User login
World first: Saliva test detects occult HPV-driven oropharyngeal cancer
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Discharge Before Return to Respiratory Baseline in Children with Neurologic Impairment
Children with neurologic impairment (NI; eg, hypoxic-ischemic encephalopathy, muscular dystrophy) are characterized by functional and/or intellectual impairments resulting from a variety of neurologic diseases.1 These children commonly have respiratory comorbidities, including central hypoventilation, impaired cough, and oromotor dysfunction, that may lead to chronic respiratory insufficiency and a need for chronic respiratory support at baseline.2,3 Baseline respiratory support modalities can include supplemental oxygen, noninvasive positive pressure ventilation, or invasive mechanical ventilation.
Acute respiratory infections (ARI; eg, pneumonia, bronchiolitis) are the most common cause of hospitalization, intensive care unit (ICU) admission, and death for children with NI.1,3 Discharge criteria for otherwise healthy children admitted to the hospital with ARI often include return to respiratory baseline.4 Children with complex chronic conditions have longer hospitalizations when hospitalized with respiratory infections,5-7 because, in part, comorbidities and complications prolong the time to return to baseline. This prolonged return to respiratory baseline in combination with family knowledge, comfort, and skill in managing respiratory support and other complexities at home may alter discharge practices in the population of children with NI. In our clinical experience, discharge before return to baseline respiratory support occurs more frequently in children with NI than in otherwise healthy children when hospitalized with ARI. However, the consequences of discharging children with NI prior to return to respiratory baseline are unknown.
In this study, we sought to determine if discharge prior to return to baseline respiratory support is associated with reutilization among children with NI hospitalized with ARI. We hypothesized that patients discharged prior to return to respiratory baseline would have higher rates of 30-day hospital reutilization.
METHODS
Study Design and Data Source
This single-center, retrospective cohort study of children hospitalized at Cincinnati Children’s Hospital Medical Center (CCHMC) used data from the Pediatric Health Information System (PHIS) and the electronic medical record (EMR). PHIS, an administrative database of 45 not-for-profit, tertiary care, US pediatric hospitals managed by Children’s Hospital Association (Lenexa, Kansas), was used to identify eligible children, examine demographic and clinical variables, and define outcomes. PHIS contains data regarding patient demographics, inpatient resource utilization, and diagnoses. Encrypted medical record numbers in PHIS allowed for local identification of patients’ medical records to complete EMR review to confirm eligibility and obtain detailed patient-level clinical information (eg, respiratory support needs) not available in PHIS.
Pilot medical record reviews allowed for standardized study definitions and procedures. All study staff underwent training with the primary investigator, including detailed review of 10 initial abstractions. Two investigators (K.M. and S.C.) performed repeat abstractions from 40 randomly selected records to enable assessment of interrater reliability. Average reliability, indicated by the κ statistic, indicated substantial to near-perfect reliability8 (κ = 0.97, 95% CI 0.90-1.00) for the primary exposure. EMR data were managed using Research Electronic Data Capture (REDCap, Nashville, Tennessee)9 and subsequently merged with PHIS data.
Study Population
Hospitalizations of children with NI aged 1 to 18 years at CCHMC between January 2010 and September 2015 were eligible for inclusion if they had a principal discharge diagnosis indicative of ARI and required increased respiratory support from baseline during hospitalization. NI was defined as a high-intensity, chronic neurological diagnosis with substantial functional impairments according to previously defined diagnosis codes.1,10 ARI was identified using codes in the Clinical Classification Software (Agency for Healthcare Research and Quality, Rockville, MD) respiratory group indicative of ARI (eg, pneumonia, bronchiolitis, influenza; Appendix Table).
Children transferred to CCHMC were excluded because records from their initial illness presentation and management were not available. Because of expected differences in management and outcomes, children with a known diagnosis of tuberculosis or human immunodeficiency virus were excluded. Because exposure criteria were dependent on hospital discharge status, hospitalizations for children who died during admission (4 of 632 hospitalizations, 0.63%) were excluded from the final cohort (Appendix Figure).
Study Definitions
Baseline respiratory support (ie, “respiratory baseline”) was defined as the child’s highest level of respiratory support needed prior to admission when well (ie, no support, supplemental oxygen, continuous positive airway pressure [CPAP] or bilevel positive airway pressure [BiPAP], or ventilator support), and further characterized by night or day/night requirement. Respiratory baseline was identified using EMR documentation of home respiratory support use at the time of index admission. Return to respiratory baseline was defined as the date on which the child achieved documented home respiratory support settings, regardless of clinical symptoms.
Children may have required increased respiratory support from baseline at any time during hospitalization. Maximum respiratory support required was categorized as one of the following: (1) initiation of supplemental oxygen or increase in oxygen flow or duration; (2) initiation of CPAP or BiPAP; (3) increase in pressure settings or duration of pressure support for those with baseline CPAP, BiPAP, or ventilator use; and (4) initiation of full mechanical ventilation. Respiratory support categories were mutually exclusive: children requiring multiple types of increased respiratory support were classified for analysis by the most invasive form of respiratory support used (eg, a child requiring increase in both oxygen flow and pressure settings was categorized as an increase in pressure settings). Children who received heated high-flow nasal cannula therapy as maximum support were categorized as initiation or increase in oxygen support.
Time to return to respiratory baseline was defined as the difference in days between date of return to respiratory baseline and date of admission. Time to return to respiratory baseline was determined only for children who were discharged at respiratory baseline.
Primary Exposure and Outcome Measures
The primary exposure was hospital discharge before return to respiratory baseline (ie, discharge on higher respiratory support than at baseline settings). At our institution, standardized discharge criteria for children with NI do not exist. The primary outcome was all-cause, 30-day hospital reutilization, including hospital readmissions and emergency department (ED) revisits. Secondary outcomes included 30-day reutilization for ARI and hospital length of stay (LOS) in days.
Patient Demographics and Clinical Characteristics
Demographic and patient characteristics that might influence hospital discharge before return to respiratory baseline or readmission were obtained from PHIS (eg, demographic information, age, insurance type, measures of clinical complexity, illness severity) and by EMR review (eg, baseline respiratory support needs, maximum respiratory support during hospitalization). Measures of clinical complexity included comorbid complex chronic conditions (CCCs)11-14 and technology dependence14-16 using previously defined diagnostic codes. Measures of illness severity included sepsis17 and ICU-level care. At our institution, children with baseline ventilator use do not require admission to the ICU unless they are clinically unstable.
Statistical Analysis
Continuous variables were described using medians and interquartile ranges (IQR). Categorical variables were described using counts and percentages. Patient characteristics and outcomes were stratified by primary exposure and compared using chi-square test or Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
To examine the independent association between discharge before return to respiratory baseline and hospital reutilization, a generalized estimating equation was used that included potential confounders while accounting for within-patient clustering. Patient demographics included age, race, ethnicity, and insurance type; measures of clinical complexity included number of CCCs, technology dependence, and baseline respiratory support; and measures of acute illness severity included ARI diagnosis, degree of increase in respiratory support during hospitalization, and ICU-level care. LOS was also included in the model as a covariate because of its expected association with both exposure and outcome.
Secondary analyses were conducted using the outcome of 30-day reutilization for ARI. Subgroup analysis excluding hospitalizations of children lost to follow-up (ie, no encounters in the 6 months after hospital discharge) was also conducted. All analyses were performed with SAS v9.3 (SAS Institute, Cary, North Carolina). P values < .05 were considered statistically significant. This study was approved by the Institutional Review Board.
RESULTS
Study Cohort
A total of 632 hospitalizations experienced by 366 children with NI who were admitted with ARI were included (Appendix Figure). Most children (66.4%) in the cohort experienced only one hospitalization, 17.5% had two hospitalizations, 7.9% had three hospitalizations, and 8.2% had four or more hospitalizations. The median age at hospitalization was 5.0 years (IQR 2.8-10.5) and most hospitalizations were for children who were male (56.6%), white (78.3%), non-Hispanic (96.0%), and publicly insured (51.7%; Table 1). More than one-quarter (28.6%) of hospitalizations were for children with four or more CCCs, and in 73.4% of hospitalizations, children were technology dependent (Table 1). Baseline respiratory support was common (46.8%), including home mechanical ventilation in 11.1% of hospitalizations (Table 1). Bacterial pneumonia, including aspiration pneumonia, was the most common discharge diagnosis (50.5%, Table 1).

Demographic and Clinical Characteristics
Children were discharged before return to respiratory baseline in 30.4% of hospitalizations (Appendix Figure). Children discharged before return to respiratory baseline were older (median age 5.7 years, IQR 3.1-11.0, vs 4.9 years, IQR 2.6-9.7; P = .04) and more likely to be privately insured (54.2% vs 43.4%; P = .04), compared with children discharged at respiratory baseline (Table 1). Children discharged before return to respiratory baseline were also more likely to have a respiratory CCC (59.9% vs 30.9%; P < .001), have a respiratory technology dependence diagnosis code (44.8% vs 24.1%; P < .001), and have baseline respiratory support needs on EMR review (67.7% vs 37.7%; P < .001), compared with children discharged at baseline (Table 1).
Children discharged before return to respiratory baseline required significantly greater escalation in respiratory support during hospitalization, compared with children discharged at respiratory baseline, including higher rates of initiation of CPAP or BiPAP, increased pressure settings from baseline (for home CPAP, BiPAP, or ventilator users), and initiation of full mechanical ventilation (Table 1). Hospitalizations in which children were discharged before return to respiratory baseline were also more likely to include ICU care than were those for children discharged at baseline (52.1% vs 35.2%; P < .001; Table 1).
Clinical Outcomes and Utilization
Reutilization within 30 days occurred after 32.1% of hospitalizations, with 26.1% requiring hospital readmission and 6.0% requiring ED revisit (Table 2). There was no statistical association in either unadjusted (Table 2) or adjusted (Table 3) analysis between children discharged before return to respiratory baseline and 30-day all-cause hospital reutilizations, hospital readmissions, or ED revisits.
In analysis of secondary outcomes, 30-day reutilization because of ARI occurred after 21.5% of hospitalizations, with 19.0% requiring hospital readmission and 2.5% requiring ED revisit. Median hospital LOS for the cohort was 4 days (IQR 2-8; Table 2). Hospitalizations in which children were discharged before return to respiratory baseline were longer than in those discharged at baseline (median 6 days, IQR 3-11, vs 4 days, IQR 2-7; P < .001; Table 2).
For hospitalizations of children discharged at respiratory baseline, the median time to return to respiratory baseline was 3 days (IQR 1-5, complete range 0-80). In these encounters, discharge occurred soon after return to respiratory baseline (median 1 day, IQR 0-1.5, complete range 0-54).
In subgroup analysis excluding the 18 hospitalizations in which children were lost to follow-up (2.8% of the total cohort), discharge before return to respiratory baseline was not associated with 30-day all-cause hospital reutilization (Table 4).

DISCUSSION
In this retrospective cohort study, children with NI hospitalized with ARI were frequently discharged using increased respiratory support from baseline. However, those discharged before return to respiratory baseline, despite their greater clinical complexity and acute illness severity, did not have increased hospital reutilization, compared with children discharged at respiratory baseline. Our findings suggest that discharge before return to baseline respiratory support after ARI may be clinically appropriate in some children with NI.
With the growing emphasis on decreasing hospital costs, concern exists that patients are being discharged from hospitals “quicker and sicker,”18,19 with shortening lengths of stay and higher patient instability at discharge. Clinical instability at discharge has been associated with adverse postdischarge outcomes in adults with pneumonia20-23; however, studies evaluating discharge readiness have not examined the population of children with NI. Our findings of no difference in reutilization for children with NI discharged before return to respiratory baseline, which would be expected to approximate one or more clinical instabilities, contrast these concerns.
Clinicians caring for children with NI hospitalized with ARI may find it difficult to determine a child’s discharge readiness, in part because many children with NI have longer times to return to respiratory baseline and some never return to their pre-illness baseline.24 In otherwise healthy children hospitalized with respiratory infections such as pneumonia, discharge criteria typically include complete wean from respiratory support prior to discharge.4,25 In our study’s more complex children, whose parents already manage respiratory support at home, we hypothesize that discharging providers may be comfortable with discharge when the child has certain types of increased respiratory support compatible with home equipment, a parent skilled with monitoring the child’s respiratory status, and the support of an experienced outpatient provider and home nursing providers. At our institution, outpatient respiratory support weans are primarily performed by pediatric pulmonologists and, for isolated weaning of supplemental oxygen or time using support, by parents and outpatient pediatricians.
Another important factor in determining a child’s discharge readiness is the perspective of the child’s parent. Berry et al found that children whose parents believe they are not healthy enough for discharge are more likely to experience unplanned hospital readmissions,24 signaling the role of child- and family-specific factors in safe discharge decisions. Therefore, parents of children with NI should be proactively involved throughout the multidisciplinary discharge process,26,27 including the decision to discharge before return to respiratory baseline. Parents have identified ongoing provider support, opportunities to practice home care skills, and written instructions with contingency plans as important components of discharge readiness.28 Further work to create partnerships with these highly skilled caregivers in discharge decision making and transition planning are needed to promote safe discharge practices in this complex population.
In our study, children discharged before return to respiratory baseline were more likely to be older and privately insured compared with children discharged at respiratory baseline. Prior studies have found that social factors including low socioeconomic status influence ED provider admissions decisions for children with pneumonia.29,30 However, the role of socioeconomic factors in provider discharge decisions for children with NI has not been assessed. These traits may also be proxy markers of other sociodemographic factors, such as parent education level, financial hardship influencing ability to participate in a child’s care at the bedside, access to comprehensive outpatient primary care, and availability of private home nursing. We hypothesize that these related characteristics directly and indirectly influence provider discharge decisions.
Discharging providers are likely more comfortable with discharge prior to return to respiratory baseline when the family has private duty nursing in the home. Home nurses can assist families in providing increased respiratory support and recognizing respiratory problems that may arise following discharge. However, home nursing shortages are common nationwide.31,32 Low-income children, children with respiratory technology use, and children without Medicaid have been found to have larger gaps in home nursing availability.31,32 Further studies are needed to understand the role of home nursing availability in provider discharge decisions in this population.
This study has several limitations. The retrospective design of this study creates the potential for residual confounding; there may be other clinical or demographic factors influencing hospital discharge decisions that we are unable to capture using EMR review, including parental knowledge and comfort managing illness, quality of discharge instructions, frequency of follow-up visits, and presence of skilled home nursing services. Categorization of children based on respiratory support status at discharge lends potential for misclassification of exposure; however, our substantial interrater reliability suggests that misclassification bias is small. This study’s primary finding indicated no difference between exposure groups; although we may be unable to detect small differences, we had sufficient power with our sample size to detect meaningful differences in reutilization outcomes.
This study was not designed to capture outpatient time to return to respiratory baseline; prospective studies are needed to identify rates of return to respiratory baseline following ARI in children with NI. We did not measure the level of respiratory support used by children at the time of discharge and, therefore, are unable to estimate the amount of respiratory support weaning needed following discharge or the compatibility of support with home equipment using our data. In addition, this study focused on respiratory support modalities and, thus, did not measure inpatient utilization of mucociliary clearance technologies that might be hypothesized to decrease the time to return to baseline respiratory support. Next steps in evaluating treatment of ARI include investigating the effect of mucociliary clearance on both exposure and outcome in this population.
There may be other clinically meaningful outcomes for this population apart from reutilization that we have not assessed in this study, including increased respiratory support required following discharge, primary care reutilization, healthcare costs, or parent satisfaction with timing of and outcomes after discharge. Finally, although our hospital has reutilization rates for children with NI that are similar to other institutions in the United States,33 our results may not be generalizable to children with NI hospitalized at other institutions because local discharge processes and systems of care may be different. Prospective, multicenter investigation is needed to evaluate the clinical consequences of discharge before return to respiratory baseline more broadly.
CONCLUSION
At our institution, approximately one-quarter of children with NI hospitalized with ARI were discharged before return to respiratory baseline, but these children were not at increased risk of reutilization, compared with children discharged at respiratory baseline. Our findings suggest that return to baseline respiratory support might not be a necessary component of hospital discharge criteria. In otherwise clinically stable children with NI, discharge before return to respiratory baseline may be reasonable if their parents are comfortable managing respiratory support at home.
Acknowledgments
The authors thank Jonathan Rodean of the Children’s Hospital Association for his assistance with abstraction of PHIS data.
1. Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. https://doi.org/10.1371/journal.pmed.1001158.
2. Srivastava R, Jackson WD, Barnhart DC. Dysphagia and gastroesophageal reflux disease: dilemmas in diagnosis and management in children with neurological impairment. Pediatr Ann. 2010;39(4):225-231. https://doi.org/10.3928/00904481-20100318-07.
3. Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88(1):75-78. https://doi.org/10.1136/adc.88.1.75.
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556.
5. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33(9):907-911. https://doi.org/10.1097/INF.0000000000000317.
6. Stagliano DR, Nylund CM, Eide MB, Eberly MD. Children with Down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr. 2015;166(3):703-709.e702. https://doi.org/10.1016/j.jpeds.2014.11.058.
7. Kaiser SV, Bakel LA, Okumura MJ, Auerbach AD, Rosenthal J, Cabana MD. Risk factors for prolonged length of stay or complications during pediatric respiratory hospitalizations. Hosp Pediatr. 2015;5(9):461-473. https://doi.org/10.1542/hpeds.2014-0246.
8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Thomson JE, Feinstein JA, Hall M, Gay JC, Butts B, Berry JG. Identification of children with high-intensity neurological impairment. JAMA Pediatr. 2019. https://doi.org/10.1001/jamapediatrics.2019.2672.
11. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington state, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209.
12. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):e99. https://doi.org/10.1542/peds.107.6.e99.
13. Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD.
14. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org//10.1186/1471-2431-14-199.
15. Berry JG HD, Kuo DZ, Cohen E, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8. https://doi.org/10.1186/1471-2431-5-8.
17. Balamuth F, Weiss SL, Hall M, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167(6):1295-1300.e4. https://doi.org/10.1016/j.jpeds.2015.09.027.
18. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983.
19. Qian X, Russell LB, Valiyeva E, Miller JE. “Quicker and sicker” under Medicare’s prospective payment system for hospitals: new evidence on an old issue from a national longitudinal survey. Bull Econ Res. 2011;63(1):1-27. https://doi.org/10.1111/j.1467-8586.2010.00369.x.
20. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279(18):1452-1457. https://doi.org/10.1001/jama.279.18.1452.
21. Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278-1284. https://doi.org/10.1001/archinte.162.11.1278.
22. Wolf RB, Edwards K, Grijalva CG, et al. Time to clinical stability among children hospitalized with pneumonia. J Hosp Med. 2015;10(6):380-383. https://doi.org/10.1002/jhm.2370.
23. Capelastegui A, España PP, Bilbao A, et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595-600. https://doi.org/10.1378/chest.07-3039.
24. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. https://doi.org/10.1093/intqhc/mzt051.
25. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531.
26. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832.
27. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5(4):219-231. https://doi.org10.1542/hpeds.2014-0097.
28. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
29. Agha MM, Glazier RH, Guttmann A. Relationship between social inequalities and ambulatory care-sensitive hospitalizations persists for up to 9 years among children born in a major Canadian urban center. Ambul Pediatr. 2007;7(3):258-262. https://doi.org/10.1016/j.ambp.2007.02.005.
30. Flores G, Abreu M, Chaisson CE, Sun D. Keeping children out of hospitals: parents’ and physicians’ perspectives on how pediatric hospitalizations for ambulatory care-sensitive conditions can be avoided. Pediatrics. 2003;112(5):1021-1030. https://doi.org/10.1542/peds.112.5.1021.
31. Weaver MS, Wichman B, Bace S, et al. Measuring the impact of the home health nursing shortage on family caregivers of children receiving palliative care. J Hosp Palliat Nurs. 2018;20(3):260-265. https://doi.org/10.1097/NJH.0000000000000436.
32. Leonard BJ, Brust JD, Sielaff BH. Determinants of home care nursing hours for technology-assisted children. Public Health Nurs. 1991;8(4):239-244. https://doi.org/10.1111/j.1525-1446.1991.tb00663.x.
33. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-1470. https://doi.org/10.1542/peds.2012-0175.
Children with neurologic impairment (NI; eg, hypoxic-ischemic encephalopathy, muscular dystrophy) are characterized by functional and/or intellectual impairments resulting from a variety of neurologic diseases.1 These children commonly have respiratory comorbidities, including central hypoventilation, impaired cough, and oromotor dysfunction, that may lead to chronic respiratory insufficiency and a need for chronic respiratory support at baseline.2,3 Baseline respiratory support modalities can include supplemental oxygen, noninvasive positive pressure ventilation, or invasive mechanical ventilation.
Acute respiratory infections (ARI; eg, pneumonia, bronchiolitis) are the most common cause of hospitalization, intensive care unit (ICU) admission, and death for children with NI.1,3 Discharge criteria for otherwise healthy children admitted to the hospital with ARI often include return to respiratory baseline.4 Children with complex chronic conditions have longer hospitalizations when hospitalized with respiratory infections,5-7 because, in part, comorbidities and complications prolong the time to return to baseline. This prolonged return to respiratory baseline in combination with family knowledge, comfort, and skill in managing respiratory support and other complexities at home may alter discharge practices in the population of children with NI. In our clinical experience, discharge before return to baseline respiratory support occurs more frequently in children with NI than in otherwise healthy children when hospitalized with ARI. However, the consequences of discharging children with NI prior to return to respiratory baseline are unknown.
In this study, we sought to determine if discharge prior to return to baseline respiratory support is associated with reutilization among children with NI hospitalized with ARI. We hypothesized that patients discharged prior to return to respiratory baseline would have higher rates of 30-day hospital reutilization.
METHODS
Study Design and Data Source
This single-center, retrospective cohort study of children hospitalized at Cincinnati Children’s Hospital Medical Center (CCHMC) used data from the Pediatric Health Information System (PHIS) and the electronic medical record (EMR). PHIS, an administrative database of 45 not-for-profit, tertiary care, US pediatric hospitals managed by Children’s Hospital Association (Lenexa, Kansas), was used to identify eligible children, examine demographic and clinical variables, and define outcomes. PHIS contains data regarding patient demographics, inpatient resource utilization, and diagnoses. Encrypted medical record numbers in PHIS allowed for local identification of patients’ medical records to complete EMR review to confirm eligibility and obtain detailed patient-level clinical information (eg, respiratory support needs) not available in PHIS.
Pilot medical record reviews allowed for standardized study definitions and procedures. All study staff underwent training with the primary investigator, including detailed review of 10 initial abstractions. Two investigators (K.M. and S.C.) performed repeat abstractions from 40 randomly selected records to enable assessment of interrater reliability. Average reliability, indicated by the κ statistic, indicated substantial to near-perfect reliability8 (κ = 0.97, 95% CI 0.90-1.00) for the primary exposure. EMR data were managed using Research Electronic Data Capture (REDCap, Nashville, Tennessee)9 and subsequently merged with PHIS data.
Study Population
Hospitalizations of children with NI aged 1 to 18 years at CCHMC between January 2010 and September 2015 were eligible for inclusion if they had a principal discharge diagnosis indicative of ARI and required increased respiratory support from baseline during hospitalization. NI was defined as a high-intensity, chronic neurological diagnosis with substantial functional impairments according to previously defined diagnosis codes.1,10 ARI was identified using codes in the Clinical Classification Software (Agency for Healthcare Research and Quality, Rockville, MD) respiratory group indicative of ARI (eg, pneumonia, bronchiolitis, influenza; Appendix Table).
Children transferred to CCHMC were excluded because records from their initial illness presentation and management were not available. Because of expected differences in management and outcomes, children with a known diagnosis of tuberculosis or human immunodeficiency virus were excluded. Because exposure criteria were dependent on hospital discharge status, hospitalizations for children who died during admission (4 of 632 hospitalizations, 0.63%) were excluded from the final cohort (Appendix Figure).
Study Definitions
Baseline respiratory support (ie, “respiratory baseline”) was defined as the child’s highest level of respiratory support needed prior to admission when well (ie, no support, supplemental oxygen, continuous positive airway pressure [CPAP] or bilevel positive airway pressure [BiPAP], or ventilator support), and further characterized by night or day/night requirement. Respiratory baseline was identified using EMR documentation of home respiratory support use at the time of index admission. Return to respiratory baseline was defined as the date on which the child achieved documented home respiratory support settings, regardless of clinical symptoms.
Children may have required increased respiratory support from baseline at any time during hospitalization. Maximum respiratory support required was categorized as one of the following: (1) initiation of supplemental oxygen or increase in oxygen flow or duration; (2) initiation of CPAP or BiPAP; (3) increase in pressure settings or duration of pressure support for those with baseline CPAP, BiPAP, or ventilator use; and (4) initiation of full mechanical ventilation. Respiratory support categories were mutually exclusive: children requiring multiple types of increased respiratory support were classified for analysis by the most invasive form of respiratory support used (eg, a child requiring increase in both oxygen flow and pressure settings was categorized as an increase in pressure settings). Children who received heated high-flow nasal cannula therapy as maximum support were categorized as initiation or increase in oxygen support.
Time to return to respiratory baseline was defined as the difference in days between date of return to respiratory baseline and date of admission. Time to return to respiratory baseline was determined only for children who were discharged at respiratory baseline.
Primary Exposure and Outcome Measures
The primary exposure was hospital discharge before return to respiratory baseline (ie, discharge on higher respiratory support than at baseline settings). At our institution, standardized discharge criteria for children with NI do not exist. The primary outcome was all-cause, 30-day hospital reutilization, including hospital readmissions and emergency department (ED) revisits. Secondary outcomes included 30-day reutilization for ARI and hospital length of stay (LOS) in days.
Patient Demographics and Clinical Characteristics
Demographic and patient characteristics that might influence hospital discharge before return to respiratory baseline or readmission were obtained from PHIS (eg, demographic information, age, insurance type, measures of clinical complexity, illness severity) and by EMR review (eg, baseline respiratory support needs, maximum respiratory support during hospitalization). Measures of clinical complexity included comorbid complex chronic conditions (CCCs)11-14 and technology dependence14-16 using previously defined diagnostic codes. Measures of illness severity included sepsis17 and ICU-level care. At our institution, children with baseline ventilator use do not require admission to the ICU unless they are clinically unstable.
Statistical Analysis
Continuous variables were described using medians and interquartile ranges (IQR). Categorical variables were described using counts and percentages. Patient characteristics and outcomes were stratified by primary exposure and compared using chi-square test or Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
To examine the independent association between discharge before return to respiratory baseline and hospital reutilization, a generalized estimating equation was used that included potential confounders while accounting for within-patient clustering. Patient demographics included age, race, ethnicity, and insurance type; measures of clinical complexity included number of CCCs, technology dependence, and baseline respiratory support; and measures of acute illness severity included ARI diagnosis, degree of increase in respiratory support during hospitalization, and ICU-level care. LOS was also included in the model as a covariate because of its expected association with both exposure and outcome.
Secondary analyses were conducted using the outcome of 30-day reutilization for ARI. Subgroup analysis excluding hospitalizations of children lost to follow-up (ie, no encounters in the 6 months after hospital discharge) was also conducted. All analyses were performed with SAS v9.3 (SAS Institute, Cary, North Carolina). P values < .05 were considered statistically significant. This study was approved by the Institutional Review Board.
RESULTS
Study Cohort
A total of 632 hospitalizations experienced by 366 children with NI who were admitted with ARI were included (Appendix Figure). Most children (66.4%) in the cohort experienced only one hospitalization, 17.5% had two hospitalizations, 7.9% had three hospitalizations, and 8.2% had four or more hospitalizations. The median age at hospitalization was 5.0 years (IQR 2.8-10.5) and most hospitalizations were for children who were male (56.6%), white (78.3%), non-Hispanic (96.0%), and publicly insured (51.7%; Table 1). More than one-quarter (28.6%) of hospitalizations were for children with four or more CCCs, and in 73.4% of hospitalizations, children were technology dependent (Table 1). Baseline respiratory support was common (46.8%), including home mechanical ventilation in 11.1% of hospitalizations (Table 1). Bacterial pneumonia, including aspiration pneumonia, was the most common discharge diagnosis (50.5%, Table 1).

