User login
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Operation Quack Hack: FDA moves to stop fraudulent COVID-19 products
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.
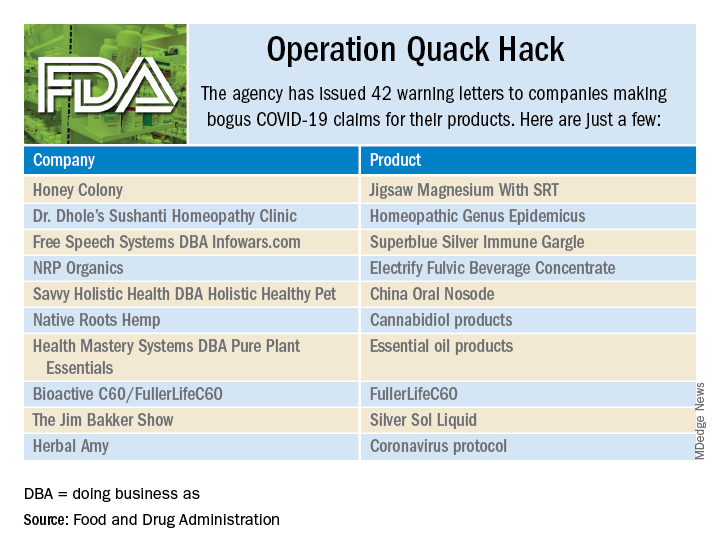
As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
COVID-19 & Mental Health
On Thursday, April 23, 2020, MDedge Psychiatry hosted a Twitter conversation at #MDedgeChats on COVID-19 and mental health.
Two psychiatrists affiliated with Johns Hopkins University, Dinah Miller, MD (@shrinkrapdinah), and Elizabeth Ryznar, MD (@RyznarMD), hosted the conversation. Throughout the conversation several themes emerged. One is that COVID-19 illustrates the connections between housing and health, and another is that stigma tied to being COVID positive is leading to suicidality, particularly in Haiti. The discussants also talked about the importance of self-care.
Questions asked during the Twitter chat:
- How are your pre-pandemic patients doing during this crisis?
- How has COVID-19 affected inpatient and outpatient care for you?
- How are our most vulnerable populations being affected by COVID-19?
- How are you doing personally and professionally as medical professionals during the pandemic?
- What psychiatric manifestations are you seeing in your patients who have had COVID-19?
The following is an edited version of the discussion. For the full experience, visit our Twitter archive, you can still join the conversation.













Some experts are predicting that the magnitude of the mental health fallout from the COVID-19 pandemic will be profound. They also have concerns that physical distancing and increased unemployment forced by the pandemic will lead to a rise in suicide risk, particularly for at-risk populations.1
For psychiatric patients, COVID-19 is expected to leave behind higher levels of anxiety, depression, and insomnia.2
And for health care workers on the front lines, the mental health toll could lead to stress, burnout, and worse.
Clinicians are also dealing with shortages of PPE and medical equipment, worrying about their safety and that of their families, while trying to save lives amid great uncertainty about SARS-CoV-2, the coronavirus that causes COVID-19.
Most recently an emergency department physician in New York who was recovering from COVID-19 herself reportedly recently ended her own life, according to her father, who is a physician.
As Dr. Sarah Candler said in a previous twitter chat with MDedge Internal Medicine, “Good doctors take care of themselves, too.” Be kind to yourself during this global crisis.
The National Suicide Prevention Lifeline is always available at 1-800-273-TALK (8255).
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis
- Podcast: Physician Suicide
1Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366(20):30171-1.
2Brain, Behav Immun. 2020 Apr 27. doi: 10.10116/j.bbi.2020.04.069).
On Thursday, April 23, 2020, MDedge Psychiatry hosted a Twitter conversation at #MDedgeChats on COVID-19 and mental health.
Two psychiatrists affiliated with Johns Hopkins University, Dinah Miller, MD (@shrinkrapdinah), and Elizabeth Ryznar, MD (@RyznarMD), hosted the conversation. Throughout the conversation several themes emerged. One is that COVID-19 illustrates the connections between housing and health, and another is that stigma tied to being COVID positive is leading to suicidality, particularly in Haiti. The discussants also talked about the importance of self-care.
Questions asked during the Twitter chat:
- How are your pre-pandemic patients doing during this crisis?
- How has COVID-19 affected inpatient and outpatient care for you?
- How are our most vulnerable populations being affected by COVID-19?
- How are you doing personally and professionally as medical professionals during the pandemic?
- What psychiatric manifestations are you seeing in your patients who have had COVID-19?
The following is an edited version of the discussion. For the full experience, visit our Twitter archive, you can still join the conversation.













Some experts are predicting that the magnitude of the mental health fallout from the COVID-19 pandemic will be profound. They also have concerns that physical distancing and increased unemployment forced by the pandemic will lead to a rise in suicide risk, particularly for at-risk populations.1
For psychiatric patients, COVID-19 is expected to leave behind higher levels of anxiety, depression, and insomnia.2
And for health care workers on the front lines, the mental health toll could lead to stress, burnout, and worse.
