User login
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
Testicular sperm may improve IVF outcomes in some cases
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
FROM ACOG 2020
Inflammatory back pain likely underrecognized in primary care
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
REPORTING FROM SPARTAN 2020
COVID-19 exacerbating challenges for Latino patients
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Disproportionate burden of pandemic complicates mental health care
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Race and location appear to play a role in the incidence of CLL and DLBCL
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Vulvar Syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.

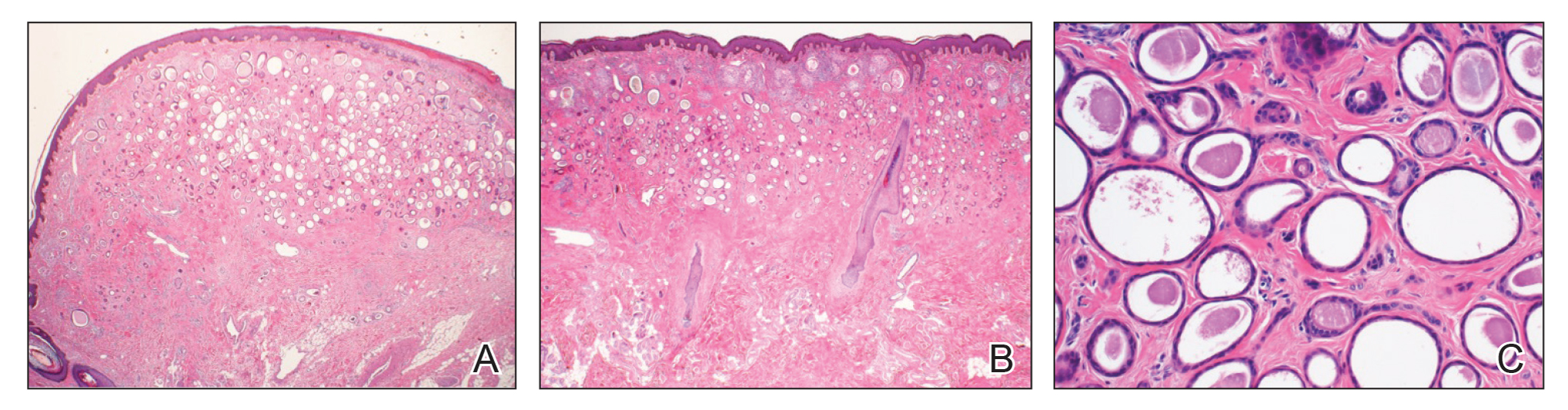
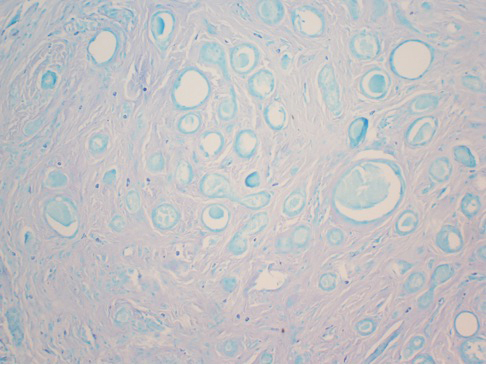
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
Practice Points
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype.
Sweet Syndrome With Marked Eosinophilic Infiltrate
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.
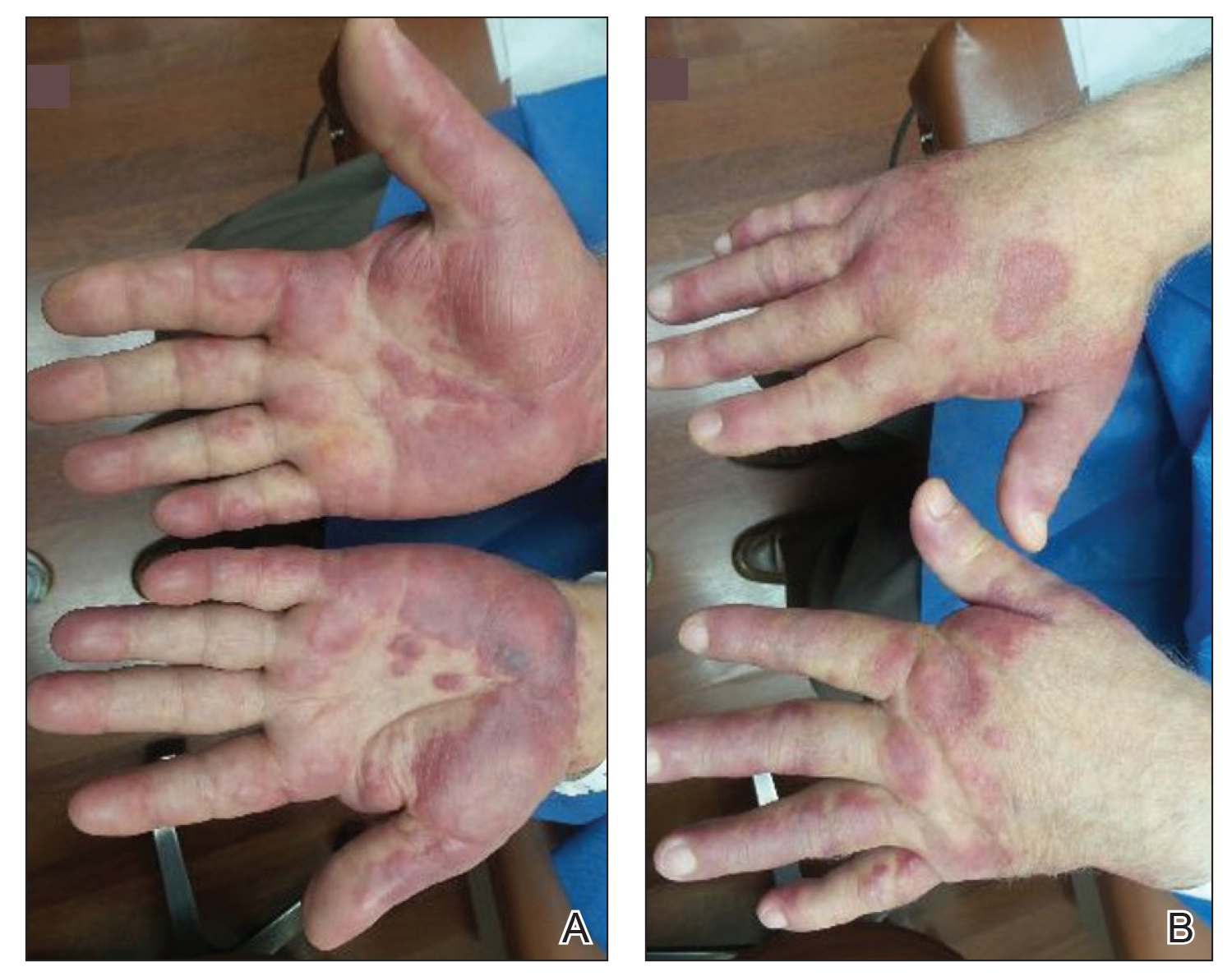
Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.
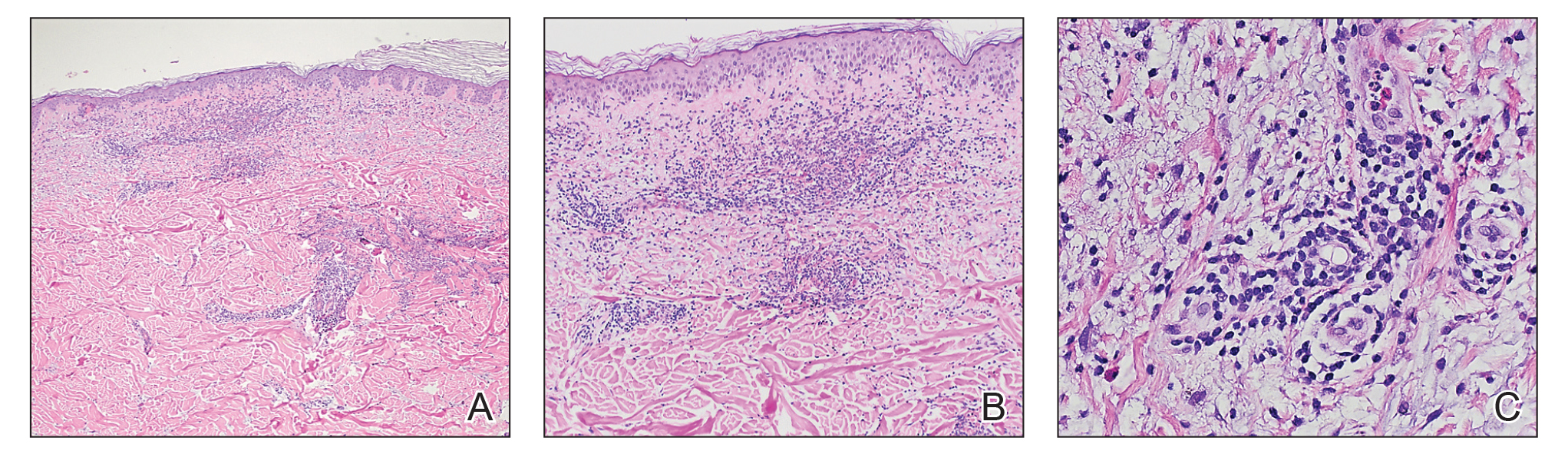
Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.

Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.

Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.

Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.

Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
Practice Points
- This report highlights a case of classical Sweet syndrome (SS) with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses.
- Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of SS.
New diagnostic CT scan model predicts pulmonary hypertension
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
FROM EUROPEAN RADIOLOGY
Inflammation, thrombosis biomarkers tied to COVID-19 deaths
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Their prospective cohort study of 1150 patients hospitalized with the disease in New York City also revealed a high proportion of racial and ethnic minorities, and confirmed high rates of critical illness and mortality.
“Of particular interest is the finding that over three quarters of critically ill patients required a ventilator and almost one third required renal dialysis support,” Max O’Donnell, MD, MPH, assistant professor of medicine and epidemiology at Columbia University in New York City, said in a press release.
O’Donnell and colleagues published the results of their study online today in The Lancet. It is the largest prospective cohort study published in the United States, they said.
“Although the clinical spectrum of disease has been characterised in reports from China and Italy, until now, detailed understanding of how the virus is affecting critically ill patients in the US has been limited to reports from a small number of cases,” said Natalie Yip, MD, assistant professor of medicine at Columbia University.
In the cohort, drawn from two NewYork-Presbyterian hospitals, the researchers focused on the 257 (22%) patients who required intensive care. When they estimated inflammation through interleukin-6 (IL-6) concentrations and thrombosis through D-dimer concentrations, they found a 10% increased risk for death with every 10% increase of IL-6 (adjusted hazard ratio [aHR], 1.11; 95% confidence interval [CI], 1.02–1.20) or D-dimer concentration (aHR, 1.10; 95% CI, 1.01–1.19).
“The association of mortality with higher concentrations of IL-6 and d-dimer is particularly relevant for two reasons,” write Giacomo Grasselli, from the Fondazione IRCCS Ca’ Granda Ospediale Maggiore Policlinico, and Alberto Zanella, from the University of Milan, Italy, in an accompanying commentary.
“First, it confirms the key pathogenic role played by the activation of systemic inflammation and endothelial-vascular damage in the development of organ dysfunction,” they write. “Second, it provides the rationale for the design of clinical trials for measuring the efficacy of treatment with immunomodulating and anticoagulant drugs.”
Seventeen percent of patients received interleukin receptor antagonists and 26% received corticosteroids, but the authors did not report any data on the effects of these treatments, or any data about anticoagulant therapies administered.
Severe disease common
The study also highlighted a high proportion of ethnic and racial minorities. Sixty-two percent of the critically ill patients were Hispanic or Latinx, 19% Black, 32% White, and 3% Asian.
Their median age was 62 years and 67% were men. Eighty-two percent had at least one chronic illness, most commonly hypertension (63%), followed by diabetes (36%). Forty-six percent were obese.
As of April 28, 2020, 101 (39%) of the critically ill patients had died following a median of 9 days (interquartile range (IQR), 5–15) in the hospital and 94 (37%) remained hospitalized. Of the 203 patients who received invasive mechanical ventilation, 84 (41%) had died.
The poor prognosis of patients requiring ventilation is consistent with data from a report on patients treated in National Health Service intensive care units in England, Wales, and Northern Ireland through May 15. Overall, 11,292 patients with COVID-19 required critical care, and 4855 needed advanced respiratory support. Approximately half of the patients receiving mechanical ventilation had died 30 days after starting critical care.
In the New York study, patients spent an average of 18 days on a ventilator (IQR, 9–28 days). This is a longer period than reported in smaller studies of cases from Washington state, but corresponds with a recent report from Italy, the researchers said.
Remarkably, O’Donnell and colleagues report that almost a third (31%) of critically ill patients developed severe kidney damage and required dialysis.
Mortality was associated with several baseline factors, including older age (aHR, 1.31 [95% CI, 1.09–1.57] per 10-year increase), chronic cardiac disease (aHR, 1.76; 95% CI, 1.08–2·86), and chronic pulmonary disease (aHR, 2.94; 95% CI, 1.48–5.84).
Authors of the New York study reported financial relationships to ICE Neurosystems, ALung Technologies, Baxter, BREETHE, Xenios, Hemovent, Gilead Sciences, Amazon, and Karyopharm Therapeutics. Grasselli reports personal fees from Biotest, Draeger, Fisher & Paykel, Maquet, Merck Sharp & Dohme, and Pfizer, all outside the area of work commented on here. Zanella has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
DOACs linked to lower fracture risk versus warfarin in AFib patients
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
FROM ANNALS OF INTERNAL MEDICINE


![“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and non-immigrant,” said Dr. Pamela Montano.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/Montano_Pamela_NY_web.jpg?itok=9aJ-niUG)



![“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and non-immigrant,” said Dr. Pamela Montano.](https://cdn.mdedge.com/files/s3fs-public/Montano_Pamela_NY_web.jpg)