Demographic and Clinical Characteristics
Children were discharged before return to respiratory baseline in 30.4% of hospitalizations (Appendix Figure). Children discharged before return to respiratory baseline were older (median age 5.7 years, IQR 3.1-11.0, vs 4.9 years, IQR 2.6-9.7; P = .04) and more likely to be privately insured (54.2% vs 43.4%; P = .04), compared with children discharged at respiratory baseline (Table 1). Children discharged before return to respiratory baseline were also more likely to have a respiratory CCC (59.9% vs 30.9%; P < .001), have a respiratory technology dependence diagnosis code (44.8% vs 24.1%; P < .001), and have baseline respiratory support needs on EMR review (67.7% vs 37.7%; P < .001), compared with children discharged at baseline (Table 1).
Children discharged before return to respiratory baseline required significantly greater escalation in respiratory support during hospitalization, compared with children discharged at respiratory baseline, including higher rates of initiation of CPAP or BiPAP, increased pressure settings from baseline (for home CPAP, BiPAP, or ventilator users), and initiation of full mechanical ventilation (Table 1). Hospitalizations in which children were discharged before return to respiratory baseline were also more likely to include ICU care than were those for children discharged at baseline (52.1% vs 35.2%; P < .001; Table 1).
Clinical Outcomes and Utilization
Reutilization within 30 days occurred after 32.1% of hospitalizations, with 26.1% requiring hospital readmission and 6.0% requiring ED revisit (Table 2). There was no statistical association in either unadjusted (Table 2) or adjusted (Table 3) analysis between children discharged before return to respiratory baseline and 30-day all-cause hospital reutilizations, hospital readmissions, or ED revisits.

In analysis of secondary outcomes, 30-day reutilization because of ARI occurred after 21.5% of hospitalizations, with 19.0% requiring hospital readmission and 2.5% requiring ED revisit. Median hospital LOS for the cohort was 4 days (IQR 2-8; Table 2). Hospitalizations in which children were discharged before return to respiratory baseline were longer than in those discharged at baseline (median 6 days, IQR 3-11, vs 4 days, IQR 2-7; P < .001; Table 2).

For hospitalizations of children discharged at respiratory baseline, the median time to return to respiratory baseline was 3 days (IQR 1-5, complete range 0-80). In these encounters, discharge occurred soon after return to respiratory baseline (median 1 day, IQR 0-1.5, complete range 0-54).
In subgroup analysis excluding the 18 hospitalizations in which children were lost to follow-up (2.8% of the total cohort), discharge before return to respiratory baseline was not associated with 30-day all-cause hospital reutilization (Table 4).

DISCUSSION
In this retrospective cohort study, children with NI hospitalized with ARI were frequently discharged using increased respiratory support from baseline. However, those discharged before return to respiratory baseline, despite their greater clinical complexity and acute illness severity, did not have increased hospital reutilization, compared with children discharged at respiratory baseline. Our findings suggest that discharge before return to baseline respiratory support after ARI may be clinically appropriate in some children with NI.
With the growing emphasis on decreasing hospital costs, concern exists that patients are being discharged from hospitals “quicker and sicker,”18,19 with shortening lengths of stay and higher patient instability at discharge. Clinical instability at discharge has been associated with adverse postdischarge outcomes in adults with pneumonia20-23; however, studies evaluating discharge readiness have not examined the population of children with NI. Our findings of no difference in reutilization for children with NI discharged before return to respiratory baseline, which would be expected to approximate one or more clinical instabilities, contrast these concerns.
Clinicians caring for children with NI hospitalized with ARI may find it difficult to determine a child’s discharge readiness, in part because many children with NI have longer times to return to respiratory baseline and some never return to their pre-illness baseline.24 In otherwise healthy children hospitalized with respiratory infections such as pneumonia, discharge criteria typically include complete wean from respiratory support prior to discharge.4,25 In our study’s more complex children, whose parents already manage respiratory support at home, we hypothesize that discharging providers may be comfortable with discharge when the child has certain types of increased respiratory support compatible with home equipment, a parent skilled with monitoring the child’s respiratory status, and the support of an experienced outpatient provider and home nursing providers. At our institution, outpatient respiratory support weans are primarily performed by pediatric pulmonologists and, for isolated weaning of supplemental oxygen or time using support, by parents and outpatient pediatricians.
Another important factor in determining a child’s discharge readiness is the perspective of the child’s parent. Berry et al found that children whose parents believe they are not healthy enough for discharge are more likely to experience unplanned hospital readmissions,24 signaling the role of child- and family-specific factors in safe discharge decisions. Therefore, parents of children with NI should be proactively involved throughout the multidisciplinary discharge process,26,27 including the decision to discharge before return to respiratory baseline. Parents have identified ongoing provider support, opportunities to practice home care skills, and written instructions with contingency plans as important components of discharge readiness.28 Further work to create partnerships with these highly skilled caregivers in discharge decision making and transition planning are needed to promote safe discharge practices in this complex population.
In our study, children discharged before return to respiratory baseline were more likely to be older and privately insured compared with children discharged at respiratory baseline. Prior studies have found that social factors including low socioeconomic status influence ED provider admissions decisions for children with pneumonia.29,30 However, the role of socioeconomic factors in provider discharge decisions for children with NI has not been assessed. These traits may also be proxy markers of other sociodemographic factors, such as parent education level, financial hardship influencing ability to participate in a child’s care at the bedside, access to comprehensive outpatient primary care, and availability of private home nursing. We hypothesize that these related characteristics directly and indirectly influence provider discharge decisions.
Discharging providers are likely more comfortable with discharge prior to return to respiratory baseline when the family has private duty nursing in the home. Home nurses can assist families in providing increased respiratory support and recognizing respiratory problems that may arise following discharge. However, home nursing shortages are common nationwide.31,32 Low-income children, children with respiratory technology use, and children without Medicaid have been found to have larger gaps in home nursing availability.31,32 Further studies are needed to understand the role of home nursing availability in provider discharge decisions in this population.
This study has several limitations. The retrospective design of this study creates the potential for residual confounding; there may be other clinical or demographic factors influencing hospital discharge decisions that we are unable to capture using EMR review, including parental knowledge and comfort managing illness, quality of discharge instructions, frequency of follow-up visits, and presence of skilled home nursing services. Categorization of children based on respiratory support status at discharge lends potential for misclassification of exposure; however, our substantial interrater reliability suggests that misclassification bias is small. This study’s primary finding indicated no difference between exposure groups; although we may be unable to detect small differences, we had sufficient power with our sample size to detect meaningful differences in reutilization outcomes.
This study was not designed to capture outpatient time to return to respiratory baseline; prospective studies are needed to identify rates of return to respiratory baseline following ARI in children with NI. We did not measure the level of respiratory support used by children at the time of discharge and, therefore, are unable to estimate the amount of respiratory support weaning needed following discharge or the compatibility of support with home equipment using our data. In addition, this study focused on respiratory support modalities and, thus, did not measure inpatient utilization of mucociliary clearance technologies that might be hypothesized to decrease the time to return to baseline respiratory support. Next steps in evaluating treatment of ARI include investigating the effect of mucociliary clearance on both exposure and outcome in this population.
There may be other clinically meaningful outcomes for this population apart from reutilization that we have not assessed in this study, including increased respiratory support required following discharge, primary care reutilization, healthcare costs, or parent satisfaction with timing of and outcomes after discharge. Finally, although our hospital has reutilization rates for children with NI that are similar to other institutions in the United States,33 our results may not be generalizable to children with NI hospitalized at other institutions because local discharge processes and systems of care may be different. Prospective, multicenter investigation is needed to evaluate the clinical consequences of discharge before return to respiratory baseline more broadly.
CONCLUSION
At our institution, approximately one-quarter of children with NI hospitalized with ARI were discharged before return to respiratory baseline, but these children were not at increased risk of reutilization, compared with children discharged at respiratory baseline. Our findings suggest that return to baseline respiratory support might not be a necessary component of hospital discharge criteria. In otherwise clinically stable children with NI, discharge before return to respiratory baseline may be reasonable if their parents are comfortable managing respiratory support at home.
Acknowledgments
The authors thank Jonathan Rodean of the Children’s Hospital Association for his assistance with abstraction of PHIS data.
Children with neurologic impairment (NI; eg, hypoxic-ischemic encephalopathy, muscular dystrophy) are characterized by functional and/or intellectual impairments resulting from a variety of neurologic diseases.1 These children commonly have respiratory comorbidities, including central hypoventilation, impaired cough, and oromotor dysfunction, that may lead to chronic respiratory insufficiency and a need for chronic respiratory support at baseline.2,3 Baseline respiratory support modalities can include supplemental oxygen, noninvasive positive pressure ventilation, or invasive mechanical ventilation.
Acute respiratory infections (ARI; eg, pneumonia, bronchiolitis) are the most common cause of hospitalization, intensive care unit (ICU) admission, and death for children with NI.1,3 Discharge criteria for otherwise healthy children admitted to the hospital with ARI often include return to respiratory baseline.4 Children with complex chronic conditions have longer hospitalizations when hospitalized with respiratory infections,5-7 because, in part, comorbidities and complications prolong the time to return to baseline. This prolonged return to respiratory baseline in combination with family knowledge, comfort, and skill in managing respiratory support and other complexities at home may alter discharge practices in the population of children with NI. In our clinical experience, discharge before return to baseline respiratory support occurs more frequently in children with NI than in otherwise healthy children when hospitalized with ARI. However, the consequences of discharging children with NI prior to return to respiratory baseline are unknown.
In this study, we sought to determine if discharge prior to return to baseline respiratory support is associated with reutilization among children with NI hospitalized with ARI. We hypothesized that patients discharged prior to return to respiratory baseline would have higher rates of 30-day hospital reutilization.
METHODS
Study Design and Data Source
This single-center, retrospective cohort study of children hospitalized at Cincinnati Children’s Hospital Medical Center (CCHMC) used data from the Pediatric Health Information System (PHIS) and the electronic medical record (EMR). PHIS, an administrative database of 45 not-for-profit, tertiary care, US pediatric hospitals managed by Children’s Hospital Association (Lenexa, Kansas), was used to identify eligible children, examine demographic and clinical variables, and define outcomes. PHIS contains data regarding patient demographics, inpatient resource utilization, and diagnoses. Encrypted medical record numbers in PHIS allowed for local identification of patients’ medical records to complete EMR review to confirm eligibility and obtain detailed patient-level clinical information (eg, respiratory support needs) not available in PHIS.
Pilot medical record reviews allowed for standardized study definitions and procedures. All study staff underwent training with the primary investigator, including detailed review of 10 initial abstractions. Two investigators (K.M. and S.C.) performed repeat abstractions from 40 randomly selected records to enable assessment of interrater reliability. Average reliability, indicated by the κ statistic, indicated substantial to near-perfect reliability8 (κ = 0.97, 95% CI 0.90-1.00) for the primary exposure. EMR data were managed using Research Electronic Data Capture (REDCap, Nashville, Tennessee)9 and subsequently merged with PHIS data.
Study Population
Hospitalizations of children with NI aged 1 to 18 years at CCHMC between January 2010 and September 2015 were eligible for inclusion if they had a principal discharge diagnosis indicative of ARI and required increased respiratory support from baseline during hospitalization. NI was defined as a high-intensity, chronic neurological diagnosis with substantial functional impairments according to previously defined diagnosis codes.1,10 ARI was identified using codes in the Clinical Classification Software (Agency for Healthcare Research and Quality, Rockville, MD) respiratory group indicative of ARI (eg, pneumonia, bronchiolitis, influenza; Appendix Table).
Children transferred to CCHMC were excluded because records from their initial illness presentation and management were not available. Because of expected differences in management and outcomes, children with a known diagnosis of tuberculosis or human immunodeficiency virus were excluded. Because exposure criteria were dependent on hospital discharge status, hospitalizations for children who died during admission (4 of 632 hospitalizations, 0.63%) were excluded from the final cohort (Appendix Figure).
Study Definitions
Baseline respiratory support (ie, “respiratory baseline”) was defined as the child’s highest level of respiratory support needed prior to admission when well (ie, no support, supplemental oxygen, continuous positive airway pressure [CPAP] or bilevel positive airway pressure [BiPAP], or ventilator support), and further characterized by night or day/night requirement. Respiratory baseline was identified using EMR documentation of home respiratory support use at the time of index admission. Return to respiratory baseline was defined as the date on which the child achieved documented home respiratory support settings, regardless of clinical symptoms.
Children may have required increased respiratory support from baseline at any time during hospitalization. Maximum respiratory support required was categorized as one of the following: (1) initiation of supplemental oxygen or increase in oxygen flow or duration; (2) initiation of CPAP or BiPAP; (3) increase in pressure settings or duration of pressure support for those with baseline CPAP, BiPAP, or ventilator use; and (4) initiation of full mechanical ventilation. Respiratory support categories were mutually exclusive: children requiring multiple types of increased respiratory support were classified for analysis by the most invasive form of respiratory support used (eg, a child requiring increase in both oxygen flow and pressure settings was categorized as an increase in pressure settings). Children who received heated high-flow nasal cannula therapy as maximum support were categorized as initiation or increase in oxygen support.
Time to return to respiratory baseline was defined as the difference in days between date of return to respiratory baseline and date of admission. Time to return to respiratory baseline was determined only for children who were discharged at respiratory baseline.
Primary Exposure and Outcome Measures
The primary exposure was hospital discharge before return to respiratory baseline (ie, discharge on higher respiratory support than at baseline settings). At our institution, standardized discharge criteria for children with NI do not exist. The primary outcome was all-cause, 30-day hospital reutilization, including hospital readmissions and emergency department (ED) revisits. Secondary outcomes included 30-day reutilization for ARI and hospital length of stay (LOS) in days.
Patient Demographics and Clinical Characteristics
Demographic and patient characteristics that might influence hospital discharge before return to respiratory baseline or readmission were obtained from PHIS (eg, demographic information, age, insurance type, measures of clinical complexity, illness severity) and by EMR review (eg, baseline respiratory support needs, maximum respiratory support during hospitalization). Measures of clinical complexity included comorbid complex chronic conditions (CCCs)11-14 and technology dependence14-16 using previously defined diagnostic codes. Measures of illness severity included sepsis17 and ICU-level care. At our institution, children with baseline ventilator use do not require admission to the ICU unless they are clinically unstable.
Statistical Analysis
Continuous variables were described using medians and interquartile ranges (IQR). Categorical variables were described using counts and percentages. Patient characteristics and outcomes were stratified by primary exposure and compared using chi-square test or Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
To examine the independent association between discharge before return to respiratory baseline and hospital reutilization, a generalized estimating equation was used that included potential confounders while accounting for within-patient clustering. Patient demographics included age, race, ethnicity, and insurance type; measures of clinical complexity included number of CCCs, technology dependence, and baseline respiratory support; and measures of acute illness severity included ARI diagnosis, degree of increase in respiratory support during hospitalization, and ICU-level care. LOS was also included in the model as a covariate because of its expected association with both exposure and outcome.
Secondary analyses were conducted using the outcome of 30-day reutilization for ARI. Subgroup analysis excluding hospitalizations of children lost to follow-up (ie, no encounters in the 6 months after hospital discharge) was also conducted. All analyses were performed with SAS v9.3 (SAS Institute, Cary, North Carolina). P values < .05 were considered statistically significant. This study was approved by the Institutional Review Board.
RESULTS
Study Cohort
A total of 632 hospitalizations experienced by 366 children with NI who were admitted with ARI were included (Appendix Figure). Most children (66.4%) in the cohort experienced only one hospitalization, 17.5% had two hospitalizations, 7.9% had three hospitalizations, and 8.2% had four or more hospitalizations. The median age at hospitalization was 5.0 years (IQR 2.8-10.5) and most hospitalizations were for children who were male (56.6%), white (78.3%), non-Hispanic (96.0%), and publicly insured (51.7%; Table 1). More than one-quarter (28.6%) of hospitalizations were for children with four or more CCCs, and in 73.4% of hospitalizations, children were technology dependent (Table 1). Baseline respiratory support was common (46.8%), including home mechanical ventilation in 11.1% of hospitalizations (Table 1). Bacterial pneumonia, including aspiration pneumonia, was the most common discharge diagnosis (50.5%, Table 1).

Demographic and Clinical Characteristics
Children were discharged before return to respiratory baseline in 30.4% of hospitalizations (Appendix Figure). Children discharged before return to respiratory baseline were older (median age 5.7 years, IQR 3.1-11.0, vs 4.9 years, IQR 2.6-9.7; P = .04) and more likely to be privately insured (54.2% vs 43.4%; P = .04), compared with children discharged at respiratory baseline (Table 1). Children discharged before return to respiratory baseline were also more likely to have a respiratory CCC (59.9% vs 30.9%; P < .001), have a respiratory technology dependence diagnosis code (44.8% vs 24.1%; P < .001), and have baseline respiratory support needs on EMR review (67.7% vs 37.7%; P < .001), compared with children discharged at baseline (Table 1).
Children discharged before return to respiratory baseline required significantly greater escalation in respiratory support during hospitalization, compared with children discharged at respiratory baseline, including higher rates of initiation of CPAP or BiPAP, increased pressure settings from baseline (for home CPAP, BiPAP, or ventilator users), and initiation of full mechanical ventilation (Table 1). Hospitalizations in which children were discharged before return to respiratory baseline were also more likely to include ICU care than were those for children discharged at baseline (52.1% vs 35.2%; P < .001; Table 1).
Clinical Outcomes and Utilization
Reutilization within 30 days occurred after 32.1% of hospitalizations, with 26.1% requiring hospital readmission and 6.0% requiring ED revisit (Table 2). There was no statistical association in either unadjusted (Table 2) or adjusted (Table 3) analysis between children discharged before return to respiratory baseline and 30-day all-cause hospital reutilizations, hospital readmissions, or ED revisits.

In analysis of secondary outcomes, 30-day reutilization because of ARI occurred after 21.5% of hospitalizations, with 19.0% requiring hospital readmission and 2.5% requiring ED revisit. Median hospital LOS for the cohort was 4 days (IQR 2-8; Table 2). Hospitalizations in which children were discharged before return to respiratory baseline were longer than in those discharged at baseline (median 6 days, IQR 3-11, vs 4 days, IQR 2-7; P < .001; Table 2).

For hospitalizations of children discharged at respiratory baseline, the median time to return to respiratory baseline was 3 days (IQR 1-5, complete range 0-80). In these encounters, discharge occurred soon after return to respiratory baseline (median 1 day, IQR 0-1.5, complete range 0-54).
In subgroup analysis excluding the 18 hospitalizations in which children were lost to follow-up (2.8% of the total cohort), discharge before return to respiratory baseline was not associated with 30-day all-cause hospital reutilization (Table 4).