Clinicians are also dealing with shortages of PPE and medical equipment, worrying about their safety and that of their families, while trying to save lives amid great uncertainty about SARS-CoV-2, the coronavirus that causes COVID-19.
Most recently an emergency department physician in New York who was recovering from COVID-19 herself reportedly recently ended her own life, according to her father, who is a physician.
As Dr. Sarah Candler said in a previous twitter chat with MDedge Internal Medicine, “Good doctors take care of themselves, too.” Be kind to yourself during this global crisis.
The National Suicide Prevention Lifeline is always available at 1-800-273-TALK (8255).
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis
- Podcast: Physician Suicide
1Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366(20):30171-1.
2Brain, Behav Immun. 2020 Apr 27. doi: 10.10116/j.bbi.2020.04.069).
On Thursday, April 23, 2020, MDedge Psychiatry hosted a Twitter conversation at #MDedgeChats on COVID-19 and mental health.
Two psychiatrists affiliated with Johns Hopkins University, Dinah Miller, MD (@shrinkrapdinah), and Elizabeth Ryznar, MD (@RyznarMD), hosted the conversation. Throughout the conversation several themes emerged. One is that COVID-19 illustrates the connections between housing and health, and another is that stigma tied to being COVID positive is leading to suicidality, particularly in Haiti. The discussants also talked about the importance of self-care.
Questions asked during the Twitter chat:
- How are your pre-pandemic patients doing during this crisis?
- How has COVID-19 affected inpatient and outpatient care for you?
- How are our most vulnerable populations being affected by COVID-19?
- How are you doing personally and professionally as medical professionals during the pandemic?
- What psychiatric manifestations are you seeing in your patients who have had COVID-19?
The following is an edited version of the discussion. For the full experience, visit our Twitter archive, you can still join the conversation.













Some experts are predicting that the magnitude of the mental health fallout from the COVID-19 pandemic will be profound. They also have concerns that physical distancing and increased unemployment forced by the pandemic will lead to a rise in suicide risk, particularly for at-risk populations.1
For psychiatric patients, COVID-19 is expected to leave behind higher levels of anxiety, depression, and insomnia.2
And for health care workers on the front lines, the mental health toll could lead to stress, burnout, and worse.
Clinicians are also dealing with shortages of PPE and medical equipment, worrying about their safety and that of their families, while trying to save lives amid great uncertainty about SARS-CoV-2, the coronavirus that causes COVID-19.
Most recently an emergency department physician in New York who was recovering from COVID-19 herself reportedly recently ended her own life, according to her father, who is a physician.
As Dr. Sarah Candler said in a previous twitter chat with MDedge Internal Medicine, “Good doctors take care of themselves, too.” Be kind to yourself during this global crisis.
The National Suicide Prevention Lifeline is always available at 1-800-273-TALK (8255).
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis
- Podcast: Physician Suicide
1Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366(20):30171-1.
2Brain, Behav Immun. 2020 Apr 27. doi: 10.10116/j.bbi.2020.04.069).
Coronary CT angiography gives superior MI risk prediction
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
FROM ACC 2020
U.S. ‘deaths of despair’ from COVID-19 could top 75,000, experts warn
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
Fewer than 20% of eligible children received the recommended two doses of flu vaccine
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
FROM JAMA PEDIATRICS
Andexanet alfa reverses factor Xa inhibitors
Background: Factor Xa inhibitors have become increasingly popular in the treatment and prevention of thrombotic events, but the lack of specific reversal agents in the event of life-threatening or uncontrolled bleeding may limit their use. Andexanet alfa is a new Food and Drug Administration–approved reversal agent which rapidly reduces anti–factor Xa activity, thereby reversing the anticoagulation effects of factor Xa inhibitors.
Study design: A prospective, open-label, single-group cohort study.
Setting: An industry-sponsored, multicenter study.
Synopsis: The study evaluated 352 adult patients who had acute major bleeding (such as intracranial hemorrhage [64%] or GI bleeding [26%] within 18 hours after administration of a factor Xa inhibitor, including apixaban, rivaroxaban, or edoxaban). Efficacy was assessed in 254 patients who met criteria for severe bleeding and elevated baseline anti–factor Xa activity. Patients were administered a bolus dose of andexanet alfa followed by a 2-hour infusion. The median anti–factor Xa activity reduced by 92% each among patients receiving apixaban or rivaroxaban. The majority (82%) of evaluable patients achieved excellent or good hemostasis at 12 hours after andexanet alfa administration, which compares favorably with the hemostatic efficacy of 72% observed with prothrombin complex concentrate used to reverse anticoagulation in patients treated with vitamin K antagonists. Of patients in the study, 10% experienced a thrombotic event during the 30-day follow-up period, and 14% died.
Limitations of the study include lack of a control group and absence of a significant relationship between a reduction in anti–factor Xa activity and hemostasis. The sponsor is planning to conduct a randomized trial with FDA guidance in the near future.
