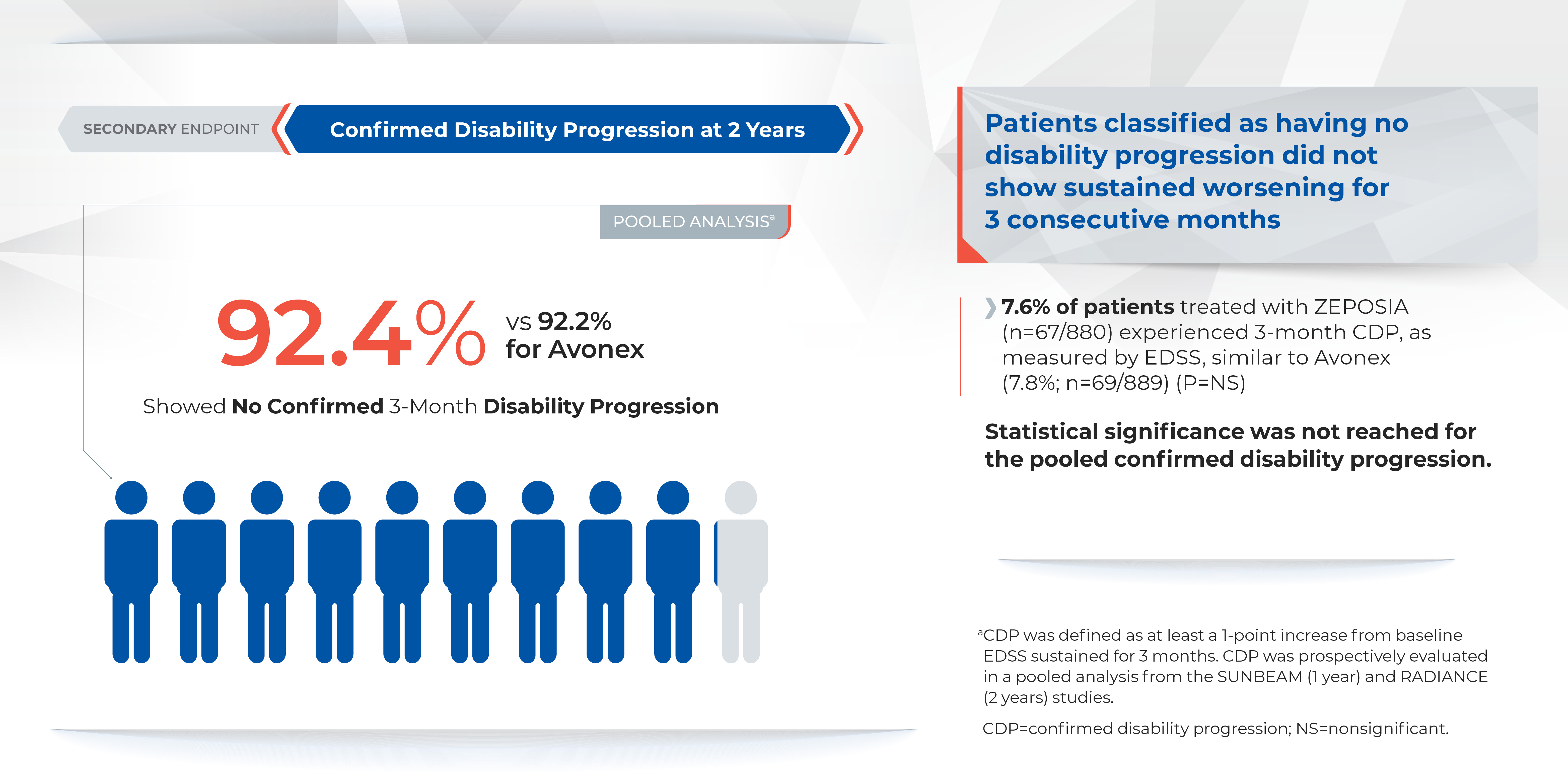
There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1
Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Paid content by 

Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1

Proven superiority in reducing brain lesions
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1

In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1

There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1

Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Paid content by 

Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1

Proven superiority in reducing brain lesions
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1

In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1

There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1

Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Tumor molecular profiling may help identify ‘exceptional responders’
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Large COVID-19 dataset: Kidney injury in >35% of those in hospital
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
AAN publishes ethical guidance on patient care during the pandemic
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
FROM NEUROLOGY