DISCUSSION
In this retrospective cohort study, children with NI hospitalized with ARI were frequently discharged using increased respiratory support from baseline. However, those discharged before return to respiratory baseline, despite their greater clinical complexity and acute illness severity, did not have increased hospital reutilization, compared with children discharged at respiratory baseline. Our findings suggest that discharge before return to baseline respiratory support after ARI may be clinically appropriate in some children with NI.
With the growing emphasis on decreasing hospital costs, concern exists that patients are being discharged from hospitals “quicker and sicker,”18,19 with shortening lengths of stay and higher patient instability at discharge. Clinical instability at discharge has been associated with adverse postdischarge outcomes in adults with pneumonia20-23; however, studies evaluating discharge readiness have not examined the population of children with NI. Our findings of no difference in reutilization for children with NI discharged before return to respiratory baseline, which would be expected to approximate one or more clinical instabilities, contrast these concerns.
Clinicians caring for children with NI hospitalized with ARI may find it difficult to determine a child’s discharge readiness, in part because many children with NI have longer times to return to respiratory baseline and some never return to their pre-illness baseline.24 In otherwise healthy children hospitalized with respiratory infections such as pneumonia, discharge criteria typically include complete wean from respiratory support prior to discharge.4,25 In our study’s more complex children, whose parents already manage respiratory support at home, we hypothesize that discharging providers may be comfortable with discharge when the child has certain types of increased respiratory support compatible with home equipment, a parent skilled with monitoring the child’s respiratory status, and the support of an experienced outpatient provider and home nursing providers. At our institution, outpatient respiratory support weans are primarily performed by pediatric pulmonologists and, for isolated weaning of supplemental oxygen or time using support, by parents and outpatient pediatricians.
Another important factor in determining a child’s discharge readiness is the perspective of the child’s parent. Berry et al found that children whose parents believe they are not healthy enough for discharge are more likely to experience unplanned hospital readmissions,24 signaling the role of child- and family-specific factors in safe discharge decisions. Therefore, parents of children with NI should be proactively involved throughout the multidisciplinary discharge process,26,27 including the decision to discharge before return to respiratory baseline. Parents have identified ongoing provider support, opportunities to practice home care skills, and written instructions with contingency plans as important components of discharge readiness.28 Further work to create partnerships with these highly skilled caregivers in discharge decision making and transition planning are needed to promote safe discharge practices in this complex population.
In our study, children discharged before return to respiratory baseline were more likely to be older and privately insured compared with children discharged at respiratory baseline. Prior studies have found that social factors including low socioeconomic status influence ED provider admissions decisions for children with pneumonia.29,30 However, the role of socioeconomic factors in provider discharge decisions for children with NI has not been assessed. These traits may also be proxy markers of other sociodemographic factors, such as parent education level, financial hardship influencing ability to participate in a child’s care at the bedside, access to comprehensive outpatient primary care, and availability of private home nursing. We hypothesize that these related characteristics directly and indirectly influence provider discharge decisions.
Discharging providers are likely more comfortable with discharge prior to return to respiratory baseline when the family has private duty nursing in the home. Home nurses can assist families in providing increased respiratory support and recognizing respiratory problems that may arise following discharge. However, home nursing shortages are common nationwide.31,32 Low-income children, children with respiratory technology use, and children without Medicaid have been found to have larger gaps in home nursing availability.31,32 Further studies are needed to understand the role of home nursing availability in provider discharge decisions in this population.
This study has several limitations. The retrospective design of this study creates the potential for residual confounding; there may be other clinical or demographic factors influencing hospital discharge decisions that we are unable to capture using EMR review, including parental knowledge and comfort managing illness, quality of discharge instructions, frequency of follow-up visits, and presence of skilled home nursing services. Categorization of children based on respiratory support status at discharge lends potential for misclassification of exposure; however, our substantial interrater reliability suggests that misclassification bias is small. This study’s primary finding indicated no difference between exposure groups; although we may be unable to detect small differences, we had sufficient power with our sample size to detect meaningful differences in reutilization outcomes.
This study was not designed to capture outpatient time to return to respiratory baseline; prospective studies are needed to identify rates of return to respiratory baseline following ARI in children with NI. We did not measure the level of respiratory support used by children at the time of discharge and, therefore, are unable to estimate the amount of respiratory support weaning needed following discharge or the compatibility of support with home equipment using our data. In addition, this study focused on respiratory support modalities and, thus, did not measure inpatient utilization of mucociliary clearance technologies that might be hypothesized to decrease the time to return to baseline respiratory support. Next steps in evaluating treatment of ARI include investigating the effect of mucociliary clearance on both exposure and outcome in this population.
There may be other clinically meaningful outcomes for this population apart from reutilization that we have not assessed in this study, including increased respiratory support required following discharge, primary care reutilization, healthcare costs, or parent satisfaction with timing of and outcomes after discharge. Finally, although our hospital has reutilization rates for children with NI that are similar to other institutions in the United States,33 our results may not be generalizable to children with NI hospitalized at other institutions because local discharge processes and systems of care may be different. Prospective, multicenter investigation is needed to evaluate the clinical consequences of discharge before return to respiratory baseline more broadly.
CONCLUSION
At our institution, approximately one-quarter of children with NI hospitalized with ARI were discharged before return to respiratory baseline, but these children were not at increased risk of reutilization, compared with children discharged at respiratory baseline. Our findings suggest that return to baseline respiratory support might not be a necessary component of hospital discharge criteria. In otherwise clinically stable children with NI, discharge before return to respiratory baseline may be reasonable if their parents are comfortable managing respiratory support at home.
Acknowledgments
The authors thank Jonathan Rodean of the Children’s Hospital Association for his assistance with abstraction of PHIS data.
1. Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. https://doi.org/10.1371/journal.pmed.1001158.
2. Srivastava R, Jackson WD, Barnhart DC. Dysphagia and gastroesophageal reflux disease: dilemmas in diagnosis and management in children with neurological impairment. Pediatr Ann. 2010;39(4):225-231. https://doi.org/10.3928/00904481-20100318-07.
3. Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88(1):75-78. https://doi.org/10.1136/adc.88.1.75.
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556.
5. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33(9):907-911. https://doi.org/10.1097/INF.0000000000000317.
6. Stagliano DR, Nylund CM, Eide MB, Eberly MD. Children with Down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr. 2015;166(3):703-709.e702. https://doi.org/10.1016/j.jpeds.2014.11.058.
7. Kaiser SV, Bakel LA, Okumura MJ, Auerbach AD, Rosenthal J, Cabana MD. Risk factors for prolonged length of stay or complications during pediatric respiratory hospitalizations. Hosp Pediatr. 2015;5(9):461-473. https://doi.org/10.1542/hpeds.2014-0246.
8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Thomson JE, Feinstein JA, Hall M, Gay JC, Butts B, Berry JG. Identification of children with high-intensity neurological impairment. JAMA Pediatr. 2019. https://doi.org/10.1001/jamapediatrics.2019.2672.
11. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington state, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209.
12. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):e99. https://doi.org/10.1542/peds.107.6.e99.
13. Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD.
14. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org//10.1186/1471-2431-14-199.
15. Berry JG HD, Kuo DZ, Cohen E, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8. https://doi.org/10.1186/1471-2431-5-8.
17. Balamuth F, Weiss SL, Hall M, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167(6):1295-1300.e4. https://doi.org/10.1016/j.jpeds.2015.09.027.
18. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983.
19. Qian X, Russell LB, Valiyeva E, Miller JE. “Quicker and sicker” under Medicare’s prospective payment system for hospitals: new evidence on an old issue from a national longitudinal survey. Bull Econ Res. 2011;63(1):1-27. https://doi.org/10.1111/j.1467-8586.2010.00369.x.
20. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279(18):1452-1457. https://doi.org/10.1001/jama.279.18.1452.
21. Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278-1284. https://doi.org/10.1001/archinte.162.11.1278.
22. Wolf RB, Edwards K, Grijalva CG, et al. Time to clinical stability among children hospitalized with pneumonia. J Hosp Med. 2015;10(6):380-383. https://doi.org/10.1002/jhm.2370.
23. Capelastegui A, España PP, Bilbao A, et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595-600. https://doi.org/10.1378/chest.07-3039.
24. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. https://doi.org/10.1093/intqhc/mzt051.
25. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531.
26. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832.
27. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5(4):219-231. https://doi.org10.1542/hpeds.2014-0097.
28. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
29. Agha MM, Glazier RH, Guttmann A. Relationship between social inequalities and ambulatory care-sensitive hospitalizations persists for up to 9 years among children born in a major Canadian urban center. Ambul Pediatr. 2007;7(3):258-262. https://doi.org/10.1016/j.ambp.2007.02.005.
30. Flores G, Abreu M, Chaisson CE, Sun D. Keeping children out of hospitals: parents’ and physicians’ perspectives on how pediatric hospitalizations for ambulatory care-sensitive conditions can be avoided. Pediatrics. 2003;112(5):1021-1030. https://doi.org/10.1542/peds.112.5.1021.
31. Weaver MS, Wichman B, Bace S, et al. Measuring the impact of the home health nursing shortage on family caregivers of children receiving palliative care. J Hosp Palliat Nurs. 2018;20(3):260-265. https://doi.org/10.1097/NJH.0000000000000436.
32. Leonard BJ, Brust JD, Sielaff BH. Determinants of home care nursing hours for technology-assisted children. Public Health Nurs. 1991;8(4):239-244. https://doi.org/10.1111/j.1525-1446.1991.tb00663.x.
33. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-1470. https://doi.org/10.1542/peds.2012-0175.
1. Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. https://doi.org/10.1371/journal.pmed.1001158.
2. Srivastava R, Jackson WD, Barnhart DC. Dysphagia and gastroesophageal reflux disease: dilemmas in diagnosis and management in children with neurological impairment. Pediatr Ann. 2010;39(4):225-231. https://doi.org/10.3928/00904481-20100318-07.
3. Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88(1):75-78. https://doi.org/10.1136/adc.88.1.75.
4. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556.
5. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33(9):907-911. https://doi.org/10.1097/INF.0000000000000317.
6. Stagliano DR, Nylund CM, Eide MB, Eberly MD. Children with Down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr. 2015;166(3):703-709.e702. https://doi.org/10.1016/j.jpeds.2014.11.058.
7. Kaiser SV, Bakel LA, Okumura MJ, Auerbach AD, Rosenthal J, Cabana MD. Risk factors for prolonged length of stay or complications during pediatric respiratory hospitalizations. Hosp Pediatr. 2015;5(9):461-473. https://doi.org/10.1542/hpeds.2014-0246.
8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
10. Thomson JE, Feinstein JA, Hall M, Gay JC, Butts B, Berry JG. Identification of children with high-intensity neurological impairment. JAMA Pediatr. 2019. https://doi.org/10.1001/jamapediatrics.2019.2672.
11. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington state, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209.
12. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):e99. https://doi.org/10.1542/peds.107.6.e99.
13. Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD.
14. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org//10.1186/1471-2431-14-199.
15. Berry JG HD, Kuo DZ, Cohen E, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8. https://doi.org/10.1186/1471-2431-5-8.
17. Balamuth F, Weiss SL, Hall M, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167(6):1295-1300.e4. https://doi.org/10.1016/j.jpeds.2015.09.027.
18. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983.
19. Qian X, Russell LB, Valiyeva E, Miller JE. “Quicker and sicker” under Medicare’s prospective payment system for hospitals: new evidence on an old issue from a national longitudinal survey. Bull Econ Res. 2011;63(1):1-27. https://doi.org/10.1111/j.1467-8586.2010.00369.x.
20. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279(18):1452-1457. https://doi.org/10.1001/jama.279.18.1452.
21. Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278-1284. https://doi.org/10.1001/archinte.162.11.1278.
22. Wolf RB, Edwards K, Grijalva CG, et al. Time to clinical stability among children hospitalized with pneumonia. J Hosp Med. 2015;10(6):380-383. https://doi.org/10.1002/jhm.2370.
23. Capelastegui A, España PP, Bilbao A, et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595-600. https://doi.org/10.1378/chest.07-3039.
24. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. https://doi.org/10.1093/intqhc/mzt051.
25. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. https://doi.org/10.1093/cid/cir531.
26. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832.
27. Desai AD, Popalisky J, Simon TD, Mangione-Smith RM. The effectiveness of family-centered transition processes from hospital settings to home: a review of the literature. Hosp Pediatr. 2015;5(4):219-231. https://doi.org10.1542/hpeds.2014-0097.
28. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. https://doi.org/10.1016/j.acap.2015.08.003.
29. Agha MM, Glazier RH, Guttmann A. Relationship between social inequalities and ambulatory care-sensitive hospitalizations persists for up to 9 years among children born in a major Canadian urban center. Ambul Pediatr. 2007;7(3):258-262. https://doi.org/10.1016/j.ambp.2007.02.005.
30. Flores G, Abreu M, Chaisson CE, Sun D. Keeping children out of hospitals: parents’ and physicians’ perspectives on how pediatric hospitalizations for ambulatory care-sensitive conditions can be avoided. Pediatrics. 2003;112(5):1021-1030. https://doi.org/10.1542/peds.112.5.1021.
31. Weaver MS, Wichman B, Bace S, et al. Measuring the impact of the home health nursing shortage on family caregivers of children receiving palliative care. J Hosp Palliat Nurs. 2018;20(3):260-265. https://doi.org/10.1097/NJH.0000000000000436.
32. Leonard BJ, Brust JD, Sielaff BH. Determinants of home care nursing hours for technology-assisted children. Public Health Nurs. 1991;8(4):239-244. https://doi.org/10.1111/j.1525-1446.1991.tb00663.x.
33. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-1470. https://doi.org/10.1542/peds.2012-0175.
© 2020 Society of Hospital Medicine
Costs and Reimbursements for Mental Health Hospitalizations at Children’s Hospitals
Increasing numbers of children and adolescents are presenting to children’s hospitals with acute mental health crises requiring emergent or inpatient treatment.1-5 As a result, children’s hospitals are experiencing additional financial challenges because specialty mental health services are often reimbursed at lower rates than other medical services.6-9 Poor reimbursement has also been cited as a deterrent to the provision of mental health specialty care, including emergency mental health crisis services.10 The cumulative financial impact of recent trends in the provision of mental health crisis services at children’s hospitals, however, is unknown. We conducted this study to assess children’s hospitals’ costs, reimbursement, and net profits or losses when delivering inpatient mental health care.
METHODS
We conducted a retrospective cohort study of the Children’s Hospital Association’s Pediatric Health Information System (PHIS) and Revenue Management Program (RMP) databases. PHIS is an administrative and billing database that collects International Classification of Disease, 10th Revision (ICD-10) diagnoses, procedure codes, and hospital charges from encounters at 52 US children’s hospitals. Costs are estimated from charges using hospital-, year-, and department-specific cost-to-charge ratios. The RMP database is an add-on module to the PHIS database that captures reimbursement data submitted quarterly from 17 participating hospitals based on actual reimbursement amounts collected for each encounter.
Among the 17 participating hospitals, we included all medical (ie, not surgical or intensive care) encounters during calendar year 2017 for children older than 6 years. We stratified encounters into three diagnosis types: primary mental health diagnosis,5 suicide attempt,11 or other medical hospitalizations. We separated suicide attempts since these encounters often require care for both mental health concerns and medical complications. Eating disorders were excluded because these programs at children’s hospitals primarily focus on medical complications, require complex multispecialty support, have significantly longer hospitalizations and made up a small volume of overall mental health hospitalizations.
We stratified all analyses by inpatient or observation encounter and determined the proportion of encounters and hospital days attributed to primary mental health, suicide attempt, and other medical conditions at each hospital. One of the 17 children’s hospitals does not use observation status billing, so the observation encounters dataset includes 16 hospitals.
We summarized patients’ demographic and clinical characteristics using frequencies and percentages, comparing across diagnosis groups using chi-square tests. We calculated mean cost per day as (total cost) ÷ (total length of stay [LOS]), reimbursement per day as (total reimbursement) ÷ (total LOS) for each hospital and patient group, and margin per day as (reimbursement per day) – (cost per day). We then determined the total margin difference of caring for mental health vs caring for other medical encounters as ([margin per day for mental health] – [margin per day other medical]) × (number of mental health days). Similarly, we calculated the total margin loss for suicide attempts vs other medical encounters. After calculating profits and losses at individual hospitals, we summed total annual profits and losses to calculate cumulative annual differences. We summarized these profits and losses across all hospitals with medians and interquartile ranges (IQR).
This study of deidentified administrative data was approved by the Internal Review Board at Vanderbilt University as non-human subjects research. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
Across the 17 included children’s hospitals, there were 8,521 (7.6%) mental health encounters, 3,247 (2.9%) suicide attempt encounters, and 99,937 (89.5%) other medical encounters. LOS was significantly longer for mental health hospitalizations than for suicide attempts and for other medical hospitalizations.
Hospital Characteristics
All 17 free-standing children’s hospitals in the study had an inpatient behavioral health/psychiatric consultation service, and 7 of the 17 had an inpatient behavioral health/psychiatric unit. The total number of discharges for mental health, suicide attempt, and other medical conditions per year varied (range, 2,868-13,214) across the hospitals.
Hospital Daily Profits and Losses for Mental Health, Suicide Attempt, and Other Medical Admissions
For inpatient status mental health hospitalizations, the median margin was $376/day (IQR, $23-$618). For inpatient status suicide attempt hospitalizations, the median margin was $685/day (IQR, $3-$1,117), and for other medical hospitalizations the median margin was $603/day (IQR, $240-$991). With regard to observation status admissions, mental health hospitalizations had a median margin of –$453/day (IQR, –$806 to $362), suicide attempts of –$103/day (IQR, –$639 to $264), and other medical conditions of $353/day (IQR, –$616 to $658; Figure).
Hospital Annual Profits and Losses for Mental Health and Suicide Attempt Admissions, Compared With Other Medical Admissions
The Table shows daily and annual profits and losses for inpatient and observation status. The total annual loss across all hospitals for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, including both inpatient and observation status, was –$26,658,255 when taking both profits and losses into account. For the seven hospitals with net profits for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net profit for combined inpatient and observation status encounters was $119,361 (IQR, $82,818-$195,543), and the total net profit was $5,872,665. For the 10 hospitals with net losses for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net loss for combined inpatient and observation status was –$2,169,357 (IQR, –$4,034,085 to –$511,755), and the total net loss was –$27,419,379.
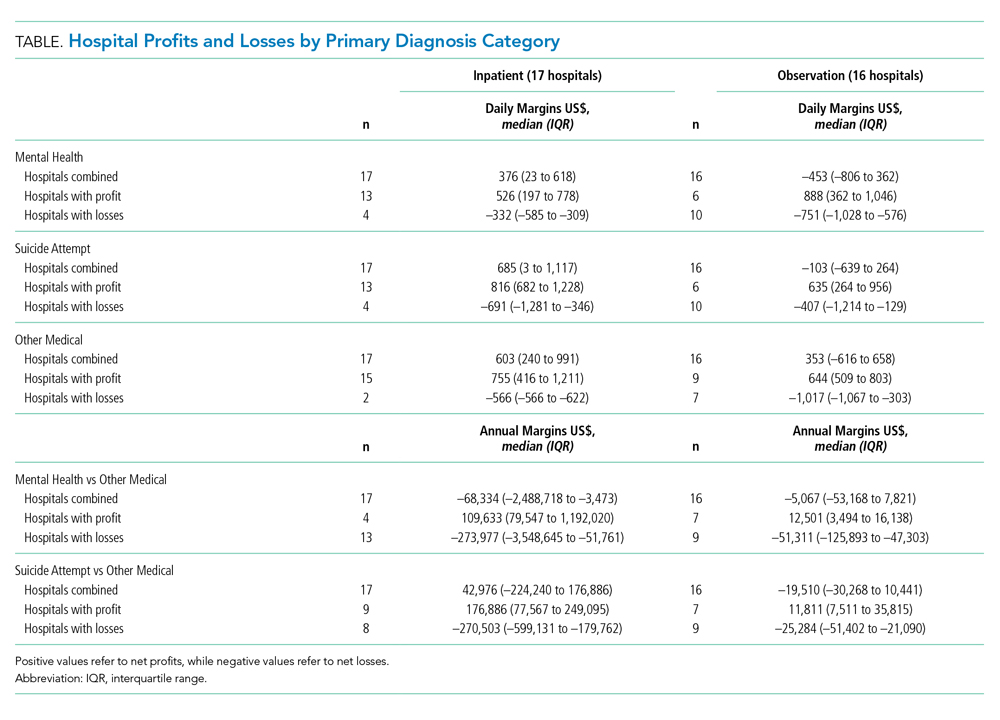
DISCUSSION
Hospitalizations for mental health disorders and suicide attempts accounted for 10.5% of hospitalizations at 17 US children’s hospitals in 2017. Overall, mental health and suicide attempt hospitalizations had lower financial margins than did other medical hospitalizations, and they accounted for a total margin loss of more than $26 million across 17 hospitals. Seven hospitals generated a profit for mental health and suicide attempt admissions; 10 hospitals reported losses. Only three hospitals generated a higher net profit for mental health admissions than for other medical admissions. More hospitals had net profits for inpatient status mental health and suicide attempt admissions than for observation status mental health and suicide attempt admissions.
For a minority of children’s hospitals, mental health hospitalizations had higher profit margins than for other medical hospitalizations. This raises questions about patient outcomes and the type of care models employed. One potential explanation is that these hospitals have negotiated favorable agreements with payers. Another possibility could be variations in case-mix and payer mix. Certain mental health services, such as crisis response teams, social workers, and child life specialists, may also be funded from nonpayer sources, so estimates may not fully reflect the cost of providing mental health services. A worst-case view is that hospitals with higher profit margins are providing less or poorer care because of lower reimbursement.
Mental health and suicide attempt hospitalizations were associated with smaller margins but counterintuitively generally wider IQRs for cost. This might be related to variation in care models, but our study was not positioned to examine reasons for this variation. The relationship between reimbursement or margins and patient outcomes, as well as specific mechanisms which may drive costs and outcomes, are areas for future research.
Health insurance plays a crucial role in mental health care. In our study, hospitals were more likely to report positive margins from inpatient status mental health hospitalizations rather than from observation status ones. This is unsurprising because payments for observation status are generally lower than for inpatient status.12 Less is known about what influences billing and payment for inpatient versus observation at individual hospitals, particularly for mental health hospitalizations. In many cases, billing status is not strictly under the hospital’s control and may be determined by payers during or after the hospitalization. Significant variability in the percentage of patients billed as observation status and the impact of lower, often negative, margins for observation mental health encounters, will have a disproportionate effect on some hospitals. Future work could investigate how these differences may influence overall costs and delivery of care.
This study has several limitations that deserve attention. Costs reported are based on cost to charge ratios, which may generate imperfect estimates. Data was limited to 17 freestanding children’s hospitals, and our findings may not generalize to other hospitals. We also compared mental health and suicide attempt hospitalizations with “other medical” hospitalizations. This broad group contains certain medical conditions that may have higher or lower profit margins than average, and estimates of the margins could be over- or underestimated. We assumed that mental health and suicide attempt admissions were displacing admissions with non–mental health medical conditions (ie, not an empty bed). If those beds would otherwise be unoccupied, raw margins are better estimates of the financial impact than margin differences between mental health/suicide attempt and other medical hospitalizations.
CONCLUSION
Children’s hospitals are more likely to have significantly lower financial margins for mental health and suicide attempt hospitalizations than for other medical hospitalizations. Future work to investigate how quality of care is associated with reimbursement can help ensure that funding for children’s acute mental health care services is commensurate with resources required to provide high quality services.
Disclosures
The authors had no financial relationships relevant to this article to disclose.
Funding Source
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH115162 (Doupnik).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008-2015. Pediatrics. 2018;141(6):e20172426. https://doi.org/10.1542/peds.2017-2426.
2. Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children--United States, 2005-2011. MMWR Suppl. 2013;62:1-35.
3. Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 2016;138(6):e20161878. https://doi.org/10.1542/peds.2016-1878.
4. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief. 2016;(241):1–8.
5. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909. https://doi.org/10.1542/peds.2016-0909.
6. Bierenbaum ML, Katsikas S, Furr A, Carter BD. Factors associated with non-reimbursable activity on an inpatient pediatric consultation-liaison service. J Clin Psychol Med Settings. 2013;20:464-72. https://doi.org/10.1007/s10880-013-9371-2.
7. Bishop TF, Press MJ, Keyhani S, Pincus HA. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71:176-81. https://doi.org/10.1001/jamapsychiatry.2013.2862.
8. McAuliffe Lines M, Tynan WD, Angalet GB, Shroff Pendley J. Commentary: the use of health and behavior codes in pediatric psychology: where are we now? J Pediatr Psychol. 2012;37:486-90. https://doi.org/10.1093/jpepsy/jss045.
9. Drotar D. Introduction to the special section: pediatric psychologists’ experiences in obtaining reimbursement for the use of health and behavior codes. J Pediatr Psychol. 2012;37:479-85. https://doi.org/10.1093/jpepsy/jss065.
10. Komers AM. “Indiana children’s hospital shutters psychiatric unit.” Becker’s Hospital Review. 2019. https://www.beckershospitalreview.com/patient-flow/indiana-children-s-hospital-shutters-psychiatric-unit.html. Accessed August 28, 2019.
11. Hedegaard H, Schoenbaum M, Claassen C, Crosby A, Holland K, Proescholdbell S. Issues in developing a surveillance case definition for nonfatal suicide attempt and intentional self-harm using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coded data. Natl Health Stat Report. 2018;(108):1-19.
12. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-8. https://doi.org/10.1542/peds.2012-2494.
Increasing numbers of children and adolescents are presenting to children’s hospitals with acute mental health crises requiring emergent or inpatient treatment.1-5 As a result, children’s hospitals are experiencing additional financial challenges because specialty mental health services are often reimbursed at lower rates than other medical services.6-9 Poor reimbursement has also been cited as a deterrent to the provision of mental health specialty care, including emergency mental health crisis services.10 The cumulative financial impact of recent trends in the provision of mental health crisis services at children’s hospitals, however, is unknown. We conducted this study to assess children’s hospitals’ costs, reimbursement, and net profits or losses when delivering inpatient mental health care.
METHODS
We conducted a retrospective cohort study of the Children’s Hospital Association’s Pediatric Health Information System (PHIS) and Revenue Management Program (RMP) databases. PHIS is an administrative and billing database that collects International Classification of Disease, 10th Revision (ICD-10) diagnoses, procedure codes, and hospital charges from encounters at 52 US children’s hospitals. Costs are estimated from charges using hospital-, year-, and department-specific cost-to-charge ratios. The RMP database is an add-on module to the PHIS database that captures reimbursement data submitted quarterly from 17 participating hospitals based on actual reimbursement amounts collected for each encounter.
Among the 17 participating hospitals, we included all medical (ie, not surgical or intensive care) encounters during calendar year 2017 for children older than 6 years. We stratified encounters into three diagnosis types: primary mental health diagnosis,5 suicide attempt,11 or other medical hospitalizations. We separated suicide attempts since these encounters often require care for both mental health concerns and medical complications. Eating disorders were excluded because these programs at children’s hospitals primarily focus on medical complications, require complex multispecialty support, have significantly longer hospitalizations and made up a small volume of overall mental health hospitalizations.
We stratified all analyses by inpatient or observation encounter and determined the proportion of encounters and hospital days attributed to primary mental health, suicide attempt, and other medical conditions at each hospital. One of the 17 children’s hospitals does not use observation status billing, so the observation encounters dataset includes 16 hospitals.
We summarized patients’ demographic and clinical characteristics using frequencies and percentages, comparing across diagnosis groups using chi-square tests. We calculated mean cost per day as (total cost) ÷ (total length of stay [LOS]), reimbursement per day as (total reimbursement) ÷ (total LOS) for each hospital and patient group, and margin per day as (reimbursement per day) – (cost per day). We then determined the total margin difference of caring for mental health vs caring for other medical encounters as ([margin per day for mental health] – [margin per day other medical]) × (number of mental health days). Similarly, we calculated the total margin loss for suicide attempts vs other medical encounters. After calculating profits and losses at individual hospitals, we summed total annual profits and losses to calculate cumulative annual differences. We summarized these profits and losses across all hospitals with medians and interquartile ranges (IQR).
This study of deidentified administrative data was approved by the Internal Review Board at Vanderbilt University as non-human subjects research. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
Across the 17 included children’s hospitals, there were 8,521 (7.6%) mental health encounters, 3,247 (2.9%) suicide attempt encounters, and 99,937 (89.5%) other medical encounters. LOS was significantly longer for mental health hospitalizations than for suicide attempts and for other medical hospitalizations.
Hospital Characteristics
All 17 free-standing children’s hospitals in the study had an inpatient behavioral health/psychiatric consultation service, and 7 of the 17 had an inpatient behavioral health/psychiatric unit. The total number of discharges for mental health, suicide attempt, and other medical conditions per year varied (range, 2,868-13,214) across the hospitals.
Hospital Daily Profits and Losses for Mental Health, Suicide Attempt, and Other Medical Admissions
For inpatient status mental health hospitalizations, the median margin was $376/day (IQR, $23-$618). For inpatient status suicide attempt hospitalizations, the median margin was $685/day (IQR, $3-$1,117), and for other medical hospitalizations the median margin was $603/day (IQR, $240-$991). With regard to observation status admissions, mental health hospitalizations had a median margin of –$453/day (IQR, –$806 to $362), suicide attempts of –$103/day (IQR, –$639 to $264), and other medical conditions of $353/day (IQR, –$616 to $658; Figure).
Hospital Annual Profits and Losses for Mental Health and Suicide Attempt Admissions, Compared With Other Medical Admissions
The Table shows daily and annual profits and losses for inpatient and observation status. The total annual loss across all hospitals for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, including both inpatient and observation status, was –$26,658,255 when taking both profits and losses into account. For the seven hospitals with net profits for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net profit for combined inpatient and observation status encounters was $119,361 (IQR, $82,818-$195,543), and the total net profit was $5,872,665. For the 10 hospitals with net losses for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net loss for combined inpatient and observation status was –$2,169,357 (IQR, –$4,034,085 to –$511,755), and the total net loss was –$27,419,379.