Bottom line: Andexanet alfa is an FDA-approved agent and appears effective in achieving hemostasis in patients with a factor Xa inhibitor–associated major acute bleeding.
Citation: Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Eng J Med. 2019 Feb 7. doi: 10.1056/NEJMoa1814051.
Dr. Vedamurthy is a hospitalist at Massachusetts General Hospital.
Background: Factor Xa inhibitors have become increasingly popular in the treatment and prevention of thrombotic events, but the lack of specific reversal agents in the event of life-threatening or uncontrolled bleeding may limit their use. Andexanet alfa is a new Food and Drug Administration–approved reversal agent which rapidly reduces anti–factor Xa activity, thereby reversing the anticoagulation effects of factor Xa inhibitors.
Study design: A prospective, open-label, single-group cohort study.
Setting: An industry-sponsored, multicenter study.
Synopsis: The study evaluated 352 adult patients who had acute major bleeding (such as intracranial hemorrhage [64%] or GI bleeding [26%] within 18 hours after administration of a factor Xa inhibitor, including apixaban, rivaroxaban, or edoxaban). Efficacy was assessed in 254 patients who met criteria for severe bleeding and elevated baseline anti–factor Xa activity. Patients were administered a bolus dose of andexanet alfa followed by a 2-hour infusion. The median anti–factor Xa activity reduced by 92% each among patients receiving apixaban or rivaroxaban. The majority (82%) of evaluable patients achieved excellent or good hemostasis at 12 hours after andexanet alfa administration, which compares favorably with the hemostatic efficacy of 72% observed with prothrombin complex concentrate used to reverse anticoagulation in patients treated with vitamin K antagonists. Of patients in the study, 10% experienced a thrombotic event during the 30-day follow-up period, and 14% died.
Limitations of the study include lack of a control group and absence of a significant relationship between a reduction in anti–factor Xa activity and hemostasis. The sponsor is planning to conduct a randomized trial with FDA guidance in the near future.
Bottom line: Andexanet alfa is an FDA-approved agent and appears effective in achieving hemostasis in patients with a factor Xa inhibitor–associated major acute bleeding.
Citation: Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Eng J Med. 2019 Feb 7. doi: 10.1056/NEJMoa1814051.
Dr. Vedamurthy is a hospitalist at Massachusetts General Hospital.
Background: Factor Xa inhibitors have become increasingly popular in the treatment and prevention of thrombotic events, but the lack of specific reversal agents in the event of life-threatening or uncontrolled bleeding may limit their use. Andexanet alfa is a new Food and Drug Administration–approved reversal agent which rapidly reduces anti–factor Xa activity, thereby reversing the anticoagulation effects of factor Xa inhibitors.
Study design: A prospective, open-label, single-group cohort study.
Setting: An industry-sponsored, multicenter study.
Synopsis: The study evaluated 352 adult patients who had acute major bleeding (such as intracranial hemorrhage [64%] or GI bleeding [26%] within 18 hours after administration of a factor Xa inhibitor, including apixaban, rivaroxaban, or edoxaban). Efficacy was assessed in 254 patients who met criteria for severe bleeding and elevated baseline anti–factor Xa activity. Patients were administered a bolus dose of andexanet alfa followed by a 2-hour infusion. The median anti–factor Xa activity reduced by 92% each among patients receiving apixaban or rivaroxaban. The majority (82%) of evaluable patients achieved excellent or good hemostasis at 12 hours after andexanet alfa administration, which compares favorably with the hemostatic efficacy of 72% observed with prothrombin complex concentrate used to reverse anticoagulation in patients treated with vitamin K antagonists. Of patients in the study, 10% experienced a thrombotic event during the 30-day follow-up period, and 14% died.
Limitations of the study include lack of a control group and absence of a significant relationship between a reduction in anti–factor Xa activity and hemostasis. The sponsor is planning to conduct a randomized trial with FDA guidance in the near future.
Bottom line: Andexanet alfa is an FDA-approved agent and appears effective in achieving hemostasis in patients with a factor Xa inhibitor–associated major acute bleeding.
Citation: Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Eng J Med. 2019 Feb 7. doi: 10.1056/NEJMoa1814051.
Dr. Vedamurthy is a hospitalist at Massachusetts General Hospital.
Neoadjuvant nivolumab ‘promising’ in Merkel cell carcinoma
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Postpropofol driving poses low risk to endoscopy patients
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
FROM DDW 2020
Key clinical point: Patients who received propofol prior to endoscopic procedures showed no significant difference in driving ability from before the procedure to postsedation recovery.
Major finding: A simulated measure of driving ability showed similar competence before and after endoscopic procedures (number of times over the speed limit, 3.2 and 3.4, respectively).
Study details: The data come from a prospective, open-label study of 41 adults who underwent endoscopic procedures at a single center.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Lal P et al. DDW 2020, Abstract 295.
UNTOUCHED: Inappropriate shocks cut by subcutaneous ICD improvements
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
FROM HEART RHYTHM 2020