DISCUSSION
Hospitalizations for mental health disorders and suicide attempts accounted for 10.5% of hospitalizations at 17 US children’s hospitals in 2017. Overall, mental health and suicide attempt hospitalizations had lower financial margins than did other medical hospitalizations, and they accounted for a total margin loss of more than $26 million across 17 hospitals. Seven hospitals generated a profit for mental health and suicide attempt admissions; 10 hospitals reported losses. Only three hospitals generated a higher net profit for mental health admissions than for other medical admissions. More hospitals had net profits for inpatient status mental health and suicide attempt admissions than for observation status mental health and suicide attempt admissions.
For a minority of children’s hospitals, mental health hospitalizations had higher profit margins than for other medical hospitalizations. This raises questions about patient outcomes and the type of care models employed. One potential explanation is that these hospitals have negotiated favorable agreements with payers. Another possibility could be variations in case-mix and payer mix. Certain mental health services, such as crisis response teams, social workers, and child life specialists, may also be funded from nonpayer sources, so estimates may not fully reflect the cost of providing mental health services. A worst-case view is that hospitals with higher profit margins are providing less or poorer care because of lower reimbursement.
Mental health and suicide attempt hospitalizations were associated with smaller margins but counterintuitively generally wider IQRs for cost. This might be related to variation in care models, but our study was not positioned to examine reasons for this variation. The relationship between reimbursement or margins and patient outcomes, as well as specific mechanisms which may drive costs and outcomes, are areas for future research.
Health insurance plays a crucial role in mental health care. In our study, hospitals were more likely to report positive margins from inpatient status mental health hospitalizations rather than from observation status ones. This is unsurprising because payments for observation status are generally lower than for inpatient status.12 Less is known about what influences billing and payment for inpatient versus observation at individual hospitals, particularly for mental health hospitalizations. In many cases, billing status is not strictly under the hospital’s control and may be determined by payers during or after the hospitalization. Significant variability in the percentage of patients billed as observation status and the impact of lower, often negative, margins for observation mental health encounters, will have a disproportionate effect on some hospitals. Future work could investigate how these differences may influence overall costs and delivery of care.
This study has several limitations that deserve attention. Costs reported are based on cost to charge ratios, which may generate imperfect estimates. Data was limited to 17 freestanding children’s hospitals, and our findings may not generalize to other hospitals. We also compared mental health and suicide attempt hospitalizations with “other medical” hospitalizations. This broad group contains certain medical conditions that may have higher or lower profit margins than average, and estimates of the margins could be over- or underestimated. We assumed that mental health and suicide attempt admissions were displacing admissions with non–mental health medical conditions (ie, not an empty bed). If those beds would otherwise be unoccupied, raw margins are better estimates of the financial impact than margin differences between mental health/suicide attempt and other medical hospitalizations.
CONCLUSION
Children’s hospitals are more likely to have significantly lower financial margins for mental health and suicide attempt hospitalizations than for other medical hospitalizations. Future work to investigate how quality of care is associated with reimbursement can help ensure that funding for children’s acute mental health care services is commensurate with resources required to provide high quality services.
Disclosures
The authors had no financial relationships relevant to this article to disclose.
Funding Source
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH115162 (Doupnik).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Increasing numbers of children and adolescents are presenting to children’s hospitals with acute mental health crises requiring emergent or inpatient treatment.1-5 As a result, children’s hospitals are experiencing additional financial challenges because specialty mental health services are often reimbursed at lower rates than other medical services.6-9 Poor reimbursement has also been cited as a deterrent to the provision of mental health specialty care, including emergency mental health crisis services.10 The cumulative financial impact of recent trends in the provision of mental health crisis services at children’s hospitals, however, is unknown. We conducted this study to assess children’s hospitals’ costs, reimbursement, and net profits or losses when delivering inpatient mental health care.
METHODS
We conducted a retrospective cohort study of the Children’s Hospital Association’s Pediatric Health Information System (PHIS) and Revenue Management Program (RMP) databases. PHIS is an administrative and billing database that collects International Classification of Disease, 10th Revision (ICD-10) diagnoses, procedure codes, and hospital charges from encounters at 52 US children’s hospitals. Costs are estimated from charges using hospital-, year-, and department-specific cost-to-charge ratios. The RMP database is an add-on module to the PHIS database that captures reimbursement data submitted quarterly from 17 participating hospitals based on actual reimbursement amounts collected for each encounter.
Among the 17 participating hospitals, we included all medical (ie, not surgical or intensive care) encounters during calendar year 2017 for children older than 6 years. We stratified encounters into three diagnosis types: primary mental health diagnosis,5 suicide attempt,11 or other medical hospitalizations. We separated suicide attempts since these encounters often require care for both mental health concerns and medical complications. Eating disorders were excluded because these programs at children’s hospitals primarily focus on medical complications, require complex multispecialty support, have significantly longer hospitalizations and made up a small volume of overall mental health hospitalizations.
We stratified all analyses by inpatient or observation encounter and determined the proportion of encounters and hospital days attributed to primary mental health, suicide attempt, and other medical conditions at each hospital. One of the 17 children’s hospitals does not use observation status billing, so the observation encounters dataset includes 16 hospitals.
We summarized patients’ demographic and clinical characteristics using frequencies and percentages, comparing across diagnosis groups using chi-square tests. We calculated mean cost per day as (total cost) ÷ (total length of stay [LOS]), reimbursement per day as (total reimbursement) ÷ (total LOS) for each hospital and patient group, and margin per day as (reimbursement per day) – (cost per day). We then determined the total margin difference of caring for mental health vs caring for other medical encounters as ([margin per day for mental health] – [margin per day other medical]) × (number of mental health days). Similarly, we calculated the total margin loss for suicide attempts vs other medical encounters. After calculating profits and losses at individual hospitals, we summed total annual profits and losses to calculate cumulative annual differences. We summarized these profits and losses across all hospitals with medians and interquartile ranges (IQR).
This study of deidentified administrative data was approved by the Internal Review Board at Vanderbilt University as non-human subjects research. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
Across the 17 included children’s hospitals, there were 8,521 (7.6%) mental health encounters, 3,247 (2.9%) suicide attempt encounters, and 99,937 (89.5%) other medical encounters. LOS was significantly longer for mental health hospitalizations than for suicide attempts and for other medical hospitalizations.
Hospital Characteristics
All 17 free-standing children’s hospitals in the study had an inpatient behavioral health/psychiatric consultation service, and 7 of the 17 had an inpatient behavioral health/psychiatric unit. The total number of discharges for mental health, suicide attempt, and other medical conditions per year varied (range, 2,868-13,214) across the hospitals.
Hospital Daily Profits and Losses for Mental Health, Suicide Attempt, and Other Medical Admissions
For inpatient status mental health hospitalizations, the median margin was $376/day (IQR, $23-$618). For inpatient status suicide attempt hospitalizations, the median margin was $685/day (IQR, $3-$1,117), and for other medical hospitalizations the median margin was $603/day (IQR, $240-$991). With regard to observation status admissions, mental health hospitalizations had a median margin of –$453/day (IQR, –$806 to $362), suicide attempts of –$103/day (IQR, –$639 to $264), and other medical conditions of $353/day (IQR, –$616 to $658; Figure).
Hospital Annual Profits and Losses for Mental Health and Suicide Attempt Admissions, Compared With Other Medical Admissions
The Table shows daily and annual profits and losses for inpatient and observation status. The total annual loss across all hospitals for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, including both inpatient and observation status, was –$26,658,255 when taking both profits and losses into account. For the seven hospitals with net profits for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net profit for combined inpatient and observation status encounters was $119,361 (IQR, $82,818-$195,543), and the total net profit was $5,872,665. For the 10 hospitals with net losses for mental health and suicide attempt hospitalizations, compared with other medical hospitalizations, the median net loss for combined inpatient and observation status was –$2,169,357 (IQR, –$4,034,085 to –$511,755), and the total net loss was –$27,419,379.

DISCUSSION
Hospitalizations for mental health disorders and suicide attempts accounted for 10.5% of hospitalizations at 17 US children’s hospitals in 2017. Overall, mental health and suicide attempt hospitalizations had lower financial margins than did other medical hospitalizations, and they accounted for a total margin loss of more than $26 million across 17 hospitals. Seven hospitals generated a profit for mental health and suicide attempt admissions; 10 hospitals reported losses. Only three hospitals generated a higher net profit for mental health admissions than for other medical admissions. More hospitals had net profits for inpatient status mental health and suicide attempt admissions than for observation status mental health and suicide attempt admissions.
For a minority of children’s hospitals, mental health hospitalizations had higher profit margins than for other medical hospitalizations. This raises questions about patient outcomes and the type of care models employed. One potential explanation is that these hospitals have negotiated favorable agreements with payers. Another possibility could be variations in case-mix and payer mix. Certain mental health services, such as crisis response teams, social workers, and child life specialists, may also be funded from nonpayer sources, so estimates may not fully reflect the cost of providing mental health services. A worst-case view is that hospitals with higher profit margins are providing less or poorer care because of lower reimbursement.
Mental health and suicide attempt hospitalizations were associated with smaller margins but counterintuitively generally wider IQRs for cost. This might be related to variation in care models, but our study was not positioned to examine reasons for this variation. The relationship between reimbursement or margins and patient outcomes, as well as specific mechanisms which may drive costs and outcomes, are areas for future research.
Health insurance plays a crucial role in mental health care. In our study, hospitals were more likely to report positive margins from inpatient status mental health hospitalizations rather than from observation status ones. This is unsurprising because payments for observation status are generally lower than for inpatient status.12 Less is known about what influences billing and payment for inpatient versus observation at individual hospitals, particularly for mental health hospitalizations. In many cases, billing status is not strictly under the hospital’s control and may be determined by payers during or after the hospitalization. Significant variability in the percentage of patients billed as observation status and the impact of lower, often negative, margins for observation mental health encounters, will have a disproportionate effect on some hospitals. Future work could investigate how these differences may influence overall costs and delivery of care.
This study has several limitations that deserve attention. Costs reported are based on cost to charge ratios, which may generate imperfect estimates. Data was limited to 17 freestanding children’s hospitals, and our findings may not generalize to other hospitals. We also compared mental health and suicide attempt hospitalizations with “other medical” hospitalizations. This broad group contains certain medical conditions that may have higher or lower profit margins than average, and estimates of the margins could be over- or underestimated. We assumed that mental health and suicide attempt admissions were displacing admissions with non–mental health medical conditions (ie, not an empty bed). If those beds would otherwise be unoccupied, raw margins are better estimates of the financial impact than margin differences between mental health/suicide attempt and other medical hospitalizations.
CONCLUSION
Children’s hospitals are more likely to have significantly lower financial margins for mental health and suicide attempt hospitalizations than for other medical hospitalizations. Future work to investigate how quality of care is associated with reimbursement can help ensure that funding for children’s acute mental health care services is commensurate with resources required to provide high quality services.
Disclosures
The authors had no financial relationships relevant to this article to disclose.
Funding Source
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH115162 (Doupnik).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008-2015. Pediatrics. 2018;141(6):e20172426. https://doi.org/10.1542/peds.2017-2426.
2. Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children--United States, 2005-2011. MMWR Suppl. 2013;62:1-35.
3. Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 2016;138(6):e20161878. https://doi.org/10.1542/peds.2016-1878.
4. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief. 2016;(241):1–8.
5. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909. https://doi.org/10.1542/peds.2016-0909.
6. Bierenbaum ML, Katsikas S, Furr A, Carter BD. Factors associated with non-reimbursable activity on an inpatient pediatric consultation-liaison service. J Clin Psychol Med Settings. 2013;20:464-72. https://doi.org/10.1007/s10880-013-9371-2.
7. Bishop TF, Press MJ, Keyhani S, Pincus HA. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71:176-81. https://doi.org/10.1001/jamapsychiatry.2013.2862.
8. McAuliffe Lines M, Tynan WD, Angalet GB, Shroff Pendley J. Commentary: the use of health and behavior codes in pediatric psychology: where are we now? J Pediatr Psychol. 2012;37:486-90. https://doi.org/10.1093/jpepsy/jss045.
9. Drotar D. Introduction to the special section: pediatric psychologists’ experiences in obtaining reimbursement for the use of health and behavior codes. J Pediatr Psychol. 2012;37:479-85. https://doi.org/10.1093/jpepsy/jss065.
10. Komers AM. “Indiana children’s hospital shutters psychiatric unit.” Becker’s Hospital Review. 2019. https://www.beckershospitalreview.com/patient-flow/indiana-children-s-hospital-shutters-psychiatric-unit.html. Accessed August 28, 2019.
11. Hedegaard H, Schoenbaum M, Claassen C, Crosby A, Holland K, Proescholdbell S. Issues in developing a surveillance case definition for nonfatal suicide attempt and intentional self-harm using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coded data. Natl Health Stat Report. 2018;(108):1-19.
12. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-8. https://doi.org/10.1542/peds.2012-2494.
1. Plemmons G, Hall M, Doupnik S, et al. Hospitalization for suicide ideation or attempt: 2008-2015. Pediatrics. 2018;141(6):e20172426. https://doi.org/10.1542/peds.2017-2426.
2. Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children--United States, 2005-2011. MMWR Suppl. 2013;62:1-35.
3. Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 2016;138(6):e20161878. https://doi.org/10.1542/peds.2016-1878.
4. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief. 2016;(241):1–8.
5. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5):e20160909. https://doi.org/10.1542/peds.2016-0909.
6. Bierenbaum ML, Katsikas S, Furr A, Carter BD. Factors associated with non-reimbursable activity on an inpatient pediatric consultation-liaison service. J Clin Psychol Med Settings. 2013;20:464-72. https://doi.org/10.1007/s10880-013-9371-2.
7. Bishop TF, Press MJ, Keyhani S, Pincus HA. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71:176-81. https://doi.org/10.1001/jamapsychiatry.2013.2862.
8. McAuliffe Lines M, Tynan WD, Angalet GB, Shroff Pendley J. Commentary: the use of health and behavior codes in pediatric psychology: where are we now? J Pediatr Psychol. 2012;37:486-90. https://doi.org/10.1093/jpepsy/jss045.
9. Drotar D. Introduction to the special section: pediatric psychologists’ experiences in obtaining reimbursement for the use of health and behavior codes. J Pediatr Psychol. 2012;37:479-85. https://doi.org/10.1093/jpepsy/jss065.
10. Komers AM. “Indiana children’s hospital shutters psychiatric unit.” Becker’s Hospital Review. 2019. https://www.beckershospitalreview.com/patient-flow/indiana-children-s-hospital-shutters-psychiatric-unit.html. Accessed August 28, 2019.
11. Hedegaard H, Schoenbaum M, Claassen C, Crosby A, Holland K, Proescholdbell S. Issues in developing a surveillance case definition for nonfatal suicide attempt and intentional self-harm using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coded data. Natl Health Stat Report. 2018;(108):1-19.
12. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-8. https://doi.org/10.1542/peds.2012-2494.
© 2020 Society of Hospital Medicine
Effect of Parental Adverse Childhood Experiences and Resilience on a Child’s Healthcare Reutilization
Adverse Childhood Experiences, or ACEs, include exposure to abuse, neglect, or household dysfunction (eg, having a parent who is mentally ill) as a child.1 Exposure to ACEs affects health into adulthood, with a dose-response relationship between ACEs and a range of comorbidities.1 Adults with 6 or more ACEs have a 20-year shorter life expectancy than do those with no ACEs.1 Still, ACEs are static; once experienced, that experience cannot be undone. However, resilience, or positive adaptation in the context of adversity, can be protective, buffering the negative effects of ACEs.2,3 Protective factors that promote resilience include social capital, such as positive relationships with caregivers and peers.3
With their clear link to health outcomes across the life-course, there is a movement for pediatricians to screen children for ACEs4 and to develop strategies that promote resilience in children, parents, and families. However, screening a child for adversity has challenges because younger children may not have experienced an adverse exposure, or they may be unable to voice their experiences. Studies have demonstrated that parental adversity, or ACEs, may be a marker for childhood adversity.5,6 Biological models also support this potential intergenerational effect of ACEs. Chronic exposure to stress, including ACEs, results in elevated cortisol via a dysregulated hypothalamic-pituitary-adrenal axis, which results in chronic inflammation.7 This “toxic stress” is prolonged, severe in intensity, and can lead to epigenetic changes that may be passed on to the next generation.8,9
Hospitalization of an ill child, and the transition to home after that hospitalization, is a stressful event for children and families.10 This stress may be relevant to parents that have a history of a high rate of ACEs or a current low degree of resilience. Our previous work demonstrated that, in the inpatient setting, parents with high ACEs (≥4) or low resilience have increased coping difficulty 14 days after their child’s hospital discharge.11 Our objective here was to evaluate whether a parent’s ACEs and/or resilience would also be associated with that child’s likelihood of reutilization. We hypothesized that more parental ACEs and/or lower parental resilience would be associated with revisits the emergency room, urgent care, or hospital readmissions.
METHODS
Participants and Study Design
We conducted a prospective cohort study of parents of hospitalized children recruited from the “Hospital-to-Home Outcomes” Studies (H2O I and H2O II).12,13 H2O I and II were prospective, single-center, randomized controlled trials designed to determine the effectiveness of either a nurse-led transitional home visit (H2O I) or telephone call (H2O II) on 30-day unplanned healthcare reutilization. The trials and this study were approved by the Cincinnati Children’s Institutional Review Board. All parents provided written informed consent.
Details of H2O I and II recruitment and design have been described previously.12,13 Briefly, children were eligible for inclusion in either study if they were admitted to our institution’s general Hospital Medicine or the Hospital Medicine Complex Care Services; for H2O I, children hospitalized on the Neurology and Neurosurgery services were also eligible.12,13 Patients were excluded if they were discharged to a residential facility, if they lived outside the home healthcare nurse service area, if they were eligible for skilled home healthcare services (eg, intravenous antibiotics), or if the participating caregiver was non-English speaking.12,13 In H2O I, families were randomized either to receive a single nurse home visit within 96 hours of discharge or standard of care. In H2O II, families enrolled were randomized to receive a telephone call by a nurse within 96 hours of discharge or standard of care. As we have previously published, randomization in both trials successfully balanced the intervention and control arms with respect to key demographic characteristics.12,13 For the analyses presented here, we focused on a subset of caregivers 18 years and older whose children were enrolled in either H2O I or II between August 2015 and October 2016. In both H2O trials, face-to-face and paper-based questionnaires were completed by parents during the index hospitalization.
Outcome and Predictors
Our primary outcome was unanticipated healthcare reutilization defined as return to the emergency room, urgent care, or unplanned readmission within 30 days of hospital discharge, consistent with the H2O trials. This was measured using the primary institution’s administrative data supplemented by a utilization database shared across regional hospitals.14 Readmissions were identified as “unplanned” using a previously validated algorithm,15 and treated as a dichotomous yes/no variable.
Our primary predictors were parental ACEs and resilience (see Appendix Tables). The ACE questionnaire addresses abuse, neglect, and household dysfunction in the first 18 years of life.1 It is composed of 10 questions, each with a yes/no response.1 We defined parents as low (ACE 0), moderate (ACE 1-3), or high (ACE ≥4) risk a priori because previous literature has described poor outcomes in adults with 4 or more ACEs.16
Given the sensitive nature of the questions, respondents independently completed the ACE questionnaire on paper instead of via the face-to-face survey. Respondents returned the completed questionnaire to the research assistant in a sealed envelope. All families received educational information on relevant hospital and community-based resources (eg, social work).
Parental resilience was measured using the Brief Resilience Scale (BRS). The BRS is 6 items, each on a 5-point Likert scale. Responses were averaged, providing a total score of 1-5; higher scores are representative of higher resilience.17 We treated the BRS score as a continuous variable. BRS has been used in clinical settings; it has demonstrated positive correlation with social support and negative correlation with fatigue.17 Parents answered BRS questions during the index pediatric hospitalization in a face-to-face interview.
Parent and Child Characteristics
Parent and child sociodemographic variables were also obtained during the face-to-face interview. Parental variables included age, gender, educational attainment, household income, employment status, and financial and social strain.11 Educational attainment was analyzed in 2 categories—high school or less vs more than high school—because most discharge instructions are written at a high school reading level.18 Parents reported their annual household income in the following categories: <$15,000; $15,000-$29,999; $30,000-$44,999; $45,000-$59,999; $60,000-$89,999; $90,000-$119,999; ≥$120,000. Employment was dichotomized as not employed/student vs any employment. Financial and social strain were assessed using a series of 9 previously described questions.19 These questions assessed, via self-report, a family’s ability to make ends meet, ability to pay rent/mortgage or utilities, need to move in with others because of financial reasons, and ability to borrow money if needed, as well as home ownership and parental marital status.15,19 Strain questions were all dichotomous (yes/no, single/not single). A composite variable was then constructed that categorized those reporting no strain items, 1 to 2 items, 3 to 4 items, and 5 or more items.20
Child variables included race, ethnicity, age, primary care access,21 payer, and H2O treatment arm. Race categories were white/Caucasian, black/African American, American Indian or Alaskan Native, Asian or Pacific Islander, and other; ethnicity categories were Hispanic/Latino, non-Hispanic/Latino, and unknown. Given relatively low numbers of children reported to be Hispanic/Latino, we combined race and ethnicity into a single variable, categorized as non-Hispanic/white, non-Hispanic/black, and multiracial/Hispanic/other. Primary care access was assessed using the access subscale to the Parent’s Perception of Primary Care questionnaire. This includes assessment of a family’s ability to travel to their doctor, to see their doctor for routine or sick care, and to get help or advice on evenings or weekends. Scores were categorized as always adequate, almost always adequate, or sometimes/never adequate.21 Payer was dichotomized to private or public/self-pay.
Statistical Analyses
We examined the distribution of outcomes, predictors, and covariates. We compared sociodemographic characteristics of those respondents and nonrespondents to the ACE screen using the chi-square test for categorical variables or the t test for continuous variables. We used logistic regression to assess for associations between the independent variables of interest and reutilization, adjusting for potential confounders. To build our adjusted, multivariable model, we decided a priori to include child race/ethnicity, primary care access, financial and social strain, and trial treatment arm. We treated the H2O I control group as the referent group. Other covariates considered for inclusion were caregiver education, household income, employment, and payer. These were included in multivariable models if bivariate associations were significant at the P < .1 level. We assessed an ACE-by-resilience interaction term because we hypothesized that those with more ACEs and lower resilience may have more reutilization outcomes than parents with fewer ACEs and higher resilience. We also evaluated interaction terms between trial arm assignment and predictors to assess effects that may be introduced by the randomization. Predictors in the final logistic regression model were significant at the P < .05 level. Logistic regression assumption of little or no multicollinearity among the independent variables was verified in the final models. All analyses were performed with Stata v16 (Stata Corp, College Station, Texas).
RESULTS
There were a total of 1,787 parent-child dyads enrolled in the H2O I and II during the study period; 1,320 parents (74%) completed the ACE questionnaire and were included the analysis. Included parents were primarily female and employed, as well as educated beyond high school (Table 1). Overall, 64% reported one or more ACEs (range 0 to 9); 45% reported 1to 3, and 19% reported 4 or more ACEs. The most commonly reported ACEs were divorce (n = 573, 43%), exposure to alcoholism (n = 306, 23%), and exposure to mental illness (n = 281, 21%; Figure 1). Parents had a mean BRS score of 3.97 (range 1.17-5.00), with the distribution shown in Figure 2.
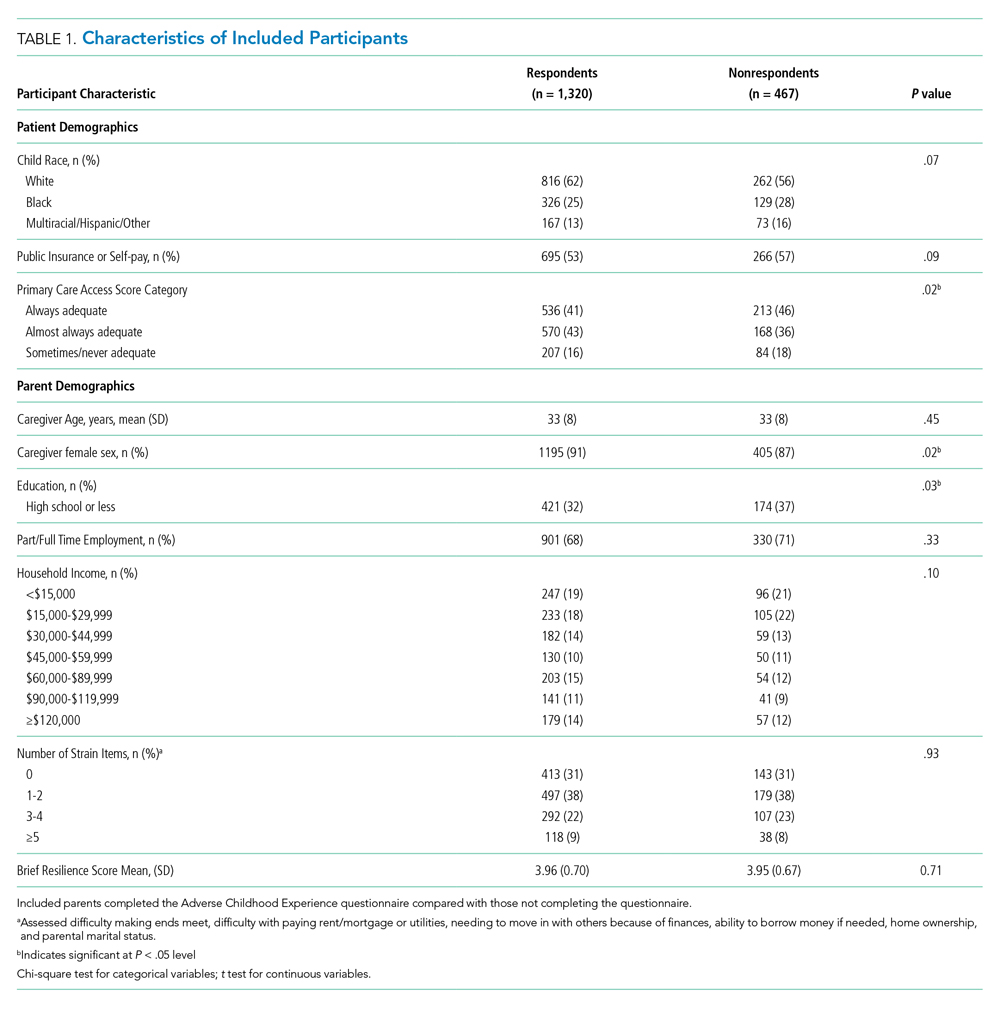
Of the 1,320 included patients, the average length of stay was 2.5 days, and 82% of hospitalizations were caused by acute medical issues (eg, bronchiolitis). A total of 211 children experienced a reutilization event within 30 days of discharge. In bivariate analysis, children with parents with 4 or more ACEs had a 2.02-times (95% CI 1.35-3.02) higher odds of experiencing a reutilization event than did those with parents reporting no ACEs. Parents with higher resilience scores had children with a lower odds of reutilization (odds ratio [OR] 0.77 95% CI 0.63-0.95).

In addition to our a priori variables, parental education, employment, and insurance met our significance threshold for inclusion in the multivariable model. The ACE-by-resilience interaction term was not significant and not included in the model. Similarly, there was no significant interaction between ACE and resilience and H2O treatment arm; the interaction terms were not included in the final adjusted model, but treatment arm assignment was kept as a covariate. A total of 1,292 children, out of the 1,320 respondents, remained in the final multivariable model; the excluded 28 had incomplete covariate data but were not otherwise different. In this final adjusted model, children with parents reporting 4 or more ACEs had a 1.69-times (95% CI 1.11-2.60) greater odds of reutilization than did those with parents reporting no ACEs (Table 2). Resilience failed to reach statistical significance in the adjusted model (OR 0.86, 95% CI 0.70-1.07).
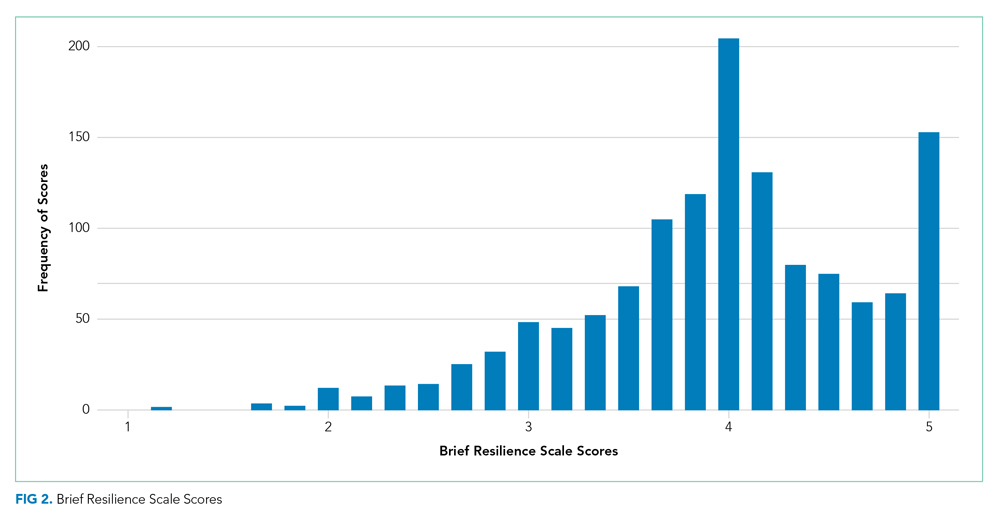
DISCUSSION
We found that high-risk parents (4 or more ACEs) had children with an increased odds of healthcare reutilization, suggesting intergenerational effects of ACEs. We did not find a similar effect relating to parental resilience. We also did not find an interaction between parental ACEs and resilience, suggesting that a parent’s reported degree of resilience does not modify the effect of ACEs on reutilization risk.
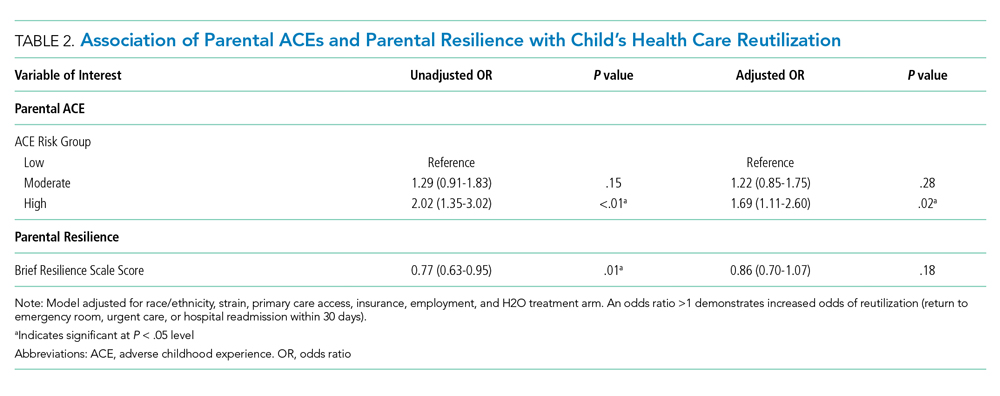
Parental adversity may be a risk factor for a child’s unanticipated reutilization. We previously demonstrated that parents with 4 or more ACEs have more coping difficulty than a parent with no ACEs after a child’s hospitalization.11 It is possible that parents with high adversity may have poorer coping mechanisms when dealing with a stressful situation, such as a child’s hospitalization. This may have resulted in inequitable outcomes (eg, increased reutilization) for their children. Other studies have confirmed such an intergenerational effect of adversity, linking a parent’s ACEs with poor developmental, behavioral, and health outcomes in their children.6,22,23 O’Malley et al showed an association of parental ACEs to current adversities,24 such as insurance or housing concerns, that affect the entirety of the household, including children. In short, it appears that parental ACEs may be a compelling predictor of current childhood adversity.
Resilience buffers the negative effects of ACEs; however, we did not find significant associations between resilience and reutilization or an interaction between ACEs and resilience. The factors that may contribute to reutilization are complex. In our previous work, parental resilience was associated with coping difficulty after discharge; but again, did not interact with parental ACEs.11 Here, we suggest that while resilience may buffer the negative effects of ACEs, that buffering may not affect the likelihood of reutilization. It is also possible that the BRS tool is of less relevance on how one handles the stress of a child’s hospitalization. While the BRS is one measure of resilience, there are many other relevant constructs to resilience, such as connection to social supports, that also may also contribute to risk of reutilization.25
Reducing the stress of a hospitalization itself and promoting a safe transition from hospital to home is critical to improving child health outcomes. Our data here, and in our previous work, demonstrate that a history of adversity and one’s current coping ability may drive a parent’s response to a child’s hospitalization and affect their capacity to care for that child after hospital discharge.11 Additional in-hospital supports like child life, behavioral health, or pastoral care could reduce the stress of the hospitalization while also building positive coping mechanisms.26-29 A meta-analysis demonstrated that such coping interventions can help alleviate the stress of a hospitalization.30 Hill et al demonstrated successful stress reduction in parents of hospitalized children using a “Coping Kit for Parents.”31 Further studies are warranted to understand which interventions are most effective for children and families and whether they could be more effectively deployed if the inpatient team knew more about parental ACEs.
Screening for parental ACEs could help to identify patients at highest risk for a poor transition to home. Therefore, screening for parental adversity in clinical settings, including inpatient settings, may be relevant and valuable.32 Additionally, by recognizing the high prevalence of ACEs in an inpatient setting, hospitals and healthcare organizations could be motivated to develop and enact trauma-informed approaches. A trauma-informed care approach recognizes the intersection of trauma with health and social problems. With this recognition, care teams can more sensitively address the trauma as they provide relevant services.33 Trauma-informed care is a secondary public health prevention approach that would help team members identify the prevalence and effects of trauma via screening, recognize the signs of a maladaptive response to stress, and respond by integrating awareness of trauma into practice management.28,34 Both the National Academy of Medicine and the Agency for Healthcare Research and Quality have called for such a trauma-informed approach in primary care.35 In response, many healthcare organizations have developed trauma-informed practices to better address the needs of the populations they serve. For example, provider training on this approach has led to improved rapport in patient-provider relationships.36
Although ACE awareness is a component of trauma-informed care, there are still limitations of the original ACE questionnaire developed by Felitti et al. The existing tool is not inclusive of all adversities a parent or child may face. Moreover, its focus is on past exposures and experiences and not current health-related social needs (eg, food insecurity) which have known linkages with a range of health outcomes and health disparities.37 Additionally, the original ACE questionnaire was created as a population level tool and not as a screening tool. If used as a screening tool, providers may view the questions as too sensitive to ask, and parents may have difficulty responding to and understanding the relevance to their child’s care. Therefore, we suggest that more evidence is required to understand how to best adapt ACE questions into a screening processes that may be implemented in a medical setting.
More evidence is also needed to determine when and where such screening may be most useful. A primary care provider would be best equipped to screen caregivers for ACEs given their established relationship with parents and patients. Given the potential relevance of such information for inpatient care provision, information could then flow from primary care to the inpatient team. However, because not all patients have established primary care providers and only 4% of pediatricians screen for ACEs,38 it is important for inpatient medical teams to understand their role in identifying and addressing ACEs during hospital stays. Development of a screening tool, with input from all stakeholders—including parents—that is valid and feasible for use in a pediatric inpatient setting would be an important step forward. This tool should be paired with training in how to discuss these topics in a trauma-informed, nonjudgmental, empathic manner. We see this as a way in which providers can more effectively elicit an accurate response while simultaneously educating parents on the relevance of such sensitive topics during an acute hospital stay. We also recommend that screening should always be paired with response capabilities that connect those who screen positive with resources that could help them to navigate the stress experienced during and after a child’s hospitalization. Furthermore, communication with primary care providers about parents that screen positive should be integrated into the transition process.
This work has several limitations. First, our study was a part of randomized controlled trials conducted in one academic setting, which thereby limits generalizability. For example, we limited our cohort to those who were English-speaking patients only. This may bias our results because respondents with limited English proficiency may have different risk profiles than their English-speaking peers. In addition, the administration of the both the ACE and resilience questionnaires occurred during an acutely stressful period, which may influence how a parent responds to these questions. Also, both of the surveys are self-reported by parents, which may be susceptible to memory and response biases. Relatedly, we had a high number of nonrespondents, particularly to the ACE questionnaire. Our results are therefore only relevant to those who chose to respond and cannot be applied to nonrespondents. Further work assessing why one does or does not respond to such sensitive questions is an important area for future inquiry. Lastly, our cohort had limited medical complexity; future studies may consider links between parental ACEs (and resilience) and morbidity experienced by children with medical complexity.
CONCLUSION
Parents history of adversity is linked to their children’s unanticipated healthcare reutilization after a hospital discharge. Screening for parental stressors during a hospitalization may be an important first step to connecting parents and children to evidence-based interventions capable of mitigating the stress of hospitalization and promoting better, more seamless transitions from hospital to home.
Acknowledgments
Group Members: The following H2O members are nonauthor contributors: JoAnne Bachus, BSN, RN; Monica Borell, BSN, RN; Lenisa V Chang, MA, PhD; Patricia Crawford, RN; Sarah Ferris, BA; Jennifer Gold, BSN, RN; Judy A Heilman, BSN, RN; Jane C Khoury, PhD; Pierce Kuhnell, MS; Karen Lawley, BSN, RN; Margo Moore, MS, BSN, RN; Lynne O’Donnell, BSN, RN; Sarah Riddle, MD; Susan N Sherman, DPA; Angela M Statile, MD, MEd; Karen P Sullivan, BSN, RN; Heather Tubbs-Cooley, PhD, RN; Susan Wade-Murphy, MSN, RN; and Christine M White, MD, MAT.
The authors also thank David Keller, MD, for his guidance on the study.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding Source
Supported by funds from the Academic Pediatric Young Investigator Award (Dr A Shah) and the Patient-Centered Outcomes Research Institute Award (IHS-1306-0081, to Dr K Auger, Dr S Shah, Dr H Sucharew, Dr J Simmons), the National Institutes of Health (1K23AI112916, to Dr AF Beck), and the Agency for Healthcare Research and Quality (1K12HS026393-01, to Dr A Shah, K08-HS024735- 01A1, to Dr K Auger). Dr J Haney received Summer Undergraduate Research Fellowship funding through the Summer Undergraduate Research Fellowship at Cincinnati Children’s Hospital Medical Center.
Disclaimer
All statements in this report, including findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or the Methodology Committee.
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. https://doi.org/10.1016/s0749-3797(98)00017-8.
2. Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff. 2014;33(12):2106-2115. https://doi.org/10.1377/hlthaff.2014.0914.
3. Masten AS. Ordinary Magic. Resilience processes in development. Am Psychol. 2001;56(3):227-238. https://doi.org/10.1037//0003-066x.56.3.227.
4. Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of C, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224-231. https://doi.org/10.1542/peds.2011-2662.
5. Randell KA, O’Malley D, Dowd MD. Association of parental adverse childhood experiences and current child adversity. JAMA Pediatrics. 2015;169(8):786-787. https://doi.org/10.1001/jamapediatrics.2015.0269.
6. Le-Scherban F, Wang X, Boyle-Steed KH, Pachter LM. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. 2018;141(6):e20174274. https://doi.org/10.1542/peds.2017-4274.
7. Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319-327. https://doi.org/10.1542/peds.2012-0469.
8. Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760-769. https://doi.org/10.1016/j.biopsych.2008.11.028.
9. Garner AS, Forkey H, Szilagyi M. Translating developmental science to address childhood adversity. Acad Pediatr. 2015;15(5):493-502. https://doi.org/10.1016/j.acap.2015.05.010.
10. Weiss M, Johnson NL, Malin S, Jerofke T, Lang C, Sherburne E. Readiness for discharge in parents of hospitalized children. J Pediatr Nurs. 2008;23(4):282-295. https://doi.org/10.1016/j.pedn.2007.10.005.
11. Shah AN, Beck AF, Sucharew HJ, et al. Parental adverse childhood experiences and resilience on coping after discharge. Pediatrics. 2018;141(4):e20172127. https://doi.org/10.1542/peds.2017-2127.
12. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: The Hospital to Home Outcomes (H2O) Trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919.
13. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
14. TheHealthCollaborative. Healthbridge analytics. http://healthcollab.org/hbanalytics/. Accessed August 11, 2017.
15. Auger K, Mueller E, Weinberg S, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org10.1016/j.jpeds.2015.11.051.
16. Felitti VJ. Belastungen in der Kindheit und Gesundheit im Erwachsenenalter: die Verwandlung von Gold in Blei [The relationship of adverse childhood experiences to adult health: turning gold into lead]. Z Psychosom Med Psychother. 2002;48(4):359-369. https://doi.org/10.13109/zptm.2002.48.4.359.
17. Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194-200. https://doi.org/10.1080/10705500802222972.
18. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798. https://doi.org/10.1046/j.1525-1497.1998.00242.x.
19. Auger KA, Kahn RS, Simmons JM, et al. Using address information to identify hardships reported by families of children hospitalized with asthma. Acad Pediatr. 2017;17(1):79-87. https://doi.org/10.1016/j.acap.2016.07.003.
20. Auger KA, Kahn RS, Davis MM, Simmons JM. Pediatric asthma readmission: asthma knowledge is not enough? J Pediatr. 2015;166(1):101-108. https://doi.org/10.1016/j.jpeds.2014.07.046.
21. Seid M, Varni JW, Bermudez LO, et al. Parents’ perceptions of primary care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264-270. https://doi:10.1542/peds.108.2.264.
22. Schickedanz A, Halfon N, Sastry N, Chung PJ. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics. 2018;142(2). https://doi.org/10.1542/peds.2018-0023.
23. Folger AT, Eismann EA, Stephenson NB, et al. Parental adverse childhood experiences and offspring development at 2 years of age. Pediatrics. 2018;141(4):e20172826. https://doi.org/10.1542/peds.2017-2826.
24. O’Malley DM, Randell KA, Dowd MD. Family adversity and resilience measures in pediatric acute care settings. Public Health Nurs. 2016;33(1):3-10. https://doi.org/10.1111/phn.12246.
25. Masten AS. Resilience in developing systems: the promise of integrated approaches. Eur J Dev Psychol. 2016;13(3):297-312. https://doi.org/10.1080/17405629.2016.1147344.
26. Burns-Nader S, Hernandez-Reif M. Facilitating play for hospitalized children through child life services. Child Health Care. 2016;45(1):1-21. https://doi.org/10.1080/02739615.2014.948161.
27. Feudtner C, Haney J, Dimmers MA. Spiritual care needs of hospitalized children and their families: a national survey of pastoral care providers’ perceptions. Pediatrics. 2003;111(1):e67-e72. https://doi.org/10.1542/peds.111.1.e67.
28. Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 2015;54(5):574-580. https://doi.org/10.3109/0284186X.2014.995774.
29. Kodish I. Behavioral health care for children who are medically hospitalized. Pediatr Ann. 2018;47(8):e323-e327. https://doi.org/10.3928/19382359-20180705-01.
30. Doupnik SK, Hill D, Palakshappa D, et al. Parent coping support interventions during acute pediatric hospitalizations: a meta-analysis. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2016-4171.
31. Hill DL, Carroll KW, Snyder KJG, et al. Development and pilot testing of a coping kit for parents of hospitalized children. Acad Pediatr. 2019;19(4):454-463. https://doi.org/10.1016/j.acap.2018.11.001.
32. Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol. 2010;35(9):966-974. https://doi.org/10.1093/jpepsy/jsq004.
33. Bowen EA, Murshid NS. Trauma-informed social policy: a conceptual framework for policy analysis and advocacy. Am J Public Health. 2016;106(2):223-229. https://doi.org/10.2105/AJPH.2015.302970.
34. Substance Abuse and Mental Health Services Administration. SAMHSA’s Concept of Trauma and Guidance for a Trauma-Informed Approach. Rockville, MD: SAMHSA; 2014.
35. Machtinger EL, Cuca YP, Khanna N, Rose CD, Kimberg LS. From treatment to healing: the promise of trauma-informed primary care. Womens Health Issues. 2015;25(3):193-197. https://doi.org/10.1016/j.whi.2015.03.008.
36. Green BL, Saunders PA, Power E, et al. Trauma-informed medical care: patient response to a primary care provider communication training. J Loss Trauma . 2016;21(2):147-159. https://doi.org/10.1080/15325024.2015.1084854.
37. McKay S, Parente V. Health Disparities in the Hospitalized Child. Hosp Pediatr. 2019;9(5):317-325. https://doi.org/10.1542/hpeds.2018-0223.
38. Kerker BD, Storfer-Isser A, Szilagyi M, et al. Do pediatricians ask about adverse childhood experiences in pediatric primary care? Acad Pediatr. 2016;16(2):154-160. https://doi.org/10.1
Adverse Childhood Experiences, or ACEs, include exposure to abuse, neglect, or household dysfunction (eg, having a parent who is mentally ill) as a child.1 Exposure to ACEs affects health into adulthood, with a dose-response relationship between ACEs and a range of comorbidities.1 Adults with 6 or more ACEs have a 20-year shorter life expectancy than do those with no ACEs.1 Still, ACEs are static; once experienced, that experience cannot be undone. However, resilience, or positive adaptation in the context of adversity, can be protective, buffering the negative effects of ACEs.2,3 Protective factors that promote resilience include social capital, such as positive relationships with caregivers and peers.3
With their clear link to health outcomes across the life-course, there is a movement for pediatricians to screen children for ACEs4 and to develop strategies that promote resilience in children, parents, and families. However, screening a child for adversity has challenges because younger children may not have experienced an adverse exposure, or they may be unable to voice their experiences. Studies have demonstrated that parental adversity, or ACEs, may be a marker for childhood adversity.5,6 Biological models also support this potential intergenerational effect of ACEs. Chronic exposure to stress, including ACEs, results in elevated cortisol via a dysregulated hypothalamic-pituitary-adrenal axis, which results in chronic inflammation.7 This “toxic stress” is prolonged, severe in intensity, and can lead to epigenetic changes that may be passed on to the next generation.8,9
Hospitalization of an ill child, and the transition to home after that hospitalization, is a stressful event for children and families.10 This stress may be relevant to parents that have a history of a high rate of ACEs or a current low degree of resilience. Our previous work demonstrated that, in the inpatient setting, parents with high ACEs (≥4) or low resilience have increased coping difficulty 14 days after their child’s hospital discharge.11 Our objective here was to evaluate whether a parent’s ACEs and/or resilience would also be associated with that child’s likelihood of reutilization. We hypothesized that more parental ACEs and/or lower parental resilience would be associated with revisits the emergency room, urgent care, or hospital readmissions.
METHODS
Participants and Study Design
We conducted a prospective cohort study of parents of hospitalized children recruited from the “Hospital-to-Home Outcomes” Studies (H2O I and H2O II).12,13 H2O I and II were prospective, single-center, randomized controlled trials designed to determine the effectiveness of either a nurse-led transitional home visit (H2O I) or telephone call (H2O II) on 30-day unplanned healthcare reutilization. The trials and this study were approved by the Cincinnati Children’s Institutional Review Board. All parents provided written informed consent.
Details of H2O I and II recruitment and design have been described previously.12,13 Briefly, children were eligible for inclusion in either study if they were admitted to our institution’s general Hospital Medicine or the Hospital Medicine Complex Care Services; for H2O I, children hospitalized on the Neurology and Neurosurgery services were also eligible.12,13 Patients were excluded if they were discharged to a residential facility, if they lived outside the home healthcare nurse service area, if they were eligible for skilled home healthcare services (eg, intravenous antibiotics), or if the participating caregiver was non-English speaking.12,13 In H2O I, families were randomized either to receive a single nurse home visit within 96 hours of discharge or standard of care. In H2O II, families enrolled were randomized to receive a telephone call by a nurse within 96 hours of discharge or standard of care. As we have previously published, randomization in both trials successfully balanced the intervention and control arms with respect to key demographic characteristics.12,13 For the analyses presented here, we focused on a subset of caregivers 18 years and older whose children were enrolled in either H2O I or II between August 2015 and October 2016. In both H2O trials, face-to-face and paper-based questionnaires were completed by parents during the index hospitalization.
Outcome and Predictors
Our primary outcome was unanticipated healthcare reutilization defined as return to the emergency room, urgent care, or unplanned readmission within 30 days of hospital discharge, consistent with the H2O trials. This was measured using the primary institution’s administrative data supplemented by a utilization database shared across regional hospitals.14 Readmissions were identified as “unplanned” using a previously validated algorithm,15 and treated as a dichotomous yes/no variable.
Our primary predictors were parental ACEs and resilience (see Appendix Tables). The ACE questionnaire addresses abuse, neglect, and household dysfunction in the first 18 years of life.1 It is composed of 10 questions, each with a yes/no response.1 We defined parents as low (ACE 0), moderate (ACE 1-3), or high (ACE ≥4) risk a priori because previous literature has described poor outcomes in adults with 4 or more ACEs.16
Given the sensitive nature of the questions, respondents independently completed the ACE questionnaire on paper instead of via the face-to-face survey. Respondents returned the completed questionnaire to the research assistant in a sealed envelope. All families received educational information on relevant hospital and community-based resources (eg, social work).
Parental resilience was measured using the Brief Resilience Scale (BRS). The BRS is 6 items, each on a 5-point Likert scale. Responses were averaged, providing a total score of 1-5; higher scores are representative of higher resilience.17 We treated the BRS score as a continuous variable. BRS has been used in clinical settings; it has demonstrated positive correlation with social support and negative correlation with fatigue.17 Parents answered BRS questions during the index pediatric hospitalization in a face-to-face interview.
Parent and Child Characteristics
Parent and child sociodemographic variables were also obtained during the face-to-face interview. Parental variables included age, gender, educational attainment, household income, employment status, and financial and social strain.11 Educational attainment was analyzed in 2 categories—high school or less vs more than high school—because most discharge instructions are written at a high school reading level.18 Parents reported their annual household income in the following categories: <$15,000; $15,000-$29,999; $30,000-$44,999; $45,000-$59,999; $60,000-$89,999; $90,000-$119,999; ≥$120,000. Employment was dichotomized as not employed/student vs any employment. Financial and social strain were assessed using a series of 9 previously described questions.19 These questions assessed, via self-report, a family’s ability to make ends meet, ability to pay rent/mortgage or utilities, need to move in with others because of financial reasons, and ability to borrow money if needed, as well as home ownership and parental marital status.15,19 Strain questions were all dichotomous (yes/no, single/not single). A composite variable was then constructed that categorized those reporting no strain items, 1 to 2 items, 3 to 4 items, and 5 or more items.20
Child variables included race, ethnicity, age, primary care access,21 payer, and H2O treatment arm. Race categories were white/Caucasian, black/African American, American Indian or Alaskan Native, Asian or Pacific Islander, and other; ethnicity categories were Hispanic/Latino, non-Hispanic/Latino, and unknown. Given relatively low numbers of children reported to be Hispanic/Latino, we combined race and ethnicity into a single variable, categorized as non-Hispanic/white, non-Hispanic/black, and multiracial/Hispanic/other. Primary care access was assessed using the access subscale to the Parent’s Perception of Primary Care questionnaire. This includes assessment of a family’s ability to travel to their doctor, to see their doctor for routine or sick care, and to get help or advice on evenings or weekends. Scores were categorized as always adequate, almost always adequate, or sometimes/never adequate.21 Payer was dichotomized to private or public/self-pay.
Statistical Analyses
We examined the distribution of outcomes, predictors, and covariates. We compared sociodemographic characteristics of those respondents and nonrespondents to the ACE screen using the chi-square test for categorical variables or the t test for continuous variables. We used logistic regression to assess for associations between the independent variables of interest and reutilization, adjusting for potential confounders. To build our adjusted, multivariable model, we decided a priori to include child race/ethnicity, primary care access, financial and social strain, and trial treatment arm. We treated the H2O I control group as the referent group. Other covariates considered for inclusion were caregiver education, household income, employment, and payer. These were included in multivariable models if bivariate associations were significant at the P < .1 level. We assessed an ACE-by-resilience interaction term because we hypothesized that those with more ACEs and lower resilience may have more reutilization outcomes than parents with fewer ACEs and higher resilience. We also evaluated interaction terms between trial arm assignment and predictors to assess effects that may be introduced by the randomization. Predictors in the final logistic regression model were significant at the P < .05 level. Logistic regression assumption of little or no multicollinearity among the independent variables was verified in the final models. All analyses were performed with Stata v16 (Stata Corp, College Station, Texas).
RESULTS
There were a total of 1,787 parent-child dyads enrolled in the H2O I and II during the study period; 1,320 parents (74%) completed the ACE questionnaire and were included the analysis. Included parents were primarily female and employed, as well as educated beyond high school (Table 1). Overall, 64% reported one or more ACEs (range 0 to 9); 45% reported 1to 3, and 19% reported 4 or more ACEs. The most commonly reported ACEs were divorce (n = 573, 43%), exposure to alcoholism (n = 306, 23%), and exposure to mental illness (n = 281, 21%; Figure 1). Parents had a mean BRS score of 3.97 (range 1.17-5.00), with the distribution shown in Figure 2.

Of the 1,320 included patients, the average length of stay was 2.5 days, and 82% of hospitalizations were caused by acute medical issues (eg, bronchiolitis). A total of 211 children experienced a reutilization event within 30 days of discharge. In bivariate analysis, children with parents with 4 or more ACEs had a 2.02-times (95% CI 1.35-3.02) higher odds of experiencing a reutilization event than did those with parents reporting no ACEs. Parents with higher resilience scores had children with a lower odds of reutilization (odds ratio [OR] 0.77 95% CI 0.63-0.95).

In addition to our a priori variables, parental education, employment, and insurance met our significance threshold for inclusion in the multivariable model. The ACE-by-resilience interaction term was not significant and not included in the model. Similarly, there was no significant interaction between ACE and resilience and H2O treatment arm; the interaction terms were not included in the final adjusted model, but treatment arm assignment was kept as a covariate. A total of 1,292 children, out of the 1,320 respondents, remained in the final multivariable model; the excluded 28 had incomplete covariate data but were not otherwise different. In this final adjusted model, children with parents reporting 4 or more ACEs had a 1.69-times (95% CI 1.11-2.60) greater odds of reutilization than did those with parents reporting no ACEs (Table 2). Resilience failed to reach statistical significance in the adjusted model (OR 0.86, 95% CI 0.70-1.07).

DISCUSSION
We found that high-risk parents (4 or more ACEs) had children with an increased odds of healthcare reutilization, suggesting intergenerational effects of ACEs. We did not find a similar effect relating to parental resilience. We also did not find an interaction between parental ACEs and resilience, suggesting that a parent’s reported degree of resilience does not modify the effect of ACEs on reutilization risk.

Parental adversity may be a risk factor for a child’s unanticipated reutilization. We previously demonstrated that parents with 4 or more ACEs have more coping difficulty than a parent with no ACEs after a child’s hospitalization.11 It is possible that parents with high adversity may have poorer coping mechanisms when dealing with a stressful situation, such as a child’s hospitalization. This may have resulted in inequitable outcomes (eg, increased reutilization) for their children. Other studies have confirmed such an intergenerational effect of adversity, linking a parent’s ACEs with poor developmental, behavioral, and health outcomes in their children.6,22,23 O’Malley et al showed an association of parental ACEs to current adversities,24 such as insurance or housing concerns, that affect the entirety of the household, including children. In short, it appears that parental ACEs may be a compelling predictor of current childhood adversity.
Resilience buffers the negative effects of ACEs; however, we did not find significant associations between resilience and reutilization or an interaction between ACEs and resilience. The factors that may contribute to reutilization are complex. In our previous work, parental resilience was associated with coping difficulty after discharge; but again, did not interact with parental ACEs.11 Here, we suggest that while resilience may buffer the negative effects of ACEs, that buffering may not affect the likelihood of reutilization. It is also possible that the BRS tool is of less relevance on how one handles the stress of a child’s hospitalization. While the BRS is one measure of resilience, there are many other relevant constructs to resilience, such as connection to social supports, that also may also contribute to risk of reutilization.25
Reducing the stress of a hospitalization itself and promoting a safe transition from hospital to home is critical to improving child health outcomes. Our data here, and in our previous work, demonstrate that a history of adversity and one’s current coping ability may drive a parent’s response to a child’s hospitalization and affect their capacity to care for that child after hospital discharge.11 Additional in-hospital supports like child life, behavioral health, or pastoral care could reduce the stress of the hospitalization while also building positive coping mechanisms.26-29 A meta-analysis demonstrated that such coping interventions can help alleviate the stress of a hospitalization.30 Hill et al demonstrated successful stress reduction in parents of hospitalized children using a “Coping Kit for Parents.”31 Further studies are warranted to understand which interventions are most effective for children and families and whether they could be more effectively deployed if the inpatient team knew more about parental ACEs.
Screening for parental ACEs could help to identify patients at highest risk for a poor transition to home. Therefore, screening for parental adversity in clinical settings, including inpatient settings, may be relevant and valuable.32 Additionally, by recognizing the high prevalence of ACEs in an inpatient setting, hospitals and healthcare organizations could be motivated to develop and enact trauma-informed approaches. A trauma-informed care approach recognizes the intersection of trauma with health and social problems. With this recognition, care teams can more sensitively address the trauma as they provide relevant services.33 Trauma-informed care is a secondary public health prevention approach that would help team members identify the prevalence and effects of trauma via screening, recognize the signs of a maladaptive response to stress, and respond by integrating awareness of trauma into practice management.28,34 Both the National Academy of Medicine and the Agency for Healthcare Research and Quality have called for such a trauma-informed approach in primary care.35 In response, many healthcare organizations have developed trauma-informed practices to better address the needs of the populations they serve. For example, provider training on this approach has led to improved rapport in patient-provider relationships.36
Although ACE awareness is a component of trauma-informed care, there are still limitations of the original ACE questionnaire developed by Felitti et al. The existing tool is not inclusive of all adversities a parent or child may face. Moreover, its focus is on past exposures and experiences and not current health-related social needs (eg, food insecurity) which have known linkages with a range of health outcomes and health disparities.37 Additionally, the original ACE questionnaire was created as a population level tool and not as a screening tool. If used as a screening tool, providers may view the questions as too sensitive to ask, and parents may have difficulty responding to and understanding the relevance to their child’s care. Therefore, we suggest that more evidence is required to understand how to best adapt ACE questions into a screening processes that may be implemented in a medical setting.
More evidence is also needed to determine when and where such screening may be most useful. A primary care provider would be best equipped to screen caregivers for ACEs given their established relationship with parents and patients. Given the potential relevance of such information for inpatient care provision, information could then flow from primary care to the inpatient team. However, because not all patients have established primary care providers and only 4% of pediatricians screen for ACEs,38 it is important for inpatient medical teams to understand their role in identifying and addressing ACEs during hospital stays. Development of a screening tool, with input from all stakeholders—including parents—that is valid and feasible for use in a pediatric inpatient setting would be an important step forward. This tool should be paired with training in how to discuss these topics in a trauma-informed, nonjudgmental, empathic manner. We see this as a way in which providers can more effectively elicit an accurate response while simultaneously educating parents on the relevance of such sensitive topics during an acute hospital stay. We also recommend that screening should always be paired with response capabilities that connect those who screen positive with resources that could help them to navigate the stress experienced during and after a child’s hospitalization. Furthermore, communication with primary care providers about parents that screen positive should be integrated into the transition process.
This work has several limitations. First, our study was a part of randomized controlled trials conducted in one academic setting, which thereby limits generalizability. For example, we limited our cohort to those who were English-speaking patients only. This may bias our results because respondents with limited English proficiency may have different risk profiles than their English-speaking peers. In addition, the administration of the both the ACE and resilience questionnaires occurred during an acutely stressful period, which may influence how a parent responds to these questions. Also, both of the surveys are self-reported by parents, which may be susceptible to memory and response biases. Relatedly, we had a high number of nonrespondents, particularly to the ACE questionnaire. Our results are therefore only relevant to those who chose to respond and cannot be applied to nonrespondents. Further work assessing why one does or does not respond to such sensitive questions is an important area for future inquiry. Lastly, our cohort had limited medical complexity; future studies may consider links between parental ACEs (and resilience) and morbidity experienced by children with medical complexity.
CONCLUSION
Parents history of adversity is linked to their children’s unanticipated healthcare reutilization after a hospital discharge. Screening for parental stressors during a hospitalization may be an important first step to connecting parents and children to evidence-based interventions capable of mitigating the stress of hospitalization and promoting better, more seamless transitions from hospital to home.
Acknowledgments
Group Members: The following H2O members are nonauthor contributors: JoAnne Bachus, BSN, RN; Monica Borell, BSN, RN; Lenisa V Chang, MA, PhD; Patricia Crawford, RN; Sarah Ferris, BA; Jennifer Gold, BSN, RN; Judy A Heilman, BSN, RN; Jane C Khoury, PhD; Pierce Kuhnell, MS; Karen Lawley, BSN, RN; Margo Moore, MS, BSN, RN; Lynne O’Donnell, BSN, RN; Sarah Riddle, MD; Susan N Sherman, DPA; Angela M Statile, MD, MEd; Karen P Sullivan, BSN, RN; Heather Tubbs-Cooley, PhD, RN; Susan Wade-Murphy, MSN, RN; and Christine M White, MD, MAT.
The authors also thank David Keller, MD, for his guidance on the study.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding Source
Supported by funds from the Academic Pediatric Young Investigator Award (Dr A Shah) and the Patient-Centered Outcomes Research Institute Award (IHS-1306-0081, to Dr K Auger, Dr S Shah, Dr H Sucharew, Dr J Simmons), the National Institutes of Health (1K23AI112916, to Dr AF Beck), and the Agency for Healthcare Research and Quality (1K12HS026393-01, to Dr A Shah, K08-HS024735- 01A1, to Dr K Auger). Dr J Haney received Summer Undergraduate Research Fellowship funding through the Summer Undergraduate Research Fellowship at Cincinnati Children’s Hospital Medical Center.
Disclaimer
All statements in this report, including findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or the Methodology Committee.
Adverse Childhood Experiences, or ACEs, include exposure to abuse, neglect, or household dysfunction (eg, having a parent who is mentally ill) as a child.1 Exposure to ACEs affects health into adulthood, with a dose-response relationship between ACEs and a range of comorbidities.1 Adults with 6 or more ACEs have a 20-year shorter life expectancy than do those with no ACEs.1 Still, ACEs are static; once experienced, that experience cannot be undone. However, resilience, or positive adaptation in the context of adversity, can be protective, buffering the negative effects of ACEs.2,3 Protective factors that promote resilience include social capital, such as positive relationships with caregivers and peers.3
With their clear link to health outcomes across the life-course, there is a movement for pediatricians to screen children for ACEs4 and to develop strategies that promote resilience in children, parents, and families. However, screening a child for adversity has challenges because younger children may not have experienced an adverse exposure, or they may be unable to voice their experiences. Studies have demonstrated that parental adversity, or ACEs, may be a marker for childhood adversity.5,6 Biological models also support this potential intergenerational effect of ACEs. Chronic exposure to stress, including ACEs, results in elevated cortisol via a dysregulated hypothalamic-pituitary-adrenal axis, which results in chronic inflammation.7 This “toxic stress” is prolonged, severe in intensity, and can lead to epigenetic changes that may be passed on to the next generation.8,9
Hospitalization of an ill child, and the transition to home after that hospitalization, is a stressful event for children and families.10 This stress may be relevant to parents that have a history of a high rate of ACEs or a current low degree of resilience. Our previous work demonstrated that, in the inpatient setting, parents with high ACEs (≥4) or low resilience have increased coping difficulty 14 days after their child’s hospital discharge.11 Our objective here was to evaluate whether a parent’s ACEs and/or resilience would also be associated with that child’s likelihood of reutilization. We hypothesized that more parental ACEs and/or lower parental resilience would be associated with revisits the emergency room, urgent care, or hospital readmissions.
METHODS
Participants and Study Design
We conducted a prospective cohort study of parents of hospitalized children recruited from the “Hospital-to-Home Outcomes” Studies (H2O I and H2O II).12,13 H2O I and II were prospective, single-center, randomized controlled trials designed to determine the effectiveness of either a nurse-led transitional home visit (H2O I) or telephone call (H2O II) on 30-day unplanned healthcare reutilization. The trials and this study were approved by the Cincinnati Children’s Institutional Review Board. All parents provided written informed consent.
Details of H2O I and II recruitment and design have been described previously.12,13 Briefly, children were eligible for inclusion in either study if they were admitted to our institution’s general Hospital Medicine or the Hospital Medicine Complex Care Services; for H2O I, children hospitalized on the Neurology and Neurosurgery services were also eligible.12,13 Patients were excluded if they were discharged to a residential facility, if they lived outside the home healthcare nurse service area, if they were eligible for skilled home healthcare services (eg, intravenous antibiotics), or if the participating caregiver was non-English speaking.12,13 In H2O I, families were randomized either to receive a single nurse home visit within 96 hours of discharge or standard of care. In H2O II, families enrolled were randomized to receive a telephone call by a nurse within 96 hours of discharge or standard of care. As we have previously published, randomization in both trials successfully balanced the intervention and control arms with respect to key demographic characteristics.12,13 For the analyses presented here, we focused on a subset of caregivers 18 years and older whose children were enrolled in either H2O I or II between August 2015 and October 2016. In both H2O trials, face-to-face and paper-based questionnaires were completed by parents during the index hospitalization.
Outcome and Predictors
Our primary outcome was unanticipated healthcare reutilization defined as return to the emergency room, urgent care, or unplanned readmission within 30 days of hospital discharge, consistent with the H2O trials. This was measured using the primary institution’s administrative data supplemented by a utilization database shared across regional hospitals.14 Readmissions were identified as “unplanned” using a previously validated algorithm,15 and treated as a dichotomous yes/no variable.
Our primary predictors were parental ACEs and resilience (see Appendix Tables). The ACE questionnaire addresses abuse, neglect, and household dysfunction in the first 18 years of life.1 It is composed of 10 questions, each with a yes/no response.1 We defined parents as low (ACE 0), moderate (ACE 1-3), or high (ACE ≥4) risk a priori because previous literature has described poor outcomes in adults with 4 or more ACEs.16
Given the sensitive nature of the questions, respondents independently completed the ACE questionnaire on paper instead of via the face-to-face survey. Respondents returned the completed questionnaire to the research assistant in a sealed envelope. All families received educational information on relevant hospital and community-based resources (eg, social work).
Parental resilience was measured using the Brief Resilience Scale (BRS). The BRS is 6 items, each on a 5-point Likert scale. Responses were averaged, providing a total score of 1-5; higher scores are representative of higher resilience.17 We treated the BRS score as a continuous variable. BRS has been used in clinical settings; it has demonstrated positive correlation with social support and negative correlation with fatigue.17 Parents answered BRS questions during the index pediatric hospitalization in a face-to-face interview.
Parent and Child Characteristics
Parent and child sociodemographic variables were also obtained during the face-to-face interview. Parental variables included age, gender, educational attainment, household income, employment status, and financial and social strain.11 Educational attainment was analyzed in 2 categories—high school or less vs more than high school—because most discharge instructions are written at a high school reading level.18 Parents reported their annual household income in the following categories: <$15,000; $15,000-$29,999; $30,000-$44,999; $45,000-$59,999; $60,000-$89,999; $90,000-$119,999; ≥$120,000. Employment was dichotomized as not employed/student vs any employment. Financial and social strain were assessed using a series of 9 previously described questions.19 These questions assessed, via self-report, a family’s ability to make ends meet, ability to pay rent/mortgage or utilities, need to move in with others because of financial reasons, and ability to borrow money if needed, as well as home ownership and parental marital status.15,19 Strain questions were all dichotomous (yes/no, single/not single). A composite variable was then constructed that categorized those reporting no strain items, 1 to 2 items, 3 to 4 items, and 5 or more items.20
Child variables included race, ethnicity, age, primary care access,21 payer, and H2O treatment arm. Race categories were white/Caucasian, black/African American, American Indian or Alaskan Native, Asian or Pacific Islander, and other; ethnicity categories were Hispanic/Latino, non-Hispanic/Latino, and unknown. Given relatively low numbers of children reported to be Hispanic/Latino, we combined race and ethnicity into a single variable, categorized as non-Hispanic/white, non-Hispanic/black, and multiracial/Hispanic/other. Primary care access was assessed using the access subscale to the Parent’s Perception of Primary Care questionnaire. This includes assessment of a family’s ability to travel to their doctor, to see their doctor for routine or sick care, and to get help or advice on evenings or weekends. Scores were categorized as always adequate, almost always adequate, or sometimes/never adequate.21 Payer was dichotomized to private or public/self-pay.
Statistical Analyses
We examined the distribution of outcomes, predictors, and covariates. We compared sociodemographic characteristics of those respondents and nonrespondents to the ACE screen using the chi-square test for categorical variables or the t test for continuous variables. We used logistic regression to assess for associations between the independent variables of interest and reutilization, adjusting for potential confounders. To build our adjusted, multivariable model, we decided a priori to include child race/ethnicity, primary care access, financial and social strain, and trial treatment arm. We treated the H2O I control group as the referent group. Other covariates considered for inclusion were caregiver education, household income, employment, and payer. These were included in multivariable models if bivariate associations were significant at the P < .1 level. We assessed an ACE-by-resilience interaction term because we hypothesized that those with more ACEs and lower resilience may have more reutilization outcomes than parents with fewer ACEs and higher resilience. We also evaluated interaction terms between trial arm assignment and predictors to assess effects that may be introduced by the randomization. Predictors in the final logistic regression model were significant at the P < .05 level. Logistic regression assumption of little or no multicollinearity among the independent variables was verified in the final models. All analyses were performed with Stata v16 (Stata Corp, College Station, Texas).
RESULTS
There were a total of 1,787 parent-child dyads enrolled in the H2O I and II during the study period; 1,320 parents (74%) completed the ACE questionnaire and were included the analysis. Included parents were primarily female and employed, as well as educated beyond high school (Table 1). Overall, 64% reported one or more ACEs (range 0 to 9); 45% reported 1to 3, and 19% reported 4 or more ACEs. The most commonly reported ACEs were divorce (n = 573, 43%), exposure to alcoholism (n = 306, 23%), and exposure to mental illness (n = 281, 21%; Figure 1). Parents had a mean BRS score of 3.97 (range 1.17-5.00), with the distribution shown in Figure 2.

Of the 1,320 included patients, the average length of stay was 2.5 days, and 82% of hospitalizations were caused by acute medical issues (eg, bronchiolitis). A total of 211 children experienced a reutilization event within 30 days of discharge. In bivariate analysis, children with parents with 4 or more ACEs had a 2.02-times (95% CI 1.35-3.02) higher odds of experiencing a reutilization event than did those with parents reporting no ACEs. Parents with higher resilience scores had children with a lower odds of reutilization (odds ratio [OR] 0.77 95% CI 0.63-0.95).

In addition to our a priori variables, parental education, employment, and insurance met our significance threshold for inclusion in the multivariable model. The ACE-by-resilience interaction term was not significant and not included in the model. Similarly, there was no significant interaction between ACE and resilience and H2O treatment arm; the interaction terms were not included in the final adjusted model, but treatment arm assignment was kept as a covariate. A total of 1,292 children, out of the 1,320 respondents, remained in the final multivariable model; the excluded 28 had incomplete covariate data but were not otherwise different. In this final adjusted model, children with parents reporting 4 or more ACEs had a 1.69-times (95% CI 1.11-2.60) greater odds of reutilization than did those with parents reporting no ACEs (Table 2). Resilience failed to reach statistical significance in the adjusted model (OR 0.86, 95% CI 0.70-1.07).

DISCUSSION
We found that high-risk parents (4 or more ACEs) had children with an increased odds of healthcare reutilization, suggesting intergenerational effects of ACEs. We did not find a similar effect relating to parental resilience. We also did not find an interaction between parental ACEs and resilience, suggesting that a parent’s reported degree of resilience does not modify the effect of ACEs on reutilization risk.

Parental adversity may be a risk factor for a child’s unanticipated reutilization. We previously demonstrated that parents with 4 or more ACEs have more coping difficulty than a parent with no ACEs after a child’s hospitalization.11 It is possible that parents with high adversity may have poorer coping mechanisms when dealing with a stressful situation, such as a child’s hospitalization. This may have resulted in inequitable outcomes (eg, increased reutilization) for their children. Other studies have confirmed such an intergenerational effect of adversity, linking a parent’s ACEs with poor developmental, behavioral, and health outcomes in their children.6,22,23 O’Malley et al showed an association of parental ACEs to current adversities,24 such as insurance or housing concerns, that affect the entirety of the household, including children. In short, it appears that parental ACEs may be a compelling predictor of current childhood adversity.
Resilience buffers the negative effects of ACEs; however, we did not find significant associations between resilience and reutilization or an interaction between ACEs and resilience. The factors that may contribute to reutilization are complex. In our previous work, parental resilience was associated with coping difficulty after discharge; but again, did not interact with parental ACEs.11 Here, we suggest that while resilience may buffer the negative effects of ACEs, that buffering may not affect the likelihood of reutilization. It is also possible that the BRS tool is of less relevance on how one handles the stress of a child’s hospitalization. While the BRS is one measure of resilience, there are many other relevant constructs to resilience, such as connection to social supports, that also may also contribute to risk of reutilization.25
Reducing the stress of a hospitalization itself and promoting a safe transition from hospital to home is critical to improving child health outcomes. Our data here, and in our previous work, demonstrate that a history of adversity and one’s current coping ability may drive a parent’s response to a child’s hospitalization and affect their capacity to care for that child after hospital discharge.11 Additional in-hospital supports like child life, behavioral health, or pastoral care could reduce the stress of the hospitalization while also building positive coping mechanisms.26-29 A meta-analysis demonstrated that such coping interventions can help alleviate the stress of a hospitalization.30 Hill et al demonstrated successful stress reduction in parents of hospitalized children using a “Coping Kit for Parents.”31 Further studies are warranted to understand which interventions are most effective for children and families and whether they could be more effectively deployed if the inpatient team knew more about parental ACEs.
Screening for parental ACEs could help to identify patients at highest risk for a poor transition to home. Therefore, screening for parental adversity in clinical settings, including inpatient settings, may be relevant and valuable.32 Additionally, by recognizing the high prevalence of ACEs in an inpatient setting, hospitals and healthcare organizations could be motivated to develop and enact trauma-informed approaches. A trauma-informed care approach recognizes the intersection of trauma with health and social problems. With this recognition, care teams can more sensitively address the trauma as they provide relevant services.33 Trauma-informed care is a secondary public health prevention approach that would help team members identify the prevalence and effects of trauma via screening, recognize the signs of a maladaptive response to stress, and respond by integrating awareness of trauma into practice management.28,34 Both the National Academy of Medicine and the Agency for Healthcare Research and Quality have called for such a trauma-informed approach in primary care.35 In response, many healthcare organizations have developed trauma-informed practices to better address the needs of the populations they serve. For example, provider training on this approach has led to improved rapport in patient-provider relationships.36
Although ACE awareness is a component of trauma-informed care, there are still limitations of the original ACE questionnaire developed by Felitti et al. The existing tool is not inclusive of all adversities a parent or child may face. Moreover, its focus is on past exposures and experiences and not current health-related social needs (eg, food insecurity) which have known linkages with a range of health outcomes and health disparities.37 Additionally, the original ACE questionnaire was created as a population level tool and not as a screening tool. If used as a screening tool, providers may view the questions as too sensitive to ask, and parents may have difficulty responding to and understanding the relevance to their child’s care. Therefore, we suggest that more evidence is required to understand how to best adapt ACE questions into a screening processes that may be implemented in a medical setting.
More evidence is also needed to determine when and where such screening may be most useful. A primary care provider would be best equipped to screen caregivers for ACEs given their established relationship with parents and patients. Given the potential relevance of such information for inpatient care provision, information could then flow from primary care to the inpatient team. However, because not all patients have established primary care providers and only 4% of pediatricians screen for ACEs,38 it is important for inpatient medical teams to understand their role in identifying and addressing ACEs during hospital stays. Development of a screening tool, with input from all stakeholders—including parents—that is valid and feasible for use in a pediatric inpatient setting would be an important step forward. This tool should be paired with training in how to discuss these topics in a trauma-informed, nonjudgmental, empathic manner. We see this as a way in which providers can more effectively elicit an accurate response while simultaneously educating parents on the relevance of such sensitive topics during an acute hospital stay. We also recommend that screening should always be paired with response capabilities that connect those who screen positive with resources that could help them to navigate the stress experienced during and after a child’s hospitalization. Furthermore, communication with primary care providers about parents that screen positive should be integrated into the transition process.
This work has several limitations. First, our study was a part of randomized controlled trials conducted in one academic setting, which thereby limits generalizability. For example, we limited our cohort to those who were English-speaking patients only. This may bias our results because respondents with limited English proficiency may have different risk profiles than their English-speaking peers. In addition, the administration of the both the ACE and resilience questionnaires occurred during an acutely stressful period, which may influence how a parent responds to these questions. Also, both of the surveys are self-reported by parents, which may be susceptible to memory and response biases. Relatedly, we had a high number of nonrespondents, particularly to the ACE questionnaire. Our results are therefore only relevant to those who chose to respond and cannot be applied to nonrespondents. Further work assessing why one does or does not respond to such sensitive questions is an important area for future inquiry. Lastly, our cohort had limited medical complexity; future studies may consider links between parental ACEs (and resilience) and morbidity experienced by children with medical complexity.
CONCLUSION
Parents history of adversity is linked to their children’s unanticipated healthcare reutilization after a hospital discharge. Screening for parental stressors during a hospitalization may be an important first step to connecting parents and children to evidence-based interventions capable of mitigating the stress of hospitalization and promoting better, more seamless transitions from hospital to home.
Acknowledgments
Group Members: The following H2O members are nonauthor contributors: JoAnne Bachus, BSN, RN; Monica Borell, BSN, RN; Lenisa V Chang, MA, PhD; Patricia Crawford, RN; Sarah Ferris, BA; Jennifer Gold, BSN, RN; Judy A Heilman, BSN, RN; Jane C Khoury, PhD; Pierce Kuhnell, MS; Karen Lawley, BSN, RN; Margo Moore, MS, BSN, RN; Lynne O’Donnell, BSN, RN; Sarah Riddle, MD; Susan N Sherman, DPA; Angela M Statile, MD, MEd; Karen P Sullivan, BSN, RN; Heather Tubbs-Cooley, PhD, RN; Susan Wade-Murphy, MSN, RN; and Christine M White, MD, MAT.
The authors also thank David Keller, MD, for his guidance on the study.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding Source
Supported by funds from the Academic Pediatric Young Investigator Award (Dr A Shah) and the Patient-Centered Outcomes Research Institute Award (IHS-1306-0081, to Dr K Auger, Dr S Shah, Dr H Sucharew, Dr J Simmons), the National Institutes of Health (1K23AI112916, to Dr AF Beck), and the Agency for Healthcare Research and Quality (1K12HS026393-01, to Dr A Shah, K08-HS024735- 01A1, to Dr K Auger). Dr J Haney received Summer Undergraduate Research Fellowship funding through the Summer Undergraduate Research Fellowship at Cincinnati Children’s Hospital Medical Center.
Disclaimer
All statements in this report, including findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or the Methodology Committee.
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. https://doi.org/10.1016/s0749-3797(98)00017-8.
2. Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff. 2014;33(12):2106-2115. https://doi.org/10.1377/hlthaff.2014.0914.
3. Masten AS. Ordinary Magic. Resilience processes in development. Am Psychol. 2001;56(3):227-238. https://doi.org/10.1037//0003-066x.56.3.227.
4. Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of C, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224-231. https://doi.org/10.1542/peds.2011-2662.
5. Randell KA, O’Malley D, Dowd MD. Association of parental adverse childhood experiences and current child adversity. JAMA Pediatrics. 2015;169(8):786-787. https://doi.org/10.1001/jamapediatrics.2015.0269.
6. Le-Scherban F, Wang X, Boyle-Steed KH, Pachter LM. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. 2018;141(6):e20174274. https://doi.org/10.1542/peds.2017-4274.
7. Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319-327. https://doi.org/10.1542/peds.2012-0469.
8. Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760-769. https://doi.org/10.1016/j.biopsych.2008.11.028.
9. Garner AS, Forkey H, Szilagyi M. Translating developmental science to address childhood adversity. Acad Pediatr. 2015;15(5):493-502. https://doi.org/10.1016/j.acap.2015.05.010.
10. Weiss M, Johnson NL, Malin S, Jerofke T, Lang C, Sherburne E. Readiness for discharge in parents of hospitalized children. J Pediatr Nurs. 2008;23(4):282-295. https://doi.org/10.1016/j.pedn.2007.10.005.
11. Shah AN, Beck AF, Sucharew HJ, et al. Parental adverse childhood experiences and resilience on coping after discharge. Pediatrics. 2018;141(4):e20172127. https://doi.org/10.1542/peds.2017-2127.
12. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: The Hospital to Home Outcomes (H2O) Trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919.
13. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
14. TheHealthCollaborative. Healthbridge analytics. http://healthcollab.org/hbanalytics/. Accessed August 11, 2017.
15. Auger K, Mueller E, Weinberg S, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org10.1016/j.jpeds.2015.11.051.
16. Felitti VJ. Belastungen in der Kindheit und Gesundheit im Erwachsenenalter: die Verwandlung von Gold in Blei [The relationship of adverse childhood experiences to adult health: turning gold into lead]. Z Psychosom Med Psychother. 2002;48(4):359-369. https://doi.org/10.13109/zptm.2002.48.4.359.
17. Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194-200. https://doi.org/10.1080/10705500802222972.
18. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798. https://doi.org/10.1046/j.1525-1497.1998.00242.x.
19. Auger KA, Kahn RS, Simmons JM, et al. Using address information to identify hardships reported by families of children hospitalized with asthma. Acad Pediatr. 2017;17(1):79-87. https://doi.org/10.1016/j.acap.2016.07.003.
20. Auger KA, Kahn RS, Davis MM, Simmons JM. Pediatric asthma readmission: asthma knowledge is not enough? J Pediatr. 2015;166(1):101-108. https://doi.org/10.1016/j.jpeds.2014.07.046.
21. Seid M, Varni JW, Bermudez LO, et al. Parents’ perceptions of primary care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264-270. https://doi:10.1542/peds.108.2.264.
22. Schickedanz A, Halfon N, Sastry N, Chung PJ. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics. 2018;142(2). https://doi.org/10.1542/peds.2018-0023.
23. Folger AT, Eismann EA, Stephenson NB, et al. Parental adverse childhood experiences and offspring development at 2 years of age. Pediatrics. 2018;141(4):e20172826. https://doi.org/10.1542/peds.2017-2826.
24. O’Malley DM, Randell KA, Dowd MD. Family adversity and resilience measures in pediatric acute care settings. Public Health Nurs. 2016;33(1):3-10. https://doi.org/10.1111/phn.12246.
25. Masten AS. Resilience in developing systems: the promise of integrated approaches. Eur J Dev Psychol. 2016;13(3):297-312. https://doi.org/10.1080/17405629.2016.1147344.
26. Burns-Nader S, Hernandez-Reif M. Facilitating play for hospitalized children through child life services. Child Health Care. 2016;45(1):1-21. https://doi.org/10.1080/02739615.2014.948161.
27. Feudtner C, Haney J, Dimmers MA. Spiritual care needs of hospitalized children and their families: a national survey of pastoral care providers’ perceptions. Pediatrics. 2003;111(1):e67-e72. https://doi.org/10.1542/peds.111.1.e67.
28. Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 2015;54(5):574-580. https://doi.org/10.3109/0284186X.2014.995774.
29. Kodish I. Behavioral health care for children who are medically hospitalized. Pediatr Ann. 2018;47(8):e323-e327. https://doi.org/10.3928/19382359-20180705-01.
30. Doupnik SK, Hill D, Palakshappa D, et al. Parent coping support interventions during acute pediatric hospitalizations: a meta-analysis. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2016-4171.
31. Hill DL, Carroll KW, Snyder KJG, et al. Development and pilot testing of a coping kit for parents of hospitalized children. Acad Pediatr. 2019;19(4):454-463. https://doi.org/10.1016/j.acap.2018.11.001.
32. Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol. 2010;35(9):966-974. https://doi.org/10.1093/jpepsy/jsq004.
33. Bowen EA, Murshid NS. Trauma-informed social policy: a conceptual framework for policy analysis and advocacy. Am J Public Health. 2016;106(2):223-229. https://doi.org/10.2105/AJPH.2015.302970.
34. Substance Abuse and Mental Health Services Administration. SAMHSA’s Concept of Trauma and Guidance for a Trauma-Informed Approach. Rockville, MD: SAMHSA; 2014.
35. Machtinger EL, Cuca YP, Khanna N, Rose CD, Kimberg LS. From treatment to healing: the promise of trauma-informed primary care. Womens Health Issues. 2015;25(3):193-197. https://doi.org/10.1016/j.whi.2015.03.008.
36. Green BL, Saunders PA, Power E, et al. Trauma-informed medical care: patient response to a primary care provider communication training. J Loss Trauma . 2016;21(2):147-159. https://doi.org/10.1080/15325024.2015.1084854.
37. McKay S, Parente V. Health Disparities in the Hospitalized Child. Hosp Pediatr. 2019;9(5):317-325. https://doi.org/10.1542/hpeds.2018-0223.
38. Kerker BD, Storfer-Isser A, Szilagyi M, et al. Do pediatricians ask about adverse childhood experiences in pediatric primary care? Acad Pediatr. 2016;16(2):154-160. https://doi.org/10.1
1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. https://doi.org/10.1016/s0749-3797(98)00017-8.
2. Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff. 2014;33(12):2106-2115. https://doi.org/10.1377/hlthaff.2014.0914.
3. Masten AS. Ordinary Magic. Resilience processes in development. Am Psychol. 2001;56(3):227-238. https://doi.org/10.1037//0003-066x.56.3.227.
4. Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of C, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224-231. https://doi.org/10.1542/peds.2011-2662.
5. Randell KA, O’Malley D, Dowd MD. Association of parental adverse childhood experiences and current child adversity. JAMA Pediatrics. 2015;169(8):786-787. https://doi.org/10.1001/jamapediatrics.2015.0269.
6. Le-Scherban F, Wang X, Boyle-Steed KH, Pachter LM. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. 2018;141(6):e20174274. https://doi.org/10.1542/peds.2017-4274.
7. Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319-327. https://doi.org/10.1542/peds.2012-0469.
8. Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760-769. https://doi.org/10.1016/j.biopsych.2008.11.028.
9. Garner AS, Forkey H, Szilagyi M. Translating developmental science to address childhood adversity. Acad Pediatr. 2015;15(5):493-502. https://doi.org/10.1016/j.acap.2015.05.010.
10. Weiss M, Johnson NL, Malin S, Jerofke T, Lang C, Sherburne E. Readiness for discharge in parents of hospitalized children. J Pediatr Nurs. 2008;23(4):282-295. https://doi.org/10.1016/j.pedn.2007.10.005.
11. Shah AN, Beck AF, Sucharew HJ, et al. Parental adverse childhood experiences and resilience on coping after discharge. Pediatrics. 2018;141(4):e20172127. https://doi.org/10.1542/peds.2017-2127.
12. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: The Hospital to Home Outcomes (H2O) Trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919.
13. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
14. TheHealthCollaborative. Healthbridge analytics. http://healthcollab.org/hbanalytics/. Accessed August 11, 2017.
15. Auger K, Mueller E, Weinberg S, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org10.1016/j.jpeds.2015.11.051.
16. Felitti VJ. Belastungen in der Kindheit und Gesundheit im Erwachsenenalter: die Verwandlung von Gold in Blei [The relationship of adverse childhood experiences to adult health: turning gold into lead]. Z Psychosom Med Psychother. 2002;48(4):359-369. https://doi.org/10.13109/zptm.2002.48.4.359.
17. Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194-200. https://doi.org/10.1080/10705500802222972.
18. Baker DW, Parker RM, Williams MV, Clark WS. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791-798. https://doi.org/10.1046/j.1525-1497.1998.00242.x.
19. Auger KA, Kahn RS, Simmons JM, et al. Using address information to identify hardships reported by families of children hospitalized with asthma. Acad Pediatr. 2017;17(1):79-87. https://doi.org/10.1016/j.acap.2016.07.003.
20. Auger KA, Kahn RS, Davis MM, Simmons JM. Pediatric asthma readmission: asthma knowledge is not enough? J Pediatr. 2015;166(1):101-108. https://doi.org/10.1016/j.jpeds.2014.07.046.
21. Seid M, Varni JW, Bermudez LO, et al. Parents’ perceptions of primary care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264-270. https://doi:10.1542/peds.108.2.264.
22. Schickedanz A, Halfon N, Sastry N, Chung PJ. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics. 2018;142(2). https://doi.org/10.1542/peds.2018-0023.
23. Folger AT, Eismann EA, Stephenson NB, et al. Parental adverse childhood experiences and offspring development at 2 years of age. Pediatrics. 2018;141(4):e20172826. https://doi.org/10.1542/peds.2017-2826.
24. O’Malley DM, Randell KA, Dowd MD. Family adversity and resilience measures in pediatric acute care settings. Public Health Nurs. 2016;33(1):3-10. https://doi.org/10.1111/phn.12246.
25. Masten AS. Resilience in developing systems: the promise of integrated approaches. Eur J Dev Psychol. 2016;13(3):297-312. https://doi.org/10.1080/17405629.2016.1147344.
26. Burns-Nader S, Hernandez-Reif M. Facilitating play for hospitalized children through child life services. Child Health Care. 2016;45(1):1-21. https://doi.org/10.1080/02739615.2014.948161.
27. Feudtner C, Haney J, Dimmers MA. Spiritual care needs of hospitalized children and their families: a national survey of pastoral care providers’ perceptions. Pediatrics. 2003;111(1):e67-e72. https://doi.org/10.1542/peds.111.1.e67.
28. Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 2015;54(5):574-580. https://doi.org/10.3109/0284186X.2014.995774.
29. Kodish I. Behavioral health care for children who are medically hospitalized. Pediatr Ann. 2018;47(8):e323-e327. https://doi.org/10.3928/19382359-20180705-01.
30. Doupnik SK, Hill D, Palakshappa D, et al. Parent coping support interventions during acute pediatric hospitalizations: a meta-analysis. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2016-4171.
31. Hill DL, Carroll KW, Snyder KJG, et al. Development and pilot testing of a coping kit for parents of hospitalized children. Acad Pediatr. 2019;19(4):454-463. https://doi.org/10.1016/j.acap.2018.11.001.
32. Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol. 2010;35(9):966-974. https://doi.org/10.1093/jpepsy/jsq004.
33. Bowen EA, Murshid NS. Trauma-informed social policy: a conceptual framework for policy analysis and advocacy. Am J Public Health. 2016;106(2):223-229. https://doi.org/10.2105/AJPH.2015.302970.
34. Substance Abuse and Mental Health Services Administration. SAMHSA’s Concept of Trauma and Guidance for a Trauma-Informed Approach. Rockville, MD: SAMHSA; 2014.
35. Machtinger EL, Cuca YP, Khanna N, Rose CD, Kimberg LS. From treatment to healing: the promise of trauma-informed primary care. Womens Health Issues. 2015;25(3):193-197. https://doi.org/10.1016/j.whi.2015.03.008.
36. Green BL, Saunders PA, Power E, et al. Trauma-informed medical care: patient response to a primary care provider communication training. J Loss Trauma . 2016;21(2):147-159. https://doi.org/10.1080/15325024.2015.1084854.
37. McKay S, Parente V. Health Disparities in the Hospitalized Child. Hosp Pediatr. 2019;9(5):317-325. https://doi.org/10.1542/hpeds.2018-0223.
38. Kerker BD, Storfer-Isser A, Szilagyi M, et al. Do pediatricians ask about adverse childhood experiences in pediatric primary care? Acad Pediatr. 2016;16(2):154-160. https://doi.org/10.1
© 2020 Society of Hospital Medicine
A Jaw-Dropping Diagnosis
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.
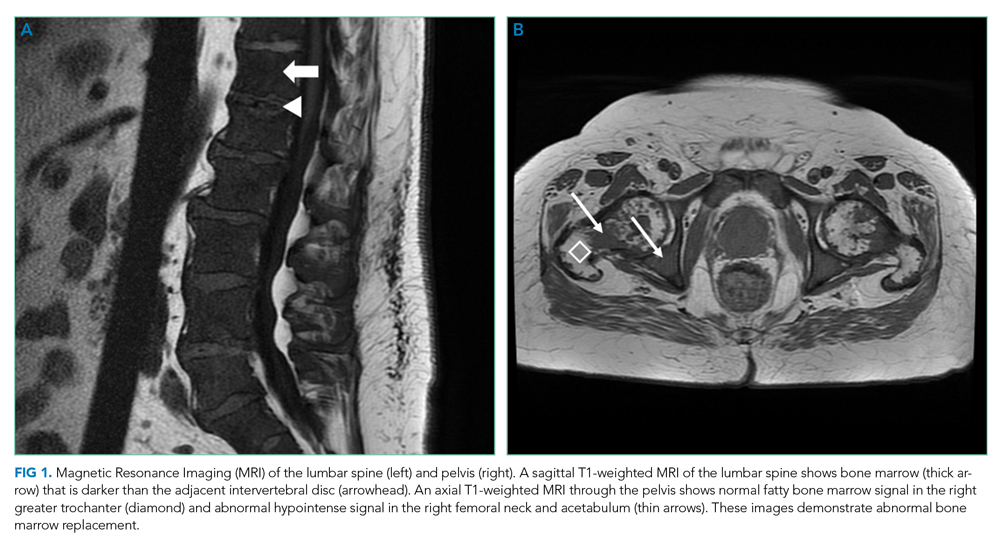
The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.
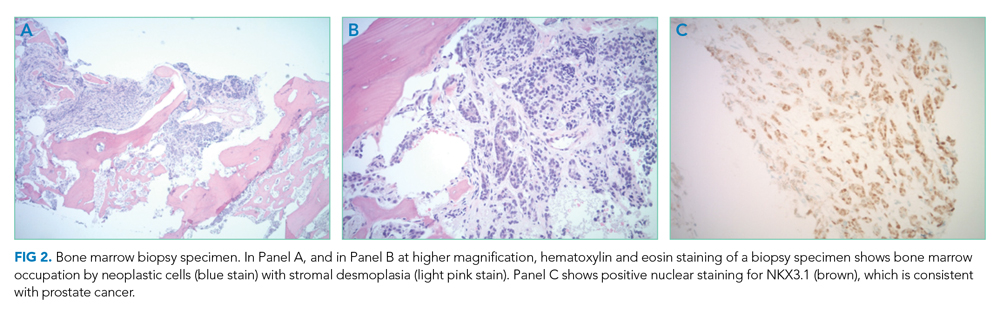
The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
© 2020 Society of Hospital Medicine
School intervention on mental illness stigma boosts treatment-seeking
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
A school curriculum–based intervention aimed at reducing the stigma of mental illness was associated with a nearly fourfold increase in the likelihood of youth with significant symptoms seeking treatment.
Writing in Pediatrics, researchers reported the outcome of a 2-year, longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes in 14 schools across Texas.
The intervention was a school-based curriculum program called Eliminating the Stigma of Differences (ESD); a 3-hour, three-module curriculum program delivered over 1 week, which contained a mix of teaching, group discussion, and homework exercises.
One module explored the idea of difference; the definition, causes and consequences of stigma; ways to end stigma; and the description, causes and treatments of mental illness as well as the barriers to seeking help. The other two modules explored specific mental illnesses in more detail but with content designed to stimulate empathy.
The study compared this with two other interventions – in-class presentations and discussions led by two young adults with a history of mental illness; or exposure to anti-stigma printed materials – and a no-intervention control.
The study found that involvement with the curriculum program was associated with a significant and sustained increase in knowledge of and attitudes to mental illness compared with the control and other interventions, and with significant decreases in social distance, which measures the extent to which children are unwilling to interact with someone who is identified as having a mental illness. This association was seen even after the researchers controlled for other factors such as a participants’ knowledge of or attitudes toward mental illness before the intervention, their age, sex, race or ethnicity, or their parents’ educational level.
“Our study, in combination with other studies, suggests strongly that youth can be positively influenced at a relatively young age, fostering changes in mental health attitudes and behaviors that last, as our study has shown, for at least 2 years,” wrote Bruce G. Link, PhD, of the School of Public Policy at the University of California, Riverside, and coauthors.
The study also found that among youth who were experiencing a high level of symptoms of mental illness, the curriculum-based intervention was associated with nearly fourfold higher odds of seeking treatment (odd ratio 3.9, P < .05), after adjustment for similar covariates.
The authors looked separately at whether this self-reported treatment-seeking was the first time that students had sought treatment, a continuation of treatment seeking, or a return to it. All three showed similar odds ratios but small sample sizes meant they did not reach statistical significance.
“We do know that negative attitudes toward mental illnesses and the exceptionally large percentage of people who experience but do not receive treatment for such illnesses are problems that have been with us for a long time,” Dr. Link and associates said. “Interventions such as ESD represent a partial but positive response to this public mental health challenge.”
The intervention didn’t lead to a significant increase in treatment-seeking behavior among students with low levels of mental illness symptoms.
There were no significant differences in the effectiveness of the intervention across race or ethnicity, sex, education level of caregivers, or the baseline attitudes toward mental illness. The only exception was seen with Latino youth, where the intervention was not associated with a decrease in social distancing.
Contact intervention, in which two young people with a history of mental illness came to talk to classes and participate in discussions, was not associated with any significant changes in attitudes.
“A potential explanation is that contact is not as effective in youth, a possibility that is supported by a meta-analysis showing diminished effects of contact compared with educational interventions in adolescents,” Dr. Link and associates said.
In an accompanying editorial, Nathaniel Beers, MD, of Children’s National Hospital in Washington, and Dr. Shashank V. Joshi, MD, of Stanford (Calif.) University, wrote that more than one-fifth of children and youth in the United States are diagnosed with behavioral health needs before they reach the age of 18, but the perception of stigma can make families reluctant to access treatment.
“Previous research has highlighted the importance of stigma reduction in school-based settings as a crucial component in changing the social norms about seeking help among diverse youth populations,” they said. Reducing stigma also can reduce detrimental outcomes from social isolation and bullying.
Dr. Beers and Dr. Joshi noted that school-based interventions can have a substantial and lasting effect, with the benefit of influencing parents and staff in addition to students.
“Combined with screening and improved access to school-based mental health services, this curriculum could add a critical component to addressing the mental health needs of children and youth in the United States,” they concluded.
The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures. Dr. Beers and Dr. Joshi received no external funding, and they said they had no relevant financial disclosures.
SOURCES: Link B. et al. Pediatrics 2020, May 20. doi: 10.1542/peds.2019-0780; Beers N and Joshi SV. Pediatrics. 2020, May 20. doi: 10.1542/peds.2020-0127.
FROM PEDIATRICS
Key clinical point: A curriculum-based intervention addressing stigma and mental illness had a significant impact on attitudes and treatment-seeking.
Major finding:
Study details: A longitudinal, cluster-randomized trial involving 416 students in sixth-grade classes across 14 schools.
Disclosures: The study was supported by the National Institute of Mental Health and National Institutes of Health. The authors said they had no relevant financial disclosures.
Source: Link B. et al. Pediatrics 2020 May 20. doi: 10.1542/peds.2019-0780.
Maskomania: Masks and COVID-19
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13
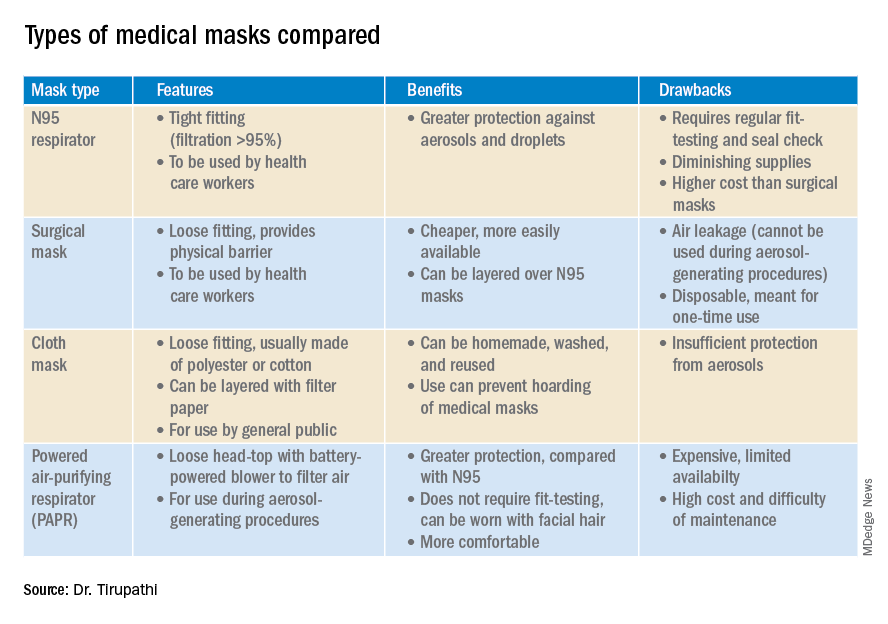
N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
A comprehensive review
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
Today’s top news highlights: COVID-19 in kids, addiction-related suicide
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Universal CAR-T therapy produces CRs in relapsed/refractory T-ALL
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
FROM AACR 2020
COVID-19: Reflections on Working Together Through a Pandemic
Dr. Tishler is Senior Vice President of Medical Services for Commonwealth Care Alliance, Boston, MA. She is also Editor-in-Chief of the Journal of Clinical Outcomes Management.
Just as we were moving toward remote work in the face of COVID-19, a nonmedical colleague said to me, “I’ve never really seen a doctor in a crisis; you’re so calm.
Let’s face it. At this point in my career, I’m not really on the front lines. I’m not running into ICU rooms, proning people with COVID-19 to stave off the need for a ventilator. I’m not holding up my iPad to enable a Zoom family conference. I’m not a caregiver in a COVID-19 isolation and recovery center for people experiencing homelessness. I’m not a member of anyone’s field team, continuing to provide home care in high-risk settings. Nope. My job now is to take care of the caregivers on the front lines who are doing all that—and the people who are supporting the caregivers doing all that. And in supporting our frontline clinicians and staff, I’m using some of the skills that I’ve gained from the relatively short time I’ve been a physician leader, but many more from the long years of being a clinician.
Late in January, I had a meeting with our chief medical officer. As our meeting was ending, I said to him, “You might think this is silly, but we need to start thinking about this new coronavirus and how it will impact our patients and our staff. I think we’ve probably got only a short time before we see a case here.” Leadership agreed, and we started our clinical Coronavirus Task Force that afternoon. Our executive leadership supported us, with consistent messaging that our organization would listen to the science and that the health of our members and employees was paramount.
Our timing and planning turned out to be correct. The first coronavirus case in Massachusetts appeared not even a week later. The infamous Biogen meeting took place late in February. By March 13, our entire workforce of more than 1000 people was at home. By March 24, we had retooled our integrated complex care organization to ensure that our most at-risk patients were still getting the home care they needed and that our staff were appropriately protected when they went into those homes. After years of debating about virtual care—telemedicine—we embraced it. As we worried deeply that our patients would be impacted by this virus in terrible ways—they are dually eligible for Medicare and Medicaid, poor, and quite sick—we discovered a level of resilience among many people that gave us great satisfaction and hope.
Over these past weeks, that Task Force has grown to become our Command Center. It’s grown from a group that was thinking about masks and hand hygiene (still important!) to a 10+ workstream, technology-enabled, working group that breaks down silos and solves problems in real time. We have made more than 1000 home visits, preserving employee health and PPE. We use dashboards to help us see trends and act appropriately. We add streams and remove them as needed. We use research (where it exists) and case studies to help inform our decisions.
When I was thinking about organizing this group and wondering how I was going to drink daily from a firehose, I heard in my head the voice of my very first resident during my internship. She said, “Present the patient by telling us the events of yesterday, followed by data—exam, vitals, and labs. Then, tell us what you need help with and your plans for tomorrow.” Suddenly, it seemed just that simple. I did know how to do this. We started what we called “rapid rounds,” and each day, each stream tells us what they’ve done, what data they have collected—that might be the number of patients seen in the field, the number of masks needed, or the number of our patients who are ill—what they need from the other members of the group, and what their plans are for tomorrow.
Working together to meet the challenges presented by the pandemic has been extraordinary. We see, every day, the power of a dedicated, diverse group of caring clinicians and nonclinicians to take a good idea and make it better. Over these past weeks, my colleagues have come up with amazing ideas that have helped us to provide excellent care for our members and for our staff. Like the best of medicine, it is science, art, and a lot of heart. New ideas abound. Many of these ideas will survive the lockdown. We have a weekly webinar to update hundreds of viewers on the ever-changing medicine and ever-changing processes related to COVID-19 as we learn more. We have developed ways to ensure people who are at the end of their lives can make appropriate choices for their goals of care. We have found ways to share, use, reuse, and decontaminate PPE. We have ensured that personal care needs for disabled members are met. We’ve informed the organization and worked closely with our Commonwealth. Along the way, we’ve become a tight team, sharing daily bright spots and some sad stories, new baby chicks and knitting projects, with pets and children making welcome cameos.
Yes, this is what we trained for. Not for a global pandemic, of course. But to be able to make sound, well-informed decisions with the best information possible, given the circumstances. Once those decisions are made, we need to share them, communicate them, and support our patients and each other. We need to acknowledge when we misstep and reorganize to be better next time. If one solution doesn’t work, we must go forward and try another. In the midst of horrible times, there is the opportunity, every day, for medicine to be at its very best.
Dr. Tishler is Senior Vice President of Medical Services for Commonwealth Care Alliance, Boston, MA. She is also Editor-in-Chief of the Journal of Clinical Outcomes Management.
Just as we were moving toward remote work in the face of COVID-19, a nonmedical colleague said to me, “I’ve never really seen a doctor in a crisis; you’re so calm.
Let’s face it. At this point in my career, I’m not really on the front lines. I’m not running into ICU rooms, proning people with COVID-19 to stave off the need for a ventilator. I’m not holding up my iPad to enable a Zoom family conference. I’m not a caregiver in a COVID-19 isolation and recovery center for people experiencing homelessness. I’m not a member of anyone’s field team, continuing to provide home care in high-risk settings. Nope. My job now is to take care of the caregivers on the front lines who are doing all that—and the people who are supporting the caregivers doing all that. And in supporting our frontline clinicians and staff, I’m using some of the skills that I’ve gained from the relatively short time I’ve been a physician leader, but many more from the long years of being a clinician.
Late in January, I had a meeting with our chief medical officer. As our meeting was ending, I said to him, “You might think this is silly, but we need to start thinking about this new coronavirus and how it will impact our patients and our staff. I think we’ve probably got only a short time before we see a case here.” Leadership agreed, and we started our clinical Coronavirus Task Force that afternoon. Our executive leadership supported us, with consistent messaging that our organization would listen to the science and that the health of our members and employees was paramount.
Our timing and planning turned out to be correct. The first coronavirus case in Massachusetts appeared not even a week later. The infamous Biogen meeting took place late in February. By March 13, our entire workforce of more than 1000 people was at home. By March 24, we had retooled our integrated complex care organization to ensure that our most at-risk patients were still getting the home care they needed and that our staff were appropriately protected when they went into those homes. After years of debating about virtual care—telemedicine—we embraced it. As we worried deeply that our patients would be impacted by this virus in terrible ways—they are dually eligible for Medicare and Medicaid, poor, and quite sick—we discovered a level of resilience among many people that gave us great satisfaction and hope.
Over these past weeks, that Task Force has grown to become our Command Center. It’s grown from a group that was thinking about masks and hand hygiene (still important!) to a 10+ workstream, technology-enabled, working group that breaks down silos and solves problems in real time. We have made more than 1000 home visits, preserving employee health and PPE. We use dashboards to help us see trends and act appropriately. We add streams and remove them as needed. We use research (where it exists) and case studies to help inform our decisions.
When I was thinking about organizing this group and wondering how I was going to drink daily from a firehose, I heard in my head the voice of my very first resident during my internship. She said, “Present the patient by telling us the events of yesterday, followed by data—exam, vitals, and labs. Then, tell us what you need help with and your plans for tomorrow.” Suddenly, it seemed just that simple. I did know how to do this. We started what we called “rapid rounds,” and each day, each stream tells us what they’ve done, what data they have collected—that might be the number of patients seen in the field, the number of masks needed, or the number of our patients who are ill—what they need from the other members of the group, and what their plans are for tomorrow.
Working together to meet the challenges presented by the pandemic has been extraordinary. We see, every day, the power of a dedicated, diverse group of caring clinicians and nonclinicians to take a good idea and make it better. Over these past weeks, my colleagues have come up with amazing ideas that have helped us to provide excellent care for our members and for our staff. Like the best of medicine, it is science, art, and a lot of heart. New ideas abound. Many of these ideas will survive the lockdown. We have a weekly webinar to update hundreds of viewers on the ever-changing medicine and ever-changing processes related to COVID-19 as we learn more. We have developed ways to ensure people who are at the end of their lives can make appropriate choices for their goals of care. We have found ways to share, use, reuse, and decontaminate PPE. We have ensured that personal care needs for disabled members are met. We’ve informed the organization and worked closely with our Commonwealth. Along the way, we’ve become a tight team, sharing daily bright spots and some sad stories, new baby chicks and knitting projects, with pets and children making welcome cameos.
Yes, this is what we trained for. Not for a global pandemic, of course. But to be able to make sound, well-informed decisions with the best information possible, given the circumstances. Once those decisions are made, we need to share them, communicate them, and support our patients and each other. We need to acknowledge when we misstep and reorganize to be better next time. If one solution doesn’t work, we must go forward and try another. In the midst of horrible times, there is the opportunity, every day, for medicine to be at its very best.
Dr. Tishler is Senior Vice President of Medical Services for Commonwealth Care Alliance, Boston, MA. She is also Editor-in-Chief of the Journal of Clinical Outcomes Management.
Just as we were moving toward remote work in the face of COVID-19, a nonmedical colleague said to me, “I’ve never really seen a doctor in a crisis; you’re so calm.
Let’s face it. At this point in my career, I’m not really on the front lines. I’m not running into ICU rooms, proning people with COVID-19 to stave off the need for a ventilator. I’m not holding up my iPad to enable a Zoom family conference. I’m not a caregiver in a COVID-19 isolation and recovery center for people experiencing homelessness. I’m not a member of anyone’s field team, continuing to provide home care in high-risk settings. Nope. My job now is to take care of the caregivers on the front lines who are doing all that—and the people who are supporting the caregivers doing all that. And in supporting our frontline clinicians and staff, I’m using some of the skills that I’ve gained from the relatively short time I’ve been a physician leader, but many more from the long years of being a clinician.
Late in January, I had a meeting with our chief medical officer. As our meeting was ending, I said to him, “You might think this is silly, but we need to start thinking about this new coronavirus and how it will impact our patients and our staff. I think we’ve probably got only a short time before we see a case here.” Leadership agreed, and we started our clinical Coronavirus Task Force that afternoon. Our executive leadership supported us, with consistent messaging that our organization would listen to the science and that the health of our members and employees was paramount.
Our timing and planning turned out to be correct. The first coronavirus case in Massachusetts appeared not even a week later. The infamous Biogen meeting took place late in February. By March 13, our entire workforce of more than 1000 people was at home. By March 24, we had retooled our integrated complex care organization to ensure that our most at-risk patients were still getting the home care they needed and that our staff were appropriately protected when they went into those homes. After years of debating about virtual care—telemedicine—we embraced it. As we worried deeply that our patients would be impacted by this virus in terrible ways—they are dually eligible for Medicare and Medicaid, poor, and quite sick—we discovered a level of resilience among many people that gave us great satisfaction and hope.
Over these past weeks, that Task Force has grown to become our Command Center. It’s grown from a group that was thinking about masks and hand hygiene (still important!) to a 10+ workstream, technology-enabled, working group that breaks down silos and solves problems in real time. We have made more than 1000 home visits, preserving employee health and PPE. We use dashboards to help us see trends and act appropriately. We add streams and remove them as needed. We use research (where it exists) and case studies to help inform our decisions.
When I was thinking about organizing this group and wondering how I was going to drink daily from a firehose, I heard in my head the voice of my very first resident during my internship. She said, “Present the patient by telling us the events of yesterday, followed by data—exam, vitals, and labs. Then, tell us what you need help with and your plans for tomorrow.” Suddenly, it seemed just that simple. I did know how to do this. We started what we called “rapid rounds,” and each day, each stream tells us what they’ve done, what data they have collected—that might be the number of patients seen in the field, the number of masks needed, or the number of our patients who are ill—what they need from the other members of the group, and what their plans are for tomorrow.
Working together to meet the challenges presented by the pandemic has been extraordinary. We see, every day, the power of a dedicated, diverse group of caring clinicians and nonclinicians to take a good idea and make it better. Over these past weeks, my colleagues have come up with amazing ideas that have helped us to provide excellent care for our members and for our staff. Like the best of medicine, it is science, art, and a lot of heart. New ideas abound. Many of these ideas will survive the lockdown. We have a weekly webinar to update hundreds of viewers on the ever-changing medicine and ever-changing processes related to COVID-19 as we learn more. We have developed ways to ensure people who are at the end of their lives can make appropriate choices for their goals of care. We have found ways to share, use, reuse, and decontaminate PPE. We have ensured that personal care needs for disabled members are met. We’ve informed the organization and worked closely with our Commonwealth. Along the way, we’ve become a tight team, sharing daily bright spots and some sad stories, new baby chicks and knitting projects, with pets and children making welcome cameos.
Yes, this is what we trained for. Not for a global pandemic, of course. But to be able to make sound, well-informed decisions with the best information possible, given the circumstances. Once those decisions are made, we need to share them, communicate them, and support our patients and each other. We need to acknowledge when we misstep and reorganize to be better next time. If one solution doesn’t work, we must go forward and try another. In the midst of horrible times, there is the opportunity, every day, for medicine to be at its very best.